Abstract
Adventitious root (AR) is a kind of later root, which derives from stems and leaf petioles of plants. Many different kinds of small signaling molecules can transmit information between cells of multicellular organisms. It has been found that small molecules can be involved in many growth and development processes of plants, including stomatal movement, flowering, fruit ripening and developing, and AR formation. Therefore, this review focuses on discussing the functions and mechanisms of small signaling molecules in the adventitious rooting process. These compounds, such as nitric oxide (NO), hydrogen gas (H2), hydrogen sulfide (H2S), carbon monoxide (CO), methane (CH4), ethylene (ETH), and hydrogen peroxide (H2O2), can be involved in the induction of AR formation or development. This review also sums the crosstalk between these compounds. Besides, those signaling molecules can regulate the expressions of some genes during AR development, including cell division genes, auxin-related genes, and adventitious rooting-related genes. We conclude that these small-molecule compounds enhance adventitious rooting by regulating antioxidant, water balance, and photosynthetic systems as well as affecting transportation and distribution of auxin, and these compounds further conduct positive effects on horticultural plants under environmental stresses. Hence, the effect of these molecules in plant AR formation and development is definitely a hot issue to explore in the horticultural study now and in the future.
Keywords: small signaling molecules, interactions, gene expression
1. Introduction
The root system of a plant is made up of the primary, lateral, and adventitious roots. The primary root originates during embryogenesis and will later elongate after germination. The AR is initiated and developed post-embryonically from differentiated cells; however, the later root is derived from pericycle cells, which is also defined anatomically as meristems [1,2]. AR is a type of lateral root, which is originally derived from stems and leaf petiole [3]. The process of AR formation can be divided into three phases: induction (when biochemical and molecular changes occur), initiation (when cells start to divide to form an internal root meristem), and expression (when the AR primordium grows and emerges from the stem) [4]. The development of AR is a vital step in the vegetative propagation of plants, which is regulated by various endogenous factors such as plant hormones, metabolic constituents, enzyme activities, and external environmental stimulations, and it plays a key function for plant adaptation to abiotic stresses, including salt, drought, heavy metal, and osmotic stresses [5]. Moreover, AR would be subjected to biotic stress, such as beneficial or pathogenic microbes during plant growth, and in AR development of artificial explants produced by wounding [6]. Therefore, understanding the mechanism of adventitious rooting is of significant importance to strategize breeding efforts to maximize its marketable yield [7].
Many different kinds of molecules transmit information between the cells of multicellular organisms, including small-molecule compounds. These molecules are produced by signaling cells and subsequently bind to receptors in target cells, acting as ligands and chemical signals to coordinate responses and a variety of biological processes both in animals and plants [8,9]. Over the past decades, carbon monoxide (CO) and hydrogen sulfide (H2S) were regarded as toxic molecule compounds in animals. Gradually, small signaling molecules have proven to be resistant to various abiotic stresses in plants, such as chilling, osmotic stress, drought stress, flooding, salt stress, heavy metal stress (aluminum stress, cadmium toxicity, and mercury toxicity), and UV-A irradiation [5,7,10]. NO was also discovered to influence the interactions between plants and microbes during pathogenesis [11,12]. Besides, they are involved in numerous plant growth and development processes, including cell division, stomatal movement, adventitious rooting, flowering, fruit ripening and development, and seed dormancy or germination [5,13,14,15]. In recent years, increasing kinds of small molecules have been found to participate in signal transduction during adventitious rooting. For example, some typical small molecules such as NO, H2, CH4, ethylene (ETH), and H2O2 have been indicated to induce AR formation and development in plants [16,17,18,19]. Also, the roles of small compounds under stresses have been widely investigated through studying their functions and the crosstalk among them. Now, multiple researchers suggest that small-molecule compounds can promote AR formation and development via mediating a variety of growth and developmental processes, which help to improve the resistance against environmental stress conditions in plants.
AR development is a complicated process and influenced by many abiotic factors such as mineral nutrition (Ca, N, Zn), light, temperature, and various biotic factors including ectomycorrhizas and agrobacterium rhizogenes [1,20]. In addition, previous reviews and studies have reported that multiple endogenous factors (aging, polyamines, enzymatic activities of peroxidases, phytohormones, and phenolic compounds) can affect the development of AR [1,20]. Furthermore, the innovational application of small-molecule compounds is found to be necessary for promoting AR development. A number of studies have demonstrated the effectiveness of these compounds when applied to multiple horticultural plant species such as cucumber, marigold, and tomato. However, there is little review about the effects of small-molecule compounds in plant AR development. The innovational application of small-molecule compounds is necessary to promote AR formation, and its great importance in horticultural study cannot be ignored. For a more in-depth study of the effects of ARs on small molecules, this review will mainly discuss the recent progresses of small-molecule compounds on AR formation and development in plants. Also, the interactions between NO and other signaling molecules are also discussed.
2. Functions of Small-Molecule Compounds during Adventitious Rooting
2.1. Nitric Oxide (NO)
NO plays a crucial role as a second messenger molecule, after Ca2+, of plant signal transduction in the growth and development of plants, such as seed germination and dormancy, stomatal movement, and photosynthesis [21]. NO also participates in plant responses to various stresses, including heavy metal, low temperature, drought, salt, and UV-B radiation [22].
In plants, NO is synthesized through two synthetic pathways: enzymatic and nonenzymatic. The enzymatic pathway includes nitrate reductase (NR) and NO synthase (NOS)-like enzymes. Previous studies of NO showed that NR played crucial roles in the process of physiological activities in NO production [10]. It was also reported that both NR and NOS could contribute to NO production and synergistically induced adventitious rooting in marigold (Tagetes erecta L. ‘Marvel’) [23] and AR development in cucumber (Cucumis sativus L.) [10], suggesting that NO could be produced by NOS substrates in plants and that NOS-like activity does exist in plants. The treatment of 10 μM NO donor sodium nitroprusside (SNP) significantly promoted AR formation in cucumber explants through NOS and NR pathways [24,25]. Additionally, cyclic guanosine monophosphate (cGMP) was involved in NO-induced adventitious rooting of marigold explants to serve as a crucial component of the NO-regulated signaling pathway [23]. Ample evidence also showed that exogenous NO dramatically triggered adventitious rooting in marigold [26,27]. These findings indicate that NOS and NR are important signaling molecules in the production of NO to promote adventitious rooting.
It has been widely reported that NO may regulate the developmental process of adventitious rooting. Application of NO significantly enhanced AR length and number in the cuttings of ground-cover chrysanthemum (Dendranthema morifolium ‘Beiguozhicun’), and the explants of cucumber and marigold [26,27,28], which indicates that NO has a positive effect on AR development in plants.
In addition, cell cycle regulation plays important roles in the xylem during the growth of plant roots. Cell cycle regulation in the xylem pericycle plays an important part in root organogenesis. Cell cycle regulation often occurs in the xylem pole of the pericycle, in which cells proceed to the G2 phase, whereas the rest of cells in pericycle remain at the G1 phase, which indicates that NO is involved in cell cycle regulation in the process of adventitious rooting in plants [7].
NO can also regulate AR development through adjusting enzyme activities, such as peroxidase (POD), polyphenol oxidase (PPO), and indoleacetic acid oxidase (IAAO). Furthermore, NO accumulation significantly increased the activity of pro-oxidants including triphosphopyridine nucleotide (NADPH oxidase) and antioxidants such as superoxide dismutase (SOD), catalase (CAT), POD, ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) enzymes in AR development in mountain ginseng (Panax ginseng C.A. Meyer) [29]. NO donor SNP increased PPO activity and reduced POD and IAAO activities during the AR development process in cucumber [30]. Exogenous NO could also increase IAAO, POD, and PPO activities in marigold, which was associated with the induction of AR [27,28]. These results indicate that NO triggers AR development by regulating enzymatic activities.
NO also regulates AR development under abiotic stresses. Exogenous NO enhanced the root number and root fresh weight in marigold explants under drought stress [16]. And NO donor SNP prompted the adapted mesophyll cell ultrastructure changes under drought conditions. Meanwhile, application of NO remarkably increased the chlorophyll content and fluorescence energy parameters, including Fv/Fm, ɸPSII, and qP parameters, and inhibited the decrease of water-soluble carbohydrate (WSC) and total soluble protein, which subsequently promoted rooting under drought stress [16]. Recently, the NO donor was reported to significantly induce AR length and number in cucumber explants under osmotic stress by strengthening the photosynthetic performance [22]. And NO treatment increased the ψw, SOD, CAT, and APX activities and chlorophyll content in cucumber explants [22]. Moreover, NO significantly induced new AR formation in rice (Oryza sativa L. cv. Komal) seedlings under arsenate (AsV) stress by increasing APX content [31]. Therefore, NO can trigger AR development and play an important role during the process of AR development under abiotic stresses.
2.2. Hydrogen Gas (H2) and Hydrogen Sulfide (H2S)
Hydrogen gas (H2) is a colorless and tasteless molecule, and hydrogen is one of the most abundant elements in the universe, constituting nearly 75% of the mass of the universe [32]. It has been widely reported in animals that H2 has come to the forefront in therapeutic medical gas research. Recently, the attention on the role of H2 has changed from animals to plants. It has been found that H2 could regulate the growth and development of plants, including seed germination, seedling growth, stomatal closure. and root elongation [33]. In addition, H2 enhances the tolerance of plants to abiotic stress conditions such as salt stress, osmotic stress, drought, cadmium toxicity, aluminum stress, mercury toxicity, and UV-A irradiation [5].
Many studies have shown that H2 has a positive effect on adventitious root development in plants. In cucumber, 50% hydrogen-rich water (HRW) significantly induced adventitious rooting and enhanced NO content in a time-dependent manner, which reached a maximum at 24 h during the treatment. Also, the application of H2 triggered transition from G1 to S phase in plant cell cycle to enter a new cell cycle in a synchronous manner [7]. And HRW remarkably induced the activities of antioxidant enzymes, such as POD, PPO, and IAAO [30]. Further studies reported that 50% HRW could prompt AR development in marigold explants by increasing relative water content (RWC) and WSC, starch, and soluble protein content, as well as POD, PPO, and IAAO activities, and it could also decrease stomatal aperture and electrolyte leakage [5].
Also, H2 exerted a positive effect on AR development under stress conditions. Fifty percent HRW significantly induced AR length and number and enhanced the RWC during rooting in cucumber explants under drought stress [18]. Also, exogenous H2 dramatically increased leaf chlorophyll content, Fv/Fm, ɸPSII, and qP as well as the activities of SOD, POD, CAT, and APX enzymes during drought [18]. Additionally, a recent study showed that under cadmium (Cd) stress, 50% HRW was the most proper dose to trigger adventitious rooting in cucumber [34]. Compared with Cd treatment, HRW + Cd treatment significantly reduced the content of malondialdehyde (MDA), hydrogen peroxide (H2O2), superoxide radical (O2−), thiobarbituric acid reactive substances (TBARS), ascorbic acid (AsA), and reduced glutathione (GSH), as well as relative electrical conductivity (REC), lipoxygenase (LOX) activity, AsA/docosahexaenoic acid (DHA) ratio, and GSH/oxidized glutathione (GSSG) ratio, while increasing DHA and GSSG content. HRW + Cd treatment also significantly increased in the activity and related gene expressions of APX, DHAR, monodehydroascorbate reductase (MDHAR), and GR. Additionally, HRW + Cd treatment increased the contents of osmotic adjustment substances, as well as the activities of POD and PPO, while significantly decreasing IAAO activity [34]. These results confirm that H2 induces adventitious rooting under Cd stress by decreasing the oxidative damage, increasing osmotic adjustment substance content, and regulating rooting-related enzyme activity.
Hydrogen sulfide (H2S) is a naturally occurring, colorless, highly soluble, flammable, and toxic gas [35]. It has been also considered as the third gaseous transmitter after NO and CO in animals [36]. Furthermore, exogenous H2S has been found to be involved in abiotic stresses of plants such as salinity, drought, extreme temperatures, and heavy metals [37]. Recent research suggested that H2S regulated many aspects of plant development, like seed germination and adventitious root induction [38]. The donor of H2S, 10 μM sodium hydrosulfide (NaHS) significantly induced AR primordia in cucumber explants [39,40], and 0.2 μM NaHS was suitable for the increase of both root number and root length in excised willow (Salix matsudana var. tortuosa Vilm) and soybean (Glycine max L.) seedlings [38].
2.3. Carbon Monoxide (CO)
CO is a low molecular weight diatomic and poisonous gas that occurs ubiquitously in nature. However, CO has recently been proven to be one of the most essential cellular components and regulates a variety of biological processes both in animals and plants. In animals, CO regulates many physiological events such as platelet aggregation, neurotransmission, and vasodilation [18]. Heme oxygenase (HO) is the rate-limiting enzyme in the process of heme catabolism. CO in the human body is mainly produced by the metabolism of HO. There are three types of HO: oxidative stress-inducible heme oxygenase-1 (HO-1), constitutive heme oxygenase-2 (HO-2), and undetermined heme oxygenase-3 (HO-3) [18]. In plants, CO plays a key role in seed germination, stomatal closure, and root development [18], and it is required for the alleviation of abiotic stresses, including salt stress, drought, heavy metal stress, and UV-B radiation [9].
A number of studies showed that CO could trigger AR development. CO treatment induced adventitious rooting in the hypocotyl of mung bean (Phaseolus radiatus L. cv. Mingguang) in dose- and time-dependent manners [41]. Also, NO fluorescence was significantly enhanced by the treatment of CO, indicating that CO triggered AR development of hypocotyl cuttings from mung bean seedlings, possibly through regulating the NO/NOS pathway. The CO artificial donor, 10 μM hemin and hematin could significantly induce AR development in cucumber [17,37,42]. Hemin (500 μM) and 30% CO aqueous solution significantly increased the AR number and length in cucumber explants under drought stress [18]. The applied polyethylene glycol (PEG) reduced leaf RWC during adventitious rooting, and CO alleviated the reduction. Furthermore, CO enhanced leaf chlorophyll content and then increased photosynthesis and promoted AR development during drought conditions. Moreover, CO treatment enhanced the activities of SOD, POD, CAT, and APX in cucumber under drought stress [18]. These findings suggest that CO can induce adventitious rooting in cucumber seedlings under drought stress by alleviating the negative effects of drought stress on RWC, chlorophyll content, and antioxidant systems.
2.4. Methane (CH4)
CH4 is a physiologic inert gas and regarded as an important greenhouse gas in the atmosphere. Recently, studies have illustrated that CH4 exhibits anti-apoptotic, anti-inflammatory, and antioxidative activities in animals [43]. CH4 also plays an important role in responses of plants to environmental stresses [44]. It was found that aerobic CH4 release could be stimulated by the increase of temperature, cutting injuries, production of reactive oxygen species (ROS), and ultraviolet radiation [45,46]. However, only few studies reported the functions of exogenously applied CH4 on various plant developmental processes. Eighty percent methane-rich water (MRW) dramatically triggered the increase of AR length and number in cucumber [19]. CH4-induced adventitious rooting in cucumber explants required γ-glutamyl cysteine synthetase (γ-ECS) [40,47]. These findings indicate that CH4 can be regarded as a crucial inducer during AR process.
2.5. Ethylene (ETH)
ETH is a gaseous plant hormone and involved in many growth and development processes in plants, such as seed germination and dormancy, cell division and expansion, leaf senescence, and fruit ripening [48,49]. Zimmerman and Hitchcock (1933) [50] first observed that ETH significantly enhanced the root length and number in AR development. Now, many studies have shown that ETH is involved in AR growth in many plant species [51,52]. In addition, exogenous ETH dramatically increased the activities of antioxidant enzymes (IAAO, POD, and PPO), resulting in AR development in marigold [27]. The ethylene donor ethephon significantly promoted AR development in cucumber and marigold explants [10,27]. But the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) could not trigger AR formation in wild-type seedlings of Arabidopsis (Arabidopsis thaliana) [53]. However, ACC was shown to have the effect on adventitious rooting in Arabidopsis mutant (max2 and max4) plants in a dose-dependent manner [53]. The application of ACC at 0.01 μM significantly increased the development of AR, but 100 μM ACC exhibited inhibition effects [53].
2.6. Hydrogen Peroxide (H2O2)
H2O2 is viewed mainly as a type of ROS and a signaling messenger. A number of studies reveal that H2O2 is involved in many biological processes of plants, such as stomatal closure [54], flowering [55], leaf senescence [56], cellular differentiation and plant morphogenesis [57], lateral root formation, and AR development [58,59].
According to Bai et al. (2012) [60], in mung bean seedlings, 3-O-C10-HL could stimulate AR formation, depending on cGMP pathways, by triggering endogenous H2O2 production, which showed that 3-O-C10-HL is likely to participate in auxin-prompted AR formation by H2O2-dependent cGMP signaling. Li et al. (2007) [59] found that 20–40 μM H2O2 could significantly increase the number of AR, while treatment with 10–50 μM H2O2 significantly increased the fresh weight of AR in cucumber. Moreover, in marigold, the treatment of 200 μM H2O2 significantly induced root length and root number, but its scavenger CAT alleviated its positive effects [23]. It has also been reported that H2O2 and the synthetic phytohormone indole-3-butyric acid (IBA) may act synergistically to regulate adventitious rooting dependent on the auxin pathway in marigold explants [26]. Exogenous NPA led to a significant decrease in the endogenous levels of auxin, which, in turn, inhibited the endogenous accumulation of H2O2, suggesting that H2O2 may be the downstream signal molecule in the auxin signaling cascade [26]. Also, 200 µM H2O2 promoted the AR length and number in marigold, and treatment with indole-3-butyric acid (IBA) enhanced the endogenous level of H2O2 in explants [26], which suggests that the promotion of AR by IBA may occur via enhancing the level of H2O2. In ground-cover chrysanthemum, 200 μM H2O2 was the most suitable dosage to promote AR development, at which this dosage increased the activities of PPO, IAAO, and WSC, as well as total nitrogen content, and decreased the total polyphenol content [28]. These results indicate that H2O2 could enhance AR development via increasing some enzyme activities, and carbohydrate and nitrogen contents, and inhibiting polyphenol production.
Under drought stress, 600 μM H2O2 treatment significantly promoted root length and number in marigold, attenuated the destruction of the mesophyll cell ultrastructure, and increased leaf chlorophyll content, chlorophyll fluorescence parameters (Fv/Fm, ɸPS II, and qP), and hypocotyl soluble carbohydrate and protein content, while decreasing starch content of marigold [16]. It implies that under drought conditions, the protection of H2O2 on the ultra-microstructure of mesophyll cells improves the photosynthetic performance of blades and lightens the adverse impacts of drought on the build-up of carbohydrates and nitrogen in explants, boosting adventitious rooting. An overview of the effects and mechanisms in plant AR-related studies of small-molecule compounds under no stress and stress conditions are shown in Table 1 and Table 2, respectively.
Table 1.
Overview of small-molecule compounds that induce adventitious root (AR) formation and development under no stress in plants.
| Small-Molecule Compounds | Plant Species | Small Signal Molecule Mediated Effects | References |
|---|---|---|---|
| NO | Marigold | NO can trigger AR development and enhances endogenous H2O2 levels | [23] |
| IAAO, POD, and PPO↑ | [27] | ||
| NR and NOS can contribute to NO production to induce AR development | [16] | ||
| Cucumber | NO induces AR formation through NOS and NR pathways | [24,25] | |
| NO can trigger AR development in a cGMP-dependent manner | [17] | ||
| NO can induce AR formation and up-regulate cell cycle-related genes | [7] | ||
| PPO↑; POD and IAAO↓ | [30] | ||
| Two NO-releasing compounds, NOS-like and DAO, trigger AR formation | [19] | ||
| NR and NOS promote AR development and up-regulate their gene relative expression levels | [10] | ||
| Ground-cover chrysanthemum | PPO, IAAO, WSC, and total nitrogen↑; total polyphenol content↓ | [28] | |
| Mountain ginseng | CAT, POD, APX, DHAR, GR, NADPH, and O2−↑ | [29] | |
| H2 | Cucumber | H2 upregulates cell cycle-related genes and promotes AR formation | [7] |
| 50% HRW significantly induces adventitious rooting and POD, PPO, and IAAO↑ | [30] | ||
| Marigold | RWC, WSC, starch, soluble protein content, POD, PPO, and IAAO↑; stomatal aperture and electrolyte leakage↓ | [5] | |
| H2S | Cucumber | 10 μM NaHS triggers AR development | [16] |
| H2S can induce AR primordia | [40] | ||
| Willow | Endogenous H2S, IAA, and NO↑ | [38] | |
| Soybean | Endogenous H2S, IAA, and NO↑ | [38] | |
| CO | Mung bean | NO fluorescence↑ | [41] |
| Cucumber | 10 μM hemin and hematin can significantly induce AR development in cucumber | [17,42] | |
| CH4 | Cucumber | 80% MRW increases root length and number | [19] |
| CH4-induced adventitious rooting of cucumber explants requires γ-glutamyl cysteine SGH | [40,47] | ||
| ETH | Cucumber | Exposure of cucumber explants to ETH up-regulated NOS and NR activity and their gene relative expression levels | [10] |
| Marigold | IAAO, POD, and PPO↑ | [27] | |
| H2O2 | Cucumber | 10–50 and 20–40 μM H2O2, respectively, increases the weight and number of AR respectively | [59] |
| Marigold | 200 μM H2O2 significantly induces root length and root number | [23] | |
| IBA and H2O2 may act synergistically to mediate adventitious rooting | [26] | ||
| Ground-cover chrysanthemum | PPO, IAAO, WSC, and total nitrogen↑; total polyphenol content↓ | [28] |
Table 2.
Overview of small-molecule compounds that induce AR formation and development under stresses in plants.
| Small-Molecule Compounds | Plant Species | Stress Condition | Small Signal Molecule Mediated Effects | References |
|---|---|---|---|---|
| NO | Cucumber | Osmotic stress | Fv/Fm, ɸPSII, qP, NPQ, SOD, CAT, and APX↑; H2O2 and O2−↓ | [22] |
| Marigold | Drought stress | chl (a+b) content, Fv/Fm, ɸPS II and qP, and soluble carbohydrate and protein content↑; starch content↓ | [16] | |
| Rice | AsV stress | APX↑ | [31] | |
| H2 | Cucumber | Drought stress | RWC, leaf chlorophyll content, Fv/Fm, ɸPSII and qP, SOD, POD, CAT, and APX↑ | [18] |
| Cd stress | DHA, GSSG, APX, DHAR, MDHAR, GR, POD, and PPO↑; MDA, H2O2, O2−, TBARS, AsA, GSH, REC, LOX, and IAAO↓ | [34] | ||
| CO | Cucumber | Drought stress | leaf chlorophyll content, SOD, POD, CAT, and APX↑; RWC↓ | [18] |
| H2O2 | Marigold | Drought stress | chl (a + b) content, Fv/Fm, ɸPS II and qP, and soluble carbohydrate and protein content↑; starch content↓ | [16] |
3. Cross-Talk between Small-Molecule Compounds during Adventitious Root Development
3.1. NO and Other Signaling Molecules
3.1.1. NO and CO
NO and CO are two toxic gases, but they function as second messenger molecules in decreasing vascular tone and inhibiting platelet aggregation by simultaneously inducing cGMP content in animals [61]. The biosynthesis enzymes of NO and CO are NOS and HO, respectively, and they share similar isoforms, requirements for activity, regulation, and localization, illustrating the coordinated function of NO and CO in animals [61]. NO and CO also work together to exert growth and development processes in plants, and here we summarize the crosstalk between them during plant adventitious rooting.
In plants, NO and CO are able to act as two typical downstream signals in auxin-induced adventitious rooting. In mung bean seedlings, addition of the CO donor hematin induced AR formation in dose- and time-dependent manners, similarly with the phenotype induced by NO donor SNP. Furthermore, the NO-specific scavenger cPTIO suppressed the entire NO induced by CO, and the root numbers of the mung bean hypocotyls were decreased by NOS inhibitor L-NAME and hematin treatments when compared to that under hematin treatment alone. However, the inhibitor of HO-1 zinc protoporphyrin IX (ZnPPIX) could not affect the SNP action, suggesting that the CO-induced adventitious rooting process is mediated by the NO/NOS pathway, and NO may act downstream of CO during AR formation in mung bean hypocotyls [41].
In cucumber, the heme oxygenase1 (CsHO1) gene was cloned and proved to exhibit HO activity, which was the inducible isoform of heme oxygenase, and it participated in the heme degradation pathway while concomitantly releasing CO. The nitric oxide donor SNP, as well as other AR inducers, could increase the level of the CsHO1 transcript and the corresponding protein, which further suggested the crosstalk between endogenous HO-1/CO and NO during adventitious rooting and their signal transduction pathway [62].
Subsequent research found that application of CO and hemin to cucumber explants induced the production of NO and the NOS-like enzyme inhibitors, and the NO scavenger blocked NO content and AR formation stimulated by CO, but not the NR inhibitors. Simultaneously, researchers found that NO might act as a downstream regulator of CO during adventitious rooting in a cGMP-dependent manner [17].
3.1.2. NO and H2
It was reported that NO had a close relation with H2 in AR development in cucumber and marigold. The NO scavenger cPTIO, NOS inhibitor NG-nitro-L-Arg methyl ester hydrochloride (l-NAME) or NR inhibitor sodium azide (NaN3), and tungstate could partly reduce the effect of H2-triggered AR in cucumber, which indicated that NO acts as the downstream signaling molecule, and that NOS and NR might be responsible for NO generation during H2-induced AR formation [7]. Moreover, NO and H2 induced AR development through regulating the cell cycle and the activity of root organogenesis-related enzymes POD, IAAO, and PPO [30].
3.1.3. NO and ETH
The marigold and cucumber explants treated with ETH and NO together exhibited a better phenotype of AR formation than those treated with ETH or NO alone. These effects of ETH were restrained by cPTIO, L-NAME and NaN3, suggesting that the positive role of ETH on AR formation was partially NO-dependent [27]. It was further shown that addition of the ETH donor ethephon increased the endogenous NO level and up-regulated activities of NOS and NR and their corresponding gene expressions in explants, suggesting that the positive role of ETH on AR formation may rely on internal accumulation of NO [10,27]. In marigold, ETH and NO could simultaneously induce the activities of IAAO, POD, and PPO during adventitious rooting [27]. In cucumber, ETH and NO could stimulate AR formation through regulating cell cycle activation, nutrient distribution, and photosynthesis [10]. Together, NO acts as downstream regulator of ETH in AR development.
3.1.4. NO and CH4
The applied CH4 in cucumber (C. sativus ‘Lufeng’) explants triggered generation of NO and was followed by adventitious rooting, which was similar to the effects of the NO donor SNP and NONOate, suggesting the involvement of NO in CH4-elicited root organogenesis. Further studies illustrated that CH4 induced endogenous NO production through NOS-like enzymes and diamine oxidases (DAOs), and it triggered AR development via controlling expressions of NO-targeted genes [19].
3.1.5. NO and H2S
In sweet potato (Ipomoea batatas L.), application of the H2S donor NaHS induced ARs in a dose-dependent manner. Treatment of NaHS increased endogenous H2S, IAA, and NO contents, and H2S mediated AR, which was blocked by NPA and cPTIO, This indicated that IAA and NO participate in H2S-induced adventitious rooting, and this regulation mechanism could also be applicable to root organogenesis in willow (S. matsudana var. tortuosa Vilm) and soybean (G. max L.) stem cuttings [38]
3.1.6. NO and H2O2
In marigold, previous studies showed that AR length and number were much higher with the application of SNP + H2O2, compared to SNP or H2O2 treatment alone. cPTIO, the scavenger of NO, inhibited H2O2-triggered adventitious root formation, while the treatment of CAT, an H2O2 inhibitor, suppressed SNP-induced AR development, suggesting that both NO and H2O2 can have independent or synergistic effects on the induction of adventitious rooting [23]. Further studies indicated that NO and H2O2 may be downstream signal molecules in the auxin signaling cascade, and NO may be involved as an upstream signaling molecule for H2O2 production [26].
In ground-cover chrysanthemum, both NO and H2O2 treatments at the proper dosage increased PPO, IAAO, WSC, and total nitrogen, and they decreased total polyphenol content, which implies that NO and H2O2 treatments enhanced AR development synergistically and independently by stimulating PPO and IAAO enzyme activities and carbohydrate and nitrogen contents while simultaneously repressing the production of polyphenol [28].
Under drought conditions, SNP and H2O2 reduced the harmful effects by changing the chlorophyll content, fluorescence parameters, and carbon and nitrogen levels, indicating that H2O2 and NO act synergistically, and H2O2 is involved in rooting promoted by NO under drought conditions [16].
3.2. CO and Other Signaling Molecules
3.2.1. CO and H2
In alfalfa seedlings, H2 alleviated oxidative stress induced by paraquat via HO-1 signaling, indicating a synergistic effect of H2 and CO during plant responses to abiotic stresses [63]. In cucumber, the inducible effect on AR by HRW mimicked that by hemin or HO-1, and the HRW-induced response was restrained by addition of NPA. The blocking of HO-1 inhibitor ZnPPIX and the reversion of CO to HRW-treated AR further suggested that HRW took part in AR formation at least partially through the HO-1/CO directed pathway [64]. In cucumber (C. Sativus ‘Xinchun No. 4′), researchers further discovered that CO may be involved in H2-triggered adventitious root formation through adjusting RWC, photosynthesis, and metabolic constituent content and alleviating oxidative damage under drought stress conditions [58].
3.2.2. CO and H2S
In cucumber, the H2S donor NaHS induced adventitious rooting, and the expression of CsHO-1 as well, and ZnPPIX significantly suppressed the effects induced by NaHS. Furthermore, NaHS regulated similar target genes of HO-1/CO in AR formation, but the H2S scavenger hypotaurine (HT) could not influence HO-1/CO AR formation, which suggested that HO-1/CO acts downstream of H2S during AR formation [40].
3.2.3. CO and CH4
In cucumber, MRW significantly induced AR formation in IAA-depleted explants, and the inducible affect was still effective in soybean and mung bean explants. Meanwhile, auxin signaling-related genes and cell cycle regulatory genes exerted by MRW during AR formation were blocked by ZnPPIX and further reversed by CO, and the involvement of the Ca2+ pathway in the MRW-induced AR process was certified. Together, CH4 could stimulate adventitious rooting through partially adjusting HO-1/CO and Ca2+ pathways [65].
3.3. Crosstalk between Other Signaling Molecules
Previous results showed that H2S and CH4 mediated adventitious rooting in cucumber explants. Further study demonstrated that CH4 induced endogenous generation of H2S during adventitious rooting, and the H2S scavenger HT restrained the development of adventitious root primordia and gene expressions as well as S-sulfhydration level elicited by CH4, indicating the important role of endogenous H2S in participating in CH4-induced cucumber adventitious root development [40].
During the adventitious root bioreactor culture of ginseng, the enrichment of O2 increased adventitious root mass, ginsenoside contents, and polysaccharides, while CO2 and C2H4 were found to operate opposite impacts as O2 on secondary metabolism in plant cell cultures [66].
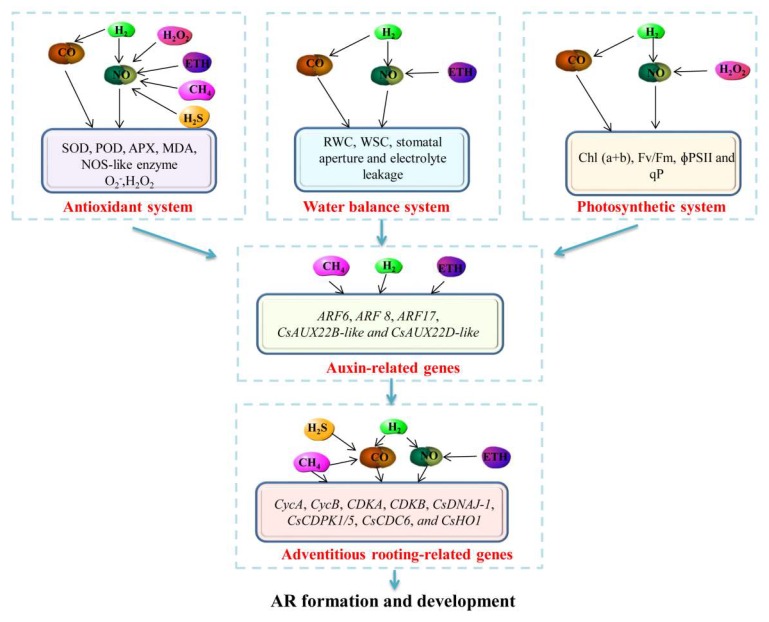
In summary, H2, NO, CO, ETH, and other small-molecule compounds work together by regulating antioxidant, water balance, and photosynthetic systems, and they affect the transportation and distribution of auxin as well as expedite plant adventitious rooting. The mechanisms of the cross-talk between NO and other small signal molecules during plant adventitious rooting are summarized in Figure 1.
Figure 1.
Schematic model of the interactions among different small signaling molecules during plant adventitious rooting through regulating different plant physiological process systems (antioxidant system, water balance system, and photosynthetic system). In the antioxidant system, NO is regarded as the downstream molecule to enhance the enzyme activities of SOD, POD, APX, and MDA and the production of O2− and H2O2 [18,22,23,29,34]. In the water balance system, NO is also located downstream to increase RWC, WSC, stomatal aperture, and electrolyte leakage of plants [5,18,28]. In the photosynthetic system, NO is involved in H2, H2O2, and CO-increased chl (a+b), Fv/Fm, ɸPSII, and qP [16,18,22]. In addition, some auxin-related genes, including ARF6, ARF8, ARF17, CsAUX22B-like, and CsAUX22D-like, can be up-regulated by the application of CH4, H2, and ETH [40,47,64,67]. Furthermore, small signaling molecules up-regulate AR-related genes such as CycA, CycB, CDKA, CDKB, CsDNAJ-1, CsCDPK1/5, CsCDC6, and CsHO1 [7,10,17,39,40,47,62,64,65] and finally conduct the formation and development of adventitious roots.
4. Some Related Genes during Adventitious Root Development Induced by Small-Molecule Compounds
It is well known that cell cycle regulation genes are often related to the regulation of cell division in plant internode growth. Two cyclins (CycA, CycB) and two CDK (CDKA and CDKB) genes are found to analyze the effect of cell division on AR formation in plants. In cucumber, NO and H2 treatment up-regulated the expression of the cell-cycle regulatory genes CycA, CycB, CDKA, and CDKB, which participate in mediating cell division in internode growth and root meristem induction to boost the process of AR development. This result suggests that both NO and H2 take part in regulating the cell cycle progress to the mitotic phase [7]. Also, NO and ETH treatments enhanced the transcription levels of CsCDPK1 and CsCDPK5 genes in cucumber explants [64]. The application of CH4 could also up-regulate cell cycle regulatory genes in cucumber, including CsCDC6, CsCDPK1, CsCDPK5, and CsDNAJ-1 [40,47].
Furthermore, molecular evidence illustrates that the corresponding genes of DNAJ-like proteins and calcium-dependent protein kinases (CDPKs) are related to the initiation and development of adventitious roots. Xu et al. (2017) [10] found that NO and ETH could induce AR development in cucumber by regulating CsDNAJ-1 and CsCDPK1/5 genes. However, when NO and ETH were applied alone, the relative expressions of CsDNAJ-1, CsCDPK1, and CsCDPK5 genes were significantly higher than that of any other treatments. The CsDNAJ-1 and CsCDPK1/5 target genes were up-regulated by the application of H2 and HO-1/CO during AR development in cucumber [64]. The H2S donor NaHS triggered up-regulation of target genes responsible for HO-1/CO-induced cucumber adventitious root formation, including CsDNAJ-1 and CsCDPK1/5 [39]. CsDNAJ-1 and CsCDPK1/5 genes were the possible target genes in the MRW-induced HO-1/CO-mediated cucumber adventitious development [66]. Also, the up-regulation of the HO-1 gene CsHO1 can inhibit antioxidant processes in some abiotic stresses. CsHO1 was found to be involved in cucumber adventitious rooting via NO, H2S, and CO treatments [17,62]. In addition, there are also some adventitious rooting-related genes. For example, the transcripts of CsmiR160 and CsmiR167 were increased or decreased, respectively, by the application of MRW, indicating that CH4 may play a positive effect on AR development in cucumber [47]. Therefore, NO, ETH, H2, CO, H2S, and CH4 are able to induce high expressions of the CsDNAJ-1, CsCDPK1/5, and CsHO1 genes to mediate adventitious rooting.
Besides, some auxin-response genes are associated with AR formation. Previous studies showed that the auxin response factors ARF6 and ARF8, which were targeted by miR167, had important effects on the regulation of AR development by adding ETH in Arabidopsis; however, ARF17, a target of miR160, was a negative regulator [67]. In cucumber, CsAUX22B-like and CsAUX22D-like were target genes to HRW-induced AR formation [64]. Recently, MRW could increase the transcripts levels of CsAux22D-like and CsAux22B-like in cucumber explants [47]. Also, CH4 could induce two auxin-signaling genes, such as CsAux22D-like and CsAux22B-like, which are responsible for AR formation in cucumber [40]. Table 3 summarizes all genes regulated by small-molecule compounds during adventitious rooting in plants.
Table 3.
Overview of gene regulation by small-molecule compounds during AR formation and development.
| Gene Functions | Plant Species | Small Signal Molecules-Mediated Genes | Small Signal Molecules | References |
|---|---|---|---|---|
| Cell cycle regulation | Cucumber | CsCDPK1, CsCDPK5 | NO and ETH | [64] |
| CycA, CycB, CDKA, and CDKB, | NO and H2 | [7] | ||
| CsCDC6, CsCDPK1, CsCDPK5, and CsDNAJ-1 | CH4 | [40,47] | ||
| Adventitious rooting-related | Cucumber | CsDNAJ-1 and CsCDPK1/5 | NO and ETH | [10] |
| CsDNAJ-1 and CsCDPK1/5 | H2 and CO | [64] | ||
| CsDNAJ-1 and CsCDPK1/5 | H2S and CO | [39] | ||
| CsDNAJ-1 and CsCDPK1/5 | CH4 and CO | [65] | ||
| CsHO1 | NO, H2S, and CO | [17,62] | ||
| CsmiR160 and CsmiR167 | CH4 | [47] | ||
| Auxin-response | Arabidopsis | ARF6, ARF 8, and ARF17 | ETH | [67] |
| Cucumber | CsAUX22B-like and CsAUX22D-like | H2 | [64] | |
| CsAux22D-like and CsAux22B-like | CH4 | [40,47] |
5. Conclusions and Perspectives
In recent years, there have been increasing studies about the effects on the small signaling molecules of AR formation and development. Some molecules, like CO and H2S, were previously considered as toxic. They recently were assumed to be the gaseous substances to induce AR development. This review is based on a number of studies to show that small-molecule compounds such as NO, H2, H2S, CO, CH4, and ETH can be necessary to trigger adventitious rooting. However, the mechanisms of these compounds in adventitious rooting should still be illustrated and consummated. Many results have reported that some small molecules exert interactions with each other. For instance, H2 is involved in CO-induced AR development, but the concrete pathway is still unclear. Therefore, the combined effects of these compounds can mediate the induction of AR development. Besides, some target genes responsible for adventitious rooting, including CsDNAJ-1 and CsCDPK1/5, could also be regulated by these small molecules.
Above all, these small-molecule compounds are involved in AR development through modifying antioxidant defenses, water balance, photosynthesis, and auxin transportation. In addition, the small compound molecules are widely and easily applied in various plant species and regulate many processes in plants, and they are more efficient and cheaper than some chemicals. Therefore, it is very meaningful to study the influence of small molecules on AR development and formation. However, the mechanism of the promotion of AR development by these small molecules has not been clear. Thus, further research is required to investigate their mechanisms. Finally, the question of whether there any other signaling compounds that can be used to promote AR formation and development should be considered. In the future, related work should be done to improve our knowledge in AR promotion and the future application of signaling molecules to horticultural products.
Abbreviations
AR: adventitious root; NO, nitric oxide; H2, hydrogen gas; H2S, hydrogen sulfide; CO, carbon monoxide; CH4, methane; ETH, ethylene; H2O2, hydrogen peroxide; NR, nitrate reductase; NOS, NO synthase; SNP, sodium nitroprusside; cPTIO, 2-(4-carboxy-2-phenyl)-4, 4, 5, 5-tetramethylimidazoline-1-oxyl-3-oxide; l-NAME, N-nitro-l-argininemethyl ester; cGMP, cyclic guanosine monophosphate; POD, peroxidase; PPO, polyphenol oxidase; IAAO, indoleacetic acid oxidase; NADPH oxidase, triphosphopyridine nucleotide; SOD, superoxide dismutase; CAT, catalase; APX, ascorbate peroxidase; DHAR, dehydroascorbate reductase; GR, glutathione reductase; WSC, water-soluble carbohydrate; As, arsenate; HRW, hydrogen-rich water; RWC, relative water content; Cd, cadmium; MDA, malondialdehyde; O2−, superoxide radical; TBARS, thiobarbituric acid reactive substances; AsA, ascorbic acid; GSH, reduced glutathione; REC, relative electrical conductivity; LOX, lipoxygenase; DHA, docosahexaenoic acid; GSSG, oxidized glutathione; MDHAR, monodehydroascorbate reductase; NaHS, sodium hydrosulfide; HO, heme oxygenase; HO-1, heme oxygenase-1; HO-2, heme oxygenase-2; HO-3, heme oxygenase-3; PEG, polyethylene glycol; ROS, reactive oxygen species; MRW, methane-rich water; SGH, γ-glutamyl cysteine synthetase; ACC, 1-aminocyclopropane-1-carboxylic acid; IBA, indole-3-butyric acid; CsHO1, heme oxygenase-1; ZnPPIX, zinc protoporphyrin IX; NaN3, sodium azide; DAO, diamine oxidases; and HT, hypotaurine.
Author Contributions
Conceptualization, W.L. (Weibiao Liao); resources, Y.D., N.W., Y.Y., L.W., and W.L. (Weifang Li); writing—original draft preparation, Y.D. and C.W.; writing—review and editing, W.L. (Weibiao Liao), Y.D. and C.W.; funding acquisition, W.L. (Weibiao Liao) and C.W.; figures creation and adaptation, Y.D. and Y.Y.
Funding
This work was supported by the Scientific research start-up funds for openly-recruited doctors (2017RCZX-29); the National Key Research and Development Program (2018YFD1000800); the National Natural Science Foundation of China (Nos. 31860568, 31560563 and 31160398); the Research Fund of Higher Education of Gansu, China (No. 2018C-14 and 2019A-051); the Post-Doctoral Foundation of China (Nos. 20100470887 and 2012T50828); and the Natural Science Foundation of Gansu Province, China (Nos. 1606RJZA073 and 1606RJZA077).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Geiss G., Gutierrez L., Bellini C. Annual Plant Reviews Online. John Wiley & Sons, Ltd.; Plymouth, UK: 2018. Adventitious root formation: New insights and perspectives; pp. 127–156. [Google Scholar]
- 2.Birnbaum K.D. How many ways are there to make a root? Curr. Opin. Plant Biol. 2016;34:61–67. doi: 10.1016/j.pbi.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Ramírez-Carvajal G.A., Morse A.M., Dervinis C., Davis J.M. The cytokinin type-B response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol. 2009;150:759–771. doi: 10.1104/pp.109.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porfirio S., da Silva M.D.G., Cabrita M.J., Azadi P., Peixe A. Reviewing current knowledge on olive (Olea europaea L.) adventitious root formation. Sci. Hortic. 2016;198:207–226. doi: 10.1016/j.scienta.2015.11.034. [DOI] [Google Scholar]
- 5.Zhu Y., Liao W. The metabolic constituent and rooting-related enzymes responses of marigold explants to hydrogen gas during adventitious root development. Theor. Exp. Plant Phys. 2017;29:77–85. doi: 10.1007/s40626-017-0085-y. [DOI] [Google Scholar]
- 6.Orozco-Cárdenas M.L., Ryan C.A. Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol. 2002;130:487–493. doi: 10.1104/pp.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Y., Liao W., Niu L., Wang M., Ma Z. Nitric oxide is involved in hydrogen gas-induced cell cycle activation during adventitious root formation in cucumber. BMC Plant Biol. 2016;16:146. doi: 10.1186/s12870-016-0834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jablonka E. Inheritance systems and the evolution of new levels of individuality. J. Theor. Biol. 1994;170:301–309. doi: 10.1006/jtbi.1994.1191. [DOI] [PubMed] [Google Scholar]
- 9.Wang M., Liao W. Carbon monoxide as a signaling molecule in plants. Front. Plant Sci. 2016;7:572. doi: 10.3389/fpls.2016.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X.T., Jin X., Liao W.B., Dawuda M.M., Li X.P., Wang M., Niu L.J., Ren P.J., Zhu Y.C. Nitric oxide is involved in ethylene-induced adventitious root development in cucumber (Cucumis sativus L.) explants. Sci. Hortic. 2017;215:65–71. doi: 10.1016/j.scienta.2016.12.006. [DOI] [Google Scholar]
- 11.Perrine-Walker F.M., Gartner E., Hocart C.H., Becker A., Rolfe B.G. Rhizobium-initiated rice growth inhibition caused by nitric oxide accumulation. Mol. Plant Microbe Interact. 2007;20:283–292. doi: 10.1094/MPMI-20-3-0283. [DOI] [PubMed] [Google Scholar]
- 12.El-Beltagi H.S., Ahmed O.K., Shehab G.M. Nitric oxide treatment and induced genes role against Phytophthora infestans in potato. Gesunde Pflanz. 2017;69:171–183. doi: 10.1007/s10343-017-0402-z. [DOI] [Google Scholar]
- 13.Hu H., Li P., Wang Y., Gu R. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 2014;156:100–109. doi: 10.1016/j.foodchem.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J., Fang H., Huo J., Huang D., Wang B., Liao W. Involvement of calcium and calmodulin in nitric oxide-regulated senescence of cut lily flowers. Front. Plant Sci. 2018;9:1284. doi: 10.3389/fpls.2018.01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xin T., Zhang Z., Li S., Zhang S., Li Q., Zhang Z., Huang S., Yang X. Genetic regulation of ethylene dosage for cucumber fruit elongation. Plant Cell. 2019;31:1063–1076. doi: 10.1105/tpc.18.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao W.B., Huang G.B., Yu J.H., Zhang M.L. Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol. Biotech. 2012;58:6–15. doi: 10.1016/j.plaphy.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Xuan W., Xu S., Li M., Han B., Zhang B., Zhang J., Lin Y., Huang J., Shen W., Cui J. Nitric oxide is involved in hemin-induced cucumber adventitious rooting process. J. Plant Physiol. 2012;169:1032–1039. doi: 10.1016/j.jplph.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y., Wang M., Hu L., Liao W., Dawuda M.M., Li C. Carbon monoxide is involved in hydrogen gas-induced adventitious root development in cucumber under simulated drought stress. Front. Plant Sci. 2017;8:128. doi: 10.3389/fpls.2017.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi F., Xiang Z., Kou N., Cui W., Xu D., Wang R., Zhu D., Shen W. Nitric oxide is involved in methane-induced adventitious root formation in cucumber. Physiol. Plantarum. 2017;159:366–377. doi: 10.1111/ppl.12531. [DOI] [PubMed] [Google Scholar]
- 20.Motte H., Vanneste S., Beeckman T. Molecular and environmental regulation of root development. Annu. Rev. Plant Biol. 2019;70:465–488. doi: 10.1146/annurev-arplant-050718-100423. [DOI] [PubMed] [Google Scholar]
- 21.Wilson I.D., Neill S.J., Hancock J.T. Nitric oxide synthesis and signalling in plants. Plant Cell Environ. 2008;31:622–631. doi: 10.1111/j.1365-3040.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 22.Niu L., Yu J., Liao W., Yu J., Zhang M., Dawuda M.M. Calcium and calmodulin are involved in Nitric Oxide-induced adventitious rooting of cucumber under simulated osmotic stress. Front. Plant Sci. 2017;8:1684. doi: 10.3389/fpls.2017.01684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao W., Xiao H., Zhang M. Role and relationship of nitric oxide and hydrogen peroxide in adventitious root development of marigold. Acta. Physiol. Plant. 2009;31:1279. doi: 10.1007/s11738-009-0367-3. [DOI] [Google Scholar]
- 24.Pagnussat G.C., Simontacchi M., Puntarulo S., Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002;129:954–956. doi: 10.1104/pp.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagnussat G.C., Lanteri M.L., Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao W., Huang G., Yu J., Zhang M., Shi X. Nitric oxide and hydrogen peroxide are involved in indole-3-butyric acid-induced adventitious root development in marigold. J. Hortic. Sci. Biotech. 2011;86:159–165. doi: 10.1080/14620316.2011.11512742. [DOI] [Google Scholar]
- 27.Jin X., Liao W.B., Yu J.H., Ren P.J., Dawuda M.M., Wang M., Niu L.J., Li X.P., Xu X.T. Nitric oxide is involved in ethylene-induced adventitious rooting in marigold (Tagetes erecta L.) Can. J. Plant Sci. 2017;97:620–631. [Google Scholar]
- 28.Liao W.B., Xiao H.L., Zhang M.L. Effect of nitric oxide and hydrogen peroxide on adventitious root development from cuttings of ground-cover chrysanthemum and associated biochemical changes. J. Plant Growth Regul. 2010;29:338–348. doi: 10.1007/s00344-010-9140-5. [DOI] [Google Scholar]
- 29.Tewari R.K., Hahn E.J., Paek K.Y. Modulation of copper toxicity-induced oxidative damage by nitric oxide supply in the adventitious roots of Panax ginseng. Plant Cell Rep. 2008;27:171–181. doi: 10.1007/s00299-007-0423-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y., Liao W., Wang M., Niu L., Xu Q., Jin X. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J. Plant Physiol. 2016;195:50–58. doi: 10.1016/j.jplph.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Kushwaha B.K., Singh S., Tripathi D.K., Sharma S., Prasad S.M., Chauhan D.K., Kumar V., Singh V.P. New adventitious root formation and primary root biomass accumulation are regulated by nitric oxide and reactive oxygen species in rice seedlings under arsenate stress. J. Hazard. Mater. 2019;361:134–140. doi: 10.1016/j.jhazmat.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Liu F., Li J., Liu Y. Molecular hydrogen can take part in phytohormone signal pathways in wild rice. Biol. Plantarum. 2016;60:311–319. doi: 10.1007/s10535-016-0591-9. [DOI] [Google Scholar]
- 33.Li C., Gong T., Bian B., Liao W. Roles of hydrogen gas in plants: A review. Funct. Plant Biol. 2018;45:783–792. doi: 10.1071/FP17301. [DOI] [PubMed] [Google Scholar]
- 34.Wang B., Bian B., Wang C., Li C., Fang H., Zhang J., Huang D., Huo J., Liao W. Hydrogen gas promotes the adventitious rooting in cucumber under cadmium stress. PLoS ONE. 2019;14:e0212639. doi: 10.1371/journal.pone.0212639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fotopoulos V., Christou A., Manganaris G.A. Molecular Approaches in Plant Abiotic Stress. University of Tehran; Boca Raton, Iran: 2013. Hydrogen sulfide as a potent regulator of plant responses to abiotic stress factors; pp. 353–373. [Google Scholar]
- 36.Li L., Bhatia M., Moore P.K. Hydrogen sulphide—a novel mediator of inflammation? Curr. Opin. Pharmacol. 2006;6:125–129. doi: 10.1016/j.coph.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Fotopoulos V., Christou A., Antoniou C., Manganaris G.A. Review article Hydrogen sulphide: A versatile tool for the regulation of growth and defence responses in horticultural crops. J. Hortic. Sci. Biotech. 2015;90:227–234. doi: 10.1080/14620316.2015.11513176. [DOI] [Google Scholar]
- 38.Zhang H., Tang J., Liu X.P., Wang Y., Yu W., Peng W.Y., Fang F., Ma D.F., Wei Z.J., Hu L.Y. Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J. Integr. Plant Biol. 2009;51:1086–1094. doi: 10.1111/j.1744-7909.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y.T., Li M.Y., Cui W.T., Lu W., Shen W.B. Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J. Plant Growth Regul. 2012;31:519–528. doi: 10.1007/s00344-012-9262-z. [DOI] [Google Scholar]
- 40.Kou N., Xiang Z., Cui W., Li L., Shen W. Hydrogen sulfide acts downstream of methane to induce cucumber adventitious root development. J. Plant Physiol. 2018;228:113–120. doi: 10.1016/j.jplph.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Xu J., Xuan W., Huang B., Zhou Y., Ling T., Xu S., Shen W. Carbon monoxide-induced adventitious rooting of hypocotyl cuttings from mung bean seedling. Chin. Sci. Bull. 2006;51:668–674. doi: 10.1007/s11434-006-0668-5. [DOI] [Google Scholar]
- 42.Xuan W., Zhu F.Y., Xu S., Huang B.K., Ling T.F., Qi J.Y., Ye M.B., Shen W.B. The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol. 2008;148:881–893. doi: 10.1104/pp.108.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L., Yao Y., He R., Meng Y., Li N., Zhang D., Xu J., Chen C., Cui J., Bian J., et al. Methane ameliorates spinal cord ischemia-reperfusion injury in rats: Antioxidant, anti-inflammatory and anti-apoptotic activity mediated by Nrf2 activation. Free Radic. Bio. Med. 2017;103:69–86. doi: 10.1016/j.freeradbiomed.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z.P., Chang S.X., Chen H., Han X.G. Widespread non-microbial methane production by organic compounds and the impact of environmental stresses. Earth-Sci. Rev. 2013;127:193–202. doi: 10.1016/j.earscirev.2013.10.001. [DOI] [Google Scholar]
- 45.Bruhn D., Mikkelsen T.N., Øbro J., Willats W.G.T., Ambus P. Effects of temperature, ultraviolet radiation and pectin methyl esterase on aerobic methane release from plant material. Plant Biol. 2009;11:43–48. doi: 10.1111/j.1438-8677.2009.00202.x. [DOI] [PubMed] [Google Scholar]
- 46.Bruhn D., Møller I.M., Mikkelsen T.N., Ambus P. Terrestrial plant methane production and emission. Physiol. Plant. 2012;144:201–209. doi: 10.1111/j.1399-3054.2011.01551.x. [DOI] [PubMed] [Google Scholar]
- 47.Jiang X., He J., Cheng P., Xiang Z., Zhou H., Wang R., Shen W. Methane control of adventitious rooting requires γ-glutamyl cysteine synthetase-mediated glutathione homeostasis. Plant Cell Physiol. 2018;60:802–815. doi: 10.1093/pcp/pcy241. [DOI] [PubMed] [Google Scholar]
- 48.Dubois M., Van den Broeck L., Inzé D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018;23:311–323. doi: 10.1016/j.tplants.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z., Guo H. Plant Senescence. Humana Press; New York, NY, USA: 2018. Ethylene Treatment in studying leaf senescence in Arabidopsis; pp. 105–112. [DOI] [PubMed] [Google Scholar]
- 50.Zimmerman P.W., Hitchcock A.E. Initiation and stimulation of adventitious roots caused by unsaturated hydrocarbon gases. Contrib. Boyce Thompson Inst. 1933;5:351–369. [Google Scholar]
- 51.Lorbiecke R., Sauter M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999;119:21–30. doi: 10.1104/pp.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steffens B., Kovalev A., Gorb S.N., Sauter M. Emerging roots alter epidermal cell fate through mechanical and reactive oxygen species signaling. Plant Cell. 2012;24:3296–3306. doi: 10.1105/tpc.112.101790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen A., Hu Y., Depaepe T., Vandenbussche F., Boyer F.D., Van Der Straeten D., Geelen D. Ethylene controls adventitious root initiation sites in Arabidopsis hypocotyls independently of strigolactones. J. Plant Growth Regul. 2017;36:897–911. doi: 10.1007/s00344-017-9692-8. [DOI] [Google Scholar]
- 54.Pei Z.M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G.J., Grill E., Schroeder J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 55.Potocký M., Jones M.A., Bezvoda R., Smirnoff N., Žárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- 56.Hung K.T., Hsu Y.T., Kao C.H. Hydrogen peroxide is involved in methyl jasmonate-induced senescence of rice leaves. Physiol. Plant. 2006;127:293–303. doi: 10.1111/j.1399-3054.2006.00662.x. [DOI] [Google Scholar]
- 57.Neill S.J., Desikan R., Clarke A., Hurst R.D., Hancock J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002;53:1237–1247. doi: 10.1093/jexbot/53.372.1237. [DOI] [PubMed] [Google Scholar]
- 58.Chen Z., Gu Q., Yu X., Huang L., Xu S., Wang R., Shen W., Shen W. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann. Bot-Lond. 2018;121:1127–1136. doi: 10.1093/aob/mcx207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S., Xue L., Xu S., Feng H., An L. Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regul. 2007;52:173–180. doi: 10.1007/s10725-007-9188-9. [DOI] [Google Scholar]
- 60.Bai X., Todd C.D., Desikan R., Yang Y., Hu X. N-3-oxo-decanoyl-L-homoserine-lactone activates auxin-induced adventitious root formation via hydrogen peroxide-and nitric oxide-dependent cyclic GMP signaling in mung bean. Plant Physiol. 2012;158:725–736. doi: 10.1104/pp.111.185769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartsfield C.L. Cross talk between carbon monoxide and nitric oxide. Antioxid. Redox Signal. 2002;4:301–307. doi: 10.1089/152308602753666352. [DOI] [PubMed] [Google Scholar]
- 62.Li M.Y., Cao Z.Y., Shen W.B., Cui J. Molecular cloning and expression of a cucumber (Cucumis sativus L.) heme oxygenase-1 gene, CsHO1, which is involved in adventitious root formation. Gene. 2011;486:47–55. doi: 10.1016/j.gene.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Jin Q., Zhu K., Cui W., Xie Y., Han B.I.N., Shen W. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ. 2013;36:956–969. doi: 10.1111/pce.12029. [DOI] [PubMed] [Google Scholar]
- 64.Lin Y., Zhang W., Qi F., Cui W., Xie Y., Shen W. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J. Plant Physiol. 2014;171:1–8. doi: 10.1016/j.jplph.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Cui W., Qi F., Zhang Y., Cao H., Zhang J., Wang R., Shen W. Methane-rich water induces cucumber adventitious rooting through heme oxygenase1/carbon monoxide and Ca2+ pathways. Plant Cell Rep. 2015;34:435–445. doi: 10.1007/s00299-014-1723-3. [DOI] [PubMed] [Google Scholar]
- 66.Jeong C.S., Chakrabarty D., Hahn E.J., Lee H.L., Paek K.Y. Effects of oxygen, carbon dioxide and ethylene on growth and bioactive compound production in bioreactor culture of ginseng adventitious roots. Biochem. Eng. J. 2006;27:252–263. doi: 10.1016/j.bej.2005.08.025. [DOI] [Google Scholar]
- 67.Gutierrez L., Bussell J.D., Păcurar D.I., Schwambach J., Păcurar M., Bellini C. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of auxin response factor transcripts and microRNA abundance. Plant Cell. 2009;21:3119–3132. doi: 10.1105/tpc.108.064758. [DOI] [PMC free article] [PubMed] [Google Scholar]