Abstract
Diffuse lung metastases have been reported in non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations. The purpose of our study was to compare the incidence of diffuse lung metastases in EGFR-mutant NSCLC and EGFR-wild type NSCLC and to assess other imaging features that may be associated with diffuse lung metastases in EGFR-mutant NSCLC. Two radiologists retrospectively reviewed pre-treatment imaging of metastatic NSCLC cases with known EGFR mutation status. We assessed the imaging features of the primary tumor and patterns of metastases. The cohort consisted of 217 patients (117 EGFR-mutant, 100 EGFR wild-type). Diffuse lung metastasis was significantly more common in EGFR-mutant NSCLC compared with wild-type (18% vs. 3%, p < 0.01). Among the EGFR-mutant group, diffuse lung metastases were inversely correlated with the presence of a nodule greater than 6 mm other than the primary lung lesion (OR: 0.13, 95% CI: 0.04–0.41, p < 0.01). EGFR mutations in NSCLC are associated with increased frequency of diffuse lung metastases. The presence of diffuse lung metastases in EGFR-mutant NSCLC is also associated with a decreased presence of other larger discrete lung metastases. EGFR mutations in NSCLC should be suspected in the setting of a dominant primary lung mass associated with diffuse lung metastases.
Keywords: epidermal growth factor receptor (EGFR) mutation, lung cancer, radiology
1. Introduction
Current guidelines recommend routine molecular testing for patients with metastatic non-small cell lung cancer (NSCLC) [1,2]. In those identified to have tumors with actionable somatic alterations, target-specific tyrosine kinase inhibitors (TKIs) are the preferred first-line treatment [1]. The most common such genetic alteration is a mutation in the epidermal growth factor receptor (EGFR) gene [3,4]. Five drugs targeting EGFR-mutant NSCLC are currently FDA-approved as front-line therapy [5,6,7,8,9].
This trend towards personalized, precision medicine in the treatment of NSCLC and the indispensable nature of imaging in the management of NSCLC have led to increased interest in radiogenomics and the correlation of radiologic features with genetic mutations. Several groups have investigated and reported the imaging findings of different genetic mutational subtypes in NSCLC [10,11,12,13,14,15]. Imaging features that have been associated with the primary tumor in EGFR-mutant NSCLC include peripheral location, pleural tagging, air bronchograms, and ground-glass opacities [16,17,18]. Several authors have also reported the increased frequency of diffuse lung metastases, also referred to as “miliary metastases” by some authors, in the setting of EGFR mutation-positive NSCLC [19,20,21].
To our knowledge, no study has investigated imaging features and patterns of metastases that may be associated with EGFR-mutant NSCLC with diffuse lung metastases. The goals of our study were to assess the incidence of diffuse lung metastases in EGFR-mutant and EGFR wild-type NSCLC on imaging and to assess other imaging features that may be associated with diffuse lung metastases in EGFR-mutant NSCLC.
2. Results
2.1. Patients
The 217 patients studied included 117 patients with EGFR-mutant NSCLC and 100 patients with EGFR wild-type NSCLC and no documented driver mutation (Table 1). The median age was 65 years (range 26–90) and the majority was female (57%) and were current or previous smokers (59%). As expected, those with EGFR-mutant NSCLC were more likely to be female, never-smokers.
Table 1.
Patients characteristics, tumor genotypes, and imaging features among all patients (n = 217).
| Characteristics | All (n = 217) | EGFR | p-Value * | |
|---|---|---|---|---|
| Mutant (n = 117) | Wild-type (n = 100) | |||
| Patients Characteristics | ||||
| Median (range) | ||||
| Age | 65 (26–90) | 63 (26–90) | 68 (42–84) | <0.01 |
| Gender | n (%) | |||
| Female | 123 (57%) | 81 (69%) | 42 (42%) | <0.01 |
| Male | 94 (43%) | 36 (31%) | 58 (58%) | |
| Race | ||||
| Caucasian | 185 (85%) | 94 (80%) | 91 (91%) | 0.03 |
| Asian | 19 (9%) | 12 (10%) | 7 (7%) | |
| African-American | 4 (2%) | 3 (3%) | 1 (1%) | |
| Hispanic | 3 (1%) | 3 (3%) | 0 (0%) | |
| Others/Unknown | 6 (3%) | 5 (4%) | 1 (1%) | |
| Smoking status | ||||
| Never | 88 (41%) | 72 (62%) | 16 (16%) | <0.01 |
| Ever | 129 (59%) | 45 (38%) | 84 (84%) | |
| Primary tumor features | ||||
| Size (mm) | 50 (10–134) | 47 (11–134) | 52 (10–115) | 0.84 |
| Primary lesion lobar zone | ||||
| Both | 51 (24%) | 29 (25%) | 22 (22%) | 0.18 |
| Central | 91 (42%) | 54 (46%) | 37 (37%) | |
| Peripheral | 75 (35%) | 34 (29%) | 41 (41%) | |
| Solid | ||||
| No | 21 (10%) | 13 (11%) | 8 (8%) | 0.5 |
| Yes | 196 (90%) | 104 (89%) | 92 (92%) | |
| Air bronchograms | ||||
| No | 156 (72%) | 84 (72%) | 72 (72%) | > 0.99 |
| Yes | 61 (28%) | 33 (28%) | 28 (28%) | |
| Cavity | ||||
| No | 198 (91%) | 111 (95%) | 87 (87%) | 0.05 |
| Yes | 19 (9%) | 6 (5%) | 13 (13%) | |
| Tumor Calcification | ||||
| No | 211 (97%) | 112 (96%) | 99 (99%) | 0.22 |
| Yes | 6 (3%) | 5 (4%) | 1 (1%) | |
| Nodal disease | ||||
| Negative | 23 (11%) | 19 (16%) | 4 (4%) | <0.01 |
| Positive | 194 (89%) | 98 (84%) | 96 (96%) | |
| Metastatic sites | ||||
| Intrathoracic | ||||
| Absent | 33 (15%) | 21 (18%) | 12 (12%) | 0.26 |
| Present | 184 (85%) | 96 (82%) | 88 (88%) | |
| Pleura | ||||
| No | 100 (46%) | 70 (60%) | 30 (30%) | <0.01 |
| Yes | 117 (54%) | 47 (40%) | 70 (70%) | |
| Lung | ||||
| No | 67 (31%) | 34 (29%) | 33 (33%) | 0.56 |
| Yes | 150 (69%) | 83 (71%) | 67 (67%) | |
| Diffuse lung | ||||
| No | 193 (89%) | 96 (82%) | 97 (97%) | <0.01 |
| Yes | 24 (11%) | 21 (18%) | 3 (3%) | |
| Extrathoracic | ||||
| Absent | 63 (29%) | 33 (28%) | 30 (30%) | 0.88 |
| Present | 154 (71%) | 84 (72%) | 70 (70%) | |
| Bone | ||||
| No | 133 (61%) | 68 (58%) | 65 (65%) | 0.33 |
| Yes | 84 (39%) | 49 (42%) | 35 (35%) | |
| Brain | ||||
| No | 140 (65%) | 70 (60%) | 70 (70%) | 0.15 |
| Yes | 77 (35%) | 47 (40%) | 30 (30%) | |
| Adrenal | ||||
| No | 170 (78%) | 101 (86%) | 69 (69%) | <0.01 |
| Yes | 47 (22%) | 16 (14%) | 31 (31%) | |
| Soft tissue | ||||
| No | 179 (82%) | 92 (79%) | 87 (87%) | 0.11 |
| Yes | 38 (18%) | 25 (21%) | 13 (13%) | |
* p-Values provided are for comparison between EGFR-mutant and EGFR-wild type groups. Significant p-Values are highlighted.
The frequency of diffuse lung metastases was significantly higher in EGFR-mutant patients (18% vs. 3%, p < 0.01). EGFR-positive patients were also more likely to have nodal disease (p < 0.01), pleural metastases (p < 0.01), and adrenal metastases (p < 0.01).
2.2. Diffuse Lung Metastases in EGFR-Mutant NSCLC
Among EGFR-mutant patients, we examined the characteristics of those with and without diffuse metastases (Table 2). The presence of a metastatic nodule >6 mm was inversely correlated with the presence of diffuse lung metastases (p < 0.01).
Table 2.
Patients characteristics and imaging features, among EGFR-mutant patients (n = 117).
| Characteristics | All (n = 117) | Diffuse Lung Metastases | p-Value * | |
|---|---|---|---|---|
| Yes (n = 21) | No (n = 96) | |||
| Patients characteristics | ||||
| Median (range) | ||||
| Age | 63 (26–90) | 58 (37–82) | 64 (26–90) | 0.16 |
| Size (mm) | 47 (11–134) | 47 (17–99) | 47 (11–134) | 0.42 |
| Age | n (%) | n (%) | ||
| <63 | 57 (49%) | 14 (67%) | 43 (45%) | 0.09 |
| ≥63 | 60 (51%) | 7 (33%) | 53 (55%) | |
| Gender | ||||
| Female | 81 (69%) | 11 (52%) | 70 (73%) | 0.07 |
| Male | 36 (31%) | 10 (48%) | 26 (27%) | |
| Ethnicity | ||||
| Caucasian | 94 (80%) | 15 (71%) | 79 (82%) | 0.36 |
| Asian | 12 (10%) | 3 (14%) | 9 (9%) | |
| African American | 3 (3%) | 1 (5%) | 2 (2%) | |
| Hispanic | 3 (3%) | 0 (0%) | 3 (3%) | |
| Others/Unknown | 5 (4%) | 2 (10%) | 3 (3%) | |
| Smoking status | ||||
| Never | 72 (62%) | 16 (76%) | 56 (58%) | 0.15 |
| Ever | 45 (38%) | 5 (24%) | 40 (42%) | |
| EGFR subtype | ||||
| Exon 19 | 61 (52%) | 12 (57%) | 49 (51%) | 0.9 |
| Exon 21 | 33 (28%) | 5 (24%) | 28 (29%) | |
| Exon 18 | 13 (11%) | 1 (5%) | 12 (13%) | |
| Exon 20 | 10 (9%) | 3 (14%) | 7 (7%) | |
| Primary tumor features | ||||
| Size >30 mm | ||||
| No | 22 (19%) | 3 (14%) | 19 (20%) | 0.76 |
| Yes | 95 (81%) | 18 (86%) | 77 (80%) | |
| Location | ||||
| Both | 29 (25%) | 9 (43%) | 20 (21%) | 0.11 |
| Central | 54 (46%) | 8 (38%) | 46 (48%) | |
| Peripheral | 34 (29%) | 4 (19%) | 30 (31%) | |
| Solid | ||||
| No | 13 (11%) | 3 (14%) | 10 (10%) | 0.7 |
| Yes | 104 (89%) | 18 (86%) | 86 (90%) | |
| Air bronchograms | ||||
| No | 84 (72%) | 15 (71%) | 69 (72%) | >0.99 |
| Yes | 33 (28%) | 6 (29%) | 27 (28%) | |
| Cavity | ||||
| No | 111 (95%) | 19 (90%) | 92 (96%) | 0.29 |
| Yes | 6 (5%) | 2 (10%) | 4 (4%) | |
| Calcification | ||||
| No | 112 (96%) | 21 (100%) | 91 (95%) | 0.58 |
| Yes | 5 (4%) | 0 (0%) | 5 (5%) | |
| Nodal disease | ||||
| Negative | 19 (16%) | 1 (5%) | 18 (19%) | 0.19 |
| Positive | 98 (84%) | 20 (95%) | 78 (81%) | |
| Metastases sites | ||||
| Pleural | ||||
| No | 70 (60%) | 11 (52%) | 59 (61%) | 0.47 |
| Yes | 47 (40%) | 10 (48%) | 37 (39%) | |
| Extra-thoracic | ||||
| Absent | 33 (28%) | 6 (29%) | 27 (28%) | >0.99 |
| Present | 84 (72%) | 15 (71%) | 69 (72%) | |
| Bone | ||||
| No | 68 (58%) | 12 (57%) | 56 (58%) | >0.99 |
| Yes | 49 (42%) | 9 (43%) | 40 (42%) | |
| Brain | ||||
| No | 70 (60%) | 11 (52%) | 59 (61%) | 0.47 |
| Yes | 47 (40%) | 10 (48%) | 37 (39%) | |
| Adrenal | ||||
| No | 101 (86%) | 17 (81%) | 84 (88%) | 0.48 |
| Yes | 16 (14%) | 4 (19%) | 12 (12%) | |
| Soft tissue | ||||
| No | 92 (79%) | 17 (81%) | 75 (78%) | >0.99 |
| Yes | 25 (21%) | 4 (19%) | 21 (22%) | |
| Other lung metastasis >6 mm | ||||
| No | 49 (42%) | 16 (76%) | 33 (34%) | <0.01 |
| Yes | 68 (58%) | 5 (24%) | 63 (66%) | |
* p-Values provided are for comparison between EGFR-mutant NSCLC patients with diffuse lung metastases versus those without diffuse lung metastases. Significant p-Values are highlighted.
2.3. Multivariable Regression Model for Presence of Diffuse Lung Metastases in The Setting of EGFR-Mutation
Age (≥63, <63), zone, and lung metastases >6mm are significant predictors of whether patients had diffuse lung disease metastases or not. When holding the other covariates fixed, among EGFR-mutant patients, the odds of having diffuse lung metastases are 87% lower in those with lung metastases >6 mm than those without lung metastasis >6 mm (OR: 0.13, 95% CI: 0.04–0.41).
3. Discussion
Our findings add to the growing evidence that there is increased frequency of diffuse lung metastases in the setting of EGFR-mutant NSCLC. We also found that the presence of diffuse lung metastases in the setting of EGFR-mutant NSCLC is inversely correlated with the presence of larger discrete metastatic nodules.
In our cohort, there was almost a six-fold increased incidence (18% vs. 3%) of diffuse lung metastases in patients with metastatic EGFR-mutant NSCLC compared to patients with EGFR-wild type NSCLC. Other studies have reported rates of 12–50% [17,21,22]. Differences in frequencies among several studies may be, at least in part, due to the differences in defining the finding of diffuse lung metastases and miliary metastases. We adhered to a stricter definition (i.e., diffuse nodules ≤6 mm in size), while others have used the size cut-off of up to 30 mm [22].
Prior clinical- and population-based studies have also reported the increased frequency of “miliary metastases” in the setting of EGFR-mutant NSCLC [19,20,21]. Another retrospective study also reported higher incidence of EGFR mutations in those who present with miliary metastases compared to those who do not have miliary metastases [23]. While several authors have used the term “miliary” metastasis in the setting of EGFR-mutant NSCLC, we propose that the designation of “diffuse lung metastases” is more accurate.
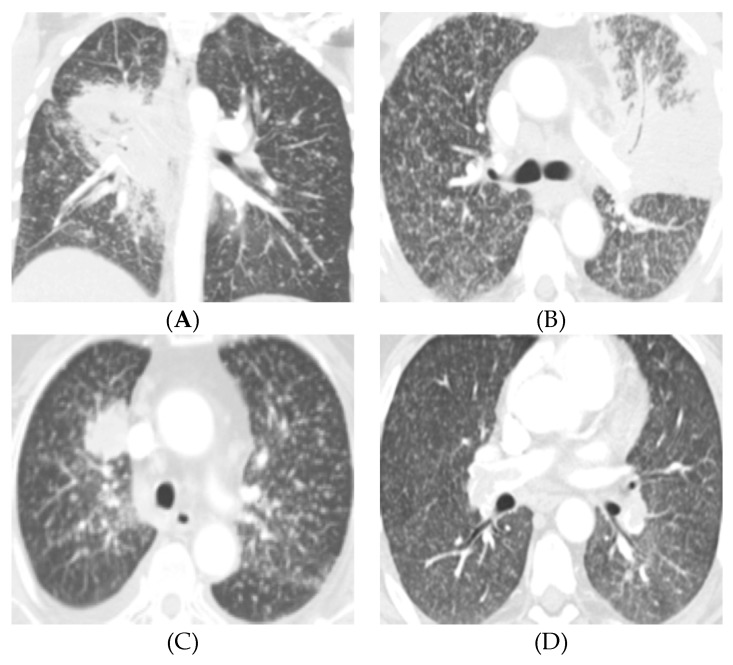
Miliary nodular pattern of disease has distinct radiologic features characterized by diffuse, bilateral infiltration of the lungs by tiny, typically 1–4 mm in size, nodules likened to millet seeds [24]. This finding is seen in numerous infectious and inflammatory etiologies, including tuberculosis, histoplasmosis, silicosis, and sarcoidosis [25,26,27,28,29,30]. Miliary nodular patterns have also been in the setting of metastatic disease, most notably with primary thyroid cancer, renal cell carcinoma, and melanoma [24,25]. While the imaging features of diffuse lung metastases in EGFR-mutant NSCLC may overlap with the other pathologies that present with miliary nodules, EGFR-mutant NSCLC with diffuse lung metastases can by distinguished by the presence of a dominant primary lung mass or nodule (Figure 1).
Figure 1.
Four patients with EGFR-mutated lung cancers and diffuse lung metastases. (A) 44-year-old female never-smoker presents with a central right hilar mass with mediastinal and right hilar lymphadenopathy and innumerable 2–3 mm pulmonary nodules bilaterally. Patient was subsequently diagnosed with non-small cell lung cancer (NSCLC) with EGFR exon 19 mutation. Note the air bronchograms in the primary tumor, a feature also described in EGFR-mutated NSCLC. (B) 66-year-old male never-smoker presents with a consolidative mass in the left upper lobe and lingula with miliary-like pattern of diffuse 2–3 mm pulmonary nodules bilaterally and mediastinal and left hilar lymphadenopathy. This patient was found to have NSCLC with EGFR exon 21 mutation. Again, note the air bronchogram in the primary tumor. (C) 74-year-old female never-smoker presenting with a dominant right upper lobe mass and diffuse innumerable 2–3 mm nodules bilaterally. Patient had an EGFR exon 19 mutation. (D) 61-year-old male never-smoker presents with diffuse 2–3 mm lung nodules with a dominant 2.3 cm left lower lobe spiculated nodule (not shown), which was positive for EGFR exon 19 mutation.
Of note, our findings also suggest that the presence of a larger (>6 mm) discrete lung metastasis is inversely correlated with diffuse lung metastases in the setting of EGFR-mutant NSCLC. It is unclear as to why those with diffuse lung metastases are less likely to have concomitant larger metastatic nodules, but it suggests a distinct mechanism of spread. We hypothesize that this may be due to the diffuse synchronous development of the nodules, which may lead to worse symptomatology and presentation before nodules have time to increase in size.
We did not find any other significant associations between the presence of diffuse lung metastases and tumor morphology, size, and location of primary tumor and the presence of lymphangitic carcinomatosis, pleural metastasis, and other distant metastases in the setting of EGFR mutations. Although some investigators have suggested that the exon-19 deletion subtype of EGFR may be associated with increased tendency for miliary metastases [23,31], our findings did not support this. A larger study cohort may be needed to validate these reports.
The single-institution, retrospective nature of our study predisposes it to selection bias and limits the findings’ generalizability to larger populations. Our relatively small cohort may also limit the study’s statistical power in discovering associations or lack thereof between diffuse lung metastases and other imaging features of EGFR-mutant NSCLC patients. Again, a larger cohort, perhaps from a collaborative multicenter study, would be helpful in validating our findings and in resolving these limitations.
Although the presence of diffuse lung metastases in the setting of NSCLC may be suggestive of an underlying EGFR mutation, this feature does not replace molecular genotyping. Testing for EGFR mutations in NSCLC traditionally depended on unmodified Sanger sequencing, which requires at least 50% malignant cellularity to be reliable [2,32]. Subsequently, however, more sensitive PCR-based targeted methods have been developed and validated, requiring as little as 10% tumor content [2,33,34,35]. Although more sensitive, these methods still require adequate tissue for accurate diagnosis. Tissue sampling and subsequent testing can take time and potentially delay initiation of treatment. Another factor that can prolong time to diagnosis and treatment is the timing of testing. In many centers, including ours, it is still customary for the treating physician to decide if a tumor specimen should be tested for genetic mutations, as opposed to reflex testing, wherein a pathologist decides which specimens should be tested for genetic mutations, which can further delay diagnosis and treatment [36,37].
The presence of diffuse lung metastasis in the setting of NSCLC should increase the suspicion for the presence of EGFR mutation. This may be assistive in determining which patients may potentially benefit from expedited molecular testing and those who may benefit from repeat alternative testing following an unexpectedly negative or discordant result initial testing.
4. Materials and Methods
4.1. Patient Selection
Under an institutional “Partners Human Research” (the Massachusetts General Hospital IRB) review board-approved protocol (protocol number: 2019P000198), we searched our clinical database for patients with biopsy-proven stage IV NSCLC who had undergone genetic testing and found to have activating EGFR mutations between January 2010 and December 2013 and who also had CT and or PET/CT scans performed prior to any anti-cancer therapy either at our hospital or at an outside facility with the images uploaded in to our picture archiving and communication system. For controls, we selected a subset of NSCLC patients with genetic testing who were wild-type for EGFR, negative for other potentially targetable mutations (e.g., ALK, ROS1, RET, etc), and had similar pre-treatment imaging available for review.
4.2. Genetic Analysis
Genotyping was performed using a multiplex PCR-based assay (SNaPshot® platform, Applied Biosystems, Foster City, CA, USA). This system detects single nucleotide polymorphisms in 14 key cancer genes and more than 50 hotspot mutations [2,38]. The genetic testing was performed on formalin-fixed paraffin-embedded biopsy specimens that were obtained from different organs via surgical, bronchoscopic, or image guided percutaneous procedures.
4.3. CT Imaging Protocol
The CT examinations of the body (chest, abdomen, and pelvis) were performed on multidetector-row CT scanners with helical acquisition mode, automatic exposure control, tube potential 100–120 kV, slice thickness of 1–2.5 mm for chest and 5 mm for abdomen. The brain MR and/or CT images were reviewed for the presence of metastases. The 18-FDG PET images, when available, were reviewed to assess for metabolic activity and were correlated with CT images.
4.4. Image Analysis
The cross-sectional imaging studies were independently reviewed by a board-certified thoracic radiologist (SRD) and a thoracic radiology fellow (DM, PGP), and discrepancies were resolved by consensus following concurrent review. The primary tumor was evaluated for size, lobar location, axial location within the lobe (inner, middle, or outer third), density (solid, pure ground glass opacity, or mixed density), cavitation, and air bronchograms. The metastatic lymph nodes were characterized as N0, N1, N2, or N3 per the American Joint Committee on Cancer (AJCC) 7th edition TNM staging manual [39]. More distant lymph nodes were classified along with distant metastases. The presence of metastases in the lung, pleura, adrenal glands, bones, soft tissues/viscera, and brain were documented for each patient. The presence of axial and septal interstitial thickening that involved greater than half a lobe and extending far beyond the primary tumor was considered as lymphangitic spread of tumor.
The lung metastases, when present, were classified as diffuse or discrete. Diffuse lung metastasis was defined as randomly distributed innumerable small nodules (less than or equal to 6 mm) of uniform size that were distributed over a wide area in both lungs (Figure 1). Diffuse lung metastases were evaluated for axial and craniocaudal distribution. Patients with metastatic lung nodules that did not meet the above criteria for diffuse pattern were further subdivided based on their location relative to the dominant lung mass as within the same lobe, ipsilateral different lobe, and contralateral lung. As most of the subjects presented prior to the adoption of the AJCC 8th edition in the United States and our institution, the criteria laid out in the AJCC 7th edition staging manual were used to designate TNM stage and overall stage category [39].
4.5. Statistical Analysis
Patient characteristics and imaging features were summarized using descriptive statistics. Continuous data were presented as medians with ranges, and categorical data were presented as frequencies with percentages. Comparisons were performed between EGFR-mutant and EGFR wild-type groups. Within the EGFR-mutant group, comparisons were also made between patients with diffuse lung metastases and those without diffuse lung metastases. Continuous characteristics between groups were compared using the Wilcoxon rank-sum test, and categorical features were compared using Fisher’s exact test. All tests were two-tailed, and p-values less than 0.05 were considered significant.
In order to investigate the variables that can predict the presence of diffuse lung metastases among patients with EGFR-mutant patients, a multivariable logistic regression model was built. The criteria for choosing candidate predictors were p-value < 0.20 based on univariate analysis and proper sample size.
5. Conclusions
In conclusion, EGFR-mutant NSCLC is associated with increased frequency of diffuse lung metastases. The presence of this diffuse pattern in EGFR-mutant NSCLC is associated with decreased propensity for the presence of other non-miliary pulmonary nodules. EGFR mutations in NSCLC have important treatment and prognostic implications and should be considered in the setting of suspected NSCLC with diffuse lung metastases. Although these distinct features cannot replace molecular testing in determining the presence of EGFR mutations, they may help identify patients who may benefit from expedited or repeat testing following unexpectedly negative genotyping.
Author Contributions
Conceptualization, S.R.D. and L.V.S.; Data curation, S.R.D., D.P.M., A.P., T.Q.C. and P.G.P.; Formal analysis, D.P.M., A.P. and T.Q.C.; Investigation, S.R.D., D.P.M., A.P., T.Q.C, P.G.P., Z.P. and L.V.S.; Methodology, S.R.D., D.P.M. and L.V.S.; Supervision, S.R.D. and L.V.S.; Validation, S.R.D.; Writing—original draft, S.R.D. and D.P.M.; Writing—review & editing, S.R.D., D.P.M., A.P., T.Q.C, P.G.P, Z.P. and L.V.S.
Funding
This research received no external funding.
Conflicts of Interest
D.P.M., A.P., and T.C.—none. S.R.D.—Provided independent image analysis through the hospital for clinical research trials programs sponsored by Merck, Pfizer, Bristol Mayer Squibb, Novartis, Roche, Polaris, Cascadian, Abbvie, Gradalis, Clinical Bay, Zai laboratories. Received honorarium from Siemens. P.G.P.—The views expressed in this article are those of the author and do not reflect the official policy of the Department of the Army, Department of Defense, or U.S. Government. Z.P.—Advisory relationships with AstraZeneca, Spectrum, Takeda, ImmunoGen and Guardant Health. Research funding from Novartis, AstraZeneca, Takeda and Spectrum. L.V.S.—Advisory relationships with AstraZeneca, Blueprint Medicines, Janssen, Merrimack Pharmaceuticals, Genentech. Research funding from Novartis, AstraZeneca, Boehringer Ingelheim, Genentech, Merrimack Pharmaceuticals, LOXO, Blueprint Medicines.
References
- 1.Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J., Chirieac L.R., D’Amico T.A., DeCamp M.M., Dilling T.J., Dobelbower M., et al. Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. J. Natl. Compr. Canc. Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 2.Lindeman N.I., Cagle P.T., Aisner D.L., Arcila M.E., Beasley M.B., Bernicker E.H., Colasacco C., Dacic S., Hirsch F.R., Kerr K., et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018;142:321–346. doi: 10.5858/arpa.2017-0388-CP. [DOI] [PubMed] [Google Scholar]
- 3.Paez J.G., Jänne P.A., Lee J.C., Tracy S., Greulich H., Gabriel S., Herman P., Kaye F.J., Lindeman N., Boggon T.J., et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 4.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Mok T.S., Wu Y.-L., Thongprasert S., Yang C.-H., Chu D.-T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 7.Sequist L.V., Yang J.C.-H., Yamamoto N., O’Byrne K., Hirsh V., Mok T., Geater S.L., Orlov S., Tsai C.-M., Boyer M., et al. Phase III Study of Afatinib or Cisplatin plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 8.Soria J.-C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 9.Mok T.S., Cheng Y., Zhou X., Lee K.H., Nakagawa K., Niho S., Lee M., Linke R., Rosell R., Corral J., et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J. Clin. Oncol. 2018;36:2244–2250. doi: 10.1200/JCO.2018.78.7994. [DOI] [PubMed] [Google Scholar]
- 10.Kim T.J., Lee C.-T., Jheon S.H., Park J.-S., Chung J.-H. Radiologic Characteristics of Surgically Resected Non-Small Cell Lung Cancer with ALK Rearrangement or EGFR Mutations. Ann. Thorac. Surg. 2016;101:473–480. doi: 10.1016/j.athoracsur.2015.07.062. [DOI] [PubMed] [Google Scholar]
- 11.Yoon H.J., Sohn I., Cho J.H., Lee H.Y., Kim J.-H., Choi Y.-L., Kim H., Lee G., Lee K.S., Kim J. Decoding Tumor Phenotypes for ALK, ROS1, and RET Fusions in Lung Adenocarcinoma Using a Radiomics Approach. Medicine. 2015;94:e1753. doi: 10.1097/MD.0000000000001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Z., Shan F., Yang Y., Shi Y., Zhang Z. CT Characteristics of Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor Mutation: A Systematic Review and Meta-Analysis. BMC Med. Imaging. 2017;17:5. doi: 10.1186/s12880-016-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpenny D.F., Riely G.J., Hayes S., Yu H., Zheng J., Moskowitz C.S., Ginsberg M.S. Are There Imaging Characteristics Associated with Lung Adenocarcinomas Harboring ALK Rearrangements? Lung Cancer. 2014;86:190–194. doi: 10.1016/j.lungcan.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plodkowski A.J., Drilon A., Halpenny D.F., O’Driscoll D., Blair D., Litvak A.M., Zheng J., Moskowitz C.S., Ginsberg M.S. From Genotype to Phenotype: Are There Imaging Characteristics Associated with Lung Adenocarcinomas Harboring RET and ROS1 Rearrangements? Lung Cancer. 2015;90:321–325. doi: 10.1016/j.lungcan.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendoza D.P., Dagogo-Jack I., Chen T., Padole A., Shepard J.-A.O., Shaw A.T., Digumarthy S.R. Imaging Characteristics of BRAF-Mutant Non-Small Cell Lung Cancer by Functional Class. Lung Cancer. 2019;129:80–84. doi: 10.1016/j.lungcan.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Koo H.J., Kim M.Y., Park S., Lee H.N., Kim H.J., Lee J.C., Kim S.-W., Lee D.H., Choi C.-M. Non-Small Cell Lung Cancer with Resistance to EGFR-TKI Therapy: CT Characteristics of T790M Mutation-Positive Cancer. Radiology. 2018;289:227–237. doi: 10.1148/radiol.2018180070. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa M., Sakai F., Ishikawa R., Kimura F., Ishida H., Kobayashi K. CT Features of Epidermal Growth Factor Receptor-Mutated Adenocarcinoma of the Lung: Comparison with Nonmutated Adenocarcinoma. J. Thorac. Oncol. 2016;11:819–826. doi: 10.1016/j.jtho.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Lee H.-J., Kim Y.T., Kang C.H., Zhao B., Tan Y., Schwartz L.H., Persigehl T., Jeon Y.K., Chung D.H. Epidermal Growth Factor Receptor Mutation in Lung Adenocarcinomas: Relationship with CT Characteristics and Histologic Subtypes. Radiology. 2013;268:254–264. doi: 10.1148/radiol.13112553. [DOI] [PubMed] [Google Scholar]
- 19.Kim H.J., Kang S.H., Chung H.W., Lee J.S., Kim S.J., Yoo K.H., Lee K.Y. Clinical Features of Lung Adenocarcinomas with Epidermal Growth Factor Receptor Mutations and Miliary Disseminated Carcinomatosis. Thorac. Cancer. 2015;6:629–635. doi: 10.1111/1759-7714.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuma Y., Kashima J., Watanabe K., Homma S. Survival Analysis and Pathological Features of Advanced Non-Small Cell Lung Cancer with Miliary Pulmonary Metastases in Patients Harboring Epidermal Growth Factor Receptor Mutations. J. Cancer Res. Clin. Oncol. 2018;144:1601–1611. doi: 10.1007/s00432-018-2681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu F., Nichol A., Toriumi T., Caluwe A.D. Miliary Metastases Are Associated with Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Population-Based Study. Acta Oncol. 2017;56:1175–1180. doi: 10.1080/0284186X.2017.1328128. [DOI] [PubMed] [Google Scholar]
- 22.Togashi Y., Masago K., Kubo T., Sakamori Y., Kim Y.H., Hatachi Y., Fukuhara A., Mio T., Togashi K., Mishima M. Association of Diffuse, Random Pulmonary Metastases, Including Miliary Metastases, with Epidermal Growth Factor Receptor Mutations in Lung Adenocarcinoma. Cancer. 2011;117:819–825. doi: 10.1002/cncr.25618. [DOI] [PubMed] [Google Scholar]
- 23.Wu S.-G., Hu F.-C., Chang Y.-L., Lee Y.-C., Yu C.-J., Chang Y.-C., Wu J.-Y., Shih J.-Y., Yang P.-C. Frequent EGFR Mutations in Nonsmall Cell Lung Cancer Presenting with Miliary Intrapulmonary Carcinomatosis. Eur. Respir. J. 2013;41:417–424. doi: 10.1183/09031936.00006912. [DOI] [PubMed] [Google Scholar]
- 24.Andreu J., Mauleón S., Pallisa E., Majó J., Martinez-Rodriguez M., Cáceres J. Miliary Lung Disease Revisited. Curr. Probl. Diagn. Radiol. 2002;31:189–197. doi: 10.1067/mdr.2002.127634. [DOI] [PubMed] [Google Scholar]
- 25.Salahuddin M., Karanth S., Ocazionez D., Estrada-Y-Martin R.M., Cherian S.V. Clinical Characteristics and Etiologies of Miliary Nodules in the US: A Single-Center Study. Am. J. Med. 2019;132:767–769. doi: 10.1016/j.amjmed.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 26.Touré N.O., Cissé M.F., Dia Kane Y., Diatta A., Bouker Bakioui B., Ndiaye E.H.M., Thiam K., Hane A.A. Miliary tuberculosis: A report of 49 cases. Rev. Mal. Respir. 2011;28:312–316. doi: 10.1016/j.rmr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Leung C.C., Yu I.T.S., Chen W. Silicosis. Lancet. 2012;379:2008–2018. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 28.Bui P.V. Disseminated Histoplasmosis with Miliary Histoplasmosis, Neurohistoplasmosis, and Histoplasma Capsulatum Bacteremia in Probable Neurosarcoidosis. Case Rep. Med. 2018;2018:3162403. doi: 10.1155/2018/3162403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuura S., Mochizuka Y., Oishi K., Miyashita K., Naoi H., Mochizuki E., Mikura S., Tsukui M., Koshimizu N., Ohata A., et al. Sarcoidosis with Pancreatic Mass, Endobronchial Nodules, and Miliary Opacities in the Lung. Intern. Med. 2017;56:3083–3087. doi: 10.2169/internalmedicine.8916-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taki M., Ikegami N., Konishi C., Nakao S., Funazou T., Ariyasu R., Yoshida M., Nakagawa K., Morita K., Hee Hwang M., et al. Pulmonary Sarcoidosis Presenting with Miliary Opacities. Intern. Med. 2015;54:2483–2486. doi: 10.2169/internalmedicine.54.4681. [DOI] [PubMed] [Google Scholar]
- 31.Laack E., Simon R., Regier M., Andritzky B., Tennstedt P., Habermann C., Verth C.Z., Thöm I., Grob T., Sauter G., et al. Miliary Never-Smoking Adenocarcinoma of the Lung: Strong Association with Epidermal Growth Factor Receptor Exon 19 Deletion. J. Thorac. Oncol. 2011;6:199–202. doi: 10.1097/JTO.0b013e3181fb7cf1. [DOI] [PubMed] [Google Scholar]
- 32.Endo K., Konishi A., Sasaki H., Takada M., Tanaka H., Okumura M., Kawahara M., Sugiura H., Kuwabara Y., Fukai I., et al. Epidermal Growth Factor Receptor Gene Mutation in Non-Small Cell Lung Cancer Using Highly Sensitive and Fast TaqMan PCR Assay. Lung Cancer. 2005;50:375–384. doi: 10.1016/j.lungcan.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Arcila M.E., Oxnard G.R., Nafa K., Riely G.J., Solomon S.B., Zakowski M.F., Kris M.G., Pao W., Miller V.A., Ladanyi M. Rebiopsy of Lung Cancer Patients with Acquired Resistance to EGFR Inhibitors and Enhanced Detection of the T790M Mutation Using a Locked Nucleic Acid-Based Assay. Clin. Cancer Res. 2011;17:1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J., Wang L., Jänne P.A., Makrigiorgos G.M. Coamplification at Lower Denaturation Temperature-PCR Increases Mutation-Detection Selectivity of TaqMan-Based Real-Time PCR. Clin. Chem. 2009;55:748–756. doi: 10.1373/clinchem.2008.113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward R., Hawkins N., O’Grady R., Sheehan C., O’Connor T., Impey H., Roberts N., Fuery C., Todd A. Restriction Endonuclease-Mediated Selective Polymerase Chain Reaction: A Novel Assay for the Detection of K-Ras Mutations in Clinical Samples. Am. J. Pathol. 1998;153:373–379. doi: 10.1016/S0002-9440(10)65581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim C., Tsao M.S., Le L.W., Shepherd F.A., Feld R., Burkes R.L., Liu G., Kamel-Reid S., Hwang D., Tanguay J., et al. Biomarker Testing and Time to Treatment Decision in Patients with Advanced Nonsmall-Cell Lung Cancer. Ann. Oncol. 2015;26:1415–1421. doi: 10.1093/annonc/mdv208. [DOI] [PubMed] [Google Scholar]
- 37.Cheema P.K., Menjak I.B., Winterton-Perks Z., Raphael S., Cheng S.Y., Verma S., Muinuddin A., Freedman R., Toor N., Perera J., et al. Impact of Reflex EGFR/ALK Testing on Time to Treatment of Patients With Advanced Nonsquamous Non–Small-Cell Lung Cancer. JOP. 2016;13:e130–e138. doi: 10.1200/JOP.2016.014019. [DOI] [PubMed] [Google Scholar]
- 38.Sequist L.V., Heist R.S., Shaw A.T., Fidias P., Rosovsky R., Temel J.S., Lennes I.T., Digumarthy S., Waltman B.A., Bast E., et al. Implementing Multiplexed Genotyping of Non-Small-Cell Lung Cancers into Routine Clinical Practice. Ann. Oncol. 2011;22:2616–2624. doi: 10.1093/annonc/mdr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstraw P., Crowley J., Chansky K., Giroux D.J., Groome P.A., Rami-Porta R., Postmus P.E., Rusch V., Sobin L., International Association for the Study of Lung Cancer International Staging Committee The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]