Abstract
The aim of this study was to evaluate the test–retest reproducibility of a non-quantitative food frequency questionnaire (acronym: 62-item FFQ-6) and the possibility of identifying dietary patterns (DPs) in 13–21-year-old females. The study involved 97 females within three age groups: 13–15, 16–18, and 19–21 years, including 31, 38, and 28 subjects, respectively. The questionnaire was completed twice with a two-week interval (test and retest). For the total sample, using a principal component analysis (PCA), two similar PCA-driven DPs (DP1 and DP2) were identified separately from test data and retest data, considering two sets of input variables. 60-item-DP1 and 60-item-DP2 were identified after excluding two items—vegetables and fruits in general—due to including single items of various kinds of vegetables and fruits. After an aggregation of some items of the questionnaire, 25-item-DP1 and 25-item-DP2 were identified. The kappa statistic (test vs. retest) in the total sample averaged at 0.52 (0.32–0.72 for food items), while within age groups, it averaged at 0.41, 0.53, and 0.65, respectively. The percentage of subjects classified into the same food frequency category (test vs. retest) in the total sample averaged at 68% (51%–89% for food items), while within age groups, it averaged at 60%, 68%, and 77%, respectively. The Spearman correlations between dietary pattern scores (test vs. retest) in the total sample were: 0.84 (within age groups 0.83, 0.81, and 0.78, respectively) for 60-item-DP1, 0.68 (within age groups 0.24, 0.79, and 0.76, respectively) for 60-item-DP2, 0.76 (within age groups 0.56, 0.82, and 0.89, respectively) for 25-item-DP1, and 0.48 (within age groups 0.40, 0.57, and 0.53, respectively) for 25-item-DP2 (p < 0.05 for all). In conclusion, the test–retest reproducibility of the 62-item FFQ-6 was good or very good for most food items, with a tendency to be higher in older age groups of females under study. Due to the acceptable-to-good reproducibility of dietary pattern identification, the use of a 62-item FFQ-6 to describe the overall diet of young Polish females can be recommended.
Keywords: FFQ, test–retest, reproducibility, reliability, dietary assessment, dietary pattern, kappa coefficient, girls, women
1. Introduction
An assessment of dietary intake is still a topic of interest due to the causal relation of many diseases with diet and the possibility of identifying individuals and subpopulations at risk of inadequate food consumption [1]. There is no gold-standard method for dietary intake assessment. The most commonly used are three methods: food record, 24-hour dietary recall, and food frequency method using food frequency questionnaires (FFQs) [2,3,4]. These methods are characterized by various levels of validity and reproducibility since the validity and reproducibility of any dietary method is a function of the measurement errors and uncertainty resulting from true variability in daily food consumption and modulating factors such as sex, age, personality, education level, family affluence, national wealth, tradition, religion, and seasonality in food supply [2,5].
FFQs are often used in dietary assessment because they are practical, easy, and quick to administer, relatively inexpensive and less engaging the respondent, and also better at describing the usual diet than other methods [1,5,6,7]. The main possible respondent-related errors, which can be attributed to FFQ use include skipping or adding food reported or inadequate assessment of the frequency and/or quantity of food consumed [2,5]. Despite this, it was shown that FFQs can be a reliable tool to assess selected food consumption or selected nutrient intake and to describe the whole diet, including dietary patterns [7,8,9,10,11]. Identifying dietary patterns (DPs) has been widely used in various subpopulations across the world to find association of DPs with health outcomes [7,12,13,14].
All newly developed or modified FFQs have to be checked for reproducibility and relevance in respect to the population under study [1,5]. In Poland, there are a few semi-quantitative food frequency questionnaires which have been developed for selected nutrients and validated in young adults, i.e., calcium [15], vitamin D [16], iron [17], iodine [18], folate [19], and zinc [20]; a short non-quantitative food frequency questionnaire (FIVeQ) developed to measure food intake variety in older people [21]; a full semi-quantitative food frequency questionnaire (165-item FFQ) compared with a 3-day food record in young females [22,23]; and a non-quantitative food frequency questionnaire (KomPAN®) whose reproducibility was assessed in adolescents and adults [24]. The 165-item FFQ is difficult to apply due to a large number of detailed questions, although it was successfully used to study dietary patterns and adverse health outcomes in adolescents [13]. The KomPAN® allows diet to be described in terms of ‘healthy’ and ‘unhealthy’ dietary behaviors with pre-defined diet quality scores (pro-healthy diet index and non-healthy diet index) but does not allow for diet in the context of diet-related diseases to be fully described due to aggregating some foods in one question (e.g., vegetable oils, margarines, and mixes of butter and margarines). Some Polish researchers use two FFQs in one study, which is inconvenient for the respondents and time-consuming for the researchers [14,25,26]. Thus, another FFQ with a more detailed food list is needed.
The study aimed to evaluate the test–retest reproducibility of a non-quantitative food frequency questionnaire (acronym: 62-item FFQ-6) to assess the whole diet and the possibility of identifying dietary patterns in 13–21-year-old females. Young females were selected as a population of interest for several reasons. Adolescent girls, more often than boys, use a variety of diets, e.g., to lose weight or follow a dietary fashion [27,28]. Although girls often make healthier dietary choices than boys [29,30], the overall diet quality decreases with their age [31]. Polish women, due to the traditional division of roles in the family, can strongly influence the dietary habits of their daughters [32,33,34,35]. Therefore, they can influence the diet and health of the next generation.
2. Materials and Methods
2.1. Ethical Approval
The study was approved by the Bioethics Committee of the Faculty of Medical Sciences, University of Warmia and Mazury in Olsztyn in June 17, 2010 (resolution no. 20/2010). Informed consent was obtained from adult study participants and from parents/legal guardians of underage girls (<18 years old).
2.2. Study Design and Sample Collection
A pilot study was carried out to examine if the questions included in the 62-item FFQ-6 questionnaire were understandable and properly formulated. The main study was carried out in October and November 2012. The questionnaire was completed by each respondent twice with a two-week interval (test and retest). The data were collected by researchers in a face-to-face situation. The researchers described the aim of the study and answering manner in detail to the respondents before starting an interview. The same researcher conducted both the test and the retest with the same respondent (each respondent was assigned an identification number to identify the respondent during the second administration of the questionnaire).
Participants were recruited by contacting students of middle and high schools located in North-Eastern Poland. The intention was to achieve the same proportion of subjects in each age category (13–15, 16–18, and 19–21 years). The inclusion criteria were female gender, age ≥ 13 and ≤21 years and the use of the Polish language in speech. A total of 100 females were recruited. Three girls did not complete at least one questionnaire and they were excluded from the study. The final sample consisted of 97 females aged 13–21 years, including 31 subjects aged 13–15 years, 38 subjects aged 16-18 years, and 28 subjects aged 19–21 years.
2.3. A Food Frequency Questionnaire
The 62-item FFQ-6 consists of a list of 62 food items and refers to the usual frequency of food consumption over the last 12 months. The grouping of foods into 62 food items (Table S1) was created based on the authors′ experience and the Food Intake Variety Questionnaire (FIVeQ) with a similar food list whose reproducibility was tested previously [21]. The 62-item FFQ-6 contains two additional questions regarding the consumption frequency of vegetables and fruits in general (questions Q40 and Q29, respectively; Table S4). These two questions can be used by researchers in two ways: (1) to interpret the consumption frequency of vegetables and/or fruits in general (without considering single items), (2) to adjust the consumption frequency of single items of vegetables and fruits, collected with separate questions (Q41–47 and Q30–37, respectively) and then to interpret the consumption frequency of single items of vegetables and fruits in detail. A manual for the adjustment of consumption frequency of single items of vegetables and fruits is included in the Supplementary Materials (Table S4). This study shows the results from crude data, without adjustment for single items of vegetables or fruits.
When answering, to indicate frequency of food consumption, respondents could choose one of six categories (next converted by researcher into daily frequency): never or very rarely (0 times/day), once a month or less (0.025 times/day), several times a month (0.1 times/day), several times a week (0.571 times/day), daily (1 time/day), or a few times a day (2 times/day) [36]. Some food items were aggregated into 25 food items by summing up their daily consumption frequencies (in times/day), and this data set was subjected to further analysis (Table S1).
2.4. Statistical Analysis
Categorical variables were presented as a sample percentage (%), and continuous variables as means and standard deviation (SD) [37,38]. The distribution of continuous variables was examined using the Kolmogorov–Smirnov normality test. The reproducibility of a questionnaire was measured by comparing the results of the first interview (test) and the second administration of the questionnaire (retest) in the total sample and by age groups [5]. To comprehensively compare test data and retest data, several statistical measures and tests were used: (i) the Wilcoxon signed rank test for two dependent samples—to verify differences in means of food consumption frequency between the test and the retest; since all continuous variables (as expected) lacked a normal distribution, a non-parametric test was chosen, (ii) Spearman’s correlation coefficient (SCC)—to compare the daily frequency of food consumption (times/day) between test-data and retest-data, (iii) the Fleiss kappa statistic, cross-classification analysis, and chi-square test—to evaluate the agreement of the subject distribution by the same food frequency categories in the test and the retest. The strength of the correlation was interpreted as fair (<0.3), moderate (0.3 to <0.5), good (0.5 to <0.7), or very good (≥0.7). The agreement (measured with the Fleiss' kappa) was interpreted as poor (≤0.2), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80), or very good (≥0.81) [39]. Two-tailed tests were applied and p-values < 0.05 were considered as significant.
The principal component analysis (PCA) with varimax normalized rotation was used to identify PCA-driven dietary patterns [40]. For the total sample (n = 97), four separate PCA for test data and retest data including a different number of input variables were performed. The input variables were consumption frequencies (in times/day) of: (i) all 60 food items, except for vegetables and fruits in general, (ii) 25 food items after aggregating some items (see Section 2.3). Factor loadings ≥ |0.40| were considered as having a significant contribution toward identifying DPs. Eigenvalues of at least 1.00, scree plot, and variance explained were considered when choosing the best solution. Based on tertile distribution of factor scores of dietary patterns, subjects were divided into three categories within each DP as follows: bottom, middle, and upper tertile. Dietary pattern scores reflecting adherence of each subject to each DP were established. The dietary pattern scores were calculated as a sum of the products of the food consumption frequency and a factor loading for 60 or 25 food items. To evaluate the agreement of DPs identified from data collected with the test and the retest, Spearman correlations for dietary pattern scores were calculated. All analyses were performed with STATISTICA software (version 13.3 PL; StatSoft Inc., Tulsa, OK, USA; StatSoft, Krakow, Poland).
3. Results
3.1. Mean Frequency of Food Consumption
There were no significant differences in mean frequency of food consumption for most food items reported in the test and the retest in the total sample (57 out of 62) and within age groups (60, 58, and 60 for 13–15, 16–18, and 19–21 years, respectively; Table 1). Significant differences (p < 0.05) in mean frequency of food consumption (test vs. retest) were found for olives, wine and cocktails, chocolates, honey, milk and milk beverages—natural in the total sample; for baked confectionery, vegetables, and vegetable-fruit juices in females aged 13–15; for olives, sweetened beverages, honey, and milk and milk beverages—natural in females aged 16–18; and for high-quality cured meats and milk and milk beverages—natural in females aged 19–21.
Table 1.
Comparison of food consumption frequency (times/day) in the test and the retest by age groups (mean and standard deviation).
| Food Items 1 | Total Sample (n = 97) | 13–15 Years (n = 31) | 16–18 Years (n = 38) | 19–21 Years (n = 28) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | Retest | p | SCC | Test | Retest | p | SCC | Test | Retest | p | SCC | Test | Retest | p | SCC | |
| Olives | 0.08 ± 0.31 | 0.05 ± 0.23 | 0.046 | 0.86 * | 0.11 ± 0.39 | 0.08 ± 0.36 | 0.893 | 0.75 * | 0.11 ± 0.35 | 0.06 ± 0.18 | 0.028 | 0.89 * | 0.02 ± 0.04 | 0.02 ± 0.04 | 0.180 | 0.94 * |
| Beer | 0.09 ± 0.18 | 0.12 ± 0.22 | 0.147 | 0.85 * | 0.05 ± 0.14 | 0.08 ± 0.22 | 0.529 | 0.71 * | 0.13 ± 0.22 | 0.15 ± 0.24 | 0.575 | 0.89 * | 0.10 ± 0.14 | 0.12 ± 0.17 | 0.109 | 0.92 * |
| Wine and cocktails | 0.04 ± 0.09 | 0.07 ± 0.23 | 0.013 | 0.83 * | 0.00 ± 0.02 | 0.07 ± 0.36 | 0.273 | 0.61 * | 0.06 ± 0.13 | 0.09 ± 0.17 | 0.051 | 0.83 * | 0.04 ± 0.04 | 0.06 ± 0.11 | 0.285 | 0.94 * |
| Spirits | 0.04 ± 0.09 | 0.05 ± 0.14 | 0.209 | 0.83 * | 0.01 ± 0.03 | 0.06 ± 0.20 | 0.109 | 0.74 * | 0.06 ± 0.13 | 0.05 ± 0.10 | 0.953 | 0.78 * | 0.04 ± 0.04 | 0.05 ± 0.11 | 0.655 | 0.98 * |
| Sugar confectionery | 0.38 ± 0.45 | 0.41 ± 0.45 | 0.449 | 0.82 * | 0.42 ± 0.41 | 0.52 ± 0.48 | 0.080 | 0.73 * | 0.35 ± 0.43 | 0.34 ± 0.31 | 0.753 | 0.81 * | 0.38 ± 0.53 | 0.40 ± 0.56 | 0.735 | 0.91 * |
| Sugar | 0.95 ± 0.71 | 1.00 ± 0.73 | 0.155 | 0.81 * | 0.79 ± 0.62 | 0.84 ± 0.65 | 0.826 | 0.56 * | 0.89 ± 0.70 | 0.94 ± 0.71 | 0.477 | 0.89 * | 1.22 ± 0.77 | 1.26 ± 0.78 | 0.673 | 0.83 * |
| Dried fruit | 0.09 ± 0.20 | 0.08 ± 0.24 | 0.381 | 0.81 * | 0.14 ± 0.28 | 0.17 ± 0.41 | 0.730 | 0.75 * | 0.06 ± 0.13 | 0.03 ± 0.03 | 0.141 | 0.78 * | 0.06 ± 0.15 | 0.03 ± 0.04 | 0.225 | 0.90 * |
| High quality cured meats | 0.51 ± 0.45 | 0.54 ± 0.45 | 0.122 | 0.80 * | 0.51 ± 0.50 | 0.48 ± 0.43 | 0.814 | 0.71 * | 0.59 ± 0.46 | 0.63 ± 0.50 | 0.333 | 0.83 * | 0.40 ± 0.34 | 0.48 ± 0.38 | 0.043 | 0.90 * |
| Other animal fats | 0.01 ± 0.02 | 0.02 ± 0.06 | 0.117 | 0.80 * | 0.01 ± 0.02 | 0.01 ± 0.03 | 0.584 | 0.74 * | 0.01 ± 0.02 | 0.03 ± 0.09 | 0.075 | 0.79 * | 0.01 ± 0.02 | 0.01 ± 0.02 | 1.000 | 0.86 * |
| Tomatoes | 0.77 ± 0.59 | 0.70 ± 0.54 | 0.084 | 0.79 * | 0.58 ± 0.45 | 0.55 ± 0.35 | 0.834 | 0.73 * | 0.77 ± 0.60 | 0.64 ± 0.58 | 0.069 | 0.69 * | 0.97 ± 0.64 | 0.94 ± 0.60 | 0.593 | 0.95 * |
| Poultry and rabbit | 0.34 ± 0.36 | 0.30 ± 0.29 | 0.163 | 0.79 * | 0.39 ± 0.36 | 0.36 ± 0.35 | 0.507 | 0.77 * | 0.35 ± 0.41 | 0.28 ± 0.27 | 0.308 | 0.80 * | 0.27 ± 0.25 | 0.25 ± 0.25 | 0.361 | 0.81 * |
| Energy drinks | 0.12 ± 0.20 | 0.15 ± 0.25 | 0.074 | 0.79 * | 0.15 ± 0.23 | 0.17 ± 0.27 | 0.594 | 0.63 * | 0.13 ± 0.21 | 0.18 ± 0.27 | 0.173 | 0.85 * | 0.07 ± 0.11 | 0.11 ± 0.17 | 0.106 | 0.86 * |
| Chocolates | 0.68 ± 0.57 | 0.61 ± 0.49 | 0.032 | 0.78 * | 0.78 ± 0.62 | 0.66 ± 0.46 | 0.266 | 0.57 * | 0.71 ± 0.55 | 0.66 ± 0.44 | 0.424 | 0.79 * | 0.55 ± 0.53 | 0.50 ± 0.57 | 0.080 | 0.91 * |
| Savory snacks | 0.34 ± 0.39 | 0.34 ± 0.43 | 1.000 | 0.78 * | 0.35 ± 0.43 | 0.37 ± 0.51 | 0.683 | 0.68 * | 0.35 ± 0.33 | 0.41 ± 0.43 | 0.196 | 0.82 * | 0.30 ± 0.44 | 0.22 ± 0.28 | 0.173 | 0.82 * |
| Mayonnaise | 0.21 ± 0.31 | 0.19 ± 0.30 | 0.453 | 0.78 * | 0.14 ± 0.20 | 0.14 ± 0.20 | 0.838 | 0.75 * | 0.27 ± 0.41 | 0.25 ± 0.40 | 0.463 | 0.72 * | 0.19 ± 0.24 | 0.17 ± 0.22 | 0.735 | 0.86 * |
| Butter | 0.98 ± 0.71 | 0.98 ± 0.67 | 0.911 | 0.77 * | 0.88 ± 0.65 | 0.83 ± 0.52 | 0.367 | 0.69 * | 1.04 ± 0.75 | 1.13 ± 0.72 | 0.255 | 0.76 * | 0.99 ± 0.74 | 0.92 ± 0.72 | 0.263 | 0.92 * |
| Fruit juices and nectars | 0.63 ± 0.55 | 0.57 ± 0.53 | 0.267 | 0.77 * | 0.70 ± 0.62 | 0.51 ± 0.52 | 0.091 | 0.64 * | 0.72 ± 0.51 | 0.70 ± 0.52 | 0.636 | 0.80 * | 0.42 ± 0.45 | 0.46 ± 0.53 | 0.441 | 0.90 * |
| Kiwi and citrus fruit | 0.44 ± 0.47 | 0.46 ± 0.47 | 0.478 | 0.77 * | 0.57 ± 0.58 | 0.56 ± 0.51 | 0.683 | 0.72 * | 0.46 ± 0.43 | 0.50 ± 0.50 | 0.861 | 0.69 * | 0.27 ± 0.32 | 0.29 ± 0.32 | 0.361 | 0.92 * |
| Leafy green vegetables | 0.36 ± 0.38 | 0.37 ± 0.37 | 0.699 | 0.77 * | 0.22 ± 0.27 | 0.28 ± 0.31 | 0.328 | 0.61 * | 0.37 ± 0.37 | 0.39 ± 0.34 | 0.450 | 0.76 * | 0.50 ± 0.46 | 0.43 ± 0.46 | 0.068 | 0.94 * |
| Sweetened beverages | 0.33 ± 0.38 | 0.37 ± 0.45 | 0.255 | 0.77 * | 0.35 ± 0.42 | 0.41 ± 0.51 | 0.638 | 0.63 * | 0.36 ± 0.42 | 0.45 ± 0.50 | 0.018 | 0.89 * | 0.26 ± 0.28 | 0.22 ± 0.23 | 0.463 | 0.77 * |
| Gourds and squashes | 0.58 ± 0.56 | 0.55 ± 0.51 | 0.451 | 0.76 * | 0.51 ± 0.45 | 0.53 ± 0.45 | 0.780 | 0.67 * | 0.55 ± 0.62 | 0.45 ± 0.50 | 0.130 | 0.71 * | 0.69 ± 0.59 | 0.69 ± 0.57 | 0.686 | 0.92 * |
| Cheese | 0.68 ± 0.48 | 0.64 ± 0.48 | 0.243 | 0.75 * | 0.55 ± 0.46 | 0.52 ± 0.45 | 0.972 | 0.84 * | 0.68 ± 0.47 | 0.64 ± 0.47 | 0.534 | 0.62 * | 0.84 ± 0.49 | 0.78 ± 0.49 | 0.141 | 0.81 * |
| Margarine | 0.26 ± 0.44 | 0.27 ± 0.44 | 0.287 | 0.74 * | 0.14 ± 0.28 | 0.16 ± 0.25 | 0.163 | 0.48 * | 0.33 ± 0.50 | 0.40 ± 0.60 | 0.477 | 0.89 * | 0.30 ± 0.47 | 0.23 ± 0.33 | 0.529 | 0.82 * |
| All kinds of fruits | 0.89 ± 0.54 | 0.88 ± 0.56 | 0.936 | 0.73 * | 1.02 ± 0.60 | 0.98 ± 0.57 | 0.834 | 0.61 * | 0.88 ± 0.54 | 0.86 ± 0.59 | 0.889 | 0.73 * | 0.77 ± 0.47 | 0.79 ± 0.52 | 0.465 | 0.87 * |
| Sausages, bacon, reconstituted meat | 0.45 ± 0.47 | 0.42 ± 0.47 | 0.194 | 0.73 * | 0.37 ± 0.32 | 0.30 ± 0.36 | 0.133 | 0.52 * | 0.44 ± 0.50 | 0.46 ± 0.51 | 0.807 | 0.73 * | 0.56 ± 0.55 | 0.52 ± 0.52 | 0.263 | 0.91 * |
| Eggs and egg dishes | 0.22 ± 0.25 | 0.26 ± 0.27 | 0.099 | 0.73 * | 0.20 ± 0.22 | 0.28 ± 0.30 | 0.142 | 0.75 * | 0.24 ± 0.25 | 0.32 ± 0.29 | 0.074 | 0.70 * | 0.22 ± 0.28 | 0.17 ± 0.19 | 0.281 | 0.76 * |
| Oily fish | 0.06 ± 0.12 | 0.06 ± 0.10 | 0.844 | 0.73 * | 0.07 ± 0.14 | 0.09 ± 0.17 | 0.209 | 0.78 * | 0.05 ± 0.09 | 0.04 ± 0.04 | 0.327 | 0.74 * | 0.07 ± 0.15 | 0.04 ± 0.04 | 0.953 | 0.70 * |
| Lean fish | 0.10 ± 0.18 | 0.08 ± 0.17 | 0.269 | 0.72 * | 0.10 ± 0.22 | 0.12 ± 0.26 | 0.583 | 0.60 * | 0.10 ± 0.17 | 0.07 ± 0.13 | 0.154 | 0.80 * | 0.10 ± 0.17 | 0.05 ± 0.04 | 0.142 | 0.81 * |
| Fruit preserves and fruit condiments | 0.30 ± 0.37 | 0.33 ± 0.42 | 0.279 | 0.71 * | 0.30 ± 0.33 | 0.32 ± 0.46 | 0.931 | 0.59 * | 0.25 ± 0.37 | 0.35 ± 0.42 | 0.055 | 0.76 * | 0.37 ± 0.41 | 0.34 ± 0.40 | 0.674 | 0.77 * |
| Honey | 0.05 ± 0.10 | 0.10 ± 0.19 | 0.003 | 0.71 * | 0.02 ± 0.03 | 0.07 ± 0.20 | 0.142 | 0.36 * | 0.05 ± 0.10 | 0.12 ± 0.20 | 0.033 | 0.85 * | 0.07 ± 0.15 | 0.10 ± 0.17 | 0.123 | 0.80 * |
| Bananas | 0.42 ± 0.43 | 0.41 ± 0.38 | 0.880 | 0.70 * | 0.56 ± 0.53 | 0.50 ± 0.42 | 0.824 | 0.63 * | 0.31 ± 0.39 | 0.33 ± 0.38 | 0.351 | 0.70 * | 0.41 ± 0.33 | 0.41 ± 0.34 | 0.933 | 0.75 * |
| Refined cereals | 0.92 ± 0.59 | 0.98 ± 0.63 | 0.237 | 0.69 * | 0.91 ± 0.61 | 0.98 ± 0.61 | 0.422 | 0.50 * | 0.86 ± 0.59 | 0.86 ± 0.61 | 0.826 | 0.81 * | 1.00 ± 0.57 | 1.13 ± 0.67 | 0.182 | 0.67 * |
| Vegetable-based oil | 0.41 ± 0.43 | 0.37 ± 0.44 | 0.190 | 0.69 * | 0.36 ± 0.34 | 0.27 ± 0.30 | 0.163 | 0.46 * | 0.50 ± 0.56 | 0.48 ± 0.60 | 0.784 | 0.82 * | 0.34 ± 0.28 | 0.33 ± 0.28 | 0.673 | 0.73 * |
| Breakfast cereals | 0.34 ± 0.36 | 0.34 ± 0.39 | 0.784 | 0.69 * | 0.43 ± 0.40 | 0.40 ± 0.47 | 0.663 | 0.67 * | 0.35 ± 0.38 | 0.36 ± 0.36 | 0.809 | 0.68 * | 0.22 ± 0.27 | 0.24 ± 0.30 | 0.735 | 0.75 * |
| Whole meal cereals | 0.61 ± 0.64 | 0.64 ± 0.61 | 0.826 | 0.68 * | 0.57 ± 0.63 | 0.73 ± 0.68 | 0.438 | 0.38 * | 0.66 ± 0.70 | 0.68 ± 0.66 | 0.610 | 0.91 * | 0.60 ± 0.57 | 0.48 ± 0.40 | 0.445 | 0.69 * |
| Baked confectionery | 0.36 ± 0.45 | 0.37 ± 0.41 | 0.466 | 0.68 * | 0.28 ± 0.29 | 0.39 ± 0.38 | 0.037 | 0.59 * | 0.30 ± 0.33 | 0.32 ± 0.31 | 0.778 | 0.63 * | 0.51 ± 0.67 | 0.43 ± 0.55 | 0.208 | 0.85 * |
| Nuts and nut spreads | 0.21 ± 0.32 | 0.20 ± 0.35 | 0.357 | 0.68 * | 0.28 ± 0.30 | 0.29 ± 0.43 | 0.824 | 0.61 * | 0.27 ± 0.40 | 0.24 ± 0.38 | 0.535 | 0.55 * | 0.06 ± 0.04 | 0.05 ± 0.04 | 0.173 | 0.80 * |
| Berries | 0.35 ± 0.45 | 0.29 ± 0.37 | 0.178 | 0.67 * | 0.45 ± 0.60 | 0.33 ± 0.42 | 0.334 | 0.42 * | 0.36 ± 0.36 | 0.32 ± 0.36 | 0.366 | 0.75 * | 0.25 ± 0.33 | 0.22 ± 0.32 | 0.834 | 0.78 * |
| Avocado | 0.03 ± 0.21 | 0.04 ± 0.23 | 0.959 | 0.67 * | 0.07 ± 0.36 | 0.10 ± 0.40 | 0.753 | 0.44 * | 0.02 ± 0.09 | 0.02 ± 0.09 | 0.800 | 0.73 * | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.789 | 0.86 * |
| Venison | 0.00 ± 0.02 | 0.01 ± 0.02 | 0.197 | 0.67 * | 0.00 ± 0.02 | 0.01 ± 0.03 | 0.109 | 0.75 * | 0.01 ± 0.02 | 0.01 ± 0.01 | 1.000 | 0.58 * | 0.00 ± 0.01 | 0.00 ± 0.01 | NA | 0.80 * |
| Potatoes | 0.79 ± 0.46 | 0.78 ± 0.47 | 0.952 | 0.66 * | 0.78 ± 0.43 | 0.83 ± 0.47 | 0.478 | 0.51 * | 0.84 ± 0.45 | 0.77 ± 0.46 | 0.295 | 0.57 * | 0.74 ± 0.50 | 0.75 ± 0.49 | 0.675 | 0.89 * |
| Apples and pears | 0.68 ± 0.46 | 0.66 ± 0.43 | 0.487 | 0.66 * | 0.86 ± 0.51 | 0.77 ± 0.43 | 0.249 | 0.56 * | 0.56 ± 0.48 | 0.62 ± 0.48 | 0.505 | 0.57 * | 0.63 ± 0.31 | 0.58 ± 0.34 | 0.173 | 0.81 * |
| Cheese curds | 0.29 ± 0.35 | 0.27 ± 0.27 | 0.887 | 0.66 * | 0.27 ± 0.33 | 0.22 ± 0.29 | 0.328 | 0.59 * | 0.29 ± 0.32 | 0.32 ± 0.25 | 0.267 | 0.65 * | 0.31 ± 0.43 | 0.25 ± 0.27 | 0.415 | 0.80 * |
| Tropical fruits | 0.15 ± 0.29 | 0.20 ± 0.37 | 0.202 | 0.65 * | 0.19 ± 0.41 | 0.27 ± 0.53 | 0.397 | 0.50 * | 0.16 ± 0.24 | 0.21 ± 0.30 | 0.327 | 0.77 * | 0.09 ± 0.17 | 0.10 ± 0.17 | 0.534 | 0.62 * |
| Flavored cheese curds | 0.13 ± 0.22 | 0.13 ± 0.28 | 0.311 | 0.65 * | 0.12 ± 0.23 | 0.18 ± 0.42 | 1.000 | 0.48 * | 0.17 ± 0.25 | 0.13 ± 0.20 | 0.507 | 0.76 * | 0.09 ± 0.14 | 0.08 ± 0.14 | 0.260 | 0.70 * |
| Fresh and tinned legumes | 0.13 ± 0.19 | 0.14 ± 0.28 | 0.531 | 0.63 * | 0.16 ± 0.21 | 0.21 ± 0.41 | 0.875 | 0.40 * | 0.14 ± 0.22 | 0.11 ± 0.19 | 0.286 | 0.70 * | 0.10 ± 0.14 | 0.11 ± 0.20 | 0.715 | 0.86 * |
| Red meat | 0.10 ± 0.17 | 0.10 ± 0.16 | 0.915 | 0.63 * | 0.05 ± 0.10 | 0.09 ± 0.17 | 0.108 | 0.70 * | 0.10 ± 0.17 | 0.09 ± 0.15 | 0.551 | 0.56 * | 0.15 ± 0.20 | 0.12 ± 0.17 | 0.173 | 0.59 * |
| Cream | 0.24 ± 0.33 | 0.24 ± 0.33 | 0.928 | 0.62 * | 0.24 ± 0.28 | 0.22 ± 0.25 | 0.925 | 0.35 | 0.26 ± 0.41 | 0.29 ± 0.42 | 0.601 | 0.65 * | 0.21 ± 0.26 | 0.19 ± 0.25 | 0.590 | 0.91 * |
| Stone fruit | 0.45 ± 0.49 | 0.38 ± 0.42 | 0.052 | 0.61 * | 0.57 ± 0.65 | 0.43 ± 0.46 | 0.246 | 0.40 * | 0.45 ± 0.42 | 0.43 ± 0.45 | 0.363 | 0.64 * | 0.32 ± 0.33 | 0.25 ± 0.30 | 0.128 | 0.84 * |
| Milk beverages—sweetened | 0.51 ± 0.44 | 0.51 ± 0.39 | 0.622 | 0.60 * | 0.58 ± 0.51 | 0.48 ± 0.41 | 0.449 | 0.29 | 0.55 ± 0.45 | 0.58 ± 0.41 | 0.334 | 0.71 * | 0.37 ± 0.32 | 0.44 ± 0.33 | 0.075 | 0.86 * |
| Ice-cream and custard | 0.24 ± 0.33 | 0.21 ± 0.36 | 0.076 | 0.60 * | 0.27 ± 0.39 | 0.21 ± 0.40 | 0.142 | 0.35 | 0.22 ± 0.31 | 0.17 ± 0.25 | 0.244 | 0.71 * | 0.22 ± 0.30 | 0.25 ± 0.43 | 0.625 | 0.72 * |
| Seeds and bran | 0.15 ± 0.26 | 0.17 ± 0.32 | 0.637 | 0.59 * | 0.20 ± 0.29 | 0.19 ± 0.29 | 0.542 | 0.26 | 0.20 ± 0.31 | 0.26 ± 0.42 | 0.328 | 0.78 * | 0.03 ± 0.03 | 0.02 ± 0.02 | 0.124 | 0.64 * |
| Milk and milk beverages—natural | 0.61 ± 0.47 | 0.52 ± 0.46 | 0.015 | 0.58 * | 0.59 ± 0.53 | 0.62 ± 0.65 | 0.811 | 0.52 * | 0.62 ± 0.48 | 0.47 ± 0.36 | 0.041 | 0.58 * | 0.62 ± 0.40 | 0.47 ± 0.30 | 0.018 | 0.76 * |
| Vegetable and vegetable-fruit juices | 0.28 ± 0.41 | 0.22 ± 0.31 | 0.075 | 0.58 * | 0.35 ± 0.51 | 0.15 ± 0.21 | 0.005 | 0.58 * | 0.29 ± 0.39 | 0.33 ± 0.41 | 0.642 | 0.52 * | 0.18 ± 0.26 | 0.13 ± 0.19 | 0.263 | 0.74 * |
| Offal products | 0.05 ± 0.11 | 0.07 ± 0.23 | 0.483 | 0.54 * | 0.06 ± 0.14 | 0.12 ± 0.38 | 0.784 | 0.52 * | 0.03 ± 0.04 | 0.06 ± 0.13 | 0.826 | 0.44 * | 0.07 ± 0.15 | 0.04 ± 0.11 | 0.142 | 0.74 * |
| Fine groats | 0.15 ± 0.21 | 0.13 ± 0.16 | 0.474 | 0.51 * | 0.16 ± 0.23 | 0.14 ± 0.17 | 0.894 | 0.36 * | 0.14 ± 0.19 | 0.12 ± 0.16 | 0.878 | 0.63 * | 0.16 ± 0.20 | 0.11 ± 0.13 | 0.333 | 0.52 * |
| All kinds of vegetables (potatoes not included) | 0.69 ± 0.47 | 0.71 ± 0.46 | 0.837 | 0.50 * | 0.68 ± 0.54 | 0.73 ± 0.56 | 0.650 | 0.35 | 0.74 ± 0.53 | 0.71 ± 0.49 | 0.496 | 0.56 * | 0.64 ± 0.30 | 0.69 ± 0.29 | 0.273 | 0.70 * |
| Coarse groats | 0.09 ± 0.16 | 0.09 ± 0.13 | 0.911 | 0.48 * | 0.17 ± 0.27 | 0.11 ± 0.16 | 0.712 | 0.36 * | 0.06 ± 0.04 | 0.10 ± 0.15 | 0.155 | 0.41 * | 0.06 ± 0.04 | 0.05 ± 0.04 | 0.059 | 0.70 * |
| Root vegetables and others | 0.39 ± 0.35 | 0.37 ± 0.37 | 0.335 | 0.45 * | 0.34 ± 0.32 | 0.33 ± 0.31 | 0.862 | 0.09 | 0.39 ± 0.36 | 0.33 ± 0.42 | 0.124 | 0.53 * | 0.46 ± 0.36 | 0.46 ± 0.36 | 0.878 | 0.74 * |
| Dry and processed pulses | 0.07 ± 0.10 | 0.08 ± 0.15 | 0.685 | 0.43 * | 0.07 ± 0.10 | 0.10 ± 0.16 | 0.679 | 0.27 | 0.07 ± 0.09 | 0.09 ± 0.18 | 0.514 | 0.48 * | 0.06 ± 0.11 | 0.04 ± 0.04 | 0.612 | 0.58 * |
| Yellow-orange vegetables | 0.50 ± 0.42 | 0.45 ± 0.36 | 0.204 | 0.33 * | 0.50 ± 0.48 | 0.43 ± 0.44 | 0.334 | 0.39 * | 0.53 ± 0.43 | 0.48 ± 0.32 | 0.799 | 0.63 * | 0.46 ± 0.32 | 0.43 ± 0.31 | 0.499 | 0.76 * |
| Cruciferous vegetables | 0.29 ± 0.34 | 0.28 ± 0.28 | 0.981 | 0.24 * | 0.20 ± 0.27 | 0.20 ± 0.23 | 0.784 | 0.48 * | 0.35 ± 0.41 | 0.32 ± 0.31 | 0.646 | 0.81 * | 0.31 ± 0.30 | 0.31 ± 0.27 | 0.787 | 0.72 * |
| Mean for all food items | - | - | 0.68 | - | - | 0.56 | - | - | 0.72 | - | - | 0.80 | ||||
1 Sorted by SCC values for the total sample; n—sample size; SCC—Spearman’s correlation coefficient: * p < 0.05; p–significance level of Wilcoxon’s test (for two dependent samples) for differences in means of food consumption frequency (times/day) between the test and the retest; NA–statistical analysis was not performed in this age group due to sample distribution (not enough respondents in a category).
The Spearman correlations (SCCs) between the frequency of food consumption reported in the test and the retest for food items ranged from 0.24 to 0.86 in the total sample, from 0.09 to 0.84 in females aged 13–15, from 0.41 to 0.91 in females aged 16–18, and from 0.52 to 0.98 in females aged 19–21 (Table 1). The SCC was more than 0.50 (good and very good) for 57 out of 62 food items (92% of total) in the total sample and for 42, 59, and 62 (68%, 95%, and 100%) within the age groups, respectively.
3.2. Categories of Food Consumption Frequency
The kappa statistic (test vs. retest) in the total sample averaged at 0.52 and ranged for food items from 0.32 (root vegetables and others) to 0.72 (spirits), while within age groups, it averaged at 0.41 (0.05–0.66 for food items), 0.53 (0.32–0.71 for food items), and 0.65 (0.37–0.89 for food items), respectively (Table 2). The kappa statistic in the total sample showed moderate agreement (0.41–0.60) for 50 out of 62 food items (81% of total) and good agreement (0.61–0.80) for 8/62 food items (13%). Within age groups, the kappa statistic showed moderate agreement for 26/62 food items (42% of total), 41/62 food items (66%), and 18/62 food items (29%), respectively, and good agreement for 5/62 food items (8% of total), 15/62 food items (24%), and 35/62 food items (57%), respectively. Very good agreement (≥0.81) was found for 7/62 food items (11% of total) in 19–21-year-old females only.
Table 2.
Classification agreement and misclassification of food consumption frequency in the test and the retest by age groups (%).
| Food Items 1 | Total Sample (n = 97) | 13–15 Years (n = 31) | 16–18 Years (n = 38) | 19–21 Years (n = 28) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compatible | Non-Compatible | k | Compatible | Non-Compatible | k | Compatible | Non-Compatible | k | Compatible | Non-Compatible | k | |||||
| ±1 | ±2 or More | ±1 | ±2 or More | ±1 | ±2 or More | ±1 | ±2 or More | |||||||||
| Venison | 89 | 10 | 1 | 0.56 | 90 | 6 | 3 | 0.58 | 82 | 18 | 0 | 0.50 | 96 | 4 | 0 | 0.78 |
| Other animal fats | 88 | 10 | 2 | 0.64 | 87 | 10 | 3 | 0.54 | 84 | 13 | 3 | 0.53 | 93 | 7 | 0 | 0.85 |
| Olives | 87 | 11 | 2 | 0.70 | 84 | 10 | 6 | 0.65 | 84 | 16 | 0 | 0.71 | 93 | 7 | 0 | 0.86 |
| Spirits | 86 | 10 | 4 | 0.72 | 90 | 3 | 6 | 0.66 | 76 | 18 | 5 | 0.63 | 93 | 7 | 0 | 0.89 |
| Avocado | 84 | 15 | 1 | 0.54 | 81 | 16 | 3 | 0.42 | 82 | 18 | 0 | 0.61 | 89 | 11 | 0 | 0.52 |
| Wine and cocktails | 84 | 12 | 4 | 0.71 | 87 | 6 | 6 | 0.50 | 76 | 18 | 5 | 0.66 | 89 | 11 | 0 | 0.84 |
| Beer | 80 | 18 | 2 | 0.70 | 81 | 13 | 6 | 0.62 | 74 | 26 | 0 | 0.64 | 89 | 11 | 0 | 0.83 |
| All kinds of fruits | 74 | 22 | 4 | 0.61 | 74 | 19 | 6 | 0.63 | 66 | 32 | 3 | 0.52 | 86 | 11 | 4 | 0.79 |
| Energy drinks | 74 | 19 | 7 | 0.63 | 65 | 26 | 10 | 0.51 | 76 | 16 | 8 | 0.68 | 82 | 14 | 4 | 0.74 |
| Eggs and egg dishes | 73 | 24 | 3 | 0.56 | 65 | 32 | 3 | 0.48 | 74 | 24 | 3 | 0.59 | 82 | 14 | 4 | 0.64 |
| Tomatoes | 73 | 18 | 9 | 0.62 | 74 | 16 | 10 | 0.63 | 61 | 24 | 16 | 0.49 | 89 | 11 | 0 | 0.85 |
| Chocolates | 72 | 23 | 5 | 0.60 | 65 | 23 | 13 | 0.48 | 71 | 26 | 3 | 0.60 | 82 | 18 | 0 | 0.76 |
| Kiwi and citrus fruit | 72 | 23 | 5 | 0.59 | 68 | 23 | 10 | 0.56 | 66 | 29 | 5 | 0.53 | 86 | 14 | 0 | 0.77 |
| Dried fruit | 72 | 23 | 5 | 0.57 | 55 | 32 | 13 | 0.37 | 79 | 18 | 3 | 0.67 | 82 | 18 | 0 | 0.73 |
| Cruciferous vegetables | 72 | 24 | 4 | 0.55 | 61 | 29 | 10 | 0.41 | 74 | 26 | 0 | 0.60 | 82 | 14 | 4 | 0.70 |
| Poultry and rabbit | 72 | 25 | 3 | 0.59 | 58 | 39 | 3 | 0.44 | 74 | 24 | 3 | 0.62 | 86 | 11 | 4 | 0.79 |
| Sugar confectionery | 71 | 26 | 3 | 0.58 | 74 | 19 | 6 | 0.60 | 66 | 32 | 3 | 0.53 | 75 | 25 | 0 | 0.67 |
| Savory snacks | 71 | 26 | 3 | 0.58 | 68 | 29 | 3 | 0.55 | 68 | 29 | 3 | 0.57 | 79 | 18 | 4 | 0.67 |
| Leafy green vegetables | 71 | 24 | 5 | 0.60 | 58 | 35 | 6 | 0.43 | 71 | 21 | 8 | 0.60 | 86 | 14 | 0 | 0.81 |
| Lean fish | 71 | 23 | 6 | 0.57 | 61 | 26 | 13 | 0.46 | 74 | 24 | 3 | 0.63 | 79 | 18 | 4 | 0.67 |
| Bananas | 70 | 26 | 4 | 0.54 | 65 | 29 | 6 | 0.49 | 71 | 26 | 3 | 0.56 | 75 | 21 | 4 | 0.62 |
| Fresh and tinned legumes | 70 | 22 | 8 | 0.54 | 55 | 29 | 16 | 0.37 | 71 | 24 | 5 | 0.56 | 86 | 11 | 4 | 0.76 |
| High quality cured meats | 70 | 27 | 3 | 0.56 | 61 | 32 | 6 | 0.46 | 74 | 24 | 3 | 0.61 | 75 | 25 | 0 | 0.65 |
| Honey | 69 | 25 | 6 | 0.53 | 65 | 23 | 13 | 0.39 | 71 | 26 | 3 | 0.59 | 71 | 25 | 4 | 0.60 |
| Mayonnaise | 69 | 27 | 4 | 0.56 | 68 | 29 | 3 | 0.54 | 66 | 26 | 8 | 0.53 | 75 | 25 | 0 | 0.64 |
| Fine groats | 68 | 23 | 9 | 0.46 | 65 | 16 | 19 | 0.42 | 74 | 21 | 5 | 0.58 | 64 | 32 | 4 | 0.38 |
| Apples and pears | 68 | 26 | 6 | 0.51 | 58 | 39 | 3 | 0.34 | 68 | 21 | 11 | 0.56 | 79 | 18 | 4 | 0.66 |
| Sugar | 67 | 27 | 6 | 0.54 | 55 | 29 | 16 | 0.39 | 71 | 26 | 3 | 0.61 | 75 | 25 | 0 | 0.63 |
| Baked confectionery | 67 | 26 | 7 | 0.50 | 68 | 19 | 13 | 0.46 | 63 | 29 | 8 | 0.47 | 71 | 29 | 0 | 0.59 |
| Cheese | 67 | 25 | 8 | 0.53 | 58 | 29 | 13 | 0.44 | 71 | 18 | 11 | 0.59 | 71 | 29 | 0 | 0.58 |
| Coarse groats | 67 | 24 | 9 | 0.47 | 52 | 35 | 13 | 0.29 | 71 | 18 | 11 | 0.51 | 79 | 18 | 4 | 0.64 |
| Butter | 67 | 23 | 10 | 0.53 | 61 | 23 | 16 | 0.36 | 68 | 21 | 11 | 0.57 | 71 | 25 | 4 | 0.63 |
| Yellow-orange vegetables | 67 | 25 | 8 | 0.49 | 52 | 29 | 19 | 0.30 | 74 | 21 | 5 | 0.61 | 75 | 25 | 0 | 0.60 |
| Nuts and nut spreads | 66 | 28 | 6 | 0.50 | 65 | 23 | 13 | 0.48 | 58 | 37 | 5 | 0.40 | 79 | 21 | 0 | 0.66 |
| Red meat | 66 | 26 | 8 | 0.51 | 58 | 39 | 3 | 0.40 | 63 | 24 | 13 | 0.48 | 79 | 14 | 7 | 0.68 |
| Oily fish | 66 | 33 | 1 | 0.48 | 61 | 39 | 0 | 0.45 | 68 | 32 | 0 | 0.51 | 68 | 29 | 4 | 0.51 |
| All kinds of vegetables (potatoes not included) | 65 | 28 | 7 | 0.47 | 52 | 35 | 13 | 0.34 | 61 | 34 | 5 | 0.42 | 86 | 11 | 4 | 0.76 |
| Potatoes | 65 | 32 | 3 | 0.46 | 52 | 45 | 3 | 0.23 | 66 | 29 | 5 | 0.47 | 79 | 21 | 0 | 0.69 |
| Sausages, bacon, reconstituted meat | 65 | 26 | 9 | 0.54 | 58 | 26 | 16 | 0.45 | 66 | 26 | 8 | 0.56 | 71 | 25 | 4 | 0.64 |
| Vegetable-based oil | 64 | 32 | 4 | 0.48 | 48 | 42 | 10 | 0.29 | 68 | 29 | 3 | 0.58 | 75 | 25 | 0 | 0.60 |
| Margarine | 64 | 27 | 9 | 0.52 | 48 | 32 | 19 | 0.31 | 71 | 26 | 3 | 0.63 | 71 | 21 | 7 | 0.62 |
| Gourds and squashes | 64 | 27 | 9 | 0.51 | 58 | 29 | 13 | 0.43 | 55 | 32 | 13 | 0.43 | 82 | 18 | 0 | 0.76 |
| Sweetened beverages | 64 | 31 | 5 | 0.50 | 55 | 35 | 10 | 0.38 | 61 | 37 | 3 | 0.49 | 79 | 18 | 4 | 0.67 |
| Flavored cheese curds | 63 | 28 | 9 | 0.48 | 55 | 29 | 16 | 0.39 | 66 | 29 | 5 | 0.54 | 68 | 25 | 7 | 0.54 |
| Whole meal cereals | 63 | 23 | 14 | 0.52 | 48 | 23 | 29 | 0.36 | 74 | 21 | 5 | 0.68 | 64 | 25 | 11 | 0.53 |
| Milk and milk beverages—natural | 62 | 25 | 13 | 0.46 | 42 | 39 | 19 | 0.26 | 68 | 18 | 13 | 0.57 | 75 | 18 | 7 | 0.58 |
| Cream | 62 | 28 | 10 | 0.47 | 55 | 26 | 19 | 0.38 | 53 | 37 | 11 | 0.39 | 82 | 18 | 0 | 0.74 |
| Tropical fruits | 62 | 30 | 8 | 0.43 | 55 | 29 | 16 | 0.37 | 68 | 24 | 8 | 0.54 | 61 | 39 | 0 | 0.37 |
| Offal products | 62 | 27 | 11 | 0.42 | 61 | 26 | 13 | 0.42 | 63 | 24 | 13 | 0.44 | 61 | 32 | 7 | 0.41 |
| Fruit juices and nectars | 62 | 34 | 4 | 0.48 | 65 | 26 | 10 | 0.52 | 55 | 42 | 3 | 0.39 | 68 | 32 | 0 | 0.56 |
| Milk beverages—sweetened | 61 | 28 | 11 | 0.44 | 45 | 26 | 29 | 0.22 | 61 | 34 | 5 | 0.45 | 79 | 21 | 0 | 0.68 |
| Refined cereals | 61 | 35 | 4 | 0.44 | 58 | 35 | 6 | 0.39 | 63 | 34 | 3 | 0.50 | 61 | 36 | 4 | 0.43 |
| Dry and processed pulses | 61 | 27 | 12 | 0.41 | 48 | 35 | 16 | 0.24 | 61 | 26 | 13 | 0.41 | 75 | 18 | 7 | 0.58 |
| Seeds and bran | 61 | 29 | 10 | 0.44 | 42 | 32 | 26 | 0.23 | 71 | 24 | 5 | 0.61 | 68 | 32 | 0 | 0.42 |
| Stone fruit | 60 | 33 | 7 | 0.42 | 45 | 39 | 16 | 0.27 | 61 | 34 | 5 | 0.42 | 75 | 25 | 0 | 0.64 |
| Berries | 60 | 31 | 9 | 0.45 | 52 | 29 | 19 | 0.32 | 58 | 37 | 5 | 0.44 | 71 | 25 | 4 | 0.61 |
| Fruit preserves and fruit condiments | 60 | 31 | 9 | 0.46 | 42 | 45 | 13 | 0.21 | 66 | 26 | 8 | 0.54 | 71 | 21 | 7 | 0.61 |
| Vegetable and vegetable-fruit juices | 60 | 23 | 18 | 0.45 | 52 | 26 | 23 | 0.34 | 58 | 24 | 18 | 0.44 | 71 | 18 | 11 | 0.61 |
| Cheese curds | 58 | 37 | 5 | 0.40 | 58 | 32 | 10 | 0.44 | 53 | 42 | 5 | 0.32 | 64 | 36 | 0 | 0.47 |
| Ice-creams and custard | 56 | 41 | 3 | 0.35 | 45 | 45 | 10 | 0.15 | 61 | 39 | 0 | 0.40 | 61 | 39 | 0 | 0.45 |
| Breakfast cereals | 55 | 36 | 9 | 0.39 | 42 | 45 | 13 | 0.25 | 50 | 42 | 8 | 0.32 | 75 | 18 | 7 | 0.65 |
| Root vegetables and others | 51 | 32 | 18 | 0.32 | 32 | 35 | 32 | 0.05 | 55 | 26 | 18 | 0.41 | 64 | 36 | 0 | 0.47 |
| Mean for all food items | 68 | 25 | 7 | 0.52 | 60 | 28 | 12 | 0.41 | 68 | 26 | 6 | 0.53 | 77 | 21 | 3 | 0.65 |
1 Sorted by compatible classification (%) for the total sample; k–the Fleiss’ kappa.
The percentage of subjects classified into the same food frequency category (test vs. retest) in the total sample was on average 68% and ranged for food items from 51% (‘root vegetables and others’) to 89% (venison), while within age groups it averaged at 60%, 68%, and 77%, respectively (Table 2).
3.3. Dietary Patterns Identified from 60 Food Items
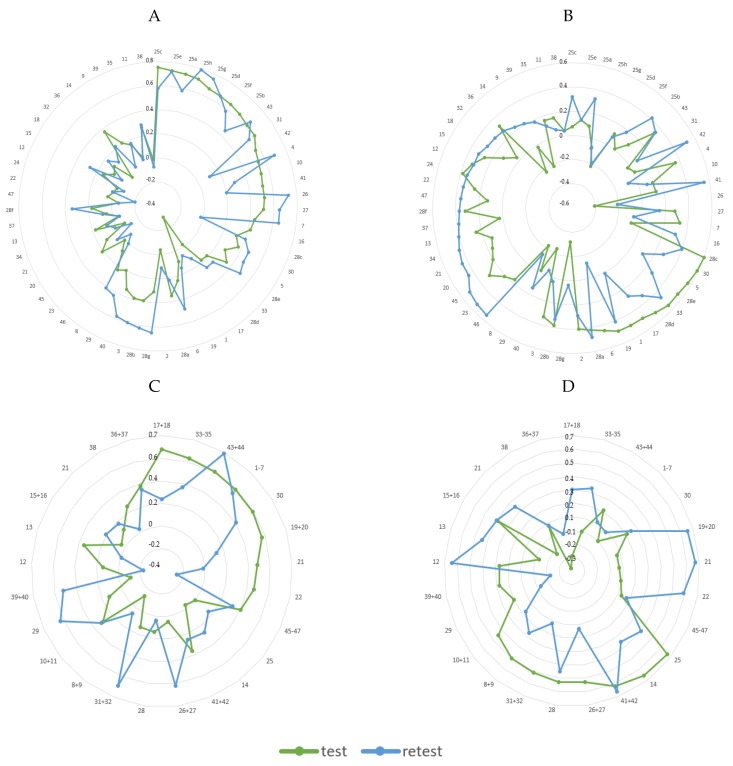
Considering 60 food items as input variables, two similar dietary patterns (DP1 and DP2) were identified from both test data and retest data (Table S2). The total variance explained for two DPs derived from test data was 23.4%, including 15.9% for the 60-item-DP1 and 7.5% for the 60-item-DP2, and from the retest data it was 25.8%, including 18.0% for the 60-item-DP1 and 7.8% for the 60-item-DP2. Figure 1 presents the factor loadings of dietary patterns identified from different numbers of food items in the test and the retest to visually present the similarities/differences between DPs.
Figure 1.
Diagrams with factor loadings of dietary patterns identified in the total sample from a different number of food items in the test and the retest: (A) 60-item-DP1, (B) 60-item-DP2, (C) 25-item-DP1, (D) 25-item-DP2. The numbers correspond to food items: 1–sugar; 2–honey; 3–chocolates; 4–sugar confectionery; 5–baked confectionery; 6–ice-creams and custard; 7–savory snacks; 8–milk and milk beverages—natural; 9–cheese curds; 10–milk beverages—sweetened; 11–flavored cheese curds; 12–cheese; 13–eggs and egg dishes; 14–breakfast cereals; 15–whole meal cereals; 16–coarse groats; 17–refined cereals; 18–fine groats; 19–butter; 20–cream; 21–other animal fats; 22–vegetable-based oil; 23–margarine; 24–mayonnaise; 25–all kinds of fruits; 25a–stone fruit; 25b–kiwi and citrus fruit; 25c–tropical fruits; 25d–berries; 25e–bananas; 25f–apples and pears; 25g–avocado; 25h–olives; 26–dried fruit; 27–fruit preserves and fruit condiments; 28–all kinds of vegetables (potatoes not included); 28a–cruciferous vegetables; 28b–yellow-orange vegetables; 28c–leafy green vegetables; 28d–tomatoes; 28e–gourds and squashes; 28f–root vegetables and others; 28g–fresh and tinned legumes; 29–dry and processed pulses; 30–potatoes; 31–nuts and nut spreads; 32–seeds and bran; 33–sausages, bacon, reconstituted meat; 34–high-quality cured meats; 35–offal products; 36–red meat; 37–venison; 38–poultry and rabbit; 39–lean fish; 40–oily fish; 41–fruit juices and nectars; 42–vegetable and vegetable-fruit juices; 43–sweetened beverages; 44–energy drinks; 45–beer; 46–wine and cocktails; 47–spirits.
The 60-item-DP1 derived from test-data was positively loaded by the frequency of consumption of tropical fruits (factor loading: 0.75), bananas (0.73); stone fruit (0.72); olives (0.71); avocado (0.67); berries (0.66); apples and pears (0.65); kiwi and citrus fruit (0.64); sweetened beverages (0.62); nuts and nut spread (0.62); vegetable and vegetable-fruit juices (0.56); sugar confectionery (0.54); milk beverages—sweetened (0.53); fruit juices and nectars (0.52); dried fruit (0.52); fruit preserves and fruit condiments (0.51); savory snacks (0.44); and coarse groats (0.42) (Table S2, Figure 1). The 60-item-DP2 derived from test-data was positively loaded by the frequency of consumption of leafy green vegetables (0.60); potatoes (0.59); baked confectionery (0.57); gourds and squashes (0.55); sausages, bacon, reconstituted meat (0.55); tomatoes (0.52); refined cereals (0.49); sugar (0.49); butter (0.49); ice-cream and custard (0.45); cruciferous vegetables (0.42); and honey (0.40) and negatively loaded by the consumption frequency of dried fruit (−0.41).
The Spearman correlation between dietary pattern scores (test vs. retest) in the total sample was 0.84 (within age groups 0.83, 0.81, and 0.78, respectively) for 60-item-DP1 and 0.68 (within age groups 0.24, 0.79, and 0.76, respectively) for the 60-item-DP2 (all p < 0.05, except for 60-item-DP2 in females aged 13–15 years; Table 3). The agreement of subject distribution by tertiles of DPs in the total sample was 59% (within age groups 68%, 63%, and 43%, respectively) for the 60-item-DP1, and it was 52% (within age groups 32%, 68%, and 50%, respectively) for the 60-item-DP2 (Table 4). The misclassification to the extreme tertiles in the total sample was 4% (within age groups 0%, 8%, and 4%, respectively) for the 60-item-DP1, and was 7% (within age groups 10%, 5%, and 7%, respectively) for the 60-item-DP2.
Table 3.
Spearman correlations for scores of dietary patterns (DP) identified in the total sample in the test and the retest by age group.
| Age Group | DP1 | p | DP2 | p | ||
|---|---|---|---|---|---|---|
| 60-Item-DP1 | 25-Item-DP1 | 60-Item-DP2 | 25-Item-DP2 | |||
| Total sample (n = 97) | 0.84 * | 0.76 * | 0.1247 | 0.68 * | 0.48 * | 0.0372 |
| 13-15 years (n = 31) | 0.83 * | 0.56 * | 0.0422 | 0.24 | 0.40 * | 0.5060 |
| 16-18 years (n = 38) | 0.81 * | 0.82 * | 0.9012 | 0.79 * | 0.57 * | 0.0804 |
| 19-21 years (n = 28) | 0.78 * | 0.89 * | 0.1889 | 0.76 * | 0.53 * | 0.1571 |
n—sample size; significance level of Spearman's rank correlation: * p < 0.05; p—significance value for comparison of Spearman correlations between DPs identified from different number of food items (60 or 25).
Table 4.
Agreement of subjects’ distribution by tertiles of dietary patterns (DPs) identified in the total sample in the test and the retest by age group (%).
| Agreement of Subjects’ Distribution | DP1 | DP2 | ||
|---|---|---|---|---|
| 60-Item-DP1 | 25-Item-DP1 | 60-Item-DP2 | 25-Item-DP2 | |
| Total sample (n = 97) | ||||
| Compatible | 59 | 54 a | 52 | 39 a |
| Non-compatible ± 1 category | 37 | 38 | 41 | 46 |
| Non-compatible ± 2 category | 4 | 8 | 7 | 14 |
| 13–15 years (n = 31) | ||||
| Compatible | 68 b | 48 | 32 b | 32 |
| Non-compatible ±1 category | 32 c | 42 | 58 c | 55 |
| Non-compatible ±2 category | 0 | 10 | 10 | 13 |
| 16–18 years (n = 38) | ||||
| Compatible | 63 | 58 | 68 | 45 |
| Non-compatible ±1 category | 29 | 37 | 26 | 42 |
| Non-compatible ±2 category | 8 | 5 | 5 | 13 |
| 19–21 years (n = 28) | ||||
| Compatible | 43 | 54 | 50 | 39 |
| Non-compatible ±1 category | 54 | 36 | 43 | 43 |
| Non-compatible ±2 category | 4 | 11 | 7 | 18 |
n—sample size; the same letters (a-a, b-b, c-c) indicate statistically significant difference (p < 0.05) in pairs (chi-square test).
3.4. Dietary Patterns Identified from 25 Food Items
Considering 25 food items as input variables, two similar dietary patterns (DP1 and DP2) were identified from both test data and retest data (Table S3, Figure 1). The total variance explained for two DPs derived from test data was 26.6%, including 16.1% for the 25-item-DP1 and 10.5% for the 25-item-DP2, and from retest data 28.2%, including 17.7% for the 25-item-DP1 and 10.5% for the 25-item-DP2.
The 25-item-DP1 derived from test data was positively loaded by the frequency of consumption of refined grain products (factor loading: 0.68), processed meats (0.63); sweetened beverages and energy drinks (0.61); sugar, sweets, and snacks (0.59); potatoes (0.59); butter and cream (0.57); other edible fats (0.48); vegetable oils (0.46); and alcohol (0.40) (Table S3, Figure 1). The 25-item-DP2 derived from test data was positively loaded by the frequency of consumption of fruits (0.67); breakfast cereals (0.66); juices (0.62); dried fruit, fruit preserves, and fruit condiments (0.53); vegetables (0.53); nuts and seeds (0.51); milk, fermented milk drinks, and curd cheese (0.50); and sweetened milk products (0.45).
The Spearman correlation between dietary pattern scores (test vs. retest) in the total sample was 0.76 (within age groups 0.56, 0.82, and 0.89, respectively) for 25-item-DP1, and it was 0.48 (within age groups 0.40, 0.57, and 0.53, respectively) for 25-item-DP2 (all p < 0.05; Table 3). The agreement of subject distribution by tertiles of DPs in the total sample was 54% (within age groups 48%, 58%, and 54%, respectively) for the 25-item-DP1, and it was 39% (within age groups 32%, 45%, and 39%, respectively) for the 25-item-DP2 (Table 4). The misclassification to the extreme tertiles in the total sample was 8% (within age groups 10%, 5%, and 11%, respectively) for 25-item-DP1, and it was 14% (within age groups 13%, 13%, and 18%, respectively) for the 25-item-DP2.
4. Discussion
4.1. General Reproducibility
It has been found that the reproducibility of the questionnaire was good or very good for most food items regardless of the statistical approach used. In the total sample, the Spearman’s correlation coefficient was on average 0.68, which is considered a good result obtained for FFQ [1]. The same interpretation can be drawn for test–retest reproducibility measured with the kappa statistic (on average 0.52), the percentage of compatible classification into the food frequency category (on average 68%) and the comparison of mean of food consumption frequency (no significant differences for 92% of items). These results are in line with those previously reported. Based on the literature review, Cade et al. [5] stated that correlation coefficients in the range of 0.5–0.7 between test and retest of the FFQs were often reported. For the example, such correlations (0.50–0.70) were found among Brazilian, Danish, Norwegian, and Chinese adolescents [41,42,43,44,45] and American adults (on average 0.70) [46], while a slightly higher correlation (on average 0.78) was noted in Polish young females [22]. In Belgian adolescents, the percentage of compatible classification between test and retest ranged from 37% to 87% [47].
Better reproducibility of the questionnaire was found in older than younger age groups of females (19–21 vs. 16–18 vs. 13–15 years). The Spearman’s correlation coefficient and the kappa statistic tended to be higher in older age groups. For all food items, the highest percentages of compatible classification into the food frequency category were found for the oldest females (19–21 years), while the lowest was for the youngest females (13–15 years). There are a few explanations for the higher reproducibility of the questionnaire in older females (19–21 years). Older females could better identify foods based on its name (given in the questionnaire without a photo gallery or 3D models) and they could better distinguish various types of foods and accurately determine the frequency of consumed food. Older females (as with most adults, in comparison with adolescents) tended to have more stable dietary habits, which are easier to report. Difficulties in precisely assessing dietary intake by adolescent respondents were previously reported [48,49,50]. This was explained by adolescents′ lower knowledge related to food perception and preparation compared to adults and also in rapid changes in dietary habits during adolescence, less eating at home and less supervision by adults. These changes result from a growing sense of independence, peer influence and awareness of social acceptance, greater emotional and financial autonomy, limited time of concentration and attention, and also a lack of interest and motivation to monitor one′s own diet [49,50].
Regardless of the statistical approach used, test–retest reproducibility was the highest for foods consumed occasionally or never (e.g., spirits, olives, venison), and the lowest for foods consumed often (e.g., root vegetables and others, cruciferous vegetables, cheese curds). It can be speculated that some of the foods with lower reproducibility could be consumed as a component of complex dishes (e.g., root vegetables and others in vegetable salad) which could cause difficulties in determining the consumption frequency. A bias in the reported food consumption frequency should be also considered, including overestimation and underestimation. Young people, especially females, may overestimate the consumption of food considered healthy (e.g., vegetables), and to the opposite, can underestimate the consumption of foods considered unhealthy (e.g., fast-foods, soft drinks, sweets, and salty snacks) [51]. In adolescence, requirements for increasing energy supply, concerns related to self-image, and following food fashions may all contribute to poor compliance in dietary reporting [49].
4.2. Reproducibility of the Identification of Dietary Patterns
Regardless of the data set used (with 60 or 25 food items), two dietary patterns were identified in the total sample. The first dietary pattern was characterized by the frequent consumption of various types of fruit, sweetened milk products, nuts and seeds, juices, dried fruit, fruit preserves and fruit condiments. This dietary pattern, having a fruit–vegetable–milk profile, can be classified as pro-healthy, although sweet. A similar dietary pattern (the fruit and vegetables) was previously found in a representative sample of Polish females aged 13–21 years [52], in which a positive attitude towards health and natural product interest were revealed. Females chose fruits presumably because of the desire to be healthy and enjoying the good taste of food [53]. Vegetables are also perceived by females as healthy, fashionable, and low-calorie [27,54,55]. Across the world and various subpopulations, healthy or prudent dietary patterns consisting of fruit and vegetables as well as low-fat dairy products, whole grains, legumes, fish and seafood, nuts, and vegetable oils were identified more often [56,57,58].
The second dietary pattern was characterized by the frequent consumption of processed meats, refined grain products, potatoes, butter, sugar and sweets. This dietary pattern can be classified as Polish traditional with a westernized profile. Traditional westernized dietary patterns were identified across the world, showing a universal trend toward diet westernization, i.e., a shift from traditional foods towards highly processed, high-fat, high-sugar, and low-fiber foods [13,14,59]. It was discussed that the taste, food availability, and also following food fashions are important factors of food choice, especially for young people [60,61,62]. Karimi-Shahanjarini et al. [61] showed that young females aged 12–15 years were eating unhealthy snacks because of the taste, easy access, and high price of healthy snacks, and the potential risk of disease in the future was not important to them.
It was found that the dietary patterns identified from retest data were similar to those identified from the test data. This was documented by considering dietary patterns scores in the test and retest. In the total sample, the Spearman’s correlation coefficients were good to very good (0.68–0.84) except one was slightly lower (0.48), and the agreement of subjects’ distribution by tertiles of dietary patterns was acceptable to good (39% to 59%). Based on this, the use of the questionnaire to identify dietary patterns in young Polish females can be recommended. It is possible that the 62-item FFQ-6 can also be used in other European countries with similar dietary habits and food availability, although further investigation is needed. There are limited data regarding the reproducibility of the dietary patterns identified with the FFQs. Such an analysis was previously conducted for American, Spanish, and Japanese adults [46,63,64]. Among American men, two dietary patterns were identified from the 131-item FFQ [46]. The correlations between first and second (1 year apart) administration of the FFQ were good: 0.70 for the Prudent pattern and 0.67 for the Western pattern. In Japanese adults, the reproducibility of dietary patterns was reported based on a systematic review of PCA-derived dietary patterns [64]. The reproducibility of dietary patterns was assessed using a congruence coefficient (CC). When high quality data, i.e., coming from a validated FFQ or multiple-day dietary records and sample size ≥ 200, were included, the median CC was high for Healthy pattern (0.89), Prudent pattern (0.86), and Japanese pattern (0.80), while it was low for the Traditional pattern (0.59), Western pattern (0.44), and Traditional Japanese pattern (0.31). This systematic review has shown that there are some dietary patterns that are relatively reproducible in different populations in a given country. The reproducibility of data-driven dietary patterns was assessed in different Spanish samples extracted from similar populations [63] using congruence coefficients similarly to the Japanese study [64]. The median of the CC was 0.90 for Western pattern, 0.77 for the Mediterranean pattern, and 0.76 for Prudent pattern. Due to a lack of similar studies covering respondents at the same age (13–21 years), and taking into account that reporting of food consumption is more biased in adolescents than adults [49], the current findings (i.e., Spearman’s correlation 0.48–0.84) cannot be directly compared with those cited above. However, it can be speculated that reproducibility of pro-healthy dietary patterns (e.g., Prudent) is better than non-healthy dietary patterns (e.g., Western).
It is difficult to comprehensively discuss the reproducibility of dietary patterns identified across the age groups (13–15 vs. 16–18 vs. 19–21 years) due to the small number of subjects (31 vs. 38 vs. 28, respectively). However, showing great caution in reasoning, it can be suggested that better reproducibility of dietary patterns identification was found in older than younger age groups of females. A possible explanation was discussed in Section 4.1.
4.3. Strengths and Limitations
The main strength of the study is applying several methods of statistical analysis as recommended, all suitable for an evaluation of the FFQs’ reproducibility [5,65]. Such an approach—the application of multiple statistical tests—allows for gaining comprehensive insights, reduces the chance of an over-interpretation of research findings, strengthens the conclusions, and increases the possibility of comparing these results with others. Secondly, using two data sets, i.e., with 60 and 25 food items, provided the possibility of using the questionnaire to identify dietary patterns from non-aggregated and aggregated food items and, indirectly, the usefulness of the questionnaire in terms of processing the dietary data obtained. This indicates the possibilities offered by this questionnaire and may be an inspiration for less advanced researchers. To facilitate future use of this questionnaire by other researchers, the manual (Table S4) is attached.
The limitation of the study is the relatively small number of subjects (97). The findings related to the age groups should be particularly interpreted with caution (as tendency) due to the small number of respondents (31, 38, and 28). However, in FFQ validation studies, similarly numbered samples (in total 48–90) were previously reported in adolescents from Denmark [43], Brazil [41], New Zealand [11], and Norway [44], and also in young Polish females [17,20]. Furthermore, similarly numbered sub-samples (40–66) were analyzed across the age, sex, or ethnicity in children and adolescents [66] and adults [39,67]. When a principal component analysis (PCA) was performed from 60 food items, the subject-to-item ratio was low (1.6:1, i.e., 97 subjects to 60 items), but for 25 food items it can be considered as sufficient (3.9:1, i.e., 97 subjects to 25 items) because it was slightly below the lower border of the recommended range of the subject-to-item ratio [68,69]. Regardless of the limitations in the PCA, it should be emphasized that the PCA was performed to assess repeatability in identifying dietary patterns, and not to interpret them comprehensively. Since only one gender group of Polish residents was selected with a narrow age range (13–21 years), these findings cannot be applied to people of different age or gender. Even so, it may be supposed that similar relations can be found in other European females of a similar age. Since dietary interviews conducted in adults are less burdened by measurement errors compared to adolescents [49], it can be speculated that this questionnaire can be used in adults of both sexes as they provide relatively reliable dietary data. In the present study, the reproducibility was assessed for a questionnaire administrated by trained interviewers, so it can be assumed that the reproducibility will be lower for a self-administered questionnaire. Previously, the better reproducibility of the interviewer-administered KomPAN® questionnaire than its self-administered version was revealed in Polish adolescents and adults [24].
5. Conclusions
The test–retest reproducibility of the 62-item FFQ-6 was good or very good for most food items, with a tendency to be higher in older age groups of females under study. Due to the acceptable-to-good reproducibility of identification of dietary patterns derived using the principal component analysis, the use of 62-item FFQ-6 to describe the overall diet of Polish young females can be recommended.
Acknowledgments
Thanks are expressed to the participants for their contribution to the study.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/9/2183/s1, Table S1: Questionnaire food items (62 items) description and foods aggregation into 25 food items, Table S2: Factor loading matrix for the two major dietary patterns identified by principal component analysis with 60 food items as input variables (excluding all kinds of fruits and all kinds of vegetables), Table S3: Factor loading matrix for the two major dietary patterns identified by principal component analysis with 25 food items as input variables, Table S4: Food Frequency Questionnaire (62-item FFQ-6) including a manual for the adjustment of consumption frequency of single items of vegetables and fruits.
Author Contributions
E.N. and L.W. were involved in the conceptualization, the study design and methodology. E.N. and L.W. developed the Food Frequency Questionnaire (62-item FFQ-6); E.N., L.W. and J.K. were involved in the development of the manual for the adjustment of consumption frequency of single items of vegetables and fruits. E.N. was involved in the data acquisition. E.N. and J.K. statistically analyzed the data; E.N. and L.W. were involved in the data visualization; E.N., L.W. and J.K. interpreted the data; E.N. wrote the manuscript. L.W. and J.K. were involved in critically revising the manuscript. L.W. was involved in the funding acquisition. All authors have given their approval to the manuscript submitted.
Funding
The project was financially supported by the Ministry of Science and Higher Education in the range of the program entitled "Regional Initiative of Excellence" for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Willett W. Nutritional Epidemiology. 3rd ed. Oxford University Press; New York, NY, USA: 2012. [Google Scholar]
- 2.Food and Agriculture Organization (FAO) Dietary Assessment: A Resource Guide to Method Selection and Application in Low Resource Settings. [(accessed on 20 June 2019)]; Available online: http://www.fao.org/3/i9940en/I9940EN.
- 3.Laureano G.H.C., Torman V.B.L., Crispim S.P., Dekkers A.L.M., Camey S.A. Comparison of the ISU, NCI, MSM, and SPADE methods for estimating usual intake: A simulation study of nutrients consumed daily. Nutrients. 2016;8:166. doi: 10.3390/nu8030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olukotun O., Seal N. A systematic review of dietary assessment tools for children age 11 years and younger. ICAN. 2015;7:139–147. doi: 10.1177/1941406415584819. [DOI] [Google Scholar]
- 5.Cade J., Thompson R., Burley V., Warm D. Development, validation and utilisation of food-frequency questionniares—A review. Public Health Nutr. 2002;5:567–587. doi: 10.1079/PHN2001318. [DOI] [PubMed] [Google Scholar]
- 6.Andersen L.F., Johansson L., Solvoll K. Usefulness of a short food frequency questionnaire for screening of low intake of fruit and vegetable and for intake of fat. Eur. J. Public Health. 2002;12:208–213. doi: 10.1093/eurpub/12.3.208. [DOI] [PubMed] [Google Scholar]
- 7.Saeedi P., Skeaff S.A., Wong J.E., Skidmore M.L. Reproducibility and relative validity of short food frequency questionnaire in 9–10 year-old children. Nutrients. 2016;8:271. doi: 10.3390/nu8050271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bel-Serrat S., Mouratidou T., Pala V., Huybrechts I., Börnhorst C., Fernández-Alvira J.M., Hadjigeorgiou C., Eiben G., Hebestreit A., Lissner L., et al. Relative validity of the children’s eating habits questionnaire-food frequency section among young European children: the IDEFICS study. Public Health Nutr. 2014;17:266–276. doi: 10.1017/S1368980012005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiwanuka S.N., Astrøm A.N., Trovik T.A. Sugar snack consumption in Ugandan schoolchildren: Validity and reliability of a food frequency questionnaire. Community Dent. Oral Epidemiol. 2006;34:372–380. doi: 10.1111/j.1600-0528.2006.00287.x. [DOI] [PubMed] [Google Scholar]
- 10.Magarey A., Golley R., Spurrier N., Goodwin E., Ong F. Reliability and validity of the Children’s Dietary Questionnaire; a new tool to measure children’s dietary patterns. Int. J. Pediatr. Obes. 2009;4:257–265. doi: 10.3109/17477160902846161. [DOI] [PubMed] [Google Scholar]
- 11.Wong J.E., Parnell W.R., Black K.E., Skidmore P.M.L. Reliability and relative validity of a food frequency questionnaire to assess food group intakes in New Zealand adolescents. Nutr. J. 2012;11:65. doi: 10.1186/1475-2891-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United States Department of Agriculture (USDA) A Series of Systematic Reviews on the Relationship Between Dietary Patterns and Health Outcomes. [(accessed on 31 July 2019)]; Available online: https://pdfs.semanticscholar.org/9dc0/ebed6c942f2be042fe359c12a801f1dbc690.pdf.
- 13.Dlugosz A. Dietary Patterns, Adverse Health Outcomes, Socio-Economic Status and Lifestyle of Adolescents from Less Urbanized Regions of Poland. 1st ed. University of Warmia and Mazury in Olsztyn; Olsztyn, Poland: 2017. pp. 1–108. [Google Scholar]
- 14.Osadnik T., Pawlas N., Lonnie M., Osadnik K., Lejawa M., Wądołowska L., Bujak K., Fronczek M., Reguła R., Gawlita M., et al. Family history of premature coronary artery disease (P-CAD)—A non-modifiable risk factor? Dietary patterns of young healthy offspring of P-CAD patients: A case-control study (MAGNETIC project) Nutrients. 2018;10:1488. doi: 10.3390/nu10101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szymelfejnik E.J., Wądołowska L., Cichon R., Przysławski J., Bolesławska I. Dairy products frequency questionnaire (ADOS-Ca) calibration for calcium intake evaluation. Pol. J. Food Nutr. Sci. 2006;15/56:229–236. [Google Scholar]
- 16.Głąbska D., Guzek D., Sidor P., Włodarek D. Vitamin D dietary intake questionnaire validation conducted among young Polish women. Nutrients. 2016;8:36. doi: 10.3390/nu8010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Głąbska D., Guzek D., Ślązak J., Włodarek D. Assessing the validity and reproducibility of an iron dietary intake questionnaire conducted in a group of young Polish women. Nutrients. 2017;9:199. doi: 10.3390/nu9030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Głąbska D., Malowaniec E., Guzek D. Validity and reproducibility of the iodine dietary intake questionnaire assessment conducted for young Polish women. Int J Environ Res Public Health. 2017;14:700. doi: 10.3390/ijerph14070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Głąbska D., Książek A., Guzek D. Development and validation of the brief folate-specific food frequency questionnaire for young women’s diet Assessment. Int. J. Env. Res. Public Health. 2017;14:1574. doi: 10.3390/ijerph14121574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Głąbska D., Staniec A., Guzek D. Assessment of validity and reproducibility of the zinc-specific dietary intake questionnaire conducted for young Polish female respondents. Nutrients. 2018;10:104. doi: 10.3390/nu10010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niedzwiedzka E., Wadolowska L. Accuracy analysis of the food intake variety questionnaire (FIVeQ). reproducibility assessment among older people. Pakistan J. Nutr. 2008;7:426–435. doi: 10.3923/pjn.2008.426.435. [DOI] [Google Scholar]
- 22.Wądołowska L. Validation of food frequency questionnaire–FFQ. Reproducibility assessment. Bromat. Chem. Toksykol. 2005;38:27–33. [Google Scholar]
- 23.Kowalkowska J., Slowinska M.A., Slowinski D., Dlugosz A., Niedzwiedzka E., Wadolowska L. Comparison of a full food-frequency questionnaire with the three-day unweighted food records in young Polish adult women: implications for dietary assessment. Nutrients. 2013;5:2747–2776. doi: 10.3390/nu5072747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowalkowska J., Wadolowska L., Czarnocinska J., Czlapka-Matyasik M., Galinski G., Jezewska-Zychowicz M., Bronkowska M., Dlugosz A., Loboda D., Wyka J. Reproducibility of a questionnaire for dietary habits, lifestyle and nutrition knowledge assessment (KomPAN®) in Polish adolescents and adults. Nutrients. 2018;10:1845. doi: 10.3390/nu10121845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krusinska B., Hawrysz I., Wadolowska L., Slowinska M.A., Biernacki M., Czerwinska A., Golota J.J. Associations of Mediterranean diet and a posteriori derived dietary patterns with breast and lung cancer risk: A case-control study. Nutrients. 2018;10:470. doi: 10.3390/nu10040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krusinska B., Wadolowska L., Slowinska M.A., Biernacki M., Drozdowski M., Chadzynski T. Associations of dietary patterns and metabolic-hormone profiles with breast cancer risk: A case-control study. Nutrients. 2018;10:2013. doi: 10.3390/nu10122013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazur J., editor. Zdrowie i zachowania zdrowotne młodzieży szkolnej w Polsce na tle wybranych uwarunkowań socjodemograficznych. Wyniki badań HBSC 2014. IMiD; Warsaw, Poland: 2015. [Google Scholar]
- 28.Wozniak A., Artych M., Wawrzyniak A. Nutritional behaviours and body self-perception in Polish pupils attending middle-school. Rocz. Panstw. Zakl. Hig. 2014;65:331–336. [PubMed] [Google Scholar]
- 29.Culter G.J., Flood A., Hannan P.J., Slavin J.L., Neumark-Sztainer D. Association between major patterns of dietary intake and weight status in adolescents. Br. J. Nutr. 2012;108:349. doi: 10.1017/S0007114511005435. [DOI] [PubMed] [Google Scholar]
- 30.Howe A.S., Black K.E., Wong J.E., Parnell W.R., Skidmore P.N. Dieting status influences associations between dietary patterns and body composition in adolescents: A cross-sectional study. Nutr. J. 2013;12:51. doi: 10.1186/1475-2891-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birch L., Savage J.S., Ventura A. Influences on the development of children’s eating behaviours: From infancy to adolescents. Can. J. Diet. Pract. Res. 2007;68:S1–S56. [PMC free article] [PubMed] [Google Scholar]
- 32.Pabjan K., Wądołowska L., Słowińska M.A., Człapka-Matyasik M., Niedźwiedzka E. Body composition of mother-daughter family pairs in relation to dairy products and calcium intake. Pol. J. Food Nutr. Sci. 2010;60:77–88. [Google Scholar]
- 33.Pabjan K., Wądołowska L., Słowińska M.A., Człapka-Matyasik M., Niedźwiedzka E., Szczepańska J. Analiza charakterystycznych modeli spożycia wapnia przez pary rodzinne matka-córka. Probl. Hig. Epidemiol. 2011;92:54–62. [Google Scholar]
- 34.Sobas K., Wadolowska L., Slowinska M.A., Czlapka-Matyasik M., Wuenstel J.W., Niedzwiedzka E. Like mother, like daughter? Dietary and non-dietary bone fracture risk factors in mothers and their daughters. Iranian J Publ Health. 2015;44:939–952. [PMC free article] [PubMed] [Google Scholar]
- 35.Wadolowska L., Ulewicz N., Sobas K., Wuenstel J.W., Slowinska M.A., Niedzwiedzka E., Czlapka-Matyasik M. Dairy-related dietary patterns, dietary calcium, body weight and composition: a study of obesity in Polish mothers and daughters, the MODAF project. Nutrients. 2018;10:90. doi: 10.3390/nu10010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lidia Wądołowska—UWM. [(accessed on 5 August 2012)]; Available online: www.uwm.edu.pl/edu/lidiawadolowska.
- 37.Armitage P., Berry G., Matthews J.N.S. Statistical Methods in Medical Research. 4th ed. Blackwell Science Ltd.; Oxford, UK: 2008. [Google Scholar]
- 38.Stanisz A. Przystępny kurs Statystyki z Zastosowaniem STATISTICA PL na Przykładach z Medycyny. Tom 1. Statystyki podstawowe. StatSoft; Kraków, Poland: 2006. [Google Scholar]
- 39.Masson L.F., McNeill G., Tomany J.O., Simpson J.A., Peace H.S., Wei L., Bolton-Smith C. Statistical approaches for assessing the relative validity of food frequency questionnaire, use of correlation coefficients and the kappa statistic. Public Health Nutr. 2003;6:313–321. doi: 10.1079/PHN2002429. [DOI] [PubMed] [Google Scholar]
- 40.Stanisz A. Przystępny kurs Statystyki z Zastosowaniem STATISTICA PL na Przykładach z Medycyny. Tom 2. Modele liniowe i nieliniowe. StatSoft; Kraków, Poland: 2007. [Google Scholar]
- 41.Marchioni D.M.L., Voci S.M., de Lima F.E.L., Fisberg R.M., Slater B. Reproducibility of a food frequency questionnaire for adolescents. Cad. Saude Publica. 2007;23:2187–2196. doi: 10.1590/S0102-311X2007000900026. [DOI] [PubMed] [Google Scholar]
- 42.Marques R.M., de Oliveira A.C., Teles S.A., Stringuini M.L., Fornes N.S., Gardenghi G. Relative validity and reproducibility of a quantitative food frequency questionnaire for adolescents with type 1 diabetes: Validity of a food frequency questionnaire. Int. J. Endocrinol. 2014;2014:1–11. doi: 10.1155/2014/976508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjerregaard A.A., Tetens I., Olsen S.F., Halldorsson T.I. Reproducibility of a web-based FFQ for 13 to 15-year-old Danish adolescents. J. Nutr. Sci. 2016;5:e5. doi: 10.1017/jns.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Øverby N.C., Johannesen E., Jensen G., Skjaevesland A.-K., Haugen M. Test-retest reliability and validity of a web-based food-frequency questionnaire for adolescents aged 13–14 to be used in the Norwegian mother and child cohort study (MoBa) Food Nutr. Res. 2014;58:23956–23966. doi: 10.3402/fnr.v58.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia W., Sun C., Zhang X., Wang J., Wu L. Reproducibility and relative validity of a food frequency questionnaire developed for female adolescents in Suihua, North China. PLoS ONE. 2011;6:e19656. doi: 10.1371/journal.pone.0019656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu F.B., Rimm E., Smith-Warner S.A., Feskanich D., Stampfer M.J., Ascherio A., Sampson L., Willett W.C. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 1999;69:243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 47.Vereecken C.A., Maes L. A Belgian study on the reliability and relative validity of the Health Nehaviour in school-aged children food-frequency questionnaire. Public Health Nutr. 2003;6:581–588. doi: 10.1079/PHN2003466. [DOI] [PubMed] [Google Scholar]
- 48.Warren J., Guelinckx I., Livingstone B., Potischman N., Nelson M., Foster E., Holmes B. Challenges in the assessment of total fluid intake in children and adolescents: A discussion paper. Eur. J. Nutr. 2018;57:43–51. doi: 10.1007/s00394-018-1745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livingstone M.B., Robson P.J., Wallace J.M. Issues in dietary intake assessment of children and adolescents. Br. J. Nutr. 2004;92:213–222. doi: 10.1079/BJN20041169. [DOI] [PubMed] [Google Scholar]
- 50.Thompson F.E., Subar A.F. Dietary assessment methodology. In: Coulston A.M., Boushey C.J., editors. Nutrition in The Prevention and Treatment of Disease. 2nd ed. Academic Press; San Diego, CA, USA: 2008. pp. 3–39. [Google Scholar]
- 51.Forrestal S.G. Energy intake misreporting among children and adolescents: a literature review. Matren. Child. Nutr. 2011;7:112. doi: 10.1111/j.1740-8709.2010.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kowalkowska J., Lonnie M., Wadolowska L., Czarnocinska J., Jezewska-Zychowicz M., Babicz-Zielinska E. Health- and taste-related attitudes associated with dietary patterns in a representative sample of Polish girls and young women: A cross-sectional study (GEBaHealth project) Nutrients. 2018;10:254. doi: 10.3390/nu10020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar G.S., Bryan M., Bayakly R., Drenzek C., Merlo C., Perry G.S. Reported motivations for and locations of healthy eating among georgia high school students. J. Sch. Health. 2017;87:353–362. doi: 10.1111/josh.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sweeting H.N. Gendered dimensions of obesity in childhood and adolescence. Nutr. J. 2008;99:S7–S14. doi: 10.1186/1475-2891-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weible D. Gender-driven food choice: Explaining school milk consumption of boys and girls. J. Consum. Policy. 2013;36:403–423. doi: 10.1007/s10603-013-9225-1. [DOI] [Google Scholar]
- 56.Wirfält E., Drake I., Wallström P. What do review papers conclude about food and dietary patterns? Food Nutr. Res. 2013;57:20523–20536. doi: 10.3402/fnr.v57i0.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulze M.B., Martínez-González M.A., Fung T.T., Lichtenstein A.H., Forouhi N.G. Food based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396. doi: 10.1136/bmj.k2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rezazadeh A., Rashidkhan B., Omidvar N. Association of major dietary patterns with socioeconomic and lifestyle factors of adult women living in Teheran, Iran. Nutrition. 2010;26:337–341. doi: 10.1016/j.nut.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 59.Morinaka T., Wozniewicz M., Jeszka J., Bajerska J., Nowaczyk P., Sone Y. Westernization of dietary patterns among young Japanese and Polish females—A comparison study. Ann. Agric. Environ Med. 2013;20:122. [PubMed] [Google Scholar]
- 60.Deliens T., Clarys P., De Bourdeaudhuij I., Deforche B. Determinants of eating behaviour in university students: a qualitative study using focus group discussions. BMC Public Health. 2014;14:53–64. doi: 10.1186/1471-2458-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karimi-Shahanjarini A., Omidvar N., Bazargan M., Rashidian A., Majdzadeh R., Shojaeizadeh D. Iranian female adolescent’s views on unhealthy snacks consumption: A qualitative study. Iran. J. Public Health. 2010;39:92–101. [PMC free article] [PubMed] [Google Scholar]
- 62.Wadolowska L., Babicz-Zielinska E., Czarnocinska J. Food choice models and their relation with food preferences and eating frequency in the Polish population: POFPRES study. Food Policy. 2008;33:122–134. doi: 10.1016/j.foodpol.2007.08.001. [DOI] [Google Scholar]
- 63.Castellŏ A., Lope V., Vioque J., Santamariňa C. Reproducibility of data-driven dietary patterns in two groups of adult Spanish women from different studies. Br. J. Nutr. 2016;116:734–742. doi: 10.1017/S000711451600252X. [DOI] [PubMed] [Google Scholar]
- 64.Murakami K., Shinozaki N., Fujiwara A., Yuan X., Hashimoto A., Fujihashi H., Wang H.C., Livingstone M.B.E., Sasaki S. A systematic review of principal component analysis-derived dietary patterns in Japanese adults: Are major dietary patterns reproducible within a country? Adv. Nutr. 2019;10:237–249. doi: 10.1093/advances/nmy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lombard M.J., Steyn N.P., Charlton K.E., Senekal M. Application and interpretation of multiple statistical tests to evaluate validity of dietary intake assessment methods. Nutr. J. 2015;14:40–50. doi: 10.1186/s12937-015-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez C.A., Smith E.R., Villamor E., Zavaleta N., Respicio-Torres G., Contreras C., Perea S., Jimenez J., Tintaya K., Lecca L., et al. Development and validation of a food frequency questionnaire to estimate intake among children and adolescents in Urban Peru. Nutrients. 2017;9:1121. doi: 10.3390/nu9101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitton C., Ho J.C.Y., Tay Z., Rebello S.A., Lu Y., Ong C.N., van Dam R.M. Relative validity and reproducibility of a food frequency questionnaire for assessing dietary intakes in a multi-ethnic Asian population using 24-h dietary recalls and biomarkers. Nutrients. 2017;9:1059. doi: 10.3390/nu9101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Comrey A.L., Lee H.B. A First Course in Factor Analysis. 2nd ed. Lawrence Erlbaum Associates Inc.; Hlilsdale, NJ, USA: 1992. [Google Scholar]
- 69.Osborne J.W., Costello A.B. Sample size and subject to item ratio in principal components analysis. Pract. Assess. Res. Eval. 2004;9:1–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.