Abstract
Trans-1-amino-3-18F-fluorocyclobutanecarboxylic-acid (anti-[18F]-FACBC) has been approved for the detection of prostate cancer (PCa) in patients with elevated prostate-specific-antigen following prior treatment. This review and meta-analysis aimed to investigate the diagnostic performance of 18F-FACBC positron emission tomography/computed-tomography (PET/CT) in the detection of primary/recurrent PCa. A bibliographic search was performed including several databases, using the following terms: “FACBC”/“fluciclovine” AND “prostate cancer”/“prostate” AND “PET”/“Positron Emission Tomography”. Fifteen and 9 studies were included in the systematic reviews and meta-analysis, respectively. At patient-based analysis, the pooled sensitivity and specificity of 18F-FACBC-PET/CT for the assessment of PCa were 86.3% and 75.9%, respectively. The pooled diagnostic odds-ratio value was 16.453, with heterogeneity of 30%. At the regional-based-analysis, the pooled sensitivity of 18F-FACBC-PET/CT for the evaluation of primary/recurrent disease in the prostatic bed was higher than in the extra-prostatic regions (90.4% vs. 76.5%, respectively); conversely, the pooled specificity was higher for the evaluation of extra-prostatic region than the prostatic bed (89% vs. 45%, respectively). 18F-FACBC-PET/CT seems to be promising in recurrent PCa, particularly for the evaluation of the prostatic bed. Additional studies to evaluate its utility in clinical routine are mandatory.
Keywords: prostate cancer, 18F-FACBC, PET/CT, recurrence, meta-analysis, review
1. Introduction
Prostate cancer (PCa) is the most frequently detected type of cancer in men and constitutes a major healthcare problem in developed countries [1], remaining the second most common cause of cancer-related death in the Western world [2].
Following initial diagnosis, the majority of men receive several treatments, such as usually a radical prostatectomy ± lymphadenectomy or radiation/brachytherapy in case of localized disease, and systemic therapy in case of widespread disease. Relapse remains common despite advances in primary treatment and improved overall survival (OS) with a biochemical recurrence developing in 20% to 40% of patients [3,4,5,6].
The management of primary and recurrent PCa patients has been completely changed after the inclusion of new imaging modalities, such as magnetic resonance imaging (MRI) and positron emission tomography (PET). MRI is a well-documented method to evaluate the extension of the primary tumor and to detect and localize recurrent cancer within the prostate [7,8,9]. However, routine multiparametric (mp) MRI is still limited by its poor specificity to differentiate significantly from indolent PCa [10].
In the last 10 years, PET/computed tomography (PET/CT) has gained an important role in the evaluation of patients with PCa. Radiolabeled choline PET/CT has demonstrated the ability to detect the presence of early recurrence of disease when conventional imaging resulted negative [11]. Furthermore, the recent introduction of radiolabeled prostate specific membrane antigen (PSMA), like 68Ga-PSMA and 18F-PSMA, has significantly improved the detection rate, also in case of early recurrence of disease (such as a prostate-specific antigen (PSA) <0.5 ng/mL) [12].
Trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid (anti-[18F]-FACBC) is an amino acid PET tracer that has shown to be promising for visualizing PCa. This tracer was developed for L-amino acid transport evaluation; it demonstrated favorable dosimetry with the liver being the critical organ [13]. Its safety, tracer stability, and uptake kinetics in patients have been reported in a phase I trial [14]. Nowadays, 18F-FACBC is approved by the Food and Drug Administration (FDA) and the European Commission (EC) to detect PCa in patients with elevated PSA following prior treatment. Approval was based on encouraging diagnostic performance and histologically confirmed data on patients with biochemical recurrence [15]. Recently it was included in the National Comprehensive Cancer National (NCCN) guidelines for the management of recurrent PCa patients.
Until now, few pooled data have been published about the role of 18F -FACBC PET/CT in patients with PCa. Ren et al. [16] collected data from six studies, published between 2011 and 2014 and including 251 patients that concluded for a good sensitivity of 18F -FACBC PET/CT for the detection of PCa recurrence. In 2015, Yu et al. [17] published a critical analysis of the available tracers for PET/CT in PCa, collecting data for 18F -FACBC from five studies (n = 84 subjects), showing a limited detection rate of this imaging technique for the recurrence of post-prostatectomy PCa (detection rate = 40%). However, in May 2016, 18F -FACBC PET/CT received the approval by the Food and Drug administration for use in patients with suspected recurrent PCa [18]. In the last years, many prospective and retrospective experiences have been performed, and therefore, a new update of the recent findings seems necessary, not only in the restaging but also in the initial staging of disease.
Therefore, the present review and meta-analysis aimed to investigate the diagnostic performance of 18F -FACBC in the detection of primary and recurrent PCa patients.
2. Materials and Methods
2.1. Search Strategy and Study Selection
A bibliographic search until 30 April 2019 was performed by including the following databases: Pubmed, Scopus, Embase, Web of Science, Cochrane library, and Google Scholar. The terms used were “FACBC” or “fluciclovine” AND “prostate cancer” or “prostate” AND “PET” or “Positron Emission Tomography”. The search was carried out with and without the addition of filters (such as English language only; type of article: original article, research article; subjects: humans only). Three reviewers (Domenico Albano, Viviana Frantelizzi and Matteo Baucknhet) performed the literature search, and two independent reviewers (Priscilla Guglielmo and Lorenzo Fantechi) selected the study inclusion and data extraction in duplicate. Any discrepancies were resolved by a consensus, when necessary. All recognized records were combined, and the full texts were retrieved. Full texts were further evaluated by four reviewers (Giovanni Argiroffi, Riccardo Laudicella, Pierpaolo Alongi and Laura Evangelista). Moreover, a search across the databases was completed by another reviewer (Anna Giulia Nappi) checking the references of the studies included to further improve the eligibility.
This systematic review was carried out using established methods [19], and the presentation of results was made according to the PRISMA guidelines [20]. All studies that fulfilled the inclusion criteria were considered eligible for the systematic review and meta-analysis: (a) a sample size more than 10 patients; (b) the index test: 18F-FACBC PET/CT; (d) the outcomes, such as detection rate (DR), true positive (TP), true negative (TN), false positive (FP), and false negative (FN), which allowed us to construct 2 × 2 contingency tables. Moreover, in the case of studies that included the same population, the report with the highest number of enrolled patients was considered for the meta-analysis. Conversely, reviews, clinical reports, meeting abstracts, and editor comments were excluded. The quality assessment included both the risk of bias assessment and applicability concerns by using QUADAS-2 evaluation [21].
2.2. Data Extraction
For each included study, general information was retrieved, such as basic data (authors, journal, year of publication, country and study design), patient characteristics (number of patients, mean or median age, Gleason score), type of treatment, mean or median PSA value at PET time, and PSA kinetic values.
2.3. Statistical Method
StatsDirect and Meta-Analyst (version Beta 3.13; [22]) were used to carry out the analysis. Heterogeneity was tested using the χ2 and the I2 tests. The χ2 -test provided an estimate of the between-study variance and the I2 test measured the proportion of inconsistency in individual studies that cannot be explained by chance. According to Higgins et al. [19], the values of 25%, 50%, and 75% for heterogeneity (I2) were considered low, moderate, and high, respectively. In accordance with the recommendation of the Cochrane Oral Health Group, the meta-analysis was carried out with the random-effect model as the number of studies was equal or superior to 4.
Data on diagnostic performance such as pooled sensitivity, pooled specificity, positive and negative likelihood ratio (LR+ and LR−), diagnostic odds ratio (DOR) with 95% confidence intervals (CIs) for the evaluation of primary and recurrent PCa, were assessed. A patient-based and a region-based meta-analyses were carried out in accordance with available data. Publication bias was assessed using a funnel plot. A symmetrical plot was indicative of the absence of publication bias.
3. Results
3.1. Search Results
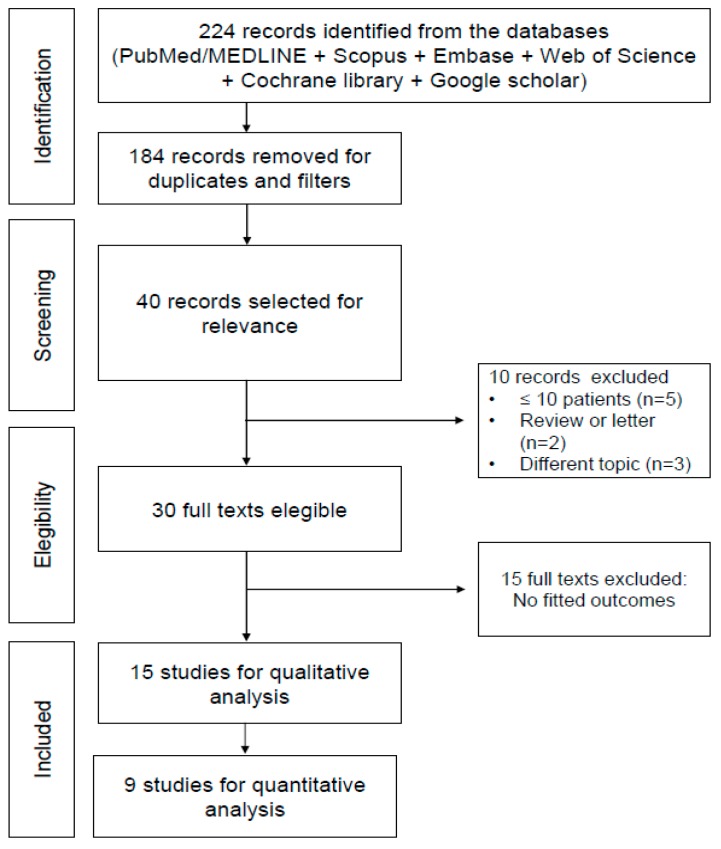
The literature search revealed 40 articles published from 1 January 2007 to 30 April 2019. Reviewing titles and abstracts, we excluded 24 articles because these did not fit with the field of interest or because these papers were letters, editorials, reviews or due to the patient data overlap. Therefore, 15 studies were selected and included in the systematic reviews and 9 articles were considered for the meta-analysis (Figure 1). Also the papers by the developers of 18F-FACBC were considered [23,24].
Figure 1.
PRISMA flow-chart.
3.2. Study Characteristics
The basics characteristics of the included studies are reported in Table 1 [15,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. The number of enrolled patients ranged from 15 to 596, and a total of 1226 PCa patients were included. The selected articles were published by researchers from Europe, USA, and Japan. Four studies were retrospective whereas 11 studies were prospective. 18F-FACBC PET/CT was performed in the preoperative setting in 6 studies (n = 178 patients), for the detection of recurrence in patients with biochemical relapse after primary treatments in 8 studies (n = 1033 patients) and in both settings in 1 study (n = 15 patients). In the restaging, the mean value of PSA ranged between 0.44 and 17.94 ng/mL.
Table 1.
Characteristics of selected studies.
| Study Characteristics | Patient Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Journal | Country | Study Design | Setting | N. pts | Mean Age (Range) | Gleason Score (n) | Type of Treatment (n) | Mean PSA (Range) | Mean PSA Doubling Time (Range) |
| Schuster et al. [23] | 2007 | JNM | USA | Prospective | Staging (n = 9) Restaging (n = 6) | 15 | 62y (45–76) | 6 (2) 7 (2) 8 (2) 9 (2) 10 (1) NA (6) |
SP (1), BCT + RT + CTR (1), BCT (2), BCT + RT (1), RP + RT (1), naive (9) |
15 ng/mL (1.9–71) | NA |
| Schuster et al. [24] | 2011 | Radiology | USA | Prospective | Restaging | 50 | 68.3y (50–90) | NA | RP (13), CTR, HFUS, EBRT, and/or BCT (37) | 6.62 ng/mL (0.11–44.74) |
NA |
| Turkbey et al. [25] | 2014 | Radiology | USA | Prospective | Staging | 21 | 62y (44–73) | 6 (3) 7 (12) 8 (5) 9 (1) |
RARP + LND (21) | 13.5 ng/mL (3.55–37.3) |
NA |
| Kairemo et al. [26] | 2014 | BioMed Research Intern | Finland | Retrospective | Restaging | 26 * | 68.1y (56–77) | 5 (3) 6 (7) 7 (7) 8 (3) 9 (5) |
RP + RT (12), RT (13), ADT (20), BT (11), CHT(5), 153Sm-EDTMP (7), Denosumab (1) |
7.9 ng/mL (0.11–69) |
positive FACBC 3.2mo (0.3–6) negative FACBC 31.2mo (8–84) |
| Nanni et al. [27] | 2014 | ClinGenitourin Cancer | Italy | Prospective | Restaging | 28 | 67y (55–78) |
6 (1) 7 (16) 8 (6) 9 (4) 10 (1) |
RP (28), RT (11), ADT (14) |
2.9 ng/mL (0.2–14.6) |
NA |
| Nanni et al. [28] | 2015 | ClinNucl Med | Italy | Prospective | Restaging | 50 | 67y (55–78) | ≤6 (4) 7 (31) 8–10 (15) |
RP (50), RT (23), ADT (21) |
3.2 ng/mL (0.24–15.6) |
NA |
| Odewole et al. [29] | 2016 | EJNMMI | USA | Retrospective | Staging | 53 | 67.57y (49–90) | 7 (49) NA (4) |
RP (7), EBRT (5), BCT (6), CTR (4), HT (1), 2 or more treatment (30) |
7.2 ng/mL (0.11–44.8) |
18.6mo ## (−31.6–357.8) |
| Bach-Gansmo et al. [15] | 2017 | J Urol | Norway Italy USA | Retrospective | Restaging | 596 | 67y (42–90) | 6.7 (110) § 7.4 (355) §§ |
RP (130), RP + other but no RT (62), RT (76), RT + other (266), other but no RT/RP (41) |
5.43 ng/mL (0.05–82.0) |
NA |
| Akin-Akintayo et al. [30] | 2017 | ClinNucl Med | USA | Prospective | Restaging | 42 | 62y (42–75) |
7 (42) # | RP (42) | 2.1 ng/mL (0.07–11.15) |
NA |
| Selnaes et al. [31] | 2018 | EurRadiol | Norway | Prospective | Staging | 26 | 66.2y (55–71.9) | 7 (11) 8 (8) 9 (7) |
RARP + LND (26) | 14.6 ng/mL (3.7–56.9) |
NA |
| Jambor et al. [32] | 2018 | EJNMMI | Finland | Prospective | Staging | 26 | 65y ** (49–76) |
6 (1) 7 (17) 8 (2) 9 (6) |
RARP + LND (26) | 12 ng/mL (4.1–35) |
NA |
| Akin-Akintayo et al. [33] | 2018 | Eur J Radiol | USA | Prospective | Staging | 24 | 70.8y (60–83) |
7 (24) # | BCT (3), RT (3), PT (1), CTR (1), CTR + HT (1), BCT + other treatment but no RP (13), other treatment but no BCT (2) |
8.5 ng/mL (2.2–29.3) |
NA |
| Andriole et al. [34] | 2019 | J Urol | USA | Prospective | Restaging | 213 | 66.4y (46–90) |
≤6 (27) 7 (134) ≥8 (50) NA (2) |
RP (121), RP + RT (43), EBRT (21), BCT (1), EBRT + BCT (2), EBRT + ADT (17), EBRT + CTR (2), CTR (1), BCT + ADT (1), EBRT + BCT + ADT (2), HIFU (1), High-dose BCT (1) |
4.24 ng/mL (0.2–93.5) |
NA |
| England et al. [35] | 2019 | Clin Nucl Med | USA | Retrospective | Restaging | 28 | 67.1y (53–77) |
7 (19) 8 (3) 9 (6) |
Primary treatment RP (22), RP+ EBRT (3), RP + EBRT + ADT (1), EBRT + ADT (2) Salvage therapy RT (6), ADT (1), RT + ADT (1), LND (1) |
0.44 ng/mL (0.1–1.0) |
6.38mo (1.6–16.8) |
| Suzuki et al. [36] | 2019 | Japanese J Clin Oncol | Japan | Prospective | Staging | 28 | 67.9 (57–77) | <6 (1) 7 (12) 8 (8) 9 (8) |
RARP + LND (28) | 17.94 ng/mL (1.20–82.38) |
NA |
RP = radical prostatectomy; RS = radical surgery; EBRT = external beam radiotherapy; RT = radiotherapy; ADT = androgen deprivation therapy; LND = lymph nodal dissection; HT = hormone therapy; RARP = robot assisted radical prostatectomy; BT = bisphosphonate therapy; CHT = chemotherapy; BCT = brachitherapy; CTR = criotherapy; HFUS = high-frequency ultrasound; SP = subtotal prostatectomy; PT = proton therapy; NA = not-available. * 1/26 patient was affected by meningioma, considered as negative; ** Median value of the initial 32 patients; § Median Gleason-score value in Recurrent Prostate Cancer; §§ Median Gleason-score value in Primary Standard of Truth; # Median Gleason-score value; ## Only for 49/53 patients.
The mean and median age of the patients ranged from 42 to 90 years. The Gleason score (GS) was ≤6 in 49 (4%) patients, 7 in 376 (30.6%) patients, ≥8 in 142 (11.6%) patients, not available in the remaining 659 (53.8%). No significant adverse effects after the administration of 18F-FACBC were reported.
3.3. Methodological Quality
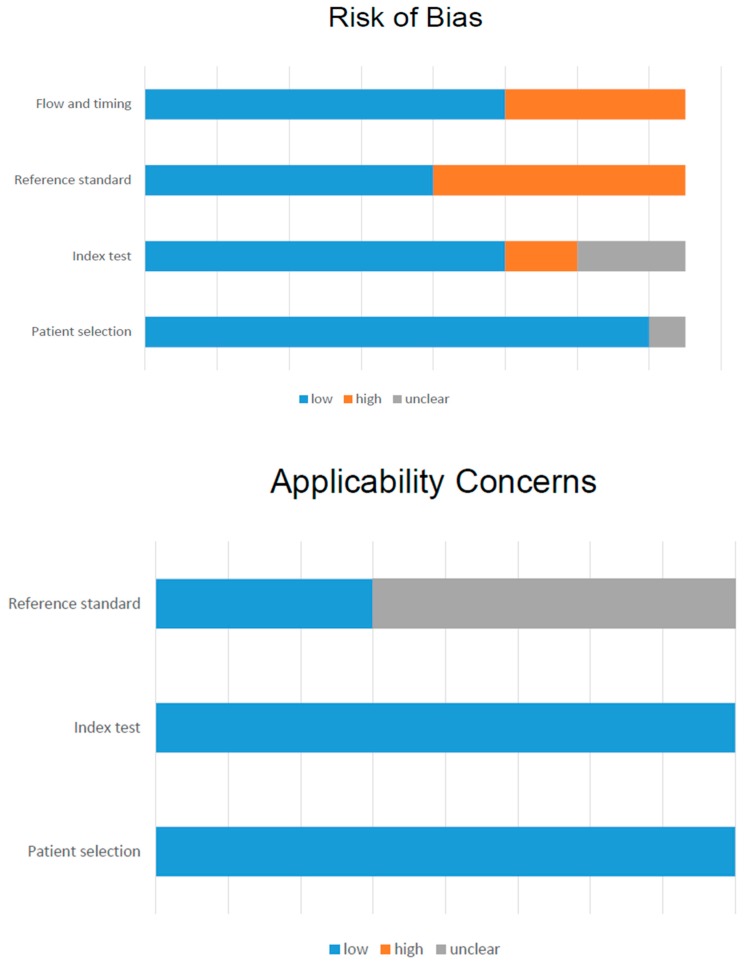
All 15 studies were evaluated qualitatively using the QUADAS-2 tool (Table S1; Figure 2). The risk of bias was unclear for patient selection in 1 study, which did not provide information regarding consecutive enrollment [15]. For the index test and reference standard, the risk of bias was low in 6 studies [24,29,31,32,33,36]. For flow and timing, many studies reported time intervals between PET/CT examinations and pathological or other imaging confirmations. The applicability of the included studies was adequate in the majority of reports, being unclear only in 1 study for the reference standard [30].
Figure 2.
QUADAS 2 score of all included studies.
3.4. Qualitative Results
PET/CT was employed in 14/15 studies, without CT contrast media injection, whereas PET/MRI was used in 2 studies [31,32]. The injected radiopharmaceutical activity and the time between radiotracer injection and image acquisition were similar across all studies.
Analysis of PET images was mostly performed using visual analysis; however, additional semi-quantitative criteria, i.e., maximal standardized uptake values (SUVmax), was performed in some reports [23,26,27,28]. 18F-FACBC PET/CT or PET/MRI identified the presence of PCa in prostatic and extra-prostatic bed, such as in the regional, distant lymph nodes and bone. The DR was available in 9/15 studies. It ranged between 36% and 90%, being different in accordance with PSA serum levels (Table 2). Andriole et al. [34] demonstrated that DR was broadly proportional to pre-scan PSA: lesions were detected in 79% patients with PSA ≥ 1.0 ng/mL and in 84% with PSA ≥ 2.0 ng/mL. On the other side, some authors found that there was no statistically significant difference in the PSA values and PSA doubling-time (PSAdt) between patients with positive and negative findings [26,35]. England et al. [35] reported that the DR was significantly higher for patients with GS > 7 than those with a score equal to 7.
Table 2.
The selection of the studies.
| Author, (Ref) | Year | Journal | Country | N pts | Outcome | DR | TP | TN | FP | FN |
|---|---|---|---|---|---|---|---|---|---|---|
| Schuster et al. [23] | 2007 | JNM | USA | 9 | Accuracy LN (patient-based) | NA | 2 | 5 | 0 | 2 |
| Schuster et al. [24] | 2011 | Radiology | USA | 50 | Accuracy (PB) FACBC (region-based) | NA | 32 | 8 | 4 | 4 |
| Acc (extra-p) FACBC (region-based) | 10 | 7 | 0 | 0 | ||||||
| Acc (PB) Capromab (region-based) | 25 | 7 | 5 | 11 | ||||||
| Ac (extra-p) Capromab (region-based) | 1 | 7 | 0 | 9 | ||||||
| Turkbey et al. [25] | 2014 | Radiology | USA | 21 | DR for primary | 19/21 (90%) | ||||
| Lesion-based | 33 | 0 | 38 | 15 | ||||||
| Accuracy MRI (les-based) | 34 | 0 | 21 | 14 | ||||||
| Kairemo et al. [26] | 2014 | BioMed Research Intern | Finland | 26 ** | DR | 17/26 (65%) | ||||
| Patient-based | 11 | 12 | 3 | 0 | ||||||
| Nanni et al. [27] | 2014 | ClinGenitourin Cancer | Italy | 28 | DR (comparison with Choline) | 10/28 (36%) | NA | NA | NA | NA |
| Nanni et al. [28] | 2015 | ClinNucl Med | Italy | 50 | DR (comparison with Choline) | 17/50 (34%) | NA | NA | NA | NA |
| Odewole et al. [29] | 2016 | EJNMMI | USA | 53 | DR (all PSA levels and clinical data) | 41/53 (77.4%) | ||||
| Accuracy (PB) FACBC | 31 | 9 | 7 | 4 | ||||||
| Accuracy (PB) CT | 4 | 14 | 2 | 31 | ||||||
| Accuracy (extra-pr) FACBC | 12 | 15 | 0 | 15 | ||||||
| Accuracy (extra-pr) CT | 3 | 15 | 0 | 23 | ||||||
| Bach-Gasmo et al. [15] | 2017 | J Urol | Norway Italy USA |
596 | DR | 403/595 (67.7%) | ||||
| Lesion-based | 153 | 216 | 93 | 91 | ||||||
| Region-based (PB) | 74 | 14 | 20 | 10 | ||||||
| Region-based (Extra-prost) | 36 | 1 | 3 | 4 | ||||||
| Patient-based | 98 | 14 | 21 | 10 | ||||||
| Akin-Akintayo et al. [30] | 2017 | ClinNucl Med | USA | 42 | DR (change in radiotherapy strategy) | 34/42 (81%) | NA | NA | NA | NA |
| Selnaes et al. [31] | 2018 | EurRadiol | Norway | 26 | Accuracy for LN | NA | ||||
| Patient-based | NA | 4 | 16 | 0 | 6 | |||||
| Region-based | NA | 6 | 185 | 0 | 14 | |||||
| Jambor et al. [32] | 2018 | EJNMMI | Finland | 26 | Accuracy LN | NA | ||||
| Patient-based | 7 | 19 | 0 | 0 | ||||||
| Region-based | NA | NA | NA | NA | ||||||
| Akin-Akintayo et al. [33] | 2018 | Eur J Radiol | USA | 24 | Accuracy (PB) FACBC * | NA | 13 | 1 | 8 | 0 |
| Accuracy (PB) MRI * | 5 | 5 | 4 | 8 | ||||||
| Accuracy (extra-p) FACBC | 7 | 9 | 1 | 1 | ||||||
| Accuracy (extra-p) MRI * | 4 | 7 | 3 | 4 | ||||||
| Andriole et al. [34] | 2019 | J Urol | USA | 213 | DR (also for PSA level) | 122/213 (57%) | NA | NA | NA | NA |
| England et al. [35] | 2019 | ClinNucl Med | USA | 28 | DR (for site and clinical data) | 13/28 (46%) | NA | NA | NA | NA |
| Suzuki et al. [36] | 2019 | Japanese J ClinOncol | Japan | 28 | Accuracy LN | NA | ||||
| Patient-based | 4 | 19 | 3 | 2 | ||||||
| Lesion-based | 4 | 28 | 5 | 3 |
DR = detection rate; NA = not available; LN = lymph node; PB = prostatic bed; * M1 reader; ** 1/26 patient affected by meningioma was considered as negative.
The performance of 18F-FACBC PET/CT was different based on the phase and the site of PCa (Table 3). In particular, in the initial staging, the sensitivity for the primary and lymph nodes metastasis was 71% [32] and 67% [36], respectively. In the restaging setting, the sensitivity for the prostatic bed and extra-prostatic bed recurrence was 89% [24] and 90% [15], respectively. Interestingly, in the study by Turkbey et al. [25], 18F-FACBC uptake in tumors was similar to that in benign prostatic hyperplasia (BPH). However, Jambor et al. [32] reported that SUVmax in the primary tumor was statistically significantly higher for patients with GS > 7 than GS = 6 or BPH, thus underlying the importance of the patient selection.
Table 3.
Accuracies based on the study setting and the type of analysis.
| Type of Analysis | Study Name (Year), Ref | Setting (Site) | TP | FN | TN | FP | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|
| Patient-based analysis | Suzuki et al. (2019), [36] | Staging (LN) | 4 | 2 | 19 | 3 | 66.6% | 86.3% |
| Selnaes et al. (2018), [31] | Staging (LN) | 4 | 6 | 16 | 0 | 45% | 80.8% | |
| Jambor et al. (2018), [32] | Staging (primary) | 7 | 0 | 19 | 0 | 70.6% | 82.8% | |
| Bach-Gasmo et al. (2017), [15] | Restaging (all) | 98 | 10 | 14 | 21 | 90.7% | 40% | |
| Kairemo et al. (2014), [26] | Restaging (all) | 11 | 0 | 12 | 3 | 76.2% | 68% | |
| Schuster et al. (2007), [23] | Staging/restaging (all) | 2 | 2 | 5 | 0 | 50% | 66.7% | |
| Region-based analysis (PB) | Schuster et al. (2011), [24] | Restaging | 32 | 4 | 8 | 4 | 88.9% | 66.7% |
| Bach-Gasmo et al. (2017), [15] | Restaging | 74 | 10 | 14 | 20 | 88.1% | 41.2% | |
| Akin-Akintayo et al. (2018), [33] | Staging | 13 | 0 | 1 | 8 | 78.3% | 31.6% | |
| Odewole et al. (2016), [29] | Staging | 31 | 4 | 9 | 7 | 88.6% | 56.3% | |
| Region-based analysis (extra-PB) | Schuster et al. (2011), [24] | Restaging | 10 | 0 | 7 | 0 | 75% | 70.6% |
| Bach-Gasmo et al. (2017), [15] | Restaging | 36 | 4 | 1 | 3 | 90% | 25% | |
| Akin-Akintayo et al. (2018), [33] | Staging | 7 | 1 | 9 | 1 | 87.5% | 90% | |
| Odewole et al. (2016), [29] | Staging | 12 | 15 | 15 | 0 | 45.9% | 80% |
LN = lymph node; TP = true positive; FN = false negative; TN = true negative; FP = false positive.
Akin-Akintayo et al. [33] compared 18F-FACBC PET/CT with mpMRI in patients with recurrent PCa showing a higher detection for the first modality (overall 94.7% vs. 36.8%); Turkbey et al. [25], instead, performed a sector-based comparison with histopathologic analysis in patients with a recent diagnosis of PCa, revealing lower sensitivity and specificity for 18F-FACBC PET/CT than for T2-weighted imaging (67% and 66% vs. 73% and 79%, respectively), but combined modalities achieved a positive predictive value of 82% for tumor localization, which was higher than that with either modality alone. Another study proved higher positivity rates with 18F -FACBC PET/CT than enhanced CT at all PSA levels, PSAdt and GS in patients with suspected recurrent PCa [29]. Furthermore, the performance of 18F-FACBC PET/CT was superior to those of 111In-capromab SPECT/CT regarding sensitivity for prostatic and extra-prostatic bed (89% vs. 69% and 100% vs. 10%, respectively) [24]. Finally, two studies directly compared 18F-FACBC with 11C-Choline PET/CT, demonstrating a greater detection rate for 18F-FACBC than 11C-Choline, either on a patient- and a lesion-based analysis and despite the PSA serum levels [27,28].
The change of management with 18F-FACBC PET/CT was reported by Andriole et al. [34], in 122 out of 213 patients (56%); the most frequent change was to withhold planned salvage or non-curative systemic therapy in favor of watchful waiting. Moreover, Akin-Akintayo et al. [30] demonstrated that 18F-FACBC PET/CT was able to modify the radiotherapy field and overall radiotherapy decision in 40.5% of patients with post-prostatectomy recurrent PCa.
3.5. Quantitative Results
In accordance with the inclusion criteria, the quantitative assessment was available in 9 studies [15,23,24,26,29,31,32,33,36] (Table 4). At patient-based analysis (n = 6 studies), the pooled sensitivity and specificity of 18F-FACBC PET/CT scan for the assessment of primary and recurrent PCa were 86.3% (95% CIs: 79.6–91.4%) and 75.9% (66.9–83.5%) with an heterogeneity of 78.6% and 88.7% (both p <0.0001), respectively. Moreover, the pooled DOR value was 16.453 (95% CI: 5.241–51.646), with heterogeneity of 30%. At the regional based-analysis (n = 4 studies), the pooled sensitivity of 18F-FACBC PET/CT for the evaluation of primary and recurrent disease in the prostatic bed was higher than that in the extra-prostatic regions (90.4% vs. 76.5%, respectively); conversely, the pooled specificity was higher for the evaluation of extra-prostatic region than the prostatic bed (89% vs. 45%, respectively). Furthermore, LR+ was high in the extra-prostatic region, while LR- was low in prostatic bed, with heterogeneity of 0%. No asymmetry in the forest plot was found; therefore, no publication bias was present across the studies.
Table 4.
The pooled diagnostic performance for 18F-FACBC (independently from the clinical setting and site).
| Meta-Analysis Results | Patient-Based Analysis (95% CI) | Region-Based Analysis (PB) (95% CI) | Region-Based Analysis (ex-PB) (95% CI) | |||
|---|---|---|---|---|---|---|
| Value | I2 | Value | I2 | Value | I2 | |
| Pooled sensitivity, % | 86.3% (79.6–91.4%) | 78.6% | 90.4% (84.8–94.4%) | 22.1% | 76.5% (66–85%) | 87.3% |
| Pooled specificity, % | 75.9% (66.9–83.5%) | 88.7% | 45.1% (33.2–57.3%) | 63.3% | 88.9% (73.9–96.9%) | 78.7% |
| DOR | 16.453 (5.241–51.646) | 29.9% | 8.026 (3.841–16.769) | 3.5% | 24.820 (3.777–163.12) | 36% |
| LR+ | 4.557 (1.685–12.324) | 72.9% | 1.598 (1.088–2.349) | 70% | 6.024 (0.568–63.943) | 85.6% |
| LR− | 0.337 (0.166–0.681) | 63.6% | 0.221 (0.130–0.375) | 0% | 0.251 (0.058–1.090) | 71.6% |
PB = prostatic bed; PPV = positive predictive value; NPV = negative predictive value; DOR = diagnostic odds ratio; LR = likelihood ratio; IC = interval of confidence; I2 = inconsistency.
4. Discussion
As previously mentioned, the meta-analysis from Ren et al. [16] reported that 18F-FACBC PET/CT had a high sensitivity (pooled sensitivity = 87%) and a moderate specificity (pooled specificity = 66%), therefore it can be considered an useful non-invasive, metabolic imaging technique for the diagnostic workup of PCa relapse. In the present meta-analysis, performed in 1226 PCa, the pooled sensitivity and specificity were 86% and 76% respectively, thus showing a slight increase for the specificity.
Furthermore, in the analysis by Yu et al. [17], FACBC showed a detection rate ranged between 22% and 61% for prostatic disease and between 19% and 33% for extra-prostatic disease, in accordance with the primary treatments (radical prostatectomy or radiotherapy). In our meta-analysis, we did not evaluate the pooled detection rate, but we calculated the pooled sensitivity and specificity. As illustrated in Table 4, the sensitivity of 18F-FACBC was equal to 90% for the identification of disease in the prostatic bed and 77% for extra-prostatic organs.
However, in the last years, PSMA-PET has rapidly been introduced in clinical practice for the management of patients with recurrent PCa, particularly in case of low PSA levels [37]. Already, the study by Yu C-Y et al. [17] reported that 18F-FACBC, Choline and Acetate-PET have similar detection rate for overall site of disease after radical prostatectomy or radiotherapy (ranged between 40% and 81%), but PSMA was able to reach a detection rate ranged between 82% and 96% in the same setting.
Two recent papers about a head-to-head comparison between 18F-FACBC and 68Ga-PSMA PET/CT have been published. The data are controversial. In the study by Pernthaler et al. [38] involving 58 patients with recurrent PCa with a PSA level ranged between 0.2 and 230 ng/mL, 18F-FACBC detected more accurately the presence of a local recurrence than 68Ga-PSMA, due to its favorable biodistribution. Furthermore, the authors found that 18F-FACBC is almost equivalent to 68Ga-PSMA-11 in detecting distant metastases of PCa recurrence. Conversely, in the study by Calais et al. [39] enrolling 50 patients with recurrent PCa, the detection rate of PSMA-PET was significantly higher than 18F-FACBC (56% vs. 26%, respectively) in case of a PSA level <1 ng/mL. However, the authors found that the detection rate for the local recurrence was higher for 18F-FACBC than 68Ga-PSMA PET/CT (38% vs. 14%, respectively). The missing data about the diagnostic performance, in terms of sensitivity and specificity in both the above-mentioned papers, represent a great limitation for the final conclusion on “the best radiopharmaceutical agent”. A recent paper by Lawhn-Heath et al. [40] reported that the sensitivity and specificity of 68Ga-PSMA-11 for recurrent PCa are equal to 89.1% and 31.2%, thus registering a high rate of false positivity.
From the present systematic review and meta-analysis arise some considerations:
18F-FACBC is more performant than 111In-capromab SPECT/CT and 11C-Choline for the detection of PCa recurrence. Therefore, if available it should be preferred in patients with a PSA increase, after primary treatments. However, data about the comparison with 18F-Choline PET/CT are missing and should be explored, also considering the radioisotope properties.
The combination of 18F-FACBC PET/CT with mpMRI (or with a PET/MRI) seems useful for the detection of primary PCa, and therefore, it would be suggested in case of undetectable tumors in patients with a negative biopsy but a persistent PSA level increase. However, the interpretation of this sophisticated imaging required a great experience and a significant learning curve.
The sensitivity for the evaluation of lymph node metastasis in the initial staging of disease is moderate (45%–66%; [31,36]), like for the other radiopharmaceuticals (radiolabeled PSMA and Choline; [41,42]). Probably the recent introduction of new imaging modalities, such as digital PET/CT or PET/MRI that has a higher spatial resolution, would improve the pathological lymph node detection.
The pooled sensitivity for the identification of recurrence in prostate bed is high, being >90% with a limited pooled specificity (about 45%), probably due to the FP findings in case of inflamed cells, as reported by Oka et al. [43]. However, the absent uptake of radiopharmaceutical in the bladder represents a great advantage for the identification of peri-anastomotic PCa recurrence. Further data about the complementary role of 18F-FACBC and MRI are required for the assessment of prostatic bed recurrence, at different PSA levels.
The recurrence in the extra-prostatic site may be assessed by 18F-FACBC PET/CT with a moderate sensitivity and specificity, independently from the PSA levels. However, the correlation with PSA kinetics is warranted in a selected large cohort of patients, thus testing the final impact on the patient management.
Despite some articles have defined a potential impact of 18F-FACBC PET/CT on therapeutic management, there is still a lack information with regard to its role in radiotherapy planning and other adapted therapy.
5. Future Researches
More data about the correlation between the detection rate of 18F-FACBC PET/CT or PET/MRI and the PSA kinetics are warranted, particularly by a site and lesion-based analysis. The complementary role of 18F-FACBC PET/CT and mpMRI for the evaluation of the prostatic bed should be largely explored. A head-to-head comparison with 18F-Choline would be used in order to definitely assess its advantages in clinical routine. Data about the utility of 18F-FACBC PET/CT in patients undergoing or not hormonal therapy are required. The evaluation of response to therapy (chemotherapy or new hormonal agents) by 18F-FACBC PET/CT should be assessed. Finally, additional data about the effect of 18F-FACBC PET/CT on patient management is required, by considering both PSA levels and histopathological PCa characteristics.
6. Conclusions
18F-FACBC PET/CT seems to be promising in recurrent PCa, particularly for the evaluation of the prostatic bed. However, additional studies are mandatory in order to evaluate its utility in clinical routine.
Acknowledgments
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.: all authors.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/11/9/1348/s1, Table S1: QUADAS 2 score for each selected study (green smile = low risk; red smile = high risk; yellow question mark = unclear). Figure S1: Forest-plots for the patient-based and region-based analysis. Figure S2: ROC curves for patient-based and region-based analyses.
Author Contributions
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: Priscilla Guglielmo, Lorenzo Fantechi, Giovanni Argiroffi, Riccardo Laudicella, Pierpaolo Alongi and Laura Evangelista. Drafting the work or revising it critically for important intellectual content: all authors. Final approval of the version to be published: all authors.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Kim M.M., Hoffman K.E., Levy L.B., Frank S.J., Pugh T.J., Choi S., Nguyen Q.N., McGuire S.E., Lee A.K., Kuban D.A. Improvement in prostate cancer survival over time: A 20-year analysis. Cancer J. 2012;18:1–8. doi: 10.1097/PPO.0b013e3182467419. [DOI] [PubMed] [Google Scholar]
- 4.Bruce J.Y., Lang J.M., McNeel D.G., Liu G. Current controversies in the management of biochemical failure in prostate cancer. Clin. Adv. Hematol. Oncol. 2012;10:716–722. [PubMed] [Google Scholar]
- 5.Roehl K.A., Han M., Ramos C.G., Antenor J.A.V., Catalona W.J. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3478 consecutive patients: Long-term results. J. Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 6.Simmons M.N., Stephenson A.J., Klein E.A. Natural history of biochemical recurrence after radical prostatectomy: Risk assessment for secondary therapy. Eur. Urol. 2007;51:1175–1184. doi: 10.1016/j.eururo.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed H.U., El-ShaterBosaily A., Brown L.C., Gabe R., Kaplan R., Parmar M.K., Collaco-Moraes Y., Ward K., Hindley R.G., Freeman A., et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet (Lond. Engl.) 2017;389:815–822. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 8.Panebianco V., Barchetti F., Grompone M.D., Colarieti A., Salvo V., Cardone G., Catalano C. Magnetic resonance imaging for localization of prostate cancer in the setting of biochemical recurrence. Urol. Oncol. Semin. Orig. Investig. 2016;34:303–310. doi: 10.1016/j.urolonc.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Abd-Alazeez M., Ramachandran N., Dikaios N., Ahmed H.U., Emberton M., Kirkham A., Arya M., Taylor S., Halligan S., Punwani S. Multiparametric MRI for detection of radiorecurrent prostate cancer: Added value of apparent diffusion coefficient maps and dynamic contrast-enhanced images. Prostate Cancer Prostatic Dis. 2015;18:128–136. doi: 10.1038/pcan.2014.55. [DOI] [PubMed] [Google Scholar]
- 10.Johnson D.C., Reiter R.E. Multi-parametric magnetic resonance imaging as a management decision tool. Transl. Androl. Urol. 2017;6:472–482. doi: 10.21037/tau.2017.05.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallitt K.L., Khan S.R., Dubash S., Tam H.H., Khan S., Barwick T.D. Clinical PET Imaging in Prostate Cancer. Radiographics. 2017;37:1512–1536. doi: 10.1148/rg.2017170035. [DOI] [PubMed] [Google Scholar]
- 12.Farolfi A., Ceci F., Castellucci P., Graziani T., Siepe G., Lambertini A., Schiavina R., Lodi F., Morganti A.G., Fanti S. 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:11–19. doi: 10.1007/s00259-018-4066-4. [DOI] [PubMed] [Google Scholar]
- 13.Nye J.A., Schuster D.M., Yu W., Camp V.M., Goodman M.M., Votaw J.R. Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti-18F-FACBC in humans. J. Nucl. Med. 2007;48:1017–1020. doi: 10.2967/jnumed.107.040097. [DOI] [PubMed] [Google Scholar]
- 14.Sörensen J., Owenius R., Lax M., Johansson S. Regional distribution and kinetics of [18F] fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:394–402. doi: 10.1007/s00259-012-2291-9. [DOI] [PubMed] [Google Scholar]
- 15.Bach-Gansmo T., Nanni C., Nieh P.T., Zanoni L., Bogsrud T.V., Sletten H., Korsan K.A., Kieboom J., Tade F.I., Odewole O., et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine (18F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J. Urol. 2017;197:676–683. doi: 10.1016/j.juro.2016.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren J., Yuan L., Wen G., Yang J. The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: A meta-analysis. Actaradiologica. 2016;57:487–493. doi: 10.1177/0284185115581541. [DOI] [PubMed] [Google Scholar]
- 17.Yu C.-Y., Desai B., Ji L., Groshen S., Jadvar H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: A critical analysis of literature. Am. J. Nucl. Med. Mol. Imaging. 2014;4:580. [PMC free article] [PubMed] [Google Scholar]
- 18.DA Approves 18F-Fluciclovine and 68Ga-DOTATATE Products. [(accessed on 1 September 2019)]; Available online: http://jnm.snmjournals.org/content/57/8/9N.full.pdf.
- 19.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Whiting P.F., Rutjes A.W.S., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M.G., Sterne J.A.C., Bossuyt P.M.M., QUADAS-2 Group QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 22.Wallace B.C., Schmid C.H., Lau J., Trikalinos T.A. Meta-Analyst: Software for meta-analysis of binary, continuous and diagnostic data. BMC Med. Res. Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster D.M., Votaw J.R., Nieh P.T., Yu W., Nye J.A., Master V., Bowman F.D., Issa M.M., Goodman M.M. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J. Nucl. Med. 2007;48:56–63. [PubMed] [Google Scholar]
- 24.Schuster D.M., Savir-Baruch B., Nieh P.T., Master V.A., Halkar R.K., Rossi P.J., Lewis M.M., Nye J.A., Yu W., Bowman F.D., et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259:852–861. doi: 10.1148/radiol.11102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkbey B., Mena E., Shih J., Pinto P.A., Merino M.J., Lindenberg M.L., Bernardo M., McKinney Y.L., Adler S., Owenius R., et al. Localized prostate cancer detection with 18F FACBC PET/CT: Comparison with MR imaging and histopathologic analysis. Radiology. 2014;270:849–856. doi: 10.1148/radiol.13130240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kairemo K., Rasulova N., Partanen K., Joensuu T. Preliminary clinical experience of trans-1-Amino-3-(18)F-fluorocyclobutanecarboxylic Acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer patients. BioMed Res. Int. 2014;2014:305182. doi: 10.1155/2014/305182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanni C., Schiavina R., Brunocilla E., Borghesi M., Ambrosini V., Zanoni L., Gentile G., Vagnoni V., Romagnoli D., Martorana G., et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: A prospective study in 28 patients. Clin. Genitourin. Cancer. 2014;12:106–110. doi: 10.1016/j.clgc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Nanni C., Schiavina R., Brunocilla E., Boschi S., Borghesi M., Zanoni L., Pettinato C., Martorana G., Fanti S. 18F-Fluciclovine PET/CT for the Detection of Prostate Cancer Relapse: A Comparison to 11C-Choline PET/CT. Clin. Nucl. Med. 2015;40:e386–e391. doi: 10.1097/RLU.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 29.Odewole O.A., Tade F.I., Nieh P.T., Savir-Baruch B., Jani A.B., Master V.A., Rossi P.J., Halkar R.K., Osunkoya A.O., Akin-Akintayo O., et al. Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: Comparison with CT. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:1773–1783. doi: 10.1007/s00259-016-3383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akin-Akintayo O.O., Jani A.B., Odewole O., Tade F.I., Nieh P.T., Master V.A., Bellamy L.M., Halkar R.K., Zhang C., Chen Z., et al. Change in Salvage Radiotherapy Management Based on Guidance With FACBC (Fluciclovine) PET/CT in Postprostatectomy Recurrent Prostate Cancer. Clin. Nucl. Med. 2017;42:e22–e28. doi: 10.1097/RLU.0000000000001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selnæs K.M., Krüger-Stokke B., Elschot M., Willoch F., Størkersen Ø., Sandsmark E., Moestue S.A., Tessem M.-B., Halvorsen D., Kjøbli E., et al. 18F-Fluciclovine PET/MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur. Radiol. 2018;28:3151–3159. doi: 10.1007/s00330-017-5213-1. [DOI] [PubMed] [Google Scholar]
- 32.Jambor I., Kuisma A., Kähkönen E., Kemppainen J., Merisaari H., Eskola O., Teuho J., Perez I.M., Pesola M., Aronen H.J., et al. Prospective evaluation of 18F-FACBC PET/CT and PET/MRI versus multiparametric MRI in intermediate- to high-risk prostate cancer patients (FLUCIPRO trial) Eur. J. Nucl. Med. Mol. Imaging. 2018;45:355–364. doi: 10.1007/s00259-017-3875-1. [DOI] [PubMed] [Google Scholar]
- 33.Akin-Akintayo O., Tade F., Mittal P., Moreno C., Nieh P.T., Rossi P., Patil D., Halkar R., Fei B., Master V., et al. Prospective evaluation of fluciclovine (18F) PET-CT and MRI in detection of recurrent prostate cancer in non-prostatectomy patients. Eur. J. Radiol. 2018;102:1–8. doi: 10.1016/j.ejrad.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andriole G.L., Kostakoglu L., Chau A., Duan F., Mahmood U., Mankoff D.A., Schuster D.M., Siegel B.A., LOCATE Study Group The Impact of Positron Emission Tomography with 18F-Fluciclovine on the Treatment of Biochemical Recurrence of Prostate Cancer: Results from the LOCATE Trial. J. Urol. 2019;201:322–331. doi: 10.1016/j.juro.2018.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.England J.R., Paluch J., Ballas L.K., Jadvar H. 18F-Fluciclovine PET/CT Detection of Recurrent Prostate Carcinoma in Patients With Serum PSA ≤ 1 ng/mL After Definitive Primary Treatment. Clin. Nucl. Med. 2019;44:e128–e132. doi: 10.1097/RLU.0000000000002432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H., Jinnouchi S., Kaji Y., Kishida T., Kinoshita H., Yamaguchi S., Tobe T., Okamura T., Kawakita M., Furukawa J., et al. Diagnostic performance of 18F-fluciclovine PET/CT for regional lymph node metastases in patients with primary prostate cancer: A multicenter phase II clinical trial. [(accessed on 1 September 2019)];Jpn. J. Clin. Oncol. 2019 doi: 10.1093/jjco/hyz072. Epub ahead of print. Available online: https://academic.oup.com/jjco/advance-article/doi/10.1093/jjco/hyz072/5490163. [DOI] [PubMed] [Google Scholar]
- 37.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M., Fossati N., Gross T., Henry A.M., Joniau S., et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Pernthaler B., Kulnik R., Gstettner C., Salamon S., Aigner R.M., Kvaternik H. A Prospective Head-to-Head Comparison of 18F-Fluciclovine with 68Ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT. Clin. Nucl. Med. 2019 doi: 10.1097/RLU.0000000000002703. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Calais J., Ceci F., Eiber M., Hope T.A., Hofman M.S., Rischpler C., Bach-Gansmo T., Nanni C., Savir-Baruch B., Elashoff D., et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019 doi: 10.1016/S1470-2045(19)30415-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawhn-Heath C., Flavell R.R., Behr S.C., Yohannan T., Greene K.L., Feng F., Carroll P.R., Hope T.A. SingleCenter Prospective Evaluation of 68Ga-PSMA-11 PET in Biochemical Recurrence of Prostate Cancer. AJR Am. J. Roentgenol. 2019:1–8. doi: 10.2214/AJR.18.20699. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Evangelista L., Guttilla A., Zattoni F., Muzzio P.C., Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: A systematic literature review and meta-analysis. Eur. Urol. 2013;63:1040–1048. doi: 10.1016/j.eururo.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 42.Yaxley J.W., Raveenthiran S., Nouhaud F.X., Samartunga H., Yaxley A.J., Coughlin G., Delahunt B., Egevad L., McEwan L., Wong D. Outcomes of Primary Lymph Node Staging of Intermediate and High Risk Prostate Cancer with 68 Ga-PSMA Positron Emission Tomography/Computerized Tomography Compared to Histological Correlation of Pelvic Lymph Node Pathology. J. Urol. 2019;201:815–820. doi: 10.1097/JU.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 43.Oka S., Hattori R., Kurosaki F., Toyama M., Williams L.A., Yu W., Votaw J.R., Yoshida Y., Goodman M.M., Ito O. A preliminary study of Anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J. Nucl. Med. 2007;48:46–55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.