Abstract
Uncomplicated healthy pregnancy is the outcome of successful fertilization, implantation of embryos, trophoblast development and adequate placentation. Any deviation in these cascades of events may lead to complicated pregnancies such as preeclampsia (PE). The current incidence of PE is 2–8% in all pregnancies worldwide, leading to high maternal as well as perinatal mortality and morbidity rates. A number of randomized controlled clinical trials observed the association between low dose aspirin (LDA) treatment in early gestational age and significant reduction of early onset of PE in high-risk pregnant women. However, a substantial knowledge gap exists in identifying the particular mechanism of action of aspirin on placental function. It is already established that the placental-derived exosomes (PdE) are present in the maternal circulation from 6 weeks of gestation, and exosomes contain bioactive molecules such as proteins, lipids and RNA that are a “fingerprint” of their originating cells. Interestingly, levels of exosomes are higher in PE compared to normal pregnancies, and changes in the level of PdE during the first trimester may be used to classify women at risk for developing PE. The aim of this review is to discuss the mechanisms of action of LDA on placental and maternal physiological systems including the role of PdE in these phenomena. This review article will contribute to the in-depth understanding of LDA-induced PE prevention.
Keywords: pregnancy, placentation, preeclampsia, low dose aspirin, exosomes
1. Introduction
Pregnancy is an important event that leads to significant changes in maternal physiology. Successful pregnancy requires involvement of a series of processes commencing from fertilization to establishment of placental and maternal vascular connection with the fetus in correct order. Adequate placentation is one of the prerequisites for maintaining a normal healthy pregnancy. New insights into the placentation process involve migration, invasion, adherence, proliferation and differentiation of the placental principal cellular component, i.e., extravillous trophoblasts (EVTs), followed by their interaction with the pre-decasualized maternal uterine blood vessels, glands and lymphatics [1]. Placentation further evolves by digestion of the extracellular matrix where the EVTs tolerate surrounding maternal circulatory oxidative stress and the effects of soluble cytokines [1]. Nonetheless, the allogenic EVTs also interact with maternal decidual immune cells to provide immune competence [2]. Any deviation in these events may lead to pathological pregnancies, i.e., preeclampsia (PE). The broad concept of PE pathophysiology includes defective trophoblast invasion and inadequate uterine spiral arterial remodeling in the first trimester that follows with reduced uteroplacental perfusion [3]. This subsequently leads to poorly perfused and stressed placental syncytiotrophoblasts that release a range of mediators causing endothelial dysfunction and PE clinical manifestations [3]. Moreover, such abnormal placentation leads to the secretion of abnormal levels of anti-angiogenic and inflammatory proteins that enter the systemic maternal circulation and impair maternal systemic vascular function, resulting in the clinical manifestations of PE. Since PE and its clinical symptoms rapidly abate after delivery (removal of the placenta), the placenta must play a central or initiating role in this pregnancy disorder.
Novel pharmacological interventions for the prevention of PE have not been developed for many years, as the complex pathophysiology, diversified clinical presentation of the disease and difficulties associated with conducting drug discovery research in pregnant women have hampered their development. Low dose aspirin (LDA) is considered to be the most effective prophylactic therapy for reducing disease prevalence in women at high risk for developing early-onset PE. The use of LDA in pregnant women is generally considered to be safe as it does not affect the pregnant mothers and/or their unborn fetuses inadvertently. It has been suggested that the principal mechanism of action by which LDA exerts its effect is via the inhibition of thromboxane production that leads to the inhibition of platelet aggregation. Additionally, LDA has a direct positive effect on the villous trophoblasts [4]. However, recent evidence suggests that LDA prevents the development of PE by promoting trophoblast invasion and migration into the uterine arteries, interfering with cytokine production and stimulating the production of proangiogenic protein placental growth factor (PlGF); thereby, inhibiting apoptosis and premature uterine arterial remodeling [5].
Recent meta-analysis suggest that LDA (≥100 mg/day) in early gestation (before 16 weeks) is beneficial in preventing common pregnancy complications; i.e., PE, fetal growth restriction, preterm birth [6,7,8,9], suggesting that aspirin may have effect on implantation and early placentation [10]. Low-dose aspirin has been utilized for many years to prevent PE [11,12,13]. A recent individual patient data meta-analysis observed that LDA can reduce the risk of PE development by 10% and small for gestational age (SGA) births by 24% [14] without posing a major safety risk to mothers or fetuses other than placental abruption in some cases [15]. Other studies reported that low dose aspirin is generally well tolerated within both preconception and early pregnancy periods [16].
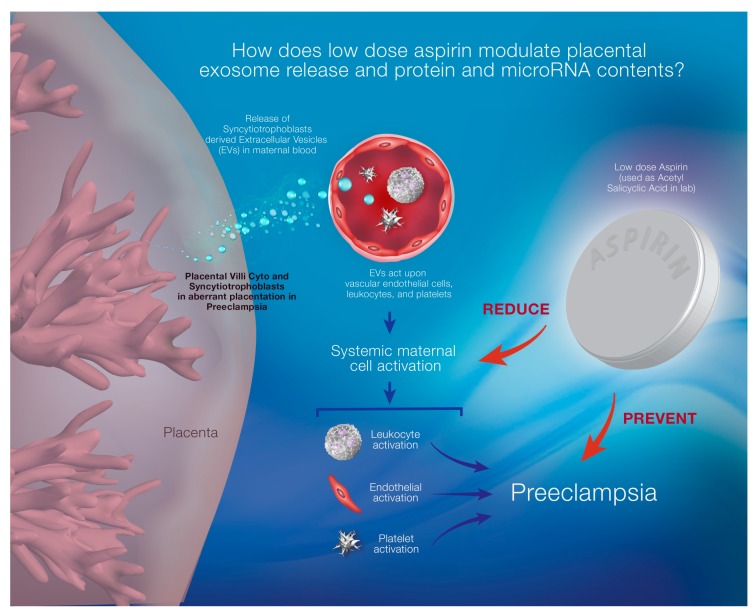
In normal healthy pregnancy, placental syncytiotrophoblast release extracellular vesicles (EVs) including exosomes into the maternal bloodstream that contain some information (i.e., micro RNA, mRNA, proteins) to convey from the originating cells to their distant target cells such as maternal immune cells in order to adapt to the pregnancy associated physiological changes [17]. This EV release is further increased from the preeclamptic placenta due to oxidative stress, causing widespread systemic endothelial dysfunction, giving rise to maternal hypertension, feto-placental circulatory compromise and damaging various maternal organs [17]. Some recently published reports have suggested that LDA could influence platelet derived EV release; however, the effect of LDA on the regulation of placental EV release is not known. Therefore, in this review, we will discuss the potential mechanisms of action of aspirin in the context of PE prevention and the potential role of extracellular vesicles released from the placenta in this phenomenon (Figure 1).
Figure 1.
Diagrammatic representation of preeclampsia (PE) development pathogenesis and mechanism of prevention by low dose aspirin (LDA). In PE, syncytiotrophoblast-derived extracellular vesicles (EVs), including exosomes, are released into the maternal circulation in increased amounts due to inadequate placental vascular remodeling. These EVs activate the vascular endothelial cells, leukocytes and platelets and cause dysfunction. LDA prevents the development of PE by reducingendothelial cell dysfunction. The proposed mechanism that was investigated; acetylsalicylic acid, the crude form of aspirin, modulates trophoblast derived exosome release and changes their proteomic and microRNA contents.
2. Physiology of Pregnancy
Pregnancy induces a number of alterations to maternal physiology for maintaining the correct course of pregnancy and it involves a cascade of processes commencing from fertilization to the establishment of feto-maternal communication and cross-talk mediated via the placenta. Preimplantation conditions, vascularisation, invasion of the embryonic cells to the maternal uterine wall and oxidative stress are the essential regulators in the function of pregnancy events [18]. Prior to implantation, there is a postovulatory surge of circulatory progesterone level that inhibits the proliferation of estrogen-dependent uterine epithelium and induces secretory transformation of uterine glands. In the early stages of development, i.e., ~day 6 of fertilization, the microvilli of the blastocyst interact with the pinopodes of the uterine endometrial luminal epithelium in order to establish apposition, which becomes stable through the increased adherence of the trophectoderm and the uterine luminal epithelium. During this interaction, a range of molecules are secreted from the immune activated cells including mucin, selectin, integrin and cadherin [18,19]. Shortly thereafter, invasion begins and trophectoderm penetrates the uterine epithelium, invading the wall of the uterine arteries where they interact with the cells of the maternal circulatory immune system and mediate the remodeling of the uterine spiral arteries that supply the placenta. This is followed by deportation of aggregates containing transcriptive materials.
There is direct evidence that platelets are involved in the placentation process. During placentation, trophoblasts invade the decidual stroma including the uterine glands and migrate into the maternal uterine spiral arteries replacing the vascular smooth muscle cells to remodel the arteries as low resistance, large caliber vessels [20]. This process ensures adequate placental perfusion by the remodeling of maternal uterine vessels. Histological examination shows deposition of maternal platelets in the trophoblast aggregates formed in the uterine spiral arteries. A number of studies discovered that these platelets are activated, releasing soluble factors enhancing the invasive capacity of trophoblast cells [21]. The trophoblasts produce a range of vasoconstrictors and vasodialators that are in balance to maintain the placental blood flow for proper fetal development during pregnancy [22].
During pregnancy, the viscosity and coagulability of maternal blood upsurges due to the increase in pro-coagulant agents, such as plasminogen activator inhibitor-1, fibrinogen, factor VII, VIII, von Willebrand factor and to the reduced fibrinolysis. The hypercoagulable state is attributed to the activation of platelets [23]. Maternal serum biochemical markers of pregnancy, namely alpha fetoprotein (AFP), human chorionic gonadotrophin (hCG), unconjugated estriol [24] and inhibin-A [25], are produced and found in higher concentration in the maternal peripheral circulation due to the implantation and placentation processes of pregnancy.
3. Pathogenesis of Preeclampsia
Preeclampsia (PE) is defined as new onset of hypertension after 20 weeks’ gestation with renal, hepatic, hematologic, neurological, pulmonary or fetal involvement. Physical signs of preeclampsia are hypertension, proteinuria, renal insufficiency, hemolysis, reduced platelet count and/or increased platelet activation [26]. It is a serious complication of pregnancy affecting ~7.6% pregnancies globally and is associated with high morbidity and mortality in affected mothers and children [27]. It is a lifelong disorder with increased risks of neonatal and child morbidity and mortality including health risks in adulthood [27]. Pregnancy induced hypertension is one of the most prevalent risk factors for the development of PE. The consequences of PE include intrauterine fetal growth restriction (IUGR) and preterm birth. [28].
In PE, there is widespread systemic endothelial dysfunction that leads to hypertension and concomitant proteinuria [29]. Clinical risk factors for developing PE assessed before 16 weeks of gestation include prior history of hypertension, chronic hypertension, pre-gestational diabetes, pre-pregnancy BMI > 30 and use of assisted reproductive technology [30]. The most commonly used screening test for early prediction of PE involves analysis of maternal characteristics, maternal mean arterial pressure, uterine arterial Doppler pulsatility index and serum biochemistry (PaPP-A and/or PlGF). This test is performed at 11–13 weeks of gestation [31]. The present management of patients with PE depends on symptom severity. Currently, some drugs are available to treat mild to severe PE (e.g., methyldopa, hydralazine, magnesium sulphate) [32]. However, the best treatment currently available for PE is delivery of the newborn and placenta as all the signs and symptoms of PE abolish when the placenta is separated from the mother.
In early-onset PE, there is defective implantation and placentation due to inadequate extravillous trophoblast invasion and partial failure of uterine arterial remodeling, resulting in high resistance and low capacitance vascular supply to the placenta and fetus [33]; however, this phenomenon is not evident in late-onset PE. Some research studies showed that during normal healthy pregnancy, the invasive trophoblast cells replace the smooth muscle and elastic lamina of the maternal uterine vessels, causing dilation and funneling at the vessel mouth and facilitating further migration of trophoblasts. In the absence of conversion of the maternal uterine vessels, there is retention of smooth muscle cells contributing to increased resistance to maternal blood flow. Nonetheless, maternal blood enters into the intervillous space as a turbulent jet that increases the risk of spontaneous vasoconstriction and ischemia-reperfusion injury, generating oxidative stress within the maternal circulation [34]. This in turn gives rise to placental villous infarcts, constriction of spiral arteries due to the mural hypertrophy and fibrin deposition, leading to the abnormal ultrasound indices and biochemical markers seen in the maternal circulation. This failure in vascular dilation has a direct impact on placental blood flow and is the primary determinant of pregnancy pathology [34]. In addition to the general concept of PE pathogenesis, where there is defective EVT invasion and uterine arterial remodeling, there is also an imbalance of angiogenic and antiangiogenic factors. These factors include vascular endothelial growth factor (VEGF), soluble endoglin, soluble fms-like tyrosine kinase-1 receptors (sFlt-1) and placental growth factor (PlGF). Abnormal production of these factors is closely associated with PE and intrauterine growth restriction [35]. At the end of the first trimester of pregnancy, the extravillous trophoblasts (EVT) invade the uterine spiral arteries and replace the vascular smooth muscle and the endothelium to remodel the arteries, which lead to the formation of low resistance and high capacitance vessels that facilitates increased placental perfusion. When there is perturbation of this process, there is reduced placental perfusion causing placental stress where platelets aggregate and accumulate in the partially damaged placenta [36]. In PE, there are interactions between maternal characteristics and risk factors and placental pathophysiological factors leading to a vicious cycle of maternal inflammation, vascular dysfunction and the activation of pro-coagulation pathways [33].
Current research on PE is focused on the role of extracellular vesicles released from the placenta [33]. Following placentation, the residual syncytiotrophoblastic material generated from placental shedding or by placental microparticles releases various vessel constricting factors that cause systemic endothelial dysfunction [37,38]. These microparticles contain a set of proteins including some pro-inflammatory and pro-coagulatory molecules that contribute to the development of PE [39,40,41] A recent article on PE stated that there was interaction between fetal Human Leukocyte Antigen-C (HLA-C) molecule and maternal natural killer cells’ killer-cell immunoglobulin-like receptor (KIR) in severe PE; these molecules are carried by the EVs released from the placenta and maternal circulatory cells [42]. Inadequate placentation causes the development of pregnancy-induced hypertension (PIH) and preeclampsia (PE) [43,44], leading to focal regions of hypoxia that are responsible for modifying the production of growth factors, cytokines [45], lipid peroxides [46] and prostaglandins by placental trophoblasts [45]. Elevated placental levels of inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1α, IL-1β and IL-6, are generally considered unfavorable to pregnancy [47]. Moreover, clinical studies have shown changes in the levels of cytokines and prostaglandins in women with PE [48,49]. Maternal circulatory neutrophils are activated in pregnancy and further activated in PE, which are the source of oxidative stress by generating reactive oxygen species such as hydrogen peroxide and superoxide anion and these molecules cause damage to the proteins, lipids and nucleic acids [50]. Neutrophil activation is initiated in the intervillous space by increased secretion of lipid peroxides by the placenta, which is abnormally increased in PE. This stimulates phospholipase A2 and cyclooxygenase enzymes to increase the production of thromboxane. Thromboxane is implicated in monocyte activation responses and plays role in mediating tumor necrosis factor alpha (TNF-α) production by neutrophils in response to oxidative stress [50].
In PE, the production of thromboxane A2 and prostaglandin I2 is altered with excessive accumulation of THXA2 metabolite in the maternal systemic circulation [51,52]. This results to the increased activation and aggregation of platelets and vasoconstriction causing impaired placental perfusion and oxidative stress [53,54,55,56,57]. The platelet count is reduced in PE due to platelet activation and aggregation under the effect of elevated levels of ThXA2 Synthase [28]. In addition [36], PE contributes to some biochemical changes in maternal circulatory system, such as, increase in phosphodiesterase-5 [58], thromboxane synthase [28] and an elevated hCG level [59]. Nonetheless, immunological changes also take place in PE. There is rise in anti β2-glycoprotein I antibodies that are related with aberrant implantation [60]. many predictive biomarkers for PE have been described including placental biomarkers (PAPP-A, PLGF, s-FLT-1, placental protein 13 (PP 13)), Free HbF, Alpha 1 Macroglobulin and Uterine Artery Doppler Pulsatility Index [61,62]. Not all studies have consistently shown value of these markers, for example changes in PP13 have not been consistently replicated [63]. Measurements of total cell free DNA and fetal fraction in maternal plasma at 11–13 and 20–24 weeks are not predictive of PE [64].
4. Pharmacology of Aspirin and Basis for Its Use in PE
The chemical name of aspirin is acetylsalicylic acid (ASA) [65,66,67,68,69]. It is a nonsteroidal anti-inflammatory drug (NSAID). It is typically used in two dose regimens—high dose (600 mg) and low dose (60–150 mg). It has anti-inflammatory, analgesic, antipyretic and antiplatelet effects [70]. The endothelial dysfunction in PE involves increased lipid peroxidation, which activates COX and inhibits prostacyclin synthase, thus inducing rapid imbalance in the TXA2/prostacyclin (PGI2) ratio in favor of TXA2 [51]. TXA2 favors systemic vasoconstriction, and increasing platelet aggregation and adhesion, which is compensated in this context by the vasodilator effect of prostacyclins, levels of which drop sharply. This imbalance is present from 13 weeks of gestation in high-risk PE patients [71]. LDA treatment for 2 weeks reverses TXA2/PGI2 imbalance by inhibiting THXA2 production [72,73]. Some studies observed that LDA can reduce the release of sFLT-1 from trophoblast cells and induce the production of vascular endothelial growth factor thereby promoting angiogenesis [74]. LDA also modulates cytokine production, reduces apoptosis and alters cell aggregation and fusion thereby improving defective trophoblast implantation [5]. LDA improves EVT migration and invasion into the maternal uterine spiral arteries and reduces placental cell apoptosis [75]. PE is associated with some augmented anti-angiogenic, oxidative and pro-inflammatory markers, as well as increasing human polymorphonuclear neutrophil (PMN)-endothelial cell adhesion [76]. LDA reduces the circulatory levels of these factors and improves the cytokine profile [5]. LDA causes retardation in leukocyte-endothelial cell adhesion and interaction and thus it prevents the endothelial cell dysfunction in PE [76]. Several reports have suggested that few biomarkers can be identified in maternal blood to be monitored for assessing treatment response after initiation of LDA treatment in pregnant women at high risk for preeclampsia; i.e., placental growth factor, placental protein 13, alpha fetoprotein [77].
The mechanism of action of aspirin involves a cascade of events. Aspirin irreversibly acetylates the platelet enzyme cyclooxygenase (COX), modifying the production of different prostaglandins and also acts as an analgesic, anti-inflammatory agent. There are three isoforms of COX enzyme upon which aspirin acts; the sources of these enzymes are mainly platelets, but they are also found in other immune cells namely leukocytes, monocytes and macrophages. Aspirin inhibits COX-1 irreversibly and COX-2 reversibly to a lesser extent. The resultant inhibition of COX-dependent generation of thromboxane A2 prevents platelet aggregation. This effect is maintained for the entire platelet lifespan of 8–9 days [78].
5. Low Dose Aspirin (LDA) and Pregnancy
Low dose aspirin reduces the mortality and morbidity in pregnant women at high risk for PE [79,80,81,82]. National guidelines typically suggest that women considered to be at high risk of developing pre-eclampsia should be treated with prophylactic low dose aspirin to reduce the prevalence of disease, although there are differences in how “high risk” is defined (NICE guidelines and ACOG recommendations (2017)). Acetylsalicylic Acid (ASA) is considered a highly attractive pharmacological agent to use in pregnancy for the prevention of maternal and perinatal mortality and morbidity worldwide due to its low cost, widespread availability, ease of administration and safety profile [83]. Aspirin is listed as a US Food and Drug Administration (FDA) category C drug during the first and second trimester and a category D drug in the third trimester of pregnancy [70]. Although some recent evidence has suggested that aspirin can affect the fetus adversely causing congenital anomaly, the FDA has assigned this drug as pregnancy category C, and treatment is relatively safe [84]. Although aspirin can cross the placenta, it is safe in low doses [85].
Low dose aspirin is a very effective treatment. Meta-analysis of a series of >30 randomized controlled trials have shown that low dose aspirin prophylaxis (any dose, any gestation) reduces the incidence of PE by 10% [11,12,14,86]. If analysis is restricted to assessment of outcomes for PE leading to delivery before 34 weeks in women who commence aspirin <16 weeks gestation and have a higher dose (>100 mg/day), then the data show a 90% reduction in early PE [87]. Aspirin also appears to be effective at reducing the prevalence of intrauterine growth restriction (IUGR); once again, this meta-analysis shows that treatment is more effective if a higher dose (>100 mg/day) is given and treatment is started before 16 weeks [88]. Other meta-analyses have also shown that low dose aspirin may be effective in preventing spontaneous preterm birth [6,7,8,9]. Other studies have demonstrated that low dose aspirin is generally well tolerated in both preconception and early pregnancy periods [16].
To date, several studies have attempted to assess the beneficial effects of aspirin treatment in gestational hypertensive disorders, in particular PE. In spite of different conflicting results on the effects of aspirin in pregnancy, one study found that aspirin administered early i.e., from the eighth week of gestation has in fact a positive effect on the pregnancy outcome without the manifestation of teratogenicity or fetotoxicity [29]. Recent studies on PE found that in high risk pregnancies, any preventative treatment should be aimed at or before 16 gestational weeks to be effective as placentation and uterine spiral arterial remodeling is completed by 20 gestational weeks [11]. Additionally, to prevent perinatal death and to improve perinatal outcomes, low dose aspirin should be prescribed before 16 gestational weeks [89]. Cost benefit analysis in a US based research study showed that aspirin prophylaxis through pregnancy would reduce morbidity and mortality, leading to a reduction in health care costs [90].
Following the preparation of a systematic review, the US Preventive Service Task Force recommended the use of low-dose aspirin (81 mg/d) as preventive medication after 12 weeks of gestation in women who are at high risk for PE [91,92,93]. The US Preventive Service Task Force also found that LDA prophylaxis in early pregnancy does not increase the chances of placental abruption, postpartum hemorrhage, fetal intracranial hemorrhage or perinatal mortality [94].
Other authors have suggested that the dose and timing of aspirin prophylaxis is also important. Ayala et al., 2013, identified that (i) 100 mg/d ASA should be the recommended minimum dose for prevention of complications in pregnancy; (ii) ingestion of low-dose ASA should be started at ≤16 weeks of gestation and (iii) low-dose ASA should be ingested at bedtime, not during the morning. Aspirin prescribed in this way significantly regulates ambulatory blood pressure (BP) and reduces the incidence of PE, gestational hypertension, preterm delivery and intrauterine growth restriction (IUGR) [95].
Other agents have been used for prophylaxis against PE in high risk women, either alone or in combination with LDA. There is a significant body of literature investigating whether low molecular weight (LMWH) or unfractionated heparin can reduce rates of PE, preterm birth, perinatal mortality and small for gestational age babies when prescribed to high risk women [96,97]. Heparin is safe from a fetal perspective and does not cross the placental barrier due to its high molecular weight [85,98]. While some observational studies that have combined the use of aspirin and LMWH show significant reduction in rates of PE in very high risk groups [99,100], an individual patient meta-analysis did not show significant benefit to this intervention [101]. Calcium (1 g/day) has also been widely investigated and appears to be particularly useful in low and middle income settings where dietary calcium intake is poor [102]. Vitamin C, D, E [103,104,105], fish oil/omega 3, statins [35], L-arginine [106] and antihypertensive drugs such as calcium channel blockers [107] have also been investigated, although there is a paucity of randomized controlled trial-based data for these investigations. The most significant ongoing research issues are to establish why aspirin is less effective in some groups of women; for example, those that have chronic hypertension and to determine whether additional agents can impact rates of term pre-eclampsia, which are not as significantly reduced using aspirin therapy.
A table on recent studies involving aspirin and pregnancy has been presented in the Table 1.
Table 1.
Recent Studies on Aspirin and Pregnancy.
| Study Design | Mode of Treatment | Outcome of Study | Reference |
|---|---|---|---|
| Randomized controlled trial (RCT) | Low dose aspirin (LDA) and/or Low Molecular Weight Heparin (LMWH) | Improved pregnancy outcomes (Less PE and IUGR incidence) | [108] |
| Prospective case-control study | LDA and LMWH in first trimester | Reduced incidence of unexplained recurrent spontaneous abortion | [109] |
| Database searching for RCTs involving LDA and placebo in PE | LDA or Placebo | LDA reduces PE risk | [110] |
| Literature searching on LDA and PE | LDA at 100 mg/day <16 gestational weeks | Reduced PE incidence due to LDA prophylaxis | [111] |
| Systematic literature searches about aspirin and PE | LDA | LDA prophylaxis in at risk patients to develop PE have higher advantages compared to negligible disadvantages i.e., feto-maternal bleeding, aspirin resistance etc. | [112] |
| Systematic review and an individual participant data meta-analysis | Antiplatelet aspirin therapy in early pregnancy | 10–15% reduction in the risk of PE | [113] |
| A systematic review and meta-analysis of randomized controlled trials | 50–150 mg/day aspirin or no treatment at <16 or >16 gestational weeks | LDA at <16 weeks, there was a significant reduction and a dose-response effect for the prevention of preeclampsia | [87] |
| A systematic review and meta-analysis through electronic database searches (PubMed, Cochrane, Embase). | LDA or placebo at <16 or >16 gestational weeks | <16 weeks, significant reduction of PE. >16 weeks, negligible impact on PE and related disorders |
[11] |
| Databases searching involving keywords ‘aspirin’ and ‘pregnancy’ | RCTs that evaluated the prophylactic use of LDA (50–150 mg/day) during pregnancy were included. | LDA initiated at ≤ 16 weeks of gestation is associated with a greater reduction of perinatal death and other adverse perinatal outcomes than when initiated at >16 weeks. | [114] |
| Meta-analysis of individual patient data recruited to 31 RCTs of PE primary prevention. | One or more antiplatelet agents (e.g., LDA or dipyridamole) versus a placebo or no antiplatelet agent. | Antiplatelet agents were associated with a significant 10% reduction in the relative risk of both PE (p = 0.004) and preterm birth before 34 weeks’ gestation (p = 0.011) compared to control cases. | [86] |
| Women at high risk for preterm PE were recruited to RCTs | 150 mg/day of aspirin was used to reduce the incidence of aspirin resistance and maximize the effect. | LDA reduced the incidence of preterm PE | [115] |
| A planned secondary analysis of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, a multicenter, block-randomized, double-blind, placebo-controlled trial investigating the effects of LDA on the incidence of live birth. | Daily LDA (81 mg, n = 615) or placebo (n = 613) and were followed for up to six menstrual cycles or through gestation if they became pregnant. | Preconception LDA appears to be well tolerated by women trying to conceive, women who become pregnant, and by their fetuses and neonates. | [16] |
| Chronological, cumulative meta-analyses of two recently published meta-analyses of RCTs examining the effects of antioxidant or LDA on the rates of PE. | Antioxidant or Low Dose Acetylsalicylic Acid (LDAA) therapy | Studies with smaller sample sizes are more likely to be biased against the null hypothesis. As such, cumulative meta-analysis is an effective tool in predicting potential bias against the null hypothesis and the need for additional studies. | [116] |
| Prospective cohort study involving 533 pregnant women in their first trimester | LDAA and LMWH | The use of ASA may be associated with an increased risk of developing a sub-chorionic hematoma (SCH) during the first trimester. | [117] |
| Multicentre RCTs involving 32 women with a previous delivery <34 weeks gestation with HD and/or SGA and aPLA were included before 12 weeks gestation. | The intervention was daily LMWH with aspirin or aspirin alone. | Combined LMWH and aspirin treatment started before 12 weeks gestation in a subsequent pregnancy did not show reduction of onset of recurrent HD either <34 weeks gestation or irrespective of gestational age, compared with aspirin alone. | [118] |
| Prospective randomized, placebo-controlled, double-blinded, multinational clinical trial | Daily administration of LDA (81 mg/day) initiated between 6 and 13 weeks of pregnancy and continued upto 36 weeks. | PTB, PE, SGA, perinatal mortality were reduced. | [119] |
| Prospective RCTs | Preconception LDA daily | It is not associated with reduction of pregnancy loss | [120] |
| Multicenter, double blind, placebo-controlled trial involving women at high risk for preterm PE | Some of them received 150 mg/day aspirin and some of them received placebo at 11–14 gestational weeks until 36 weeks of gestation | Primary outcome was delivery with PE before 37 weeks of gestation. Treatment with aspirin reduced the incidence of preterm preeclampsia. | [121] |
6. Effects of LDA on Placental and Maternal Body System Function
To date, a number of studies have attempted to elucidate the role of aspirin in the prevention of adverse pregnancy outcomes. However, the particular function of LDA in preventing PE and other pregnancy-induced hypertension is not clearly understood. Some in-vitro studies found that there is no specific effect of LDA or LMWH on BeWo choriocarcinoma cells when treated with forskolin except cell fusion due to the placental protein level 13 increase [122]. Some studies reported that thromboxane has been found to be involved with vasoconstriction leading to placental ischemia, thrombosis and platelet aggregation [123]. Other research studies reported that aspirin can negatively act on COX2 enzyme, thereby inhibiting thromboxane A2 production from arachidonic acid [124,125]. Interestingly, there are also data suggesting that aspirin can reduce the release of thromboxane from the trophoblasts [22].
Low-dose aspirin, which selectively inhibits TXA2 production, is used to prevent high-risk PE [28]. Low-dose aspirin, a common antiplatelet agent, usually restores prostacyclin and thromboxane levels that prevent vasoconstriction, and therefore, has been targeted as an intervention to reduce PE in at-risk women [124,126]. LDA increases the production of prostaglandin I2 by blocking the synthesis of thromboxane A2 [73]. This PGI2 increases vasodilatation and prevent thromboxane mediated damage [127]. Some studies have shown that TXA2 analogues cause hypertension in pregnancy and TXAS depletion prevents hypertension and IUGR [128]. Urine specimens of PE women show the presence of thromboxane B2, which is the metabolite of thromboxane A2 and LDA shifts the balance between THXA2 and PGI2 favoring the production of PGI2 that increases the blood flow to the placenta [129].
In normal, healthy pregnancies, uterine spiral arterial remodeling occurs at around 8 weeks of gestation and is complete by 16–20 weeks [130]. However, in PE, placentation is inadequate and under stress due to impaired uterine spiral arterial remodeling [131]. Some randomized controlled clinical trials observed that LDA is associated with improvement in uterine arterial pulsatility index when started in the first trimester of pregnancy [132,133]. Another study observed that low dose aspirin reduces the UtA Doppler pulsatility index, indicating improved blood flow [134].
In-vitro studies found an association of LDA treated trophoblast cells and an improvement in cytokine profile that prevents trophoblast apoptosis and promotes angiogenesis by increasing the production of placental growth factor (PlGF) [75]. Another similar study by Panagodage, et al. identified a number of factors that are involved in preeclampsia prevention with low dose aspirin (LDA) treatment. The authors observed that placental growth factor is significantly decreased in preeclamptic women’s sera compared to normotensive women’s sera; LDA increases trophoblast secretion of PlGF and restores abnormal cytokine (Activated Leukocyte cell adhesion molecule ALCAM, CXCL-16 and ErbB3) production by trophoblasts in PE [5]. Soluble fms-like tyrosine kinase-1 (sFLT1) is an antiangiogenic factor and its expression is increased in preeclamptic placentas and in cytotrophoblast exposed to hypoxia. Aspirin inhibits the production of sFLT1 in CTBs and this effect is mediated by the inhibition of COX-1 [74].
Preeclampsia is associated with some augmented anti-angiogenic, oxidative and pro-inflammatory markers, as well as increasing human polymorphonuclear neutrophil (PMN)-endothelial cell adhesion. This cell adhesion is reduced when human PMN are incubated with ATL (aspirin triggered lipoxin A4) [76]. This aspirin triggered lipoxin is similar to endogenously produced lipoxins but the duration of action is prolonged [135]. ATL acts as an anti-inflammatory agent; it promotes angiogenesis and causes immunosuppression and it also blocks the generation of reactive oxygen species in the endothelial cells, inhibits chemotaxis of polymorphonuclear neutrophil and the leukocyte-endothelial interaction [136,137,138,139,140] causes nuclear factor kappa B activation [137,141] and secretion of tumor necrosis factor alpha (TNF-α) in activated T cells [142]. Additionally, ATL can increase nitric oxide synthesis where the heme oxygenase-1 enzyme is also involved [143] and this effect is responsible for resolving inflammation [143]. Heme oxygenase enzyme-1 degrades heme to generate bilirubin, carbon monoxide and iron, exerting their anti-oxidant, antiapoptotic and cytoprotective actions [144]. Additionally, another recently conducted study identified that aspirin prevents TNF-alpha-induced endothelial cell dysfunction by regulating the NF-kappa B-dependent miR-155/eNOS pathway in preeclampsia [145].
The pathophysiology of PE also involves the genetic expression of the STOX1 transcription factor by extravillous trophoblasts that modulate trophoblast proliferation [146,147]. The STOX1 gene is overexpressed in human placental extravillous trophoblasts and is associated with PE pathogenesis [147,148,149]. Founds et al. [150] showed, in transcriptomic analysis, that STOX1 is overexpressed during the first trimester of pregnancies that had a preeclamptic outcome. Other studies have performed functional assays to determine the function of the STOX1 gene; using an in-vivo mouse model, this gene was found to cause severe gestational hypertension, proteinuria, an increased circulatory level of antiangiogenic factors and histological alterations in the kidney as well as the placenta [151]. These researchers also demonstrated that low dose aspirin improved maternal PE-like symptoms [152]. LDA improves uterine perfusion and favourably affects aspects of reproduction [153]. In addition, empirical introduction of LDA during in vitro fertilization (IVF) treatment improves the quality of oocytes and embyros [154]. Low dose aspirin and heparin in combination improve the live birth rate in IVF for unexplained implantation failure [155]. Low-dose aspirin effectively improves perifollicular artery blood flow and enhances oocyte quality and clinical pregnancy rates [156].
7. Complications of LDA for Fetuses and Mothers
A systematic evidence review by the US Preventive Services Task Force (USPSTF) identified no adverse impact on the mother or offspring during the perinatal period following aspirin use for prevention of preeclampsia [157] including no documented adverse effect on neonatal platelets [158]. However, some studies have identified adverse effects with the antenatal and perinatal use of aspirin, albeit taken at a higher dose. Potential risks associated with aspirin therapy during the third trimester include premature closure of the ductus arteriosus and hemorrhagic complications [159], subchorionic hematoma if administered in first trimester of pregnancy [117], fetal loss [160], endocrine disturbances in the human fetal testis and interference in the testicular descent [161], childhood asthma [162] and fetal complications [110]. Some research studies observed that high doses of aspirin may affect fertility, increases the risk of miscarriages and may cause fetal cryptorchidism [163,164,165]. Additionally, LDA therapy in the late gestational age has on rare occasion been reported to cause renal injury, cardiovascular abnormality such as closure of the ductus arteriosus, necrotizing enterocolitis and intracranial hemorrhage in the fetus as well as reduced breast milk supply in the mother, likely due to the inhibition of cyclooxygenase enzyme pathways [164]. The common adverse effects of aspirin in adults are significantly associated with gastrointestinal or cerebral bleeding episodes [166]. Given the risks of aspirin therapy, it is better to reserve treatment for women deemed high-risk of deep placentation related disorders rather than to prescribe it universally.
8. Predictive Biomarkers for Preeclampsia Cases Treated with Low Dose Aspirin
Few biomarkers have been identified in maternal blood as candidates for monitoring treatment response after initiation of low dose aspirin treatment in pregnant women at high risk for preeclampsia:
-
(i)
Maternal serum concentrations of placental growth factor (PlGF) level are generally low in preeclampsia.
-
(ii)
Low-dose aspirin reduces adverse pregnancy outcome such as PE and delivery before 34 weeks of gestation in pregnant women with unexplained elevated levels of alpha-fetoprotein (AFP) [167,168].
-
(iii)
Normotension in the first trimester is associated with reduced risk of PE [169].
-
(iv)
In a randomized controlled clinical trial conducted by Asemi Z. et al., low dose aspirin (80 mg) was administered with calcium supplementation (500 mg) in pregnant women who were at risk for PE. The treatment was continued for nine consecutive weeks before measuring high sensitivity C-reactive protein (hs-CRP), total antioxidant capacity (TAC), total glutathione (GSH) in plasma and serum glucose and insulin level. The study showed a significant difference in serum hs-CRP level and increased levels of plasma TAC and total GSH in pregnant women at risk for preeclampsia as compared to those that took placebo (did not receive any treatment), but serum insulin levels were not affected at all [170].
9. Extracellular Vesicles (EVs)
Extracellular vesicles (EVs) are mediators that can modify the function of target cells by transferring proteins and genomic materials to other cells; thus, EVs have an active participation in cell-to-cell communication [171]. EVs shred from a variety of cells and have a number of important physiological as well as pathological functions as they are capable of trafficking and transfecting the genetic material from cell to cell. The biogenesis and contents of EVs predominantly depends on the originating cell type and their surrounding microenvironment [172]. Several studies using electron microscopy analysis to characterize the morphology of EVs demonstrate that are spherical with lipid bilayer membrane [173,174]. The correct classification of EVs still a manner of debate and the majority of the information in the literature classify them according to their size and the different biogenesis pathways. Typically, EVs are categorized as exosomes (~40–100 nm), microvesicles (~100–1000 nm) and apoptotic bodies (~1000–5000 nm) based on their size and origin. Microvesicles and apoptotic bodies are formed directly via budding of the plasma membrane, whereas exosomes are produced via an endocytic pathway [174]. Distinction between different EVs subgroups is difficult, due to the minimal physical and morphological differences, to the lack of specific markers, and to the fact that the same cellular source may dynamically produce different class of EVs in response to different conditions [175]. Recently, the international society of extracellular vesicles has recommended classifying the vesicles according to the their size in small EVs (<100–200 nm) and medium/large EVs (>200 nm), or density (low, middle, high, with each range will defined) or their biochemical composition (e.g., CD63+ve) [176]. Currently, there is no single method allowing for accurate characterization and discrimination of the different EVs classes [175]. EVs can be ordinarily isolated from different biological fluids using the differential and buoyant density centrifugation methods followed by ultrafiltration/size exclusion chromatography or flow cytometry or precipitation using polymers or antibodies to enrich the pure EVs population [177]. EVs play an important role in cell-to-cell communication and influence a variety of cellular functions, including cytokine production modulation, cell proliferation, apoptosis and metabolism, by transferring their protein, lipid or messenger RNA and micro RNA molecules [173,178]. EVs can be isolated both biological fluids (e.g., plasma/serum, urine, cerebrospinal fluid, saliva, etc.) and in vitro from cell-conditioned media. Moreover, as EVs are natural carriers of bioactive molecules, different research studies are addressing the therapeutic potential of EVs due to their specific genetic material packaging capabilities [175]. Several groups have identified EVs in maternal biofluids during normal and complication of pregnancies and the potential role of EV during pregnancy have been reviewed in details by our group previously [179,180,181,182].
10. Extracellular Vesicles/Exosomes in Normal and Preeclamptic Pregnancies
Different research studies utilized a variety of experimental models (i.e., biological fluids, primary placental trophoblasts, trophoblast cell line, placental explant, placental perfusate etc.) to isolate different subpopulations (exosomes, microvesicles) of EVs and studied their role in the context of healthy as well as in pathological pregnancies. Small and large EVs originating from the placenta have been identified in maternal plasma. Concentrations of both total and placenta-derived exosomes present in maternal circulation increase across gestation [183] and are higher in complicated pregnancies (such as those affected by PE) as compared to normal pregnancies [184,185]. Interestingly, the global miRNA profile within small vesicles such as exosomes differs between normal and PE pregnancies across gestation and it is likely that PE is not only associated with changes in the circulating levels of exosomes, but also in their miRNA content [186]. Recently, Biro et al. identified that hsa-miR-210 level increased in the circulating exosomes isolated from PE pregnancies [187]. Poor placentation is associated with hypoxia and oxidative stress, which are features of PE and affects the invasion of extravillous trophoblast (EVT) and the uterine spiral arterial remodeling. Truong et al. studied whether low oxygen tension alters exosome release and the exosomal miRNA profile from HTR-8/SVneo cell line and examined their interaction with endothelial cells [188]. HTR-8/SVneo cells are commonly used as a model for EVT cells, although they are not ideal, as they contain a heterogenous population of trophoblast and stromal cells [189]. In this study, low oxygen tension to exosomes from EVTs cultured under normoxic conditions. Moreover, a specific set of miRNAs within exosomes from EVTs cultured under hypoxia were identified, and these miRNAs are present in circulating exosomes at early gestation from women who develop PE later in pregnancy. This data suggests that aberrant extracellular vesicle signaling is one of the common factors in the development of PE. In normal healthy pregnancy, syncytiotrophoblast derived EVs release into the maternal blood stream where they act upon their target endothelial cells and circulating immune cells [33,190,191,192]. Placental EVs carry different proteins, lipids and nucleic acids that play a crucial role in feto-maternal communication to maintain pregnancy [193]. Interestingly, concentrations of large EVs gradually increase through pregnancy irrespective of their origin [186] and these EVs convey pro-inflammatory and pro-thrombotic antigens that might contribute to the hypercoagulable state observed in the last trimester of pregnancy [186]. Chang et al. identified that high levels of preeclamptic exosomes contain abundant sFlt-1 and sEng that can induce vascular dysfunction as these proteins were captured by vascular endothelial cells [194]. Tannetta et al. investigated the level of expression of placental protein 13 in syncytiotrophoblast derived extracellular vesicles (STBEVs) isolated from PE and normal pregnancy placental perfusate and found it was low in PE placenta [195]. Tong et al. described a novel mechanism by which placental EVs can attenuate PE pathogenesis in the presence of antiphospholipid antibody (aPL), which can induce the synthesis of toll-like receptors on placental EVs to increase the level of expression of mitochondrial DNA in these vesicles [196]. Thus, placenta-derived EVs are involved in gene regulation, placental homeostasis and cellular function that overall reflect the placental-maternal crosstalk [197]. Placental exosomes were also observed in fetal blood and their concentration correlated with fetal growth [198]. The concentration of placental exosomes in the fetal circulation was higher than that found in the maternal circulation and was also higher in pregnancies affected by PE [199]. Interestingly, not only the concentration of circulation exosomes in PE is different compared with normal pregnancies, and specific changes in the protein cargo of exosomes in PE have been identified [200].
Another recent study measured the level of different biomarkers including copeptin, annexin V and placental growth factor in maternal serum derived microparticles at 10–14 gestational weeks in women with PE and compared with that of normal healthy pregnancy [201]. Interestingly, the levels of nitric oxide synthase enzyme in the STBEVs were lower in STBEVs from PE compared to normal pregnancies [202]. In this regard, in a similar study, the levels of the protein neprilysin were increased in EVs of PE placenta [203].
Kohli et al. identified a novel pathway by which the placental EVs interact and causes release of EVs from endothelial cells and platelets that further activate the inflammasome in the trophoblast resulting in the development of PE [204]. The role of EVs in relation to PE pathophysiology including their different contents has been summarized in Table 2.
Table 2.
Updated Research Studies on EVs in PE Pathophysiology.
| EVs | Sample Type | Gestational Age | Isolation Method | Pregnancy Condition | Biological Process/Results | Reference |
|---|---|---|---|---|---|---|
| Maternal Blood Stream and other Body Fluids | ||||||
| Trophoblast derived exosomal micro RNA (has-miR-210) | Plasma and HTR-8 cell culture conditioned media | Third trimester | Membrane affinity spin column method | Normal and PE | This micro RNA is responsible for PE pathogenesis | [187] |
| Exosomes | Plasma | Third trimester (before cesarean section) | Commercial kit (ExoQuick) | Normal and PE | Vascular dysfunction | [194] |
| Exosomal micro RNAs | Placental mesenchymal stem cells culture conditioned media and peripheral blood | During cesarean section delivery | Ultracentrifugation followed by Real Time PCR | Normal Pregnancy (NP) and PE | High level of exosomal miRNA-136, 494, 495 in PE | [205] |
| Urinary Exosomal proteins | Urine | After 20 weeks | Centrifugation | Healthy non-pregnant, Normal pregnancy, PE | Phosphorylation of renal tubular sodium transporter proteins that enhance sodium reabsorption in PE compared to NP | [206] |
| Exosomes | Human umbilical cord mesenchymal stem cells (MSC) | After delivery | Flow cytometry based detection of MSC surface markers | PE | Effect on placental tissue morphology and angiogenesis in rat PE placenta | [207,208] |
| Placental syncytiotrophoblast derived extracellular vesicles (STBEVs) | Placental perfusate | Following cesarean section delivery | Centrifugation | Normal and PE pregnancy | Lower level of placental protein 13 was found in STBEVs of PE placenta | [195] |
| Placental extracellular vesicles | Cultured human placental villi explant and Maternal serum | First trimester placenta | Sequential centrifugation and ultracentrifugation | Normal pregnancy | Presence of antiphospholipid antibody increases the level of mitochondrial DNA in the placental EVs and increases the risk to develop PE | [196] |
| Microparticles | Maternal serum | 10–14 weeks | Centrifugation | Normal, PE, IUGR | Serum copeptin, annexin V were higher and placental growth factor was low in PE | [201] |
| Macovesicles/placental debris | Placental explant and maternal serum | First trimester (8–10 weeks) | Centrifugation | PE | Melatonin is secreted from placental explant that reduce PE sera induced production of endothelial cell activating placental EVs | [209] |
| Nanovesicles | Placenta | First trimester and term placenta | Differential centrifugation | PE | Transthyretin is increased in amount and incorporated in placental nanovesicles | [210] |
| EVs | Urine | Maternal urine | EVs were stained for annexin, nephrin and podocin proteins | PE and Normotensive pregnant women | Nephrin protein was packaged in increased amount in urinary EVs of PE women | [211] |
| Syncytiotrophoblast derived extracellular vesicles (STBEV) | Placental perfusion and maternal plasma | Gestational age matched | Differential centrifugation | Normal and PE | Less nitric oxide synthase in STBEVs of PE women | [202] |
| EVs | Placental explant | First and second trimester | Sequential centrifugation | Normal and PE | Endothelial dysfunction in severe early onset PE is via soluble angiogenic factors, not by EVs | [212] |
| Exosomes | Maternal plasma | First, second and third trimester | Differential centrifugation, ultracentrifugation followed by density gradient centrifugation | Normal and PE | The concentration of exosomes is higher and miRNA content is different in PE compared to normal pregnancy | [184] |
| Microparticles | Placental trophoblasts | At term (>37 weeks) | Two-step centrifugation | Uncomplicated and preeclamptic | Increase MP shedding from PE placenta; upregulation of caveolin-1 and downregulation of eNOS in these MPs which is modulated by vitamin-D | [213] |
| EVs | Endothelial cells and Platelets | Not mentioned | Differential centrifugation | Normal and PE | Inflammasome activation in placental trophoblasts results in PE development | [204] |
| Fetal Circulation | ||||||
| Exosomes | Umbilical cord blood | At delivery | Differential Centrifugation + Density gradient centrifugation | Normal | No difference in concentration of exosomes in term, small for gestational age, fetal growth restricted neonates | [198] |
| Microparticle (MPs) | Umbilical cord blood | At delivery | MPs were identified by size and annexin V fluorescein isothiocyanate (FITC) labelling | Normal and PE | MP levels is higher compared to maternal blood in PE | [199] |
| Exosomes | Umbilical cord blood | At delivery | Differential centrifugation + Filtration | Normal and PE | Altered protein expression profile that are involved with PE etiology | [200] |
A number of drugs that can be used to treat PE appear to modulate EV expression. Some studies also addressed the mode of action of different antihypertensives, including thiazide diuretics that are used to treat the hypertension in PE. Hu et al. identified some changes in the sodium transporters in the renal tubule that were incorporated in the urinary exosomes isolated from PE women [206]. Another very interesting study by Chamley L. et al. identified melatonin as an effective agent that can reduce the endothelial cell activating placental EVs release in PE [209]. In a similar study, transthyretin which is the thyroxin binding protein, was found in aggregated form and packaged in the small placental EVs in PE [210]. Xu et al. identified potential molecular mechanisms by which vitamin-D can reduce oxidative-stress induced PE [213]. Among the different therapeutic agents, the efficacy of aspirin was evaluated due to its availability and cost-effectiveness. However, there is lack of understanding in the mechanism of action of aspirin in the context of EV secretion regulation.
11. Effects of LDA on Exosomal Secretion
Up until now, there has been very limited evidence on the potential effect of aspirin on the release and content of EVs. Goetzl E.J. et al., discovered that in the presence of some coagulation factors (e.g., thrombin/ collagen) induces changes in the plasmatic levels of platelet-derived exosomes and their protein content (i.e., α-granule chemokines CXCL4 and CXCL7 and cytoplasmic high-mobility group box 1 (HMGB1)) [214]. Incubation of normal platelets with aspirin significantly inhibits arachidonic acid (AA)-induced platelet reactivity, EV formation and pro-coagulant activity [215]. Interestingly, aspirin therapy can significantly reduce microparticle (MP) shredding from erythrocytes, monocytes and vascular smooth muscle cells, reversing the effects of diabetes-induced stress on these cells [216]. Other studies have identified that aspirin changes the miRNA profile and EV release from platelet [217]. Syncytiotropholast-derived extracellular vesicles that are placental alkaline phosphatase (PLAP) positive inhibit the aggregation of platelets that were treated with aspirin [218]. Tannetta et al. observed that STBEV that are placental alkaline phosphatase (PLAP) positive inhibit the aggregation of platelets that were treated with aspirin [218]. On the contrary, platelets were activated and thrombus formation was increased by the STBEV isolated from preeclamptic placentas. Another study observed the effect of anticoagulant therapy (treatment with either unfractionated heparin (UFH) or low molecular weight heparin (LMWH), and/or LDA) on cell derived microparticles and outcome of pregnancy [219]. These findings indicate that placenta-derived extracellular vesicles may provide understanding in their potential role in low dose aspirin induced placental functions. Although there is significant evidence for dysregulation of both concentrations and bioactivity of circulating placental EVs in PE compared to normal pregnancies, no studies have described the potential effect of aspirin on EVs released from placental cells and their bioactivity.
12. Summary
Several studies focused on EVs (mainly small EVs called exosomes) highlighting their extraordinary characteristics as natural carriers of bioactive molecules, which can be used as biomarker for several pathological conditions including PE. These vesicles are unique in terms of their cell trafficking and transfecting capabilities. These nanovesicles are released from almost all types of cells into different human body fluids. Their release and contents are dependent on the microenvironment where the cells are exposed and the origin of the cells [172]. Therefore, EVs can be used as a diagnostic tool as well as prognostic marker for several pathologies, and we have proposed that the analysis of placental vesicles in maternal plasma can function as a liquid biopsy to establish placental function during pregnancy. During pregnancy, placenta and other cells, such as platelets and immune cells, secrete exosomes into the maternal circulation; this process is exaggerated in pathological pregnancies (i.e., PE, PIH, IUGR) in an attempt to modulate the pathology [43,190,191]. Very few studies [218,219] have been conducted to identify the particular mechanism of action that exosomes can produce on the placenta and overall maternal physiological system when treated with low dose aspirin and other antithrombotic medication. An avenue is open to explore the placental and other cell derived exosomal functions in placental dysfunctional disorders when treated with antithrombotic medications including low dose aspirin. A better understanding of these processes may lead to the development of novel prognostic markers utilizing placenta specific exosomes or for monitoring the response to aspirin treatment for placental pathologies.
Funding
Carlos Salomon is supported by The Lions Medical Research Foundation, National Health and Medical Research Council (NHMRC; 1114013) and the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1170809). Suchismita Dutta received the Australian postgraduate award scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mendes S. New Insights into the Process of Placentation and the Role of Oxidative Uterine Microenvironment. Oxid. Med. Cell. Longev. 2019;2019:9174521. doi: 10.1155/2019/9174521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solano M.E. Decidual immune cells: Guardians of human pregnancies. Best Pr. Res. Clin. Obs. Gynaecol. 2019 doi: 10.1016/j.bpobgyn.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Burton G.J. Pre-eclampsia: Pathophysiology and clinical implications. BMJ. 2019;366:12381. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 4.Bose P. Heparin and aspirin attenuate placental apoptosis in vitro: Implications for early pregnancy failure. Am. J. Obstet. Gynecol. 2005;192:23–30. doi: 10.1016/j.ajog.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 5.Panagodage S. Low-Dose Acetylsalicylic Acid Treatment Modulates the Production of Cytokines and Improves Trophoblast Function in an in Vitro Model of Early-Onset Preeclampsia. Am. J. Pathol. 2016;186:3217–3224. doi: 10.1016/j.ajpath.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Villa P.M., Kajantie E., Laivuori H. Acetylsalicylic acid and prevention of preeclampsia. Duodecim. 2014;130:243–250. [PubMed] [Google Scholar]
- 7.Oyola S., Kirley K. Another good reason to recommend Low-Dose Aspirin. J. Fam. Pract. 2015;64:301–303. [PMC free article] [PubMed] [Google Scholar]
- 8.Yao S., Wu H., Yu Y. Early intervention with aspirin for preventing preeclampsia in high-risk women: A meta-analysis. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:868–873. [PubMed] [Google Scholar]
- 9.Andreoli L., Bertsias G.K., Agmon-Levin N., Brown S., Cervera R., Costedoat-Chalumeau N., Doria A., Fischer-Betz R., Forger F., Moraes-Fontes M.F., et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 2016;76:476–485. doi: 10.1136/annrheumdis-2016-209770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L. Efficacy evaluation of low-dose aspirin in IVF/ICSI patients evidence from 13 RCTs: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7720. doi: 10.1097/MD.0000000000007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bujold E. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: A meta-analysis. Obstet. Gynecol. 2010;116:402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 12.Villa P.M. Aspirin in the prevention of pre-eclampsia in high-risk women: A randomised placebo-controlled PREDO Trial and a meta-analysis of randomised trials. Bjog. 2013;120:64–74. doi: 10.1111/j.1471-0528.2012.03493.x. [DOI] [PubMed] [Google Scholar]
- 13.Fantasia H.C. Low-Dose Aspirin for the Prevention of Preeclampsia. Nurs. Womens Health. 2018;22:87–92. doi: 10.1016/j.nwh.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Roberge S. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: A systematic review and meta-analysis. Am. J. Perinatol. 2012;29:551–556. doi: 10.1097/01.ogx.0000425641.72994.b5. [DOI] [PubMed] [Google Scholar]
- 15.Xu T.T., Zhou F., Deng C.Y., Huang G.Q., Li J.K., Wang X.D. Low-Dose Aspirin for Preventing Preeclampsia and Its Complications: A Meta-Analysis. J. Clin. Hypertens (Greenwich) 2015;17:567–573. doi: 10.1111/jch.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahrens K.A. Complications and Safety of Preconception Low-Dose Aspirin Among Women WITH Prior Pregnancy Losses. Obstet. Gynecol. 2016;127:689–698. doi: 10.1097/AOG.0000000000001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cronqvist T. Syncytiotrophoblast derived extracellular vesicles transfer functional placental miRNAs to primary human endothelial cells. Sci. Rep. 2017;7:4558. doi: 10.1038/s41598-017-04468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holcberg G. Recurrent Pregnancy Loss. Springer International Publishing; Basel, Switzerland: 2016. Implantation, Physiology of Placentation. [Google Scholar]
- 19.Haller-Kikkatalo K. Autoimmune activation toward embryo implantation is rare in immune-privileged human endometrium. Semin. Reprod. Med. 2014;32:376–384. doi: 10.1055/s-0034-1376356. [DOI] [PubMed] [Google Scholar]
- 20.Moser G., Huppertz B. Implantation and extravillous trophoblast invasion: From rare archival specimens to modern biobanking. Placenta. 2017;56:19–26. doi: 10.1016/j.placenta.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato Y., Fujiwara H., Konishi I. Role of platelets in placentation. Med. Mol. Morphol. 2010;43:129–133. doi: 10.1007/s00795-010-0508-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhao S. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta. 2008;29:81–88. doi: 10.1016/j.placenta.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mierzynski R. Anticoagulant therapy in pregnant patients with metabolic syndrome: A review. Curr. Pharm. Biotechnol. 2014;15:47–63. doi: 10.2174/1389201015666140330194049. [DOI] [PubMed] [Google Scholar]
- 24.Graves J.C., Miller K.E., Sellers A.D. Maternal serum triple analyte screening in pregnancy. Am. Fam. Physician. 2002;65:915–920. [PubMed] [Google Scholar]
- 25.Understanding Genetics. Genetic Alliance; Washington, DC, USA: 2010. Maternal Serum Marker Screening. [Google Scholar]
- 26.Nwanodi O.B. Preeclampsia-Eclampsia Adverse Outcomes Reduction: The Preeclampsia-Eclampsia Checklist. Healthcare. 2016;4:26. doi: 10.3390/healthcare4020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helou A. Management of pregnancies complicated by hypertensive disorders of pregnancy: Could we do better? Aust. N. Z. J. Obstet. Gynaecol. 2016;57:253–269. doi: 10.1111/ajo.12499. [DOI] [PubMed] [Google Scholar]
- 28.Pai C.H. Lack of Thromboxane Synthase Prevents Hypertension and Fetal Growth Restriction after High Salt Treatment during Pregnancy. PLoS ONE. 2016;11:e0151617. doi: 10.1371/journal.pone.0151617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakhti A., Vaiman D. Prevention of gravidic endothelial hypertension by aspirin treatment administered from the 8th week of gestation. Hypertens Res. 2011;34:1116–1120. doi: 10.1038/hr.2011.111. [DOI] [PubMed] [Google Scholar]
- 30.Bartsch E. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:1753. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolaides K.H. A model for a new pyramid of prenatal care based on the 11 to 13 weeks’ assessment. Prenat. Diagn. 2011;31:3–6. doi: 10.1002/pd.2685. [DOI] [PubMed] [Google Scholar]
- 32.McCoy S., Baldwin K. Pharmacotherapeutic options for the treatment of preeclampsia. Am. J. Health Syst. Pharm. 2009;66:337–344. doi: 10.2146/ajhp080104. [DOI] [PubMed] [Google Scholar]
- 33.Gilani S.I. Preeclampsia and Extracellular Vesicles. Curr. Hypertens. Rep. 2016;18:1–11. doi: 10.1007/s11906-016-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burton G.J. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman A.M., Cleary K.L. Prediction and prevention of ischemic placental disease. Semin. Perinatol. 2014;38:177–182. doi: 10.1053/j.semperi.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Cuckle H., von Dadelszen P., Ghidini A. Current controversies in prenatal diagnosis 4: Pregnancy complications due to placental vascular disease (pre-eclampsia, FGR): Are we ready for prevention? Prenat. Diagn. 2013;33:17–20. doi: 10.1002/pd.4016. [DOI] [PubMed] [Google Scholar]
- 37.Armant D.R. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development. 2006;133:751–759. doi: 10.1242/dev.02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redman C.W., Sargent I.L. Placental stress and pre-eclampsia: A revised view. Placenta. 2009;30:S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Blumenstein M. A proteomic approach identifies early pregnancy biomarkers for preeclampsia: Novel linkages between a predisposition to preeclampsia and cardiovascular disease. Proteomics. 2009;9:2929–2945. doi: 10.1002/pmic.200800625. [DOI] [PubMed] [Google Scholar]
- 40.Auer J. Serum profile in preeclampsia and intra-uterine growth restriction revealed by iTRAQ technology. J. Proteom. 2010;73:1004–1017. doi: 10.1016/j.jprot.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Chelbi S.T. Expressional and epigenetic alterations of placental serine protease inhibitors: SERPINA3 is a potential marker of preeclampsia. Hypertension. 2007;49:76–83. doi: 10.1161/01.HYP.0000250831.52876.cb. [DOI] [PubMed] [Google Scholar]
- 42.Larsen T.G. Fetal human leukocyte antigen-C and maternal killer-cell immunoglobulin-like receptors in cases of severe preeclampsia. Placenta. 2019;75:27–33. doi: 10.1016/j.placenta.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Redman C.W., Sargent I.L. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 44.Furuya M. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vasc. Health Risk Manag. 2008;4:1301–1313. doi: 10.2147/VHRM.S4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Granger J.P. Pathophysiology of preeclampsia: Linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 46.Lyall F., Myatt L. The role of the placenta in pre-eclampsia--a workshop report. Placenta. 2002;23:142–145. doi: 10.1053/plac.2002.0803. [DOI] [PubMed] [Google Scholar]
- 47.Lockwood C.J. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am. J. Pathol. 2008;172:1571–1579. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benyo D.F. Expression of inflammatory cytokines in placentas from women with preeclampsia. J. Clin. Endocrinol. Metab. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 49.Raghupathy R. Cytokines as key players in the pathophysiology of preeclampsia. Med. Princ. Pr. 2013;22:8–19. doi: 10.1159/000354200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaughan J.E., Walsh S.W., Ford G.D. Thromboxane mediates neutrophil superoxide production in pregnancy. Am. J. Obstet. Gynecol. 2006;195:1415–1420. doi: 10.1016/j.ajog.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 51.Walsh S.W. Eicosanoids in preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids. 2004;70:223–232. doi: 10.1016/j.plefa.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Walsh S.W. Preeclampsia: An imbalance in placental prostacyclin and thromboxane Production. Am. J. Obstet. Gynecol. 1985;152:335–340. doi: 10.1016/S0002-9378(85)80223-4. [DOI] [PubMed] [Google Scholar]
- 53.Reslan O.M., Khalil R.A. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc. Hematol. Agents Med. Chem. 2010;8:204–226. doi: 10.2174/187152510792481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yusuf K. Thromboxane A2 Limits Differentiation and Enhances Apoptosis of Cultured Human Trophoblasts. Pediatr. Res. 2001;50:203–209. doi: 10.1203/00006450-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Ally A.I., Horrobin D.F. Thromboxane A2 in blood vessel walls and its physiological significance: Relevance to thrombosis and hypertension. Prostaglandins Med. 1980;4:431–438. doi: 10.1016/0161-4630(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 56.Sellers M.M., Stallone J.N. Sympathy for the devil: The role of thromboxane in the regulation of vascular tone and blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1978–H1986. doi: 10.1152/ajpheart.01318.2007. [DOI] [PubMed] [Google Scholar]
- 57.Gilbert J.S. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 58.Downing J.W. Hypothesis: Selective phosphodiesterase-5 inhibition improves outcome in preeclampsia. Med. Hypotheses. 2004;63:1057–1064. doi: 10.1016/j.mehy.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 59.Euser A.G. Low-dose aspirin for pre-eclampsia prevention in twins with elevated human chorionic gonadotropin. J. Perinatol. 2016;36:601–605. doi: 10.1038/jp.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Simone N. Pathogenic role of anti-beta2-glycoprotein I antibodies on human placenta: Functional effects related to implantation and roles of heparin. Hum. Reprod. Update. 2007;13:189–196. doi: 10.1093/humupd/dml051. [DOI] [PubMed] [Google Scholar]
- 61.Anderson U.D. First trimester prediction of preeclampsia. Curr. Hypertens Rep. 2015;17:584. doi: 10.1007/s11906-015-0584-7. [DOI] [PubMed] [Google Scholar]
- 62.Meiri H. Prediction of preeclampsia by placental protein 13 and background risk factors and its prevention by aspirin. J. Perinat. Med. 2014;42:591–601. doi: 10.1515/jpm-2013-0298. [DOI] [PubMed] [Google Scholar]
- 63.Seravalli V. Relationship between first-trimester serum placental protein-13 and maternal characteristics, placental Doppler studies and pregnancy outcome. J. Perinat. Med. 2016;44:543–549. doi: 10.1515/jpm-2015-0324. [DOI] [PubMed] [Google Scholar]
- 64.Rolnik D.L. Maternal plasma cell-free DNA in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2015;45:106–111. doi: 10.1002/uog.14671. [DOI] [PubMed] [Google Scholar]
- 65.Schrör K. Acetylsalicylic Acid. John Wiley and Sons; Weinheim, Germany: 2010. Acetylsalicylic Acid. [Google Scholar]
- 66.Cadavid A.P. Aspirin: The Mechanism of Action Revisited in the Context of Pregnancy Complications. Front. Immunol. 2017;8:261. doi: 10.3389/fimmu.2017.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lafont O. From the willow to aspirin. Rev. Hist. Pharm. (Paris) 2007;55:209–216. doi: 10.3406/pharm.2007.6334. [DOI] [PubMed] [Google Scholar]
- 68.Botting R.M. Vane’s discovery of the mechanism of action of aspirin changed our understanding of its clinical pharmacology. Pharm. Rep. 2010;62:518–525. doi: 10.1016/S1734-1140(10)70308-X. [DOI] [PubMed] [Google Scholar]
- 69.West G.B. Aspirin and the prostaglandins. Chem. Drug. 1972;198:196–197. [PubMed] [Google Scholar]
- 70.Finkel R.M.A.C., Luigi X., editors. Antiinflammatory Drugs and Autacoids in Lippincott’s Illustrated Reviews: Pharmacology. 4th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2009. p. 499. [Google Scholar]
- 71.Walsh S.W. Low-dose aspirin: Treatment for the imbalance of increased thromboxane and decreased prostacyclin in preeclampsia. Am. J. Perinatol. 1989;6:124–132. doi: 10.1055/s-2007-999562. [DOI] [PubMed] [Google Scholar]
- 72.Perneby C. Thromboxane Metab. Excretion Dur. Pregnancy--Influ. Preeclampsia Aspirin Treat. Thromb. Res. 2011;127:605–606. doi: 10.1016/j.thromres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Sibai B.M. Low-dose aspirin in pregnancy. Obstet. Gynecol. 1989;74:551–557. doi: 10.1097/00132582-199004000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Li C. Aspirin inhibits expression of sFLT1 from human cytotrophoblasts induced by hypoxia, via cyclo-oxygenase 1. Placenta. 2015;36:446–453. doi: 10.1016/j.placenta.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Da Silva Costa F. 274: Low-dose aspirin improves trophoblastic function in early-onset pre-eclampsia. Am. J. Obstet. Gynecol. 2014;210:S145. doi: 10.1016/j.ajog.2013.10.307. [DOI] [Google Scholar]
- 76.Gil-Villa A.M. Aspirin triggered-lipoxin A4 reduces the adhesion of human polymorphonuclear neutrophils to endothelial cells initiated by preeclamptic plasma. Prostaglandins Leukot. Essent. Fat. Acids. 2012;87:127–134. doi: 10.1016/j.plefa.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sentilhes L., Azria E., Schmitz T. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017;377:2399–2400. doi: 10.1056/NEJMc1713798. [DOI] [PubMed] [Google Scholar]
- 78.Navaratnam K., Alfirevic A., Alfirevic Z. Low dose aspirin and pregnancy: How important is aspirin resistance? Bjog. 2016;123:1481–1487. doi: 10.1111/1471-0528.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bujold E. Low-dose aspirin reduces morbidity and mortality in pregnant women at high-risk for preeclampsia. Evid. Based Nurs. 2015;18:71. doi: 10.1136/ebnurs-2014-101915. [DOI] [PubMed] [Google Scholar]
- 80.Sibai B.M. Therapy: Low-dose aspirin to reduce the risk of pre-eclampsia? Nat. Rev. Endocrinol. 2015;11:6–8. doi: 10.1038/nrendo.2014.199. [DOI] [PubMed] [Google Scholar]
- 81.Henderson J.T., Whitlock E.P., O’Connor E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann. Intern. Med. 2014;160:695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- 82.Roberge S., Demers S., Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann. Intern. Med. 2014;161:613. doi: 10.7326/L14-5020-4. [DOI] [PubMed] [Google Scholar]
- 83.Bartsch E. Risk threshold for starting low dose aspirin in pregnancy to prevent preeclampsia: An opportunity at a low cost. PLoS ONE. 2015;10:e0116296. doi: 10.1371/journal.pone.0116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toyoda K. Antithrombotic therapy for pregnant women. Neurol. Med. Chir. (Tokyo) 2013;53:526–530. doi: 10.2176/nmc.53.526. [DOI] [PubMed] [Google Scholar]
- 85.Yurdakok M. Fetal and neonatal effects of anticoagulants used in pregnancy: A review. Turk. J. Pediatr. 2012;54:207–215. [PubMed] [Google Scholar]
- 86.Askie L.M. Antiplatelet agents for prevention of pre-eclampsia: A meta-analysis of individual patient data. Lancet. 2007;369:1791–1798. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 87.Roberge S. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017;216:110–120. doi: 10.1016/j.ajog.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 88.Summaries for patients: Aspirin to prevent preeclampsia-related complications and death: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014;161:I28. doi: 10.7326/P14-9041. [DOI] [PubMed] [Google Scholar]
- 89.Roberge S., Demers S., Bujold E. Initiation of aspirin in early gestation for the prevention of pre-eclampsia. Bjog. 2013;120:773–774. doi: 10.1111/1471-0528.12170. [DOI] [PubMed] [Google Scholar]
- 90.Ortved D. Cost-effectiveness of first-trimester screening with early preventative use of aspirin in women at high risk of early-onset pre-eclampsia. Ultrasound Obstet. Gynecol. 2019;53:239–244. doi: 10.1002/uog.19076. [DOI] [PubMed] [Google Scholar]
- 91.LeFevre M.L. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014;161:819–826. doi: 10.7326/M14-1884. [DOI] [PubMed] [Google Scholar]
- 92.Bond S. US Preventive Services Task Force guideline supports low-dose aspirin for prevention of preeclampsia. J. Midwifery Womens Health. 2015;60:222–223. doi: 10.1111/jmwh.12301_4. [DOI] [PubMed] [Google Scholar]
- 93.Sidaway P. Pre-eclampsia: Low-dose aspirin for pre-eclampsia. Nat. Rev. Nephrol. 2014;10:613. doi: 10.1038/nrneph.2014.183. [DOI] [PubMed] [Google Scholar]
- 94.Voelker R. USPSTF: Low-dose aspirin may help reduce risk of preeclampsia. JAMA. 2014;311:2055. doi: 10.1001/jama.2014.5390. [DOI] [PubMed] [Google Scholar]
- 95.Ayala D.E., Ucieda R., Hermida R.C. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol. Int. 2013;30:260–279. doi: 10.3109/07420528.2012.717455. [DOI] [PubMed] [Google Scholar]
- 96.Mutlu I. Effects of anticoagulant therapy on pregnancy outcomes in patients with thrombophilia and previous poor obstetric history. Blood Coagul. Fibrinolysis. 2015;26:267–273. doi: 10.1097/MBC.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 97.Dodd J.M. Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction. Cochrane Database Syst. Rev. 2013;24:Cd006780. doi: 10.1002/14651858.CD006780.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chauleur C. News on antithrombotic therapy and pregnancy. Therapie. 2011;66:437–443. doi: 10.2515/therapie/2011061. [DOI] [PubMed] [Google Scholar]
- 99.De Vries J.I. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: The FRUIT-RCT. J. Thromb. Haemost. 2012;10:64–72. doi: 10.1111/j.1538-7836.2011.04553.x. [DOI] [PubMed] [Google Scholar]
- 100.Gris J.C. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre-eclampsia. The pilot randomised controlled NOH-PE trial. Thromb. Haemost. 2011;106:1053–1061. doi: 10.1160/TH11-05-0340. [DOI] [PubMed] [Google Scholar]
- 101.Rodger M.A. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: A meta-analysis of individual patient data from randomised controlled trials. Lancet. 2016;388:2629–2641. doi: 10.1016/S0140-6736(16)31139-4. [DOI] [PubMed] [Google Scholar]
- 102.Souza E.V. Aspirin plus calcium supplementation to prevent superimposed preeclampsia: A randomized trial. Braz. J. Med. Biol. Res. 2014;47:419–425. doi: 10.1590/1414-431X20143629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Browne J.L. Prevention of Hypertensive Disorders of Pregnancy: A Novel Application of the Polypill Concept. Curr. Cardiol. Rep. 2016;18:1–11. doi: 10.1007/s11886-016-0725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grandone E., Villani M., Tiscia G.L. Aspirin and heparin in pregnancy. Expert Opin. Pharm. 2015;16:1793–1803. doi: 10.1517/14656566.2015.1066335. [DOI] [PubMed] [Google Scholar]
- 105.Ghesquiere L. Can we prevent preeclampsia? Presse. Med. 2016;45:403–413. doi: 10.1016/j.lpm.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 106.Kane S.C., Da Silva Costa F., Brennecke S.P. New directions in the prediction of pre-eclampsia. Aust. N. Z. J. Obstet. Gynaecol. 2014;54:101–107. doi: 10.1111/ajo.12151. [DOI] [PubMed] [Google Scholar]
- 107.Jiang N. The effect of calcium channel blockers on prevention of preeclampsia in pregnant women with chronic hypertension. Clin. Exp. Obstet. Gynecol. 2015;42:79–81. [PubMed] [Google Scholar]
- 108.Neykova K. Antithrombotic Medication in Pregnant Women with Previous Intrauterine Growth Restriction. Akush Ginekol. (Sofiia) 2016;55:3–9. [PubMed] [Google Scholar]
- 109.Maged A.M. The role of prophylactic use of low dose aspirin and calheparin in patients with unexplained recurrent abortion. Gynecol. Endocrinol. 2016;32:970–972. doi: 10.1080/09513590.2016.1203408. [DOI] [PubMed] [Google Scholar]
- 110.Gan J., He H., Qi H. Preventing preeclampsia and its fetal complications with low-dose aspirin in East Asians and non-East Asians: A systematic review and meta-analysis. Hypertens Pregnancy. 2016;35:426–435. doi: 10.1080/10641955.2016.1178772. [DOI] [PubMed] [Google Scholar]
- 111.Tong S., Mol B.W., Walker S.P. Preventing preeclampsia with aspirin: Does dose or timing matter? Am. J. Obstet. Gynecol. 2017;216:95–97. doi: 10.1016/j.ajog.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 112.Mone F. Should we recommend universal aspirin for all pregnant women? Am. J. Obstet. Gynecol. 2017;216:e1–e141. doi: 10.1016/j.ajog.2016.09.086. [DOI] [PubMed] [Google Scholar]
- 113.Meher S. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: An individual participant data meta-analysis. Am. J. Obstet. Gynecol. 2017;216:121–128. doi: 10.1016/j.ajog.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 114.Roberge S. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: A meta-analysis. Ultrasound Obstet. Gynecol. 2013;41:491–499. doi: 10.1002/uog.12421. [DOI] [PubMed] [Google Scholar]
- 115.O’Gorman N. Study protocol for the randomised controlled trial: Combined multimarker screening and randomised patient treatment with ASpirin for evidence-based PREeclampsia prevention (ASPRE) BMJ Open. 2016;6:e011801. doi: 10.1136/bmjopen-2016-011801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Etwel F., Koren G. When positive studies of novel therapies are subsequently nullified: Cumulative meta-analyses in preeclampsia. Clin. Investig. Med. 2015;38:274–283. doi: 10.25011/cim.v38i5.25684. [DOI] [PubMed] [Google Scholar]
- 117.Truong A. Subchorionic hematomas are increased in early pregnancy in women taking low-dose aspirin. Fertil. Steril. 2016;105:1241–1246. doi: 10.1016/j.fertnstert.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 118.Van Hoorn M.E. Low-molecular-weight heparin and aspirin in the prevention of recurrent early-onset pre-eclampsia in women with antiphospholipid antibodies: The FRUIT-RCT. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;197:168–173. doi: 10.1016/j.ejogrb.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 119.Hoffman M.K. A description of the methods of the aspirin supplementation for pregnancy indicated risk reduction in nulliparas (ASPIRIN) study. BMC Pregnancy Childbirth. 2017;17:135. doi: 10.1186/s12884-017-1312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mumford S.L. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum. Reprod. 2016;31:657–665. doi: 10.1093/humrep/dev329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rolnik D.L., Wright D., Poon L.C., O’Gorman N., Syngelaki A., de Paco Matallana C., Akolekar R., Cicero S., Janga D., Singh M., et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017;377:613–622. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 122.Orendi K. Effects of vitamins C and E, acetylsalicylic acid and heparin on fusion, beta-hCG and PP13 expression in BeWo cells. Placenta. 2010;31:431–438. doi: 10.1016/j.placenta.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 123.Rigourd V. Re-evaluation of the role of STOX1 transcription factor in placental development and preeclampsia. J. Reprod. Immunol. 2009;82:174–181. doi: 10.1016/j.jri.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 124.Benigni A. Effect of low-dose aspirin on fetal and maternal generation of thromboxane by platelets in women at risk for pregnancy-induced hypertension. N. Engl. J. Med. 1989;321:357–362. doi: 10.1056/NEJM198908103210604. [DOI] [PubMed] [Google Scholar]
- 125.Schiff E. The use of aspirin to prevent pregnancy-induced hypertension and lower the ratio of thromboxane A2 to prostacyclin in relatively high risk pregnancies. N. Engl. J. Med. 1989;321:351–356. doi: 10.1056/NEJM198908103210603. [DOI] [PubMed] [Google Scholar]
- 126.Sibai B.M. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med. 1993;329:1213–1218. doi: 10.1056/NEJM199310213291701. [DOI] [PubMed] [Google Scholar]
- 127.Knight M. Antiplatelet agents for preventing and treating pre-eclampsia. Cochrane Database Syst. Rev. 2000:Cd000492. doi: 10.1002/14651858.CD000492. [DOI] [PubMed] [Google Scholar]
- 128.Woodworth S.H. Eicosanoid biosynthetic enzymes in placental and decidual tissues from preeclamptic pregnancies: Increased expression of thromboxane-A2 synthase gene. J. Clin. Endocrinol. Metab. 1994;78:1225–1231. doi: 10.1210/jcem.78.5.8175982. [DOI] [PubMed] [Google Scholar]
- 129.Katsi V. Aspirin vs Heparin for the Prevention of Preeclampsia. Curr. Hypertens Rep. 2016;18:57. doi: 10.1007/s11906-016-0664-3. [DOI] [PubMed] [Google Scholar]
- 130.Pijnenborg R. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/S0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- 131.Brosens I. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jamal A., Milani F., Al-Yasin A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran. J. Reprod. Med. 2012;10:265–270. [PMC free article] [PubMed] [Google Scholar]
- 133.Turan O. 284: Starting aspirin (ASA) in the first trimester (T1) promotes placental invasion in low-risk pregnancy. Am. J. Obstet. Gynecol. 2014;210:S149–S150. doi: 10.1016/j.ajog.2013.10.317. [DOI] [Google Scholar]
- 134.Haapsamo M., Martikainen H., Rasanen J. Low-dose aspirin reduces uteroplacental vascular impedance in early and mid gestation in IVF and ICSI patients: A randomized, placebo-controlled double-blind study. Ultrasound Obstet. Gynecol. 2008;32:687–693. doi: 10.1002/uog.6215. [DOI] [PubMed] [Google Scholar]
- 135.Serhan C.N., Yacoubian S., Yang R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nascimento-Silva V. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: A novel antioxidative mechanism. Thromb. Haemost. 2007;97:88–98. [PubMed] [Google Scholar]
- 137.Cezar-de-Mello P.F. ATL-1, an analogue of aspirin-triggered lipoxin A4, is a potent inhibitor of several steps in angiogenesis induced by vascular endothelial growth factor. Br. J. Pharmcol. 2008;153:956–965. doi: 10.1038/sj.bjp.0707650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fiorucci S. Evidence that 5-lipoxygenase and acetylated cyclooxygenase 2-derived eicosanoids regulate leukocyte-endothelial adherence in response to aspirin. Br. J. Pharmacol. 2003;139:1351–1359. doi: 10.1038/sj.bjp.0705356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morris T. Effects of low-dose aspirin on acute inflammatory responses in humans. J. Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 140.Morris T. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc. Natl. Acad. Sci. USA. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jozsef L. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc. Natl. Acad. Sci. USA. 2002;99:13266–13271. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ariel A. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J. Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 143.Levy B.D. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 144.Nascimento-Silva V. Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am. J. Physiol. Cell Physiol. 2005;289:C557–C563. doi: 10.1152/ajpcell.00045.2005. [DOI] [PubMed] [Google Scholar]
- 145.Kim J. Aspirin prevents TNF-alpha-induced endothelial cell dysfunction by regulating the NF-kappaB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free Radic. Biol. Med. 2017;104:185–198. doi: 10.1016/j.freeradbiomed.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 146.Van Dijk M. The STOX1 genotype associated with pre-eclampsia leads to a reduction of trophoblast invasion by alpha-T-catenin upregulation. Hum. Mol. Genet. 2010;19:2658–2667. doi: 10.1093/hmg/ddq152. [DOI] [PubMed] [Google Scholar]
- 147.Van Dijk M., Drewlo S., Oudejans C.B.M. Differential methylation of STOX1 in human placenta. Epigenetics. 2010;5:736–742. doi: 10.4161/epi.5.8.13084. [DOI] [PubMed] [Google Scholar]
- 148.Fenstad M.H. STOX2 but not STOX1 is differentially expressed in decidua from pre-eclamptic women: Data from the Second Nord-Trondelag Health Study. Mol. Hum. Reprod. 2010;16:960–968. doi: 10.1093/molehr/gaq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van Dijk M., Oudejans C.B. STOX1: Key player in trophoblast dysfunction underlying early onset preeclampsia with growth retardation. J. Pregnancy. 2011;2011:521826. doi: 10.1155/2011/521826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Founds S.A. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Erlandsson L. Alpha-1 microglobulin as a potential therapeutic candidate for treatment of hypertension and oxidative stress in the STOX1 preeclampsia mouse model. Sci. Rep. 2019;9:8561. doi: 10.1038/s41598-019-44639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Doridot L. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension. 2013;61:662–668. doi: 10.1161/HYPERTENSIONAHA.111.202994. [DOI] [PubMed] [Google Scholar]
- 153.Schisterman E.F. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: Design and baseline characteristics. Paediatr Perinat Epidemiol. 2013;27:598–609. doi: 10.1111/ppe.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gizzo S. Could empirical low-dose-aspirin administration during IVF cycle affect both the oocytes and embryos quality via COX 1-2 activity inhibition? J. Assist. Reprod. Genet. 2014;31:261–268. doi: 10.1007/s10815-014-0174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Clark D.A. Aspirin and heparin to improve live birth rate in IVF for unexplained implantation failure? Reprod. Biomed. Online. 2013;26:538–541. doi: 10.1016/j.rbmo.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 156.Zhao Y. Effects of combining lowdose aspirin with a Chinese patent medicine on follicular blood flow and pregnancy outcome. Mol. Med. Rep. 2014;10:2372–2376. doi: 10.3892/mmr.2014.2570. [DOI] [PubMed] [Google Scholar]
- 157.Leeson P. Updated review identifies no adverse impact on mother or offspring during the perinatal period of aspirin use for prevention of preeclampsia. Evid. Based Med. 2015;20:11. doi: 10.1136/ebmed-2014-110056. [DOI] [PubMed] [Google Scholar]
- 158.Dasari R. Effect of maternal low dose aspirin on neonatal platelet function. Indian Pediatr. 1998;35:507–511. [PubMed] [Google Scholar]
- 159.Levy G., Garrettson L.K. Kinetics of salicylate elimination by newborn infants of mothers who ingested aspirin before delivery. Pediatrics. 1974;53:201–210. [PubMed] [Google Scholar]
- 160.Zarek S.M. Antimullerian hormone and pregnancy loss from the Effects of Aspirin in Gestation and Reproduction trial. Fertil. Steril. 2016;105:946–952. doi: 10.1016/j.fertnstert.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mazaud-Guittot S. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. J. Clin. Endocrinol. Metab. 2013;98:E1757–E1767. doi: 10.1210/jc.2013-2531. [DOI] [PubMed] [Google Scholar]
- 162.Chu S. In Utero Exposure to Aspirin and Risk of Asthma in Childhood. Epidemiology. 2016;27:726–731. doi: 10.1097/EDE.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 163.Kristensen D.M. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid) and indomethacin are anti-androgenic in the rat foetal testis. Int. J. Androl. 2012;35:377–384. doi: 10.1111/j.1365-2605.2012.01282.x. [DOI] [PubMed] [Google Scholar]
- 164.Bloor M., Paech M. Nonsteroidal anti-inflammatory drugs during pregnancy and the initiation of lactation. Anesth. Analg. 2013;116:1063–1075. doi: 10.1213/ANE.0b013e31828a4b54. [DOI] [PubMed] [Google Scholar]
- 165.Jensen M.S. Analgesics during pregnancy and cryptorchidism: Additional analyses. Epidemiology. 2011;22:610–612. doi: 10.1097/EDE.0b013e31821eca69. [DOI] [PubMed] [Google Scholar]
- 166.De Berardis G. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286–2294. doi: 10.1001/jama.2012.5034. [DOI] [PubMed] [Google Scholar]
- 167.Khazardoost S. Effect of aspirin in prevention of adverse pregnancy outcome in women with elevated alpha-fetoprotein. J. Matern. Fetal. Neonatal. Med. 2014;27:561–565. doi: 10.3109/14767058.2013.822483. [DOI] [PubMed] [Google Scholar]
- 168.Demers S., Roberge S., Bujold E. Low-dose aspirin for the prevention of adverse pregnancy outcomes in women with elevated alpha-fetoprotein. J. Matern. Fetal. Neonatal. Med. 2015;28:726. doi: 10.3109/14767058.2014.930428. [DOI] [PubMed] [Google Scholar]
- 169.Block-Abraham D.M. First-trimester risk factors for preeclampsia development in women initiating aspirin by 16 weeks of gestation. Obs. Gynecol. 2014;123:611–617. doi: 10.1097/AOG.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 170.Asemi Z. A randomized controlled clinical trial investigating the effect of calcium supplement plus low-dose aspirin on hs-CRP, oxidative stress and insulin resistance in pregnant women at risk for pre-eclampsia. Pak. J. Biol. Sci. 2012;15:469–476. doi: 10.3923/pjbs.2012.469.476. [DOI] [PubMed] [Google Scholar]
- 171.Durcin M. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles. 2017;6:1305677. doi: 10.1080/20013078.2017.1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morel O. Microparticles: A critical component in the nexus between inflammation, immunity, and thrombosis. Semin. Immunopathol. 2011;33:469–486. doi: 10.1007/s00281-010-0239-3. [DOI] [PubMed] [Google Scholar]
- 173.Gho Y.S., Lee C. Emergent properties of extracellular vesicles: A holistic approach to decode the complexity of intercellular communication networks. Mol. Biosyst. 2017;13:1291–1296. doi: 10.1039/C7MB00146K. [DOI] [PubMed] [Google Scholar]
- 174.Borges F.T., Reis L.A., Schor N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013;46:824–830. doi: 10.1590/1414-431X20132964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Di Rocco G., Baldari S., Toietta G. Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis. Stem Cells Int. 2016;2016:5029619. doi: 10.1155/2016/5029619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thery C. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Momen-Heravi F. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013;394:1253–1262. doi: 10.1515/hsz-2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang-Doran I., Zhang C.Y., Vidal-Puig A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol. Metab. 2017;28:3–18. doi: 10.1016/j.tem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 179.Mitchell M.D. Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 2015;213:S173–S181. doi: 10.1016/j.ajog.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 180.Adam S. Review: Fetal-maternal communication via extracellular vesicles Implications for complications of pregnancies. Placenta. 2017;54:83–88. doi: 10.1016/j.placenta.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 181.Salomon C., Rice G.E. Role of Exosomes in Placental Homeostasis and Pregnancy Disorders. Prog. Mol. Biol. Transl. Sci. 2017;145:163–179. doi: 10.1016/bs.pmbts.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 182.Nair S., Salomon C. Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin. Immunopathol. 2018;40:425–437. doi: 10.1007/s00281-018-0680-2. [DOI] [PubMed] [Google Scholar]
- 183.Sarker S. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014;12:204. doi: 10.1186/1479-5876-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Salomon C.G.D., Romero K.S., Longo S., Correa P., Illanes S.E., Rice G.E. Placental exosomes as early biomarker of preeclampsia—Potential role of exosomal microRNAs across gestation. J. Clin. Endocrinol. Metab. 2017;102:3182–3194. doi: 10.1210/jc.2017-00672. [DOI] [PubMed] [Google Scholar]
- 185.Salomon C. The role of placental exosomes in gestational diabetes mellitus. In: Sobrevia L., editor. Gestational Diabetes-Causes, Diagnosis and Treatment. IntechOpen; London, UK: 2013. [Google Scholar]
- 186.Radu C.M. Origin and levels of circulating microparticles in normal pregnancy: A longitudinal observation in healthy women. Scand. J. Clin. Lab. Investig. 2015;75:487–495. doi: 10.3109/00365513.2015.1052551. [DOI] [PubMed] [Google Scholar]
- 187.Biro O. Circulating exosomal and Argonaute-bound microRNAs in preeclampsia. Gene. 2019;692:138–144. doi: 10.1016/j.gene.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 188.Truong G. Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells—Liquid biopsies for monitoring complications of pregnancy. PLoS ONE. 2017;12:e0174514. doi: 10.1371/journal.pone.0174514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abou-Kheir W. HTR-8/SVneo cell line contains a mixed population of cells. Placenta. 2017;50:1–7. doi: 10.1016/j.placenta.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 190.Tannetta D.S. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: Expression of Flt-1 and endoglin. PLoS ONE. 2013;8:e56754. doi: 10.1371/journal.pone.0056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Germain S.J. Systemic inflammatory priming in normal pregnancy and preeclampsia: The role of circulating syncytiotrophoblast microparticles. J. Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 192.Redman C.W. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33:S48–S54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 193.Tong M., Chamley L.W. Placental Extracellular Vesicles and Feto-Maternal Communication. Cold Spring Harb. Perspect. Med. 2015;5:023028. doi: 10.1101/cshperspect.a023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chang X. Exosomes From Women With Preeclampsia Induced Vascular Dysfunction by Delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to Endothelial Cells. Hypertension. 2018;72:1381–1390. doi: 10.1161/HYPERTENSIONAHA.118.11706. [DOI] [PubMed] [Google Scholar]
- 195.Sammar M. Reduced placental protein 13 (PP13) in placental derived syncytiotrophoblast extracellular vesicles in preeclampsia A novel tool to study the impaired cargo transmission of the placenta to the maternal organs. Placenta. 2018;66:17–25. doi: 10.1016/j.placenta.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 196.Tong M. Antiphospholipid antibodies increase the levels of mitochondrial DNA in placental extracellular vesicles: Alarmin-g for preeclampsia. Sci. Rep. 2017;7:16556. doi: 10.1038/s41598-017-16448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Aharon A. The role of extracellular vesicles in placental vascular complications. Thromb. Res. 2015;135:S23–S25. doi: 10.1016/S0049-3848(15)50435-0. [DOI] [PubMed] [Google Scholar]
- 198.Miranda J. Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction—Liquid biopsies to monitoring fetal growth. Placenta. 2018;64:34–43. doi: 10.1016/j.placenta.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 199.Campello E. Circulating microparticles in umbilical cord blood in normal pregnancy and pregnancy with preeclampsia. Thromb. Res. 2015;136:427–431. doi: 10.1016/j.thromres.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 200.Jia R. Comparative Proteomic Profile of the Human Umbilical Cord Blood Exosomes between Normal and Preeclampsia Pregnancies with High-Resolution Mass Spectrometry. Cell Physiol. Biochem. 2015;36:2299–2306. doi: 10.1159/000430193. [DOI] [PubMed] [Google Scholar]
- 201.Jadli A. Combination of copeptin, placental growth factor and total annexin V microparticles for prediction of preeclampsia at 10-14 weeks of gestation. Placenta. 2017;58:67–73. doi: 10.1016/j.placenta.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 202.Motta-Mejia C. Placental Vesicles Carry Active Endothelial Nitric Oxide Synthase and Their Activity is Reduced in Preeclampsia. Hypertension. 2017;70:372–381. doi: 10.1161/HYPERTENSIONAHA.117.09321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gill M. Placental Syncytiotrophoblast-Derived Extracellular Vesicles Carry Active NEP (Neprilysin) and Are Increased in Preeclampsia. Hypertension. 2019;73:1112–1119. doi: 10.1161/HYPERTENSIONAHA.119.12707. [DOI] [PubMed] [Google Scholar]
- 204.Kohli S. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood. 2016;128:2153–2164. doi: 10.1182/blood-2016-03-705434. [DOI] [PubMed] [Google Scholar]
- 205.Motawi T.M.K. Role of mesenchymal stem cells exosomes derived microRNAs; miR-136, miR-494 and miR-495 in pre-eclampsia diagnosis and evaluation. Arch. Biochem. Biophys. 2018;659:13–21. doi: 10.1016/j.abb.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 206.Hu C.C. Pre-eclampsia is associated with altered expression of the renal sodium transporters NKCC2, NCC and ENaC in urinary extracellular vesicles. PLoS ONE. 2018;13:e0204514. doi: 10.1371/journal.pone.0204514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Moro L. Placental Microparticles and MicroRNAs in Pregnant Women with Plasmodium falciparum or HIV Infection. PLoS ONE. 2016;11:e0146361. doi: 10.1371/journal.pone.0146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xiong Z.H. Protective effect of human umbilical cord mesenchymal stem cell exosomes on preserving the morphology and angiogenesis of placenta in rats with preeclampsia. Biomed. Pharmacother. 2018;105:1240–1247. doi: 10.1016/j.biopha.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 209.Zhao M. Melatonin prevents preeclamptic sera and antiphospholipid antibodies inducing the production of reactive nitrogen species and extrusion of toxic trophoblastic debris from first trimester placentae. Placenta. 2017;58:17–24. doi: 10.1016/j.placenta.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 210.Tong M. Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia. Sci. Rep. 2017;7:6694. doi: 10.1038/s41598-017-07017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gilani S.I. Urinary Extracellular Vesicles of Podocyte Origin and Renal Injury in Preeclampsia. J. Am. Soc. Nephrol. 2017;28:3363–3372. doi: 10.1681/ASN.2016111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.O’Brien M., Baczyk D., Kingdom J.C. Endothelial Dysfunction in Severe Preeclampsia is Mediated by Soluble Factors, Rather than Extracellular Vesicles. Sci. Rep. 2017;7:5887. doi: 10.1038/s41598-017-06178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xu J. Vitamin D Reduces Oxidative Stress-Induced Procaspase-3/ROCK1 Activation and MP Release by Placental Trophoblasts. J. Clin. Endocrinol. Metab. 2017;102:2100–2110. doi: 10.1210/jc.2016-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Goetzl E.J. Human plasma platelet-derived exosomes: Effects of aspirin. Faseb. J. 2016;30:2058–2063. doi: 10.1096/fj.201500150R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Connor D.E. Effects of antiplatelet therapy on platelet extracellular vesicle release and procoagulant activity in health and in cardiovascular disease. Platelets. 2016;27:805–811. doi: 10.1080/09537104.2016.1190008. [DOI] [PubMed] [Google Scholar]
- 216.Chiva-Blanch G. Microparticle Shedding by Erythrocytes, Monocytes and Vascular Smooth Muscular Cells Is Reduced by Aspirin in Diabetic Patients. Rev. Esp. Cardiol. 2016;69:672–680. doi: 10.1016/j.recesp.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 217.Ambrose A.R. Comparison of the release of microRNAs and extracellular vesicles from platelets in response to different agonists. Platelets. 2017;29:446–454. doi: 10.1080/09537104.2017.1332366. [DOI] [PubMed] [Google Scholar]
- 218.Tannetta D.S. Syncytiotrophoblast Extracellular Vesicles from Pre-Eclampsia Placentas Differentially Affect Platelet Function. PLoS ONE. 2015;10:e0142538. doi: 10.1371/journal.pone.0142538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Patil R. Effect of anticoagulant therapy on cell-derived microparticles and pregnancy outcome in women with pregnancy loss. Br. J. Haematol. 2015;171:892–896. doi: 10.1111/bjh.13460. [DOI] [PubMed] [Google Scholar]