Abstract
Consumption of organic products is increasing yearly due to perceived health-promoting qualities. Several studies have shown higher amounts of phytochemicals such as polyphenols and carotenoids in foods produced by this type of agriculture than in conventional foods, but whether this increase has an impact on humans still needs to be assessed. A randomized, controlled and crossover study was carried out in nineteen healthy subjects aged 18–40 years, who all followed an organic and conventional healthy diet, both for a 4-week period. Analysis of biological samples revealed a significant increase on the excretion of 4-hydroxybenzoic acid (4-HBA), a phenolic metabolite with biological activity, after the organic intervention. However, no changes were observed in the other variables analyzed.
Keywords: healthy diet, phenolic acid, 4-HBA, crossover study, carotenes, microbiota metabolites, intervention, humans, metals
1. Introduction
Organic food consumption has been increasing yearly over the last decade due to growing public awareness of its environmental benefits and alleged healthy properties [1,2]. The general belief that organic produce is healthier due to a lower use of chemical agents, such as pesticides, fertilizers and antibiotics, [3] is supported by studies reporting lower concentrations of pesticide residues in individuals consuming organic food [4,5,6,7,8]. Differences in nutritional composition associated with the cropping system have also been found, but more studies are needed to draw conclusions [9]. Factors known to influence the nutritional composition of food include crop variety, geographical location, climatic conditions, soil type, season and state of maturity from harvest to storage. Organic food seems to have higher amounts of bioactive compounds such as polyphenols and carotenoids than conventionally produced food [10,11,12,13,14,15,16]. When exposed to a stressful environment, plants activate defense mechanisms. Accordingly, a lack of synthetic protectors (pesticides, chemical fertilizers, etc.) induces organic crops to produce phytochemicals. Phenolic acids represent one third of the phenol group in a diet, but also many of them are produced from dietary polyphenols through microbiota metabolism. Approximately 90% of polyphenols are not absorbed in the small intestine reaching the colon, where they are transformed to other compounds such as the phenolic acids [17,18]. In addition, lower concentrations of cadmium have been observed in organic versus conventional cereals [19], as well as differences in the content of fatty acids and proteins [20,21,22,23]. Among foods of animal origin, total polyunsaturated fatty acid (PUFA) and n-3 PUFA concentrations are higher in organically rather than conventionally produced milk [21]. A similar profile has been observed in meat, although the evidence is weak [23].
The few studies to evaluate the effect of organic foods on human biochemical parameters and health have employed methodologies with some limitations and provide inconclusive results [9], so further intervention studies are needed to corroborate their possible beneficial effects. Consumers of organic produce are associated with having a higher quality diet, lower body mass index (BMI), greater physical activity [24,25] and a generally healthier and more holistic lifestyle [26,27]. Thus, the question is the following: Are the consumers of organic food healthier due to their lifestyle or also because their diet has a superior nutritional value?
The aim of this study was to evaluate the effect of an intervention with organic diet versus a conventional one on biological parameters, inorganic elements, bioactive compounds, and phenolic acids and carotenes in healthy subjects.
2. Materials and Methods
2.1. Study Subjects
Twenty-one healthy volunteers aged 18–40 years were included in the intervention, 19 of whom completed the study and two dropped out alleging personal reasons. Participants had previous interest in healthy diets and organic food, and they were recruited from the Food and Nutrition Torribera Campus of the University of Barcelona and surroundings. Exclusion criteria were history of cancer, cardiovascular diseases, hypertension and dyslipidemia, chronic illness or homeostatic disorder, as well as toxic habits such as tobacco and other drugs and an excessive alcohol intake.
After approval of the protocol by the Ethics Committee of Clinical Investigation of the University of Barcelona (Barcelona, Spain), the study was registered (ISRCTN29145931). Each participant signed an informed consent prior to the start, which was conducted according to the principles of the Declaration of Helsinki.
2.2. Study Design
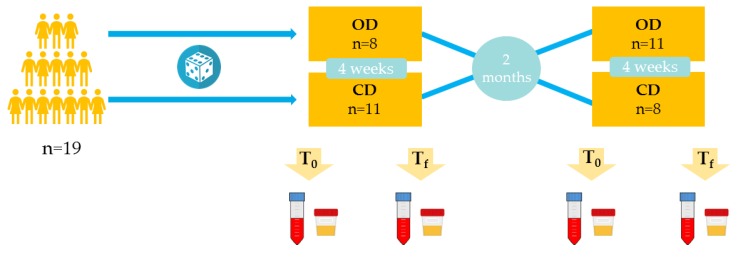
An open, crossover, randomized and controlled study was carried out (Figure 1). Each volunteer consumed an organic diet (OD) and a conventional diet (CD), both for 4 weeks, and received dietary advice to support adherence. Organic products represented at least 80% of the OD and no organic foods were allowed in the CD. In both diets, subjects were encouraged to follow a healthy Mediterranean diet with a similar food pattern. Additionally, during the OD intervention participants were given weekly vouchers from Ecoveritas S.A., as well as products (oil, wine, snacks and canned vegetables) from other organic food companies to facilitate dietary compliance. At the end of each intervention, the absence of differential dietary patterns was checked. Interventions were separated by a washout period of two months. The study was run in the Department of Nutrition, Food Science and Gastronomy of the Food Science and Nutrition Torribera Campus of the University of Barcelona (Spain).
Figure 1.
Study design. OD: Organic diet; CD: Conventional diet; T0: Initial time point (before interventions); Tf: Final time point (after interventions).
2.3. Assessment of Diet and Physical Activity
Before the study, adherence to the Mediterranean diet and physical activity were measured through a 14-item questionnaire [28] and the validated Spanish version of the Minnesota Leisure-Time Physical Activity Questionnaire [29], respectively. Also, at baseline, participants were asked about the frequency of organic food and beverage intake. After each intervention, a 137-item semi-quantitative food frequency questionnaire was filled in with the help of the study staff to assess nutrient and food intake [30].
2.4. Anthropometric and Clinical Data Measurements
Body weight was measured using an electronic scale and height with a stadiometer. The BMI was calculated from body weight and height. Waist and hip circumferences were measured with a measuring tape accurate to 0.1 cm. The waist-hip ratio (WHR) was calculated from these parameters.
Diastolic and systolic blood pressure (DBP and SBP) and heart rate were measured in fasting conditions with an OMRON M6 monitor in triplicate at each visit.
2.5. Sample Collection
Fasting blood was collected before and after each intervention. Blood samples were collected from the arm via venipuncture using tubes containing ethylenediaminetetraacetic acid (EDTA). After centrifugation of blood samples at 1902× g for 15 min at 4 °C, plasma was obtained. In addition, 24 h urine was collected at each visit. Plasma and urine were stored at −80 °C.
2.6. Laboratory Evaluations
Biochemical analyses were performed by an external accredited laboratory (mdb.lad Durán Bellido) as follows. C-reactive protein (CRP) was assayed by an immunoturbidimetry method. The lipid parameters (high density lipoprotein (HDL), low density lipoprotein (LDL) and total cholesterol and triglycerides) were tested by an enzymatic method. Urea and uric acid were measured by enzymatic and enzymatic/chromogen methods, respectively, and creatinine by the Jaffe method (as modified by Larsen) [31]. The concentration of total proteins was quantified by a Biuret reaction to the final point and amount of albumin by a bromocresol green method.
2.7. Analysis of Inorganic Elements in Plasma
Plasma samples were digested with nitric acid (HNO3) (Instra, J.T. Baker) in Teflon reactors. After incubation at 90 °C overnight, Milli-Q water was added to the reactors. An aliquot was transferred into assay tubes and stored at 4 °C for the chromatographic analyses. The inorganic compounds (Inorganic ventures, Christiansburg, VA, USA) used as standards were the following: Iron (Fe), arsenic (As), copper (Cu), cadmium (Cd), uranium (U), lead (Pb), zinc (Zn), calcium (Ca), magnesium (Mg), potassium (K) and sodium (Na). Fe, As, Cu, Cd, U, Pb and Zn were analyzed by ICP-MS (NexIon 350D. Perkin Elmer, Waltham, MA, USA) and Ca, Mg, K, P and Na, by ICP-OES (Optima8300. Perkin, Waltham, MA, USA). The analyses were performed in the facilities of the CCIT (Centres Científics i Tecnològics) of the University of Barcelona.
2.8. Extraction and Quantification of Phenolic Acids from Urine
Urinary phenolic compounds were extracted by solid phase extraction using a Waters Oasis HLB 96-well plate 30 µm (30 mg; Waters Oasis, Milford, MA, USA) [32]. Chromatographic analysis of phenolic compounds was performed by ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS), using an API 3000 triple-quadrupole mass spectrometer (Sciex, Framingham, MA, USA). The separation was carried out with Milli-Q water and acetonitrile (Panreac Quimica S.A., Barcelona, Spain) with 0.025% formic acid in both solvents (Scharlau Chemie S.A., Barcelona, Spain), according to a method validated by our group [32]. A Waters BEH C18 column 1.7 µm (50 mm × 2.1 mm) and an Acquity UPLC BEH C18 VanGuard pre-column 1.7 µm (2.1 mm × 2.0 mm) were used.
The pool of standards was prepared in synthetic urine and included 3-(4-hydroxyphenyl) propionic acid (3,4-HPPA), 4-hydroxybenzoic acid (4-HBA), 3,4-dihydroxyphenylacetic acid (3,4-DHPAA), 3-hydroxyphenylacetic acid (3-HPAA), dihydrocaffeic acid (DHCA), hippuric acid, homovanillic acid, caffeic acid (CA), m-coumaric acid (m-Cou), p-coumaric (p-Cou) and gallic acid (GA) (Sigma-Aldrich, St. Louis, MO, USA) and 4-hydroxyhippuric acid (4-HH) (Bachem Americas Inc, Torrance, CA, USA). Ethylgallate (Extrasynthese, Genay, France) was the internal standard.
2.9. Extraction and Quantification of Carotenoids from Plasma
Carotenoids were extracted from plasma samples by liquid–liquid extraction [33]. Chromatographic analysis of carotenoids was performed by high performance liquid chromatography with ultraviolet diode-array detector (HPLC-UV-DAD), using an HP 1100 HPLC system (Hewlett-105 Packard, Waldbronn, Germany) containing a quaternary pump, coupled to a DAD G1315B. The separation was carried out with Milli-Q water, methanol and methyl-tert-butyl ether (Panreac Quimica S.A., Barcelona, Spain), according to a procedure previously validated by our group [33]. A Waters reversed-phase column YMC Carotenoid S-5 µm (250 mm × 4.6 mm) and a precolumn YMC Guard Cartridge Carotenoid S-5 µm (20 mm × 4.0 mm) were used.
The standards used were α-carotene, β-carotene, and all-E-lycopene (Sigma-Aldrich, St. Louis, MO, USA) and 5-Z-licopene (CaroteNature GmbH, Ostermundigen, Switzerland). These were pooled and prepared in synthetic human plasma (Sigma-Aldrich, St. Louis, MO, USA).
2.10. Statistical Analysis
Normality of distribution was assessed by a Shapiro-Wilk test. A non-parametric Wilcoxon signed-rank test was used for all statistical analysis due to the small sample size and the non-normality distribution. First, baseline measures were compared to corroborate similar pre-intervention conditions. As no significant differences between interventions at baseline were observed, the final analysis was performed with post-intervention measures (n = 19). Baseline values of variables were calculated from the mean of 38 observations (2 measurements for each subject). Differences were considered statistically significant when p < 0.05. Statistical analysis was performed using SPSS Version 23.0 for Windows (SPSS Inc, Chicago, IL, USA).
3. Results
3.1. Participant Characteristics
Table 1 shows the baseline characteristics of participants. Nineteen healthy subjects (9 males and 10 females) completed the study. Approximately three out of every four individuals were occasional consumers of organic products (foods and beverages). The mean age was 30 years and subjects were physically active. The baseline adherence to the Mediterranean diet was high in 7 individuals (≥ 10 points); moderate in 11 (6–9 points) and low in 1 (≤ 5 points).
Table 1.
Baseline characteristics of the participants (n = 38).
| Characteristics | |
|---|---|
| Males, n (%) | 9 (47) |
| Occasional intake of organic products, n (%) | 14 (74) |
| Age (years) | 30 ± 1 |
| Physical activity in leisure time (METS-min/week) | 3814 ± 489 |
| 14-item MedDiet score (points) | 9 ± 0.3 |
| Weight (kg) | 63 ± 2 |
| BMI (kg/m2) | 22.1 ± 0.4 |
| Waist (cm) | 76 ± 1 |
| WHR | 0.79 ± 0.01 |
| DBP (mmHg) | 75 ± 2 |
| SBP (mmHg) | 116 ± 2 |
| Heart rate (bpm) | 68 ± 2 |
| CRP (mg/dL) | 0.14 ± 0.03 |
| HDL (mg/dL) | 62 ± 3 |
| LDL (mg/dL) | 93 ± 5 |
| Total cholesterol (mg/dL) | 169 ± 6 |
| Triglycerides (mg/dL) | 69 ± 4 |
| Urea (mg/dL) | 29 ± 1 |
| Creatinine (mg/dL) | 0.80 ± 0.02 |
| Uric acid (mg/dL) | 4.60 ± 0.18 |
| Total proteins (g/L) | 72 ± 1 |
| Albumin (g/L) | 44 ± 0 |
Data are mean ± SEM unless otherwise specified. BMI: body mass index, WHR: waist–hip ratio, DBP: diastolic blood pressure, SBP: systolic blood pressure, CRP: C-reactive protein, HDL: high density lipoprotein, LDL: low density lipoprotein.
Baseline anthropometric (weight, BMI, waist and WHR), clinical (DBP, SBP and heart rate) and biochemical (CRP, HDL, LDL, total cholesterol, triglycerides, urea, creatinine, uric acid, total protein and albumin) measurements are also given in Table 1. The baseline concentrations of inorganic elements and bioactive compounds (phenolic acids and carotenes) are available as Supplementary Material Tables S1, S2 and S3, respectively.
3.2. Mean Dietary Composition of Participants During the Interventions
Participants followed a similar dietary pattern in both interventions (Table 2), although the OD was lower in protein (p = 0.036) and fish/seafood (p = 0.042). The mean proportion of macronutrients was the same in both diets (57% carbohydrates, 24% fats and 19% proteins). A borderline p was obtained comparing dairy products and vegetables (p = 0.051 and 0.055). However, the differences between both diets considering individual food were not significant (data not shown). In addition, a significantly lower amount of calcium and phosphorus was ingested in the OD.
Table 2.
Nutrient and food intake of participants in both diets (n = 19).
| OD | CD | p | |
|---|---|---|---|
| Nutrient intake | |||
| Energy (kcal/d) | 1965 ± 203 | 2062 ± 204 | 0.070 |
| Carbohydrates (g/d) | 211 ± 21 | 220 ± 22 | 0.260 |
| Total fat (g/d) | 88 ± 9 | 92 ± 9 | 0.091 |
| SFA (g/d) | 22 ± 3 | 23 ± 3 | 0.064 |
| MUFA (g/d) | 45 ± 4 | 46 ± 4 | 0.136 |
| PUFA (g/d) | 12 ± 2 | 12 ± 1 | 0.136 |
| Protein (g/d) | 68 ± 9 | 72 ± 9 | 0.036 * |
| Ca (mg/d) | 780 ± 111 | 847 ± 110 | 0.024 * |
| Mg (mg/d) | 344 ± 37 | 353 ± 39 | 0.376 |
| P (mg/d) | 1352 ± 171 | 1433 ± 169 | 0.018 * |
| Fe (mg/d) | 16 ± 1 | 16 ± 2 | 0.376 |
| Food intake | |||
| Dairy products (g/d) | 192 ± 52 | 207 ± 50 | 0.051 |
| Meat (g/d) | 98 ± 20 | 102 ± 19 | 0.202 |
| Eggs (g/d) | 28 ± 3 | 31 ± 3 | 0.180 |
| Fish and seafood (g/d) | 56 ± 16 | 66 ± 16 | 0.042 * |
| Vegetables (g/d) | 296 ± 32 | 366 ± 39 | 0.055 |
| Fruits (g/d) | 360 ± 68 | 377 ± 70 | 0.650 |
| Nuts (g/d) | 13 ± 5 | 12 ± 5 | 0.950 |
| Legumes (g/d) | 26 ± 5 | 26 ± 5 | 0.528 |
| Cereals (g/d) | 98 ± 11 | 98 ± 10 | 0.717 |
| Oils (g/d) | 40 ± 4 | 40 ± 4 | 0.317 |
| Cocoa (g/d) | 18 ± 5 | 21 ± 8 | 0.812 |
| Coffee (g/d) | 62 ± 16 | 59 ± 16 | 0.600 |
| Tea (g/d) | 22 ± 7 | 17 ± 7 | 0.106 |
| Wine (g/d) | 54 ± 23 | 62 ± 28 | 0.634 |
Data are mean ± SEM. *p-value < 0.05. SFA: Saturated fatty acid, MUFA: monounsaturated fatty acid, PUFA: polyunsaturated fatty acid.
3.3. Physiological Parameters of Participants After the Interventions
Table 3 shows anthropometric, clinical and biochemical data of the participants after following the OD and CD.
Table 3.
Anthropometric, clinical and biochemical measurements after the interventions (n = 19).
| OD | CD | p | |
|---|---|---|---|
| Anthropometric measurements | |||
| Weight (kg) | 64 ± 2 | 63 ± 2 | 0.365 |
| BMI | 22.1 ± 0.6 | 22.2 ± 0.6 | 0.352 |
| Waist (cm) | 76 ± 1 | 76 ± 1 | 0.549 |
| WHR | 0.80 ± 0.01 | 0.79 ± 0.01 | 0.822 |
| Clinical measurements | |||
| DBP (mmHg) | 79 ± 2 | 73 ± 2 | 0.074 |
| SBP (mmHg) | 119 ± 4 | 118 ± 3 | 0.979 |
| Heart rate (bpm) | 70 ± 3 | 66 ± 2 | 0.326 |
| Biochemical measurements | |||
| CRP (mg/dL) | 0.17 ± 0.07 | 0.26 ± 0.11 | 0.438 |
| HDL (mg/dL) | 62 ± 4 | 60 ± 4 | 0.301 |
| LDL (mg/dL) | 92 ± 9 | 90 ± 7 | 0.653 |
| Total cholesterol (mg/dL) | 168 ± 9 | 164 ± 7 | 0.494 |
| Triglycerides (mg/dL) | 66 ± 4 | 68 ± 4 | 0.421 |
| Urea (mg/dL) | 29 ± 2 | 29 ± 2 | 0.913 |
| Creatinine (mg/dL) | 0.80 ± 0.03 | 0.79 ± 0.02 | 0.763 |
| Uric acid (mg/dL) | 4.55 ± 0.29 | 4.68 ± 0.26 | 0.456 |
| Total proteins (g/L) | 73 ± 1 | 71 ± 1 | 0.145 |
| Albumin (g/L) | 44 ± 1 | 43 ± 1 | 0.136 |
Data are mean ± SEM. BMI: body mass index, WHR: waist–hip ratio, DBP: diastolic blood pressure, SBP: systolic blood pressure, CRP: C-reactive protein, HDL: high density lipoprotein, LDL: low density lipoprotein.
3.4. Inorganic Elements in Plasma
No significant differences were observed in the plasmatic concentration of minerals and heavy metals between the two diets (Table 4).
Table 4.
Inorganic elements in plasma after the interventions (n = 19).
| OD | CD | p | |
|---|---|---|---|
| Na (ppm) | 2991 ± 20 | 2992 ± 19 | 0.445 |
| K (ppm) | 839 ± 14 | 844 ± 11 | 0.778 |
| Ca (ppm) | 88 ± 1 | 88 ± 1 | 0.717 |
| Mg (ppm) | 18 ± 0 | 18 ± 0 | 0.778 |
| P (ppm) | 104 ± 3 | 100 ± 3 | 0.136 |
| Fe (ppb) | 1252 ± 127 | 1339 ± 107 | 0.601 |
| Zn (ppb) | 778 ± 98 | 785 ± 46 | 0.376 |
| Cu (ppb) | 858 ± 64 | 856 ± 71 | 0.904 |
| As (ppb) | 4.35 ± 2.27 | 3.99 ± 0.95 | 0.221 |
| Pb (ppb) | BLD | BLD | - |
| Cd (ppb) | BLD | BLD | - |
| U (ppb) | BLD | BLD | - |
Data are mean ± SEM. BLD: Below limit of detection.
3.5. Phenolic Acids in Urine
Several polyphenols, mainly phenolic acids generated by microbiota metabolism and their derivatives, were evaluated in urine after the interventions (Table 5). A significant increase was observed in 4-HBA (p = 0.028) after the OD compared to the CD, but no changes were detected in the rest of the phenols.
Table 5.
Urinary phenolic acids excretion after the interventions (n = 19).
| OD | CD | p | |
|---|---|---|---|
| Phenylacetic acids | |||
| 3,4-DHPAA (nmol) | 90 ± 35 | 35 ± 9 | 0.42 |
| 3-HPAA (nmol) | 943 ± 594 | 941 ± 440 | 0.717 |
| Homovanillic (nmol) | 154 ± 60 | 108 ± 27 | 0.868 |
| Phenylpropionic acids | |||
| 3,4-HPPA (nmol) | 10 ± 3 | 27 ± 12 | 0.407 |
| DHCA (nmol) | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.955 |
| Hydroxybenzoic and derivatives | |||
| 4-HBA (nmol) | 205 ± 123 | 70 ± 35 | 0.028 * |
| 4-HH (nmol) | 471 ± 225 | 212 ± 85 | 0.306 |
| Hippuric (nmol) | 1281 ± 235 | 1463 ± 211 | 0.231 |
| Hydroxycinnamic and derivatives | |||
| CA (nmol) | 7 ± 2 | 10 ± 2 | 0.349 |
| m-Cou (nmol) | 0.5 ± 0.3 | 0.26 ± 0.07 | 0.501 |
| p-Cou (nmol) | 0.3 ± 0.7 | 0.54 ± 0.19 | 0.554 |
| GA (nmol) | 0.48 ± 0.45 | 0.07 ± 0.03 | 0.878 |
Data are mean ± SEM. * p-value < 0.05. 3,4-DHPAA: 3,4-dihydroxyphenylacetic acid, 3-HPAA: 3-hydroxyphenylacetic acid, 3,4-HPPA: 3-(4-hydroxyphenyl) propionic acid, DHCA: dihydrocaffeic acid, 4-HBA: 4-hydroxybenzoic acid, 4-HH: 4-hydroxyhippuric, CA: caffeic acid, m-Cou: m-coumaric acid, p-Cou: p-coumaric acid, GA: gallic acid.
3.6. Carotenoids in Plasma
No significant differences were observed in plasmatic concentrations of carotenes (Table 6).
Table 6.
Plasmatic carotenoids after the interventions (n = 19).
| OD | CD | p | |
|---|---|---|---|
| α-carotene (nmol/mL) | 0.39 ± 0.09 | 0.27 ± 0.06 | 0.552 |
| β-carotene (nmol/mL) | 1.03 ± 0.24 | 0.95 ± 0.22 | 0.744 |
| E-lycopene (nmol/mL) | 0.7 ± 0.17 | 0.78 ± 0.18 | 0.913 |
| Z-lycopene (nmol/mL) | 0.15 ± 0.04 | 0.20 ± 0.05 | 0.379 |
Data are mean ± SEM.
4. Discussion
A randomized, controlled and crossover pilot study with nineteen healthy subjects was carried out to assess whether following an OD for 4 weeks changes health parameters and biomarkers compared to a CD.
In this study, the phenol 4-HBA increased approximately three times at the end of the OD compared to the CD (p = 0.028). 4-HBA can come from a diet, nevertheless, the intake of food rich in this phenol, such as berries, beer, etc., did not change significantly between both interventions (data not shown). However, this compound is produced from anthocyanins catabolism, as a metabolite of pelargonidin [34,35,36], and it can be formed by the colonic microbiota [36,37]. The metabolite 4-HBA has shown anticancer and neuroprotective effects [37,38,39,40]. Moreover, this compound is a precursor of the coenzyme Q10, showing cardioprotective properties [41,42].
No significant differences in the urinary concentration of the rest of the phenols were observed between the two diets, although vegetable intake was borderline lower in the OD. Stracke et al. carried out a study in which healthy men consumed 500 g of organic or conventional apples for four weeks. Twenty-four hours after the last intake, polyphenol concentrations in plasma and urine were not higher in the organic consumers [43].
Studies on the carotenoid content in organically grown fruits have provided inconclusive results [13,19,44]. In the present work, no effects of the OD on carotenoid levels were detected. In contrast to our results, a previous observational study reported significant differences in both carotenes and other fat-soluble micronutrients after consumption of organic food [45].
No changes in the concentration of inorganic elements in plasma were observed after either intervention. According to other authors, organic agriculture does not affect dietary copper [45,46] or zinc absorption [45]. However, a cohort from the NutriNet-Santé study presented a higher level of magnesium after following an OD, whereas no differences were found in iron absorption [45]. Higher magnesium, iron and phosphorus levels have been described in organic versus conventional plant-derived foods [47]. In contrast, concentrations of cadmium have been reported to be lower in organic food, due to the type of plant fertilizer used, but lower levels in consumers of organic produce were not observed [19,45,48]. In the present study, cadmium was not detected in plasma, nor was lead or uranium. Marchioni et al. showed that the content of cadmium and lead in coffee was influenced by temperature and mass, respectively, but not by the type of crop [49]. Although uranium is used more in conventional than in organic agriculture [50,51], a higher uranium content was not evidenced in conventional produce [52]. We found calcium and phosphate intake was lower in the OD, likely due to a lower consumption of dairy products. Although previous studies have described a higher concentration of phosphate in conventional foods due to crop fertilizers [53], here no differences were detected in the plasma levels between the two diets. Previous findings from the Environmental Defense Fund indicate that organic foods are as likely as conventional foods to contain heavy metals, because the organic standard is focused on pesticides and not these contaminants [54].
ODs are generally believed to be healthier and to provide more bioactive compounds. Some authors have observed a higher concentration of some phytochemicals in organic food, but without considering their bioavailability. In addition, when assessing the nutritional value of food, other influential factors need to be considered, including crop variety, maturity, soil and climate. On the other hand, consumers of organic products tend to be more concerned with health-related issues than the general population, which can bias the results of observational studies.
Organic foods are appreciated for the limited use of synthetic compounds (fertilizers, pesticides and antibiotics) in their production. Nevertheless, conventional crops are also regulated in this respect, and long-term studies are required to corroborate the effect of these compounds on health. To date, evidence suggesting that organic products are more nutritive or healthier is still lacking. Therefore, further carefully designed research is needed to evaluate the effect of an OD on bioactive compounds in biological fluids and health-related biomarkers.
The strongest point of the current study is its crossover design and the evaluation of a dietary pattern instead of only one or a few foods. Also, few such clinical assays have been conducted to date, with most studies being observational. Limitations of the work include a small sample size, the short duration of interventions and some differences in dietary patterns between the two interventions. However, this may be considered a pilot study to assess the short-term effects of organic food consumption. The increase of the phenolic compound arising from microbiota metabolism (4-HBA) in consumers following the OD need to be corroborated by further research with a higher number of subjects, which may shed light on a potential mechanism and possible health beneficial effects. In addition, a better control of factors as crop variety, maturity, soil and climate would provide more reliable results.
5. Conclusions
This intervention study for only one month found a significant difference in the concentration of a phenolic acid, the 4-HBA, after the OD. No changes were observed in the rest of the bioactive compounds analyzed nor in the other health-related biomarkers considered, neither in the results of minerals and heavy metals. The relation between the organic or conventional foods consumed and the concentration of bioactive compounds in the organism should be further researched. Longer studies and with larger sample sizes could reach significant values in other biochemical and heathy variables, demonstrating the health benefits of an OD.
Acknowledgments
First: we thank all the participants of the study and sponsors (Ecoveritas S.A., Conservas José Salcedo Soria S.L., Paul & Pippa Gourmet Food S.L., Artfood S.L., Can Feixes, Grupo Codorniu Aceites Borges Pont S.A. and Molí dels Torms, S.L.) who kindly provided organic wine, olive oil, snacks and canned vegetables, and weekly vouchers to shop in organic supermarkets. S.H.B. and M.M.M. received support from the Ministerio de Educación, Cultura y deporte (MECD) through predoctoral scholarship FPU (FPU14/01715 and FPU17/00513, respectively). P.Q.-R. is thankful for the Sara Borrell postdoctoral program from the Instituto de Salud Carlos III (ISCIII). J.F.R.d.A. is grateful to the Science without Borders program for the predoctoral scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—Brazil (233576/2014-2). A.T.-R. thanks the Juan de la Cierva postdoctoral program (FJCI-2016-28694) from the Ministerio de Economía, Industria y Competitividad.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/8/9/340/s1, Table S1. Baseline concentrations of inorganic elements in plasma; Table S2. Baseline concentrations of urinary excretion of phenolic acids; Table S3. Baseline concentrations of carotenes in plasma.
Author Contributions
Conceptualization, S.H.-B., P.Q.-R., J.F.R.d.A., A.T.-R. and R.M.L.-R.; data curation, S.H.-B. and P.Q.-R.; formal analysis, S.H.-B., P.Q.-R. and A.T.-R.; investigation, S.H.-B.; methodology, S.H.-B., M.M.-M. and J.F.R.d.A.; supervision, R.M.L.-R.; writing—original draft, S.H.-B.; all participants reviewed and approved the paper; and R.M.L.-R. was the main person responsible for the project and the final content.
Funding
This study was supported by CYCIT from the Ministerio de Ciencia, Innovación y Universidades (grant number AGL2016-75329-R), the Instituto de Salud Carlos III—CIBEROBN (C03-01) and Generalitat de Catalunya (SGR 2017)—Departament d’Agricultura, Ramaderia, Pesca i Alimentació—Direcció General d’Agricultura i Ramaderia under grant 53 05012 2016.
Conflicts of Interest
Dra. R.M.L.-R. reports receiving lecture fees from Cerveceros de España and receiving lecture fees and travel support from Adventia. Moreover, weekly vouchers and other organic products have been provided by Ecoveritas S.A. and sponsors previously named. Nevertheless, these foundations and sponsors were not involved in the study design, the collection, analysis and interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication.
References
- 1.Magnusson M.K., Arvola A., Koivisto Hursti U.-K., Åberg L., Sjödén P.-O. Choice of organic foods is related to perceived consequences for human health and to environmentally friendly behaviour. Appetite. 2003;40:109–117. doi: 10.1016/S0195-6663(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 2.Dean M., Lampila P., Shepherd R., Arvola A., Saba A., Vassallo M., Claupein E., Winkelmann M., Lähteenmäki L. Perceived relevance and foods with health-related claims. Food Qual. Prefer. 2012;24:129–135. doi: 10.1016/j.foodqual.2011.10.006. [DOI] [Google Scholar]
- 3.Hoefkens C., Verbeke W., Aertsens J., Mondelaers K., Van Camp J. The nutritional and toxicological value of organic vegetables. Br. Food J. 2009;111:1062–1077. doi: 10.1108/00070700920992916. [DOI] [Google Scholar]
- 4.Curl C.L., Fenske R.A., Elgethun K. Organophosphorus pesticide exposure of urban and suburban preschool children with organic and conventional diets. Environ. Health Perspect. 2003;111:377–382. doi: 10.1289/ehp.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu C., Toepel K., Irish R., Fenske R.A., Barr D.B., Bravo R. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ. Health Perspect. 2006;114:260–263. doi: 10.1289/ehp.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradman A., Quirós-Alcalá L., Castorina R., Schall R.A., Camacho J., Holland N.T., Barr D.B., Eskenazi B. Effect of Organic Diet Intervention on Pesticide Exposures in Young Children Living in Low-Income Urban and Agricultural Communities. Environ. Health Perspect. 2015;123:1086–1093. doi: 10.1289/ehp.1408660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oates L., Cohen M., Braun L., Schembri A., Taskova R. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ. Res. 2014;132:105–111. doi: 10.1016/j.envres.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Baudry J., Debrauwer L., Durand G., Limon G., Delcambre A., Vidal R., Taupier-Letage B., Druesne-Pecollo N., Galan P., Hercberg S., et al. Urinary pesticide concentrations in French adults with low and high organic food consumption: Results from the general population-based NutriNet-Santé. J. Expo. Sci. Environ. Epidemiol. 2019;29:366–378. doi: 10.1038/s41370-018-0062-9. [DOI] [PubMed] [Google Scholar]
- 9.Hurtado-Barroso S., Tresserra-Rimbau A., Vallverdú-Queralt A., Lamuela-Raventós R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2019;59:704–714. doi: 10.1080/10408398.2017.1394815. [DOI] [PubMed] [Google Scholar]
- 10.Vallverdú-Queralt A., Lamuela-Raventós R.M. Foodomics: A new tool to differentiate between organic and conventional foods. Electrophoresis. 2016;37:1784–1794. doi: 10.1002/elps.201500348. [DOI] [PubMed] [Google Scholar]
- 11.Vallverdú-Queralt A., Medina-Remón A., Casals-Ribes I., Amat M., Lamuela-Raventós R.M. A metabolomic approach differentiates between conventional and organic ketchups. J. Agric. Food Chem. 2011;59:11703–11710. doi: 10.1021/jf202822s. [DOI] [PubMed] [Google Scholar]
- 12.Vallverdú-Queralt A., Medina-Remón A., Casals-Ribes I., Lamuela-Raventos R.M. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012;130:222–227. doi: 10.1016/j.foodchem.2011.07.017. [DOI] [Google Scholar]
- 13.Vallverdú-Queralt A., Martínez-Huélamo M., Casals-Ribes I., Lamuela-Raventós R.M. Differences in the carotenoid profile of commercially available organic and conventional tomato-based products. J. Berry Res. 2014;4:69–77. doi: 10.3233/JBR-140069. [DOI] [Google Scholar]
- 14.Borguini R.G. Avaliação do Potencial Antioxidante e de Algumas Características Físico-Químicas do Tomate (Lycopersicon Esculentum) Orgânico em Comparação ao Convencional. Biblioteca Digital de Teses e Dissertações, Universidade de São Paulo; São Paulo, Brazil: 2006. [Google Scholar]
- 15.Györe-Kis G., Deák K., Lugasi A., Csúr-Vargaa A., Helyes L. Comparison of conventional and organic tomato yield from a three-year-term experiment. Acta Aliment. 2012;41:486–493. doi: 10.1556/AAlim.41.2012.4.10. [DOI] [Google Scholar]
- 16.Roussos P.A., Gasparatos D. Apple tree growth and overall fruit quality under organic and conventional orchard management. Sci. Hortic. 2009;123:247–252. doi: 10.1016/j.scienta.2009.09.011. [DOI] [Google Scholar]
- 17.Williamson G., Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 18.Thilakarathna S., Rupasinghe H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients. 2013;5:3367–3387. doi: 10.3390/nu5093367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barański M., Średnicka-Tober D., Volakakis N., Seal C., Sanderson R., Stewart G.B., Benbrook C., Biavati B., Markellou E., Giotis C., et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014;112:794–811. doi: 10.1017/S0007114514001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palupi E., Jayanegara A., Ploeger A., Kahl J. Comparison of nutritional quality between conventional and organic dairy products: A meta-analysis. J. Sci. Food Agric. 2012;92:2774–2781. doi: 10.1002/jsfa.5639. [DOI] [PubMed] [Google Scholar]
- 21.Średnicka-Tober D., Barański M., Seal C.J., Sanderson R., Benbrook C., Steinshamn H., Gromadzka-Ostrowska J., Rembiałkowska E., Skwarło-Sona K., Eyre M., et al. Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: A systematic literature review and meta-and redundancy analyses. Br. J. Nutr. 2016;115:1043–1060. doi: 10.1017/S0007114516000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magkos F., Arvaniti F., Zampelas A. Organic food: Nutritious food or food for thought? A review of the evidence. Int. J. Food Sci. Nutr. 2003;54:357–371. doi: 10.1080/09637480120092071. [DOI] [PubMed] [Google Scholar]
- 23.Średnicka-Tober D., Barański M., Seal C., Sanderson R., Benbrook C., Steinshamn H., Gromadzka-Ostrowska J., Rembiałkowska E., Skwarło-Sońta K., Eyre M., et al. Composition differences between organic and conventional meat: A systematic literature review and meta-analysis. Br. J. Nutr. 2016;23:1–18. doi: 10.1017/S0007114515005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baudry J., Allès B., Péneau S., Touvier M., Méjean C., Hercberg S., Galan P., Lairon D., Kesse-Guyot E. Dietary intakes and diet quality according to levels of organic food consumption by French adults: Cross-sectional findings from the NutriNet-Santé Cohort Study. Public Health Nutr. 2016;20:638–648. doi: 10.1017/S1368980016002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisinger-Watzl M., Wittig F., Heuer T., Hoffmann I., Verhagen H., Scientific Advisor S. Customers Purchasing Organic Food—Do They Live Healthier? Results of the German National Nutrition Survey II. Eur. J. Nutr. Food Saf. 2015;5:59–71. doi: 10.9734/EJNFS/2015/12734. [DOI] [Google Scholar]
- 26.Baudry J., Méjean C., Allès B., Péneau S., Touvier M., Hercberg S., Lairon D., Galan P., Kesse-Guyot E. Contribution of organic food to the diet in a large sample of French adults (The NutriNet-Santé cohort study) Nutrients. 2015;7:8615–8632. doi: 10.3390/nu7105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetzke B., Nitzko S., Spiller A. Consumption of organic and functional food. A matter of well-being and health? Appetite. 2014;77:96–105. doi: 10.1016/j.appet.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Schröder H., Fitó M., Estruch R., Martínez-González M.A., Corella D., Salas-Salvadó J., Lamuela-Raventós R., Ros E., Salaverría I., Fiol M., et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011;141:1140–1145. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 29.Elosua R., Marrugat J., Molina L., Pons S., Pujol E. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am. J. Epidemiol. 1994;139:1197–1209. doi: 10.1093/oxfordjournals.aje.a116966. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Ballart J.D., Piñol J.L., Zazpe I., Corella D., Carrasco P., Toledo E., Perez-Bauer M., Martínez-González M.Á., Salas-Salvadó J., Martín-Moreno J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010;103:1808–1816. doi: 10.1017/S0007114509993837. [DOI] [PubMed] [Google Scholar]
- 31.Larsen K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta. 1972;41:209–217. doi: 10.1016/0009-8981(72)90513-X. [DOI] [PubMed] [Google Scholar]
- 32.Martínez-Huélamo M., Tulipani S., Jáuregui O., Valderas-Martinez P., Vallverdú-Queralt A., Estruch R., Torrado X., Lamuela-Raventós R. Sensitive and Rapid UHPLC-MS/MS for the Analysis of Tomato Phenolics in Human Biological Samples. Molecules. 2015;20:20409–20425. doi: 10.3390/molecules201119702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colmán-Martínez M., Martínez-Huélamo M., Miralles E., Estruch R., Lamuela-Raventós R.M. A New Method to Simultaneously Quantify the Antioxidants: Carotenes, Xanthophylls, and Vitamin A in Human Plasma. Oxid. Med. Cell Longev. 2016;2016:1–10. doi: 10.1155/2016/9268531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Ferrars R.M., Czank C., Zhang Q., Botting N.P., Kroon P.A., Cassidy A., Kay C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014;171:3268–3282. doi: 10.1111/bph.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amini A.M., Muzs K., Spencer J.P., Yaqoob P. Pelargonidin-3-O-glucoside and its metabolites have modest anti-inflammatory effects in human whole blood cultures. Nutr. Res. 2017;46:88–95. doi: 10.1016/j.nutres.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Mohsen M.A., Marks J., Kuhnle G., Moore K., Debnam E., Kaila Srai S., Rice-Evans C., Spencer J.P.E. Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br. J. Nutr. 2006;95:51–58. doi: 10.1079/BJN20051596. [DOI] [PubMed] [Google Scholar]
- 37.Sannino F., Sansone C., Galasso C., Kildgaard S., Tedesco P., Fani R., Marino G., de Pascale D., Ianora A., Parrilli E., et al. Pseudoalteromonas haloplanktis TAC125 produces 4-hydroxybenzoic acid that induces pyroptosis in human A459 lung adenocarcinoma cells. Sci. Rep. 2018;8:1190. doi: 10.1038/s41598-018-19536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X.-N., Wang K.-Y., Zhang X.-S., Yang C., Li X.-Y. 4-Hydroxybenzoic acid (4-HBA) enhances the sensitivity of human breast cancer cells to adriamycin as a specific HDAC6 inhibitor by promoting HIPK2/p53 pathway. Biochem. Biophys. Res. Commun. 2018;504:812–819. doi: 10.1016/j.bbrc.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 39.Winter A.N., Brenner M.C., Punessen N., Snodgrass M., Byars C., Arora Y., Linseman D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell Longev. 2017;2017:1–13. doi: 10.1155/2017/6297080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Distelmaier F. 4-Hydroxybenzoic acid for multiple system atrophy? Parkinsonism Relat. Disord. 2018;50:119–120. doi: 10.1016/j.parkreldis.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Timoshchuk S.V., Vavilova H.L., Strutyns’ka N.A., Talanov S.A., Petukhov D.M., Kuchmenko O.B., Donchenko H.V., Sahach V.F. Cardioprotective action of coenzyme Q in conditions of its endogenous synthesis activation in cardiac ischemia-reperfusion in old rats. BMC Pharmacol. Texicol. 2009;55:58–63. [PubMed] [Google Scholar]
- 42.Kumchenko E.B., Petukhov D.N., Donchenko G.V., Mkhitarian L.S., Timoshchuk S.V., Strutinskaia N.A., Vavilova G.L., Sagach V.F. Effect of precursors and modulators of coenzyme Q biosynthesis on the heart mitochondria function in aged rats. Biomed. Khim. 2010;56:244–256. [PubMed] [Google Scholar]
- 43.Stracke B.A., Rüfer C.E., Bub A., Seifert S., Weibel F.P., Kunz C., Watzl B. No effect of the farming system (organic/conventional) on the bioavailability of apple (Malus domestica Bork., cultivar Golden Delicious) polyphenols in healthy men: A comparative study. Eur. J. Nutr. 2010;49:301–310. doi: 10.1007/s00394-009-0088-9. [DOI] [PubMed] [Google Scholar]
- 44.Cardoso P.C., Tomazini A.P.B., Stringheta P.C., Ribeiro S.M.R., Pinheiro-Sant’Ana H.M. Vitamin C and carotenoids in organic and conventional fruits grown in Brazil. Food Chem. 2011;126:411–416. doi: 10.1016/j.foodchem.2010.10.109. [DOI] [Google Scholar]
- 45.Baudry J., Ducros V., Druesne-Pecollo N., Galan P., Hercberg S., Debrauwer L., Amiot M.J., Lairon D., Kesse-Guyot E. Some Differences in Nutritional Biomarkers are Detected Between Consumers and Nonconsumers of Organic Foods: Findings from the BioNutriNet Project. Curr. Dev. Nutr. 2019;3:nzy090. doi: 10.1093/cdn/nzy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mark A.B., Kápolna E., Laursen K.H., Halekoh U., Rasmussen S.K., Husted S., Larsen E.H., Bügel S. Consumption of organic diets does not affect intake and absorption of zinc and copper in men-evidence from two cross-over trials. Food Funct. 2013;4:409–419. doi: 10.1039/C2FO30247K. [DOI] [PubMed] [Google Scholar]
- 47.Rembiałkowska E. Review Quality of plant products from organic agriculture. J. Sci. Food Agric. 2007;87:2757–2762. doi: 10.1002/jsfa.3000. [DOI] [Google Scholar]
- 48.Kratz S., Schick J., Schnug E. Trace elements in rock phosphates and P containing mineral and organo-mineral fertilizers sold in Germany. Sci. Total Environ. 2016;542:1013–1019. doi: 10.1016/j.scitotenv.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 49.Marchioni C., de Oliveira F.M., de Magalhães C.S., Luccas P.O. Assessment of Cadmium and Lead Adsorption in Organic and Conventional Coffee. Anal. Sci. 2015;31:165–172. doi: 10.2116/analsci.31.165. [DOI] [PubMed] [Google Scholar]
- 50.Schnug E., Haneklaus N. Uranium in Phosphate Fertilizers—Review and Outlook. Uranium—Past and Future Challenges. Springer International Publishing; Cham, Switzerland: 2015. pp. 123–130. [Google Scholar]
- 51.De Kok L.J., Luit J., Schnug E. Loads and Fate of Fertilizer-Derived Uranium. Backhuys Publishers; 2008. p. 229. [Google Scholar]
- 52.Mie A., Andersen H.R., Gunnarsson S., Kahl J., Kesse-Guyot E., Rembiałkowska E., Quaglio G., Grandjean P. Human health implications of organic food and organic agriculture: A comprehensive review. Environ. Health. 2017;16:111. doi: 10.1186/s12940-017-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Syers J.K., Johnston A.E., Curtin D. Efficiency of Soil and Fertilizer Phosphorus Use Reconciling Changing Concepts of Soil Phosphorus Behaviour with Agronomic Information. [(accessed on 10 June 2019)];2008 Available online: http://www.fao.org/3/a-a1595e.pdf.
- 54.Environmental Defense Fund (EDF) Consumer Reports Study Finds “Concerning Levels” of Heavy Metals in Baby and Toddler Foods. [(accessed on 10 June 2019)]; Available online: https://www.edf.org/media/consumer-reports-study-finds-concerning-levels-heavy-metals-baby-and-toddler-foods.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.