Abstract
Dendritic cells (DCs) and leukemia-derived DC (DCleu) are potent stimulators of various immunoreactive cells and they play a pivotal role in the (re-) activation of the immune system. As a potential treatment tool for patients with acute myeloid leukemia, we developed and analyzed two new PGE1-containing protocols (Pici-PGE1, Kit M) to generate DC/DCleu ex vivo from leukemic peripheral blood mononuclear cells (PBMCs) or directly from leukemic whole blood (WB) to simulate physiological conditions. Pici-PGE1 generated significantly higher amounts of DCs from leukemic and healthy PBMCs when compared to control and comparable amounts as the already established protocol Pici-PGE2. The proportions of sufficient DC-generation were even higher after DC/DCleu-generation with Pici-PGE1. With Kits, it was possible to generate DCs and DCleu directly from leukemic and healthy WB without induction of blast proliferation. The average amounts of generated DCs and DCleu-subgroups were comparable with all Kits. The PGE1 containing Kit M generated significantly higher amounts of mature DCs when compared to the PGE2-containing Kit K and increased the anti-leukemic-activity. In summary PGE1-containing protocols were suitable for generating DC/DCleu from PBMCs as well as from WB, which reliably (re-) activated immunoreactive cells, improved the overall ex vivo anti-leukemic activity, and influenced cytokine-release-profiles.
Keywords: PGE1, AML, leukemia-derived dendritic cells, immunotherapy, dendritic cells
1. Introduction
Acute myeloid leukemia (AML) is a clonal disease that is characterized by an uncontrolled proliferation and an impaired differentiation of myeloid progenitor cells (blasts) with overall five-year-survival rates of about 28.3 % [1,2]. In the last few decades, the therapy with antigen presenting cells (APCs), such as dendritic cells (DCs) revolutionized immunotherapy of AML [3,4,5].
APCs play a pivotal role in connecting the innate and the adaptive immune system with the properties to migrate into different tissues and activate different immune reactive cells. They internalize and process antigens, present antigen-fragments via major histocompatibility complex (MHC), and form immunological synapses with T cells, resulting in a clonally restricted and potent T cell-activation [6,7,8,9,10].
Two different DC-based immunotherapy strategies have been developed. Monocyte (CD14+) derived DCs can be generated with different response modifiers in cultures and they are loaded with leukemic-associated-antigens (LAA) by the electroporation of messenger ribonucleic acid (mRNA) or by peptide pulsing [11,12,13]. After expensive ex vivo manipulation and the production of cells under Good Manufacturing Practice (GMP), DCs can be (re-) administrated to patients as a vaccine [14,15].
Moreover, leukemic blasts can be converted ex vivo directly to leukemia derived DC (DCleu), presenting the whole leukemic antigen repertoire. DCleu simultaneously express DC-antigens and -individual patients’ blast markers (blast-antigens) [16].
DCs and DCleu can be generated ex vivo from leukemic peripheral blood mononuclear cells (PBMCs) or whole blood (WB) without the induction of blast proliferation [17,18,19]. DC/DCleu-generating-protocols contain combinations of different response modifiers, including (1) cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) or Interleukin 4 (IL-4) that induce differentiation of myeloid progenitor cells; or (2) Calcium-Inophore (A23187) as a cytokine free DC/DCleu-generating method; (3) bacterial or nucleic stimulation with danger signaling effects, such as Picibanil (OK432), a lysis product from the streptococcus pyogenes or Polyinosinic:polycytidylic acid (poly I:C); and, (4) substances inducing maturation of DC/DCleu, such as Prostaglandin E2 (PGE2) or Tumor-necrosis-factor alpha (TNF-α) [17,20]. Furthermore, Interleukin 1 beta (IL-1β), Interleukin 6 (IL-6), Interferon gamma (IFN-γ), Interferon alpha (IFN-α), and Fms-related tyrosine kinase 3 ligand (FLT3-L) were used and analyzed in different protocols to generate DCs and/or DCleu from leukemic or healthy PBMCs [17,20,21,22,23,24].
The stimulation of T cell enriched immunoreactive cells with DC/DCleu ex vivo in a mixed lymphocyte culture (MLC) regularly results in the (re-) activation of T cells against leukemic blasts, although depending on the DC/DCleu protocol used for the generation of DC/DCleu [17,25,26]. We could already show that proliferating T cells (Tprol CD71+, CD69+), non-naïve T cells (Tnon-naïve, CD45RO+), regulatory T cells (Treg, CD25++CD127low), β-integrin+ T cells and T cells with effector function, such as central-memory T cells (Tcm, CD45RO+CCR7+), effector (memory) T cells (Teff-em, CD45RO+CCR7−) increase, while naïve T cells (Tnaive, CD45RO−) decrease during MLC [27,28]. DC/DCleu probably contribute to stimulating and activating cells from the innate immune system and cells on the interface of the innate and the adaptive immune system, such as natural killer cells, invariant natural killer cells, or cytokine induced killer cells [29].
Soluble factors (e.g., cytokines) are involved in the leukemogenesis, as well as in anti-leukemic immunoreactions, thereby influencing the persistence or elimination of AML-cells and patients’ treatment outcome [30]. Monocyte chemotactic protein 1 (MCP-1, also known as CC-chemokine ligand 2, CCL-2) is an inflammatory cytokine, with antitumor activity [31]. Interleukin 17A (IL17-A) is classified as an anti-tumor response related cytokine and Interleukin 10 (IL-10) is characterized as an anti-inflammatory cytokine [32,33,34,35].
Prostaglandins are responsible and involved in different physiological functions, such as inflammation, regulation of renal-blood circulation, induction of fever, and the protection of the gastric mucosa from gastric acid [36,37,38,39]. PGE2 and Prostaglandin E1 (PGE1) are arachidonic acid derivatives, which are synthesized via the cyclooxygenase 1 and 2 pathway (COX1 and COX2) [40]. The biochemical differences between PGE1 and PGE2 are caused by different amounts of double bounds in the side chain [41]. PGE2 (e.g., Dinoproston) is approved by the US Food and Drug Administration (FDA) for the induction of labor in cases with medical or obstetrical indication. Drugs that contain PGE1 are approved for the risk reduction of gastrointestinal ulcers during the treatment with nonsteroidal anti-inflammatory drugs (NSAIR) (e.g., Misoprostol) and to treat erectile dysfunction (e.g., Alprostadil). Furthermore, PGE1 is used to treat peripheral arterial disease and to maintain the patency of the ductus arteriosus in patients with ductal-dependent cardiac lesions [42,43,44]. PGE1 was also analyzed in a combination with heparin to prevent liver veno-occlusive disease (VOD), which is a life-threatening obliteration of hepatic venules, in patients with AML after bone marrow transplantation (BMT) [45,46].
To improve the DC/DCleu-treatment of patients with AML, we have developed minimalized Kits, containing combinations of at least two response modifiers. Following our hypotheses Kits should be able to convert leukemic blasts directly to DC/DCleu in WB cultures. With respect to clinical applications this could mean, that patients could be directly treated with Kits, thereby inducing DC/DCleu-generation in vivo, which would render an adoptive transfer of ex vivo generated DC/DCleu unnecessary.
The aim of this study was
-
(1)
to develop and to functionally evaluate a new PGE1-containing DC/DCleu generating protocol to produce DCs and DCleu from healthy and leukemic PBMCs;
-
(2)
to develop and to functionally evaluate an immunomodulatory Kit M (containing GM-CSF and PGE1) to produce DCs and DCleu directly from healthy and leukemic WB, thereby simulating in vivo conditions;
-
(3)
to deduce an optimized protocol for the ex vivo generation of DC/DCleu which might be used for an adoptive cell transfer; and,
-
(4)
to deduce immunomodulatory Kits that might be able to convert myeloid leukemic blasts in vivo to DC/DCleu.
2. Results
2.1. Prolog
In the first part of this manuscript, we present a new PGE1-containing protocol (Pici-PGE1) for the generation of DC/DCleu from healthy and leukemic PBMCs.
In the second part, we simulated physiological conditions and generated DC/DCleu with the DC/DCleu-generating protocols Pici-PGE1 and Pici-PGE2 and immunomodulatory Kits directly from healthy and leukemic WB. The compositions of Picis and Kits are shown in Table 1 We correlated data with the DC/DCleu stimulatory potential of T cell enriched immunoreactive cells and with the potential to generate anti-leukemia directed T cells as well as with cytokine-release-profiles.
Table 1.
Compositions of DC/DCleu-generating protocols.
| DC/DCleu-Generating Protocols | Composition | Concentration | Sources of DC/DCleu | Mode of Action | Culture Time | Reference |
|---|---|---|---|---|---|---|
| Picibanil-PGE1 | GM-CSF | 500 U/mL | PBMC WB |
GM-CSF: induction of myeloid (DC-) differentiation IL-4: induction of DC-differentiation Picibanil (OK-432): lysis product from streptococcus pyogenes; stimulates DC-differentiation PGE2: increases CCR7-expression and enhances DC-migration PGE1: effects are comparable to PGE2 |
7–10 days | |
| (Pici-PGE1) | IL-4 | 250 U/mL | ||||
| OK-432 | 10 µg/mL | |||||
| PGE1 | 1 µg/mL | |||||
| Picibanil-PGE2 | GM-CSF | 500 U/mL | PBMC WB |
7–10 days | [17,20] | |
| (Pici-PGE2) | IL-4 | 250 U/mL | ||||
| OK-432 | 10 µg/mL | |||||
| PGE2 | 1 µg/mL | |||||
| Kit M# | GM-CSF | 800 U/mL | WB | 7–10 days | [47] | |
| PGE1 | 10 µg/mL | |||||
| Kit K# | GM-CSF | 800 U/mL | WB | 7–10 days | [47] | |
| PGE2 | 1 µg/mL | |||||
| Kit I# | GM-CSF | 800 U/mL | WB | 7–10 days | [47] | |
| OK-432 | 1 µg/mL |
DC dendritic cells; DCleu dendritic cells of leukemic origin; GM-CSF granulocyte macrophage colony stimulating factor; IL-4 interleukin 4; OK-432 picibanil; PGE2 prostaglandin E2; PGE1 prostaglandin E1; PBMC peripheral blood mononuclear cells, WB whole blood; # 15 801 987.7-1118 European Patent.
2.2. DC/DCleu-Generation from Healthy and Leukemic PBMCs
2.2.1. Significantly Higher Amounts of DCs Generated from Healthy and Leukemic PBMCs with Pici-PGE1 and Pici-PGE2 Compared to Controls
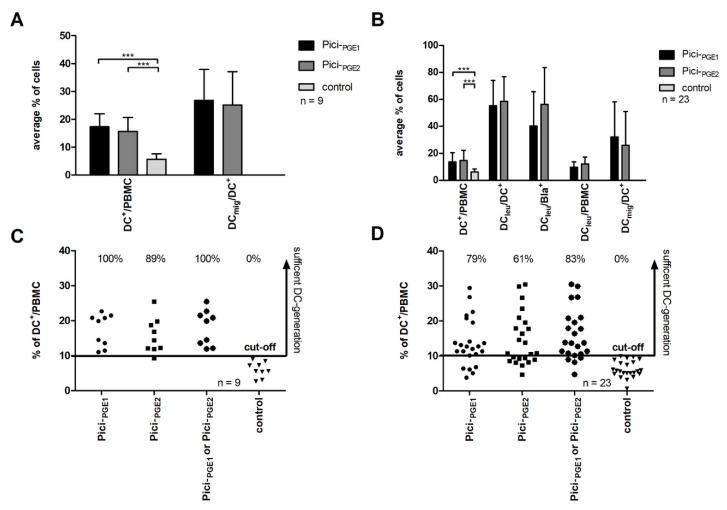
With Pici-PGE1 and Pici-PGE2 we generated on average significantly*** higher amounts of DCs from healthy PBMCs as compared to controls (n = 9) (Pici-PGE1: 17.4 ± 4.7% DC+/PBMC, p < 0.00003; Pici-PGE2: 15.6 ± 5.1% DC+/PBMC, p < 0.0003; control: 6.0 ± 2.2% DC+/PBMC). Although differences were not significant, we found, on average, higher amounts of DC+/PBMC after the stimulation of healthy PBMCs with Pici-PGE1 when compared to Pici-PGE2. No significant differences were found in amounts of DCmig/DC+ with Pici-PGE1 and Pici-PGE2 (26.8 vs. 25.1% DCmig/DC+, p < 0.77) (Figure 1A).
Figure 1.
DC/DCleu-generation from healthy (left side) and leukemic peripheral blood mononuclear cells (PBMCs) (right side). (A) shows the average amounts ± standard deviation of generated dendritic cells (DCs) in the PBMC-fraction and mature DCs in the DC-fraction [CD197+DC+, (DCmig/DC+)] from healthy PBMCs with Pici-PGE1, Pici-PGE2 and control without added cytokines. (B) presents the average amounts ± standard deviation of generated DCs in the PBMC-fraction, DCleu-subgroups [including DCleu in the DC-fraction (DCleu/DC+), DCleu in the blast-fraction (to quantify amounts of leukemic blasts converted to DCleu) (DCleu/Bla+), DCleu in the PBMC-fraction (DCleu/PBMC)] and DCmig in the DC-fraction (DCmig/DC+) from leukemic PBMCs with Pici-PGE1, Pici-PGE2 and control without added cytokines. (C) and (D) show the percentages of sufficient DC-generation from healthy (C) and leukemic (D) PBMCs with Pici-PGE1, Pici-PGE2, Pici-PGE1 or Pici-PGE2 and control without added response modifiers according to cut-off-values (≥10% DC+/PBMC). Each dot (● ▪ ● ▼) represents DC-proportions generated from each individual healthy volunteer or AML-patient. DCs dendritic cells; DCleu leukemic derived dendritic cells; PBMCs peripheral blood mononuclear cells. The differences were considered as significant*** with p values <0.005.
We generated DCs and DCleu from leukemic PBMCs and found, on average, significantly*** higher amounts of DC+/PBMC after culture with Pici-PGE1 and Pici-PGE2 compared to controls (n = 23) (Pici-PGE1: 13.7 ± 6.8% DC+/PBMC, p < 0.00003; Pici-PGE2: 14.7 ± 7.5% DC+/PBMC, p < 0.00002, control 6.1 ± 2.3% DC+/PBMC). No significant differences in the amounts of DC+/PBMC were found between Pici-PGE1 and Pici-PGE2 (p < 0.65). We found (not significantly) higher amounts of DCmig/DC+ after culture with Pici-PGE1 compared to Pici-PGE2 (32.1 vs. 25.9% DCmig/DC+, p < 0.35) (Figure 1B). Moreover, we could show that subtype (primary or secondary AML) and stage of the AML did not have an impact on the generation of DCs and DCleu from leukemic PBMCs with Pici-PGE1 or Pici-PGE2 (data not shown).
In summary, we conclude that DCs and DCmig can be generated with Pici-PGE1 and Pici-PGE2 in comparable amounts from healthy and leukemic PBMCs.
2.2.2. Efficiency of Sufficient DC-Generation is Higher with Pici-PGE1 Compared to Pici-PGE2 from Leukemic PBMCs
In healthy and leukemic control groups, we found, in every given case, less than 10% DC+/PBMC. Therefore, we defined a cut-off value of ≥10% DC+/PBMC as a successful DC-generation from healthy and leukemic PBMCs. According to this cut-off value a successful DC-generation from healthy PBMCs was possible in 100% of cases (nine of nine cases) with Pici-PGE1 and in 89% of cases (eight of nine cases) with Pici-PGE2 (Figure 1C).
A sufficient DC-generation from leukemic PBMCs was possible in 79% of cases (18 of 23 cases) with Pici-PGE1 and in 61% of cases (14 of 23 cases) with Pici-PGE2. In 83% of cases, a sufficient DC-generation was possible with Pici-PGE1 or Pici-PGE2 (19 of 23 cases) (Figure 1D). In all cases with successful DC-generation, the amounts of DCleu were comparable with Pici-PGE1 (n = 18) and Pici-PGE2 (n = 14). With Pici-PGE1 we generated on average 55.3 ± 18.8% DCleu/DC+ and Pici-PGE2 58.5 ± 18.2% DCleu/DC+. The average amounts of blasts converted to DCleu (DCleu/Bla+) were 40.3 ± 25.4% DCleu/Bla+ with Pici-PGE1 and 56.3 ± 27.2% DCleu/Bla+ with Pici-PGE2. 9.7 ± 4.0% DCleu/PBMC could be generated with Pici-PGE1 and 12.2 ± 5.1% DCleu/PBMC with Pici-PGE2 (Figure 1B).
In summary, the efficiencies of a sufficient DC-generation from leukemic PBMCs are higher with Pici-PGE1 as compared to Pici-PGE2 and comparable to healthy PBMCs. In four cases, no sufficient DC-generation was possible with both protocols.
2.2.3. Pici-PGE1 and Pici-PGE2 Do Not Induce Blast Proliferation During DC/DCleu-Culture from Leukemic PBMCs
After DC/DCleu-culture from leukemic PBMCs, we found on average comparable amounts of proliferating blasts that were not converted to DCleu (Blaprol-CD71) with Pici-PGE1 or Pici-PGE2 as compared to control: Pici-PGE1: 26.8 ± 19.9%, p < 0.29; Pici-PGE2: 25.6 ± 16.8%, p < 0.39; control: 21.3 ± 14.7%. Comparable distributions were found for Blaprol-Ipo-38 (data not shown).
We conclude that neither Pici-PGE1 nor Pici-PGE2 induce proliferation of blasts not converted to DCleu.
2.3. DC/DCleu-Generation from Healthy and Leukemic WB
2.3.1. Comparable DC-Amounts can be Generated with Immunomodulatory Kits and Picis
We compared the DC+/WB values generated with Kit M, Kit K, and Kit I (Kits n = 27) and DC+/WB values that were generated with Pici-PGE1 and Pici-PGE2 from leukemic WB (Picis n = 18).
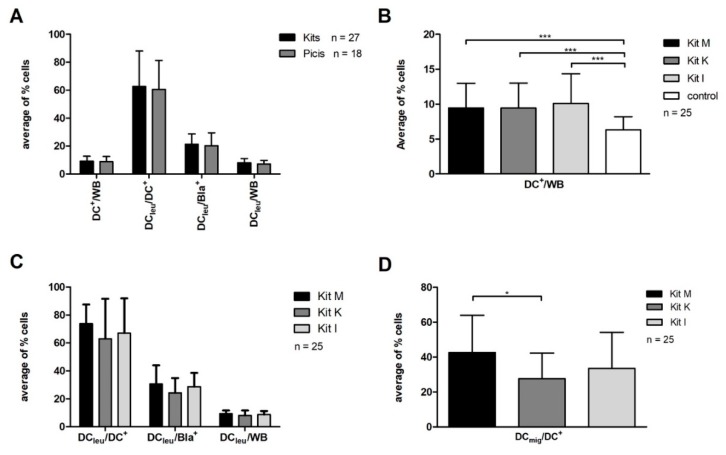
Amounts of generated DC+/WB from leukemic WB-samples were not significantly different in both groups (Kits: 9.3 ± 3.4% DC+/WB vs. Picis: 8.8 ± 3.7% DC+/WB, p < 0.62). Comparable results were found after the DC-generation from healthy WB (data not shown). Moreover, we found comparable amounts of DCleu/DC+, DCleu/Bla+, and DCleu/WB with Kits as compared to Picis (DCleu/DC+: 62.7 ± 25.30% vs. 60.6 ± 20.8%; DCleu/Bla+: 21.3 ± 7.4% vs. 20.3 ± 9.1%; DCleu/WB: 8.1 ± 3.0% vs. 7.2 ± 2.6%) (Figure 2A).
Figure 2.
DC/DCleu-generation from leukemic whole blood (WB). (A) shows average amounts ± standard deviation of DC- and DCleu-proportions [including DCleu-subgroups: DCleu in the DC-fraction (DCleu/DC+), DCleu in the blast-fraction (to quantify amounts of leukemic blasts converted to DCleu) (DCleu/Bla+) and DCleu in the WB-fraction (DCleu/WB)] from leukemic WB with Kits (including Kit M, Kit K, Kit I) compared to protocols Picis (including Pici-PGE1, Pici-PGE2). (B) shows average amounts ± standard deviation of generated DCs with Kit M, Kit K and Kit I compared to control. (C) presents average amounts ± standard deviation of generated DCleu subgroups [including DCleu-subgroups DCleu in the DC-fraction (DCleu/DC+), DCleu in the blast-fraction (to quantify amounts of leukemic blasts converted to DCleu) (DCleu/Bla+) and DCleu in the WB-fraction (DCleu/WB)] with Kit M, Kit K and Kit I. (D) shows average amounts ± standard deviation of DCmig/DC+ generated with Kit M, Kit K and Kit I. DCmig are characterized by the expression of CCR7. DCs dendritic cells; DCleu leukemic derived dendritic cells; WB whole blood. The differences were considered as significant*, with p values between 0.1 and 0.05 and as significant*** with p values <0.005.
We summarize that DC/DCleu-generation (including subgroups) is possible in comparable amounts with Kits from leukemic and healthy WB when compared to Picis.
2.3.2. Significantly Higher Amounts of DC+/WB Generated from Healthy and Leukemic WB with Immunomodulatory Kit M, Kit K and Kit I Compared to Control
We generated DCs from leukemic WB and found, on average, significantly*** higher amounts of DC+/WB after WB-DC/DCleu-cultures with Kit M, Kit K, and Kit I as compared to control (n = 25) (Kit M: 9.5 ± 3.6% DC+/WB, p < 0.0004; Kit K: 9.5 ± 3.6% DC+/WB, p < 0.0004; Kit I: 10.1 ± 4.4% DC+/WB, p < 0.0003; control: 6.3 ± 1.9% DC+/WB) (Figure 2B). Comparable distributions were found after DC-cultures from healthy WB (n = 9, data not shown). In the comparison of Kit M, Kit K, and Kit I we found, on average, comparable amounts of generated DCs in the WB-fraction from healthy and leukemic WB. In DCleu-subgroups, including DCleu/Bla+, DCleu/DC+, as well as DCleu/WB no significant differences were found (Figure 2C).
With Kit M, significantly* higher amounts of DCmig/DC+ could be generated when compared to Kit K (n = 25): (42.6 ± 21.5% DCmig/DC+ vs. 27.6 ± 14.7% DCmig/DC+; p < 0.09). In the comparison of Kit M and Kit I, no significant differences were found (Kit I: 33.5 ± 20.7% DCmig/DC+; p < 0.35). (Figure 2D).
In summary, significantly higher amounts of DC+/WB can be generated with immunomodulatory Kits from healthy and leukemic WB as compared to control. The amounts of generated DC and DCleu-subgroups are comparable with all three Kits, with the exception of significantly higher frequencies of DCmig/DC+ after DC/DCleu-culture with Kit M as compared to Kit K.
2.3.3. Kits Do Not Induce Blast-Proliferation Compared to Control
We found comparable amounts of proliferating blasts that were not converted to DCleu (Blaprol-CD71) in the WB-fraction after culture of leukemic WB with Kit M, Kit K, Kit I, and control (n = 24): Kit M: 8.5 ± 13.0%, p < 0.48; Kit K: 8.4 ± 11.0%, p < 0.45; Kit I: 9.9 ± 10.7%, p < 0.18 and control: 6.5 ± 6.1%. Comparable distributions were found for Blaprol-Ipo-38 (data not shown).
In summary, Kits do not induce blast proliferation during DC/DCleu-culture from leukemic WB when compared to control.
2.4. Stimulatory Effect of DC/DCleu (Generated with Kits from Leukemic WB) on T Cell Enriched Immunoreactive Cells in MLC and the Corresponding Blast-Lysis Activity
2.4.1. Comparison of T Cell Amounts, Phenotypes, and Blast Lysis Activity in Uncultured Cells, after MLCWB-DC Kits and after MLCWB
As shown above, DC- and DCleu-generation was possible from healthy and leukemic WB with Kits. Here we studied the (potential) stimulating effect of generated DCs and DCleu on T cell enriched immunoreactive cells in MLC. Therefore, we compared T cell compositions in uncultured cells (n = 11) (uncultured cells) with those after stimulation in MLCWB-DC-Kit M, MLCWB-DC-Kit K, and MLCWB-DC-Kit I (n = 24) (MLCWB-DC Kits) and after stimulation in MLCWB (n = 11) (MLCWB) as control.
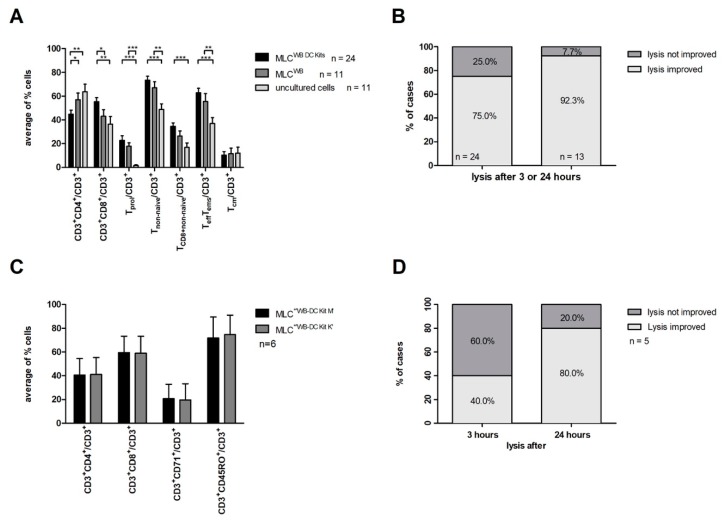
In general, we found a significantly higher activation status of immunoreactive T cells after MLCWB-DC Kits as compared to MLCWB and uncultured T cells. The main findings were characterized by increased amounts of CD3+CD8+/CD3+ and the corresponding decrease of CD3+CD4+/CD3+ after MLCWB-DC Kits when compared to MLCWB. We found after MLCWB-DC Kits as well as after MLCWB increased amounts of proliferating T cells when compared to uncultured cells, and also a shift from naive to non-naïve T cell subsets. Detailed results are shown in Table 2A and in Figure 3A. Since IL-2 was added to all MLC-experiments (including MLCWB), amounts of T cell subsets also increased after MLCWB.
Table 2.
Results of T cell compositions after MLCWB-DC Kits, MLCWB, or in uncultured cells.
(A)
| MLCWB-DC Kits | MLCWB | Uncultured Cells | p-Values | |||
|---|---|---|---|---|---|---|
| % of Cells in the Corresponding Subgroup | MLCWB-DC Kits vs. MLCWB | MLCWB-DC Kits vs. Uncultured Cells | MLCWB vs. Uncultured Cells | |||
| T Cell Subtypes | ||||||
| CD3+CD4+/CD3+ | 44.7 ± 16.1 | 57.0 ± 18.6 | 63.6 ± 21.4 | <0.08* | <0.02** | <0.44 |
| CD3+CD8+/CD3+ | 55.3 ± 16.1 | 43.0 ± 18.6 | 36.4 ± 21.4 | <0.08* | <0.02** | <0.44 |
| Proliferating T Cells | ||||||
| CD3+CD71+/CD3+ | 22.8 ± 18.3 | 17.8 ± 9.2 | 1.1 ± 0.6 | <0.3 | <0.000001*** | <0.00001*** |
| CD3+CD69+/CD3+ | 23.0 ± 16.7 | 18.4 ± 15.5 | 3.1 ± 4.4 | <0.4 | <0.00001*** | <0.009*** |
| Non-Naïve or Naïve T cells | ||||||
| CD3+CD45RO+/CD3+ | 73.5 ± 15.9 | 67.0 ± 16.9 | 48.8 ± 15.6 | <0.3 | <0.0003*** | <0.02** |
| CD3+CD8+CD45RO+/CD3+ | 34.6 ± 13.4 | 26.4 ± 14.6 | 16.8 ± 12.4 | <0.13 | <0.001*** | <0.14 |
| CD3+CD4+CD45RO+/CD3+ | 38.9 ± 17.3 | 40.7 ± 16.1 | 32.0 ± 12.5 | <0.19 | <0.17 | |
| CD3+CD45RO− (including subsets) |
26.2 ± 15.6 | 32.7 ± 16.9 | 51.1 ± 15.5 | <0.3 | <0.0003*** | <0.02** |
| Effector (Memory) T Cells | ||||||
| CD3+CCR7−CD45RO+/CD3+ | 62.8 ± 18.9 | 55.5 ± 21.8 | 36.8 ± 16.6 | <0.23 | <0.0005*** | <0.04** |
| CD3+ CCR7+CD45RO+/CD3+ | 10.3 ± 14.0 | 11.5 ± 15.5 | 11.9 ± 16.7 | <0.84 | <0.79 | <0.95 |
(B)
| MLCWB-DC Kit M | MLCWB-DC Kit K | |
|---|---|---|
| T Cell Subtypes | ||
| CD3+CD8+/CD3+ | 59.4 ± 13.9 | 59.0 ± 14.3 |
| CD3+CD4+/CD3+ | 40.6 ± 13.9 | 41.0 ± 14.3 |
| Proliferating T Cells | ||
| CD3+CD71+/CD3+ | 20.7 ± 12.1 | 19.7 ± 13.5 |
| Non-Naïve or Naïve T Cells | ||
| CD3+CD45RO+/CD3+ | 71.8 ± 17.7 | 74.6 ± 16.4 |
MLC mixed lymphocyte culture; MLCWB mixed lymphocyte culture with WB not preincubated with DC/DCleu-generating protocols; MLCWB-DC mixed lymphocyte culture with WB preincubated with DC/DCleu-generating protocols, highest average values are presented in bold figures.
Figure 3.
Stimulatory effect of DC/DCleu generated with Kits from leukemic WB on T cell enriched immunoreactive cells in MLC and the corresponding blast-lysis activity. (A) shows average amounts ± standard deviation of T cell subsets after stimulation of T cell enriched immunoreactive cells with DC/DCleu generated with Kits from leukemic WB (MLCWB-DC Kits), after stimulation of T cell enriched immunoreactive cells with a WB cell suspension not pretreated with Kits (MLCWB) and in uncultured cells. Cells were analyzed by flow cytometry and referred to the CD3+ cell fraction. (B) shows the improvement of blast-lysis-activity compared to controls after 3 or 24 h of co-culture of target and effector cells (left column) and of cases with improved blast lysis with at least one MLCWB-DC Kit proportions compared to MLCWB in each individual patient (right column). (C) shows average amounts ± standard deviation of different T cell subsets after MLCWB-DC Kit M and MLCWB-DC Kit K. (D) shows percentage of cases with improved blast-lysis after MLCWB-DC Kit M compared to MLCWB-DC Kit K after 3 h (left column) and after 24 h (right column). MLC mixed lymphocyte culture; WB whole blood. The differences were considered as significant*, with p values between 0.1 and 0.05, as significant** with p values between 0.05 and 0.005 and as significant*** with p values <0.005.
Furthermore, we pooled all the results obtained with the cytotoxicity assay after MLCWB-DC Kit (including MLCWB-DC Kit M, MLCWB-DC Kit K, MLCWB-DC Kit I) and compared the results to MLCWB after 3 or 24 h (n = 24) of incubation of effector with target cells. Anti-leukemic activity was defined as lysis of blast-target-cells obtained either after 3 or 24 h. Moreover, we analyzed the anti-leukemic activity in each individual patient (n = 13).
After MLCWB-DC lysis of blasts target cells could be improved in 75.0% of cases (18 of 24 cases) as compared to MLCWB after 3 or 24 h.
Furthermore, in 92.3% of patients (12 of 13 patients) we could select at least one MLCWB-DC Kit in each individual patient, which improved the blasts lysis after 3 h or 24 h when compared to MLCWB (Figure 3B).
In summary, we regularly demonstrate an increase of T cells’ anti-leukemic activity after stimulation with DC/DCleu generated with Kits from leukemic WB.
2.4.2. Comparison of T Cell Amounts, Phenotypes and Blast Lytic Activity after MLCWB-DC Kit M and MLCWB-DC Kit K
DC/DCleu-generation was possible with Kits from leukemic WB and the stimulation of T cell enriched immunereactive cells with these DC/DCleu resulted in T cell activation. The premise for this analysis was that MLCWB-DC Kit M and MLCWB-DC Kit K (n = 6) were performed in parallel in patients with the corresponding leukemic WB-samples.
As shown in Figure 3C and Table 2B, we found, on average, comparable amounts of T cells (subsets), including CD3+CD8+/CD3+, CD3+CD4+/CD3+ cells and CD3+CD71+/CD3+ cells after MLCWB-DC Kit M as compared to MLWB-DC Kit K from leukemic WB. Furthermore, no significant differences were found for the non-naïve or naïve T cells as well as CD3+CD45RO+CCR7−/CD3+ and CD3+CD45RO+CCR7+/CD3+ after MLCWB-DC Kit M and MLCWB-DC Kit K (data not shown). Comparable distributions were found after MLCWB-DC Kit I.
After 3 h of incubation of DC/DCleu stimulated effector cells with blast target cells we found an improved lysis after MLCWB-DC Kit M in 40% of cases (two of five cases) when compared to MLCWB-DC Kit K. Blast lysis was improved in 80% of cases (four of five cases) after 24 h after MLCWB-DC Kit M compared to MLCWB-DC Kit (Figure 3D).
We conclude that DC/DCleu in a MLCWB–DC have an impact on the amounts of different T cell subsets after stimulation as compared to MLCWB and uncultured cells, however T cell compositions after MLCWB-DC Kits were comparable with Kits used to generate DC/DCleu. Furthermore, we conclude that blast-lysis was improved in all cases (except one) after MLCWB-DC Kit M when compared to MLCWB-DC Kit K after 24 h.
2.5. Cytokine-Release-Profiles in Serum and after WB DC/DCleu-Culture
Cytokine releases by blasts, DCs, as well as by immunoreactive cells are known to influence immunological as well as immune-escape-reactions. Therefore, we correlated cytokine releases in DC/DCleu-culture-supernatants as well as in serum.
2.5.1. Significantly Higher Concentrations of the Inflammatory Cytokine and Antitumor Response Related Cytokine Found after WB-DC/DCleu-Culture with Kits Compared to Serum
We studied the cytokine release levels after WB-DC/DCleu-culture with Kits and in serum. Therefore, we pooled all of the results after WB-DC/DCleu-culture with Kits (Kit M, Kit K and Kit I) (n = 27) from leukemic WB and compared it to cytokine concentrations in serum (n = 8).
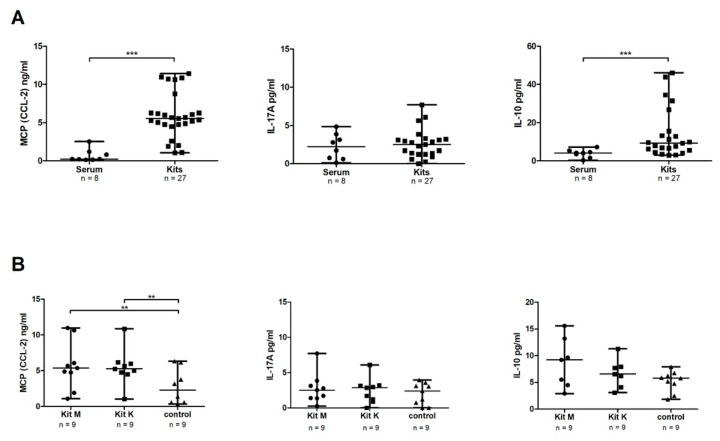
We found significantly*** higher median concentrations of MCP-1 (CCL-2) after WB-DC/DCleu-culture with Kits as compared to serum [5.5 ng/mL (range: 1.0–11.4) vs. 0.2 ng/mL (range: 0.1–2.5), p < 0.000001]. Furthermore, we found significantly*** higher median concentrations of the anti-inflammatory cytokine IL-10 after DC/DCleu-culture [9.2 pg/mL (range: 2.9–46.1) vs. 4.1 pg/mL (range: 0.3–4.1), p < 0.01]. No significant differences were found for the cytokine IL-17A (p < 0.6). Comparable results were found after DC-generation from healthy WB (data not shown) (Figure 4A).
Figure 4.
Cytokine-release-profiles after DC/DCleu-culture from leukemic WB. (A) shows concentrations of MCP-1 (CCL-2) (ng/mL), IL-17A (pg/mL) and IL-10 (pg/mL) in serum and after DC/DCleu-culture from leukemic WB with Kits (including Kit M, Kit K, and Kit I). (B) shows the comparison of concentrations of MCP-1 (CCL-2), IL-17A, and IL-10 after DC/DCleu-culture from leukemic WB with Kit M, Kit K and control without added cytokines. All cytokines were measured with ELISA. MCP-1 Monocyte chemotactic protein 1; CCL-2 CC-chemokine ligand 2; IL17-A Interleukin 17A; IL-10 Interleukin 10. The differences were considered as significant** with p values between 0.05 and 0.005 and as significant*** with p values <0.005.
Furthermore, we compared the cytokine concentrations in supernatants after DC/DCleu-cultures from leukemic WB with Kits and with Picis. In both groups, comparable concentrations of MCP-1 (CCL-2), IL-17A, and IL-10 were found (data not shown).
2.5.2. Significantly Higher Concentrations of MCP-1 (CCL-2) Found after DC/DCleu-Culture with Kit M and Kit K Compared to Control
We compared cytokine secretion after DC/DCleu-culture of leukemic WB with Kits when compared to control without added response modifiers. We found significantly** higher median concentrations of MCP-1 (CCL-2) after DC/DCleu-culture with Kit M and Kit K as compared to control (n = 9) [Kit M: 5.4 ng/mL (range: 1.1–11.0), p < 0.06; Kit K: 5.3 ng/mL (range: 1.0–10.9), p < 0.04; control: 2.3 ng/mL (range: 0.4–6.3)]. For the cytokines IL-17A and IL-10, no significant differences were found as compared to controls (Figure 4B). Comparable results were found for the Kit I (data not shown).
We conclude that the addition of Kits to leukemic WB influences the cytokine release profiles when compared to control. Kit M and Kit K produce comparable cytokine-release-profiles.
3. Discussion
3.1. DC and DCleu Based Immunotherapy for Patients with AML
DCs play a crucial role in the (re-) activation of the immune system and in linking the innate and adaptive immune system. These professional APCs have the ability to migrate into different tissues and to induce an immunological memory. During the last decades, different strategies have been developed to utilize DCs as a treatment tool for patients with AML. DCs can be generated ex vivo from CD14+ monocytes, loaded with different LAAs or tumor antigens, and they can be re-administrated to the patient as vaccine. It was already reported that vaccination with ex vivo generated monocyte-derived DCs increased the amounts of leukemia specific T cells in AML-patients and stabilized complete remission [15,48,49]. Unfortunately, the production of monocyte-derived DCs ex vivo is time-consuming, expensive, has to be performed under GMP-conditions, and the cell production is limited by the selection of LAA [11,14,15,50].
On the other hand, we and others could already show that clonal leukemic blasts can be regularly converted to DCleu with different DC/DCleu-generating protocols, independent from age, FAB-classification, mutation or hematopoietic stem cell transplantation (HSCT) status, mutations of the disease, and FAB classification [17,51]. The clonal leukemic origin of DCleu was already confirmed with fluorescence in situ hybridization (FISH) analysis [52].
3.2. The PGE1-Containing Protocol Pici-PGE1 is More Reliable to Generate DCs in Sufficient Amounts from Healthy and Leukemic PBMCs Compared to the PGE2-Containing Protocol Pici-PGE2
It was already shown that PGE2 produced by the enzyme cyclooxygenase 2 (COX-2) has a crucial role in tumor genesis in colorectal cancers in vivo [53,54]. It was reported that PGE2 influences the apoptosis, induces the angiogenesis, and activates the proliferation of cancer cells [55,56,57]. Whereas, in highly metastatic melanoma cells, it was shown that PGE1 promotes anti-tumor effects, such as the inhibition of invasion and cell growth [58]. Moreover, PGE1 increased the differentiation of tumor cells and decreased the expression levels of metalloproteinase (MMP) 2 and 9 [59]. Furthermore, it was shown that PGE1 increased the concentrations of Cis-Platin in cancers cells after treating rats with peritoneal carcinomatosis [60]. Transferring these findings into the AML treatment, we hypothesize that PGE1 could influence anti-cancer reactions in a positive way. Therefore, we analyzed the potential of PGE1-containing DC/DCleu-generating protocols to produce DCs and DCleu from healthy and leukemic PBMCs as well as from WB. In the past few years it was shown, that it is possible to generate mature DCs and DCleu from leukemic and healthy PBMCs with the PGE2-containing protocol Pici-PGE2 [17,20]. We could confirm this and we add, in addition, that the new protocol Pici-PGE1 produced comparable amounts of DCs and DCleu with a higher efficiency of sufficient DC generation from healthy and leukemic PBMCs when compared to Pici-PGE2. These results suggest, that PGE1 mediates the maturation of DCs and DCleu with comparable efficiency as PGE2, but might be also involved in the differentiation of myeloid progenitor cells and the production of DC/DCleu. This effect might be explained by the different mode of action of PGE1 and PGE2 on prostaglandin receptors (EP receptors) with different down streaming effects due to their different biochemical reactivities [61]. The generation of DC/DCleu was possible with both protocols, independent of patients’ FAB-classification, stage (first diagnosis vs. relapse), or status (primary vs. secondary AML) of the disease. Moreover, we could confirm that neither Pici-PGE1 nor Pici-PGE2 induced the proliferation of non-converted blasts during DC/DCleu-cultures [19]. In four AML-cases, no sufficient DC/DCleu-generation was possible from leukemic PBMCs with Pici-PGE2 and Pici-PGE1, which might be due to various expressions of cytokine-receptors (e.g., GM-CSF receptors, stem cell-receptors) or prostaglandin-receptors (EP-receptors) on leukemic blasts [62,63]. These patients did not receive chemotherapy before conducting DC/DCleu culture experiments, however spontaneous apoptosis or cell death of blood cells has to be discussed. Technical limitations were excluded.
3.3. Successful Generation of DCs and DCleu from Healthy and Leukemic WB Cultures
We established a WB-model to generate DC/DCleu from leukemic WB containing all soluble and cellular factors of the individual patient to simulate physiological conditions. According to our hypothesis, DCs and DCleu could also be generated in vivo by modulating myeloid blasts in their natural microenvironment after administrating a combination of different response modifiers (e.g., Kit M, Kit K, Kit I) to the patient. Therefore, well known DC/DCleu generating protocols (e.g., Pici-PGE1, Pici-PGE2) were applied in WB settings and results that were obtained with Kit M, Kit K, or/and Kit I compared: our results show that DCs and DCleu (including subgroups) could be generated in comparable amounts with Kits and Picis directly from WB [17,20]. Additionally, DCleu subgroups did not significantly differ. We conclude that DCs and DCleu can be generated with Kits from leukemic WB ex vivo. The fact that significantly more DC/DCleu could be generated with Kit M, Kit K, and Kit I when compared to control without added response modifiers points to the fact that the combination of two response modifiers is necessary to generate DCs and DCleu in sufficient amounts directly from leukemic WB: the induction of hematopoietic differentiation is induced by GM-CSF, danger signaling, and/or maturation signaling of DC/DCleu is caused by PGE1, PGE2, or Picibanil. When compared to PBMC—cultures, response modifiers, such as IL-4 and other/unknown cytokines, are physiological components of the microenvironment in WB, and therefore they have not to be added or included in DC/DCleu-generating protocols [35]. The average amounts of generated DCs (including DCleu subgroups) were comparable with all three Kits, although amounts of matured DCs were significantly higher after WB-treatment with the PGE1-containing Kit M as compared to the PGE2-containing Kit K. This finding might also be explained by the different mode of action of PGE1 and PGE2 on EP receptors with different down streaming effects [61]. Especially, for the in vivo use of DC/DCleu, the expression of the lymph-node-homing-receptor CCR7 (marker for mature DCs) is crucial for the migratory capacity of DCs and DCleu to the lymph node, where they activate T cells and other immunoreactive cells and induce anti-tumor/anti-leukemic activity [64,65,66]. Comparable results were already found under hypoxic conditions [67]. The proliferation of blasts (not converted to DCleu) was not induced with Kits during DC/DCleu-cultures. Therefore, we conclude that Kits might be safe tools for an in vivo treatment.
We could already show that, after the stimulation of T cell, enriched immunoreactive cells with DCs and DCleu generated from leukemic PBMCs composition of T cells could be shifted and influenced in a positive way and that anti-leukemic activity could be induced [17,25,27,28]. T cells directly kill cancer-cells via the Granzyme B and Perforin pathway and/or indirectly through the secretion of IFN-γ or tumor-necrosis-factor-alpha (TNF-α). We found after MLCWB DC-Kits a higher activation status with increased amounts of proliferating T cells, as well as a shift from naive to non-naïve T cell subsets when compared to cells before culture. When compared to MLCWB, amounts of CD3+CD8+ significantly increased. After MLCWB higher amounts of different T cell subsets could also be found and they can be explained by IL-2 added to all MLC, which is a potent endogenous T cell and NK cell activating cytokine [68]. The induction of an immunological memory can be postulated due to the DC concept. We found comparable amounts of central memory T cells after MLCWB-Kits, MLCWB and in uncultured cells. However, we found significantly more effector memory T cells after MLCWB-Kits, pointing to an induction of these specialized memory cells, which can enter different tissues to initiate inflammation and cytotoxicity [69]. We speculate that, in our setting, the incubation time to produce memory T cells after DC/DCleu-stimulation is too short, therefore only effector memory T cells are produced. The induction of memory T cells is crucial for the induction of long term remission in patients with AML.
The composition of Kits to produce DC/DCleu, used for the stimulation of T cell enriched immunoreactive cells has no impact on the average amounts and phenotypes of T cell subsets after MLC, but on the anti-leukemic activity. The most important result of our study was that we could demonstrate that anti-leukemic activity could be improved after MLCWB-DC-Kits compared to MLCWB. Increased production of leukemia-specific cells after MLCWB-DC-Kits as compared to MLCWB was shown in the proof of concept-assays. Extended leukemia-specific evaluations are part of our ongoing research. These results suggest that DC/DCleu generated with Kits from leukemic WB induce the immune system and can activate specific anti-leukemic activity against leukemic blasts after MLC. Furthermore, we could show that the anti-leukemic activity is comparable and it might be superior after MLC DC/DCleu generated with the PGE1-containing Kit M when compared to the PGE2-containing Kit K, especially after 24 h of simultaneous incubation of the effector and target cells. This finding might be explained by the fact that PGE2 could induce the expression of indoleamin 2,3-dioxgenase-1 (IDO1), an immunoregulatory enzyme that activates immunosuppressive Treg, but does not impair the antigen presentation capacity of DCs [70,71,72,73,74].
Moreover, during WB DC/DCleu-cultures with Kits as well as Picis cytokine-release-profiles were influenced compared to serum as well as compared to control. We can conclude that Kits induce the production of anti-tumor response related cytokines as well as inflammatory cytokines during DC/DCleu cultures, and therefore improve anti-leukemic reactivity of T cells after stimulation with Kit treated WB.
4. Material and Methods
4.1. Sample Collection
After obtaining written informed consent, in accordance with the Helsinki protocol and the local Ethic Committee (Pettenkoferstraße 8a, 80336 Munich, Germany, Ludwigs-Maximilians-University-Hospital in Munich; VoteNo 33905), the heparinized peripheral WB samples were taken from patients in acute phases of AML and from healthy volunteers. The University-Hospitals of Oldenburg, Tuebingen, Munich and Augsburg provided samples. Anticoagulation was performed with Lithium-heparin-tubes (7.5 mL, Sarstedt, Nuernberg, Germany) containing standardized concentrations of Heparin.
PBMCs were isolated from WB-samples by density gradient centrifugation while using the Ficoll-Hypaque-Technique (Biocoll-Separating-solution, Biochrom, Berlin, Germany) with a density gradient of 1.077 g/mL. PBMCs were washed and then suspended in phosphate-buffered saline (PBS, Biochrom, Berlin, Germany). CD3+T cells were enriched while using the MACS-technology (Milteney Biotech, Bergisch Gladbach, Germany). The purity of the viable T cells was, on average, 87.9% (range 77.4–96.1%). The viable cells were quantified while using Trypan Blue (Biochrom, Berlin, Germany) and they were counted with Neubauer-counting-chambers. PBMCs were directly used to set up DC/DCleu-cultures. T cells and the remaining PBMCs were used for subsequent experiments, therefore cells were frozen at −80 °C (using DMSO) and then thawed according to standardized protocols. Furthermore, the serum was frozen for subsequent ELISA-experiments.
4.2. Patients’ Characteristics and Diagnostics
DC/DCleu were generated from PBMC- and WB-samples that were obtained from AML patients (n = 29) and healthy volunteers (n = 10). The average age of AML patients was 58.6 years (range 21–79) and of healthy volunteers 28.1 years (range 20–56). The female to male ratio of AML patients was 1:1.2 and of healthy 1:0.4. The ages of healthy volunteers and AML patients were not age-matched, since no direct comparisons of those two groups were needed.
The diagnosis and classification of AML patients was based on the French-American-British (FAB) classification: AML without maturation (M1: n = 2), AML with granulocytic maturation (M2: n = 1), acute myelomonocytic leukemia (M4: n = 6), and acute monocytic leukemia (M5: n = 6). No FAB-classification was available in 14 AML cases. Patients presented with primary AML [pAML (n = 17)] or with secondary AML [sAML (n = 12)]. Patients’ stages were: first diagnosis (n = 20), relapse (n = 2) or relapse after HSCT (n = 4), and persisting disease (n = 3). Table 3 gives patients’ characteristics.
Table 3.
Characteristics of AML-patients and healthy volunteers included in this study are presented.
| Age at | Subtype | Blasts | Conducted | Conducted | |||||
|---|---|---|---|---|---|---|---|---|---|
| Stage | Pat. # | dgn. | Sex | FAB | Blast Phenotype (CD) | in PB % | DC/DCleu-Cultures | Experiments | |
| AML | First Diagnosis | P1419 | 64 | f | p/M1 | 34, 117, 33, 15, 13 | 93 | PBMC | |
| P1426 | 61 | f | s/M5 | 34, 117, 64, 33, 13 | 40 | PBMC, WB | MLCWB, MLCWB-DC, CTX | ||
| P1430 | 79 | m | p/M5 | 34, 117, 33, 13 | 70 | PBMC, WB | MLCWB, MLCWB-DC, CTX | ||
| P1432 | 34 | m | p/M5 | 34, 64, 33, 13 | 81 | PBMC, WB | MLCWB, MLCWB-DC, CTX | ||
| P1434 | 61 | f | s/n.d. | 34, 117, 64, 56, 33, 13, 7 | 61 | PBMC, WB | MLCWB, MLCWB-DC, CTX | ||
| P1439 | 61 | f | s/M5 | 34, 117, 33, 13 | 17 | PBMC, WB | MLCWB, MLCWB-DC | ||
| P1441 | 60 | m | s/M4 | 117, 65, 64, 33, 13, 14, | 81 | PBMC, WB | MLCWB, MLCWB-DC, CTX | ||
| P1442 | 73 | f | s/M4 | 117, 138, 61, 33 | 14 | PBMC, WB | MLCWB, MLCWB-DC, CTX | ||
| P1443 | 64 | m | p/n.d. | 34, 117, 33, 13 | 50 | PBMC, WB | |||
| P1447 | 21 | m | p/M5 | 56, 33, 45 | 65 | WB | CTX | ||
| P1449 | 78 | m | s/n.d. | 15, 65, 64, 45, 4 | 62 | WB | MLCWB, MLCWB-DC, CTX | ||
| P1452 | 44 | m | p/n.d. | 34, 117, 45, 33, 13 | 55 | PBMC, WB | CTX | ||
| P1453 | 54 | f | p/M4 | 15, 64, 56, 33, 14 | 52 | PBMC, WB | MLCWB, MLCWB-DC | ||
| P1454 | 60 | f | s/n.d. | 34, 117, 61, 20 | 33 | WB | |||
| P1459 | 54 | m | p/M4 | 56, 64, 38, 33, 11c, 11b | 14 | PBMC, WB | MLCWB, MLCWB-DC, CTX | ||
| P1460 | 78 | f | p/M4 | 15, 34, 117 | 68 | PBMC, WB | MLCWB, MLCWB-DC | ||
| P1462 | 49 | f | p/M5 | 34, 56, 64, 45, 33, 13 | 60 | WB | |||
| P1466 | 47 | f | p/n.d. | 34, 117, 33, 15, 13 | 15 | PBMC | |||
| P1471 | 40 | m | p/M1 | 34, 117, 33, 13 | 69 | PBMC, WB | |||
| P1472 | 33 | f | p/M2 | 117, 34, 15, 13 | 30 | WB | |||
| Relapse Before or after HSCT | P1474 | 70 | m | p/n.d. | 117, 34, 56, 33 | 80 | PBMC, WB | ||
| P1475 | 77 | m | s/n.d. | 117, 34, 33, 13 | 20 | PBMC, WB | |||
| P1455 | 63 | m | s/n.d. | 34, 117, 13 | 12 | WB | |||
| P1463 | 60 | f | s/n.d. | 34, 56, 33, 13, 2 | 8 | PBMC, WB | CTX | ||
| P1469 | 49 | m | p/M4 | 34, 117, 65, 33, 13 | 94 | PBMC | |||
| P1470 | 67 | m | p/n.d. | 56, 117, 34, 33, 13, | 9 | PBMC, WB | |||
| Persisting Disease | P1464 | 72 | m | s/n.d. | 34, 117, 71, 20 | 44 | PBMC, WB | MLCWB, MLCWB-DC, CTX | |
| P1467 | 59 | f | s/n.d. | 34, 117, 33, 15, 13 | 30 | PBMC, WB | |||
| P1468 | 66 | m | p/n.d. | 117, 56, 34, 33 | 75 | PBMC, WB | |||
| Healthy | P1418 | 22 | m | PBMC, WB | |||||
| P1420 | 26 | f | PBMC, WB | ||||||
| P1421 | 27 | f | PBMC | ||||||
| P1422 | 20 | f | PBMC, WB | ||||||
| P1428 | 56 | f | PBMC, WB | ||||||
| P1429 | 22 | f | WB | ||||||
| P1436 | 25 | m | PBMC, WB | ||||||
| P1438 | 31 | f | PBMC, WB | ||||||
| P1445 | 27 | f | PBMC, WB | ||||||
| P1446 | 25 | m | PBMC, WB |
Pat.# Patient’s number; f female; m male; p primary AML; s secondary AML; FAB French-American-British classification; PB peripheral blood; n.d. no data; dgn. diagnosis; PBMC peripheral blood mononuclear cells; WB whole blood; HSCT hematopoietic stem cell transplantation; MLCWB mixed lymphocyte culture with WB not preincubated with DC/DCleu-generating protocols; MLCWB-DC mixed lymphocyte culture with WB preincubated with DC/DCleu-generating protocols; CTX cytotoxicity (fluorolysis) assay; bold blasts markers used to detect DCleu at the day of harvest
The cellular composition of AML- and healthy-peripheral-WB-samples as well as of AML- and healthy-PBMCs-samples are shown in Table 4. In cases with the aberrant expression of T-, B-, or monocytoid-antigens or CD56 on leukemic blasts, proportions of the corresponding cells were not included in the analysis.
Table 4.
Cellular composition of Acute myeloid leukemia (AML) and healthy samples.
| Cell Type | Average of Cells in WB/PBMCs (%) | Range of Cells in WB/PBMCS (%) | |
|---|---|---|---|
| AML | Leukemic blasts * | 46.2/45.3 | 8.0–81.0/8.0–93.0 |
| CD3+T cells | 9.9/6.3 | 1.5–23.3/0.4–24.0 | |
| CD19+B cells | 2.8/2.4 | 0.1–8.4/0.2–7.6 | |
| CD56+CD3-NK-cells | 4.8/1.9 | 0.4–9.8/0.2–6.3 | |
| CD14+monocytes | 3.4/1.8 | 0.1–11.5/0.1–5.7 | |
| Heathy | CD14+monocytes | 5.6/7.0 | 4.4–8.5/2.6–12.7 |
| CD3+T cells | 19.4/36.3 | 13.6–26.3/21.1–46.7 | |
| CD56+CD3-NK-cells | 3.4/4.8 | 2.3–6.9/3.5–6.6 | |
| CD19+B cells | 2.3/6.0 | 0.8–4.8/1.7–11.8 |
* expression of CD15+, CD33+, CD34+, CD65+ and/or CD117+; AML acute myeloid leukemia; WB whole blood; PBMCs peripheral blood mononuclear cells.
4.3. DC/DCleu-Generation from Isolated PBMCs
DC/DCleu were generated from 3–4 × 106 isolated healthy and leukemic PBMCs with the DC/DCleu-generating protocols Pici-PGE1 and Pici-PGE2 [17,20]. Therefore, cells were pipetted into 12-multiwell-tissue-culture-plates (ThermoFisher Scientific, Darmstadt, Germany) and they were diluted in 2 mL serum-free X-Vivo-15-medium (Lonza, Basel, Swiss). Cytokines were added, as described below. Half medium exchange was carried out after 3–4 cell culture days. A culture without added response modifiers served as a control.
4.4. DC/DCleu-Generation from WB
DC/DCleu were generated from healthy and leukemic WB (presenting the physiological cellular and soluble composition of the individual samples) with the DC/DCleu-generating protocols Pici-PGE2, Pici-PGE1, Kit M, Kit K and Kit I [47]. Therefore, 500 μL WB were pipetted in 12-multiwell-plates and then diluted 1:2 in X-Vivo-15-medium (Lonza, Basel, Swiss) to imitate the physiological conditions. Response modifiers and immune-modulating factors were added to cultures, as described below. A culture without added response modifiers served as a control. All of the response modifiers used for the DC/DCleu-generation are approved for human treatment. Table 1 provides the compositions of DC/DCleu-generating protocols.
All of the cell-culture-experiments were conducted at standard laboratory conditions comprising 37°C, 21% O2 and 5% CO2. At the day of harvest, cell culture supernatants were collected from DC/DCleu-cultures and MLC-cultures and frozen, according to the standardized protocols for subsequent ELISA-experiments.
4.4.1. Picibanil-PGE1 (Pici-PGE1)
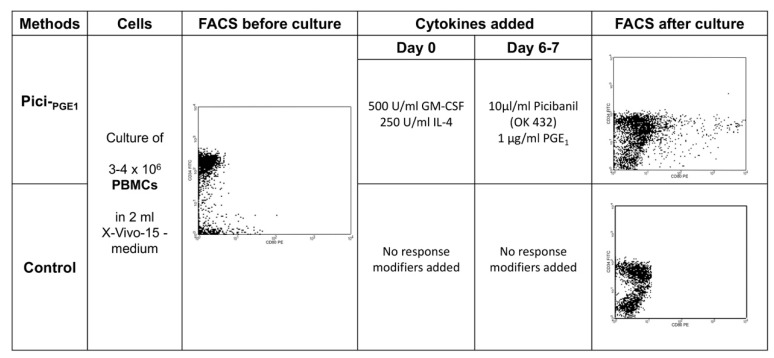
DC/DCleu were generated from PBMCs and WB with the DC/DCleu-generating protocol Pici-PGE1 -containing 500 U/mL granulocyte-macrophage colony-stimulation factor (GM-CSF, Sanofi-Aventis, Frankfurt, Germany) and 250 U/mL Interleukin-4 (IL-4) (PeproTech, Berlin, Germany). After 6–7 days, 10μg/mL Picibanil (OK 432), a lysis product from Streptococcus pyogenes that has unspecific immune modulatory effects (Chugai Pharmaceutical Co., Kajiwara, Japan) and 1μg/mL Prostaglandin E1 (PGE1) (PeproTech, Berlin, Germany) were added. After 7–10 days of incubation, the cells were harvested and used for subsequent experiments.
4.4.2. Picibanil-PGE2 (Pici-PGE2)
DC/DCleu were generated from PBMCs and WB with the Pici-PGE2 DC/DCleu-generating protocol, with the same composition, as given above, for Pici-PGE1, however substituting PGE1 by PGE2 (PeproTech, Berlin, Germany) [17,20].
4.4.3. Kit M:
The generation of DC/DCleu from WB with Kit M was performed while using 800 U/mL GM-CSF and 1μg/mL PGE1 [47]. After 2–3 days the same amounts of cytokines were added and after in total 7–10 days of incubation cells were harvested and used for subsequent experiments.
4.4.4. Kit K:
Kit K consisted of 800 U/mL GM-CSF and 1μg/mL PGE2 and it was used to generate DC/DCleu from WB [47]. Incubations were performed in analogy to Kit M.
4.4.5. Kit I:
DC/DCleu were generated with Kit I from WB using 800 U/mL GM-CSF and 10 μg/mL Picibanil [47]. The incubations were performed in analogy to Kit M.
4.5. Cell-Characterization by Flow Cytometry
Flow cytometric analyses were carried out to evaluate and quantify the amounts and phenotypes of leukemic blasts, T cell subsets, B cells, monocytes and DC/DCleu subsets in the PBMC- and WB-fractions before and after different cultures. Panels with several monoclonal antibodies (moAbs) labeled with Fluorescein isothiocyanat (FITC), phycoerythrin (PE), tandem Cy7-PE conjugation (Cy7-PE), or allophycocyanin (APC) were used. The antibodies were provided by Beckman Coulter, Krefeld, Germany (a), Becton Dickinson, Heidelberg, Germany (b), Miltenyi Biotech, Bergisch Gladbach, Germany (c), Thermo Fisher, Darmstadt, Germany (d) and Santa Cruz Biotechnology, Heidelberg, Germany (e). FITC-conjugated moAbs against CD3a, CD15a, CD33a, CD34a, CD45ROa, CD65a, CD71a, CD83a, and IPO-38e were used. To detect CD3a, CD4a, CD19a, CD33a, CD34a, CD56a, CD80b, CD83a, CD117a, and CD206a PE-conjugated moAbs were used. MoAbs against CD3a, CD4a, CD14b, CD15b, CD19a, CD33a, CD34a, CD56a, CD65c, CD80b, CD117a, and CD197b were labeled with Cy7-PE. APC-labeled moAbs against CD3a, CD4b, CD14a, CD15b, CD34a, CD45ROd, CD56a, CD65c, CD69b, CD83b, CD86g, CD117a, CD206b, and CD209b were used. 7AADb was used to detect dead cells. To stain intracellular antigens (e.g., IPO-38) the FIX & PERM® Cell Fixation and Cell Permeabilization Kit (ThermoFisher Scientific, Darmstadt, Germany) was used.
Erythrocytes in WB samples were lysed while using Lysing-Buffer (BD, Heidelberg, Germany), according to the manufacturer’s instructions. To stain cells with moAbs, they were resuspended in PBS (Biochrom, Berlin, Germany), containing 10% fetal calf-serum (FCS, Biochrome, Berlin, Germany) to avoid unspecific bindings and they were incubated for 15 min. in the dark at room temperature. Afterwards, the cells were washed, centrifuged, and resuspended in 100–200 μL PBS. At least 5000 events were evaluated with a fluorescence-activated cell sorting Flow-Cytometer (FACSCaliburTM) and Cell-Quest-data-acquisition and analysis software (Becton Dickson, Heidelberg, Germany). The isotype controls were conducted according to manufacturer’s instructions.
To quantify generated DCleu, the cells were stained with patient-specific blast-staining antibodies (e.g., CD15, CD34, CD65, and CD117), according to diagnostic reports in combination with DC-staining antibodies (e.g., CD80, CD83, CD86, CD206, and CD209), which were not expressed on blasts before culture. For the analysis and quantification of DCs and DCleu in the total- or in subtype-cell fractions after DC/DCleu-cultures, we used a refined gating strategy [16,17]. DC/DCleu subgroup analyses were only conducted in cases with ≥10% DCs in the PBMC- and WB-fractions (premise for analysis). DCleu were quantified in the total cell fraction (DCleu/PBMC or WB), in the DC-fraction (DCleu/DC+) or in the blast fraction, to quantify the amount of blasts converted to DCleu (DCleu/bla+). The workflow and FACS analysis of DC/DCleu-generation with Pici-PGE1 as compared to control is exemplarily illustrated in Figure 5 The amount of mature DCs [DC co-expressing the migration marker CCR7 (CD197)] in the DC fraction after culture (DCmig/DC+) was quantified in the cases with ≥10% DC+/cells. A refined gating strategy was used to detect non-converted proliferating blasts in the PBMC- or WB-fractions (Blaprol/PBMC or WB) after DC/DCleu-culture [19]. The proliferating blasts were characterized by the co-expression of CD71 or IPO-38 without the co-expression of DC-markers. (Table 5).
Figure 5.
Workflow and FACS analysis of DC/DCleu generated with Pici-PGE1 compared to control from leukemic PBMCs in a case of AML. 3–4 × 106 PBMCs were cultured in 12-multiwell-tissue-culure-plates and diluted in 2 mL serum-free X-vivo-15-medium. For the generation of DC/DCleu with Pici-PGE1 500 U/mL GM-CSF and 250 U/mL IL-4 were added on day 0. After 6–7 days, 10µg/mL Picibanil a lysis product from Streptococcus pyogenes, which has unspecific immune modulatory effects and 1 µg/mL PGE1 were added. No response modifiers were added to the control culture. Cells were harvested after 7–10 days of incubation. Half medium exchange was carried out after 3–4 days. x-axis: CD80; y-axis: CD34.
Table 5.
Subtypes of DC/DCleu and T cells as evaluated by flow cytometry.
| Names of Subgroups | Referred to | Surface Marker | Abbreviation | Explanatory Note Premise for Analysis | Reference | |
|---|---|---|---|---|---|---|
| DC/DCleu | leukemic blasts | cells (PBMC, WB) | CD15, CD34, CD65, CD117 | Bla+/cells [PBMC, WB] | [16] | |
| dentritic cells | cells (PBMC, WB) | CD80, CD83, CD86, CD206, CD209 | DC+/cells [PBMC, WB] | [16] | ||
| DCleu in DC fraction | DC+ | DC+Bla+ | DCleu/DC | ≥ 10% DC+ in cells | [16] | |
| blasts converted to DCleu | Bla+ | DC+Bla+ | DCleu/Bla+ | ≥ 10% DC+ in cells | [16] | |
| leukemia derived DC | cells (PBMC, WB) | DC+Bla+ | DCleu/cells [PBMC, WB] | ≥ 10% DC+ in cells | [16] | |
| migratory mature DC in DC fraction | DC+ | DC+CCR7+ | DCmig/DC | ≥ 10% DC+ in cells | [25] | |
| proliferating blasts | cells (PBMC, WB) | Bla+DC−CD71+ | Blaprol-CD71/cells [PBMC, WB] | [19] | ||
| proliferating blasts | cells (PBMC, WB) | Bla+DC−IPO-38+ | Blaprol-IPO38/cells [PBMC, WB] | [19] | ||
| T cell subsets | CD3+ pan T cells | gated cells | CD3+ | CD3+/cells | [27] | |
| CD4+ coexpressing T cells | CD3+ | CD3+CD4+ | CD4+CD3+/CD3+ | CD4+ T cells | [27] | |
| CD8+ coexpressing T cells | CD3+ | CD3+CD8+ | CD8+CD3+/CD3+ | CD8+ T cells | [27] | |
| early proliferating T cells | CD3+ | CD3+CD69+ | Tprol/CD3+ | proliferating T cells | [27] | |
| proliferating T cells | CD3+ | CD3+CD71+ | Tprol/CD3+ | proliferating T cells | [27] | |
| naive T cells | CD3+ | CD3+CD45RO− | Tnaive/CD3+ | Unprimed T cells | [28] | |
| naive CD4+ T cells | CD3+ | CD3+CD4+CD45RO− | TCD4-naive/CD3+ | Unprimed CD4+ T cells | [28] | |
| naive CD8+ T cells | CD3+ | CD3+CD8+CD45RO− | TCD8-naive/CD3+ | Unprimed CD8+ T cells | [28] | |
| non-naive T cells | CD3+ | CD3+CD45RO+ | Tnon-naive/CD3+ | Memory + effector T cells | [28] | |
| non-naive CD4+ T cells | CD3+ | CD3+CD4+CD45RO+ | TCD4-non-naive/CD3+ | Memory + effector CD4+ T cells | [28] | |
| non-naive CD8+ T cells | CD3+ | CD3+CD8+CD45RO+ | TCD8-non-naive/CD3+ | Memory + effector CD8+ T cells | [28] | |
| central (memory) T cells | CD3+ | CD3+CCR7+CD45RO+ | Tcm/CD3+ | Long-term immunity | [28] | |
| effector (memory) T cells | CD3+ | CD3+CCR7−CD45RO+ | TeffTems/CD3+ | [28] |
Surface marker combinations for the analysis of DC and DCleu (including subsets) and T cell subtypes after flow cytometric staining with fluorochrome-labelled-antibodies are given. Cells were analyzed before and after different cultures.
4.6. Mixed-Lymphocyte-Culture (MLC) of T Cell-Enriched Immunoreactive Cells with WB-Stimulator-Cell-Suspensions, Preincubated or Not Preincubated with DC/DCleu-Generating Protocols
1 × 106 CD3+T cells (effector cells) from AML patients were co-cultured in 24-multiwell-tissue-culture-plates (ThermoFisher Scientific, Darmstadt, Germany) with a stimulator cell suspension containing approximately 2.5 × 105 DC/DCleu (MLCWB-DC), which were generated with different DC/DCleu-generating protocols from leukemic WB or PBMCs. A MLC of T cell enriched immunoreactive cells with a stimulator cell suspension without preincubation with different DC/DCleu-generating protocols (MLCWB) severed as a control. The total volume of the cell culture was adjusted to 1 mL with RPMI-1640 medium (Biochrom, Berlin, Germany) containing 1% Penicillin (Biochrom, Berlin, Germany) and 50 U/mL Interleukin 2 (IL-2, PeproTech, Berlin, Germany). After 2–3 days, 50 U/mL IL-2 was added to all cultures. PBMC-cultures contained 15% human serum (Healthcare Europa GmbH, Vienna, Austria). The cells were harvested after 6–7 days and they were used for cytotoxicity-fluorolysis-assay, as described below. Before and after culture different T cell subsets in MLC were quantified by flow cytometry (Table 5).
4.7. Cytotoxicity (Fluorolysis) Assay
A Fluorolysis assay was performed to analyze the blast lytic activity of T cell-enriched immunoreactive cells after MLCWB-DC and MLCWB [25]. Therefore, effector cells were co-cultured 1:1 with thawed blast-containing target cells for 3 and 24 h in standard laboratory conditions, as described above. As a control effector- and target-cells were cultured separately for the same time and mingled on ice shortly before flow cytometric analyses were carried out. Before culture, the target-cells were stained for 15 min. with FITC-, PE-, or APC-conjugated blast specific target cell antibodies. To evaluate viable cells and the lytic activity of effector cells, the cultures were harvested after 3 h and 24 h and then resuspended in PBS containing 7AAD (Becton Dickson, Heidelberg, Germany) and a defined number of Fluorosphere beads (Beckman Coulter, Krefeld, Germany). A refined gating was used to analyse blast lytic activity (anti-leukemic activity) [25]. Therefore, viable target cells were gated in a Forward Scatter (FSC) 7AAD− gate. The cells were analyzed with a fluorescence-activated cell sorting Flow-Cytometer (FACSCaliburTM) and Cell-Quest-data-acquisition and analysis software (Becton Dickson, Heidelberg, Germany). The lytic activity of effector cells was calculated and defined as the difference in the percentage of viable target cells in the culture with co-cultured effector and target cells (for 3 h and 24 h) as compared to control.
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
Serum and WB-DC/DCleu-culture supernatants were analyzed for the concentration of Interleukin 10 (IL-10), Interleukin-17A (IL-17A), and Monocyte Chemoattractant Protein-1 (MCP-1, also known as CC-chemokine ligand 2, CCL-2) while using the human IL-10 (detection limit: 1.0 pg/mL), IL-17A (detection limit: 0.5 pg/mL) and MCP-1 (detection limit: 2.3 pg/mL) immunoassay kits (DRG Instruments GmbH, Marburg, Germany). The samples were evaluated with a Tristar LB941 ELISA reader (Berthold Company, Bad Wildbach, Germany) and the concentrations of the different cytokines were evaluated with the corresponding standard curve.
4.9. Statistical Methods
Data were presented as mean ± standard-deviations. The cytokine-concentrations were presented as median and the corresponding range. Statistical comparisons of two groups were performed while using the two-tailed t test (in cases with data normally distributed) and the Mann-Whitney-Wilcoxon-Test (in cases with data not normally distributed). Statistical analyses were performed with Microsoft Excel 2013® (Microsoft, Redmond, Washington, USA) and SSPS Statistic 24 software© (IBM, Armonk, USA). The differences were considered as ‘not significant’ in cases with p values >0.1, as ‘borderline significant’ (significant*), with p values between 0.1 and 0.05, as ‘significant’ (significant**) with p values between 0.05 and 0.005 and as ‘highly significant’ (significant***) with p values <0.005. The figures were created with GraphPad Prism7© (GraphPad Software, California, USA).
5. Conclusion: DC/DCleu Based Treatment Protocols for AML-Patients
We developed a DC/DCleu-generating protocol Pici-PGE1 and demonstrated (by replacing PGE2 with PGE1) that the efficiency of sufficient DC/DCleu generation is superior from leukemic PBMCs. Furthermore, the PGE1-containing Kit M is a reliable tool for generating ex vivo mature DC/DCleu from healthy and leukemic WB, containing the complete patient individual soluble and cellular microenvironment. These ‘new PGE1 generated’ DC/DCleu reliably (re-) activate immunoreactive cells, improve the overall ex vivo anti-leukemic activity, and influence cytokine-release-profiles compared to the PGE2-containing Kit K.
We conclude that
-
(1)
PGE1-containing protocols qualify to generate and to maturate monocyte derived DCs from healthy or even patients’ PBMCs ex vivo that could, in consequence, be manipulated (e.g., pulsed with LAA) and (re-) administrated to the patients as a vaccine.
-
(2)
PGE1-containing protocols qualify to produce ex vivo DCleu from leukemic PBMCs. These DC/DCleu could be (re-) administered to the patient in the course of an adaptive cell transfer.
-
(3)
GM-CSF, PGE1, and Picibanil are drugs that are approved for human treatment, and so we conclude that, for example, the PGE1-containing Kit M qualify to convert (residual) myeloid blasts to DCs and DCleu in vivo after the application of Kits to AML-patients. This could contribute to stabilize remission or the disease by presentation of the complete leukemic antigen repertoire to T cells and other immunoreactive cells independent of mutation or transplantation status, cytogenetic markers, FAB-classification, as well as sex or age of the patients.
-
(4)
In vivo trials with PGE1-containing Kits (in animals and humans with AML) have to be performed to study safety, the efficiency of DC/DCleu-generation, the mediation of anti-leukemic reactions, and the establishment of immunological effects in vivo.
6. Patent
HMS is the inventor of the European Patent 15 801 987.7-1118 ‘Use of immunomodulatory Kits for immunotherapeutic treatment of patients with myeloid leukemia’s’. No financial conflicts of interest have to be declared.
Acknowledgments
The authors thank patients, nurses, and physicians on the wards for their support and the diagnostic laboratories as well as the treating institutions for the patients’ diagnostic reports. The results presented in this manuscript were worked out in the medical doctoral thesis of Daniel C. Amberger at the University Hospital Großhadern of the Ludwig-Maximilian-University Munich.
Author Contributions
D.C.A. conducted cell-culture experiments (including DC/DCleu-cultures, MLC- and CTX-experiments) and all FACS- and statistical-analysis. F.D.-G., C.G., M.W., C.B., S.M., C.K., N.R. performed DC/DCleu-culture-, MLC- and CTX-experiments, which were analyzed by D.C.A. D.C.A. and C.G. conducted ELISA-experiments. U.K. was responsible for the measurement of ELISA-experiments. J.-O.W., D.K., A.R., C.S. provided leukemic samples and corresponding patients’ diagnostic reports. B.E.-V. supported functionality assays. H.M.S. designed the study and was responsible for the funding. D.C.A. and H.M.S. drafted this manuscript.
Funding
This research was funded in part by grants of the DAAD (ID 21520154 and scholarship 2016-2017 of the Ludwigs Maximilians University Munich).
Conflicts of Interest
All authors declare, that there are no financial conflicts in regard to this work.
References
- 1.Lowenberg B., Downing J.R., Burnett A. Acute myeloid leukemia. N. Engl. J. Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Cancer Stat Facts. [(accessed on 7 July 2019)]; Available online: https://seer.cancer.gov/statfacts/html/amyl.html.
- 3.Schurch C.M., Riether C., Ochsenbein A.F. Dendritic cell-based immunotherapy for myeloid leukemias. Front. Immunol. 2013;4:496. doi: 10.3389/fimmu.2013.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenegger F.S., Krupka C., Haubner S., Kohnke T., Subklewe M. Recent developments in immunotherapy of acute myeloid leukemia. J. Hematol. Oncol. 2017;10:142. doi: 10.1186/s13045-017-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anguille S., Smits E.L., Bryant C., Van Acker H.H., Goossens H., Lion E., Fromm P.D., Hart D.N., Van Tendeloo V.F., Berneman Z.N. Dendritic Cells as Pharmacological Tools for Cancer Immunotherapy. Pharmacol. Rev. 2015;67:731–753. doi: 10.1124/pr.114.009456. [DOI] [PubMed] [Google Scholar]
- 6.Palucka K., Banchereau J. Dendritic cells: A link between innate and adaptive immunity. J. Clin. Immunol. 1999;19:12–25. doi: 10.1023/A:1020558317162. [DOI] [PubMed] [Google Scholar]
- 7.Wan H., Dupasquier M. Dendritic cells in vivo and in vitro. Cell. Mol. Immunol. 2005;2:28–35. [PubMed] [Google Scholar]
- 8.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Lanzavecchia A., Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/S0092-8674(01)00455-X. [DOI] [PubMed] [Google Scholar]
- 10.Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S., Allen P.M., Dustin M.L. The immunological synapse: A molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 11.Smits E.L., Berneman Z.N., Van Tendeloo V.F. Immunotherapy of acute myeloid leukemia: Current approaches. Oncologist. 2009;14:240–252. doi: 10.1634/theoncologist.2008-0165. [DOI] [PubMed] [Google Scholar]
- 12.Rein L.A., Chao N.J. WT1 vaccination in acute myeloid leukemia: New methods of implementing adoptive immunotherapy. Expert Opin. Investig. Drugs. 2014;23:417–426. doi: 10.1517/13543784.2014.889114. [DOI] [PubMed] [Google Scholar]
- 13.Osman Y., Takahashi M., Zheng Z., Toba K., Liu A., Furukawa T., Narita M., Aizawa Y., Koike T., Shibata A. Dendritic cells stimulate the expansion of PML-RAR alpha specific cytotoxic T-lymphocytes: Its applicability for antileukemia immunotherapy. J. Exp. Clin. Cancer Res. 1999;18:485–492. [PubMed] [Google Scholar]
- 14.Van Driessche A., Van de Velde A.L., Nijs G., Braeckman T., Stein B., De Vries J.M., Berneman Z.N., Van Tendeloo V.F. Clinical-grade manufacturing of autologous mature mRNA-electroporated dendritic cells and safety testing in acute myeloid leukemia patients in a phase I dose-escalation clinical trial. Cytotherapy. 2009;11:653–668. doi: 10.1080/14653240902960411. [DOI] [PubMed] [Google Scholar]
- 15.Van Tendeloo V.F., Van de Velde A., Van Driessche A., Cools N., Anguille S., Ladell K., Gostick E., Vermeulen K., Pieters K., Nijs G., et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl. Acad. Sci. USA. 2010;107:13824–13829. doi: 10.1073/pnas.1008051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmetzer H.M., Kremser A., Loibl J., Kroell T., Kolb H.J. Quantification of ex vivo generated dendritic cells (DC) and leukemia-derived DC contributes to estimate the quality of DC, to detect optimal DC-generating methods or to optimize DC-mediated T-cell-activation-procedures ex vivo or in vivo. Leukemia. 2007;21:1338–1341. doi: 10.1038/sj.leu.2404639. [DOI] [PubMed] [Google Scholar]
- 17.Kremser A., Dressig J., Grabrucker C., Liepert A., Kroell T., Scholl N., Schmid C., Tischer J., Kufner S., Salih H., et al. Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: An evaluation of different methods. J. Immunother. 2010;33:185–199. doi: 10.1097/CJI.0b013e3181b8f4ce. [DOI] [PubMed] [Google Scholar]
- 18.Johnson L.A., Jackson D.G. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis. 2014;17:335–345. doi: 10.1007/s10456-013-9407-0. [DOI] [PubMed] [Google Scholar]
- 19.Plett C., Amberger D.C., Rabe A., Deen D., Stankova Z., Hirn Lopez A., Vokac Y., Werner J.O., Krämer D., Rank A., et al. Kits do not induce AML-blasts’ proliferation ex vivo. IPO-38 is an appropriate and reliable marker to detect and quantify proliferating blasts. J. Immunother. Cancer. 2017;52:S398. [Google Scholar]
- 20.Sato M., Takayama T., Tanaka H., Konishi J., Suzuki T., Kaiga T., Tahara H. Generation of mature dendritic cells fully capable of T helper type 1 polarization using OK-432 combined with prostaglandin E(2) Cancer Sci. 2003;94:1091–1098. doi: 10.1111/j.1349-7006.2003.tb01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee A.W., Truong T., Bickham K., Fonteneau J.F., Larsson M., Da Silva I., Somersan S., Thomas E.K., Bhardwaj N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: Implications for immunotherapy. Vaccine. 2002;20(Suppl. 4):A8–A22. doi: 10.1016/S0264-410X(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 22.Houtenbos I., Westers T.M., Stam A.G., de Gruijl T.D., Scheper R.J., Ossenkoppele G.J., van de Loosdrecht A.A. Serum-free generation of antigen presenting cells from acute myeloid leukaemic blasts for active specific immunisation. Cancer Immunol. Immunother. 2003;52:455–462. doi: 10.1007/s00262-003-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woiciechowsky A., Regn S., Kolb H.J., Roskrow M. Leukemic dendritic cells generated in the presence of FLT3 ligand have the capacity to stimulate an autologous leukemia-specific cytotoxic T cell response from patients with acute myeloid leukemia. Leukemia. 2001;15:246–255. doi: 10.1038/sj.leu.2402013. [DOI] [PubMed] [Google Scholar]
- 24.Hirn Lopez A., Deen D., Fischer Z., Rabe A., Ansprenger C., Stein K., Vogt V., Schick J., Kroell T., Kraemer D., et al. Role of Interferon (IFN)alpha in “Cocktails” for the Generation of (Leukemia-derived) Dendritic Cells (DCleu) from Blasts in Blood from Patients (pts) with Acute Myeloid Leukemia (AML) and the Induction of Antileukemic Reactions. J. Immunother. 2019;42:143–161. doi: 10.1097/CJI.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 25.Grabrucker C., Liepert A., Dreyig J., Kremser A., Kroell T., Freudenreich M., Schmid C., Schweiger C., Tischer J., Kolb H.J., et al. The quality and quantity of leukemia-derived dendritic cells from patients with acute myeloid leukemia and myelodysplastic syndrome are a predictive factor for the lytic potential of dendritic cells-primed leukemia-specific T cells. J. Immunother. 2010;33:523–537. doi: 10.1097/CJI.0b013e3181d87ffd. [DOI] [PubMed] [Google Scholar]
- 26.Liepert A., Grabrucker C., Kremser A., Dreyssig J., Ansprenger C., Freudenreich M., Kroell T., Reibke R., Tischer J., Schweiger C., et al. Quality of T-cells after stimulation with leukemia-derived dendritic cells (DC) from patients with acute myeloid leukemia (AML) or myeloid dysplastic syndrome (MDS) is predictive for their leukemia cytotoxic potential. Cell. Immunol. 2010;265:23–30. doi: 10.1016/j.cellimm.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Schick J., Vogt V., Zerwes M., Kroell T., Kraemer D., Kohne C.H., Hausmann A., Buhmann R., Tischer J., Schmetzer H. Antileukemic T-cell responses can be predicted by the composition of specific regulatory T-cell subpopulations. J. Immunother. 2013;36:223–237. doi: 10.1097/CJI.0b013e31829180e7. [DOI] [PubMed] [Google Scholar]
- 28.Vogt V., Schick J., Ansprenger C., Braeu M., Kroell T., Kraemer D., Kohne C.H., Hausmann A., Buhmann R., Tischer J., et al. Profiles of activation, differentiation-markers, or beta-integrins on T cells contribute to predict T cells’ antileukemic responses after stimulation with leukemia-derived dendritic cells. J. Immunother. 2014;37:331–347. doi: 10.1097/CJI.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 29.Boeck C.L., Amberger D.C., Doraneh-Gard F., Sutanto W., Guenther T., Schmohl J., Schuster F., Salih H., Babor F., Borkhardt A., et al. Significance of Frequencies, Compositions, and/or Antileukemic Activity of (DC-stimulated) Invariant NKT, NK and CIK Cells on the Outcome of Patients With AML, ALL and CLL. J. Immunother. 2017;40:224–248. doi: 10.1097/CJI.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 30.Kupsa T., Horacek J.M., Jebavy L. The role of cytokines in acute myeloid leukemia: A systematic review. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc. 2012;156:291–301. doi: 10.5507/bp.2012.108. [DOI] [PubMed] [Google Scholar]
- 31.Driss V., Quesnel B., Brinster C. Monocyte chemoattractant protein 1 (MCP-1/CCL2) contributes to thymus atrophy in acute myeloid leukemia. Eur. J. Immunol. 2015;45:396–406. doi: 10.1002/eji.201444736. [DOI] [PubMed] [Google Scholar]
- 32.Ersvaer E., Liseth K., Skavland J., Gjertsen B.T., Bruserud O. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC Immunol. 2010;11:38. doi: 10.1186/1471-2172-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J., Paul W.E. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozlowska A., Mackiewicz J., Mackiewicz A. Therapeutic gene modified cell based cancer vaccines. Gene. 2013;525:200–207. doi: 10.1016/j.gene.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 35.Fischbacher D., Merle M., Liepert A., Grabrucker C., Kroell T., Kremser A., Dreyssig J., Freudenreich M., Schuster F., Borkhardt A., et al. Cytokine Release Patterns in Mixed Lymphocyte Culture (MLC) of T-Cells with Dendritic Cells (DC) Generated from AML Blasts Contribute to Predict anti-Leukaemic T-Cell Reactions and Patients’ Response to Immunotherapy. Cell Commun. Adhes. 2015;22:49–65. doi: 10.1080/15419061.2016.1223634. [DOI] [PubMed] [Google Scholar]
- 36.Hao C.M., Breyer M.D. Physiological regulation of prostaglandins in the kidney. Annu. Rev. Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 37.Breyer M.D., Harris R.C. Cyclooxygenase 2 and the kidney. Curr. Opin. Nephrol. Hypertens. 2001;10:89–98. doi: 10.1097/00041552-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Smith W.L. Prostanoid biosynthesis and mechanisms of action. Pt 2Am. J. Physiol. 1992;263:F181–F191. doi: 10.1152/ajprenal.1992.263.2.F181. [DOI] [PubMed] [Google Scholar]
- 39.Dubois R.N., Abramson S.B., Crofford L., Gupta R.A., Simon L.S., Van De Putte L.B., Lipsky P.E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. doi: 10.1096/fasebj.12.12.1063. [DOI] [PubMed] [Google Scholar]
- 40.Simmons D.L., Botting R.M., Hla T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 41.Simmet T., Peskar B.A. Prostaglandin E1 and arterial occlusive disease: Pharmacological considerations. Eur. J. Clin. Investig. 1988;18:549–554. doi: 10.1111/j.1365-2362.1988.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 42.Reiter M., Bucek R.A., Stumpflen A., Minar E. Prostanoids for intermittent claudication. Cochrane Database Syst. Rev. 2004;1:CD000986. doi: 10.1002/14651858.CD000986.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Akkinapally S., Hundalani S.G., Kulkarni M., Fernandes C.J., Cabrera A.G., Shivanna B., Pammi M. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst. Rev. 2018;2:CD011417. doi: 10.1002/14651858.CD011417.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy S.C., Saxena A. Prostaglandin E1: First stage palliation in neonates with congenital cardiac defects. Indian J. Pediatr. 1998;65:211–216. doi: 10.1007/BF02752297. [DOI] [PubMed] [Google Scholar]
- 45.Gluckman E., Jolivet I., Scrobohaci M.L., Devergie A., Traineau R., Bourdeau-Esperou H., Lehn P., Faure P., Drouet L. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br. J. Haematol. 1990;74:277–281. doi: 10.1111/j.1365-2141.1990.tb02583.x. [DOI] [PubMed] [Google Scholar]
- 46.Shulman H.M., McDonald G.B., Matthews D., Doney K.C., Kopecky K.J., Gauvreau J.M., Thomas E.D. An analysis of hepatic venocclusive disease and centrilobular hepatic degeneration following bone marrow transplantation. Gastroenterology. 1980;79:1178–1191. doi: 10.1016/0016-5085(80)90911-7. [DOI] [PubMed] [Google Scholar]
- 47.Kugler C. (Medical Department 3, Working-group: Immune-Modulation, University Hospital Munich, 81377 Munich, Germany), Deen D. (Medical Department 3, Working-group: Immune-Modulation, University Hospital Munich, 81377 Munich, Germany), Stankova Z. (Medical Department 3, Working-group: Immune-Modulation, University Hospital Munich, 81377 Munich, Germany), Hirn Lopez A. (Medical Department 3, Working-group: Immune-Modulation, University Hospital Munich, 81377 Munich, Germany), Vokac Y. (Medical Department 3, Working-group: Immune-Modulation, University Hospital Munich, 81377 Munich, Germany), Rabe A. (Medical Department 3, Working-group: Immune-Modulation, University Hospital Munich, 81377 Munich, Germany), Kroell T. (Medical Department 3, Working-group: Immune-Modulation, University Hospital Munich, 81377 Munich, Germany), Krämer D. (Department for Hematology and Oncology, University Hospital of Oldenburg, 26133 Oldenburg, Germany), Kolb H.J. (Department for Hematology-Oncology Immunology Infectious Diseases, Klinikum München-Schwabing, Munich, Germany), Tischer J. (Medical Department 3, University Hospital Munich, 81377 Munich, Germany), et al. Immunmodulation of blasts in AML-patients (pts) with clinically approved response modifiers to improve antileukemic T-cell reactivity: An ex vivo simulation of the clinical situation. Personal Communication. 2019. in preparation.
- 48.Rosenblatt J., Stone R.M., Uhl L., Neuberg D., Joyce R., Levine J.D., Arnason J., McMasters M., Luptakova K., Jain S., et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci. Transl. Med. 2016;8:368ra171. doi: 10.1126/scitranslmed.aag1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinstock M., Rosenblatt J., Avigan D. Dendritic Cell Therapies for Hematologic Malignancies. Mol. Ther. Methods Clin. Dev. 2017;5:66–75. doi: 10.1016/j.omtm.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subklewe M., Geiger C., Lichtenegger F.S., Javorovic M., Kvalheim G., Schendel D.J., Bigalke I. New generation dendritic cell vaccine for immunotherapy of acute myeloid leukemia. Cancer Immunol. Immunother. 2014;63:1093–1103. doi: 10.1007/s00262-014-1600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brouwer R.E., van der Hoorn M., Kluin-Nelemans H.C., van Zelderen-Bhola S., Willemze R., Falkenburg J.H. The generation of dendritic-like cells with increased allostimulatory function from acute myeloid leukemia cells of various FAB subclasses. Hum. Immunol. 2000;61:565–574. doi: 10.1016/S0198-8859(00)00111-7. [DOI] [PubMed] [Google Scholar]
- 52.Kremser A., Kufner S., Konhaeuser E., Kroell T., Hausmann A., Tischer J., Kolb H.J., Zitzelsberger H., Schmetzer H. Combined immunophenotyping and fluorescence in situ hybridization with chromosome-specific DNA probes allows quantification and differentiation of ex vivo generated dendritic cells, leukemia-derived dendritic cells and clonal leukemic cells in patients with acute myeloid leukemia. Leuk. Lymphoma. 2013;54:1297–1308. doi: 10.3109/10428194.2012.751490. [DOI] [PubMed] [Google Scholar]
- 53.Hull M.A., Ko S.C., Hawcroft G. Prostaglandin EP receptors: Targets for treatment and prevention of colorectal cancer? Mol. Cancer Ther. 2004;3:1031–1039. [PubMed] [Google Scholar]
- 54.Eisinger A.L., Prescott S.M., Jones D.A., Stafforini D.M. The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat. 2007;82:147–154. doi: 10.1016/j.prostaglandins.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 55.Montrose D.C., Nakanishi M., Murphy R.C., Zarini S., McAleer J.P., Vella A.T., Rosenberg D.W. The role of PGE2 in intestinal inflammation and tumorigenesis. Prostaglandins Other Lipid Mediat. 2015;116–117:26–36. doi: 10.1016/j.prostaglandins.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leone V., di Palma A., Ricchi P., Acquaviva F., Giannouli M., Di Prisco A.M., Iuliano F., Acquaviva A.M. PGE2 inhibits apoptosis in human adenocarcinoma Caco-2 cell line through Ras-PI3K association and cAMP-dependent kinase A activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G673–G681. doi: 10.1152/ajpgi.00584.2006. [DOI] [PubMed] [Google Scholar]
- 57.Greenhough A., Smartt H.J., Moore A.E., Roberts H.R., Williams A.C., Paraskeva C., Kaidi A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 58.Xu Y., Qian S.Y. Anti-cancer activities of omega-6 polyunsaturated fatty acids. Biomed. J. 2014;37:112–119. doi: 10.4103/2319-4170.131378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tabolacci C., Lentini A., Provenzano B., Gismondi A., Rossi S., Beninati S. Similar antineoplastic effects of nimesulide, a selective COX-2 inhibitor, and prostaglandin E1 on B16-F10 murine melanoma cells. Melanoma Res. 2010;20:273–279. doi: 10.1097/CMR.0b013e328339d8ac. [DOI] [PubMed] [Google Scholar]
- 60.Ikeguchi M., Maeta M., Kaibara N. Cisplatin combined with prostaglandin E1 chemotherapy in rat peritoneal carcinomatosis. Int. J. Cancer. 2000;88:474–478. doi: 10.1002/1097-0215(20001101)88:3<474::AID-IJC22>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 61.Araki Y., Suganami A., Endo S., Masuda Y., Fukushima K., Regan J.W., Murayama T., Tamura Y., Fujino H. PGE1 and E3 show lower efficacies than E2 to beta-catenin-mediated activity as biased ligands of EP4 prostanoid receptors. FEBS Lett. 2017;591:3771–3780. doi: 10.1002/1873-3468.12878. [DOI] [PubMed] [Google Scholar]
- 62.Graf M., Hecht K., Reif S., Pelka-Fleischer R., Pfister K., Schmetzer H. Expression and prognostic value of hemopoietic cytokine receptors in acute myeloid leukemia (AML): Implications for future therapeutical strategies. Eur. J. Haematol. 2004;72:89–106. doi: 10.1046/j.0902-4441.2003.00184.x. [DOI] [PubMed] [Google Scholar]
- 63.Malissein E., Reynaud S., Bordessoule D., Faucher J.L., Turlure P., Trimoreau F., Denizot Y. PGE(2) receptor subtype functionality on immature forms of human leukemic blasts. Leuk. Res. 2006;30:1309–1313. doi: 10.1016/j.leukres.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Platt A.M., Randolph G.J. Dendritic cell migration through the lymphatic vasculature to lymph nodes. Adv. Immunol. 2013;120:51–68. doi: 10.1016/B978-0-12-417028-5.00002-8. [DOI] [PubMed] [Google Scholar]
- 65.Randolph G.J., Angeli V., Swartz M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 66.Forster R., Davalos-Misslitz A.C., Rot A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 67.Doraneh-Gard F., Amberger D.C., Gunsilius C., Kugler C., Schmetzer H. (Medical Department 3, Working-group: Immune-Modulation, University Hospital Munich, 81377 Munich, Germany). Influence of Hypoxia on immunereactive processes during DC-stimulation of T-cells. Personal Communication. 2019. in preparation.
- 68.Brune M., Castaigne S., Catalano J., Gehlsen K., Ho A.D., Hofmann W.K., Hogge D.E., Nilsson B., Or R., Romero A.I., et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: Results of a randomized phase 3 trial. Blood. 2006;108:88–96. doi: 10.1182/blood-2005-10-4073. [DOI] [PubMed] [Google Scholar]
- 69.Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 70.Trabanelli S., Lecciso M., Salvestrini V., Cavo M., Ocadlikova D., Lemoli R.M., Curti A. PGE2-induced IDO1 inhibits the capacity of fully mature DCs to elicit an in vitro antileukemic immune response. J. Immunol. Res. 2015;2015:253191. doi: 10.1155/2015/253191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braun D., Longman R.S., Albert M.L. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mellor A.L., Munn D.H. Tryptophan catabolism and T-cell tolerance: Immunosuppression by starvation? Immunol. Today. 1999;20:469–473. doi: 10.1016/S0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 73.Wobser M., Voigt H., Houben R., Eggert A.O., Freiwald M., Kaemmerer U., Kaempgen E., Schrama D., Becker J.C. Dendritic cell based antitumor vaccination: Impact of functional indoleamine 2,3-dioxygenase expression. Cancer Immunol. Immunother. 2007;56:1017–1024. doi: 10.1007/s00262-006-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krause P., Singer E., Darley P.I., Klebensberger J., Groettrup M., Legler D.F. Prostaglandin E2 is a key factor for monocyte-derived dendritic cell maturation: Enhanced T cell stimulatory capacity despite IDO. J. Leukoc. Biol. 2007;82:1106–1114. doi: 10.1189/jlb.0905519. [DOI] [PubMed] [Google Scholar]