Abstract
It has been suggested that diet may influence the risk of melanoma, but few studies are available on this topic. We assessed the relation between food consumption and the risk of cutaneous melanoma in a Northern Italy population. We carried out a population-based case-control study involving 380 cases of melanoma and 719 age- and sex-matched controls. Dietary habits were established through a self-administered semi-quantitative food frequency questionnaire. We computed the odds ratios (ORs) of melanoma and the corresponding 95% confidence intervals (CIs) according to tertiles of daily intake of each food item, using multiple logistic regression models adjusted for major confounding factors. We observed an indication of a positive association between melanoma risk and consumption of cereals and cereal products (OR = 1.32; 95% CI 0.89–1.96, higher vs. lowest tertile), sweets (OR = 1.22; 95% CI 0.84–1.76), chocolate, candy bars. etc., (OR = 1.51; 95% CI 1.09–2.09) and cabbages (OR = 1.51; 95% CI 1.09–2.09). Conversely, an inverse association with disease risk was found for the intake of legumes (OR = 0.77; 95% CI 0.52–1.13), olive oil (OR = 0.77; 95% CI 0.51–1.16), eggs (OR = 0.58; 95% CI 0.41–0.82), and onion and garlic (OR = 0.80; 95% CI 0.52–1.14). No relationship was observed with beverage consumption. Our results suggest potentially adverse effects on melanoma risk of foods characterized by high contents of refined flours and sugars, while suggesting a protective role for eggs and two key components of the Mediterranean diet, legumes and olive oil. These associations warrant further investigation and, if confirmed, they might have important public health implications for the reduction of melanoma incidence through dietary modification.
Keywords: diet, food, melanoma, risk, case-control study, epidemiology
1. Introduction
Cutaneous malignant melanoma is the most serious type of skin cancer [1]. In Europe, its incidence has recently increased more rapidly than any other cancer, with an annual incidence of 13.5 new cases per 100,000 inhabitants in Northern and Western Europe [2]. The few established risk factors for melanoma include unconscionable and intermittent sun exposure, tanning bed use, severe sunburns, as well as phenotypical characteristics such as light skin, light eye color, red/blonde hair and low tanning ability [3].
In recent years, attention has been paid to the role of dietary habits and nutrient intake in melanoma risk [4,5,6,7,8,9]. Several epidemiological investigations have shown a trend towards reduced melanoma risk associated with a higher intake of beta-carotene and vitamins A, C, D, E [10,11,12]. In addition, healthy dietary patterns such as the Mediterranean diet, characterized by high consumption of vegetables, fruit, olive oil, moderate consumption of fish and wine, and low dairy and meat consumption, have been suggested to exert a protective effect [7,13]. In particular, vegetables, fruit, fish [14] and caffeine [15] showed a protective effect, while alcohol [16,17] as well as a diet rich in sugars/carbohydrates [18] and characterized by high glycemic load [6] may play detrimental roles.
In this study, we aimed at assessing the relation between consumption of principal food items and melanoma risk in a Northern Italy population, generally characterized by Mediterranean-like eating habits [7] and in which we have already investigated the effect of a number of dietary constituents on disease risk.
2. Methods
Details of our population-based case-control study on dietary risk factors of melanoma in the population of five provinces of the Emilia Romagna region in Northern Italy (more than 3 m residents), have been provided elsewhere [6,7]. Briefly, in the years 2005–2006, we attempted to recruit all patients with newly diagnosed melanoma residing in the provinces of Bologna, Ferrara, Modena, Parma and Reggio Emilia and attending the local dermatological clinics. Inclusion criteria were a histologically-confirmed diagnosis of melanoma without clinical evidence of metastasis. Overall, 572 eligible patients were contacted by their dermatologists to participate in the study, and 394 (69%) agreed to participate and completed the study questionnaires (see detail below). Six referents matched to each case for sex, year of birth (±5 years) and province of residence were randomly selected from the database of Emilia-Romagna within the National Health Service directory. An envelope containing the study questionnaires and a pre-paid return envelope was mailed to 2,825 potential controls and 747 (26%) agreed to participate in the study and returned the questionnaires. Fourteen cases and 28 controls were excluded from the subsequent analysis due to data incompleteness or extreme values derived from the food-frequency questionnaire (ratio of total energy intake and sex-specific basal metabolic rate calculated through Harris-Benedict formula <0.5th percentile or >99.5th percentile) [19]. Informed consent was obtained from all individual participants included in the study. This study was conducted according to the guidelines laid down in the Declaration of Helsinki. Written informed consent was obtained from all subjects/patients.
2.1. Dietary Assessment
Dietary habits during the year prior to enrolment were established using a self-administered semi-quantitative food frequency questionnaire, designed and validated to capture eating behaviors in Italy, and specifically developed as part of the European Prospective Investigation into Cancer and Nutrition (EPIC) study for the Northern Italy population [20,21]. Participants were asked to respond to 248 questions about 188 different food items, in order to assess frequency and quantity of daily consumption for each food item. Foods and beverages were categorized into major food groups and sub-groups based on the common EPIC-SOFT classification, as previously reported [22,23]. In addition, total energy and detailed nutrient intake were also calculated for each participant on the basis of the Italian food composition tables [21,24].
2.2. Additional Variables
Each participant provided information on place and date of birth, province of residence, educational level (≤5, 6–8, 9–13 or >13 years), marital status (married, unmarried/single, divorced or widowed), weight and height, phenotypic characteristics (eye, hair and skin color), sunburn history (never, first before or after 18 years of age) and skin sun reaction (speed of tan and tendency to burn). In detail, eye color was classified as follows: light (blue/green), light brown and dark (brown/black); hair color was classified as blond, red, light brown or dark brown/black at 20 years; skin color was classified as white, light brown, brown/olive or dark brown/ebony; skin sun reaction was classified as high tendency to burn and never tan, high tendency to burn and moderate tan, moderate tendency to burn and gradual tan, no tendency to burn and golden tan, no tendency to burn and intense tan [10,11]. Based on these categories, each subject was assigned to a phototype using the Fitzpatrick phototyping scale. We computed body mass index (BMI) as weight/height2 (kg/m2) and we categorized subjects as underweight (≤19 kg/m2), normal (20–24 kg/m2), overweight (25–29 kg/m2) and obese (≥30 kg/m2).
For each study participant, we computed the score for the a priori defined diet quality index, the Greek variant of Mediterranean diet Index (GMI). GMI scores (ranking 0 to 9) were formulated as Mediterranean diet scales [25], taking into account extensive evidence supporting the notion of beneficial effects of the Mediterranean diet in preventing cancer [26], including melanoma [7]. Additionally, we included the following factors as possible confounders: the daily average values for dietary glycemic load (GL) and dietary glycemic index (GI) [6].
2.3. Statistical Analysis
We adjusted the food item daily intake for total energy according to the Willet regression-residual method [27], and we categorized it into tertiles based on the distribution of residuals in the control group. Subjects who did not consume a food item were placed in the lowest tertile. We used multivariate conditional logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of melanoma for tertiles of intake, with the lowest tertile as a reference. Along with matching variables (sex, age and province of residence), we included the subsequent variables as possible confounders: phototype (four categories), sunburn history (three categories), education (four categories), BMI (four categories), and non-alcohol energy intake (continuous). Additional dietary factors shown to affect melanoma risk in the study population are included as additional confounding factors, namely vitamin C (continuous), vitamin D (continuous), GMI score (continuous) and dietary GL value (continuous) [28]. Tests of linear trend were performed according to 1-g or 10-g increments of daily intake. We also carried out stratified analysis by sex, age, phototype and quality of diet as potential effect modifiers. Finally, we modeled the relation between food intake and melanoma risk using restricted cubic splines, computed with the ‘mkspline’ and ‘xblc’ routines of the Stata-15.1 statistical package (Stata Corp., College Station, TX, USA, 2017) [29], by selecting the optimal number of knots according to Akaike’s information criterion (AIC) and using the knot placement method recommended by Harrell [30].
3. Results
A total of 380 patients (175 males and 205 females, mean age 58 ± 16 and 53 ± 15 years, respectively) and 719 referents matched for age, sex and province of residence were included in the analysis. Baseline characteristics, and median with interquartile range of daily intake are shown in Table 1; Table 2 for each food item, within case and control groups.
Table 1.
Baseline characteristics of study participants.
| Cases | Controls | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Participants | 380 | (34.6) | 719 | (65.4) |
| Sex | ||||
| Male | 175 | (46.1) | 319 | (44.4) |
| Female | 205 | (53.9) | 400 | (55.6) |
| Age (years) | ||||
| <50 | 146 | (38.4) | 272 | (37.8) |
| ≥50 | 234 | (61.6) | 447 | (62.2) |
| Education (years) | ||||
| ≤5 | 91 | (24.1) | 170 | (23.8) |
| 6–8 | 95 | (25.1) | 176 | (24.6) |
| 9–13 | 136 | (36.0) | 266 | (37.2) |
| ≥14 | 56 | (14.8) | 103 | (14.4) |
| Marital status | ||||
| Married | 257 | (67.6) | 493 | (68.7) |
| Unmarried/single | 68 | (17.9) | 103 | (14.3) |
| Divorced | 23 | (6.0) | 48 | (6.6) |
| Widowed | 31 | (8.2) | 74 | (10.3) |
| Unknown | 1 | (0.3) | 1 | (0.1) |
| Body mass index (kg/m2) | ||||
| ≤19 | 31 | (8.2) | 45 | (6.3) |
| 20–24 | 164 | (43.3) | 306 | (42.5) |
| 25–29 | 133 | (35.0) | 287 | (39.9) |
| ≥30 | 52 | (13.7) | 81 | (11.3) |
| Phototype a | ||||
| I | 105 | (27.6) | 109 | (15.2) |
| II | 136 | (35.8) | 238 | (33.1) |
| III | 122 | (32.1) | 312 | (43.4) |
| IV | 17 | (4.5) | 60 | (8.3) |
| Sunburn history | ||||
| Never | 182 | (47.9) | 452 | (62.9) |
| Before 18 years | 108 | (28.4) | 164 | (22.8) |
| After 18 years | 90 | (23.7) | 103 | (14.3) |
| Greek Mediterranean index | ||||
| ≤2 | 61 | (16.1) | 102 | (14.2) |
| 3–4 | 140 | (36.8) | 250 | (34.8) |
| 5–6 | 128 | (33.7) | 282 | (39.2) |
| ≥7 | 51 | (13.4) | 85 | (11.8) |
| Glycemic load | ||||
| 50th (IQR) | 118.1 | (89.7–150.1) | 112.7 | (87.0–148.1) |
| Vitamin C (mg/day) | ||||
| 50th (IQR) | 113.7 | (78.0–154.1) | 121.0 | (83.66–162.6) |
| Vitamin D (mg/day) | ||||
| 50th (IQR) | 2.3 | (1.7–3.1) | 2.4 | (1.8–3.4) |
| Energy (kcal/day) | ||||
| 50th (IQR) | 1.928 | (1490–2443) | 1.906 | (1538–2365) |
a Phototype I, eyes/hair/skin light, high tendency to burn and never/moderate tan; Phototype II, eyes/hair/skin light, moderate tendency to burn and gradual tan or eyes/hair/skin brown, high tendency to burn and moderate tan; Phototype III, eyes/hair/skin brown, moderate/no tendency to burn and gradual/golden tan; Phototype IV, no tendency to burn and intense tan.
Table 2.
Daily intake (g/day) of different food items or categories for cases and controls. Median (50th) and interquartile range (IQR) values calculated only in consumers.
| Food Items | Cases | Controls | ||||
|---|---|---|---|---|---|---|
| % Non Consumers | 50th | IQR | % Non Consumers | 50th | IQR | |
| Cereals and cereal products | 0.0 | 162.6 | 119.2–201.9 | 0.0 | 147.8 | 110.5–190.4 |
| Pasta, other grain | 0.5 | 56.0 | 37.8–80.0 | 1.3 | 55.9 | 37.1–76.9 |
| Rice | 17.9 | 3.9 | 2.4–6.9 | 20.4 | 3.8 | 1.9–6.3 |
| Bread | 5.5 | 86.4 | 45.6–124.0 | 8.1 | 76.4 | 44.4–113.7 |
| Crackers, crispbread, salty snacks | 9.5 | 4.6 | 2.2–17.4 | 10.4 | 4.5 | 2.1–16.3 |
| Meat and meat products | 0.0 | 117.3 | 88.7–157.6 | 0.1 | 126.2 | 90.6–161.5 |
| Red meat | 1.1 | 61.3 | 37.8–87.2 | 1.8 | 64.5 | 40.4–89.9 |
| White meat | 4.2 | 22.2 | 13.1–42.1 | 4.7 | 24.5 | 13.4–42.4 |
| Processed meat | 0.5 | 27.5 | 18.1–39.7 | 0.3 | 26.4 | 16.9–39.9 |
| Offal | 59.5 | 0.7 | 0.3–1.9 | 56.2 | 0.7 | 0.3–2.5 |
| Milk and dairy products | 0.0 | 176.9 | 100.0–298.6 | 0.0 | 209.9 | 111.3–306.9 |
| Milk | 35.3 | 110.8 | 30.3–224.3 | 32.7 | 134.4 | 36.5–223.4 |
| Yogurt | 36.3 | 27.4 | 13.8–70.6 | 33.8 | 41.2 | 13.5–108.3 |
| Cheeses (including fresh cheeses) | 0.0 | 42.4 | 27.9–58.0 | 0.8 | 38.4 | 27.1–54.9 |
| Eggs | 0.8 | 11.4 | 7.1–17.1 | 2.1 | 13.5 | 7.9–20.1 |
| Fish and seafood | 2.4 | 28.8 | 15.9–42.7 | 2.1 | 29.9 | 17.7–45.2 |
| Fish | 2.6 | 22.7 | 12.8–34.9 | 2.5 | 23.4 | 13.8–35.9 |
| Crustaceans and molluscs | 22.6 | 3.9 | 1.8–9.7 | 21.8 | 4.6 | 1.8–11.4 |
| Vegetables | 0.0 | 136.7 | 98.8–197.5 | 0.0 | 147.4 | 105.1–204.0 |
| Leafy vegetables | 0.5 | 23.7 | 12.3–43.5 | 0.1 | 25.5 | 14.6–44.2 |
| Other vegetables | 0.8 | 19.4 | 10.8–29.8 | 0.8 | 20.5 | 11.0–34.2 |
| Tomatoes | 0.8 | 53.3 | 31.8–85.4 | 0.7 | 54.3 | 33.5–86.5 |
| Root vegetables | 11.8 | 8.2 | 3.9–18.1 | 12.0 | 8.8 | 3.8–20.9 |
| Cabbages | 21.1 | 2.5 | 0.9–7.2 | 26.0 | 2.2 | 0.7–6.3 |
| Mushrooms | 22.4 | 1.6 | 0.8–3.3 | 20.7 | 1.7 | 0.8–3.8 |
| Onion and garlic | 0.5 | 10.8 | 6.4–20.5 | 0.3 | 12.6 | 7.3–21.9 |
| Legumes | 4.5 | 13.9 | 7.82–22.4 | 3.6 | 15.5 | 8.3–24.1 |
| Potatoes | 1.3 | 19.3 | 12.0–31.6 | 1.4 | 20.4 | 12.5–32.7 |
| Fresh fruit | 0.3 | 244.7 | 167.8–357.5 | 0.8 | 257.1 | 174.4–361.8 |
| Citrus fruits | 4.2 | 53.7 | 30.0–81.2 | 3.8 | 59.7 | 31.7–85.9 |
| All other fruits | 0.3 | 191.2 | 126.8–286.3 | 0.8 | 199.5 | 130.5–279.5 |
| Dried fruit, nuts and seeds | 10.0 | 0.5 | 0.3–1.63 | 11.0 | 0.5 | 0.3–1.8 |
| Sweets | 1.3 | 84.6 | 56.7–113.1 | 1.9 | 80.0 | 55.3–113.5 |
| Chocolate, candy bars, etc. | 25.0 | 4.4 | 2.6–8.5 | 31.4 | 3.6 | 2.1–7.4 |
| Sugar, honey, jam, confectionery | 13.2 | 14.9 | 7.2–27.61 | 12.2 | 14.5 | 7.8–27.6 |
| Ice-cream | 11.3 | 11.7 | 6.4–20.4 | 12.5 | 10.8 | 5.7–19.6 |
| Cakes, pies and pastries | 16.6 | 30.5 | 17.9–54.4 | 20.2 | 30.4 | 16.5–56.2 |
| Biscuits, dry cakes | 22.6 | 12.2 | 3.7–27.9 | 28.8 | 10.1 | 3.2–24.5 |
| Oils and fats | 0.0 | 25.2 | 19.2–32.4 | 0.1 | 26.3 | 20.6–33.2 |
| Vegetable fats and non-olive oils | 26.8 | 0.7 | 0.4–1.4 | 19.9 | 0.8 | 0.4–1.8 |
| Olive oil | 2.6 | 20.3 | 13.2–28.0 | 1.3 | 21.2 | 15.3–28.5 |
| Butter and other animal fats | 8.7 | 2.3 | 1.3–4.2 | 5.3 | 2.4 | 1.3–3.9 |
| Coffee | 8.2 | 79.1 | 49.7–116.6 | 9.9 | 83.9 | 49.3–123.2 |
| Tea | 39.7 | 21.8 | 2.7–86.5 | 47.3 | 10.6 | 1.7–65.4 |
| Red wine | 34.2 | 42.3 | 22.3–135.7 | 32.7 | 40.6 | 18.5–134.1 |
| White wine | 38.2 | 20.5 | 11.0–60.6 | 37.8 | 19.6 | 9.6–71.9 |
| Aperitif wines and beers | 31.6 | 26.1 | 13.3–41.2 | 30.2 | 26.0 | 13.8–50.7 |
| Spirits and liqueurs | 56.3 | 1.4 | 0.7–2.3 | 57.7 | 1.3 | 0.7–2.2 |
| Fruit juices | 38.7 | 45.9 | 25.7–107.3 | 39.6 | 43.5 | 21.8–102.0 |
| Soft drinks | 57.4 | 30.7 | 15.3–55.6 | 55.8 | 30.4 | 15.2–67.4 |
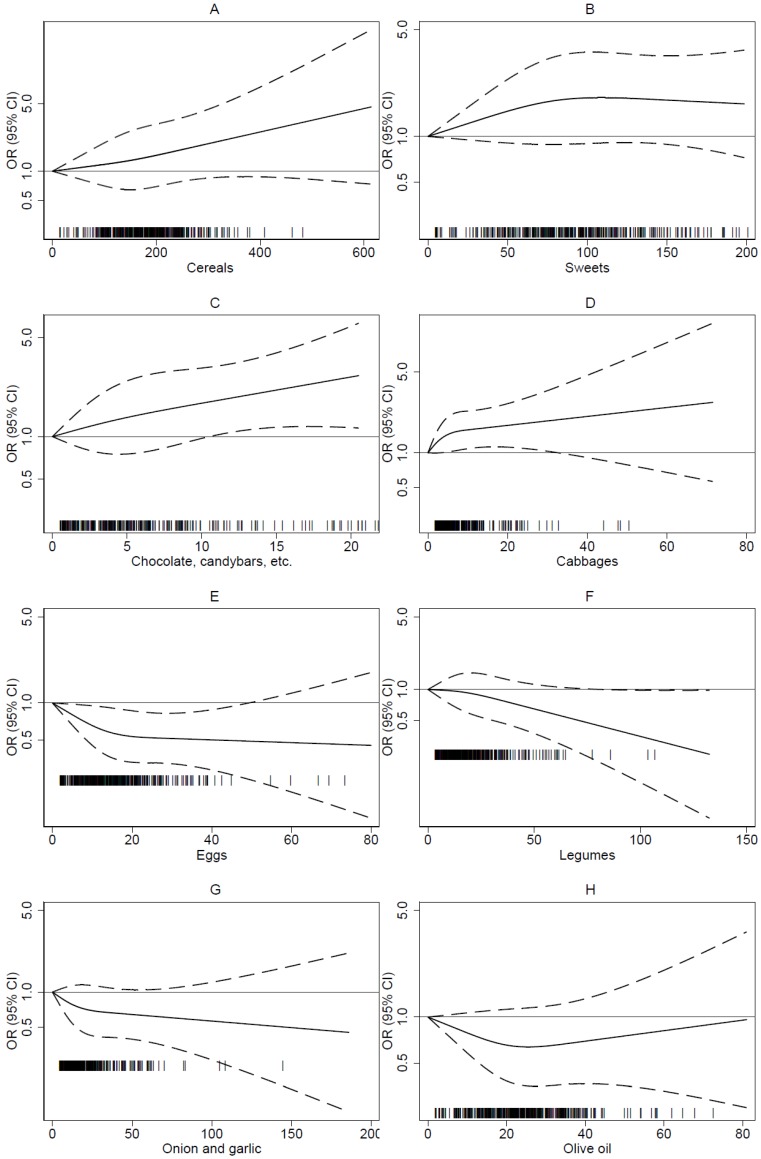
Table 3 provides ORs and 95% CIs for developing melanoma according to tertiles of daily intake for each food item, and controlling for potential confounders. In the fully adjusted model, we observed a direct association for higher consumers of the subsequent food categories: ‘Cereals and cereal products’ (OR = 1.32, 95% CI 0.89–1.96 higher vs. lowest tertile and OR = 1.02, 95% CI 1.00–1.05 linear trend), ‘Sweets’ (OR = 1.22, 95% CI 0.84–1.76 higher vs. lowest tertile and OR = 1.01, 95% CI 0.98–1.04 linear trend), particularly ‘Chocolate, candy bars etc.’ (OR = 1.51, 95% CI 1.09–2.09 higher vs. lowest tertile and OR = 1.05, 95% CI 0.88–1.26 linear trend) and ‘Cabbages’ (OR = 1.51, 95% CI 1.09–2.09 higher vs. lowest tertile and OR = 1.02, 95% CI 1.00–1.04 linear trend). Conversely, an inverse correlation with melanoma risk was observed for high consumption of the following food items: ‘Legumes’ (OR = 0.77, 95% CI 0.52–1.13 higher vs. lowest tertile and OR = 0.91, 95% CI 0.82–1.00 linear trend), ‘Olive oil’ (OR = 0.77, 95% CI 0.51–1.16 higher vs. lowest tertile and OR = 0.95, 95% CI 0.81–1.10 linear trend), ‘Eggs’ (OR = 0.58, 95% CI 0.41–0.82 higher vs. lowest tertile and OR = 0.98, 95% CI 0.73–0.97 linear trend), and ‘Onion and garlic’ (OR = 0.80, 95% CI 0.52–1.14 higher vs. lowest tertile and OR = 0.94, 95% CI 0.87–1.02 linear trend). No clear association emerged for other food and beverage categories. No substantial difference was detected between partially and fully adjusted model. Graphical plots of the aforementioned associations, obtained using regression spline analysis, are depicted in Figure 1. These show a substantially linear trend in all selected categories, except for risk going toward the null at a very high intake (>60 g/day) of olive oil.
Table 3.
Overall odds ratios (OR) and 95% confidence intervals (CI) for developing cutaneous malignant melanoma according to tertiles of daily intake (residual of regression on energy), obtained from conditional multiple logistic regression models. Linear trend for 10-g increments of daily intake.
| Food Items | Cases/Controls | Median | OR a | (95% CI) | OR b | (95% CI) |
|---|---|---|---|---|---|---|
| Cereals and cereal products | ||||||
| 1st tertile (ref.) | 111/249 | 92.1 | 1.00 | - | 1.00 | - |
| 2nd tertile | 105/235 | 147.9 | 0.90 | (0.64–1.27) | 0.84 | (0.58–1.21) |
| 3nd tertile | 164/235 | 215.0 | 1.57 | (1.14–2.17) | 1.32 | (0.89–1.96) |
| Linear trend | 1.03 | (1.01–1.05) | 1.02 | (1.00–1.05) | ||
| Pasta, other grain | ||||||
| 1st tertile (ref.) | 129/259 | 30.5 | 1.00 | - | 1.00 | - |
| 2nd tertile | 123/230 | 55.5 | 1.13 | (0.81–1.56) | 1.06 | (0.76–1.48) |
| 3nd tertile | 128/230 | 90.2 | 1.33 | (0.95–1.85) | 1.15 | (0.82–1.63) |
| Linear trend | 1.04 | (1.00–1.08) | 1.02 | (0.98–1.06) | ||
| Rice | ||||||
| 1st tertile (ref.) | 133/273 | 1.2 | 1.00 | - | 1.00 | - |
| 2nd tertile | 122/223 | 3.8 | 1.14 | (0.83–1.56) | 1.11 | (0.80–1.54) |
| 3nd tertile | 125/223 | 9.7 | 1.20 | (0.88–1.64) | 1.20 | (0.88–1.65) |
| Linear trend | 1.01 | (0.84–1.21) | 1.02 | (0.85–1.22) | ||
| Bread | ||||||
| 1st tertile (ref.) | 131/273 | 35.0 | 1.00 | - | 1.00 | - |
| 2nd tertile | 101/223 | 76.7 | 0.89 | (0.63–1.26) | 0.89 | (0.62–1.27) |
| 3nd tertile | 148/223 | 133.2 | 1.33 | (0.97–1.82) | 1.21 | (0.83–1.77) |
| Linear trend | 1.02 | (1.00–1.04) | 1.01 | (0.99–1.04) | ||
| Crackers, crispbread, salty snacks | ||||||
| 1st tertile (ref.) | 137/296 | 1.5 | 1.00 | - | 1.00 | - |
| 2nd tertile | 127/212 | 4.5 | 1.44 | (1.04–2.01) | 1.37 | (0.98–1.92) |
| 3nd tertile | 116/211 | 22.7 | 1.20 | (0.87–1.65) | 1.11 | (0.79–1.55) |
| Linear trend | 1.04 | (0.95–1.14) | 1.01 | (0.92–1.12) | ||
| Meat and meat products | ||||||
| 1st tertile (ref.) | 151/247 | 76.7 | 1.00 | - | 1.00 | - |
| 2nd tertile | 117/236 | 125.9 | 0.85 | (0.61–1.19) | 0.83 | (0.58–1.19) |
| 3nd tertile | 112/236 | 177.6 | 0.80 | (0.57–1.12) | 0.77 | (0.53–1.14) |
| Linear trend | 0.99 | (0.97–1.02) | 1.00 | (0.97–1.03) | ||
| Red meat | ||||||
| 1st tertile (ref.) | 150/253 | 32.9 | 1.00 | - | 1.00 | - |
| 2nd tertile | 116/233 | 64.5 | 0.91 | (0.66–1.26) | 0.90 | (0.64–1.26) |
| 3nd tertile | 114/233 | 104.9 | 0.90 | (0.65–1.24) | 0.87 | (0.60–1.25) |
| Linear trend | 1.00 | (0.96–1.03) | 1.00 | (0.96–1.04) | ||
| White meat | ||||||
| 1st tertile (ref.) | 144/258 | 10.2 | 1.00 | - | 1.00 | - |
| 2nd tertile | 112/231 | 23.8 | 0.88 | (0.64–1.22) | 0.91 | (0.65–1.26) |
| 3nd tertile | 124/230 | 51.4 | 0.97 | (0.70–1.33) | 1.09 | (0.78–1.52) |
| Linear trend | 0.99 | (0.94–1.04) | 1.01 | (0.95–1.06) | ||
| Processed meat | ||||||
| 1st tertile (ref.) | 130/257 | 13.6 | 1.00 | - | 1.00 | - |
| 2nd tertile | 131/231 | 26.7 | 1.19 | (0.85–1.67) | 1.11 | (0.79–1.57) |
| 3nd tertile | 119/231 | 46.5 | 1.03 | (0.74–1.44) | 1.01 | (0.70–1.44) |
| Linear trend | 1.00 | (0.93–1.06) | 0.99 | (0.92–1.06) | ||
| Offal | ||||||
| 1st tertile (ref.) | 192/354 | 0.2 | 1.00 | - | 1.00 | - |
| 2nd tertile | 106/183 | 0.7 | 1.07 | (0.76–1.51) | 1.10 | (0.77–1.55) |
| 3nd tertile | 82/182 | 4.0 | 0.89 | (0.64–1.25) | 0.98 | (0.70–1.38) |
| Linear trend c | 1.00 | (0.97–1.03) | 1.01 | (0.98–1.04) | ||
| Milk and dairy products | ||||||
| 1st tertile (ref.) | 170/259 | 79.9 | 1.00 | - | 1.00 | - |
| 2nd tertile | 104/230 | 210.4 | 0.65 | (0.47–0.90) | 0.67 | (0.48–0.92) |
| 3nd tertile | 106/230 | 365.8 | 0.70 | (0.50–0.97) | 0.72 | (0.51–1.01) |
| Linear trend | 1.00 | (0.99–1.00) | 1.00 | (0.99–1.00) | ||
| Milk | ||||||
| 1st tertile (ref.) | 188/313 | 23.8 | 1.00 | - | 1.00 | - |
| 2nd tertile | 82/203 | 131.5 | 0.68 | (0.48–0.96) | 0.71 | (0.50–1.01) |
| 3nd tertile | 110/203 | 256.8 | 0.93 | (0.68–1.29) | 0.96 | (0.69–1.33) |
| Linear trend | 1.00 | (0.99–1.01) | 1.00 | (0.99–1.01) | ||
| Yogurt | ||||||
| 1st tertile (ref.) | 174/318 | 9.0 | 1.00 | - | 1.00 | - |
| 2nd tertile | 133/201 | 36.8 | 1.21 | (0.88–1.66) | 1.28 | (0.93–1.77) |
| 3nd tertile | 73/200 | 128.1 | 0.69 | (0.48–0.98) | 0.76 | (0.53–1.09) |
| Linear trend | 0.98 | (0.97–1.00) | 0.99 | (0.97–1.00) | ||
| Cheeses (including fresh cheeses) | ||||||
| 1st tertile (ref.) | 125/259 | 20.9 | 1.00 | - | 1.00 | - |
| 2nd tertile | 118/230 | 38.7 | 1.03 | (0.74–1.45) | 1.01 | (0.71–1.45) |
| 3nd tertile | 137/230 | 65.0 | 1.11 | (0.80–1.54) | 1.17 | (0.81–1.70) |
| Linear trend | 1.03 | (0.98–1.07) | 1.03 | (0.98–1.09) | ||
| Eggs | ||||||
| 1st tertile (ref.) | 176/249 | 6.2 | 1.00 | - | 1.00 | - |
| 2nd tertile | 120/235 | 13.5 | 0.71 | (0.52–0.96) | 0.73 | (0.53–0.99) |
| 3nd tertile | 84/235 | 23.4 | 0.53 | (0.38–0.74) | 0.58 | (0.41–0.82) |
| Linear trend c | 0.98 | (0.97–0.99) | 0.98 | (0.97–1.00) | ||
| Fish and seafood | ||||||
| 1st tertile (ref.) | 143/250 | 13.6 | 1.00 | - | 1.00 | - |
| 2nd tertile | 123/235 | 30.0 | 0.88 | (0.64–1.20) | 1.01 | (0.72–1.43) |
| 3nd tertile | 114/234 | 54.6 | 0.77 | (0.56–1.07) | 1.11 | (0.73–1.70) |
| Linear trend | 0.95 | (0.90–1.00) | 1.01 | (0.94–1.08) | ||
| Fish | ||||||
| 1st tertile (ref.) | 141/251 | 10.7 | 1.00 | - | 1.00 | - |
| 2nd tertile | 124/234 | 23.4 | 0.90 | (0.66–1.23) | 1.08 | (0.77–1.51) |
| 3nd tertile | 115/234 | 43.9 | 0.81 | (0.58–1.11) | 1.24 | (0.80–1.92) |
| Linear trend | 0.94 | (0.88–1.01) | 1.04 | (0.94–1.14) | ||
| Crustaceans and molluscs | ||||||
| 1st tertile (ref.) | 162/290 | 1.3 | 1.00 | - | 1.00 | - |
| 2nd tertile | 117/215 | 4.6 | 1.02 | (0.74–1.42) | 1.08 | (0.77–1.50) |
| 3nd tertile | 101/214 | 14.9 | 0.85 | (0.62–1.18) | 0.97 | (0.69–1.37) |
| Linear trend c | 0.99 | (0.97–1.00) | 0.99 | (0.98–1.01) | ||
| Vegetables | ||||||
| 1st tertile (ref.) | 148/243 | 87.3 | 1.00 | - | 1.00 | - |
| 2nd tertile | 111/238 | 147.0 | 0.74 | (0.54–1.02) | 0.80 | (0.57–1.14) |
| 3nd tertile | 121/238 | 235.3 | 0.81 | (0.58–1.12) | 0.95 | (0.63–1.43) |
| Linear trend | 0.99 | (0.97–1.00) | 1.00 | (0.98–1.02) | ||
| Leafy vegetables | ||||||
| 1st tertile (ref.) | 144/248 | 10.6 | 1.00 | - | 1.00 | - |
| 2nd tertile | 121/236 | 25.2 | 0.80 | (0.58–1.10) | 0.85 | (0.60–1.21) |
| 3nd tertile | 115/235 | 54.5 | 0.80 | (0.57–1.11) | 0.94 | (0.64–1.37) |
| Linear trend | 0.98 | (0.93–1.04) | 1.02 | (0.96–1.09) | ||
| Other vegetables | ||||||
| 1st tertile (ref.) | 137/250 | 8.4 | 1.00 | - | 1.00 | - |
| 2nd tertile | 141/235 | 20.5 | 1.12 | (0.81–1.55) | 1.24 | (0.88–1.73) |
| 3nd tertile | 102/234 | 40.6 | 0.81 | (0.58–1.13) | 0.96 | (0.66–1.39) |
| Linear trend | 0.95 | (0.88–1.02) | 0.98 | (0.90–1.07) | ||
| Tomatoes | ||||||
| 1st tertile (ref.) | 131/255 | 25.1 | 1.00 | - | 1.00 | - |
| 2nd tertile | 130/232 | 54.1 | 1.08 | (0.78–1.48) | 1.13 | (0.81–1.57) |
| 3nd tertile | 119/232 | 103.1 | 0.96 | (0.70–1.32) | 1.02 | (0.72–1.46) |
| Linear trend | 0.99 | (0.97–1.02) | 1.00 | (0.97–1.03) | ||
| Root vegetables | ||||||
| 1st tertile (ref.) | 145/284 | 2.6 | 1.00 | - | 1.00 | - |
| 2nd tertile | 135/218 | 8.6 | 1.23 | (0.88–1.70) | 1.31 | (0.93–1.83) |
| 3nd tertile | 100/217 | 28.9 | 0.82 | (0.58–1.16) | 0.90 | (0.62–1.32) |
| Linear trend c | 1.00 | (0.99–1.00) | 1.00 | (0.99–1.01) | ||
| Cabbages | ||||||
| 1st tertile (ref.) | 140/297 | 0.4 | 1.00 | - | 1.00 | - |
| 2nd tertile | 120/211 | 2.3 | 1.22 | (0.89–1.67) | 1.38 | (1.00–1.92) |
| 3nd tertile | 120/211 | 8.9 | 1.25 | (0.91–1.73) | 1.52 | (1.08–2.14) |
| Linear trend c | 1.01 | (0.99–1.03) | 1.02 | (1.00–1.04) | ||
| Mushrooms | ||||||
| 1st tertile (ref.) | 151/291 | 0.6 | 1.00 | - | 1.00 | - |
| 2nd tertile | 130/214 | 1.6 | 1.25 | (0.90–1.73) | 1.34 | (0.96–1.86) |
| 3nd tertile | 99/214 | 4.7 | 0.90 | (0.64–1.25) | 1.09 | (0.76–1.55) |
| Linear trend c | 0.97 | (0.93–1.01) | 0.99 | (0.95–1.03) | ||
| Onion and garlic | ||||||
| 1st tertile (ref.) | 176/270 | 5.7 | 1.00 | - | 1.00 | - |
| 2nd tertile | 98/225 | 12.5 | 0.67 | (0.48–0.94) | 0.70 | (0.50–0.98) |
| 3nd tertile | 106/224 | 27.9 | 0.71 | (0.51–1.00) | 0.80 | (0.56–1.14) |
| Linear trend | 0.92 | (0.86–0.99) | 0.94 | (0.87–1.02) | ||
| Legumes | ||||||
| 1st tertile (ref.) | 148/256 | 6.0 | 1.00 | - | 1.00 | - |
| 2nd tertile | 130/232 | 15.4 | 0.97 | (0.71–1.34) | 1.01 | (0.73–1.41) |
| 3nd tertile | 102/231 | 29.6 | 0.71 | (0.50–1.00) | 0.77 | (0.52–1.13) |
| Linear trend | 0.90 | (0.82–0.98) | 0.91 | (0.82–1.00) | ||
| Potatoes | ||||||
| 1st tertile (ref.) | 142/262 | 9.5 | 1.00 | - | 1.00 | - |
| 2nd tertile | 134/229 | 20.1 | 1.11 | (0.80–1.53) | 1.14 | (0.82–1.58) |
| 3nd tertile | 104/228 | 41.6 | 0.81 | (0.58–1.12) | 0.83 | (0.60–1.16) |
| Linear trend | 0.98 | (0.92–1.04) | 0.99 | (0.93–1.05) | ||
| Fresh fruit | ||||||
| 1st tertile (ref.) | 138/245 | 137.9 | 1.00 | - | 1.00 | - |
| 2nd tertile | 125/237 | 255.0 | 0.88 | (0.63–1.22) | 0.98 | (0.69–1.39) |
| 3nd tertile | 117/237 | 411.6 | 0.83 | (0.60–1.16) | 1.09 | (0.72–1.66) |
| Linear trend | 0.99 | (0.98–1.00) | 1.00 | (0.99–1.01) | ||
| Citrus fruits | ||||||
| 1st tertile (ref.) | 150/255 | 19.5 | 1.00 | - | 1.00 | - |
| 2nd tertile | 122/232 | 58.7 | 0.91 | (0.66–1.26) | 0.97 | (0.70–1.36) |
| 3nd tertile | 108/232 | 104.5 | 0.77 | (0.55–1.07) | 0.93 | (0.64–1.37) |
| Linear trend | 0.99 | (0.96–1.01) | 1.01 | (0.97–1.04) | ||
| All other fruits | ||||||
| 1st tertile (ref.) | 140/245 | 103.1 | 1.00 | - | 1.00 | - |
| 2nd tertile | 112/237 | 198.4 | 0.81 | (0.58–1.14) | 0.90 | (0.64–1.27) |
| 3nd tertile | 128/237 | 319.7 | 0.88 | (0.63–1.23) | 1.13 | (0.76–1.69) |
| Linear trend | 0.99 | (0.98–1.00) | 1.00 | (0.99–1.01) | ||
| Dried fruit, nuts and seeds | ||||||
| 1st tertile (ref.) | 145/277 | 0.2 | 1.00 | - | 1.00 | - |
| 2nd tertile | 123/221 | 0.5 | 1.16 | (0.83–1.62) | 1.21 | (0.86–1.70) |
| 3nd tertile | 112/221 | 3.2 | 1.00 | (0.72–1.38) | 1.08 | (0.77–1.52) |
| Linear trend c | 1.01 | (0.97–1.04) | 1.01 | (0.98–1.05) | ||
| Sweets | ||||||
| 1st tertile (ref.) | 128/258 | 42.3 | 1.00 | - | 1.00 | - |
| 2nd tertile | 124/231 | 81.2 | 1.20 | (0.85–1.70) | 1.19 | (0.84–1.69) |
| 3nd tertile | 128/230 | 130.7 | 1.21 | (0.86–1.69) | 1.22 | (0.84–1.76) |
| Linear trend | 1.01 | (0.98–1.03) | 1.01 | (0.98–1.04) | ||
| Chocolate, candy bars, etc. | ||||||
| 1st tertile (ref.) | 133/321 | 1.5 | 1.00 | - | 1.00 | - |
| 2nd tertile | 121/199 | 3.7 | 1.54 | (1.08–2.17) | 1.50 | (1.05–2.13) |
| 3nd tertile | 126/199 | 10.5 | 1.55 | (1.12–2.13) | 1.51 | (1.09–2.09) |
| Linear trend | 1.06 | (0.89–1.26) | 1.05 | (0.88–1.26) | ||
| Sugar, honey, jam, confectionery | ||||||
| 1st tertile (ref.) | 155/277 | 5.8 | 1.00 | - | 1.00 | - |
| 2nd tertile | 112/221 | 14.8 | 0.98 | (0.70–1.36) | 1.00 | (0.71–1.41) |
| 3nd tertile | 113/221 | 34.0 | 0.88 | (0.63–1.23) | 0.82 | (0.58–1.16) |
| Linear trend | 1.00 | (0.94–1.07) | 0.98 | (0.92–1.05) | ||
| Ice-cream | ||||||
| 1st tertile (ref.) | 139/275 | 4.3 | 1.00 | - | 1.00 | - |
| 2nd tertile | 123/222 | 11.0 | 1.23 | (0.89–1.70) | 1.24 | (0.89–1.71) |
| 3nd tertile | 118/222 | 25.7 | 1.08 | (0.77–1.51) | 1.12 | (0.79–1.58) |
| Linear trend | 1.03 | (0.94–1.13) | 1.04 | (0.94–1.14) | ||
| Cakes, pies and pastries | ||||||
| 1st tertile (ref.) | 152/309 | 12.0 | 1.00 | - | 1.00 | - |
| 2nd tertile | 116/205 | 30.3 | 1.18 | (0.84–1.67) | 1.23 | (0.87–1.74) |
| 3nd tertile | 112/205 | 70.8 | 1.14 | (0.83–1.57) | 1.26 | (0.89–1.78) |
| Linear trend | 1.00 | (0.97–1.03) | 1.01 | (0.98–1.05) | ||
| Biscuits, dry cakes | ||||||
| 1st tertile (ref.) | 147/300 | 2.3 | 1.00 | - | 1.00 | - |
| 2nd tertile | 112/210 | 10.1 | 1.09 | (0.78–1.51) | 1.10 | (0.79–1.53) |
| 3nd tertile | 121/209 | 31.8 | 1.22 | (0.89–1.68) | 1.14 | (0.82–1.58) |
| Linear trend | 1.04 | (0.96–1.13) | 1.02 | (0.95–1.11) | ||
| Oils and fats | ||||||
| 1st tertile (ref.) | 142/245 | 17.7 | 1.00 | - | 1.00 | - |
| 2nd tertile | 124/237 | 26.3 | 0.83 | (0.60–1.14) | 0.85 | (0.61–1.20) |
| 3nd tertile | 114/237 | 36.8 | 0.81 | (0.59–1.13) | 0.89 | (0.59–1.34) |
| Linear trend | 0.92 | (0.82–1.04) | 0.95 | (0.81–1.13) | ||
| Vegetable fats and non-olive oils | ||||||
| 1st tertile (ref.) | 180/322 | 0.3 | 1.00 | - | 1.00 | - |
| 2nd tertile | 119/199 | 0.8 | 1.09 | (0.77–1.54) | 1.10 | (0.77–1.57) |
| 3nd tertile | 81/198 | 6.0 | 0.75 | (0.52–1.06) | 0.72 | (0.50–1.04) |
| Linear trend c | 1.00 | (0.98–1.02) | 1.00 | (0.98–1.03) | ||
| Olive oil | ||||||
| 1st tertile (ref.) | 147/248 | 12.1 | 1.00 | - | 1.00 | - |
| 2nd tertile | 121/236 | 21.3 | 0.81 | (0.59–1.11) | 0.81 | (0.58–1.14) |
| 3nd tertile | 112/235 | 32.4 | 0.74 | (0.53–1.03) | 0.77 | (0.51–1.16) |
| Linear trend | 0.92 | (0.82–1.03) | 0.95 | (0.81–1.10) | ||
| Butter and other animal fats | ||||||
| 1st tertile (ref.) | 164/282 | 0.9 | 1.00 | - | 1.00 | - |
| 2nd tertile | 99/219 | 2.3 | 0.87 | (0.63–1.21) | 0.87 | (0.62–1.21) |
| 3nd tertile | 117/218 | 4.8 | 0.94 | (0.69–1.29) | 0.91 | (0.64–1.28) |
| Linear trend c | 1.00 | (0.96–1.04) | 1.00 | (0.96–1.05) | ||
| Coffee | ||||||
| 1st tertile (ref.) | 138/264 | 36.4 | 1.00 | - | 1.00 | - |
| 2nd tertile | 138/228 | 83.5 | 1.20 | (0.88–1.63) | 1.22 | (0.89–1.67) |
| 3nd tertile | 104/227 | 149.0 | 0.90 | (0.65–1.24) | 0.89 | (0.64–1.24) |
| Linear trend | 0.99 | (0.98–1.01) | 0.99 | (0.98–1.01) | ||
| Tea | ||||||
| 1st tertile (ref.) | 143/321 | 1.1 | 1.00 | - | 1.00 | - |
| 2nd tertile | 116/199 | 12.3 | 1.31 | (0.95–1.80) | 1.32 | (0.95–1.83) |
| 3nd tertile | 121/199 | 148.8 | 1.32 | (0.96–1.81) | 1.32 | (0.96–1.82) |
| Linear trend | 1.00 | (0.98–1.01) | 1.00 | (0.98–1.01) | ||
| Red wine | ||||||
| 1st tertile (ref.) | 168/323 | 13.0 | 1.00 | - | 1.00 | - |
| 2nd tertile | 103/198 | 39.5 | 1.09 | (0.76–1.57) | 1.18 | (0.81–1.71) |
| 3nd tertile | 109/198 | 206.7 | 1.13 | (0.79–1.61) | 1.17 | (0.80–1.69) |
| Linear trend | 1.00 | (0.99–1.01) | 1.00 | (0.99–1.01) | ||
| White wine | ||||||
| 1st tertile (ref.) | 165/333 | 6.4 | 1.00 | - | 1.00 | - |
| 2nd tertile | 123/193 | 19.7 | 1.44 | (1.01–2.06) | 1.38 | (0.95–1.99) |
| 3nd tertile | 92/193 | 128.1 | 1.03 | (0.73–1.45) | 1.05 | (0.73–1.49) |
| Linear trend | 0.99 | (0.98–1.01) | 0.99 | (0.98–1.01) | ||
| Aperitif wines and beers | ||||||
| 1st tertile (ref.) | 187/347 | 10.3 | 1.00 | - | 1.00 | - |
| 2nd tertile | 103/186 | 26.2 | 0.94 | (0.66–1.36) | 0.93 | (0.64–1.34) |
| 3nd tertile | 90/186 | 72.0 | 0.83 | (0.58–1.19) | 0.81 | (0.56–1.18) |
| Linear trend | 0.99 | (0.97–1.00) | 0.99 | (0.97–1.00) | ||
| Spirits and liqueurs | ||||||
| 1st tertile (ref.) | 204/372 | 0.5 | 1.00 | - | 1.00 | - |
| 2nd tertile | 91/174 | 1.4 | 0.93 | (0.64–1.36) | 0.93 | (0.63–1.36) |
| 3nd tertile | 85/173 | 4.5 | 0.92 | (0.63–1.35) | 0.94 | (0.63–1.39) |
| Linear trend c | 0.99 | (0.98–1.01) | 0.93 | (0.79–1.09) | ||
| Fruit juices | ||||||
| 1st tertile (ref.) | 172/331 | 15.6 | 1.00 | - | 1.00 | - |
| 2nd tertile | 101/194 | 42.9 | 1.04 | (0.73–1.48) | 1.11 | (0.77–1.59) |
| 3nd tertile | 107/194 | 136.1 | 1.07 | (0.77–1.49) | 1.29 | (0.90–1.86) |
| Linear trend | 0.99 | (0.98–1.01) | 0.99 | (0.98–1.01) | ||
| Soft drinks | ||||||
| 1st tertile (ref.) | 194/361 | 10.7 | 1.00 | - | 1.00 | - |
| 2nd tertile | 101/179 | 30.7 | 1.04 | (0.73–1.49) | 1.00 | (0.70–1.44) |
| 3nd tertile | 85/179 | 91.7 | 0.82 | (0.58–1.17) | 0.75 | (0.52–1.08) |
| Linear trend | 0.99 | (0.98–1.01) | 0.99 | (0.97–1.00) | ||
a Adjusted for phototype, sunburn history, education, body mass index and non-alcohol energy, b Further adjusted for vitamin C and vitamin D intake, Greek Mediterranean index and glycemic index, c 1-g increments of daily intake.
Figure 1.
Spline regression analysis of the odds of being a case according to food item consumption (g/day), adjusting for phototype, sunburn history, education, body mass index, non-alcohol energy, vitamin C and vitamin D intake, Greek Mediterranean index and glycemic index; dotted lines, 95% confidence limits; reference line at 1.0. (A) Cereals; (B) Sweets; (C) Chocolate, candy bars, etc.; (D) Cabbages; (E) Eggs; (F) Legumes; (G) Onion and garlic; (H) Olive oil.
In sex and age stratified analyses (Table 4 and Supplementary Table S1), we observed a direct association between melanoma risk and consumption of ‘Cabbages’, ‘Chocolate, candy bars, etc.,’ and ‘Ice-cream’ in older subjects (≥50 years), particularly in men. On the other hand, the protective effect on melanoma risk yielded by ‘Legumes’ was higher in women (OR = 0.64, 95% CI 0.38–1.08 higher vs. lowest tertile and OR = 0.84, 95% CI 0.72–0.98 linear trend) and seemed to be limited to older subjects (OR = 0.75, 95% CI 0.47–1.21 higher vs. lowest tertile and OR = 0.87, 95% CI 0.77–0.98 linear trend). In addition, a sex and age specific direct association has emerged for ‘Dried fruits, nuts and seeds’ in men (OR = 1.34, 95% CI 0.78–2.31 highest vs. lowest and OR = 1.03, 95% CI 0.97–1.09 linear trend), especially in younger subjects (OR = 1.65, 95% CI 0.92–2.98 highest vs. lowest tertile and OR = 1.06, 95% CI 0.96–1.17 linear trend), and for ‘Fruit juices’ in women (OR = 1.91, 95% CI 1.16–3.15 highest vs. lowest tertile and OR = 1.02, 95% CI 1.00–1.04 linear trend).
Table 4.
Overall adjusted a odds ratios (OR) and 95% confidence intervals (CI) for developing cutaneous malignant melanoma associated to 10-g increments of daily intake (residual of regression on energy) according to sex or age.
| Cases/Controls (n) | Men | Women | <50 years | ≥50 years | ||||
|---|---|---|---|---|---|---|---|---|
| 175/319 | 205/400 | 146/272 | 234/447 | |||||
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Cereals and cereal products | 1.03 | (0.99–1.07) | 1.02 | (0.98–1.06) | 1.02 | (0.98–1.07) | 1.03 | (0.99–1.06) |
| Pasta, other grain | 1.01 | (0.96–1.07) | 1.03 | (0.97–1.10) | 1.00 | (0.93–1.07) | 1.02 | (0.97–1.08) |
| Rice | 1.01 | (0.78–1.31) | 1.04 | (0.79–1.38) | 0.98 | (0.71–1.35) | 1.08 | (0.86–1.35) |
| Bread | 1.03 | (0.98–1.08) | 1.00 | (0.96–1.04) | 1.04 | (0.99–1.09) | 1.01 | (0.97–1.05) |
| Crackers, crispbread, salty snacks | 0.98 | (0.84–1.15) | 1.05 | (0.92–1.20) | 0.89 | (0.73–1.09) | 1.06 | (0.94–1.19) |
| Meat and meat products | 1.01 | (0.97–1.06) | 0.99 | (0.95–1.03) | 1.00 | (0.96–1.04) | 1.00 | (0.96–1.03) |
| Red meat | 1.02 | (0.97–1.07) | 0.98 | (0.92–1.03) | 1.00 | (0.94–1.06) | 0.99 | (0.94–1.04) |
| White meat | 1.02 | (0.94–1.10) | 1.01 | (0.93–1.09) | 1.03 | (0.94–1.12) | 1.00 | (0.93–1.08) |
| Processed meat | 1.01 | (0.91–1.12) | 0.98 | (0.88–1.09) | 0.93 | (0.83–1.05) | 1.02 | (0.92–1.13) |
| Offal b | 0.95 | (0.90–1.01) | 1.04 | (1.00–1.09) | 1.06 | (0.99–1.14) | 0.99 | (0.95–1.03) |
| Milk and dairy products | 1.00 | (0.98–1.01) | 1.00 | (0.99–1.01) | 1.00 | (0.99–1.01) | 0.99 | (0.98–1.00) |
| Milk | 1.00 | (0.98–1.01) | 1.00 | (0.99–1.01) | 1.01 | (0.99–1.02) | 0.99 | (0.98–1.01) |
| Yogurt | 0.99 | (0.97–1.02) | 0.98 | (0.96–1.00) | 0.97 | (0.94–1.00) | 0.99 | (0.97–1.01) |
| Cheeses (including fresh cheeses) | 1.06 | (0.98–1.16) | 1.02 | (0.95–1.10) | 1.02 | (0.94–1.11) | 1.07 | (0.99–1.16) |
| Eggs b | 0.98 | (0.96–1.00) | 0.98 | (0.96–1.00) | 0.99 | (0.97–1.02) | 0.98 | (0.96–0.99) |
| Fish and seafood | 0.99 | (0.88–1.10) | 1.03 | (0.93–1.15) | 0.96 | (0.85–1.09) | 1.02 | (0.92–1.12) |
| Fish | 0.97 | (0.84–1.12) | 1.10 | (0.96–1.26) | 0.93 | (0.79–1.11) | 1.08 | (0.95–1.22) |
| Crustaceans and molluscs b | 1.00 | (0.98–1.03) | 0.99 | (0.97–1.01) | 1.00 | (0.98–1.02) | 0.99 | (0.96–1.01) |
| Vegetables | 0.98 | (0.95–1.02) | 1.00 | (0.97–1.03) | 1.01 | (0.97–1.04) | 0.99 | (0.97–1.02) |
| Leafy vegetables | 0.97 | (0.87–1.07) | 1.04 | (0.96–1.13) | 0.99 | (0.89–1.10) | 1.06 | (0.97–1.15) |
| Other vegetables | 1.01 | (0.87–1.17) | 0.98 | (0.88–1.08) | 0.99 | (0.85–1.16) | 0.97 | (0.87–1.07) |
| Tomatoes | 0.98 | (0.94–1.02) | 1.01 | (0.97–1.06) | 1.02 | (0.97–1.07) | 0.98 | (0.94–1.03) |
| Root vegetables b | 1.00 | (0.99–1.01) | 1.00 | (0.99–1.01) | 1.00 | (0.99–1.01) | 1.00 | (0.98–1.01) |
| Cabbages b | 1.02 | (0.99–1.05) | 1.02 | (0.99–1.05) | 1.02 | (0.99–1.06) | 1.03 | (1.00–1.06) |
| Mushrooms b | 0.97 | (0.91–1.03) | 1.00 | (0.95–1.05) | 1.07 | (0.99–1.16) | 0.95 | (0.89–1.01) |
| Onion and garlic | 0.93 | (0.83–1.05) | 0.94 | (0.83–1.05) | 0.94 | (0.82–1.08) | 0.94 | (0.85–1.04) |
| Legumes | 0.97 | (0.84–1.11) | 0.84 | (0.72–0.98) | 1.03 | (0.86–1.24) | 0.87 | (0.77–0.98) |
| Potatoes | 1.02 | (0.94–1.11) | 0.93 | (0.84–1.03) | 1.09 | (0.98–1.20) | 0.91 | (0.83–1.00) |
| Fresh fruit | 1.01 | (0.99–1.03) | 0.99 | (0.98–1.01) | 0.99 | (0.97–1.02) | 1.01 | (0.99–1.02) |
| Citrus fruits | 1.03 | (0.98–1.08) | 1.00 | (0.96–1.05) | 1.03 | (0.97–1.09) | 1.00 | (0.95–1.04) |
| All other fruits | 1.01 | (0.99–1.04) | 0.99 | (0.97–1.01) | 0.99 | (0.96–1.01) | 1.01 | (0.99–1.03) |
| Dried fruit, nuts and seeds b | 1.03 | (0.97–1.09) | 0.99 | (0.93–1.06) | 1.06 | (0.96–1.17) | 1.01 | (0.97–1.05) |
| Sweets | 0.99 | (0.95–1.04) | 1.02 | (0.99–1.06) | 1.01 | (0.97–1.05) | 1.01 | (0.97–1.05) |
| Chocolate, candy bars, etc. | 1.02 | (0.76–1.36) | 1.08 | (0.86–1.37) | 0.97 | (0.73–1.27) | 1.11 | (0.86–1.43) |
| Sugar, honey, jam, confectionery | 0.94 | (0.83–1.06) | 1.00 | (0.93–1.08) | 1.06 | (0.93–1.21) | 0.96 | (0.88–1.04) |
| Ice-cream | 1.07 | (0.94–1.23) | 1.01 | (0.88–1.16) | 0.92 | (0.79–1.07) | 1.15 | (1.01–1.31) |
| Cakes, pies and pastries | 0.99 | (0.93–1.05) | 1.02 | (0.97–1.07) | 1.01 | (0.96–1.07) | 1.00 | (0.95–1.06) |
| Biscuits, dry cakes | 0.98 | (0.85–1.13) | 1.08 | (0.98–1.20) | 1.00 | (0.88–1.14) | 1.05 | (0.95–1.17) |
| Oils and fats | 0.90 | (0.70–1.15) | 0.95 | (0.75–1.20) | 1.01 | (0.77–1.33) | 0.91 | (0.73–1.13) |
| Vegetable fats and non-olive oils | 1.00 | (0.97–1.03) | 1.01 | (0.97–1.04) | 1.02 | (0.98–1.06) | 1.00 | (0.97–1.03) |
| Olive oil | 0.93 | (0.74–1.18) | 0.93 | (0.76–1.15) | 0.93 | (0.72–1.22) | 0.94 | (0.77–1.14) |
| Butter and other animal fats b | 0.99 | (0.93–1.05) | 1.01 | (0.95–1.08) | 1.00 | (0.93–1.07) | 1.00 | (0.94–1.06) |
| Coffee | 1.00 | (0.97–1.03) | 0.99 | (0.97–1.01) | 1.01 | (0.98–1.03) | 0.98 | (0.96–1.01) |
| Tea | 1.01 | (0.99–1.03) | 0.99 | (0.98–1.01) | 1.00 | (0.97–1.02) | 1.00 | (0.98–1.01) |
| Red wine | 1.00 | (0.98–1.01) | 1.00 | (0.97–1.02) | 1.00 | (0.97–1.03) | 1.00 | (0.98–1.01) |
| White wine | 1.00 | (0.98–1.01) | 0.99 | (0.96–1.01) | 1.00 | (0.97–1.03) | 0.99 | (0.98–1.01) |
| Aperitif wines and beers | 1.00 | (0.97–1.02) | 0.97 | (0.94–1.01) | 0.98 | (0.96–1.01) | 0.99 | (0.96–1.01) |
| Spirits and liqueurs b | 0.99 | (0.98–1.01) | 0.93 | (0.86–1.00) | 0.94 | (0.90–1.00) | 1.00 | (0.98–1.02) |
| Fruit juices | 0.97 | (0.94–1.00) | 1.02 | (1.00–1.04) | 1.00 | (0.97–1.02) | 1.01 | (0.99–1.04) |
| Soft drinks | 0.98 | (0.96–1.01) | 0.99 | (0.97–1.01) | 0.98 | (0.96–1.00) | 1.00 | (0.97–1.02) |
a Adjusted for phototype, sunburn history, education, body mass index, non-alcohol energy, vitamin C, and vitamin D intake, Greek Mediterranean index and glycemic index, b 1-g increments of daily intake.
Table 5 and Supplementary Table S2 summarize results for the analysis stratified by level of adherence to the Mediterranean diet (GMI score 0–4 vs. 5–9). In subjects with higher GMI score, no food item seemed to have a clear direct association with melanoma risk, except for ‘Processed meat’ and a small and statistically unstable relation for some types of ‘Sweets’ and ‘White wine’. In subjects with lower GMI score, the intake of ‘Meat and meat products’, especially ‘Red meat’, ‘Cheese’ and ‘Mushrooms’ appeared to qualify as a risk factor for melanoma. On the other hand, ‘Tomato’ consumption showed a protective effect.
Table 5.
Overall adjusted a odds ratios (OR) and 95% confidence intervals (CI) for developing cutaneous malignant melanoma associated to 10-g increments of daily intake (residual of regression on energy) according to adherence to the Mediterranean diet.
| Cases/Controls (n) | Greek Mediterranean Index = 0–4 | Greek Mediterranean Index = 5–9 | ||
|---|---|---|---|---|
| 201/352 | 179/367 | |||
| OR | (95% CI) | OR | (95% CI) | |
| Cereals and cereal products | 1.04 | (0.99–1.08) | 1.00 | (0.95–1.06) |
| Pasta, other grain | 1.04 | (0.97–1.13) | 0.98 | (0.91–1.06) |
| Rice | 1.55 | (1.04–2.31) | 0.79 | (0.54–1.15) |
| Bread | 1.01 | (0.96–1.06) | 1.01 | (0.95–1.07) |
| Crackers, crispbread, salty snacks | 1.05 | (0.87–1.27) | 1.08 | (0.90–1.28) |
| Meat and meat products | 1.07 | (1.01–1.13) | 1.00 | (0.95–1.05) |
| Red meat | 1.09 | (1.01–1.17) | 0.95 | (0.88–1.02) |
| White meat | 1.10 | (0.99–1.21) | 0.99 | (0.89–1.10) |
| Processed meat | 0.91 | (0.80–1.03) | 1.20 | (1.04–1.37) |
| Offal b | 1.02 | (0.97–1.06) | 0.97 | (0.90–1.05) |
| Milk and dairy products | 1.00 | (0.99–1.01) | 0.98 | (0.96–1.00) |
| Milk | 1.00 | (0.99–1.02) | 0.99 | (0.96–1.01) |
| Yogurt | 1.00 | (0.97–1.03) | 0.97 | (0.93–1.01) |
| Cheeses (including fresh cheeses) | 1.04 | (0.96–1.14) | 1.00 | (0.89–1.12) |
| Eggs b | 0.98 | (0.95–1.01) | 0.99 | (0.96–1.01) |
| Fish and seafood | 0.91 | (0.76–1.09) | 1.05 | (0.92–1.19) |
| Fish | 0.92 | (0.74–1.13) | 1.08 | (0.92–1.27) |
| Crustaceans and molluscs b | 0.99 | (0.95–1.02) | 1.00 | (0.97–1.02) |
| Vegetables | 0.97 | (0.93–1.01) | 0.99 | (0.95–1.03) |
| Leafy vegetables | 0.97 | (0.85–1.10) | 1.06 | (0.95–1.18) |
| Other vegetables | 1.06 | (0.86–1.31) | 0.85 | (0.73–1.00) |
| Tomatoes | 0.93 | (0.87–0.99) | 0.99 | (0.94–1.05) |
| Root vegetables b | 1.00 | (0.98–1.02) | 1.00 | (0.99–1.01) |
| Cabbages b | 1.03 | (0.98–1.09) | 1.03 | (0.99–1.06) |
| Mushrooms b | 1.14 | (1.03–1.25) | 0.96 | (0.89–1.03) |
| Onion and garlic | 0.98 | (0.81–1.19) | 0.93 | (0.82–1.04) |
| Legumes | 0.99 | (0.82–1.20) | 0.84 | (0.70–1.01) |
| Potatoes | 0.89 | (0.78–1.02) | 1.03 | (0.92–1.14) |
| Fresh fruit | 1.01 | (0.98–1.03) | 1.01 | (0.99–1.03) |
| Citrus fruits | 1.06 | (0.99–1.14) | 1.03 | (0.97–1.09) |
| All other fruits | 1.00 | (0.97–1.03) | 1.00 | (0.98–1.03) |
| Dried fruit, nuts and seeds b | 1.03 | (0.94–1.14) | 1.00 | (0.95–1.04) |
| Sweets | 0.99 | (0.95–1.04) | 1.00 | (0.96–1.05) |
| Chocolate, candy bars, etc. | 0.90 | (0.67–1.23) | 1.24 | (0.89–1.72) |
| Sugar, honey, jam, confectionery | 1.13 | (0.97–1.31) | 0.95 | (0.87–1.05) |
| Ice-cream | 0.96 | (0.81–1.14) | 1.07 | (0.88–1.31) |
| Cakes, pies and pastries | 0.98 | (0.92–1.05) | 1.02 | (0.96–1.09) |
| Biscuits, dry cakes | 0.96 | (0.84–1.10) | 1.00 | (0.86–1.17) |
| Oils and fats | 0.88 | (0.63–1.25) | 0.91 | (0.68–1.22) |
| Vegetable fats and non-olive oils b | 1.01 | (0.97–1.06) | 1.00 | (0.96–1.04) |
| Olive oil | 0.77 | (0.56–1.07) | 0.97 | (0.75–1.25) |
| Butter and other animal fats b | 1.06 | (0.98–1.15) | 0.97 | (0.88–1.07) |
| Coffee | 1.01 | (0.98–1.03) | 0.99 | (0.96–1.02) |
| Tea | 0.99 | (0.96–1.01) | 0.99 | (0.97–1.01) |
| Red wine | 0.98 | (0.96–1.01) | 0.99 | (0.97–1.02) |
| White wine | 0.99 | (0.96–1.02) | 1.03 | (1.00–1.07) |
| Aperitif wines and beers | 0.98 | (0.96–1.01) | 1.01 | (0.98–1.05) |
| Spirits and liqueurs b | 0.99 | (0.96–1.02) | 1.00 | (0.97–1.02) |
| Fruit juices | 1.01 | (0.98–1.05) | 0.99 | (0.96–1.02) |
| Soft drinks | 1.00 | (0.98–1.01) | 0.99 | (0.94–1.03) |
a Adjusted for phototype, sunburn history, education, body mass index, no-alcohol energy, vitamin C and vitamin D intake, Greek Mediterranean index and glycemic index, b 1-g increments of daily intake.
4. Discussion
The main findings of our large population-based case-control study lay in an association between higher intake of cereal products, sweets, and cabbages and slightly increased melanoma risk. While higher consumption of legumes, olive oil, eggs, and onion and garlic was linked to decreased risk.
An association between increased cancer risk and high intake of foods such as cereal products and sweets, characterized by high contents of refined flours and sugars and therefore by a high glycemic index, has been observed at several sites such as colon-rectum, breast and endometrium [18,31,32], possibly due to increased postprandial glucose and insulin levels [33]. In addition, Gogas and colleagues suggest a possible role in melanoma development of high circulating levels of leptin, a factor involved in glucose metabolism and directly related with obesity, insulin levels and female sex [34]. Accordingly, we highlighted that a high intake of cereals and sweets could increase melanoma risk in our study population, especially in women, which confirms previous findings in the study population based on an assessment of the glycemic load of the diet [6].
High vegetable consumption has an established beneficial influence upon cancer risk, possibly including melanoma [35]. In our population, we found some indication of a protective effect on melanoma risk due to onion and garlic consumption as well as vegetables, particularly in women and in younger participants. On the other hand, cabbage consumption was associated with higher melanoma risk. This apparently conflicts with previous findings that highlighted a possible protective role of the intake of Brassicas species including all types of cabbages, broccoli, cauliflower and Brussels sprouts, through their high contents of glucosinolate compounds, especially isothiocyanates [36,37]. However, cabbages are also a possible source of heavy metals including cadmium [38], which has been associated to increased melanoma risk [39,40].
We found an inverse correlation between melanoma risk and consumption of legumes, olive oil and eggs. Eggs, with an average content of 3 µg/100 g, are among the major dietary sources of vitamin D, which could have a protective effect on melanoma risk [11]. In our population, however, the consumption of other foods rich in vitamin D such as fish, and dairy products, showed no clear relation with disease risk. Similarly, legumes, mainly beans and peas in our population, provide large quantities of folate, phytochemicals, sterols and several substances with putative antioxidant and anticancer properties such as glutathione, tocopherols and phenolic compounds, which might inhibit the process of melanoma carcinogenesis in both initiation and progression phases [41]. Olive oil consumption might have a protective role against cancer and other inflammation-related diseases [42]. The preventive activity of olive oil has been attributed to phenolic compounds. Several studies have demonstrated their ability to protect against DNA damage initiated by free radicals, inhibit proliferation and induce apoptosis in different tumor cell lines [43,44]. Another possible mechanism may be mediated by regulation of the ketogenic pathway, since it has been noted that the melanoma cells may promote proliferation and tumor growth by upregulating the expression of ketogenic enzymes [45].
In our study, we found an inverse correlation with melanoma risk in all age and sex groups, mostly in subjects with low GMI. When we stratified the analysis for level of adherence to the Mediterranean diet, we observed that in subjects with a high GMI score, no specific food consumption appeared to have a positive association with melanoma risk, except for processed meat and possibly some types of sweets and white wines. The traditional Mediterranean diet is associated with long life, lower prevalence of cardiovascular disease and cancers [46,47]. Its main components include a high intake of vegetables, fruit, olive oil, a moderate intake of fish and wine, and a low dairy and meat intake. Recently, it has been suggested that the low phosphorus content of the Mediterranean diet is linked to reduced cancer risk [48]. In addition, it may be that high adherence to the Mediterranean diet counteracts the unfavorable effects of other food categories. Conversely, in subjects with lower GMI score, the positive association between melanoma risk by meat and meat products (especially red meat), and mushrooms, appeared to be enhanced.
Some investigations suggested a protective effect on melanoma risk linked to fish and fruit intake [35], while citrus fruit consumption has been associated with an increased risk of melanoma [49]. In our study, none of these food categories were associated with melanoma risk. Similarly, we have not been able to detect a clear relation of meat and dairy products with disease risk, despite the positive association found in other studies with melanoma and cancer risk [35].
None of the beverages assessed in our study showed any clear effect on melanoma risk. This is partially at odds with previous studies, which found some negative and positive associations with coffee, fruit juices, and alcohol [15,16,50].
Our study was one of the largest population-based case-control studies investigating the association between diet and melanoma in Italy. A major strength of this investigation appears to be the validity and completeness of the EPIC food questionnaire. This had been specifically developed and validated in the Northern Italy population, including traditional seasoning and local dishes [21]. It also allowed us to conduct a very accurate assessment of exposure, including several pictures for portion anchors and the implementation of the Willet regression-residual method, and the exclusion of subjects with implausible total energy intake [51].
This study had some limitations, which must be acknowledged. We lacked information about type and number of dysplastic nevi, family history of melanoma and details about occupational history. However, in the most adjusted model, we took account of many other potential confounders such as phototype, sunburn history, education and BMI.
Moreover, our study was based on a case-control design. Therefore, we recognized that the study could have the general limitations concerning observational studies, i.e. unmeasured residual confounding, and in addition those inherent in the case-control design, such as the inability to assess disease incidence, the possibility of selection bias, and in cases recall bias. However, diet is not generally regarded by the general population as a risk factor for melanoma, thus making recall bias rather unlikely.
In addition, the questionnaire did not ascertain consumption of organic foods, such as organically-grown legumes, oil, and eggs. Therefore, we could not examine the association between organic food consumption and melanoma risk. Organic foods contain fewer pesticides and heavy metals [52], and organic food consumption has been associated with reduced cancer risk [53]. Therefore, our inability to account for consumption of such foods may have led to confounding. The impact of such bias is likely to be small because consumption of organic foods was uncommon during the study period [54].
Finally, the risk estimates we computed were generally statistically unstable, and such limited precision suggests additional caution in evaluating our findings, as well as the need to address these issues in larger studies.
5. Conclusions
In conclusion, our findings appear to confirm that dietary factors may play a role in melanoma risk, specifically suggesting potentially adverse effects of high consumption of cereal products, sweets and cabbages, and a possible protective role for legumes, olive oil, eggs, onion and garlic. In light of the rising melanoma incidence worldwide, and the current trend of some Western populations including the Italian one to deviate from the Mediterranean diet [55], these associations warrant further investigation for their potential public health and preventive medicine implications.
Acknowledgments
The authors are grateful to the patients and the controls who participated in the study and made it possible.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/9/2206/s1, Table S1. Overall adjusted odds ratios (OR) and 95% confidence intervals (CI) for developing cutaneous malignant melanoma according to tertiles of daily intake by sex or age. Lowest tertile as referent. Sex- and age-specific tertiles of food intake obtained from residual of regression on energy. Table S2. Overall adjusted a odds ratios (OR) and 95% confidence intervals (CI) for developing cutaneous malignant melanoma according to tertiles of daily intake by level of adherence to the Mediterranean diet. Lowest tertile as referent. Greek Mediterranean Index specific tertiles of food intake obtained from residual of regression on energy.
Author Contributions
M.V., C.M. and G.P. designed the original study; C.M., M.M., T.F. and M.V. analysed and interpreted the data and drafted the article; C.M. and M.V. recruited controls and collected their data; F.F., C.L. and G.P. enrolled melanoma patients and collected their clinical, lifestyle and dietary data.
Funding
The study was supported by Lega Contro i Tumori-LILT, Section of Reggio Emilia (M.V.) and Modena Policlinico Hospital-Modena Local Health Unit (M.V. and G.P.). All these funders had no role in the design, analysis or writing of this article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Bray F., Ferlay J., Laversanne M., Brewster D.H., Gombe Mbalawa C., Kohler B., Pineros M., Steliarova-Foucher E., Swaminathan R., Antoni S., et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer. 2015;137:2060–2071. doi: 10.1002/ijc.29670. [DOI] [PubMed] [Google Scholar]
- 2.Dimitriou F., Krattinger R., Ramelyte E., Barysch M.J., Micaletto S., Dummer R., Goldinger S.M. The World of Melanoma: Epidemiologic, Genetic, and Anatomic Differences of Melanoma across the Globe. Curr. Oncol. Rep. 2018;20:87. doi: 10.1007/s11912-018-0732-8. [DOI] [PubMed] [Google Scholar]
- 3.Haenssle H.A., Mograby N., Ngassa A., Buhl T., Emmert S., Schon M.P., Rosenberger A., Bertsch H.P. Association of Patient Risk Factors and Frequency of Nevus-Associated Cutaneous Melanomas. JAMA Dermatol. 2016;152:291–298. doi: 10.1001/jamadermatol.2015.3775. [DOI] [PubMed] [Google Scholar]
- 4.Vinceti M., Ballotari P., Steinmaus C., Malagoli C., Luberto F., Malavolti M., Giorgi Rossi P. Long-term mortality patterns in a residential cohort exposed to inorganic selenium in drinking water. Environ. Res. 2016;150:348–356. doi: 10.1016/j.envres.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Vinceti M., Vicentini M., Wise L.A., Sacchettini C., Malagoli C., Ballotari P., Filippini T., Malavolti M., Rossi P.G. Cancer incidence following long-term consumption of drinking water with high inorganic selenium content. Sci. Total Environ. 2018;635:390–396. doi: 10.1016/j.scitotenv.2018.04.097. [DOI] [PubMed] [Google Scholar]
- 6.Malavolti M., Malagoli C., Crespi C.M., Brighenti F., Agnoli C., Sieri S., Krogh V., Fiorentini C., Farnetani F., Longo C., et al. Glycaemic index, glycaemic load and risk of cutaneous melanoma in a population-based, case-control study. Br. J. Nutr. 2017;117:432–438. doi: 10.1017/S000711451700006X. [DOI] [PubMed] [Google Scholar]
- 7.Malagoli C., Malavolti M., Agnoli C., Crespi C.M., Fiorentini C., Farnetani F., Longo C., Ricci C., Albertini G., Lanzoni A., et al. Diet Quality and Risk of Melanoma in an Italian Population. J. Nutr. 2015;145:1800–1807. doi: 10.3945/jn.114.209320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong L.X., Young L.C. Nutrition: The future of melanoma prevention? J. Am. Acad. Dermatol. 2014;71:151–160. doi: 10.1016/j.jaad.2014.01.910. [DOI] [PubMed] [Google Scholar]
- 9.Vinceti M., Malagoli C., Iacuzio L., Crespi C.M., Sieri S., Krogh V., Marmiroli S., Pellacani G., Venturelli E. Serum Fatty acids and risk of cutaneous melanoma: A population-based case-control study. Dermatol. Res. Pr. 2013;2013:659394. doi: 10.1155/2013/659394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malavolti M., Malagoli C., Fiorentini C., Longo C., Farnetani F., Ricci C., Albertini G., Lanzoni A., Reggiani C., Virgili A., et al. Association between dietary vitamin C and risk of cutaneous melanoma in a population of northern Italy. Int. J. Vitam. Nutr. Res. 2013;83:291–298. doi: 10.1024/0300-9831/a000171. [DOI] [PubMed] [Google Scholar]
- 11.Vinceti M., Malagoli C., Fiorentini C., Longo C., Crespi C.M., Albertini G., Ricci C., Lanzoni A., Reggiani M., Virgili A., et al. Inverse association between dietary vitamin D and risk of cutaneous melanoma in a northern Italy population. Nutr. Cancer. 2011;63:506–513. doi: 10.1080/01635581.2011.539314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohmann C.B., Bonamigo R.R., Segatto M.M., Costa M.M., Mastroeni S., Fortes C. Could a specific dietary intake be a risk factor for cutaneous melanoma? Cutis. 2016;97:421–425. [PubMed] [Google Scholar]
- 13.Fortes C., Mastroeni S., Melchi F., Pilla M.A., Antonelli G., Camaioni D., Alotto M., Pasquini P. A protective effect of the Mediterranean diet for cutaneous melanoma. Int. J. Epidemiol. 2008;37:1018–1029. doi: 10.1093/ije/dyn132. [DOI] [PubMed] [Google Scholar]
- 14.De Waure C., Quaranta G., Gualano M.R., Cadeddu C., Jovic-Vranes A., Djikanovic B., La Torre G., Ricciardi W. Systematic review of studies investigating the association between dietary habits and cutaneous malignant melanoma. Public Health. 2015;129:1099–1113. doi: 10.1016/j.puhe.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Caini S., Masala G., Saieva C., Kvaskoff M., Savoye I., Sacerdote C., Hemmingsson O., Hammer Bech B., Overvad K., Tjonneland A., et al. Coffee, tea and melanoma risk: Findings from the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer. 2017;140:2246–2255. doi: 10.1002/ijc.30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandini S., Masala G., Palli D., Cavicchi B., Saieva C., Ermini I., Baldini F., Gnagnarella P., Caini S. Alcohol, alcoholic beverages, and melanoma risk: A systematic literature review and dose-response meta-analysis. Eur. J. Nutr. 2018;57:2323–2332. doi: 10.1007/s00394-018-1613-5. [DOI] [PubMed] [Google Scholar]
- 17.Yang K., Fung T.T., Nan H. An Epidemiological Review of Diet and Cutaneous Malignant Melanoma. Cancer Epidemiol. Biomarkers Prev. 2018;27:1115–1122. doi: 10.1158/1055-9965.EPI-18-0243. [DOI] [PubMed] [Google Scholar]
- 18.Sieri S., Agnoli C., Pala V., Grioni S., Brighenti F., Pellegrini N., Masala G., Palli D., Mattiello A., Panico S., et al. Dietary glycemic index, glycemic load, and cancer risk: Results from the EPIC-Italy study. Sci. Rep. 2017;7:9757. doi: 10.1038/s41598-017-09498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris J.A., Benedict F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisani P., Faggiano F., Krogh V., Palli D., Vineis P., Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997;26(Suppl. 1):S152–S160. doi: 10.1093/ije/26.suppl_1.S152. [DOI] [PubMed] [Google Scholar]
- 21.Pala V., Sieri C., Palli D., Salvini S., Berrino F., Bellegotti M., Frasca G., Tumino R., Sacerdote C., Fiorini L., et al. Diet in the Italian EPIC cohorts: Presentation of data and methodological issues. Tumori J. 2003;89:594–607. doi: 10.1177/030089160308900603. [DOI] [PubMed] [Google Scholar]
- 22.DNFCS Dutch National Food Consumption Survey 2007–2010—Part 2, Foods NEVO Codes. [(accessed on 26 August 2019)]; Available online: https://www.rivm.nl/en/Documents_and_publications/Scientific/Tables_graphs/DNFCS/DNFCS_Core_survey_Part_2_Foods_NEVO_codes.
- 23.Filippini T., Michalke B., Wise L.A., Malagoli C., Malavolti M., Vescovi L., Salvia C., Bargellini A., Sieri S., Krogh V., et al. Diet composition and serum levels of selenium species: A cross-sectional study. Food Chem. Toxicol. 2018;115:482–490. doi: 10.1016/j.fct.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 24.Salvini S., Parpinel M., Gnagnarella P. Banca Dati di Composizione Degli Alimenti Per Studi Epidemiologici in Italia. 269th ed. European Institute of Oncology; Milan, Italy: 1998. [Google Scholar]
- 25.Trichopoulou A., Costacou T., Bamia C., Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 26.Schwingshackl L., Hoffmann G. Adherence to Mediterranean diet and risk of cancer: A systematic review and meta-analysis of observational studies. Int. J. Cancer. 2014;135:1884–1897. doi: 10.1002/ijc.28824. [DOI] [PubMed] [Google Scholar]
- 27.Willett W., Stampfer M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 28.Rothman K.J. Six persistent research misconceptions. J. Gen. Intern. Med. 2014;29:1060–1064. doi: 10.1007/s11606-013-2755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orsini N., Greenland S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J. 2011;11:1–29. doi: 10.1177/1536867X1101100101. [DOI] [Google Scholar]
- 30.Harrell F.E. Regression Modeling Strategies. Springer; New York, NY, USA: 2001. [Google Scholar]
- 31.Sieri S., Krogh V., Agnoli C., Ricceri F., Palli D., Masala G., Panico S., Mattiello A., Tumino R., Giurdanella M.C., et al. Dietary glycemic index and glycemic load and risk of colorectal cancer: Results from the EPIC-Italy study. Int. J. Cancer. 2015;136:2923–2931. doi: 10.1002/ijc.29341. [DOI] [PubMed] [Google Scholar]
- 32.Sieri S., Krogh V. Dietary glycemic index, glycemic load and cancer: An overview of the literature. Nutr. Metab. Cardiovasc. Dis. NMCD. 2017;27:18–31. doi: 10.1016/j.numecd.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Wu D., Hu D., Chen H., Shi G., Fetahu I.S., Wu F., Rabidou K., Fang R., Tan L., Xu S., et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559:637–641. doi: 10.1038/s41586-018-0350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gogas H., Trakatelli M., Dessypris N., Terzidis A., Katsambas A., Chrousos G.P., Petridou E.T. Melanoma risk in association with serum leptin levels and lifestyle parameters: A case-control study. Ann. Oncol. 2008;19:384–389. doi: 10.1093/annonc/mdm464. [DOI] [PubMed] [Google Scholar]
- 35.Grasgruber P., Hrazdira E., Sebera M., Kalina T. Cancer Incidence in Europe: An Ecological Analysis of Nutritional and Other Environmental Factors. Front. Oncol. 2018;8:151. doi: 10.3389/fonc.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verhoeven D.T., Verhagen H., Goldbohm R.A., van den Brandt P.A., van Poppel G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem. Biol. Interact. 1997;103:79–129. doi: 10.1016/S0009-2797(96)03745-3. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis. 2012;33:2–9. doi: 10.1093/carcin/bgr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filippini T., Cilloni S., Malavolti M., Violi F., Malagoli C., Tesauro M., Bottecchi I., Ferrari A., Vescovi L., Vinceti M. Dietary intake of cadmium, chromium, copper, manganese, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. 2018;50:508–517. doi: 10.1016/j.jtemb.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Matthews N.H., Fitch K., Li W.Q., Morris J.S., Christiani D.C., Qureshi A.A., Cho E. Exposure to Trace Elements and Risk of Skin Cancer: A Systematic Review of Epidemiologic Studies. Cancer Epidemiol. Biomark. Prev. 2019;28:3–21. doi: 10.1158/1055-9965.EPI-18-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filippini T., Malagoli C., Wise L.A., Malavolti M., Pellacani G., Vinceti M. Dietary cadmium intake and risk of cutaneous melanoma: An Italian population-based case-control study. J. Trace Elem. Med. Biol. 2019;56:100–106. doi: 10.1016/j.jtemb.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Kouris-Blazos A., Belski R. Health benefits of legumes and pulses with a focus on Australian sweet lupins. Asia Pac. J. Clin. Nutr. 2016;25:1–17. doi: 10.6133/apjcn.2016.25.1.23. [DOI] [PubMed] [Google Scholar]
- 42.Visioli F., Franco M., Toledo E., Luchsinger J., Willett W.C., Hu F.B., Martinez-Gonzalez M.A. Olive oil and prevention of chronic diseases: Summary of an International conference. Nutr. Metab. Cardiovasc. Dis. NMCD. 2018;28:649–656. doi: 10.1016/j.numecd.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Ruzzolini J., Peppicelli S., Andreucci E., Bianchini F., Scardigli A., Romani A., la Marca G., Nediani C., Calorini L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients. 2018;10:1950. doi: 10.3390/nu10121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fogli S., Arena C., Carpi S., Polini B., Bertini S., Digiacomo M., Gado F., Saba A., Saccomanni G., Breschi M.C., et al. Cytotoxic Activity of Oleocanthal Isolated from Virgin Olive Oil on Human Melanoma Cells. Nutr. Cancer. 2016;68:873–877. doi: 10.1080/01635581.2016.1180407. [DOI] [PubMed] [Google Scholar]
- 45.Kang H.B., Fan J., Lin R., Elf S., Ji Q., Zhao L., Jin L., Seo J.H., Shan C., Arbiser J.L., et al. Metabolic Rewiring by Oncogenic BRAF V600E Links Ketogenesis Pathway to BRAF-MEK1 Signaling. Mol. Cell. 2015;59:345–358. doi: 10.1016/j.molcel.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becerra-Tomas N., Blanco Mejia S., Viguiliouk E., Khan T., Kendall C.W.C., Kahleova H., Rahelic D., Sievenpiper J.L., Salas-Salvado J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019 doi: 10.1080/10408398.2019.1565281. [DOI] [PubMed] [Google Scholar]
- 47.Galbete C., Schwingshackl L., Schwedhelm C., Boeing H., Schulze M.B. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analyses. Eur. J. Epidemiol. 2018;33:909–931. doi: 10.1007/s10654-018-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown R.B. Vitamin D, cancer, and dysregulated phosphate metabolism. Endocrine. 2019;65:238–243. doi: 10.1007/s12020-019-01985-y. [DOI] [PubMed] [Google Scholar]
- 49.Wu S., Han J., Feskanich D., Cho E., Stampfer M.J., Willett W.C., Qureshi A.A. Citrus Consumption and Risk of Cutaneous Malignant Melanoma. J. Clin. Oncol. 2015;33:2500–2508. doi: 10.1200/JCO.2014.57.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feskanich D., Willett W.C., Hunter D.J., Colditz G.A. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br. J. Cancer. 2003;88:1381–1387. doi: 10.1038/sj.bjc.6600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gavrieli A., Trichopoulou A., Valsta L.M., Ioannidou S., Berry R., Roe M., Harvey L., Finglas P., Glibetic M., Gurinovic M., et al. Identifying sources of measurement error in assessing dietary intakes—Results of a multi-country ring-trial. Nutr. Metab. Cardiovasc. Dis. NMCD. 2019;29:127–134. doi: 10.1016/j.numecd.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Johansson E., Hussain A., Kuktaite R., Andersson S.C., Olsson M.E. Contribution of organically grown crops to human health. Int. J. Environ. Res. Public Health. 2014;11:3870–3893. doi: 10.3390/ijerph110403870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baudry J., Assmann K.E., Touvier M., Alles B., Seconda L., Latino-Martel P., Ezzedine K., Galan P., Hercberg S., Lairon D., et al. Association of Frequency of Organic Food Consumption With Cancer Risk: Findings From the NutriNet-Sante Prospective Cohort Study. JAMA Intern. Med. 2018;178:1597–1606. doi: 10.1001/jamainternmed.2018.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.SINAB–Sistema D’Informazione Nazionale sull’Agricoltura Biologica. Facts and Figures on Organic Farming in Italy. [(accessed on 26 August 2019)];2018 Available online: http://www.sinab.it/sites/default/files/share/EN%20Bio%20in%20cifre%202018%20231118_%20Preview%20FINALE.pdf.
- 55.Giampaoli S., Krogh V., Grioni S., Palmieri L., Gulizia M.M., Stamler J., Vanuzzo D. Eating behaviours of italian adults: Results of the Osservatorio epidemiologico cardiovascolare/Health Examination Survey. Epidemiol. Prev. 2015;39:373–379. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.