Abstract
Diabetic neuropathy is a serious complication of chronic hyperglycemia in diabetes patients. This complication can involve both peripheral sensorimotor and autonomic nervous system. The precise nature of injury to the peripheral nerves mediated by chronic hyperglycemia is unknown; however, several mechanisms have been proposed including polyol pathway activation, enhanced glycation of proteins and lipids, increased oxidative stress, and cytokine release in the site of injury. MicroRNAs (miRNAs) are small non-coding RNAs that mediate RNA interference by post-transcriptionally modulating gene expression and protein synthesis. Therefore, they have been implicated in several developmental, physiological, and pathophysiological processes where they modulate the expression of different proteins. Recently, miRNAs gained an increasing attention also for their role as diagnostic test in many diseases due to their stability in serum and their easy detection. Furthermore, recent studies suggest that miRNAs may be involved in diabetic neuropathy although their role in the onset and the development of this complication is not fully understood. In this review, we discuss the most recent literature providing evidence for miRNAs role in diabetic neuropathy opening new pathways to improve both early diagnosis and treatment of this complication.
Keywords: diabetes mellitus, diabetes complications, diabetes neuropathy, microRNAs, peripheral nervous system, central nervous system
1. Types of Diabetes
Diabetes mellitus (DM) is a chronic metabolic disease whose incidence worldwide is increasing; overall, about 100 million people are affected. Classically, two forms of DM are distinguished. Type 1 diabetes (T1D), defined also as insulin-dependent diabetes mellitus, is a multifactorial disorder characterized by the organ-specific autoimmune destruction of pancreatic insulin producing beta cells in human leukocyte antigen (HLA) genetically predisposed subjects [1]. This form occurs in 5%–10% of DM patients [1]. T1D derives from a breakdown in immune regulation that leads to expansion of autoreactive CD4+ and CD8+ T cells, autoantibody-producing B-lymphocytes and activation of the innate immune system [1]. The presence of a chronic inflammatory infiltrate within pancreatic islets leading to insulitis is the main histopathological finding in T1D [2]. Another important aspect is that, in patients with longstanding disease, the remaining pancreatic β-cells resistant to autoimmune destruction are incapable of regeneration [3]. Nevertheless, in contrast to adolescents and adults, recent studies confirm that β-cell regeneration occurs in infants and very young children [3,4].
Type 2 diabetes (T2D), caused by altered insulin function [5], and also defined as non-insulin-dependent diabetes mellitus occurs in 90%–95% of DM patients. In T2D there is a normal production of insulin from pancreatic β-cells; however, peripheral tissues show resistance to the insulin-mediated action resulting in pathological glucose intolerance. In terms of pathogenesis, nowadays, T2D is considered the result of both genetic predisposition and environmental factors such as high calorie diet, low energy expenditure, and generally a more modern lifestyle. β-cell loss and dysfunction through genetic or cytotoxic factors is also predisposing to glucose intolerance together with insulin resistance (IR) in target tissues (American Diabetes Association, ADA 2014) [5]. IR is a pathological condition in which insulin-dependent cells do not respond to normal circulating levels of insulin [6]. Since insulin mediates the entry of glucose into cells, any alteration of its transduction pathway leads to hyperglycemia due to inability of target cells to uptake glucose [6]. However, insulin signal transduction is complex and involves many enzymes and modulatory proteins, therefore an impairment of its signaling may happen at different levels making the exact pathophysiology of IR unclear [6]. Oxidative stress, inflammation, insulin receptor mutations, endoplasmic reticulum stress, and mitochondrial dysfunctions all contribute to IR [6].
In this review, we discuss the most recent literature on diabetes neuropathy. In particular, we aim to underline the implications of miRNAs in the development of this complication and their potential role as biomarkers and therapeutic targets for future treatments.
2. Diabetes Complications
In both types of diabetes, deregulation of glucose metabolism is accompanied by characteristic long-term degenerative effects [7]. Commonly, the complications of chronic hyperglycemia are divided into macrovascular complications including endothelial dysfunction (ED) and cardiovascular diseases (CVD); microvascular complications of diabetic retinopathy, nephropathy, and peripheral neuropathy (PN) [7].
CVD is the major cause of morbidity and mortality in patients with DM. Cardiac risk factors, including hypertension, dyslipidemia, smoking, genetic factors, hyperglycemia, insulin resistance/hyperinsulinemia contribute to the pathogenesis [8]. Cardiovascular dysfunction during deregulated glucose metabolism is associated with alterations in factors that modulate vascular function such as nitric oxide (NO), endothelin-1, and endothelial growth factors involved in angiogenesis [9]. Cardiac autonomic neuropathy is manifested as tachycardia and postural hypotension [7]. Cardiovascular autonomic dysfunction is associated with increased risk of silent myocardial ischemia and mortality.
Diabetic nephropathy, leading to chronic kidney disease and renal failure, is characterized by altered urinary albumin excretion, glomerular lesions, and loss of glomerular filtration rate [10]. Nephropathy, strictly related to hyperglycemia, develops in only 35–45 percent of patients with T1D and less than 20 percent of those with T2D [11,12]. Hyperglycemia induces renal damage directly or through hemodynamic modifications such as glomerular hyperfiltration, shear stress, and microalbuminuria contributing to abnormal stimulation of resident renal cells that produce more transforming growth factor (TGF)-β1 [13]. This cytokine causes augmented extracellular matrix protein deposition at the glomerular level, mesangial expansion, and glomerular basement membrane thickening [13].
Retinopathy is the most common microvascular complication of diabetes. Around 10,000 new cases of blindness are reported every year in the United States [14]. Development of retinopathy in T2D is related to both severity of hyperglycemia and presence of hypertension; many T1D patients develop symptoms of retinopathy within 20 years of diagnosis [15]. Background retinopathy is characterized by small hemorrhages in the middle layers of the retina, microaneurysms, retinal edema, and proliferative retinopathy by the formation of new retinal blood vessels leading to vitreous hemorrhage [16]. Osmotic and oxidative stress due to the hyperglycemia may play an important role in cellular injury.
3. MicroRNAs and Diabetic Neuropathy
3.1. Diabetic Neuropathy
Diabetic neuropathy (DN) is the most common and troublesome DM complication. ADA defines DN as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes”. The exact prevalence is unknown varying from 10% to 90% of reported cases according to definition criteria [17]. DN can involve both peripheral sensorimotor and autonomic systems, although other forms include cranial and peripheral motor neuropathies [7]. Similarly to microvascular complications, duration of hyperglycemia influences the possibility of developing DN [16]. Although neuropathies appear a consequence of long-lasting diabetes, early-onset polyneuropathy has been reported [18]. Electro-physiologic studies have demonstrated slowed motor and sensory nerve conduction in several patients after 5–10 years of diabetes [19,20].
Diabetic patients affected by PN may manifest sensory, focal/multifocal, and autonomic neuropathies. Since high morbidity and mortality are associated with DN, its early diagnosis is critical for prevention and treatment.
Chronic sensorimotor distal symmetric polyneuropathy (DSPN) is the most common form of DN [16]. The main symptom is paresthesia that progresses to hypoesthesia and is manifested as reduction or absence of reflexes, and decreased sensation to vibration and light touch [21]. Typically, patients experience burning, tingling, pain that feels like a shock and sometimes simple numbness that is worse at night [7]. Loss of sensation in the feet and altered foot morphology that is asymptomatic can result in foot ulceration, which is the principal risk of PN; it is important to realize that lack of symptoms does not exclude presence of neuropathy [7]. In fact, more than 80% of amputations occur after a foot ulceration or injury, which goes untreated because the patient has distal neuropathy [22]. Some patients have a painful PN characterized by dysesthesia with lancinating or burning pain that in the worst case leads to anorexia and depression [17]. Focal motor (cranial and peripheral) and compression neuropathies are less common than sensorimotor neuropathies. They are accompanied by cranial or peripheral nerve palsy such as carpal tunnel syndrome and foot drop, and the symptoms include weakness or loss of sensation in nerve distribution [7]. Usually, these neuropathies improve spontaneously in six weeks to six months and only in cases of nerve compression lesions, a surgical intervention of decompression is adopted [7].
Gastrointestinal symptoms can be detected within 5–10 years after the onset of T1D although clinical autonomic neuropathies are less frequent [7]. Autonomic neuropathies affecting gastrointestinal tract comprise gastroparesis (early satiety, nausea, vomiting) and diarrhea (nocturnal with incontinence) [23]. Meanwhile genitourinary neuropathies are characterized by impotence, retrograde ejaculation, and overflow incontinence [23]. Treatment of autonomic neuropathy in diabetics is targeted toward the organ system that is affected, but also includes optimization of glycemic control. Except for the sensorimotor neuropathy that may lead to diabetic foot, there are no available treatments apart from foot care to prevent trauma and ulcers [21].
3.2. Functions of MicroRNAs
MicroRNAs (miRNAs, miRs) are single-stranded, small noncoding RNAs of 19–25 nucleotides that mediate RNA interference (RNAi) by post-transcriptional gene silencing [24]. Thus, the miRNAs are involved in several developmental, physiological, and pathophysiological processes where they alter and modulate the expression of different proteins [25]. miRNAs act through two distinct mechanisms sequence-dependent: cleavage of their respective target mRNA (perfect complementary mechanism) or by inhibiting gene translation after complete or only partial binding to their target sequence (imperfect complementary mechanism), respectively [26]. Each miRNA may target many different genes, and many genes can be regulated by different miRNAs [27,28]. Recently, miRNAs have obtained an increasing attention as potential useful diagnostic test in many diseases thanks to their stability in serum and their easy detection [29]. Therefore, circulating miRNAs have been already considered as predictive biomarkers for aging [30], cancer [29], and neurological disorders such as Alzheimer’s disease (AD) [31], multiple sclerosis [32], Parkinson’s disease (PD) [33], and epilepsy [34]. In addition, miRNAs have been also proposed as potential biomarkers for diabetes and its vascular complications [35]. Indeed, alterations of specific miRNA levels can lead not only to the development of chronic inflammation observed in diabetic patients but also to β-cell loss/dysfunction and dysregulation of insulin secretion and signaling along with insulin-resistance and its chronic complications [36]. Therefore, the development of specific antagonists that inhibit specific miRNAs may offer new therapeutic options for diabetes complications.
3.3. Role of MiRNAs in the Nervous System
miRNAs play an important role in neurogenesis, neuron survival, dendritic outgrowth, and spine formation [37]. Accordingly, animal models in which Dicer, the enzyme responsible for the cleavage of the pre-miRNA into mature miRNAs, has been knocked down in different brain areas leading to apoptosis and neuronal degeneration [38,39]. Furthermore, dysregulation of miRNAs affect the expression of specific proteins associated with neurodegenerative diseases such as AD, Tourette syndrome, or PD [33,37]. Our knowledge is still limited regarding the role of miRNAs in mature neurons although they may contribute to the maintenance of synaptic plasticity [40]. This evidence was supported by the studies on Dicer knockdown mice in which miRNAs loss in mature neurons of the forebrain, induced an increase of learning and memory skills associated with an overexpression of plasticity-related genes [41].
3.4. MiRNAs Involved in the Pathogenesis of DN
Recent studies highlight that miRNAs play a role in the development of DN. On a speculative basis, miRNAs involved in retinopathy and nephropathy inflammation also affect neuropathic inflammation, i.e., miRNAs able to regulate nuclear factor κB (NF-κB) [42]. Moreover, the release of cytokines such as IL-1β, MCP (monocyte chemoattractant protein)-1 and TNF-α from neuropathic tissues following long-term hyperglycemic state, suggests the infiltration of M1 macrophages and the consequential involvement of miRNAs in this process [43]. Furthermore, miR-214 and miR-21 targeting PTEN (phosphatase and tensin homologue deleted on chromosome 10) in the kidney may also regulate inflammatory gene expression and signaling following NF-κB activation in DN [44]. Hyperglycemia often damages endothelial cells thus contributing to vascular complications. Several studies have highlighted the importance of VEGF and its alteration during the development of diabetic retinopathy [45]. Similarly, VEGF and its regulating miRNAs may be involved in the progression of DN and therefore may be attractive targets for treatment. In fact, a clinical trial using intramuscular VEGF gene delivery showed efficacy on pain perception in a significant number of subjects [46]. However, since VEGF treatment induces several side effects, the use of VEGF-associated miRNAs could be a valid therapeutic alternative to the VEGF gene delivery. TGF-β has been highlighted as an important mediator of neuronal function and growth through regulation of several miRNAs that could be involved in neuropathy and represent therapeutic targets [47].
3.5. MiRNAs Contribute in Animal Models of DN
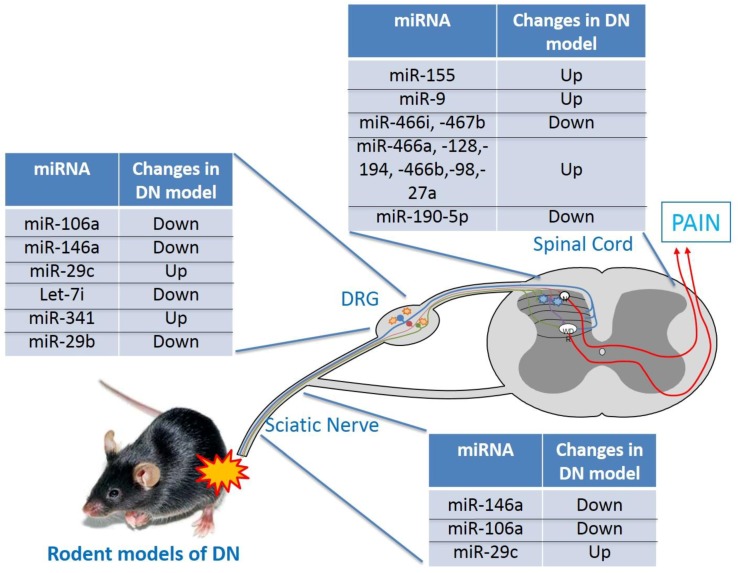
Both hyperglycemia and hypoxia are diabetes hallmarks, together with metabolic pathway alterations, may lead to complications such as DN [48]. Although the pathogenic mechanisms of DN remain to be fully elucidated, a correlation between specific miRNAs and its onset and development was suggested (Figure 1).
Figure 1.
Schematic illustration of miRNAs involved in animal model of DN.
As regard DPN was induced in rats fed with a high-fat/sugar diet for 6 weeks and subjected to intraperitoneal injection of streptozotocin (STZ) followed by further 6 weeks of high-sugar feeding [49]. Thus, 6 weeks of uncontrolled diabetes reduced miR-146a expression in the sciatic nerve of DPN rats. Furthermore, the high-sugar diet prolonged for additional 6 weeks induced a significant reduction of both motor and sensory nerve conduction velocity. These changes were not accompanied by NF-κB downregulation in the sciatic nerve indicating a possible link between miR-146a and this pro-inflammatory transcription factor [49]. Indeed miR-146a gene presents a binding site for NF-κB that induces its expression during inflammation-related hyperglycemia [50]. Conversely, miR-146a inhibits post-transcriptionally the interleukin (IL)-1 receptor-associated kinase 1 (IRAK1) and TNF-receptor-associated factor 6 (TRAF6) that mediates NF-κB activation [51]. Accordingly, hyperglycemia might activate NF-κB causing miR-146a upregulation. This leads to IRAK1 and TRAF6 downregulation and subsequent NF-κB inhibition [49]. However, long-term hyperglycemia induces miR-146a downregulation in the sciatic nerve of DPN rats causing a loss of NF-κB inhibition, tissue damage, TNF-α, and IL-1β release [49]. Similar results were obtained when 20 weeks old db/db mice were used as model of T2D [52]. In this study, downregulation of miR-146 in the sciatic nerve compared to the control group was observed meanwhile the miR-146 systemic administration mimics suppressed inflammatory genes in the sciatic nerve through NF-κB inhibition and improved neurological functions [52]. Moreover, Liu XS et al. [52] confirmed that IRAK 1 and TRAF6 are putative targets of miR-146a but in addition they used bioinformatics to predict ADAMST3 (a disintegrin and metalloproteinase with thrombospondin motifs 3) as additional target gene confirming its role in the inflammatory processes [53]. Therefore, miR-146a mimics significantly improved sciatic nerve neurovascular functions and reduced inflammation suggesting its therapeutic application for the DPN treatment [52]. In the same animal model, hyperglycemia induced miR-146a downregulation and a concomitant increase of IRAK1 and TRAF6 in dorsal root ganglia (DRG) neurons. These effects were reverted by treatment with sildenafil [54]. Conversely, miR-146a and NF-κB expressions were found increased in the sciatic nerve after 2 months of uncontrolled diabetes induced in rats by a single intraperitoneal injection of nicotinamide followed by STZ [55]. Furthermore, TRAF6 mRNA levels in the sciatic nerve did not decrease in proportion to miR-146a overexpression; downregulation of IRAK1 mRNA was not significant [55]. Therefore, in these experimental conditions, miR-146a upregulation was unable to inhibit NF-κB through IRAK1 and TRAF6 downregulation. However, the levels of pro-inflammatory cytokines were significantly increased in the sciatic nerve of diabetic rats compared to control group, and the reaction time in response to the tail immersion and the hot plate tests were significantly decreased [55]. The reasons for this discrepancy compared with other studies may depend on the different DPN experimental models. In fact, 2 months post-injection of streptozotocin is enough time to establish a PN accompanied by upregulation of pro-inflammatory mediators such as NF-κB and its regulated cytokines. In this phase, the NF-κB-miR-146a negative feedback loop may be deregulated and the NF-κB upregulation induced by a hyperglycemic state leads to an increase of miR-146a. Conversely, prolonged exposure to hyperglycemia (more than 2 months) is responsible for miR-146a downregulation and uncontrolled NF-κB activation. Therefore, a steady state for miR-146a levels in the sciatic nerve is important to avoid hyperactivation of inflammatory pathways leading to nerve damage and DN.
Other miRNAs have been involved the development of DN. For example, in diabetic mice affected by PN, miR-106a reduces the oxidative/nitrosative stress induced by hyperglycemia in DRG and sciatic nerve improving both sensor and motor nerve conduction velocity [56]. Furthermore, the authors suggested that miR-106a might exert these effects by targeting the 12/15 lipoxygenase [56]. In another study performed on diabetic rats, the prophylactic treatment with L-arginine or ibuprofen or their combination reduced miR-155 expression and the levels of nitric oxide in the spinal cord decreasing mechanical allodynia [57]. In the same diabetes model of PN, miR-9 levels were found increased in the dorsal horn (DH) of spinal cord of neuropathic rats [58]. This upregulation was positively correlated to the expression of calcium homeostasis modulator 1 (CALHM1) even if TargetScan analysis did not confirm this gene as potential target of miR-9. However, miR-9 could contribute to the DPN by indirectly increasing CALHM1 expression and is a mechanism that still needs further investigation [58]. The upregulation of CALHM1 in the dorsal horn neurons induced the expression of purinergic receptor P2X, a ligand-gated ion channel 7 (P2X7) receptor that is expressed in various cells of nervous system and mediates ATP release and calcium concentration regulation in neuropathic pain. Therefore, the authors suggested that P2X7 receptor overexpression mediated by miR-9 upregulation sustained the mechanical hypersensitivity in diabetic rats [58].
The potential role of miRNAs in the regulation of DN was further confirmed by a microarray analysis of lumbar dorsal horn of spinal cords isolated from diabetic mice in which neuropathy was confirmed by the reduction of paw withdrawal thresholds [59]. In this study, miR-466i and miR-467b were downregulated in the lumbar dorsal horns of neuropathic mice meanwhile miR-466a, miR-128, miR-194, miR-466b, miR-98, miR-27a, and miR-194 were upregulated. All these miRNAs have been shown to target both pro- and anti-inflammatory cytokines that may contribute to the DN [59].
In another microarray analysis of miRNAs in DRG tissues of DPN rats, 37 miRNAs were differentially expressed in the diabetic group. Specifically, 15 and 22 miRNAs were upregulated or downregulated, respectively [60]. In particular, using a miRanda database, 10,011 predicted target genes were found from the 37 dysregulated miRNAs; a miRNA-gene-network analysis revealed that among these miRNAs, miR-330-5p, miR-17-1- 3p, and miR-346 had a high degree of correlation [60]. Meanwhile, podocalyxin-like (Podxl, inhibiting cell-cell adhesion) and homeobox A1 (Hoxa1, sequence specific transcription factor) were the most common target mRNAs with the highest degrees of connectivity [60].
Furthermore, by using the KEGG database, 29 enriched signaling pathways including metabolic, HIF-1, calcium signaling, PI3K-Akt, as well as p53-signaling pathways were discovered. The top downregulated pathways included the ECM-receptor interaction, focal adhesion, and biosynthesis of unsaturated fatty acids, while the top upregulated pathways included HIF-1 signaling pathway, neuroactive ligand-receptor interaction and metabolic pathways.
In streptozotocin-induced model of DPN, miR-190-5p downregulation was detected in the lumbar dorsal horn accompanied by an increase of both gene and protein of solute carrier family 17A6 (SLC17A6) also known as VGLUT2 [61]. This transporter is responsible of glutamate packaging into synaptic vesicles and plays a key role in the fast-excitatory synaptic transmission in the vertebrate nervous system (reviewed (rev) in [61]). SLC17A6 is significantly up-regulated in rat DRG and spinal cord following nerve injury [62]. In fact, deletion of SLC17A6 in related nociceptors reduces acute heat, mechanical and chemical pain responsiveness in neuropathic pain models (rev in [61]). Therefore, SLC17A6 was shown to be direct target of miR-190-5p and subarachnoid injections of a SLC17A6 inhibitor or a Lentivirus carrying miR-190-5p mimic were able to reduce the mechanical hypersensitivity and the levels of IL-6 and IL-1β in lumbar spinal dorsal horn samples [61].
MicroRNAs are considered important players of intercellular communication and found encapsulated into extracellular vesicles (EVs) including exosomes released by all living cells [63,64,65]. On this regard exosomes derived from Schwann cells exposed to high concentration of glucose were found enriched of miR-28, -31a and -130a. In vitro, treatment of DRG distal axons with high glucose (HG) exosomes resulted in the reduction of axonal growth, associated with elevation of these miRNAs and reduction in axons of their DNA target proteins methyltransferase-3a (NDNMT3A), NUMB (an endocytic adaptor protein), synaptosome associated protein 25 (SNAP25), and growth-associated protein-43 (GAP43) [66]. This suggests the involvement of specific miRNAs in the regulation of genes mediating distal axonal damage under diabetic conditions. Furthermore, administration of HG exosomes to sciatic nerves of diabetic 7-week-old db/db mice promoted occurrence of PN characterized by impairment of nerve conduction velocity and induction of mechanic and thermal hypoesthesia. This was associated with substantial decreases in intra-epidermal nerve fibers confirming a functional role of exosomes derived from HG-stimulated Schwann cells in mediating DPN development [66]. In order to elucidate the molecular mechanisms underpinning the hyperglycemia-mediated distal axonal damage of peripheral nerves, another study showed that miR-29c was upregulated in DRG neurons, sciatic nerve and foot pad tissues of diabetic mice [66]. A putative target of miR-29c was identified, i.e., the PRKCI gene that encodes for the isoform iota of PKC related to the regulation of axonal growth [67]. In their study, concomitantly with miR-29s upregulation, levels of PRKCI protein were reduced in DRG neurons and sciatic nerve. These effects resulted in the suppression of axonal growth of DRG neurons as demonstrated by in vitro experiments [66]. Therefore, miR-29c is a negative regulator of axonal growth of DRG neurons by targeting PRKCI under hyperglycemia, confirming the involvement of miRNAs in the molecular mechanisms underlying hyperglycemia-induced axonal damage [66].
miR-29b was also related to the axonal growth and protection of mature neurons against apoptosis induced by diverse insults [68]. miR-29b levels were downregulated in DRG primary sensory neurons of diabetic rats [69]. This downregulation worsened in time dependent manner, associated with higher apoptosis rate and axonal swelling [69]. Furthermore, the in vitro transfection of DRG neurons from diabetic rats with a miR-29b mimic stimulated axon regeneration and inhibited neuron degeneration-related genes expression [69]. SMAD family member 3 has been already reported as potential target of miR-29b and its inhibition ameliorated diabetic nephropathy [70]. Consistently, a Smad3 inactivation was observed after miR-29b restoration in DN [69].
Let-7i and miR-341 have been identified as miRNAs potentially involved in the progression of PN in diabetic mice [71]. A microarray profiling of miRNA expressed in DRG sensory neurons revealed significant changes in diabetic mice with downregulation of let-7i and increase of miR-341 [71]. Furthermore, to counteract this variation in miRNA levels, a let-7i mimic or a miR-341 inhibitor were delivered in two independent experiments into CNS by intranasal injection. Both approaches independently improved electrophysiological, structural, and behavioral changes without altering hyperglycemia [71]. A trophic action for miRNA let-7i was shown in in vitro experiments on dissociated adult sensory neurons exposed to it. Let-7i is predicted to target at least 46 apoptotic cell death pathway mRNAs, 42 cardiovascular and diabetes-related mRNAs, 84 growth, 80 inflammation-related pathway mRNAs, 21 metabolism and diabetes pathway mRNAs, and 59 neurotransmitter and nervous system mRNAs (ingenuity pathway analysis) [71]. Therefore, the authors concluded that central manipulation of DRG neurons is effective in altering the behavior of the entire neuronal tree, including its distal terminals; these data indicate a potential new, non-viral approach to target CNS or peripheral nervous system gene function [71] (Table 1).
Table 1.
miRNAs studied in animal models of diabetic neuropathy.
| miRNA | DN Model | Changes in DN | Target (s) | Reference (s) |
|---|---|---|---|---|
| miR-146a | DPN induced by intraperitoneal injection of STZ in HFD-fed rats | Down | IRAK1; TRAF6 | Feng Y et al., 2018 [49] |
| miR-146a | Db/db mice as model of T2D and DN | Down | IRAK1; TRAF6; ADAMTS3 | Liu XS et al., 2017 [52] |
| miR-146a | Db/db mice as model of T2D and DN | Down | IRAK1; TRAF6 | Wang L et al., 2014 [54] |
| miR-146a | Diabetes was induced in rats by a single-dose injection of nicotinammide followed by STZ | Up | IRAK1; TRAF6 | Yousefzadeh N et al., 2015 [55] |
| miR-106a | DPN model was established in mice following single injection of STZ | Down | 12/15-Lipoxygenase (12/15-LOX) | Wu Y et al., 2017 [56] |
| miR-155 | DN was established in rats following injection of STZ | Up | ND | El Lithy GM et al., 2016 [57] |
| miR-9 | DN was established in rats following injection of STZ | Up | CALHM1 is indirect target of miR-9 | Liu W et al., 2016 [58] |
| miR-466i | DPN was induced in mice following injection of STZ | Down | IL-1β; TNF-α; IL-6 | Gong Q et al., 2015 [59] |
| miR-467b | Down | |||
| miR-466a | Up | IL-1β | ||
| miR-128; miR-194; miR-466b; miR-98 | Up | IL-1β | ||
| miR-27a | Up | IL-10 | ||
| miR-194 | Up | IL-13 | ||
| 37 miRNAs were differently expressed in DRG | DN was established in rats following injection of STZ | 15 miRNAs were upregulated whereas 22 were downregulated | Podx1 and Hoxa1 were the most common targets | Guo G et al., 2018 [60] |
| miR-190-5p | DPN model was established in mice following single injection of STZ | Down | SLC17A6 | Yang D et al., 2017 [61] |
| miR-28, miR-31a and miR-130a | In vitro exosomes isolated from high glucose stimulated Swann Cells contained high levels of miRs-28, -31a, -130 | Up | NDNMT3A NUMB, SNAP25 and GAP43 | Jia L et al., 2018 [66] |
| miR-29b | DN was established in rats following injection of STZ | Down | TGF-β/Smad3 | Zhang X et al., 2014 [69] |
| Let-7i and miR-341 | DPN model was established in mice following single injection of STZ | Down | NF-kB | Cheng C et al., 2015 [71] |
DPN: Diabetic Peripheral Neuropathy; STZ: Streptozotocin; T2D: Type 2 Diabetes; IRAK1: Interleukin-1 receptor associated kinase 1; TRAF6: TNF-receptor associated factor 6; DRG: Dorsal root ganglion; NO: Nitric oxide; DH: Dorsal horn; ADAMTS3: A disintegrin and metalloproteinase with thrombospondin motifs 3; SLC17A6: Solute carrier family 17A6; CALHM1: Calcium homeostasis modulator 1; Podx1: Podocalyxin; Hoxa1: Homebox A1; NDNMT3A: DNA methyltransferase-3a; NUMB: an endocytic adaptor protein; SNAP25: Synaptosome associated protein 25; GAP43: Growth-associated protein-43; TGF-β: Transforming growth factor-β; Smad3: SMAD family member 3.
3.6. Human Studies of MicroRNAs in DN
Several clinical studies show that specific miRNAs alterations may contribute to DN progression in patients and may represent a valid diagnostic tool for this complication.
Single nucleotide polymorphisms (SNPs) of miR-128a, miR-146a, and miR-27a were analyzed in order to correlate potential variations to the risk of developing peripheral or autonomic neuropathies in T2D [72]. MiR-128a rs11888095 was significantly correlated to a higher risk of developing DPN. Similarly, miR-146a variation rs2910164 was associated to a lower risk of DPN and protection in respect to early cardiovascular autonomic neuropathy (CAN). Conversely, miR-27a rs895819 SNP was linked to a higher susceptibility of developing early CAN in patients. T2D patients carrying the miR-499a rs3746444 SNP have higher predisposition to develop severe CAN [73].
MiR-199a-3p levels were increased in plasma and lower limb skin samples of diabetic patients affected by PN and with family history of diabetes [74]. Further upregulation of miR-199a-3p could act as a pro-coagulating factor in DN by targeting the extracellular serine protease inhibitor E2 (SERPINE2), contributing to the tissue vascular damage associated with PN. These studies suggest that miRNAs may be involved in the pathogenesis and progression of DN, and may serve as both biomarkers in early diagnosis and therapeutic target.
A total of 749 differentially expressed genes (DEGs) between non-progressing and progressing DN nerve samples, including 370 upregulated DEGs and 379 downregulated DEGs, was screened [75]. Using miR2Disease database, three specific miRNAs were associated with diabetes: miR-377, miR-216a and miR-217. The study predicted 1052 target genes of miR-377, 962 of miR-261a, and 1025 of miR-217 [75]. Inflammation was the most significantly represented biological process [75]. Furthermore, the Kyoto Encyclopedia of Genes and Genomes enrichment analysis revealed that peroxisome proliferator-activated receptor (PPAR) and adenosine monophosphate (AMP)-activated protein kinase (AMPK) signaling pathways were significantly regulated and enriched with PPAR gamma (PPARG), stearoyl-CoA desaturase (SCD), cluster of differentiation 36 (CD36), and phosphoenolpyruvate carboxykinase 1 (PCK1). This suggests their involvement in DN progression as possible biomarkers and potential therapeutic targets [75] (Table 2).
Table 2.
MiRNAs studied in human diabetic neuropathy.
| MiRNA | Target (s) | Changes in DN Patients | Clinical Manifestations | Reference |
|---|---|---|---|---|
| miR-499a; -128a; -146a; -27a | ND | Polymorphisms in the miRNA gene sequence | High incidence of developing CAN and DPN in T2D | Ciccacci C et al. [72,73] |
| miR-199a-3p | SerpinE2 | Up in skin biopsies | Promote coagulation in peripheral skin circulation in T2D | Li YB et al., 2017 [74] |
| miR-216a | ANXA9 | Up in nerve tissue samples | Positive association with progressing DN | Li YB et al., 2016 [75] |
| ANGPTL4 | ||||
| CHI3L2 | ||||
| miR-217 | ADRBK2 | |||
| CSN1S1 | ||||
| GALM | ||||
| miR-377 | EN1 | |||
| GAP43 | ||||
| FAM89A |
CAN: Cardiovascular autonomic neuropathy; SerpinE2: Serine protease inhibitor E2; ANXA9: Annexin A9; ANGPTL4: Angiopoietin-like 4; CHI3L2: Chitinase 3-Like Protein 2; ADRBK2: Beta-adrenergic receptor kinase 2; CSN1S1: Gene encoding for Alpha-S1-casein; GALM: Gene encoding for Aldose 1-epimerase; EN1: Gene encoding for homeobox protein engrailed-1; GAP43: Growth associated protein 43; FAM89A: Family with sequence similarity 89 member A.
4. Conclusions
Neuropathy is considered the worst and most common complication of diabetes leading to greatest morbidity and mortality, more hospitalizations, therefore huge economic impact for diabetes care. About 50%–70% of non-traumatic amputations are due to DN [17]. This highlights the importance of early and accurate diagnosis for prevention and urgency to find novel therapeutic targets.
Since their initial discovery, miRNAs have rapidly gained attention due to their role of post-transcriptional gene regulators with effect on several developmental, physiological, and pathophysiological processes where they silence genes and modulate the expression of different proteins [25]. The role of miRNAs in diabetes [33] and its complications [34] is the object of intensive investigations not only for their involvement in the pathogenesis, but also as potential disease biomarkers. Therefore, in this review we discussed the most recent literature about miRNAs involved in the development of DN and the mechanisms by which they could regulate its onset and progression. In particular, Table 3 summarizes the main pathways regulated by these miRNAs according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. In fact, both animal and human studies performed in the last 10 years show an increasing attention of the scientific community to the link between miRNAs and DN. This aspect not only increases our knowledge about the pathogenic mechanisms but also provides insights on novel and potential therapeutic targets for the treatment or the prevention of this complication. In fact, the use of selective miRNAs mimics or antagomiRs could represent a potential therapeutic tool with the aim to restore or counteract the reduction or the increase, respectively, of miRNAs levels in the nervous system. Moreover, thanks to their stability in serum and their easy detection, miRNAs may also represent a novel diagnostic tool for several diseases including DN [29]. Therefore, the detection of specific circulating miRNAs may be considered as a predictive biomarker for this complication. However, more investigations are required in future to pinpoint a whole miRNAs signature of DN and investigate its effectiveness of miRNAs and limitation in clinical practice for the management of this complication.
Table 3.
miRNAs involved in preclinical and clinical studies of diabetic neuropathy and the relative most regulated pathways.
| MiRNA | Target Genes | Most Regulated Pathways | KEGG Pathway |
|---|---|---|---|
| miR-146a | IRAK1 | NF-kB signaling pathway | mmu04064 |
| Toll-like receptor signaling pathway | mmu04620 | ||
| MAPK signaling pathway | mmu04010 | ||
| Neurotrophin signaling pathway | mmu04722 | ||
| miR-146a | TRAF6 | NF-kB signaling pathway | mmu04064 |
| Toll-like receptor signaling pathway | mmu04620 | ||
| Neurotrophin signaling pathway | mmu04722 | ||
| IL-17 signaling pathway | mmu04657 | ||
| NOD-like receptor signaling pathway | mmu04621 | ||
| MAPK signaling pathway | mmu04010 | ||
| Ubiquitin mediated proteolysis | mmu04120 | ||
| RIG-I-like receptor signaling | mmu04622 | ||
| Endocytosis | mmu04144 | ||
| miR-106a | 12/15-Lipoxygenase (12/15-LOX) | Arachidonic acid metabolism | mmu00590 |
| miR-190-5p | SLC17A6 | Synaptic vesicle cycle | mmu04721 |
| Glutamatergic synapse | mmu04724 | ||
| miR-31a; miR-130a |
NUMB; SNAP25 |
Notch signaling pathway | mmu04330 |
| Synaptic vesicle cycle | mmu04721 | ||
| Insulin secretion | mmu04911 | ||
| miR-29b | Smad3 | TGF-beta signaling pathway | mmu04350 |
| FoxO signaling pathway | mmu04068 | ||
| Wnt signaling pathway | mmu04310 | ||
| Th17 cell differentiation | mmu04659 | ||
| Cell cycle | mmu04110 | ||
| miR-29c | PRKCI | Tight junction | mmu04530 |
| Rap1 signaling pathway | mmu04015 | ||
| Endocytosis | mmu04144 | ||
| Insulin signaling pathway | mmu04910 | ||
| miR-466i; miR-467b | TNF-α; Il-6 |
NF-kB signaling pathway | mmu04064 |
| Apoptosis | mmu04210 | ||
| TNF signaling pathway | mmu04668 | ||
| HIF-1 signaling pathway | mmu04066 | ||
| PI3K-Akt signaling pathway | mmu04151 | ||
| Insulin resistance | mmu04931 | ||
| Toll-like receptor signaling pathway | mmu04620 | ||
| NOD-like receptor signaling pathway | mmu04621 | ||
| miR-466a; miR-128; -194; -466b; -98 |
IL-1β | NOD-like receptor signaling pathway | mmu04621 |
| NF-kB signaling pathway | mmu04064 | ||
| IL-17 signaling pathway | mmu04657 | ||
| Th17 cell differentiation | mmu04659 | ||
| Toll-like receptor signaling pathway | mmu04620 | ||
| Inflammatory mediator regulation of TRP channels | mmu04750 | ||
| miR-27; miR-194 | IL-10; IL-13 |
FoxO signaling pathway | mmu04068 |
| TCR signaling pathway | mmu04660 | ||
| IL-17 signaling pathway | mmu04657 | ||
| Th1 and Th2 cell differentiation | mmu04658 | ||
| miR-216a | ANGPTL4 | PPAR signaling pathway | hsa03320 |
| Cholesterol metabolism | hsa04979 | ||
| miR-217 | ADRBK2 GALM |
Glutamatergic synapse | hsa04724 |
| Glycolysis and gluconeogenesis | hsa00010 | ||
| Galactose metabolism | hsa00052 |
PRKCI: Isoform iota of Protein kinase C; MAPK: Mitogen-Activated Protein Kinase; FoxO: Forkhead Box O; HIF-1: Hypoxia Inducible Factor 1; PI3K: Phosphoinositide-3-kinase; ANGPTL4: Angiopoietin-like 4; ADRBK2: Beta-adrenergic receptor kinase 2; GALM: Gene encoding for Aldose 1-epimerase; PPAR: Peroxisome proliferator-activated receptor.
Abbreviations
| miRNAs | microRNAs |
| DM | Diabetes mellitus |
| T1D | Type 1 diabetes |
| HLA | Human leukocyte antigen |
| T2D | Type 2 diabetes |
| IR | Insulin resistance |
| ADA | American Diabetes Association |
| ED | Endothelial dysfunction |
| CVD | Cardiovascular diseases |
| PN | Peripheral neuropathy |
| NO | Nitric oxide |
| TGF | Transforming growth factor |
| DN | Diabetic neuropathy |
| DSPN | Distal symmetric polyneuropathy |
| DPN | Diabetic peripheral neuropathy |
| STZ | Streptozotocin |
| miRs | microRNAs |
| RNAi | RNA interference |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| NF-κB | Nuclear factor κB |
| MCP | Monocyte chemoattractant protein |
| PTEN | Phosphatase and tensin homologue |
| VEGF | Vascular endothelial growth factor |
| IL | Interleukin 2 |
| IKAK 1 | Interleukin-1 receptor-associated kinase 1 |
| TRAF6 | Tumor necrosis factor receptor-associated kinase 1 |
| ADAMTS3 | A disintegrin and metalloproteinase with thrombospondin motifs 3 |
| 12/15-LOX | 12/15 lipoxygenase |
| DRG | Dorsal root ganglia |
| DH | Dorsal horn |
| CALHM1 | Calcium homeostasis modulator 1 |
| P2X7 | Purinergic receptor P2X ligand gated ion channel 7 |
| Podxl | Podocalyxin-like |
| Hoxa1 | Homeobox A1 |
| SLC17A6 | Solute carrier family 17A6 |
| VGLUT2 | Vesicular-glutamate transporter 2 |
| rev | Reviewed |
| EVs | Extracellular vesicles |
| NDNMT3A | DNA target proteins methyltransferase-3a |
| NUMB | Endocytic adaptor protein |
| SNAP25 | Synaptosome associated protein 25 |
| GAP43 | Growth-associated protein-43 |
| HG | High glucose |
| PRKCI | Protein kinase C iota |
| SMAD | Small mother against decapentaplegic |
| CNS | Central nervous system |
| SNPs | Single nucleotide polymorphisms |
| CAN | Cardiovascular autonomic neuropathy |
| SERPINE2 | Serine protease inhibitor E2 |
| DEGs | Differentially expressed genes |
| PPAR | Peroxisome proliferator-activated receptor |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| PPARG | PPAR gamma |
| SCD | Stearoyl-CoA desaturase |
| CD36 | Cluster of differentiation 36 |
| PCK1 | Phosphoenolpyruvate carboxykinase 1 |
| ANXA9 | Annexin A9 |
| ANGPTL4 | Angiopoietin like 4 |
| CHI3L2 | Chitinase 3 Like 2 |
| ADRBK2 | Adrenoceptor Beta 2 |
| CSN1S1 | Casein Alpha S1 |
| GALM | Galactose mutarotase |
| EN1 | Homeobox protein engrailed-1 |
| FAM89A | Family with sequence similarity 89 member A |
Author Contributions
A.F. wrote the manuscript and supervised literature search. R.S. conducted literature search and wrote the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.In’t Veld P. Insulitis in human type 1 diabetes: The quest for an elusive lesion. Islets. 2011;3:131–138. doi: 10.4161/isl.3.4.15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg B.E., Moore P.C., Demozay D., Hall B.A., Li M., Husain A., Wright A.J., Atkinson M.A., Rhodes C.J. Formation of a human beta-cell population within pancreatic islets is set early in life. J. Clin. Endocrinol. Metab. 2012;97:3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keenan H.A., Sun J.K., Levine J., Doria A., Aiello L.P., Eisenbarth G., Bonner-Weir S., King G.L. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen A., Horton E.S. Progress in the treatment of type 2 diabetes: New pharmacologic approaches to improve glycemic control. Curr. Med. Res. Opin. 2007;23:905–917. doi: 10.1185/030079907X182068. [DOI] [PubMed] [Google Scholar]
- 6.Yaribeygi H., Farrokhi F.R., Butler A.E., Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 2019;234:8152–8161. doi: 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 7.Nathan D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 8.De Mattia G., Bravi M.C., Laurenti O., Moretti A., Cipriani R., Gatti A., Mandosi E., Morano S. Endothelial dysfunction and oxidative stress in type 1 and type 2 diabetic patients without clinical macrovascular complications. Diabetes Res. Clin. Pract. 2008;79:337–342. doi: 10.1016/j.diabres.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 9.He Z., Rask-Madsen C., King G.L. Managing heart disease and potential new pharmacological therapies. Eur. Heart J. Suppl. 2003;5:B51–B57. doi: 10.1016/S1520-765X(03)90041-1. [DOI] [Google Scholar]
- 10.Lim A. Diabetic nephropathy-complications and treatment. Int. J. Nephrol. Renovasc. Dis. 2014;7:361–381. doi: 10.2147/IJNRD.S40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deckert T., Poulsen J.E., Larsen M. Prognosis of diabetics with diabetes onset before the age of thirtyone. Diabetologia. 1978;14:363–370. doi: 10.1007/BF01228130. [DOI] [PubMed] [Google Scholar]
- 12.Ballard D.J., Humphrey L.L., Melton L.J., III, Frohnert P.P., Chu P.C., O’Fallon W.M., Palumbo P.J. Epidemiology of persistent proteinuria in type II diabetes mellitus: Population-based study in Rochester, Minnesota. Diabetes. 1988;37:405–412. doi: 10.2337/diab.37.4.405. [DOI] [PubMed] [Google Scholar]
- 13.Schena F.P., Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J. Am. Soc. Nephrol. 2005;16:S30–S33. doi: 10.1681/ASN.2004110970. [DOI] [PubMed] [Google Scholar]
- 14.Fong D.S., Aiello L., Gardner T.W., King G.L., Blankenship G., Cavallerano J.D., Ferris F.L., III, Klein R. Retinopathy in diabetes. Diabetes Care. 2004;27:S84–S87. doi: 10.2337/diacare.27.2007.S84. [DOI] [PubMed] [Google Scholar]
- 15.Keenan H.A., Costacou T., Sun J.K., Doria A., Cavellerano J., Coney J., Orchard T.J., Aiello L.P., King G.L. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: The 50-year medalist study. Diabetes Care. 2007;30:1995–1997. doi: 10.2337/dc06-2222. [DOI] [PubMed] [Google Scholar]
- 16.Fowler M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes. 2008;26:77–82. doi: 10.2337/diaclin.26.2.77. [DOI] [Google Scholar]
- 17.Vinik A., Casellini C., Nevoret M.L., Feingold K.R., Anawalt B., Boyce A., Chrousos G., Dungan K., Grossman A., Hershman J.M., et al. Endotext [Internet] [(accessed on 12 September 2019)]; Available online: MDText.com.
- 18.Said G., Goulon-Goeau C., Slama G., Tchobroutsky G. Severe early-onset polyneuropathy in insulin-dependent diabetes mellitus: A clinical and pathological study. N. Engl. J. Med. 1992;326:1257–1263. doi: 10.1056/NEJM199205073261905. [DOI] [PubMed] [Google Scholar]
- 19.Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT) Diabetes Care. 1988;11:725–732. doi: 10.2337/diacare.11.9.725. [DOI] [PubMed] [Google Scholar]
- 20.Yagihashi S., Yamagishi S., Wada R. Pathology and pathogenetic mechanisms of diabetic neuropathy: Correlation with clinical signs and symptoms. Diabetes Res. Clin. Pract. 2007;77:S184–S189. doi: 10.1016/j.diabres.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 21.Litzelman D.K., Slemenda C.W., Langefeld C.D., Hays L.M., Welch M.A., Bild D.E., Ford E.S., Vinicor F. Reduction of lower extremity clinical abnormalities in patients with non-insulin-dependent diabetes mellitus: A randomized, controlled trial. Ann. Intern. Med. 1993;119:36–41. doi: 10.7326/0003-4819-119-1-199307010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Boulton A.J., Vinik A.I., Arezzo J.C., Bril V., Feldman E.L., Freeman R., Malik R.A., Maser R.E., Sosenko J.M., Ziegler D. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal Z., Azmi S., Yadav R., Ferdousi M., Kumar M., Cuthbertson D.J., Lim J., Malik R.A., Alam U. Diabetic peripheral neuropathy: Epidemiology, diagnosis, and pharmacotherapy. Clin. Ther. 2018;40:828–849. doi: 10.1016/j.clinthera.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 25.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 26.Niederberger E., Kynast K., Lotsch J., Geisslinger G. MicroRNAs as new players in the pain game. Pain. 2011;152:1455–1458. doi: 10.1016/j.pain.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 27.Friedman R.C., Farh K.K.H., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krek A., Grun D., Poy M.N., Wolf R., Rosenberg L., Epstein E.J., MacMenamin P., da Piedade I., Gunsalus K.C., Stoffel M., et al. Combinatorial microRNA target predictions. Nat. Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 29.Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K., Guo J., Zhang Y., Chen J., Guo X., et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 30.ElSharawy A., Keller A., Flachsbart F., Wendschlag A., Jacobs G., Kefer N., Brefort T., Leidinger P., Backes C., Meese E., et al. Genome-wide miRNA signatures of human longevity. Aging Cell. 2012;11:607–616. doi: 10.1111/j.1474-9726.2012.00824.x. [DOI] [PubMed] [Google Scholar]
- 31.Grasso M., Piscopo P., Confaloni A., Denti M.A. Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules. 2014;19:6891–6910. doi: 10.3390/molecules19056891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris V.K., Tuddenham J.F., Sadiq S.A. Biomarkers of multiple sclerosis: Current findings. Degener. Neurol. Neuromuscul. Dis. 2017;7:19–29. doi: 10.2147/DNND.S98936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert S.S., De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci. 2009;32:199–206. doi: 10.1016/j.tins.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Wang J., Tan L., Tan L., Tian Y., Ma J., Tan C.C., Wang H.F., Liu Y., Tan M.S., Jiang T., et al. Circulating microRNAs are promising novel biomarkers for drug-resistant epilepsy. Sci. Rep. 2015;5:10201. doi: 10.1038/srep10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Marca V., Fierabracci A. Insights into the diagnostic potential of extracellular vesicles and their miRNA signature from liquid biopsy as early biomarkers of diabetic micro/macrovascular complications. Int. J. Mol. Sci. 2017;18:1974. doi: 10.3390/ijms18091974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClelland A.D., Kantharidis P. microRNA in the development of diabetic complications. Clin. Sci. 2014;126:95–110. doi: 10.1042/CS20130079. [DOI] [PubMed] [Google Scholar]
- 37.Kosik K.S. The neuronal microRNA system. Nat. Rev. Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 38.Davis T.H., Cuellar T.L., Koch S.M., Barker A.J., Harfe B.D., McManus M.T., Ullian E.M. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaefer A., O’Carroll D., Tan C.L., Hillman D., Sugimori M., Llinas R., Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. J. Exp. Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smalheiser N.R., Lugli G. MicroRNA regulation of synaptic plasticity. Neuromol. Med. 2009;11:133–140. doi: 10.1007/s12017-009-8065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konopka W., Kiryk A., Novak M., Herwerth M., Parkitna J.R., Wawrzyniak M., Kowarsch A., Michaluk P., Dzwonek J., Arnsperger T., et al. MicroRNA loss enhances learning and memory in mice. J. Neurosci. 2010;30:14835–14842. doi: 10.1523/JNEUROSCI.3030-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovacs B., Lumayag S., Cowan C., Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest. Ophthalmol. Vis. Sci. 2011;52:4402–4409. doi: 10.1167/iovs.10-6879. [DOI] [PubMed] [Google Scholar]
- 43.Wilson N., Wright D. Inflammatory mediators in diabetic neuropathy. J. Diabetes Metab. 2011;S5:4. doi: 10.4172/2155-6156.S5-004. [DOI] [Google Scholar]
- 44.Denby L., Ramdas V., McBride M.W., Wang J., Robinson H., McClure J., Crawford W., Lu R., Hillyard D.Z., Khanin R., et al. miR-21 and miR-214 are consistently modulated during renal injury in rodent models. Am. J. Pathol. 2011;179:661–672. doi: 10.1016/j.ajpath.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aiello L.P., Pierce E.A., Foley E.D., Takagi H., Chen H., Riddle L., Ferrara N., King G.L., Smith L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ropper A.H., Gorson K.C., Gooch C.L., Weinberg D.H., Pieczek A., Ware J.H., Kershen J., Rogers A., Simovic D., Schratzberger P., et al. Vascular endothelial growth factor gene transfer for diabetic polyneuropathy: A randomized, double-blinded trial. Ann. Neurol. 2009;65:386–393. doi: 10.1002/ana.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kantharidis P., Wang B., Carew R.M., Lan H.Y. Diabetes complications: The microRNA perspective. Diabetes. 2011;60:1832–1837. doi: 10.2337/db11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ristoiu V., Shibasaki K., Uchida K., Zhou Y., Ton B.H., Flonta M.L., Tominaga M. Hypoxia-induced sensitization of transient receptor potential vanilloid 1 involves activation of hypoxia-inducible factor-1 alpha and PKC. Pain. 2011;152:936–945. doi: 10.1016/j.pain.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Feng Y., Chen L., Luo Q., Wu M., Chen Y., Shi X. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Des. Devel. Ther. 2018;12:171–177. doi: 10.2147/DDDT.S157109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taganov K.D., Boldin M.P., Chang K.J., Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou J., Wang P., Lin L., Liu X., Ma F., An H., Wang Z., Cao X. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 52.Liu X.S., Fan B., Szalad A., Jia L., Wang L., Wang X., Pan W., Zhang L., Zhang R., Hu J., et al. MicroRNA-146a mimics reduce the peripheral neuropathy in type 2 diabetic mice. Diabetes. 2017;66:3111–3121. doi: 10.2337/db16-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemarchant S., Pruvost M., Montaner J., Emery E., Vivien D., Kanninen K., Koistinaho J. ADAMTS proteoglycanases in the physiological and pathological central nervous system. J. Neuroinflammation. 2013;10:133. doi: 10.1186/1742-2094-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L., Chopp M., Szalad A., Zhang Y., Wang X., Zhang R.L., Liu X.S., Jia L., Zhang Z.G. The role of miR-146a in dorsal root ganglia neurons of experimental diabetic peripheral neuropathy. Neuroscience. 2014;259:155–163. doi: 10.1016/j.neuroscience.2013.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yousefzadeh N., Alipour M.R., Soufi F.G. Deregulation of NF-small ka, CyrillicB-miR-146a negative feedback loop may be involved in the pathogenesis of diabetic neuropathy. J. Physiol. Biochem. 2015;71:51–58. doi: 10.1007/s13105-014-0378-4. [DOI] [PubMed] [Google Scholar]
- 56.Wu Y., Xu D., Zhu X., Yang G., Ren M. MiR-106a Associated with diabetic peripheral neuropathy through the regulation of 12/15-LOX-mediated oxidative/nitrative stress. Curr. Neurovasc. Res. 2017;14:117–124. doi: 10.2174/1567202614666170404115912. [DOI] [PubMed] [Google Scholar]
- 57.El-Lithy G.M., El-Bakly W.M., Matboli M., Abd-Alkhalek H.A., Masoud S.I., Hamza M. Prophylactic L-arginine and ibuprofen delay the development of tactile allodynia and suppress spinal miR-155 in a rat model of diabetic neuropathy. Transl. Res. 2016;177:85–97. doi: 10.1016/j.trsl.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Liu W., Ao Q., Guo Q., He W., Peng L., Jiang J., Hu X. miR-9 mediates CALHM1-activated ATP-P2X7R signal in painful diabetic neuropathy rats. Mol. Neurobiol. 2017;54:922–929. doi: 10.1007/s12035-016-9700-1. [DOI] [PubMed] [Google Scholar]
- 59.Gong Q., Lu Z., Huang Q., Ruan L., Chen J., Liang Y., Wang H., Yue Y., Feng S. Altered microRNAs expression profiling in mice with diabetic neuropathic pain. Biochem. Biophys. Res. Commun. 2015;456:615–620. doi: 10.1016/j.bbrc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Guo G., Liu Y., Ren S., Kang Y., Duscher D., Machens H.G., Chen Z. Comprehensive analysis of differentially expressed microRNAs and mRNAs in dorsal root ganglia from streptozotocin-induced diabetic rats. PLoS ONE. 2018;13:e0202696. doi: 10.1371/journal.pone.0202696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang D., Yang Q., Wei X., Liu Y., Ma D., Li J., Wan Y., Luo Y. The role of miR-190a-5p contributes to diabetic neuropathic pain via targeting SLC17A6. J. Pain Res. 2017;10:2395–2403. doi: 10.2147/JPR.S133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H.S., Yu G., Wang Z.T., Yi S.P., Su R.B., Gong Z.H. Changes in VGLUT1 and VGLUT2 expression in rat dorsal root ganglia and spinal cord following spared nerve injury. Neurochem. Int. 2016;99:9–15. doi: 10.1016/j.neuint.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 63.Simeoli R., Montague K., Jones H.R., Castaldi L., Chambers D., Kelleher J.H., Vacca V., Pitcher T., Grist J., Al-Ahdal H., et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017;8:1778. doi: 10.1038/s41467-017-01841-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fierabracci A., Del Fattore A., Luciano R., Muraca M., Teti A., Muraca M. Recent advances in mesenchymal stem cell immunomodulation: The role of microvesicles. Cell Transplant. 2015;24:133–149. doi: 10.3727/096368913X675728. [DOI] [PubMed] [Google Scholar]
- 65.Del Fattore A., Luciano R., Pascucci L., Goffredo B.M., Giorda E., Scapaticci M., Fierabracci A., Muraca M. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T Lymphocytes. Cell Transplant. 2015;24:2615–2627. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 66.Jia L., Chopp M., Wang L., Lu X., Szalad A., Zhang Z.G. Exosomes derived from high-glucose-stimulated schwann cells promote development of diabetic peripheral neuropathy. FASEB J. 2018;32:6911–6922. doi: 10.1096/fj.201800597R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanabe K., Kani S., Shimizu T., Bae Y.K., Abe T., Hibi M. Atypical protein kinase C regulates primary dendrite specification of cerebellar purkinje cells by localizing Golgi apparatus. J. Neurosci. 2010;30:16983–16992. doi: 10.1523/JNEUROSCI.3352-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kole A.J., Swahari V., Hammond S.M., Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X., Gong X., Han S., Zhang Y. MiR-29b protects dorsal root ganglia neurons from diabetic rat. Cell Biochem. Biophys. 2014;70:1105–1111. doi: 10.1007/s12013-014-0029-y. [DOI] [PubMed] [Google Scholar]
- 70.Lynch M.P., Stein J.L., Stein G.S., Lian J.B. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: Modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp. Cell Res. 1995;216:35–45. doi: 10.1006/excr.1995.1005. [DOI] [PubMed] [Google Scholar]
- 71.Cheng C., Kobayashi M., Martinez J.A., Ng H., Moser J.J., Wang X., Singh V., Fritzler M.J., Zochodne D.W. Evidence for epigenetic regulation of gene expression and function in chronic experimental diabetic Neuropathy. J. Neuropathol. Exp. Neurol. 2015;74:804–817. doi: 10.1097/NEN.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 72.Ciccacci C., Morganti R., Di Fusco D., D’Amato C., Cacciotti L., Greco C., Rufini S., Novelli G., Sangiuolo F., Marfia G.A., et al. Common polymorphisms in MIR146a, MIR128a and MIR27a genes contribute to neuropathy susceptibility in type 2 diabetes. Acta Diabetol. 2014;51:663–671. doi: 10.1007/s00592-014-0582-2. [DOI] [PubMed] [Google Scholar]
- 73.Ciccacci C., Latini A., Greco C., Politi C., D’Amato C., Lauro D., Novelli G., Borgiani P., Spallone V. Association between a MIR499A polymorphism and diabetic neuropathy in type 2 diabetes. J. Diabetes Complicat. 2018;32:11–17. doi: 10.1016/j.jdiacomp.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 74.Li Y.B., Wu Q., Liu J., Fan Y.Z., Yu K.F., Cai Y. miR-199a-3p is involved in the pathogenesis and progression of diabetic neuropathy through downregulation of SerpinE2. Mol. Med. Rep. 2017;16:2417–2424. doi: 10.3892/mmr.2017.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y.B., Ma W., Xie C., Zhang M., Yin X., Wang F., Xu J., Shi B. Identification of genes and signaling pathways associated with diabetic neuropathy using a weighted correlation network analysis: A consort study. Medicine. 2016;95:e5443. doi: 10.1097/MD.0000000000005443. [DOI] [PMC free article] [PubMed] [Google Scholar]