Abstract
Myogenesis is a complex biological process, and understanding the regulatory network of skeletal myogenesis will contribute to the treatment of human muscle related diseases and improvement of agricultural animal meat production. Long noncoding RNAs (lncRNAs) serve as regulators in gene expression networks, and participate in various biological processes. Recent studies have identified functional lncRNAs involved in skeletal muscle development and disease. These lncRNAs regulate the proliferation, differentiation, and fusion of myoblasts through multiple mechanisms, such as chromatin modification, transcription regulation, and microRNA sponge activity. In this review, we presented the latest advances regarding the functions and regulatory activities of lncRNAs involved in muscle development, muscle disease, and meat production. Moreover, challenges and future perspectives related to the identification of functional lncRNAs were also discussed.
Keywords: lncRNA, myogenesis, muscle disease, meat production
1. Introduction
Skeletal muscle is a heterogeneous organ composed of muscle fibers, the basement membrane, muscle satellite cells, and nerves [1]. In animal production, skeletal muscles are the main resources of animal protein for human consumption, and the growth and development of skeletal muscle directly influence animal meat quantity and quality. In medicine, abnormal regulation of skeletal muscle leads to many types of muscle disease, such as muscular atrophy, muscular dystrophy, muscular hypertrophy, and myosarcoma. Therefore, understanding the regulatory network of myogenesis could contribute to improved agricultural animal meat production, and to treating muscle diseases. During embryonic development, skeletal muscle originates from the myotome, and further forms myogenic progenitor cells under the regulation of the Shh, Notch and Wnt signaling pathways [2]. Muscle progenitor cells express paired box 3 (Pax3) and paired box 7 (Pax7) genes, and migrate to the limbs and trunk. Pax3 and Pax7 are upstream regulators that can induce expression of the myogenic factor 5 (Myf5) and myogenic differentiation 1 (MyoD), thus promoting the differentiation of muscle progenitor cells to myoblasts [3,4,5]. Overexpression of Pax3 and Pax7 can lead to excessive proliferation of myoblasts [6]. Skeletal myogenesis is an orderly process regulated by a series of muscle-specific transcription factors, including MyoD, myogenin (MyoG), Myf5, myogenic regulatory factor 4 (MRF4), and myocyte enhancer factor 2 (MEF2) [7,8]. Myogenesis requires these transcription factors to be expressed at the right time and location [9,10]. For example, MyoD overexpression converts fibroblasts into myoblasts and leads to the subsequent fusion into myotubes [11,12]. MyoG knockdown reverses terminal muscle cell differentiation [13,14]. In addition, myogenesis is also regulated by epigenetic modification. For example, myogenesis is accompanied by dynamic changes in global chromosome modification, especially histone modification in myogenic genes [15,16,17,18,19]. During postnatal muscle development, muscle satellite cells are divided into two groups; one of which continues to proliferate and differentiate to form new muscle fibers. The other group is stored in the basement membrane as muscle stem cells, which are quiescent under normal conditions [20,21]. Once the muscle is injured or stimulated, resting muscle satellite cells are activated immediately to express the Pax7 gene. These satellite cells then begin to proliferate, migrate, and differentiate, fusing to form new muscle fibers to supplement the injured site [22,23]. Moreover, muscle regeneration is regulated by myogenic regulatory factors, the immune system, epigenetic modification, and the satellite cell microenvironment [17,19,22,24,25].
Although 80% of the eukaryotic genome is transcribed, only 2% of transcripts are translated into proteins [26]. Noncoding transcripts account for the vast majority of eukaryotic transcripts. The noncoding RNAs mainly include microRNAs (miRNAs), Piwi-interacting RNAs (piRNAs), circular RNAs (circRNAs), and long noncoding RNAs (lncRNAs). In this article, we focus primarily on lncRNAs, which are more than 200 nucleotides in length and have no protein-coding capacity [27]. Most lncRNAs are transcribed by RNA polymerase II, 5′ end capped, 3′ end poly(A) tailed, and post-transcription spliced [28,29,30]. Although lncRNAs are less abundant, less evolutionarily conserved, and have fewer exons compared to mRNA, their expression patterns are more spatio-temporally specific [31,32,33]. In the last decade, an increasing number of studies have indicated that lncRNAs participate in diverse cell and tissue development processes, such as X chromosome inactivation, genomic imprinting, stem cell maintenance, embryonic development, myogenesis, immunity, and tumorigenesis [34,35,36,37,38,39]. lncRNAs exert their functions through diverse mechanisms, including by regulating chromosome structures, gene transcription, mRNA stability and translation, and post-translational modification [32]. Here, we review recent advances regarding the importance of lncRNAs in skeletal muscle development, regeneration, and disease.
2. Functions and Mechanisms of lncRNAs in Muscle Development and Regeneration
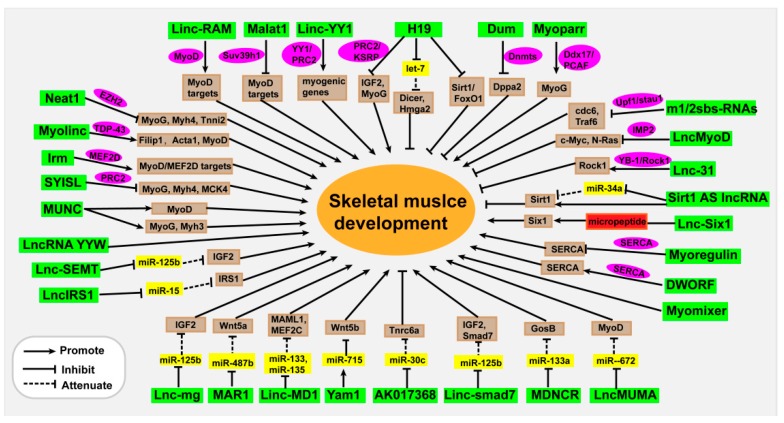
Thousands of lncRNAs have been detected in skeletal muscles. However, the function of most lncRNAs in muscle is still unclear, and only a small fraction of lncRNAs have been characterized. These lncRNAs exert functional roles through multiple mechanisms, including chromosome modification, transcription activation, molecular sponge activity, competitive binding, mRNA translation, and protein stability (Figure 1 and Table 1).
Figure 1.
Functional lncRNAs in skeletal muscle development.
Table 1.
Functional lncRNAs in skeletal muscle development and muscle disease.
| LncRNAs | Location | Function | Mechanism | Muscle Disease | Ref. |
|---|---|---|---|---|---|
| SYISL | Nucleus/ Cytoplasm |
Promotes proliferation, inhibits differentiation and muscle regeneration | Interacts with PRC2 | Unknown | [40] |
| Neat1 | Nucleus | Promotes proliferation and regeneration, inhibits differentiation | Interacts with EZH2 | Unknown | [14] |
| Malat1 | Nucleus | Inhibits differentiation and regeneration | Interacts with Suv39h1 | Unknown | [41] |
| Linc-YY1 | Nucleus | Promotes differentiation and regeneration | Interacts with YY1/PRC2 | Unknown | [42] |
| Linc-RAM | Nucleus/ Cytoplasm |
Promotes muscle growth and regeneration | Interacts with MyoD | Unknown | [43] |
| Dum | Nucleus/ Cytoplasm |
Promotes differentiation and regeneration | Interacts with Dnmts | Unknown | [44] |
| Myolinc | Nucleus | Promotes differentiation and regeneration | Interacts with TDP-43 | Unknown | [45] |
| Myoparr | Nucleus | Inhibits proliferation, promotes differentiation | Interacts with Ddx17/PCAF | Muscle atrophy | [46] |
| Irm | Nucleus | Promotes differentiation and regeneration | Interacts with MEF2D | Unknown | [47] |
| MUNC | Nucleus | Promotes differentiation | Induces MyoD, MyoG, Myh3 expression | Unknown | [48,49] |
| Meg3 | Nucleus | Promotes skeletal development during embryogenesis | Interacts with PRC2 | Unknown | [50,51] |
| SRA | Nucleus | Promotes differentiation | Assembly of p68/p72/MyoD coregulators | Unknown | [52] |
| Lnc-MD1 | Cytoplasm | Promotes differentiation | MiR-133, miRNA-135 molecular sponge | Muscle atrophy | [53] |
| MAR1 | Promotes differentiation and muscle growth | MiR-487b molecular sponge | Muscle atrophy | [54,55] | |
| Lnc-mg | Nucleus/ Cytoplasm |
Promotes differentiation and regeneration | MiR-125b molecular sponge | Muscle hypertrophy | [56,57] |
| Linc-smad7 | Nucleus/ Cytoplasm |
Inhibits proliferation, promotes differentiation and regeneration | MiR-125b molecular sponge | Unknown | [58,59] |
| AK017368 | Nucleus/ Cytoplasm |
Promotes proliferation, inhibits differentiation | MiR-30c molecular sponge | Muscle hypertrophy | [60,61] |
| Yam1 | Nucleus/ Cytoplasm |
Inhibits differentiation | Activates miR-715 expression | Unknown | [62] |
| m1/2sbs-RNAs | Cytoplasm | Regulates myogenesis | STAU1-mediated degradation of mRNA | Unknown | [63] |
| LncMyoD | Nucleus/ Cytoplasm |
Promotes differentiation | Competitively binds to IMP2 protein | Unknown | [58] |
| Lnc-31 | Nucleus/ Cytoplasm |
Promotes proliferation, inhibits differentiation | Interacts with ROCK1/YB-1 | Muscle atrophy | [64,65] |
| Myoregulin | SR/ER membrane | Reduces muscle performance | Binds to SERCA and inhibits its activity | Unknown | [66] |
| DWORF | SR membrane | Improves muscle contraction capacity | Binds to SERCA and increases its activity | Unknown | [67] |
| Myomixer | Membrane | Promotes fusion and regeneration and muscle formation during embryogenesis | Interacts with Myomaker | Unknown | [68,69,70,71] |
| LINC00961 | Endosome/ Lysosome |
Inhibits muscle regeneration | Interacts with the lysosomal v-ATPase | Unknown | [72,73] |
| H19 | Nucleus/ Cytoplasm |
Regulates differentiation and regeneration | Interacts with PRC2 or KSRP, miR-let7 molecular sponge, encodes miR-675 | Muscle hypertrophy |
[51,74,75,76,77,78,79] |
| Sirt1 AS lncRNA | Nucleus/ Cytoplasm |
Promotes proliferation, inhibits differentiation | MiR-34a molecular sponge, stabilizes Sirt1 mRNA | Unknown | [80] |
| LncIRS1 | Nucleus/ Cytoplasm |
Promotes proliferation and differentiation | MiR-15 molecular sponge | Muscle atrophy | [81] |
| LncMUMA | Promotes differentiation | MiR-672 molecular sponge | Muscle atrophy | [82] | |
| DBE-T | Nucleus | De-repressed muscle dystrophin mRNA isoforms | Interacts with ASH1L protein | Muscle atrophy | [83,84] |
| Atrolnc-1 | Promotes muscle wasting | Interacts with ABIN-1 | Muscle atrophy | [85] | |
| Chornos | Inhibits muscle hypertrophy | Interacts with EZH2 | Muscle hypertrophy | [86] |
2.1. lncRNAs Regulate Chromosome Modification
lncRNA function is associated with their subcellular localization. Nuclear-retained lncRNAs play important roles in regulating gene transcription [33,87]. Nuclear lncRNAs can influence chromosome states by interacting with chromosome modification complexes, such as Polycomb Repressive Complex 2 (PRC2), and Switch/Sucrose nonfermentable (SWI/SNF) [88,89,90]. Some lncRNAs can regulate myogenesis by recruiting chromosome modification complexes to target gene promoters. Jin et al. (2018) identified and characterized a new lncRNA, SYNPO2 intron sense-overlapping lncRNA (SYISL), involved in myoblast differentiation. Overexpression of SYISL significantly delays cell differentiation and promotes proliferation for C2C12 cells. Furthermore, knockout of SYISL significantly increases muscle mass and number of muscle fibers, and accelerates injury-induced muscle regeneration in vivo. Mechanistically, SYISL recruits PRC2 to the promoters of the target gene (e.g., p21, MyoG, or myh4), leading to H3K27me3 deposition [40]. Similarly, the Neat1 lncRNA modulates myogenesis by recruiting PRC2 to epigenetic-silenced target genes [14]. lncRNA Malat1, which was discovered in cancer cells, can promote the proliferation of cancer cells and tumor progression [91,92]. Malat1 regulates myoblast differentiation and muscle regeneration by recruiting the Suv39h1 protein to the binding site of MyoD, resulting in trimethylation of lysine 9 of histone 3 (H3K9me3) at the binding site, inhibiting myogenic gene expression [41]. Moreover, lncRNAs also regulate myogenesis by detaching chromosome modification complexes from target gene promoters. Linc-YY1 is transcribed upstream of the YY1 promoter and interacts with YY1. This interaction causes dissociation of the YY1/PRC2 complex from the promoters of the target gene, including miR-29, miR-1, MyHC, and Troponin, and reactivates their expression, promoting myoblast differentiation and regeneration [42].
2.2. lncRNAs Influence Transcription Activation
In addition to interacting with chromosome modification complexes, lncRNAs can also bind to transcription factors or RNA binding proteins to influence transcription activation. Linc-RAM, which is induced by MyoD, recruits MyoD to myogenic marker gene promoters to activate their transcription, thereby promoting muscle growth and regeneration [43]. Inhibition of Linc-RAM is essential for epidermal growth factor-related protein 2 (EGF2)-mediated suppression of myogenic differentiation [93]. An lncRNA called Myolinc is muscle-enriched and accelerates myogenesis by regulating its neighboring protein-coding gene, Filip1 in cis, and interacting with TAR DNA-binding protein 43 (TDP-43), a DNA/RNA-binding protein that regulates muscle-related gene expression (e.g., Acta1 and MyoD) [45]. A lncRNA called Myoparr, which is expressed from the MyoG gene promoter region, can promote myoblast differentiation and inhibit myoblast proliferation. Myoparr is essential for increasing the interaction between Ddx17 and PCAF, and promotes binding of the Ddx17/PCAF complex to the MyoG promoter; then, it recruits Pol II to the MyoG promoter to further promote MyoG transcription and myoblast differentiation [46]. lncRNA Irm is upregulated upon myoblast differentiation and promotes myogenic differentiation and regeneration by directly binding to MEF2D and promoting the assembly of MyoD/MEF2D on the regulatory elements of target genes [47].
Enhancer RNAs (eRNAs) are a large class of lncRNAs that are transcribed from known DNA enhancer regions, and play important roles in transcriptional activation of neighboring genes by recruiting core transcription factors or accelerating the interaction between enhancers and promoters [94,95]. The myogenic eRNA MyoD upstream noncoding RNA (MUNC), also known as DRReRNA, is reported to promote myoblast differentiation through at least two different mechanisms. First, MUNC acts as a typical eRNA to induce MyoD expression in cis. Second, MUNC can also act as an atypical eRNA to regulate MyoG, Myh3, and many other myogenic genes [48,49].
2.3. lncRNAs Serve as miRNA Molecular Sponges
The expression of lncRNA is highly associated with miRNA, suggesting that lncRNAs and miRNAs have co-regulatory functions in biological processes [96,97,98]. A lncRNA can act as a miRNA molecular sponge and weaken the inhibitory effects of miRNAs on target genes [99]. Many lncRNAs have been reported to regulate myogenesis by functioning as molecular sponges for miRNAs.
Linc-MD1 exhibits tissue-specific expression in skeletal muscle, and can promote the differentiation of skeletal muscle cells. During myogenic differentiation, linc-MD1 serves as a molecular sponge of miR-133 and miR-135 to attenuate the repression of their target genes, MAML1 and MEF2C. This increases MAML1 and MEF2C expression, thus promoting the differentiation of skeletal muscle [53]. LncRNA MAR1 is also highly expressed in muscle; overexpression of MAR1 can significantly promote myogenic differentiation and muscle growth. MAR1 can serve as a molecular sponge of miR-487b, weakening the effects of miR-487b upon its target gene, Wnt5a, and thereby promoting myogenic differentiation [54]. Lnc-mg is induced during myogenic differentiation and promotes myogenic differentiation and muscle regeneration by sponging miR-125b [56]. Lnc-mg can also regulate the expression of miR-351-5p, which can regulate myogenesis by targeting beta lactamase [57]. Linc-smad7 is a transcript of lncRNA-smad7 which has been reported to repress breast cancer cell apoptosis [100]. A transcriptome analysis of C2C12 cells demonstrated that Linc-smad7 is upregulated upon myoblast differentiation [58]. Overexpression of Linc-smad7 inhibits myoblast proliferation but promotes myoblast differentiation and regeneration. Linc-smad7 interacts with miR-125b and weakens the inhibitory effects of miR-125b upon its target genes, IGF2 and smad7 [59]. The lncRNA AK017368 promotes myoblast proliferation but inhibits myoblast differentiation by acting as a competing endogenous RNA of miR-30c [60,61].
2.4. lncRNAs Function at Post-Transcriptional Levels
Cytoplasm-located lncRNAs can regulate the expression of target genes at the post-transcriptional level by affecting the stability, splicing, and translation of mRNAs and the stability of proteins [101]. m1/2sbs-RNA is a type of lncRNA containing several SINE sequences that can complement genes containing the same SINE sequences, such as Cdc6 and Traf6. It forms a STAU1-binding site (SBS), which leads to STAU1-mediated degradation of mRNA [63]. In addition to affecting the stability of mRNA, lncRNA can also regulate mRNA translation. LncMyoD, transcribed from the upstream region of the MyoD gene, can competitively bind the IMP2 protein. This reduces the binding ability of IMP2 for the target genes, c-Myc and N-Ras, inhibiting their translation and promoting cell differentiation [58]. Lnc-31 is a cytoplasmic long noncoding RNA that is downregulated during myoblast differentiation. Knockdown of lnc-31 expression inhibits myoblast proliferation but enhances myoblast differentiation [64]. Lnc-31 affects myogenesis by binding both Rock1 (a known myogenesis suppressor) mRNA and YB-1 (a translational regulator) protein, and promotes the positive effects of YB-1 on Rock1 translation activation [65].
2.5. lncRNAs Encode Micropeptides
Although lncRNAs have little protein coding ability compared to mRNAs, some lncRNAs can give rise to functional micropeptides [102,103,104,105]. LncRNA-Six1 is located in the upstream of the protein-coding gene Six1, and has a role in promoting chicken skeletal muscle growth by regulating Six1 in cis. LncRNA-Six1 can produce a 7.26 kDa micropeptide, which plays an important role in lncRNA-Six1 cis-acting regulation of Six1 [106]. Myoregulin (MLN) is a conserved micropeptide encoded by a skeletal muscle-specific putative lncRNA, and its expression is regulated by MyoD and MEF2. MLN binds directly to sarco-endoplasmic reticulum Ca2+ adenosine triphosphatase (SERCA), inhibiting SERCA activity and hindering the uptake of Ca2+ into the sarcoplasmic reticulum. Genetic deletion of MLN increases Ca2+ release in skeletal muscle, and improves muscle performance [66]. DWORF is a micropeptide of 34 amino acids encoded by a putative lncRNA that is specifically expressed in the heart and soleus. In mice, DWORF interacts with SERCA and increases SERCA activity, affecting muscle contraction. Knockout of DWORF in slow skeletal muscle leads to delayed Ca+ release and reduced SERCA activity [67]. Myomixer, also named Minion [70] and Myomerger [68], is an 84-amino acid muscle-specific micropeptide that interacts with Myomaker to promote cell fusion and skeletal muscle formation during embryogenesis [69]. A recent study demonstrated that Myomixer is also required for muscle regeneration [71]. The small regulatory polypeptide of amino acid response (SPAR) is encoded by the conserved lncRNA LINC00961. SPAR is located in the late endosome/lysosome and negatively regulates mTORC1 activation by binding to lysosomal v-ATPase in mammals. LINC00961 is highly expressed in skeletal muscle and is downregulated upon acute injury by CTX injection. Knocking out SPAR expression in mice while maintaining host lncRNA expression using CRISPR/Cas9 engineering significantly increases muscle regeneration after CTX injection by activating mTORC1, which has a positive effect on satellite cell proliferation, differentiation, and myofiber maturation [72,73].
Many lncRNAs have been shown to regulate myogenesis via multiple mechanisms. H19 can inhibit myoblast differentiation by recruiting PRC2 to the promoters of target genes [51,107,108], by serving as a molecular sponge of miRNA let-7 [74], or by recruiting KSRP protein to the 3′ end of MyoG mRNA to decrease the stability of MyoG mRNA [75]. Furthermore, H19 promotes muscle regeneration by producing two conserved miRNAs, miR-675-3p and miR-675-5p [76]. Another lncRNA, Sirt1 AS lncRNA, which is transcribed from the Sirt1 antisense strand, accelerates myoblast proliferation and represses myoblast differentiation by attenuating the inhibition of miR-34a to Sirt1 translation, and enhancing the stability of Sirt1 mRNA [80].
3. lncRNAs in Skeletal Muscle Disease
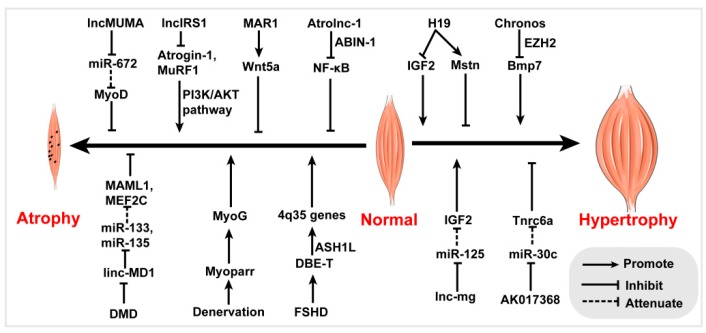
Alterations in myogenesis and muscle regeneration may lead to numerous muscle diseases, such as sarcopenia, muscle hypertrophy, and muscular dystrophy. The abnormal expression of lncRNAs is associated with various muscle diseases, and rescue of their normal expression levels in skeletal muscle can alleviate the disease phenotype (Figure 2). Here, we have summarized the latest progress on lncRNAs in human muscle disease and animal muscular disease models.
Figure 2.
Functional lncRNAs involved in skeletal muscle disease.
3.1. LncRNAs in Human Skeletal Muscle Disease
Duchenne muscular dystrophy (DMD) is one of the most common and serious forms of muscular dystrophy, and is caused by losing functional dystrophin protein [109]. The DMD locus harbors multiple lncRNAs, and these lncRNAs repress the expression of the dystrophin mRNA isoforms through interacting with the dystrophin promoter [110]. Several lncRNAs exhibit expression changes in skeletal muscles in DMD patients relative to normal people. For example, lnc-31 is up-regulated in the skeletal muscles of DMD patients [64]. Linc-MD1 is down-regulated in myoblasts derived from muscles of DMD patients [53]. The myoblasts from DMD patients exhibit impaired cell differentiation, suggesting that the aberrant expression of lnc-31 and linc-MD1 is associated with DMD disease.
Facioscapulohumeral muscular dystrophy (FSHD) is the third most prevalent muscular dystrophy type, and results in progressive weakness and loss of skeletal muscles [111,112]. FSHD is linked to a reduction in the copy number of the 3.3 kb D4Z4 repeat mapping to 4q35, but is not associated with a classical mutation within a protein-coding gene. In FSHD patients, lncRNA DBE-T interacts with the Trithorax group protein Ash1L and recruits it to the FSHD gene locus, leading to H3K36me2 and de-repression of FSHD genes, and thus promoting FSHD pathogenesis [83].
The clinical symptoms of idiopathic inflammatory myopathies (IIM) include muscle weakness and inflammation (myositis). Next generation sequencing was employed to examine the transcriptome in muscle biopsies obtained from two histologically distinct patient populations: body myositis (IBM) patients and anti-Jo-1-associated myositis (Jo-1) patients. The results showed that 55 and 46 lncRNAs are differentially expressed in IBM and Jo-1 myositis patients compared to controls, respectively. Of these lncRNAs, 16 lncRNAs, including H19, lncMyoD and MALAT1, are differentially expressed in both IBM and Jo-1 myositis patients. These differentially expressed lncRNA may be involved in myositis [113].
3.2. LncRNAs in Skeletal Muscle Disease Models
3.2.1. lncRNAs in Muscle Atrophy
Muscular dystrophy is the most common muscle disorder in humans, and is accompanied by muscle weakness and muscle wasting [114]. Several lncRNAs are differentially expressed between muscle dystrophy patients and normal individuals, including linc-MD1, lnc-31, Atrolnc-1, LncIRS1, and MAR1 [53,54,64,81,85]. Compared to normal cells, linc-MD1 expression levels are strongly reduced in Duchenne muscular dystrophy (DMD) myoblasts. Following overexpression of linc-MD1 in DMD myoblasts, the expression levels of MyoG, MyHC, MEF2C and MAML1 return to normal [53].
Chronic kidney disease is commonly associated with cachexia, and causes skeletal muscle wasting. Sun et al. (2018) found that the expression of eight lncRNAs simultaneously increased in atrophying muscles in three mouse catabolic models, and nine lncRNAs were downregulated in atrophying muscles. One of the identified lncRNAs, Atrolnc-1, is abundantly expressed in skeletal muscle and its expression is markedly increased in atrophying muscles. Overexpression of Atrolnc-1 in muscle causes myofiber atrophy, while inhibition of Atrolnc-1 ameliorates muscle wasting in mice. Mechanistically, Atrolnc-1 strongly binds to ABIN-1, inhibiting NF-κB signaling and causing protein degradation in muscle cells [85].
LncIRS1 has also been identified as a regulator of muscle development, and can promote myogenic differentiation, muscle mass, and muscle cross-sectional area via sponging the miR-15 family to activate the IGF1-PI3K/AKT pathway. Importantly, in a dexamethasone-induced myotube atrophy model in vitro, lncIRS1 regulated the expression of muscle atrophy-related genes such as p-Foxo1, p-Foxo3, p-Foxo4, p-AKT, and Atrogin-1, and rescued dexamethasone-induced muscle atrophy in cultured myotubes [81].
The lncRNA MAR1 has been found to be downregulated in aged mice and mechanically unloaded mice. Enforced MAR1 expression attenuates muscle atrophy in mouse models of age-related muscle atrophy and mechanical unloading-induced muscle atrophy, suggesting that MAR1 could be a novel therapeutic target for treating muscle atrophy induced by aging or mechanical unloading [54]. MAR1 also affects myogenesis by enhancing Wnt5a function [54]. Wnt5a may contribute to age-related skeletal muscle atrophy in rats [55]. These studies suggest that MAR1 may be involved in the Wnt5a-regulated muscular atrophy pathway.
Another atrophy-related lncRNA, mechanical unloading-induced muscle atrophy-related lncRNA (lncMUMA), is the most downregulated lncRNA during muscle atrophy development in hindlimb suspension mice. LncMUMA promotes myogenesis by acting as an miR-762 molecular sponge to regulate MyoD expression. Therapeutically, the enforced expression of lncMUMA prevents muscle atrophy development and reverses established skeletal muscle atrophy following mechanical unloading [82].
The lncRNA Myoparr is transcribed from the upstream domain of the MyoG promoter, and promotes myogenic differentiation by regulating the association between Ddx17 and the histone acetyltransferase PCAF. Overexpression of Myoparr also promotes skeletal muscle atrophy caused by denervation, and knockdown of Myoparr rescues muscle wasting, suggesting that Myoparr may be a potential therapeutic target for neurogenic atrophy [46].
3.2.2. lncRNAs in Muscle Hypertrophy
Muscle hypertrophy is associated with increased intracellular RNA and protein synthesis, and decreased protein degradation. The balance between protein synthesis and degradation is regulated by many pathways and regulators, such as the mTOR, IGF, and AMPK pathways, myostatin, and myogenic regulatory factors [115,116,117,118]. In addition, muscle hypertrophy requires activation of satellite cells [119,120]. Recent studies have indicated that several lncRNAs, such as H19, Chronos, lnc-mg and AK017368, are associated with muscle hypertrophy [56,60,77,79,87].
H19 is one of the earliest known examples of imprinted lncRNA, and plays a prominent role in regulating myogenic differentiation, which is fully repressed after birth except in skeletal muscle [77,121]. Deletion or mutation of H19 (H19Δ3) results in muscle hypertrophy and hyperplasia via reactivation of the imprinted gene network, particularly IGF2 upregulation following H19 deletion. Moreover, loss-of-function of H19 decreases myostatin (Mstn) expression [77,78,79]. These results suggest that H19 regulates muscle hypertrophy and hyperplasia mainly by influencing IGF2 and Mstn expression.
The Bmp7 signaling pathway positively regulates skeletal muscle hypertrophy through activation of Smad1/5 [122]. The muscle-enriched lncRNA Chronos is negatively regulated by Akt signaling and positively correlated with advancing age. Chronos epigenetically inhibits the expression of Bmp7 by recruiting EZH2. Knockdown of Chronos significantly increases the cross-sectional area of myofibers, and results in muscle hypertrophy in vivo [86].
4. Identification of lncRNAs in Agricultural Animal Meat Production
Muscle growth rate and muscle mass are two economically important traits in agricultural animal production. Compared with lncRNAs in model animals, the functions and mechanisms of lncRNAs affecting animal production are relatively unknown, although thousands of lncRNAs have been identified in livestock and poultry muscle (Table 2).
Table 2.
LncRNAs involved in agriculture animal muscle development.
| LncRNA | Location | Function | Mechanism | Ref. |
|---|---|---|---|---|
| MEG3 | Mainly in cytoplasm | Promotes bovine myoblast differentiation; involved in pig meat production traits | MiR-135 molecular sponge | [123,124] |
| LncMD | Mainly in nucleus | Promotes bovine myoblast differentiation | MiR-125b molecular sponge | [125] |
| Lnc133b | Mainly in nucleus | Regulates bovine skeletal muscle satellite cell proliferation and differentiation | MiR-133b molecular sponge | [126] |
| MDNCR | Promotes bovine myoblast differentiation, inhibits cell proliferation | MiR-133a molecular sponge | [127] | |
| H19 | Nucleus/Cytoplasm | Promotes bovine skeletal muscle satellite cell differentiation | Represses Sirt1/FoxO1 | [128] |
| YYW | Mainly in cucleus | Promotes bovine myoblast proliferation and differentiation | [129] | |
| LncKBTBD10 | Mainly in cucleus | Involved in bovine skeletal satellite cell proliferation and differentiation | [130] | |
| Lnc-SEMT | Promotes sheep myoblast differentiation and muscle growth | MiR-125b molecular sponge | [131] | |
| LncRNA-Six1 | Nucleus/Cytoplasm | Promotes chicken myoblast proliferation and differentiation, and involved in skeletal muscle fiber types transformation | MiR-1611 molecular sponge | [132] |
| LncIRS1 | Nucleus/Cytoplasm | Promotes the proliferation and differentiation of chicken myoblast | MiR-15 molecular sponge | [81] |
4.1. lncRNAs in Pig Skeletal Muscle Development
Tens of thousands of lncRNAs have been detected in the porcine genome through RNA sequencing (RNA-seq) and other technologies [133,134], most of which have been involved in pig skeletal muscle development [135,136,137,138,139,140]. Ren et al. (2009) isolated and identified the first pig lncRNA, trophoblast-derived noncoding RNA (TncRNA), which is differentially expressed in skeletal muscle in 90-day embryos of Tongcheng and Landrace pigs [139]. Zhao et al. (2015) identified more than 570 lncRNAs by systematically analyzing lncRNA expression in skeletal muscle at different times, and found an lncRNA, CUFF.8631, that is conserved among humans, mice, and pigs. This lncRNA contains four transcripts, and the transcripts CUFF.8631.1 and CUFF.8631.3 are differentially expressed during muscle development, suggesting that they may play a role in this process [140]. LncRNA MEG3 is differentially expressed in postnatal skeletal muscle development and conserved among humans, mice, and pigs. Four single nucleotide polymorphisms of MEG3 have been identified in Large White pigs and are associated with meat-producing traits [124].
4.2. lncRNAs in Bovine Skeletal Muscle Development
About 8000 lncRNAs expressed in bovine muscle have been identified and analyzed [141,142,143]. Several lncRNAs have been reported to play important roles in bovine myoblast proliferation and differentiation. For example, lncMD promotes bovine myoblast differentiation by acting as a molecular sponge of miR-125b [125]. Lnc133b regulates bovine skeletal muscle satellite cell proliferation and differentiation by sponging miR-133b [126]. LncRNA MDNCR promotes bovine myoblast differentiation but inhibits proliferation by acting as a molecular sponge of miR-133a, and thus weakens the inhibitory effects of miR-133a upon its target gene, GosB [127]. LncRNA H19 promotes bovine skeletal muscle satellite cell differentiation by repressing Sirt1/FoXO1 [128]. LncRNA MEG3 has a functional role in promoting bovine skeletal differentiation by sponging miR-135, attenuating the suppressive effects of miR-135 upon MEF2C [123]. LncRNA YYW is highly expressed in muscle, and promotes bovine myoblast proliferation and differentiation [129]. The lncRNA lncKBTBD10 is also induced during myogenic differentiation and plays a role in bovine skeletal muscle myogenesis [130].
4.3. lncRNAs in Sheep and Goat Skeletal Muscle Development
Zhan et al. (2016) identified 3981 lncRNAs in goat muscle tissues at different embryonic stages and three days after birth by RNA-seq, of which 577 lncRNAs were differentially expressed among the different stages of muscle development [144]. Ren et al. (2017) used Ribo-Zero RNA-seq technology to analyze the muscle lncRNA of Hu sheep at the fetal, lamb, and adult stages, and identified 6924 differentially expressed lncRNAs. GO analysis revealed that these differentially expressed lncRNAs are involved in muscle development and organ formation [145]. Li et al. (2019) identified 404 differentially expressed lncRNAs in sheep muscle from the prenatal to postnatal developmental stages using RNA-seq [146]. Lnc-SEMT, which is specifically expressed in muscle, can regulate IGF2 expression by sponging miR-125b, thus promoting muscle growth and development. Lnc-SEMT transgenic sheep exhibit significant muscle hypertrophy and weight gain [131].
4.4. lncRNAs in Chicken Skeletal Muscle Development
A total of 8072 chicken skeletal muscle-related lncRNAs have been detected by RNA-seq [147,148,149,150,151]. Among them, lncRNA-Six1 has a functional role in regulating chicken muscle development by sponging miRNA [132], and by encoding a micropeptide [106]. Another lncRNA, lncIRS1, is involved in regulating chicken muscle atrophy by acting as a molecular sponge for the miR-15 family to activate the IGF1-PI3K/AKT pathway [81].
5. Challenges and Future Perspectives
All of the above studies have shown that lncRNAs regulate multiple aspects of skeletal muscle development and disease by various regulatory mechanisms. Although the functions and mechanisms of some lncRNAs have been clearly studied, the research of lncRNAs in skeletal muscle is far from complete. Current studies regarding lncRNAs in muscles mainly focus on their roles in muscle atrophy and hypertrophy, muscle growth, and development after birth. Further attention should be paid to the regulation of muscle development during the embryonic stage, conversion of different types of muscle fibers, muscle aging, muscle metabolism, and muscle tumors. In addition, due to large number of lncRNA transcripts and low sequence conservation, the functions and mechanisms of lncRNAs are more complex than those of protein-coding genes. Therefore, there are still many unsolved problems and challenges ahead, including the following:
As tens of thousands of lncRNAs have been identified in muscles, their functions should be further explored by high-throughput methods. Recently, the development of genome editing techniques such as CRISPR/Cas9 system has provided powerful tools to identify functional lncRNAs in vivo and in vitro [152,153,154]. Thus, construction of sgRNA library targeting lncRNAs and establishment of efficient screening systems for muscle cells will be beneficial to the screening of key functional lncRNAs in skeletal muscles.
Continual innovation in data analysis tools has accelerated the investigation and identification of lncRNAs in myogenesis [155]. The development of computer models and algorithms provides an important basis for the functional prediction of lncRNAs [156,157,158,159]. Several databases, such as LncATLAS, starBase v2.0, CatRAPID, and RPISeq, have been established to predict the functions of lncRNAs, such as subcellular location, binding proteins, and miRNAs [160,161,162,163]. The computer aided functional characterizations of lncRNAs need to be further verified by experiments, as computer model-assisted predictions are mainly based on probability and statistics. Moreover, the annotation information in these databases is still incomplete, especially information relating to different transcripts. Non-poly (A) or other forms of lncRNAs, such as sno lncRNAs, are often ignored, as RNA-seq technology is mainly based on poly (A) sequencing techniques. Therefore, developing more advanced RNA-seq technologies and corresponding analysis tools will help us to recognize lncRNAs more comprehensively.
lncRNAs can play regulatory roles by interacting with DNA, RNA and proteins, and systematic identification of molecules interacting with lncRNAs is essential to elucidating their molecular mechanisms of action. Thus, more efficient techniques such as ChIRP (Chromatin isolation by RNA purification) and dChIRP (domain-specific ChIRP) should be further developed to study lncRNA interactomes.
lncRNAs can serve as biomarkers and therapeutic targets of several diseases, such as cancer, cardiopathy, neurologic diseases, and immunological diseases [164,165,166,167,168]. However, few lncRNAs have been identified and used as therapeutic targets for skeletal muscle diseases. Therefore, identifying more key lncRNAs related to skeletal muscle diseases will contribute to the treatment of skeletal muscle diseases in the future.
In this review, we presented the latest advances in the regulation network of lncRNAs in skeletal muscle development and muscle diseases, as well as the recent progress in agricultural animal meat production. Moreover, challenges and future perspectives were also discussed in the identification of novel muscle-related lncRNAs. Since the methods of studying lncRNAs have been reviewed in many studies, this review does not cover this aspect in much detail. In summary, lncRNAs play key roles in muscle development and regeneration, and in muscle diseases. The development of new tools and technologies will enable more functional lncRNAs to be identified in the future. Further studies will help to achieve an in-depth understanding of the functions and mechanisms of lncRNAs, and ultimately lead to the application of lncRNAs as therapeutic targets for muscle diseases or biomarkers for animal production.
Funding
This work was financially supported by the Natural Science Foundation of Hubei Province (Grant No. 2018CFA015), the Fundamental Research Funds for the Central Universities (Program No. 2662018PY037, 2662018PY045), the National Key Project for Transgenic Grant Nos. 2016ZX08006-002 and 2018ZX08010-12B, the Agricultural Innovation Fund of Hubei Province (2016-620-000-001-043), and the China Postdoctoral Science Foundation (Program No. 590319103).
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Giordani L., He G.J., Negroni E., Sakai H., Law J.Y.C., Siu M.M., Wan R., Corneau A., Tajbakhsh S., Cheung T.H., et al. High-Dimensional Single-Cell Cartography Reveals Novel Skeletal Muscle-Resident Cell Populations. Mol. Cell. 2019;74:609–621.e6. doi: 10.1016/j.molcel.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Musumeci G., Castrogiovanni P., Coleman R., Szychlinska M.A., Salvatorelli L., Parenti R., Magro G., Imbesi R. Somitogenesis: From somite to skeletal muscle. Acta Histochem. 2015;117:313–328. doi: 10.1016/j.acthis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Maroto M., Reshef R., Munsterberg A.E., Koester S., Goulding M., Lassar A.B. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–148. doi: 10.1016/S0092-8674(00)80190-7. [DOI] [PubMed] [Google Scholar]
- 4.Buckingham M., Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu. Rev. Cell Dev. Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- 5.Lagha M., Kormish J.D., Rocancourt D., Manceau M., Epstein J.A., Zaret K.S., Relaix F., Buckingham M.E. Pax3 regulation of FGF signaling affects the progression of embryonic progenitor cells into the myogenic program. Genes Dev. 2008;22:1828–1837. doi: 10.1101/gad.477908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins C.A., Gnocchi V.F., White R.B., Boldrin L., Perez-Ruiz A., Relaix F., Morgan J.E., Zammit P.S. Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS ONE. 2009;4:e4475. doi: 10.1371/journal.pone.0004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun T., Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011;12:349–361. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- 8.Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr. Opin. Genet. Dev. 2006;16:525–532. doi: 10.1016/j.gde.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Buckingham M., Rigby P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell. 2014;28:225–238. doi: 10.1016/j.devcel.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Bentzinger C.F., Wang Y.X., Rudnicki M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapscott S.J., Davis R.L., Thayer M.J., Cheng P.F., Weintraub H., Lassar A.B. MyoD1: A nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- 12.Choi J., Costa M.L., Mermelstein C.S., Chagas C., Holtzer S., Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc. Natl. Acad. Sci. USA. 1990;87:7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastroyiannopoulos N.P., Nicolaou P., Anayasa M., Uney J.B., Phylactou L.A. Down-regulation of myogenin can reverse terminal muscle cell differentiation. PLoS ONE. 2012;7:e29896. doi: 10.1371/journal.pone.0029896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S., Zuo H., Jin J., Lv W., Xu Z., Fan Y., Zhang J., Zuo B. Long noncoding RNA Neat1 modulates myogenesis by recruiting Ezh2. Cell Death Dis. 2019;10:505. doi: 10.1038/s41419-019-1742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barreiro E., Tajbakhsh S. Epigenetic regulation of muscle development. J. Muscle Res. Cell Motil. 2017;38:31–35. doi: 10.1007/s10974-017-9469-5. [DOI] [PubMed] [Google Scholar]
- 16.Bharathy N., Ling B.M., Taneja R. Epigenetic regulation of skeletal muscle development and differentiation. Subcell. Biochem. 2013;61:139–150. doi: 10.1007/978-94-007-4525-4_7. [DOI] [PubMed] [Google Scholar]
- 17.Brand-Saberi B. Genetic and epigenetic control of skeletal muscle development. Ann. Anat. 2005;187:199–207. doi: 10.1016/j.aanat.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Brand-Saberi B., Christ B. Genetic and epigenetic control of muscle development in vertebrates. Cell Tissue Res. 1999;296:199–212. doi: 10.1007/s004410051281. [DOI] [PubMed] [Google Scholar]
- 19.Jin W., Peng J., Jiang S. The epigenetic regulation of embryonic myogenesis and adult muscle regeneration by histone methylation modification. Biochem. Biophys. Rep. 2016;6:209–219. doi: 10.1016/j.bbrep.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Relaix F., Zammit P.S. Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- 21.Persson P.B. Skeletal muscle satellite cells as myogenic progenitors for muscle homoeostasis, growth, regeneration and repair. Acta Physiol. (Oxf.) 2015;213:537–538. doi: 10.1111/apha.12451. [DOI] [PubMed] [Google Scholar]
- 22.Dumont N.A., Bentzinger C.F., Sincennes M.C., Rudnicki M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015;5:1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- 23.Sambasivan R., Yao R., Kissenpfennig A., Van Wittenberghe L., Paldi A., Gayraud-Morel B., Guenou H., Malissen B., Tajbakhsh S., Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 24.Tidball J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017;17:165–178. doi: 10.1038/nri.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endo T. Molecular mechanisms of skeletal muscle development, regeneration, and osteogenic conversion. Bone. 2015;80:2–13. doi: 10.1016/j.bone.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Kapranov P., Cheng J., Dike S., Nix D.A., Duttagupta R., Willingham A.T., Stadler P.F., Hertel J., Hackermuller J., Hofacker I.L., et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 27.Wilusz J.E., Sunwoo H., Spector D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabili M.N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., Rinn J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia H., Osak M., Bogu G.K., Stanton L.W., Johnson R., Lipovich L. Genome-wide computational identification and manual annotation of human long noncoding RNA genes. RNA. 2010;16:1478–1487. doi: 10.1261/rna.1951310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark M.B., Johnston R.L., Inostroza-Ponta M., Fox A.H., Fortini E., Moscato P., Dinger M.E., Mattick J.S. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22:885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H., Guernec G., Martin D., Merkel A., Knowles D.G., et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao R.W., Wang Y., Chen L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- 33.Chen L.L. Linking Long Noncoding RNA Localization and Function. Trends Biochem. Sci. 2016;41:761–772. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Flynn R.A., Chang H.Y. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014;14:752–761. doi: 10.1016/j.stem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guttman M., Donaghey J., Carey B.W., Garber M., Grenier J.K., Munson G., Young G., Lucas A.B., Ach R., Bruhn L., et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satpathy A.T., Chang H.Y. Long noncoding RNA in hematopoiesis and immunity. Immunity. 2015;42:792–804. doi: 10.1016/j.immuni.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Jandura A., Krause H.M. The New RNA World: Growing Evidence for Long Noncoding RNA Functionality. Trends Genet. 2017;33:665–676. doi: 10.1016/j.tig.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt A.M., Chang H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y., Chen X., Sun H., Wang H. Long non-coding RNAs in the regulation of skeletal myogenesis and muscle diseases. Cancer Lett. 2018;417:58–64. doi: 10.1016/j.canlet.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Jin J.J., Lv W., Xia P., Xu Z.Y., Zheng A.D., Wang X.J., Wang S.S., Zeng R., Luo H.M., Li G.L., et al. Long noncoding RNA SYISL regulates myogenesis by interacting with polycomb repressive complex 2. Proc. Natl. Acad. Sci. USA. 2018;115:E9802–E9811. doi: 10.1073/pnas.1801471115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X., He L., Zhao Y., Li Y., Zhang S., Sun K., So K., Chen F., Zhou L., Lu L., et al. Malat1 regulates myogenic differentiation and muscle regeneration through modulating MyoD transcriptional activity. Cell Discov. 2017;3:17002. doi: 10.1038/celldisc.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou L., Sun K., Zhao Y., Zhang S., Wang X., Li Y., Lu L., Chen X., Chen F., Bao X., et al. Linc-YY1 promotes myogenic differentiation and muscle regeneration through an interaction with the transcription factor YY1. Nat. Commun. 2015;6:10026. doi: 10.1038/ncomms10026. [DOI] [PubMed] [Google Scholar]
- 43.Yu X., Zhang Y., Li T., Ma Z., Jia H., Chen Q., Zhao Y., Zhai L., Zhong R., Li C., et al. Long non-coding RNA Linc-RAM enhances myogenic differentiation by interacting with MyoD. Nat. Commun. 2017;8:14016. doi: 10.1038/ncomms14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Zhao Y., Bao X., Zhu X., Kwok Y.K., Sun K., Chen X., Huang Y., Jauch R., Esteban M.A., et al. LncRNA Dum interacts with Dnmts to regulate Dppa2 expression during myogenic differentiation and muscle regeneration. Cell Res. 2015;25:335–350. doi: 10.1038/cr.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Militello G., Hosen M.R., Ponomareva Y., Gellert P., Weirick T., John D., Hindi S.M., Mamchaoui K., Mouly V., Doring C., et al. A novel long non-coding RNA Myolinc regulates myogenesis through TDP-43 and Filip1. J. Mol. Cell Biol. 2018;10:102–117. doi: 10.1093/jmcb/mjy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hitachi K., Nakatani M., Takasaki A., Ouchi Y., Uezumi A., Ageta H., Inagaki H., Kurahashi H., Tsuchida K. Myogenin promoter-associated lncRNA Myoparr is essential for myogenic differentiation. EMBO Rep. 2019;20 doi: 10.15252/embr.201847468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sui Y., Han Y., Zhao X., Li D., Li G. Long non-coding RNA Irm enhances myogenic differentiation by interacting with MEF2D. Cell Death Dis. 2019;10:181. doi: 10.1038/s41419-019-1399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cichewicz M.A., Kiran M., Przanowska R.K., Sobierajska E., Shibata Y., Dutta A. MUNC, an Enhancer RNA Upstream from the MYOD Gene, Induces a Subgroup of Myogenic Transcripts in trans Independently of MyoD. Mol. Cell Biol. 2018;38 doi: 10.1128/MCB.00655-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueller A.C., Cichewicz M.A., Dey B.K., Layer R., Reon B.J., Gagan J.R., Dutta A. MUNC, a long noncoding RNA that facilitates the function of MyoD in skeletal myogenesis. Mol. Cell Biol. 2015;35:498–513. doi: 10.1128/MCB.01079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y., Cheunsuchon P., Nakayama Y., Lawlor M.W., Zhong Y., Rice K.A., Zhang L., Zhang X., Gordon F.E., Lidov H.G., et al. Activation of paternally expressed genes and perinatal death caused by deletion of the Gtl2 gene. Development. 2010;137:2643–2652. doi: 10.1242/dev.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J., Ohsumi T.K., Kung J.T., Ogawa Y., Grau D.J., Sarma K., Song J.J., Kingston R.E., Borowsky M., Lee J.T. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caretti G., Schiltz R.L., Dilworth F.J., Di Padova M., Zhao P., Ogryzko V., Fuller-Pace F.V., Hoffman E.P., Tapscott S.J., Sartorelli V. The RNA helicases p68/p72 and the noncoding RNA SRA are coregulators of MyoD and skeletal muscle differentiation. Dev. Cell. 2006;11:547–560. doi: 10.1016/j.devcel.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z.K., Li J., Guan D., Liang C., Zhuo Z., Liu J., Lu A., Zhang G., Zhang B.T. A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J. Cachexia Sarcopenia Muscle. 2018;9:613–626. doi: 10.1002/jcsm.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mumford P.W., Romero M.A., Mao X., Mobley C.B., Kephart W.C., Haun C.T., Roberson P.A., Young K.C., Martin J.S., Yarrow J.F., et al. Cross talk between androgen and Wnt signaling potentially contributes to age-related skeletal muscle atrophy in rats. J. Appl. Physiol. (1985) 2018;125:486–494. doi: 10.1152/japplphysiol.00768.2017. [DOI] [PubMed] [Google Scholar]
- 56.Zhu M., Liu J., Xiao J., Yang L., Cai M., Shen H., Chen X., Ma Y., Hu S., Wang Z., et al. Lnc-mg is a long non-coding RNA that promotes myogenesis. Nat. Commun. 2017;8:14718. doi: 10.1038/ncomms14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du J., Zhang P., Zhao X., He J., Xu Y., Zou Q., Luo J., Shen L., Gu H., Tang Q., et al. MicroRNA-351-5p mediates skeletal myogenesis by directly targeting lactamase-beta and is regulated by lnc-mg. FASEB J. 2019;33:1911–1926. doi: 10.1096/fj.201701394RRR. [DOI] [PubMed] [Google Scholar]
- 58.Gong C., Li Z., Ramanujan K., Clay I., Zhang Y., Lemire-Brachat S., Glass D.J. A long non-coding RNA, LncMyoD, regulates skeletal muscle differentiation by blocking IMP2-mediated mRNA translation. Dev. Cell. 2015;34:181–191. doi: 10.1016/j.devcel.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Song C., Wang J., Ma Y., Yang Z., Dong D., Li H., Yang J., Huang Y., Plath M., Ma Y., et al. Linc-smad7 promotes myoblast differentiation and muscle regeneration via sponging miR-125b. Epigenetics. 2018;13:591–604. doi: 10.1080/15592294.2018.1481705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang T., Zhou B., Shi L., Wang H., Chu Q., Xu F., Li Y., Chen R., Shen C., Schinckel A.P. lncRNA AK017368 promotes proliferation and suppresses differentiation of myoblasts in skeletal muscle development by attenuating the function of miR-30c. FASEB J. 2018;32:377–389. doi: 10.1096/fj.201700560RR. [DOI] [PubMed] [Google Scholar]
- 61.Guess M.G., Barthel K.K., Harrison B.C., Leinwand L.A. miR-30 family microRNAs regulate myogenic differentiation and provide negative feedback on the microRNA pathway. PLoS ONE. 2015;10:e0118229. doi: 10.1371/journal.pone.0118229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu L., Sun K., Chen X., Zhao Y., Wang L., Zhou L., Sun H., Wang H. Genome-wide survey by ChIP-seq reveals YY1 regulation of lincRNAs in skeletal myogenesis. EMBO J. 2013;32:2575–2588. doi: 10.1038/emboj.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J., Gong C., Maquat L.E. Control of myogenesis by rodent SINE-containing lncRNAs. Genes Dev. 2013;27:793–804. doi: 10.1101/gad.212639.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ballarino M., Cazzella V., D’Andrea D., Grassi L., Bisceglie L., Cipriano A., Santini T., Pinnaro C., Morlando M., Tramontano A., et al. Novel long noncoding RNAs (lncRNAs) in myogenesis: A miR-31 overlapping lncRNA transcript controls myoblast differentiation. Mol. Cell Biol. 2015;35:728–736. doi: 10.1128/MCB.01394-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dimartino D., Colantoni A., Ballarino M., Martone J., Mariani D., Danner J., Bruckmann A., Meister G., Morlando M., Bozzoni I. The Long Non-coding RNA lnc-31 Interacts with Rock1 mRNA and Mediates Its YB-1-Dependent Translation. Cell Rep. 2018;23:733–740. doi: 10.1016/j.celrep.2018.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson D.M., Anderson K.M., Chang C.L., Makarewich C.A., Nelson B.R., McAnally J.R., Kasaragod P., Shelton J.M., Liou J., Bassel-Duby R., et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelson B.R., Makarewich C.A., Anderson D.M., Winders B.R., Troupes C.D., Wu F., Reese A.L., McAnally J.R., Chen X., Kavalali E.T., et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–275. doi: 10.1126/science.aad4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quinn M.E., Goh Q., Kurosaka M., Gamage D.G., Petrany M.J., Prasad V., Millay D.P. Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 2017;8:15665. doi: 10.1038/ncomms15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bi P., Ramirez-Martinez A., Li H., Cannavino J., McAnally J.R., Shelton J.M., Sanchez-Ortiz E., Bassel-Duby R., Olson E.N. Control of muscle formation by the fusogenic micropeptide myomixer. Science. 2017;356:323–327. doi: 10.1126/science.aam9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q., Vashisht A.A., O’Rourke J., Corbel S.Y., Moran R., Romero A., Miraglia L., Zhang J., Durrant E., Schmedt C., et al. The microprotein Minion controls cell fusion and muscle formation. Nat. Commun. 2017;8:15664. doi: 10.1038/ncomms15664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bi P., McAnally J.R., Shelton J.M., Sanchez-Ortiz E., Bassel-Duby R., Olson E.N. Fusogenic micropeptide Myomixer is essential for satellite cell fusion and muscle regeneration. Proc. Natl. Acad. Sci. USA. 2018;115:3864–3869. doi: 10.1073/pnas.1800052115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsumoto A., Pasut A., Matsumoto M., Yamashita R., Fung J., Monteleone E., Saghatelian A., Nakayama K.I., Clohessy J.G., Pandolfi P.P. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017;541:228–232. doi: 10.1038/nature21034. [DOI] [PubMed] [Google Scholar]
- 73.Tajbakhsh S. lncRNA-Encoded Polypeptide SPAR(s) with mTORC1 to Regulate Skeletal Muscle Regeneration. Cell Stem Cell. 2017;20:428–430. doi: 10.1016/j.stem.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 74.Kallen A.N., Zhou X.B., Xu J., Qiao C., Ma J., Yan L., Lu L., Liu C., Yi J.S., Zhang H., et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giovarelli M., Bucci G., Ramos A., Bordo D., Wilusz C.J., Chen C.Y., Puppo M., Briata P., Gherzi R. H19 long noncoding RNA controls the mRNA decay promoting function of KSRP. Proc. Natl. Acad. Sci. USA. 2014;111:E5023–E5028. doi: 10.1073/pnas.1415098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dey B.K., Pfeifer K., Dutta A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014;28:491–501. doi: 10.1101/gad.234419.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinet C., Monnier P., Louault Y., Benard M., Gabory A., Dandolo L. H19 controls reactivation of the imprinted gene network during muscle regeneration. Development. 2016;143:962–971. doi: 10.1242/dev.131771. [DOI] [PubMed] [Google Scholar]
- 78.Gabory A., Ripoche M.A., Le Digarcher A., Watrin F., Ziyyat A., Forne T., Jammes H., Ainscough J.F., Surani M.A., Journot L., et al. H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development. 2009;136:3413–3421. doi: 10.1242/dev.036061. [DOI] [PubMed] [Google Scholar]
- 79.Park K.S., Mitra A., Rahat B., Kim K., Pfeifer K. Loss of imprinting mutations define both distinct and overlapping roles for misexpression of IGF2 and of H19 lncRNA. Nucleic Acids Res. 2017;45:12766–12779. doi: 10.1093/nar/gkx896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang G.Q., Wang Y., Xiong Y., Chen X.C., Ma M.L., Cai R., Gao Y., Sun Y.M., Yang G.S., Pang W.J. Sirt1 AS lncRNA interacts with its mRNA to inhibit muscle formation by attenuating function of miR-34a. Sci. Rep. 2016;6:21865. doi: 10.1038/srep21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Z., Cai B., Abdalla B.A., Zhu X., Zheng M., Han P., Nie Q., Zhang X. LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J. Cachexia Sarcopenia Muscle. 2019;10:391–410. doi: 10.1002/jcsm.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Z.K., Li J., Guan D., Liang C., Zhuo Z., Liu J., Lu A., Zhang G., Zhang B.T. Long Noncoding RNA lncMUMA Reverses Established Skeletal Muscle Atrophy following Mechanical Unloading. Mol. Ther. 2018;26:2669–2680. doi: 10.1016/j.ymthe.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cabianca D.S., Casa V., Bodega B., Xynos A., Ginelli E., Tanaka Y., Gabellini D. A long ncRNA links copy number variation to a polycomb/trithorax epigenetic switch in FSHD muscular dystrophy. Cell. 2012;149:819–831. doi: 10.1016/j.cell.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vizoso M., Esteller M. The activatory long non-coding RNA DBE-T reveals the epigenetic etiology of facioscapulohumeral muscular dystrophy. Cell Res. 2012;22:1413–1415. doi: 10.1038/cr.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun L., Si M., Liu X., Choi J.M., Wang Y., Thomas S.S., Peng H., Hu Z. Long-noncoding RNA Atrolnc-1 promotes muscle wasting in mice with chronic kidney disease. J. Cachexia Sarcopenia Muscle. 2018;9:962–974. doi: 10.1002/jcsm.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neppl R.L., Wu C.L., Walsh K. lncRNA Chronos is an aging-induced inhibitor of muscle hypertrophy. J. Cell Biol. 2017;216:3497–3507. doi: 10.1083/jcb.201612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun Q., Hao Q., Prasanth K.V. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet. 2018;34:142–157. doi: 10.1016/j.tig.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawaguchi T., Tanigawa A., Naganuma T., Ohkawa Y., Souquere S., Pierron G., Hirose T. SWI/SNF chromatin-remodeling complexes function in noncoding RNA-dependent assembly of nuclear bodies. Proc. Natl. Acad. Sci. USA. 2015;112:4304–4309. doi: 10.1073/pnas.1423819112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu Y., Rowley M.J., Bohmdorfer G., Wierzbicki A.T. A SWI/SNF chromatin-remodeling complex acts in noncoding RNA-mediated transcriptional silencing. Mol. Cell. 2013;49:298–309. doi: 10.1016/j.molcel.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., Tsai M.C., Hung T., Argani P., Rinn J.L., et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ji P., Diederichs S., Wang W., Boing S., Metzger R., Schneider P.M., Tidow N., Brandt B., Buerger H., Bulk E., et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 92.Lin R., Maeda S., Liu C., Karin M., Edgington T.S. A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene. 2007;26:851–858. doi: 10.1038/sj.onc.1209846. [DOI] [PubMed] [Google Scholar]
- 93.Zhao Y., Cao F., Yu X., Chen C., Meng J., Zhong R., Zhang Y., Zhu D. Linc-RAM is required for FGF2 function in regulating myogenic cell differentiation. RNA Biol. 2018;15:404–412. doi: 10.1080/15476286.2018.1431494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mousavi K., Zare H., Koulnis M., Sartorelli V. The emerging roles of eRNAs in transcriptional regulatory networks. RNA Biol. 2014;11:106–110. doi: 10.4161/rna.27950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orom U.A., Shiekhattar R. Long noncoding RNAs usher in a new era in the biology of enhancers. Cell. 2013;154:1190–1193. doi: 10.1016/j.cell.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoon J.H., Abdelmohsen K., Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He L., Chen Y., Hao S.Q., Qian J.Q. Uncovering novel landscape of cardiovascular diseases and therapeutic targets for cardioprotection via long noncoding RNA-miRNA-mRNA axes. Epigenomics. 2018;10:661–671. doi: 10.2217/epi-2017-0176. [DOI] [PubMed] [Google Scholar]
- 98.Militello G., Weirick T., John D., Doring C., Dimmeler S., Uchida S. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief. Bioinform. 2017;18:780–788. doi: 10.1093/bib/bbw053. [DOI] [PubMed] [Google Scholar]
- 99.Thomson D.W., Dinger M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016;17:272–283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 100.Arase M., Horiguchi K., Ehata S., Morikawa M., Tsutsumi S., Aburatani H., Miyazono K., Koinuma D. Transforming growth factor-beta-induced lncRNA-Smad7 inhibits apoptosis of mouse breast cancer JygMC(A) cells. Cancer Sci. 2014;105:974–982. doi: 10.1111/cas.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dykes I.M., Emanueli C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genom. Proteom. Bioinform. 2017;15:177–186. doi: 10.1016/j.gpb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ruiz-Orera J., Messeguer X., Subirana J.A., Alba M.M. Long non-coding RNAs as a source of new peptides. Elife. 2014;3:e03523. doi: 10.7554/eLife.03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andrews S.J., Rothnagel J.A. Emerging evidence for functional peptides encoded by short open reading frames. Nat. Rev. Genet. 2014;15:193–204. doi: 10.1038/nrg3520. [DOI] [PubMed] [Google Scholar]
- 104.Bazzini A.A., Johnstone T.G., Christiano R., Mackowiak S.D., Obermayer B., Fleming E.S., Vejnar C.E., Lee M.T., Rajewsky N., Walther T.C., et al. Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 2014;33:981–993. doi: 10.1002/embj.201488411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rion N., Ruegg M.A. LncRNA-encoded peptides: More than translational noise? Cell Res. 2017;27:604–605. doi: 10.1038/cr.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cai B., Li Z., Ma M., Wang Z., Han P., Abdalla B.A., Nie Q., Zhang X. LncRNA-Six1 Encodes a Micropeptide to Activate Six1 in Cis and Is Involved in Cell Proliferation and Muscle Growth. Front. Physiol. 2017;8:230. doi: 10.3389/fphys.2017.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.da Rocha S.T., Edwards C.A., Ito M., Ogata T., Ferguson-Smith A.C. Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends Genet. 2008;24:306–316. doi: 10.1016/j.tig.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 108.Davis E., Jensen C.H., Schroder H.D., Farnir F., Shay-Hadfield T., Kliem A., Cockett N., Georges M., Charlier C. Ectopic expression of DLK1 protein in skeletal muscle of padumnal heterozygotes causes the callipyge phenotype. Curr. Biol. 2004;14:1858–1862. doi: 10.1016/j.cub.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 109.Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 110.Bovolenta M., Erriquez D., Valli E., Brioschi S., Scotton C., Neri M., Falzarano M.S., Gherardi S., Fabris M., Rimessi P., et al. The DMD locus harbours multiple long non-coding RNAs which orchestrate and control transcription of muscle dystrophin mRNA isoforms. PLoS ONE. 2012;7:e45328. doi: 10.1371/journal.pone.0045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tonini M.M., Passos-Bueno M.R., Cerqueira A., Matioli S.R., Pavanello R., Zatz M. Asymptomatic carriers and gender differences in facioscapulohumeral muscular dystrophy (FSHD) Neuromuscul. Disord. 2004;14:33–38. doi: 10.1016/j.nmd.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 112.Nie M., Deng Z.L., Liu J., Wang D.Z. Noncoding RNAs, Emerging Regulators of Skeletal Muscle Development and Diseases. BioMed Res. Int. 2015;2015:676575. doi: 10.1155/2015/676575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamann P.D., Roux B.T., Heward J.A., Love S., McHugh N.J., Jones S.W., Lindsay M.A. Transcriptional profiling identifies differential expression of long non-coding RNAs in Jo-1 associated and inclusion body myositis. Sci. Rep. 2017;7:8024. doi: 10.1038/s41598-017-08603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tabebordbar M., Wang E.T., Wagers A.J. Skeletal muscle degenerative diseases and strategies for therapeutic muscle repair. Annu. Rev. Pathol. 2013;8:441–475. doi: 10.1146/annurev-pathol-011811-132450. [DOI] [PubMed] [Google Scholar]
- 115.Thomson D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018;19:3125. doi: 10.3390/ijms19103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Philippou A., Halapas A., Maridaki M., Koutsilieris M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J. Musculoskelet Neuronal Interact. 2007;7:208–218. [PubMed] [Google Scholar]
- 117.Zanou N., Gailly P. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell. Mol. Life Sci. 2013;70:4117–4130. doi: 10.1007/s00018-013-1330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blaauw B., Schiaffino S., Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr. Physiol. 2013;3:1645–1687. doi: 10.1002/cphy.c130009. [DOI] [PubMed] [Google Scholar]
- 119.Blaauw B., Reggiani C. The role of satellite cells in muscle hypertrophy. J. Muscle Res. Cell Motil. 2014;35:3–10. doi: 10.1007/s10974-014-9376-y. [DOI] [PubMed] [Google Scholar]
- 120.Lee S.J., Huynh T.V., Lee Y.S., Sebald S.M., Wilcox-Adelman S.A., Iwamori N., Lepper C., Matzuk M.M., Fan C.M. Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc. Natl. Acad. Sci. USA. 2012;109:E2353–E2360. doi: 10.1073/pnas.1206410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brannan C.I., Dees E.C., Ingram R.S., Tilghman S.M. The Product of the H19 Gene May Function as an Rna. Mol. Cell. Biol. 1990;10:28–36. doi: 10.1128/MCB.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sartori R., Schirwis E., Blaauw B., Bortolanza S., Zhao J., Enzo E., Stantzou A., Mouisel E., Toniolo L., Ferry A., et al. BMP signaling controls muscle mass. Nat. Genet. 2013;45:1309–1318. doi: 10.1038/ng.2772. [DOI] [PubMed] [Google Scholar]
- 123.Liu M., Li B., Peng W., Ma Y., Huang Y., Lan X., Lei C., Qi X., Liu G.E., Chen H. LncRNA-MEG3 promotes bovine myoblast differentiation by sponging miR-135. J. Cell. Physiol. 2019;234:18361–18370. doi: 10.1002/jcp.28469. [DOI] [PubMed] [Google Scholar]
- 124.Yu X., Wang Z., Sun H., Yang Y., Li K., Tang Z. Long non-coding MEG3 is a marker for skeletal muscle development and meat production traits in pigs. Anim. Genet. 2018;49:571–578. doi: 10.1111/age.12712. [DOI] [PubMed] [Google Scholar]
- 125.Sun X., Li M., Sun Y., Cai H., Lan X., Huang Y., Bai Y., Qi X., Chen H. The developmental transcriptome sequencing of bovine skeletal muscle reveals a long noncoding RNA, lncMD, promotes muscle differentiation by sponging miR-125b. Biochim. Biophys. Acta. 2016;1863:2835–2845. doi: 10.1016/j.bbamcr.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 126.Jin C.F., Li Y., Ding X.B., Li X., Zhang L.L., Liu X.F., Guo H. lnc133b, a novel, long non-coding RNA, regulates bovine skeletal muscle satellite cell proliferation and differentiation by mediating miR-133b. Gene. 2017;630:35–43. doi: 10.1016/j.gene.2017.07.066. [DOI] [PubMed] [Google Scholar]
- 127.Li H., Yang J., Jiang R., Wei X., Song C., Huang Y., Lan X., Lei C., Ma Y., Hu L., et al. Long Non-coding RNA Profiling Reveals an Abundant MDNCR that Promotes Differentiation of Myoblasts by Sponging miR-133a. Mol. Ther. Nucleic Acids. 2018;12:610–625. doi: 10.1016/j.omtn.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu X., Ji S., Li W., Yi B., Li H., Zhang H., Ma W. LncRNA H19 promotes the differentiation of bovine skeletal muscle satellite cells by suppressing Sirt1/FoxO1. Cell. Mol. Biol. Lett. 2017;22:10. doi: 10.1186/s11658-017-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yue Y., Jin C., Chen M., Zhang L., Liu X., Ma W., Guo H. A lncRNA promotes myoblast proliferation by up-regulating GH1. In Vitro Cell. Dev. Biol. Anim. 2017;53:699–705. doi: 10.1007/s11626-017-0180-z. [DOI] [PubMed] [Google Scholar]
- 130.Chen M., Li X., Zhang X., Li Y., Zhang J., Liu M., Zhang L., Ding X., Liu X., Guo H. A novel long non-coding RNA, lncKBTBD10, involved in bovine skeletal muscle myogenesis. In Vitro Cell. Dev. Biol. Anim. 2019;55:25–35. doi: 10.1007/s11626-018-0306-y. [DOI] [PubMed] [Google Scholar]
- 131.Wei C., Wu M., Wang C., Liu R., Zhao H., Yang L., Liu J., Wang Y., Zhang S., Yuan Z., et al. Long Noncoding RNA Lnc-SEMT Modulates IGF2 Expression by Sponging miR-125b to Promote Sheep Muscle Development and Growth. Cell. Physiol. Biochem. 2018;49:447–462. doi: 10.1159/000492979. [DOI] [PubMed] [Google Scholar]
- 132.Ma M., Cai B., Jiang L., Abdalla B.A., Li Z., Nie Q., Zhang X. lncRNA-Six1 Is a Target of miR-1611 that Functions as a ceRNA to Regulate Six1 Protein Expression and Fiber Type Switching in Chicken Myogenesis. Cells. 2018;7:243. doi: 10.3390/cells7120243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liang G., Yang Y., Li H., Yu H., Li X., Tang Z., Li K. LncRNAnet: A comprehensive Sus scrofa lncRNA database. Anim. Genet. 2018;49:632–635. doi: 10.1111/age.12720. [DOI] [PubMed] [Google Scholar]
- 134.Tang Z., Wu Y., Yang Y., Yang Y.T., Wang Z., Yuan J., Yang Y., Hua C., Fan X., Niu G., et al. Comprehensive analysis of long non-coding RNAs highlights their spatio-temporal expression patterns and evolutional conservation in Sus scrofa. Sci. Rep. 2017;7:43166. doi: 10.1038/srep43166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zou C., Li J., Luo W., Li L., Hu A., Fu Y., Hou Y., Li C. Transcriptome analysis reveals long intergenic non-coding RNAs involved in skeletal muscle growth and development in pig. Sci. Rep. 2017;7:8704. doi: 10.1038/s41598-017-07998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kern C., Wang Y., Chitwood J., Korf I., Delany M., Cheng H., Medrano J.F., Van Eenennaam A.L., Ernst C., Ross P., et al. Genome-wide identification of tissue-specific long non-coding RNA in three farm animal species. BMC Genom. 2018;19:684. doi: 10.1186/s12864-018-5037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun J., Xie M., Huang Z., Li H., Chen T., Sun R., Wang J., Xi Q., Wu T., Zhang Y. Integrated analysis of non-coding RNA and mRNA expression profiles of 2 pig breeds differing in muscle traits. J. Anim. Sci. 2017;95:1092–1103. doi: 10.2527/jas2016.0867. [DOI] [PubMed] [Google Scholar]
- 138.Zou C., Li S., Deng L., Guan Y., Chen D., Yuan X., Xia T., He X., Shan Y., Li C. Transcriptome Analysis Reveals Long Intergenic Noncoding RNAs Contributed to Growth and Meat Quality Differences between Yorkshire and Wannanhua Pig. Genes (Basel) 2017;8:203. doi: 10.3390/genes8080203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ren H., Li Y., Tang Z., Yang S., Mu Y., Cui W., Ao H., Du L., Wang L., Li K. Genomic structure, chromosomal localization and expression profile of a porcine long non-coding RNA isolated from long SAGE libraries. Anim. Genet. 2009;40:499–508. doi: 10.1111/j.1365-2052.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- 140.Zhao W., Mu Y., Ma L., Wang C., Tang Z., Yang S., Zhou R., Hu X., Li M.H., Li K. Systematic identification and characterization of long intergenic non-coding RNAs in fetal porcine skeletal muscle development. Sci. Rep. 2015;5:8957. doi: 10.1038/srep08957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Billerey C., Boussaha M., Esquerre D., Rebours E., Djari A., Meersseman C., Klopp C., Gautheret D., Rocha D. Identification of large intergenic non-coding RNAs in bovine muscle using next-generation transcriptomic sequencing. BMC Genom. 2014;15:499. doi: 10.1186/1471-2164-15-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koufariotis L.T., Chen Y.P., Chamberlain A., Vander Jagt C., Hayes B.J. A catalogue of novel bovine long noncoding RNA across 18 tissues. PLoS ONE. 2015;10:e0141225. doi: 10.1371/journal.pone.0141225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu X.F., Ding X.B., Li X., Jin C.F., Yue Y.W., Li G.P., Guo H. An atlas and analysis of bovine skeletal muscle long noncoding RNAs. Anim. Genet. 2017;48:278–286. doi: 10.1111/age.12539. [DOI] [PubMed] [Google Scholar]
- 144.Zhan S., Dong Y., Zhao W., Guo J., Zhong T., Wang L., Li L., Zhang H. Genome-wide identification and characterization of long non-coding RNAs in developmental skeletal muscle of fetal goat. BMC Genom. 2016;17:666. doi: 10.1186/s12864-016-3009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ren C., Deng M., Fan Y., Yang H., Zhang G., Feng X., Li F., Wang D., Wang F., Zhang Y. Genome-Wide Analysis Reveals Extensive Changes in LncRNAs during Skeletal Muscle Development in Hu Sheep. Genes (Basel) 2017;8:191. doi: 10.3390/genes8080191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li C.Y., Li X., Liu Z., Ni W., Zhang X., Hazi W., Ma Q., Zhang Y., Cao Y., Qi J., et al. Identification and characterization of long non-coding RNA in prenatal and postnatal skeletal muscle of sheep. Genomics. 2019;111:133–141. doi: 10.1016/j.ygeno.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 147.Li T., Wang S., Wu R., Zhou X., Zhu D., Zhang Y. Identification of long non-protein coding RNAs in chicken skeletal muscle using next generation sequencing. Genomics. 2012;99:292–298. doi: 10.1016/j.ygeno.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 148.Li Z., Ouyang H., Zheng M., Cai B., Han P., Abdalla B.A., Nie Q., Zhang X. Integrated Analysis of Long Non-coding RNAs (LncRNAs) and mRNA Expression Profiles Reveals the Potential Role of LncRNAs in Skeletal Muscle Development of the Chicken. Front. Physiol. 2016;7:687. doi: 10.3389/fphys.2016.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li T., Zhang G., Wu P., Duan L., Li G., Liu Q., Wang J. Dissection of Myogenic Differentiation Signatures in Chickens by RNA-Seq Analysis. Genes (Basel) 2018;9:34. doi: 10.3390/genes9010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li D., Li F., Jiang K., Zhang M., Han R., Jiang R., Li Z., Tian Y., Yan F., Kang X., et al. Integrative analysis of long noncoding RNA and mRNA reveals candidate lncRNAs responsible for meat quality at different physiological stages in Gushi chicken. PLoS ONE. 2019;14:e0215006. doi: 10.1371/journal.pone.0215006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ren T., Li Z., Zhou Y., Liu X., Han R., Wang Y., Yan F., Sun G., Li H., Kang X. Sequencing and characterization of lncRNAs in the breast muscle of Gushi and Arbor Acres chickens. Genome. 2018;61:337–347. doi: 10.1139/gen-2017-0114. [DOI] [PubMed] [Google Scholar]
- 152.Evers B., Jastrzebski K., Heijmans J.P., Grernrum W., Beijersbergen R.L., Bernards R. CRISPR knockout screening outperforms shRNA and CRISPRi in identifying essential genes. Nat. Biotechnol. 2016;34:631–633. doi: 10.1038/nbt.3536. [DOI] [PubMed] [Google Scholar]
- 153.Gilbert L.A., Horlbeck M.A., Adamson B., Villalta J.E., Chen Y., Whitehead E.H., Guimaraes C., Panning B., Ploegh H.L., Bassik M.C., et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell. 2014;159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goyal A., Myacheva K., Gross M., Klingenberg M., Duran Arque B., Diederichs S. Challenges of CRISPR/Cas9 applications for long non-coding RNA genes. Nucleic Acids Res. 2017;45:e12. doi: 10.1093/nar/gkw883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cao H., Wahlestedt C., Kapranov P. Strategies to Annotate and Characterize Long Noncoding RNAs: Advantages and Pitfalls. Trends Genet. 2018;34:704–721. doi: 10.1016/j.tig.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 156.Chen X., Yan C.C., Zhang X., You Z.H. Long non-coding RNAs and complex diseases: From experimental results to computational models. Brief. Bioinform. 2017;18:558–576. doi: 10.1093/bib/bbw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen X., Sun Y.Z., Guan N.N., Qu J., Huang Z.A., Zhu Z.X., Li J.Q. Computational models for lncRNA function prediction and functional similarity calculation. Brief. Funct. Genom. 2019;18:58–82. doi: 10.1093/bfgp/ely031. [DOI] [PubMed] [Google Scholar]
- 158.Zhang Z., Zhang J., Fan C., Tang Y., Deng L. KATZLGO: Large-Scale Prediction of LncRNA Functions by Using the KATZ Measure Based on Multiple Networks. IEEE/ACM Trans. Comput. Biol. Bioinform. 2019;16:407–416. doi: 10.1109/TCBB.2017.2704587. [DOI] [PubMed] [Google Scholar]
- 159.Yotsukura S., duVerle D., Hancock T., Natsume-Kitatani Y., Mamitsuka H. Computational recognition for long non-coding RNA (lncRNA): Software and databases. Brief. Bioinform. 2017;18:9–27. doi: 10.1093/bib/bbv114. [DOI] [PubMed] [Google Scholar]
- 160.Mas-Ponte D., Carlevaro-Fita J., Palumbo E., Hermoso Pulido T., Guigo R., Johnson R. LncATLAS database for subcellular localization of long noncoding RNAs. RNA. 2017;23:1080–1087. doi: 10.1261/rna.060814.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chang T.H., Huang H.Y., Hsu J.B., Weng S.L., Horng J.T., Huang H.D. An enhanced computational platform for investigating the roles of regulatory RNA and for identifying functional RNA motifs. BMC Bioinform. 2013;14(Suppl. 2) doi: 10.1186/1471-2105-14-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li J.H., Liu S., Zhou H., Qu L.H., Yang J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Muppirala U.K., Honavar V.G., Dobbs D. Predicting RNA-protein interactions using only sequence information. BMC Bioinform. 2011;12:489. doi: 10.1186/1471-2105-12-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bar C., Chatterjee S., Thum T. Long Noncoding RNAs in Cardiovascular Pathology, Diagnosis, and Therapy. Circulation. 2016;134:1484–1499. doi: 10.1161/CIRCULATIONAHA.116.023686. [DOI] [PubMed] [Google Scholar]
- 165.Beermann J., Piccoli M.T., Viereck J., Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 166.McMullen J.R., Drew B.G. Long non-coding RNAs (lncRNAs) in skeletal and cardiac muscle: Potential therapeutic and diagnostic targets? Clin. Sci. (Lond.) 2016;130:2245–2256. doi: 10.1042/CS20160244. [DOI] [PubMed] [Google Scholar]
- 167.Boon R.A., Jae N., Holdt L., Dimmeler S. Long Noncoding RNAs: From Clinical Genetics to Therapeutic Targets? J. Am. Coll. Cardiol. 2016;67:1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 168.Chandra Gupta S., Nandan Tripathi Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer. 2017;140:1955–1967. doi: 10.1002/ijc.30546. [DOI] [PubMed] [Google Scholar]