Abstract
Circular RNAs (circRNAs) have recently emerged as a novel class of transcripts, characterized by covalently linked 3′–5′ ends that result in the so-called backsplice junction. During the last few years, thousands of circRNAs have been identified in different organisms. Yet, despite their role as disease biomarker started to emerge, depicting their function remains challenging. Different studies have shown that certain circRNAs act as miRNA sponges, but any attempt to generalize from the single case to the “circ-ome” has failed so far. In this review, we explore the potential to define miRNA “sponging” as a more general function of circRNAs and describe the different approaches to predict miRNA response elements (MREs) in known or novel circRNA sequences. Moreover, we discuss how experiments based on Ago2-IP and experimentally validated miRNA:target duplexes can be used to either prioritize or validate putative miRNA-circRNA associations.
Keywords: circRNA, miRNA, target prediction, miRNA sponge
1. Introduction
During the past decades, the field of RNA biology experienced an incredible evolution dictated by the discovery of long non-coding RNAs, the elucidation of the silencing pathways of short non-coding RNAs and, more importantly, of their regulatory functions [1]. Recently, a new class of non-coding RNAs has taken the scene: circular RNAs (circRNAs) [2]. Interestingly, the existence of RNAs with a circular form has been known for many years, but they were associated only with viruses and viroids (e.g., hepatitis δ virus [3]). Although few examples of circRNAs from transcribed genes were reported (e.g., Sry, [4,5,6], DCC [7], CYP450 [8]), only recently their abundance and regulatory functions have been described openly [9,10,11,12]. circRNAs consists in covalently closed RNA molecules with the 3′- and the 5′-ends linked in a non-collinear way resulting in the so-called backsplice junction (Figure 1) [13]. They result from an unusual splicing event that is believed to be mediated either by the pairing of long flanking introns (containing repetitive elements in an inverted orientation) or by an intra-lariat splicing [13,14,15,16,17,18,19]. As linear RNA, circRNAs can undergo alternative splicing that generates different classes of circRNAs (intron-containing, single exon, multiple exon, intergenic, intronic) and increases the “circ-ome” overall complexity [12,19,20,21,22,23]. This particular splicing event causes circRNAs to lack the 3′ poly(A) tail and the 5′ capping, a feature that confers resistance to exonuclease activity (e.g., RNase R [24,25]) and results, on average, in a longer half-life as compared to linear RNAs [11]. Since the first reports of circRNAs expression in humans and mice, thousands of potential circular RNAs have been predicted in different species (like Drosophila, Caenorhabditis elegans and plants) [9,11,12,15,26,27,28,29,30,31,32]. Despite the great attention that this elusive class of ncRNA has gathered, only a handful of transcripts have been fully functionally characterized. Nevertheless, the high stability combined with their identification in human body fluids (e.g., plasma [33] and saliva [34]) has greatly increased the interest toward circRNAs as potential disease biomarkers [35] and, following this idea, dozens of studies identified circRNAs in different pathological conditions [36] such as Alzheimer’s disease, atherosclerosis, myocardial infarction and, most importantly, cancer [37,38,39,40,41,42].
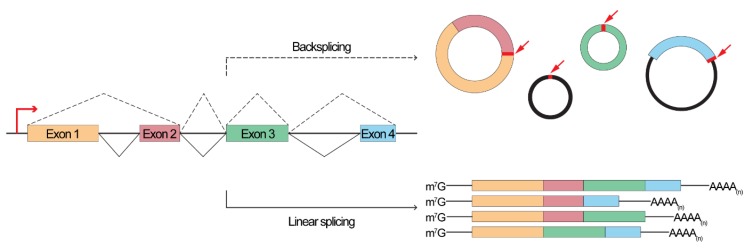
Figure 1.
Linear vs Circular splicing. Circular RNAs (circRNAs) are formed from an unusual splicing event that results in covalently linked 3′–5′ ends termed as a backsplice junction (top, indicated by a red arrow). As for linear transcripts (bottom), circRNAs can undergo alternative splicing, resulting in different classes of transcripts (mono or multi exonic, intronic, exon-intron structure).
In this review, we explore one of the hypothesized functions for circRNA, i.e., miRNA binding. Particularly, we will first address the definition of “sponging”, which, so far, has been quite an appealing but misleading term, and then discuss the different computational approaches to predict miRNA-circRNA binding sites and the strategies to prioritize/validate such interactions.
2. To Sponge or Not to Sponge, That is the Question
The high stability and the presence in different body fluids make circRNAs extremely promising disease biomarkers. Given their diagnostic relevance, a lot of efforts have been put in the functional characterization of circRNAs, as this is critical to understand their role in disease development or progression and to provide crucial insights into their physiological role. It has been shown that nuclear circRNAs can be involved in regulating mRNA expression at the level of transcription by interacting with RNA polymerase or with members of the spliceosome machinery [43], for example. Conversely, cytoplasmic circRNAs seem to be involved in post-transcriptional regulation, sequester RNA-binding proteins [14,44] or even can be translated into small peptides [45,46]. Considering post-transcriptional regulation, one of the first and most investigated functions of circRNAs is miRNA sponging (Figure 2) [10,12,38,47,48]. In fact, in the past couple of years the number of papers involving circRNA-miRNA interaction has grown almost exponentially and, in 2018, represented ~60% of circRNA-related publications (Figure 3). Despite the increasing number of studies focusing on circRNA-miRNA interactions, to generalize this specific function to the entire “circ-ome” still remains challenging. The first, and most important, issue in this regard is the definition of “sponging” or, more appropriately, of competing endogenous RNAs (ceRNAs). Whether the ceRNAs hypothesis [49] is sufficient to explain the function of thousands of poorly characterized ncRNAs is still an argument worthy of great debate (refer to Thomson and Dinger [50] for more details). Nevertheless, it is crucial to consider the evidence that the expression alone of a ceRNA (in our case specifically, circRNAs) might not be sufficient to have a measurable effect on highly expressed miRNA and, therefore, on its downstream targets [51], while the impact on lowly expressed ones could be more significant [52]. This has a major consequence in the definition of the minimum characteristics that a circRNA must hold (e.g., expression, number of possible miRNA response elements—MREs—and miRNA expression itself) to be considered a miRNA sponge. Taking into account that, overall, circRNAs are expressed at lower levels than other RNAs [11,12,20,53] and that the expression is tissue- and cell-type-specific [20,23,30,54,55,56,57], the presence of a relatively high number of MREs for the same miRNA within the sequence of a single circular RNA would be expected. Different studies have shown that, beside CDR1as, only a very limited number of circRNA exhibit this property [30,53], pointing strongly toward the idea that sponging is an exception, rather than a general function.
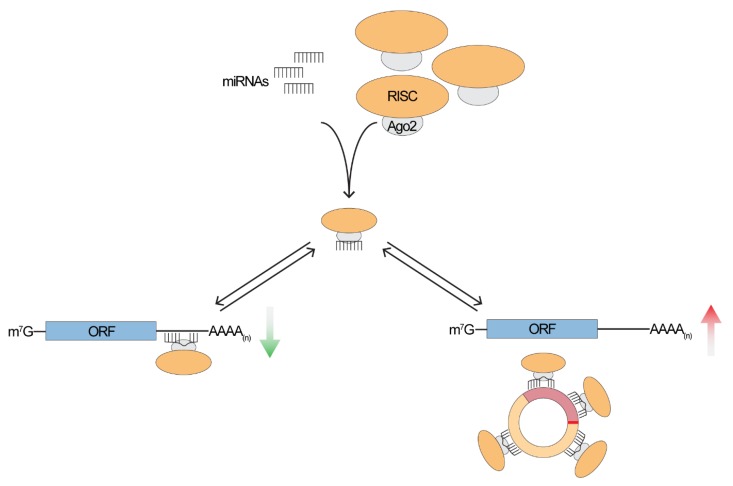
Figure 2.
A miRNA-circRNA-mRNA network. It has been proposed that circRNA can act as a miRNA sponge, therefore competing with a linear target for the binding of the RISC complex. In the absence of circRNA, miRNAs are free to bind to their linear target, determining their repression. When the circRNA is expressed, the miRNA will guide the RISC complex to bind the circRNA, ultimately causing the de-repression of the mRNA. mRNA is depicted as an Open Reading Frame (ORF) with a 5′ cap (m7G) and a 3′ poly(A) tail.
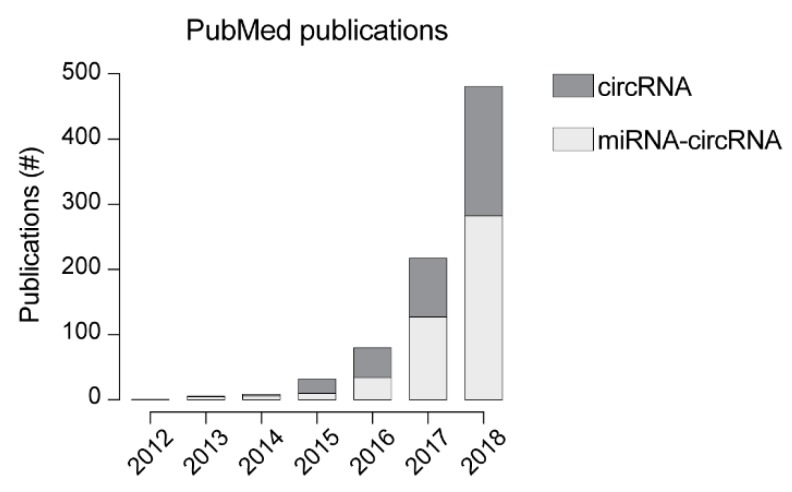
Figure 3.
Per year number of publication indexed in PubMed. Dark grey represents the number of publications resulting by the search term “circRNA” while light grey represents the number of papers resulting from the combined search of “circRNA miRNA”.
3. Predicting circRNA-miRNA Binding Sites
Although circRNAs cannot be considered “sponges”, it is clear that these molecules fulfill their regulatory function also through the interaction with miRNAs [58,59,60,61,62,63,64,65]. To this end, knowing the sequence and the expression levels of circRNAs in a given tissue is essential. The most common approach to obtain this information is based on microarrays with probes specifically designed to target the most updated collection of human/mouse/rat circRNAs [66,67]. This allows the detection of even very lowly expressed circRNAs with high reproducibility (ideally, down to one copy), and facilitates the identification of differentially expressed circRNAs. Additionally, the development of more sophisticated RNA sequencing protocols (e.g., RPAD [68]) provides the possibility to identify highly pure circRNAs, overcoming the limit of relying only on annotated transcripts. Obviously, in the latter case, not only the sequencing protocol, but also which tool is used to identify the backsplice junction are critical issues and possible sources of variability between experiments [69]. No matter which approach is used for the identification of circRNAs, the bioinformatic prediction of MREs can be done in many different ways, mainly depending whether the circRNA is already known or a novel transcript.
3.1. Investigating Known circRNAs
In the past years, several databases have been released with the main goal of collecting all possible information regarding known circRNAs in different species (e.g., circBase [70], Table 1). These databases have expanded to meet the increasing complexity of circRNA expression patterns and to collect all possible information about functional predictions and associations with diseases (e.g., circNet [71], CircInteractome [72], circ2Traits [73]). One of the features that has been included is the miRNAs binding sites for all available circRNAs. These are obtained either using miRNA target prediction tools (as TargetScan [74], RNA22 [75], PITA [76], miRanda/miRSVR [77,78], etc.), like in the case of circNet and CircInteractome, or combining Ago binding sites with miRNA target predictions (as, for instance, starBase v2.0 [79,80] and its most updated version, ENCORI), although these approaches might result in multiple putative miRNAs hits per single circRNA. A possible strategy to reduce the number of candidate circRNA-miRNA associations is to consider also the downstream mRNAs (usually from databases including experimentally validated miRNA targets like TarBase v.8 [81] or miRTarBase [82]) and create a circRNA–miRNA–mRNA network. This procedure builds on the idea that an up-regulated circRNA will cause a down-regulation of its interacting miRNA that will ultimately determine an up-regulation of the target mRNA [83,84,85,86,87,88,89,90]. Networks that satisfy these expression criteria are selected finally for functional validation: first, the MREs predicted within the circRNA are validated primarily by luciferase assay and then the expression of the target miRNA and mRNA are evaluated upon circRNA depletion (or overexpression, according to the initial transcription pattern). Although quite successful, this approach presents some limitations, for instance, the databases providing miRNA binding predictions are dealing only with human circRNAs, with the exception of starBase [79,80] which includes also data for mouse and C. elegans. Additionally, this workflow is effective only when the circRNA has been previously identified and annotated in other databases (like circBase) and, finally, it also requires a differential expression analysis for all components of the network (circRNA, miRNA and mRNA). Moreover, validating MREs with luciferase assay has two major drawbacks: i) it does not always provide a clear proof of direct interaction [91]; and ii) it implies that the MREs on the circRNA have to be sufficiently strong to cause a significant variation in either luminescence or luciferase mRNA levels, which is not necessarily the case. These issues can be overcome by an RNA Immunoprecipitation assay (RIP, [92]) that will provide information of what is directly binding to the circRNA, with no regard to a functional output [93,94,95,96,97,98,99,100].
Table 1.
List of relevant circRNA-related databases including the available organisms and their general features.
| Database | Website | Organisms | Features |
|---|---|---|---|
| circBase | http://www.circbase.org | Human Mouse Fly Worm Fish Planaria |
Most updated catalogue of predicted circRNAs. Beside human and mouse, it also collects data from several other organisms |
| circInteractome | https://circinteractome.nia.nih.gov | Human | Enables the prediction and mapping of binding sites for RNA binding proteins and miRNA on known circRNAs. It includes also a module for siRNA design for knock-down experiments and primer design for PCR |
| circNet | http://syslab5.nchu.edu.tw/CircNet/ | Human | Provides tissue-specific expression patterns, integrated miRNA-circRNA-mRNA networks, circRNA isoform expression and genomic annotation |
|
ENCORI
StarBase v2 |
http://starbase.sysu.edu.cn/index.php
http://starbase.sysu.edu.cn/starbase2/index.php |
Human Mouse Worm |
Designed for investigating interaction networks of lncRNAs, miRNAs, ceRNA, RNA binding proteins and mRNAs from public CLIP-Seq data. It also allows to browse for circRNA-miRNA interactions. |
| circ2Traits | http://gyanxet-beta.com/circdb/ | Human | Link of circRNA with disease inferred by miRNA-disease associations |
3.2. Characterizing Novel circRNAs
As mentioned previously, circRNAs have been shown to have a time- and tissue-specific expression pattern [20,23,30]. This results in the need, due to the complexity of organisms, to perform comprehensive assessments to investigate specific tissues and developmental stages. The best way to address this issue is through RNA sequencing, since this method is not limited by an a priori knowledge of circRNA sequences and expression. In turn, when it comes to MREs prediction, the bioinformatic approach becomes less straightforward. Assuming that the full sequence of novel circRNAs has been assessed, the first issue is represented by the choice of an appropriate tool to predict MREs. Although it has been shown that more than 80% of circRNAs are overlapping coding genes, less than 10% include a 3′UTR, making it almost useless to take advantage of available databases (like TargetScan [74], Table 2) that contain information on 3′UTRs only. On the other hand, databases that provide information on MREs on the entire sequence (e.g., microRNA.org [101]) and also include experimental validation information (like TarBase v8 [81] or STarMirDB [102]) do not allow to browse by target sequence in addition to gene name, therefore becoming useless for the analysis of novel circRNAs. There are some tools that have been designed to also search by custom sequences (e.g., STarMir [103]), but they show limitation in the length of the queried sequence, allowing the analysis only of few transcripts and making it difficult to perform a comprehensive assessment. One way to overcome these limitations is to use the stand-alone versions of the algorithms behind the prediction database (when available). Thus, the direct application of the algorithms allows the analysis of any given sequence for any given list of miRNAs. Unfortunately, results obtained with this approach show an extremely high rate of false positives, requiring either a systematic validation of the targets (e.g., with RIP assays) or the integration with known interactions.
Table 2.
List of the most common databases and algorithms for predicting miRNA binding sites together with the organisms for which the prediction can be browsed (by sequence and/or by gene ID) and if they share the standalone version.
| Tool | Website | Organisms | Browse by Sequenc/Gene ID | Standalone Version |
|---|---|---|---|---|
| STarMir | http://sfold.wadsworth.org/cgi-bin/starmirtest2.pl | Human Mouse Worm Other |
Sequence/ Gene ID |
no |
| STarMirDB | http://sfold.wadsworth.org/starmirDB.php | Human Mouse Worm |
Gene ID | no |
| PITA | https://genie.weizmann.ac.il/pubs/mir07/index.html | Human Mouse Fly Worm |
Gene ID | yes |
|
miRanda/
mirSVR |
http://www.microrna.org/microrna/home.do | Human Mouse Rat Fly Worm |
Gene ID | yes |
| TargetScan | http://www.targetscan.org/vert_72/ | Human Mouse Fly Worm Zebrafish |
Gene ID | yes |
| RNAhybrid | https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/ | Any | Sequence | yes |
| TarBase v8 | http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php | Human Mouse Rat Chicken Zebrafish Fly Worm Chimpanzees Macaque Soy Maize Barrelclover Grape wine Earthmoss Epstein–Barr virus KSHV |
Gene ID | no |
4. Integrating Seed Prediction on Custom Sequences with Experimental Data
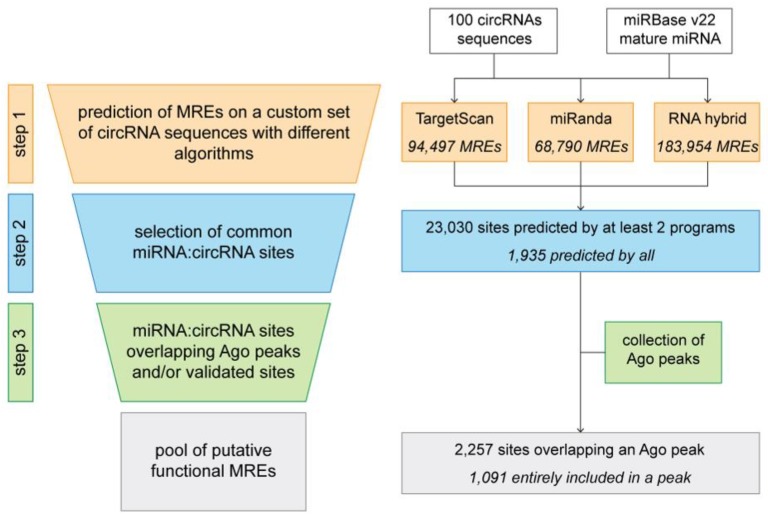
No matter which method is used to design the prediction algorithm, the major reason behind the high rate of observed false positives and false negatives is the fact that miRNA-target recognition already is effective with a seed length of six nucleotides [104,105]. Reducing and prioritizing the predicted interaction is not trivial, as each possible approach has several pros and cons. For example, to consider conservation of seed and MREs across species dramatically reduces the number of predictions, although this approach does not consider non-canonical, as well as non-conserved, binding sites [106]. Moreover, using the free energy of miRNA:target duplexes is effective at the cost of an incredibly high number of putative very stable false positive interactions. Given that no gold standard has been identified nor do any algorithms outperform the others, there are some steps that can be undertaken to “manually” predict circRNA-miRNA sites while limiting the possibly overwhelming list of predicted binding interactions. This workflow (depicted in Figure 4, left) can be divided into three main steps. To show how each step influences the final outcome, we analyzed 100 randomly chosen mouse circRNA from previous work [30]. As done in some databases for gene-miRNA target mining (e.g., miRWalk [107]), the first step consists in performing the analysis with the same input (circRNAs) and miRNA sequences using different algorithms (for this example, TargetScan [74], miRanda [77] and RNAhybrid [108] were used, Figure 4, right). Using default options, we obtained for our cohort of circRNAs an average of 115,747 putative MREs where RNAhybrid predicted the highest number of sites (183,954) while miRanda the lowest (68,790). The second step consists in retrieving only the predictions that have been identified by at least half of the programs (in our case we selected MREs predicted by at least two programs). This first filtering step reduced the initial list to approximately 23,000 MREs, with only 1935 sites predicted by all programs. In particularly, for TargetScan only ~19% of predicted sites were common to at least another algorithm, while for miRanda the sites were reduced to approximately 30%. Regarding RNAhybrid, we observed the most severe reduction, as only 5% of all the predicted MREs were kept for the last step. To further reduce the amount of possible false positives, a valid approach is to make use of complementary experimental data. Specifically, since the binding of the RISC complex is mediated by the interaction of the miRNA with members of the Argonaute protein family [109,110], it is fundamental, for a predicted miRNA-circRNA site to be real, that Ago proteins also are binding in the same positions. The development of various CLIP-Seq protocols (Cross-linking and Immunoprecipitation followed by sequencing) provides an extremely valuable source of high-throughput data of Ago binding sites [111,112,113,114,115,116,117]. These data can be directly used to eliminate all the predicted sites for which there is no binding of Ago protein [118], considering this a sine qua non condition for a true binding of miRNA on the target circRNA. Considering this, the last step consists in the retrieval of all the MREs that are overlapped also by Ago and to this end we used a collection of publicly available Ago-binding sites from mouse brains [115,119]. We obtained a final set of 2257 sites partially overlapping an Ago peak and among these, 1091 MREs were included entirely in a peak. Using this final filtering, we could reduce the number of putative circRNA:miRNA sites down to 0.9% and 1.5% for TargetScan and miRanda predictions, respectively. Again, the most dramatic decrease was observed for RNAhybrid predictions as only 0.2% of MREs were included in the final list. Since Ago CLIP-Seq experiments are not available for all cell types, tissues and organisms, for this last step accessible data also can be used indirectly, for instance, by creating a pool of Ago-binding motifs and exploiting sequence similarity to quantify existence probability of custom miRNA:circRNA duplexes.
Figure 4.
A possible pipeline for the comprehensive assessment of circRNA:miRNA binding sites starting from a custom set of expressed circRNA sequences (left) and a practical example on the outcome of each step on a set of randomly chosen sequences from previous work [30] (right).
Ranking MREs
The approach presented in this example uses a basic step-wise filtering system that takes the output of different prediction programs and sequentially reduces the pool of MREs according to the presence/absence of specific criteria (prediction by at least 2 out of 3 algorithms; overlap with an Ago peak). As shown, this system already is effective in reducing the amount of data that can be considered for further validation and functional characterization, but we still might be missing valuable information given by the pool of MREs that are specific to each algorithm but that still overlap an Ago peak (~12,000 additional MREs in total). To overcome this limitation, a possible alternative is the construction of a scoring function that evaluates the probability of a predicted MRE to be real by considering the validated data (presence of an Ago peak/binding motif) and the initial prediction information (observation that the same site is identified by one or more algorithms) [120]. Weighting these two aspects differently, the resulting score would allow the ranking of all predicted MREs and to prioritize those that include both the experimental data and the predictions by multiple algorithms while not excluding all the sites identified by single programs that still retain a correspondence among validated data.
5. Concluding Remarks
Circular RNAs have recently emerged as a novel class of transcripts characterized by covalently linked 3′–5′ ends called backsplice junctions. Studies have shown their relevance in physiological and pathological conditions, in particular as disease biomarkers and potential therapeutic targets. However, the functional characterization of these sequences is still in its infancy and the role of relatively few circRNAs has been described to date. Recently, great effort has been put toward understanding one specific mechanism, i.e., miRNA sponging. Due to the combined overall low expression of circRNAs and the low number of MREs predicted within their sequence, only a handful of transcripts really can be considered true “sponges”. Nevertheless, it is undeniable that circRNAs are capable of binding miRNAs but, more than a “sponge” for a single small RNA, they might function as a scaffold for several different ones. Regarding this, it is crucial to develop appropriate pipelines that allow a more accurate prediction of the miRNA targets, thus facilitating an overall assessment of miRNA binding and, possibly, leading to the identification of a more general function.
Acknowledgments
We thank members of our lab for helpful discussion and suggestion.
Author Contributions
M.D. conceived the project; M.D. and S.B. wrote and revised the manuscript.
Funding
This work was funded by the Italian Epigenomics Flagship Project (Epigen).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Carthew R.W., Sontheimer E.J. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosik K.S. Circles reshape the RNA world. Nature. 2013;495:322–324. doi: 10.1038/nature11956. [DOI] [PubMed] [Google Scholar]
- 3.Kos A., Dijkema R., Arnberg A.C., van der Meide P.H., Schellekens H. The hepatitis delta (δ) virus possesses a circular RNA. Nature. 1986;323:558. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- 4.Capel B., Swain A., Nicolis S., Hacker A., Walter M., Koopman P., Goodfellow P., Lovell-Badge R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell. 1993;73:1019–1030. doi: 10.1016/0092-8674(93)90279-Y. [DOI] [PubMed] [Google Scholar]
- 5.Hacker A., Capel B., Goodfellow P., Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- 6.Jeske Y.W., Bowles J., Greenfield A., Koopman P. Expression of a linear Sry transcript in the mouse genital ridge. Nat. Genet. 1995;10:480–482. doi: 10.1038/ng0895-480. [DOI] [PubMed] [Google Scholar]
- 7.Nigro J.M., Cho K.R., Fearon E.R., Kern S.E., Ruppert J.M., Oliner J.D., Kinzler K.W., Vogelstein B. Scrambled exons. Cell. 1991;64:607–613. doi: 10.1016/0092-8674(91)90244-S. [DOI] [PubMed] [Google Scholar]
- 8.Zaphiropoulos P.G. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: Correlation with exon skipping. Proc. Natl. Acad. Sci. USA. 1996;93:6536–6541. doi: 10.1073/pnas.93.13.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salzman J., Gawad C., Wang P.L., Lacayo N., Brown P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 11.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M., et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 13.Lasda E., Parker R. Circular RNAs: Diversity of form and function. RNA. 2014;20:1829–1842. doi: 10.1261/rna.047126.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashwal-Fluss R., Meyer M., Pamudurti N.R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov A., Memczak S., Wyler E., Torti F., Porath H.T., Orejuela M.R., Piechotta M., Levanon E.Y., Landthaler M., Dieterich C., et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Starke S., Jost I., Rossbach O., Schneider T., Schreiner S., Hung L.H., Bindereif A. Exon circularization requires canonical splice signals. Cell Rep. 2015;10:103–111. doi: 10.1016/j.celrep.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21:172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A., Roslan S., Schreiber A.W., Gregory P.A., Goodall G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X.O., Dong R., Zhang Y., Zhang J.L., Luo Z., Zhang J., Chen L.L., Yang L. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016;26:1277–1287. doi: 10.1101/gr.202895.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salzman J., Chen R.E., Olsen M.N., Wang P.L., Brown P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/annotation/f782282b-eefa-4c8d-985c-b1484e845855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Zhang X.O., Chen T., Xiang J.F., Yin Q.F., Xing Y.H., Zhu S., Yang L., Chen L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Li F., Zhang L., Li W., Deng J., Zheng J., An M., Lu J., Zhou Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/β-catenin pathway. Oncotarget. 2015;6:6001–6013. doi: 10.18632/oncotarget.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rybak-Wolf A., Stottmeister C., Glazar P., Jens M., Pino N., Giusti S., Hanan M., Behm M., Bartok O., Ashwal-Fluss R., et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H., Zuo Y., Wang J., Zhang M.Q., Malhotra A., Mayeda A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006;34:e63. doi: 10.1093/nar/gkl151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent H.A., Deutscher M.P. Substrate recognition and catalysis by the exoribonuclease RNase R. J. Biol. Chem. 2006;281:29769–29775. doi: 10.1074/jbc.M606744200. [DOI] [PubMed] [Google Scholar]
- 26.Westholm J.O., Miura P., Olson S., Shenker S., Joseph B., Sanfilippo P., Celniker S.E., Graveley B.R., Lai E.C. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep. 2014;9:1966–1980. doi: 10.1016/j.celrep.2014.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conn V.M., Hugouvieux V., Nayak A., Conos S.A., Capovilla G., Cildir G., Jourdain A., Tergaonkar V., Schmid M., Zubieta C., et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat. Plants. 2017;3:17053. doi: 10.1038/nplants.2017.53. [DOI] [PubMed] [Google Scholar]
- 28.Tang B., Hao Z., Zhu Y., Zhang H., Li G. Genome-wide identification and functional analysis of circRNAs in Zea mays. PLoS ONE. 2018;13:e0202375. doi: 10.1371/journal.pone.0202375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Gao Y., Zhang H., Wang H., Liu X., Xu X., Zhang Z., Kohnen M.V., Hu K., Wang H., et al. Genome-wide profiling of circular RNAs in the rapidly growing shoots of moso bamboo (Phyllostachys edulis) Plant Cell Physiol. 2019;60:1354–1373. doi: 10.1093/pcp/pcz043. [DOI] [PubMed] [Google Scholar]
- 30.Dori M., Haj Abdullah Alieh L., Cavalli D., Massalini S., Lesche M., Dahl A., Calegari F. Sequence and expression levels of circular RNAs in progenitor cell types during mouse corticogenesis. Life Sci. Alliance. 2019;2:e201900354. doi: 10.26508/lsa.201900354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragan C., Goodall G.J., Shirokikh N.E., Preiss T. Insights into the biogenesis and potential functions of exonic circular RNA. Sci. Rep. 2019;9:2048. doi: 10.1038/s41598-018-37037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu K., Chen D., Wang Z., Ma J., Zhou J., Chen N., Lv L., Zheng Y., Hu X., Zhang Y., et al. Annotation and functional clustering of circRNA expression in rhesus macaque brain during aging. Cell Discov. 2018;4:48. doi: 10.1038/s41421-018-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P., Chen S., Chen H., Mo X., Li T., Shao Y., Xiao B., Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin. Chim. Acta. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Bahn J.H., Zhang Q., Li F., Chan T.M., Lin X., Kim Y., Wong D.T., Xiao X. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin. Chem. 2015;61:221–230. doi: 10.1373/clinchem.2014.230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Memczak S., Papavasileiou P., Peters O., Rajewsky N. Identification and characterization of circular RNAs as a new class of putative biomarkers in human blood. PLoS ONE. 2015;10:e0141214. doi: 10.1371/journal.pone.0141214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyu D., Huang S. The emerging role and clinical implication of human exonic circular RNA. RNA Biol. 2017;14:1000–1006. doi: 10.1080/15476286.2016.1227904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holdt L.M., Stahringer A., Sass K., Pichler G., Kulak N.A., Wilfert W., Kohlmaier A., Herbst A., Northoff B.H., Nicolaou A., et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen T.B., Kjems J., Damgaard C.K. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–5612. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 39.Haque S., Harries L.W. Circular RNAs (circRNAs) in health and disease. Genes. 2017;8:353. doi: 10.3390/genes8120353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu N., Jamal R. Circular RNAs as promising biomarkers: A mini-review. Front. Physiol. 2016;7:355. doi: 10.3389/fphys.2016.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonizzato A., Gaffo E., Te Kronnie G., Bortoluzzi S. CircRNAs in hematopoiesis and hematological malignancies. Blood Cancer J. 2016;6:e483. doi: 10.1038/bcj.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulcheski F.R., Christoff A.P., Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J. Biotechnol. 2016;238:42–51. doi: 10.1016/j.jbiotec.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 44.Du W.W., Yang W., Liu E., Yang Z., Dhaliwal P., Yang B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Legnini I., Di Timoteo G., Rossi F., Morlando M., Briganti F., Sthandier O., Fatica A., Santini T., Andronache A., Wade M., et al. Circ-ZNF609 Is a Circular rna that can be translated and functions in myogenesis. Mol. Cell. 2017;66:22–37.e29. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pamudurti N.R., Bartok O., Jens M., Ashwal-Fluss R., Stottmeister C., Ruhe L., Hanan M., Wyler E., Perez-Hernandez D., Ramberger E., et al. Translation of CircRNAs. Mol. Cell. 2017;66:9–21.e27. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang S., Yang B., Chen B.J., Bliim N., Ueberham U., Arendt T., Janitz M. The emerging role of circular RNAs in transcriptome regulation. Genomics. 2017;109:401–407. doi: 10.1016/j.ygeno.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Verduci L., Strano S., Yarden Y., Blandino G. The circRNA-microRNA code: Emerging implications for cancer diagnosis and treatment. Mol. Oncol. 2019;13:669–680. doi: 10.1002/1878-0261.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomson D.W., Dinger M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016;17:272–283. doi: 10.1038/nrg.2016.20. [DOI] [PubMed] [Google Scholar]
- 51.Denzler R., Agarwal V., Stefano J., Bartel D.P., Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell. 2014;54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosson A.D., Zamudio J.R., Sharp P.A. Endogenous miRNA and target concentrations determine susceptibility to potential ceRNA competition. Mol. Cell. 2014;56:347–359. doi: 10.1016/j.molcel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo J.U., Agarwal V., Guo H., Bartel D.P. Expanded identification and charachterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alhasan A.A., Izuogu O.G., Al-Balool H.H., Steyn J.S., Evans A., Colzani M., Ghevaert C., Mountford J.C., Mareneah L., Elliott D.J., et al. Circular RNA enrichment in platelets is a signature of transcriptome degradation. Blood. 2016;127:e1–e11. doi: 10.1182/blood-2015-06-649434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piwecka M., Glazar P., Hernandez-Miranda L.R., Memczak S., Wolf S.A., Rybak-Wolf A., Filipchyk A., Klironomos F., Cerda Jara C.A., Fenske P., et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science. 2017;357:eaam8526. doi: 10.1126/science.aam8526. [DOI] [PubMed] [Google Scholar]
- 56.Kaur S., Mirza A.H., Pociot F. Cell type-selective expression of circular RNAs in human pancreatic islets. Noncoding RNA. 2018;4:38. doi: 10.3390/ncrna4040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holdt L.M., Kohlmaier A., Teupser D. Molecular functions and specific roles of circRNAs in the cardiovascular system. Noncoding RNA Res. 2018;3:75–98. doi: 10.1016/j.ncrna.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang G., Liu W., Zou Y., Wang G., Deng Y., Luo J., Zhang Y., Li H., Zhang Q., Yang Y., et al. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a-MET pathway. EBioMedicine. 2019;40:432–445. doi: 10.1016/j.ebiom.2018.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y., Liu H., Li W., Yu J., Shen Z., Ye G., Qi X., Li G. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging. 2017;9:1585–1594. doi: 10.18632/aging.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang W.L., Yang Z., Zhang Y.J., Lu P., Ni Y.K., Sun C.F., Liu F.Y. Competing endogenous RNA analysis reveals the regulatory potency of circRNA_036186 in HNSCC. Int. J. Oncol. 2018;53:1529–1543. doi: 10.3892/ijo.2018.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jost I., Shalamova L.A., Gerresheim G.K., Niepmann M., Bindereif A., Rossbach O. Functional sequestration of microRNA-122 from Hepatitis C Virus by circular RNA sponges. RNA Biol. 2018;15:1032–1039. doi: 10.1080/15476286.2018.1435248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang K., Gan T.Y., Li N., Liu C.Y., Zhou L.Y., Gao J.N., Chen C., Yan K.W., Ponnusamy M., Zhang Y.H., et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017;24:1111–1120. doi: 10.1038/cdd.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang R., Zhang S., Chen X., Li N., Li J., Jia R., Pan Y., Liang H. CircNT5E acts as a sponge of miR-422a to promote glioblastoma tumorigenesis. Cancer Res. 2018;78:4812–4825. doi: 10.1158/0008-5472.CAN-18-0532. [DOI] [PubMed] [Google Scholar]
- 64.Zhang F., Zhang R., Zhang X., Wu Y., Li X., Zhang S., Hou W., Ding Y., Tian J., Sun L., et al. Comprehensive analysis of circRNA expression pattern and circRNA-miRNA-mRNA network in the pathogenesis of atherosclerosis in rabbit. Aging. 2018;10:2266–2283. doi: 10.18632/aging.101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai X., Zhao Z., Dong J., Lv Q., Yun B., Liu J., Shen Y., Kang J., Li J. Circular RNA circBACH2 plays a role in papillary thyroid carcinoma by sponging miR-139-5p and regulating LMO4 expression. Cell Death Dis. 2019;10:184. doi: 10.1038/s41419-019-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Go Beyond RNA. [(accessed on 15 May 2019)]; Available online: https://www.arraystar.com/circular-rna-research/
- 67.Li S., Teng S., Xu J., Su G., Zhang Y., Zhao J., Zhang S., Wang H., Qin W., Lu Z.J., et al. Microarray is an efficient tool for circRNA profiling. Brief. Bioinform. 2018 doi: 10.1093/bib/bby006. [DOI] [PubMed] [Google Scholar]
- 68.Pandey P.R., Rout P.K., Das A., Gorospe M., Panda A.C. RPAD (RNase R treatment, polyadenylation, and poly(A)+ RNA depletion) method to isolate highly pure circular RNA. Methods. 2019;155:41–48. doi: 10.1016/j.ymeth.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen T.B., Veno M.T., Damgaard C.K., Kjems J. Comparison of circular RNA prediction tools. Nucleic Acids Res. 2016;44:e58. doi: 10.1093/nar/gkv1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glazar P., Papavasileiou P., Rajewsky N. circBase: A database for circular RNAs. RNA. 2014;20:1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y.C., Li J.R., Sun C.H., Andrews E., Chao R.F., Lin F.M., Weng S.L., Hsu S.D., Huang C.C., Cheng C., et al. CircNet: A database of circular RNAs derived from transcriptome sequencing data. Nucleic Acids Res. 2016;44:D209–D215. doi: 10.1093/nar/gkv940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dudekula D.B., Panda A.C., Grammatikakis I., De S., Abdelmohsen K., Gorospe M. CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 2016;13:34–42. doi: 10.1080/15476286.2015.1128065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosal S., Das S., Sen R., Basak P., Chakrabarti J. Circ2Traits: A comprehensive database for circular RNA potentially associated with disease and traits. Front. Genet. 2013;4:283. doi: 10.3389/fgene.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agarwal V., Bell G.W., Nam J.W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miranda K.C., Huynh T., Tay Y., Ang Y.-S., Tam W.-L., Thomson A.M., Lim B., Rigoutsos I. A Pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 76.Kertesz M., Iovino N., Unnerstall U., Gaul U., Segal E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007;39:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 77.Enright A.J., John B., Gaul U., Tuschl T., Sander C., Marks D.S. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Betel D., Koppal A., Agius P., Sander C., Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J.H., Li J.H., Shao P., Zhou H., Chen Y.Q., Qu L.H. starBase: A database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res. 2011;39:D202–D209. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J.H., Liu S., Zhou H., Qu L.H., Yang J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karagkouni D., Paraskevopoulou M.D., Chatzopoulos S., Vlachos I.S., Tastsoglou S., Kanellos I., Papadimitriou D., Kavakiotis I., Maniou S., Skoufos G., et al. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018;46:D239–D245. doi: 10.1093/nar/gkx1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chou C.H., Shrestha S., Yang C.D., Chang N.W., Lin Y.L., Liao K.W., Huang W.C., Sun T.H., Tu S.J., Lee W.H., et al. miRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46:D296–D302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng S., Song C., Li H., Cao X., Ma Y., Wang X., Huang Y., Lan X., Lei C., Chaogetu B., et al. Circular RNA SNX29 sponges miR-744 to regulate proliferation and differentiation of myoblasts by activating the Wnt5a/Ca(2+) signaling pathway. Mol. Ther. Nucleic Acids. 2019;16:481–493. doi: 10.1016/j.omtn.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L., Liu X., Che S., Cui J., Ma X., An X., Cao B., Song Y. Endometrial epithelial cell apoptosis is inhibited by a ciR8073-miR181a-neurotensis pathway during embryo implantation. Mol. Ther. Nucleic Acids. 2019;14:262–273. doi: 10.1016/j.omtn.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shang J., Chen W.M., Wang Z.H., Wei T.N., Chen Z.Z., Wu W.B. CircPAN3 mediates drug resistance in acute myeloid leukemia through the miR-153-5p/miR-183-5p-XIAP axis. Exp. Hematol. 2019;70:42–54.e43. doi: 10.1016/j.exphem.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 86.Liu J., Kong F., Lou S., Yang D., Gu L. Global identification of circular RNAs in chronic myeloid leukemia reveals hsa_circ_0080145 regulates cell proliferation by sponging miR-29b. Biochem. Biophys. Res. Commun. 2018;504:660–665. doi: 10.1016/j.bbrc.2018.08.154. [DOI] [PubMed] [Google Scholar]
- 87.Huang L., Chen M., Pan J., Yu W. Circular RNA circNASP modulates the malignant behaviors in osteosarcoma via miR-1253/FOXF1 pathway. Biochem. Biophys. Res. Commun. 2018;500:511–517. doi: 10.1016/j.bbrc.2018.04.131. [DOI] [PubMed] [Google Scholar]
- 88.Feng C., Li Y., Lin Y., Cao X., Li D., Zhang H., He X. CircRNA-associated ceRNA network reveals ErbB and Hippo signaling pathways in hypopharingeal cancer. Int. J. Mol. Med. 2019;43:127–142. doi: 10.3892/ijmm.2018.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin X., Feng C.Y., Xiang Z., Chen Y.P., Li Y.M. CircRNA expression pattern and circRNA-miRNA-mRNA network in the pathogenesis of nonalcoholic steatohepatitis. Oncotarget. 2016;7:66455–66467. doi: 10.18632/oncotarget.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang C., Xu D., You Z., Xu K., Tian W. Dysregulated circRNAs and ceRNA network in esophageal squamous cell carcinoma. Front. Biosci. 2019;1:277–290. doi: 10.2741/4717. [DOI] [PubMed] [Google Scholar]
- 91.Campos-Melo D., Droppelmann C.A., Volkening K., Strong M.J. Comprehensive luciferase-based reporter gene assay reveals previously masked up-regulatory effects of miRNAs. Int. J. Mol. Sci. 2014;15:15592–15602. doi: 10.3390/ijms150915592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan S.M., Lieberman J. Capture and identification of miRNA targets by biotin pulldown and RNA-seq. Methods Mol. Biol. 2016;1358:211–228. doi: 10.1007/978-1-4939-3067-8_13. [DOI] [PubMed] [Google Scholar]
- 93.Xie B., Zhao Z., Liu Q., Wang X., Ma Z., Li H. CircRNA has_circ_0078710 acts as the sponge of microRNA-31 involved in hepatocellular carcinoma progression. Gene. 2019;683:253–261. doi: 10.1016/j.gene.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 94.Ni H., Li W., Zhuge Y., Xu S., Wang Y., Chen Y., Shen G., Wang F. Inhibition of circHIPK3 prevents angiotensin II-induced cardiac fibrosis by sponging miR-29b-3p. Int. J. Cardiol. 2019;292:188–196. doi: 10.1016/j.ijcard.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 95.Guo J., Duan H., Li Y., Yang L., Yuan L. A novel circular RNA circ-ZNF652 promotes hepatocellular carcinoma metastasis through inducing snail-mediated epithelial-mesenchymal transition by sponging miR-203/miR-502-5p. Biochem. Biophys. Res. Commun. 2019;513:812–819. doi: 10.1016/j.bbrc.2019.03.214. [DOI] [PubMed] [Google Scholar]
- 96.Li Y., Wan B., Liu L., Zhou L., Zeng Q. Circular RNA circMTO1 suppresses bladder cancer metastasis by sponging miR-221 and inhibiting epithelial-to-mesenchymal transition. Biochem. Biophys. Res. Commun. 2019;508:991–996. doi: 10.1016/j.bbrc.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 97.Wang S., Li Q., Wang Y., Li X., Wang R., Kang Y., Xue X., Meng R., Wei Q., Feng X. Upregulation of circ-UBAP2 predicts poor prognosis and promotes triple-negative breast cancer progression through the miR-661/MTA1 pathway. Biochem. Biophys. Res. Commun. 2018;505:996–1002. doi: 10.1016/j.bbrc.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 98.Han D., Li J., Wang H., Su X., Hou J., Gu Y., Qian C., Lin Y., Liu X., Huang M., et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151–1164. doi: 10.1002/hep.29270. [DOI] [PubMed] [Google Scholar]
- 99.Cherubini A., Barilani M., Rossi R.L., Jalal M.M.K., Rusconi F., Buono G., Ragni E., Cantarella G., Simpson H., Peault B., et al. FOXP1 circular RNA sustains mesenchymal stem cell identity via microRNA inhibition. Nucleic Acids Res. 2019;47:5325–5340. doi: 10.1093/nar/gkz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu Z., Huang W., Wang X., Wang T., Chen Y., Chen B., Liu R., Bai P., Xing J. Circular RNA CEP128 acts as a sponge of miR-145-5p in promoting the bladder cancer progression via regulating SOX11. Mol. Med. 2018;24:40. doi: 10.1186/s10020-018-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Betel D., Wilson M., Gabow A., Marks D.S., Sander C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rennie W., Kanoria S., Liu C., Mallick B., Long D., Wolenc A., Carmack C.S., Lu J., Ding Y. STarMirDB: A database of microRNA binding sites. RNA Biol. 2016;13:554–560. doi: 10.1080/15476286.2016.1182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rennie W., Liu C., Carmack C.S., Wolenc A., Kanoria S., Lu J., Long D., Ding Y. STarMir: A web server for prediction of microRNA binding sites. Nucleic Acids Res. 2014;42:W114–W118. doi: 10.1093/nar/gku376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bartel D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 105.Grimson A., Farh K.K., Johnston W.K., Garrett-Engele P., Lim L.P., Bartel D.P. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol. Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loeb G.B., Khan A.A., Canner D., Hiatt J.B., Shendure J., Darnell R.B., Leslie C.S., Rudensky A.Y. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol. Cell. 2012;48:760–770. doi: 10.1016/j.molcel.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sticht C., De La Torre C., Parveen A., Gretz N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE. 2018;13:e0206239. doi: 10.1371/journal.pone.0206239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rehmsmeier M., Steffen P., Hochsmann M., Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hammond S.M., Bernstein E., Beach D., Hannon G.J. An RNA-directed nuclease mediates post-transcritpional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 110.Hutvágner G., Zamore P.D. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 111.Hafner M., Landthaler M., Burger L., Khorshid M., Hausser J., Berninger P., Rothballer A., Ascano M., Jr., Jungkamp A.C., Munschauer M., et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Helwak A., Kudla G., Dudnakova T., Tollervey D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell. 2013;153:654–665. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jungkamp A.-C., Stoeckius M., Mecenas D., Grün D., Mastrobuoni G., Kempa S., Rajewsky N. In vivo and transcriptome-wide identification of RNA binding protein target sites. Mol. Cell. 2011;44:828–840. doi: 10.1016/j.molcel.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grosswendt S., Filipchyk A., Manzano M., Klironomos F., Schilling M., Herzog M., Gottwein E., Rajewsky N. Unambiguous identification of miRNA:target site interactions by different types of ligation reactions. Mol. Cell. 2014;54:1042–1054. doi: 10.1016/j.molcel.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chi S.W., Zang J.B., Mele A., Darnell R.B. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leung A.K., Young A.G., Bhutkar A., Zheng G.X., Bosson A.D., Nielsen C.B., Sharp P.A. Genome-wide identification of Ago2 binding sites from mouse embryonic stem cells with and without mature microRNAs. Nat. Struct. Mol. Biol. 2011;18:237–244. doi: 10.1038/nsmb.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clark P.M., Loher P., Quann K., Brody J., Londin E.R., Rigoutsos I. Argonaute CLIP-Seq reveals miRNA targetome diversity across tissue types. Sci. Rep. 2014;4:5947. doi: 10.1038/srep05947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boeckel J.N., Jae N., Heumuller A.W., Chen W., Boon R.A., Stellos K., Zeiher A.M., John D., Uchida S., Dimmeler S. Identification and characterization of hypoxia-regulated endothelial circular RNA. Circ. Res. 2015;117:884–890. doi: 10.1161/CIRCRESAHA.115.306319. [DOI] [PubMed] [Google Scholar]
- 119.Moore M., Scheel T., Luna J., Park C., Fak J., Nishiuchi E., Rice C., Darnell R. miRNA-target chimeras reveal miRNA 3′-end pairing as a major determinant of Argonaute target specificity. Nat. Commun. 2015;6:8864. doi: 10.1038/ncomms9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ceci M., Pio G., Kuzmanovski V., Dzeroski S. Semi-supervised multi-view learning for gene network reconstruction. PLoS ONE. 2015;10:e0144031. doi: 10.1371/journal.pone.0144031. [DOI] [PMC free article] [PubMed] [Google Scholar]