Abstract
The stingless bee, Melipona fasciculata Smith (Apidae, Meliponini), is a native species from Brazil. Their products have high biotechnological potential, however there are no studies about the biological activities of pollen collected by M. fasciculata. In this context, the present study investigated the chemical composition, anti-oxidant, anti-inflammatory, and analgesic activities of hydroethanolic pollen extracts collected by M. fasciculata in three cities in Maranhão State, Brazil. We verified the antioxidant activity of the extracts and inhibitory activity against the cyclooxygenase enzyme using in vitro assays and in allowed to select the extract with higher efficiency to be used on in vivo assays. In these trials, the selected extract showed high anti-inflammatory activity as well as nociceptive effects at central and peripheral level, suggesting that this extract acts on inhibition of histamine release and decreased synthesis of prostaglandins and the in-silico study suggested that polyphenols and acids fatty acids in the extract may be associated with these activities. The results of the present study report the high biological potential of pollen extract and we conclude that the pollen collected by M. fasciculata can be considered as the object of research for new pharmacological alternatives.
Keywords: pollen, extract, pain, anti-inflammatory, natural products, molecular docking
1. Introduction
Natural products are sources of discovery and development of drugs to treat different diseases for centuries. The search for increasingly powerful and less toxic molecules is constant, where secondary metabolites, especially from medicinal plants, are a promising source for the selection of compounds with pharmacological interest [1,2,3,4]. In this context, natural bee products such as honey, propolis, geopropolis, wax, royal jelly, and pollen have been extensively employed since ancient times due to their wide pharmacological and nutritional activity [5]; thus demonstrating high biological potential which justifies its use for the development of new drugs.
Stingless bee products have long been used in herbal medicine and diet for their positive health consequences. Currently, products (honey, royal jelly, propolis, beeswax or bee pollen) are gaining prominence due to the presence of bioactive compounds that are associated with health-beneficial properties [6,7].
Bee pollen is gaining attention as a functional food for human consumption due to its high content of compounds with health promoting effects such as essential amino acids, enzymes, fatty acids, antioxidants, vitamins, minerals, lipids, carbohydrates, and polyphenols [7,8]. It is used as an alternative and complementary therapy to cure prostatitis, stomach ulcers and infectious diseases. A wide range of therapeutic properties have been suggested, such as antimicrobial, antioxidant, hepatoprotective, chemopreventive and anti-carcinogenic, anti-atherosclerotic, anti-inflammatory, anti-allergic and immunomodulatory [7,9,10,11].
Melipona fasciculata Smith 1858 (Apidae, Meliponini), is a species of stingless bee, traditionally cultivated in the state of Maranhão, Brazil, by small and medium producers primarily for the production and marketing of honey [12]. However, other products of this species, such as pollen can be explored, being an important product with potential for chemical and biological exploitation.
The anti-inflammatory pollen and bee pollen activity has been described both in vitro and in vivo models. Lee et al. [13] investigated the effects of pollen extract on the modulation of pro-inflammatory mediators production in RAW 264.7 macrophages lipopolysaccharide-activated (LPS) and observed inhibition in the production of nitric oxide (NO), tumor necrosis factor-α (TNF- α), IL-1 and IL-6. Studies have shown that pollen’s anti-inflammatory activity is due to the presence of phenolic compounds such as flavonoids and phenolic acids and fatty acids and phytosterols. Kaempferol, which is a flavonoid that inhibits the activity of two enzymes: hyaluronidase, which is an enzyme that catalyzes the depolymerization of hyaluronic acid and elastase, which hydrolyses elastin, strengthens connective tissue and seals blood, resulting in decreased transudates, reactions. inflammatory and edema. It has also been demonstrated that the anti-edematous, anti-inflammatory and analgesic action of quercetin, which inhibits the activity of histidine decarboxylase, reduces the level of histamine in the organism, and may also inhibit the cascade of arachidonic acid metabolism, decreasing the level of pro-inflammatory prostaglandins, having an anti-inflammatory effect due to eliminating local pain and preventing platelet aggregation [14,15].
Our research group previously reported that extracts from M. fasciculata geopropolis have antioxidant activity [16,17], leishmanicidal [18], antimicrobial and immunomodulatory [19] anti-tumor [20] and anthelmintic [21], but there are no records regarding the anti-inflammatory and antinociceptive activity of pollen collected by M. fasciculata. Considering the bioactivity and chemical composition of the pollen of honey bees and stingless bees of the genus Melipona, especially M. fasciculata, we support the hypothesis that pollen collected from M. fasciculata contains bioactive substances that can be used to treat pain and inflammation.
Thus, this study evaluated the anti-inflammatory and antinociceptive activity and the hydroethanolic pollen extract collected by M. fasciculata from different places, identified the compounds present in the material and correlated which compounds are associated with bioactivity through in silico, in vitro and in vivo assays.
2. Results
2.1. Total Phenolic Content, Total Flavonoids Content and Antioxidant Activity
The Total Phenolic Content, Total Flavonoids Content and Antioxidant Activity (DPPH•, FRAP and ABTS•+ methods) are present on Table 1. In this moment, was utilized four samples from Palmeirândia (EHPP1–4), three samples from Viana (EHPV1–3) and one sample from Chapadinha (EHPC) cities of pollen extract collected by M. fasciculata. The TPC ranging to 6.10% to 11.40%. The TFC ranging to 0.30 to 2.09%. The extract that show highest total phenolic content, total flavonoid content and minor DPPH• IC50 is the extract from EHPC, followed by the EHPP1 and EHPP3. Regarding the FRAP and ABTS•+ assays, the extracts with the highest potential were EHPP1, EHPC and EHPP3.
Table 1.
Values of polyphenols and total flavonoids contents, activity antioxidant (DPPH•, FRAP and ABTS•+ methods) from pollen extracts collected by Melipona fasciculata Smith.
| Extrato | CPT (%) a,b | CFT (%) a,c | DPPH• IC50 (μg/mL) | FRAP (mmol Fe2+/g) | ABTS•+ IC50 (μg/mL) |
|---|---|---|---|---|---|
| EHPP1 | 11.06 ± 0.08 | 1.47 ± 0.06 | 205.17 ± 0.08 c | 0.99 ± 0.05 b | 34.30 ± 0.22 b |
| EHPP2 | 8.36 ± 0.82 | 0.94 ± 0.01 | 373.56 ± 1.32 d | 0.83 ± 0.08 b | - |
| EHPP3 | 10.22 ± 0.54 | 1.16 ± 0.03 | 178.91 ± 1.09 e | 0.84 ± 0.09 b | 103.93 ± 0.14 d |
| EHPP4 | 8.87 ± 0.22 | 0.65 ± 0.01 | 269.73 ± 0.05 f | 0.62 ± 0.05 c | - |
| EHPV1 | 6.10 ± 0.31 | 0.40 ± 0.00 | 557.53 ± 0.61 g | 0.15 ± 0.08 e | 235.19 ± 0.19 f |
| EHPV2 | 9.01 ± 1.05 | 0.35 ± 001 | 597.93 ± 0.96 h | 0.34 ± 0.03 d | - |
| EHPV3 | 9.01 ± 0.02 | 0.30 ± 002 | 560.82 ± 0.20 i | 0.30 ± 0.03 d | 202.60 ± 0.15 e |
| EHPC | 11.4 ± 0.31 | 2.09 ± 0.02 | 117 ± 0.03 b | 0.84 ± 0.03 b | 70.77 ± 0.15 c |
| Trolox | - | - | 3.05 ± 0.61 a | 8.74 ± 0.13 a | 3.42 ± 0.41 a |
Values represent the mean of triplicate measurements ± standard deviation. Different letters in the same column indicate a significant difference (Tukey, p < 0.05). EHPP1–4, hydroethanolic pollen extract from M. fasciculata Smith, Palmeirândia-MA; EHPV1-3, hydroethanolic pollen extract from M. fasciculata, Viana-MA; EHPC, hydroethanolic pollen extract from M. fasciculata, Chapadinha-MA; (a) Results expressed as means ± standard deviation of quantitative evaluation tests for total polyphenols and total flavonoids in M. fasciculata pollen extracts (n = 3), (b) expressed as gallic acid equivalent; (c) expressed as quercetin equivalent. DPPH•, 2,2-diphenyl-1-picrylhydrazyl radical; FRAP, ferric reducing antioxidant power; ABTS•+, 2,2′-azinobis-3-ethylbenzotiazoline-6-sulfonic acid. (-) unrealized.
2.2. COX–1 and 2 Inhibition Assay
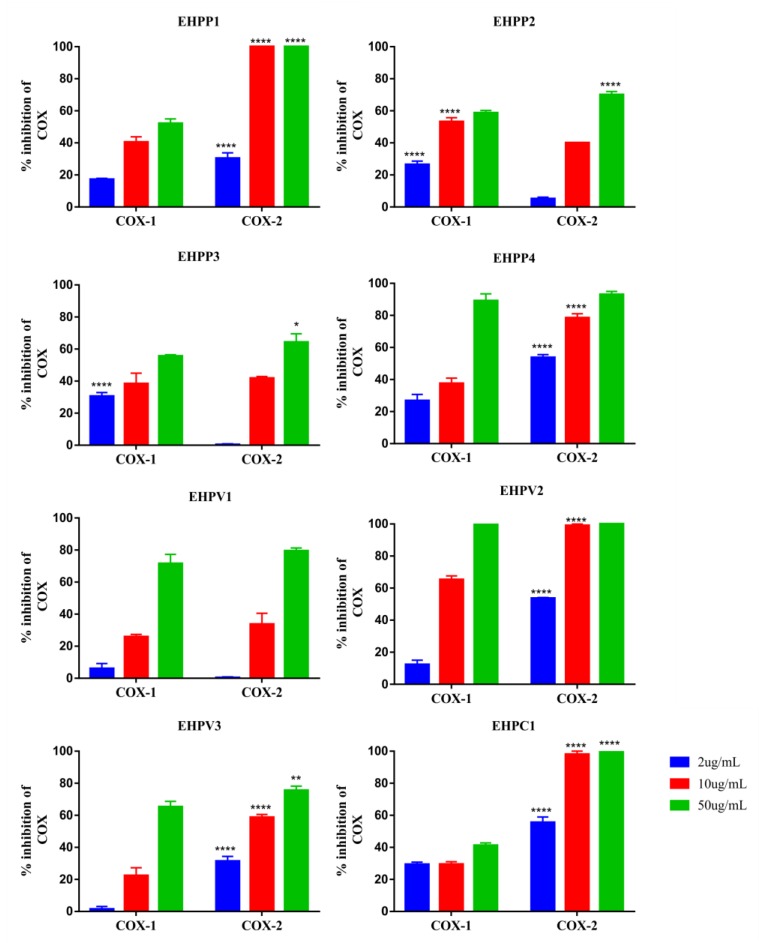
All extracts were evaluated for inhibition potential of COX-1 and 2 enzymes. EHPC was the extract that showed the best activity, inhibiting 100% of COX-2 and only 27% of COX-1 at 10 µg/mL. EHPP1 extract also inhibited 100% of COX-2, but inhibited COX-1 by 38% also at 10 µg/mL, a value about 30% higher than that observed in EHPC. The other extracts tested strongly inhibited both enzymes (EHPV2 and EHPP4) or had a maximum inhibitory activity of less than 75% (EHPP2-3; EHV1-3) at a concentration of 50 µg/ mL (Figure 1).
Figure 1.
Percentage of in vitro inhibition of COX-1 and 2 produced by hydroethanolic pollen extract collected by M. fasciculata was obtained in Chapadinha (EHPC), Palmeirândia (EHPP1-4) and Viana (EHPV1-3) municipalities. Tested at three concentrations: 2 μg/mL, 10 μg/mL and 50 μg/mL. * Represents significant differences, * with p < 0.05; ** with p < 0.005; **** with p < 0.0001 comparing inhibition of cyclooxygenase 1 (COX-1) with 2 (COX-2). (Two-way ANOVA; Sidak).
Due it had the best TPC, TFC, DPPH• IC50, was the second best in FRAP and ABTS•+ results, and had a higher affinity for COX–2 over COX–1, we chose EHPC for the posterior in vivo phase of the study and chemical characterization.
2.3. In Vivo Anti–Inflammatory Activity
2.3.1. Carrageenan–Induced Paw Edema Test
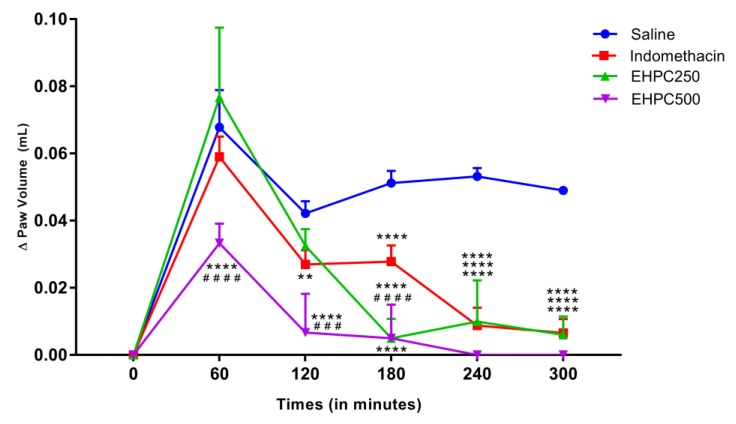
Treatment with EHPC at 500 mg/kg significantly reduced mice paw edema in 52% compared with the saline group from the first hour of test (p < 0.0001). Thereafter, these treatments reduced edema by 89%, 94%, and 100% at 2, 3, and 4 h, respectively (p < 0.0001 at all times) compared to saline. Treatment with the 250 mg/kg dose of EHPC no showed statistical difference in reducing edema within 1 and 2 h, but was able to reduce edema by 94%, 91%, and 100% at 3, 4, and 5 h, respectively, being statistically different from the saline group (p < 0.0001). Treatment with EHPC 250 mg/kg showed a statistically significant reduction in edema reduction 3 h after induction compared with indomethacin (p < 0.0001) and provides statistically similar effects for this drug at 4 and 5 h. The treatment with 500 mg/kg of EHPC, was significant (p < 0.001) better than indomethacin in the first three hours of evaluation and statistically equal in the last two hours (Figure 2).
Figure 2.
Carrageenan–Induced Paw Edema by subplantar administration of 1% carrageenan in orally treated mice with 0.9% NaCl, indomethacin 10 mg/kg, EHPC 250 and 500 mg/kg. ** p < 0.01; **** p < 0.0001 vs. CTRL; ### p < 0.001; #### p < 0.0001 vs. Indomethacin (ANOVA; Tukey).
2.3.2. Dextran–Induced Paw Edema Test
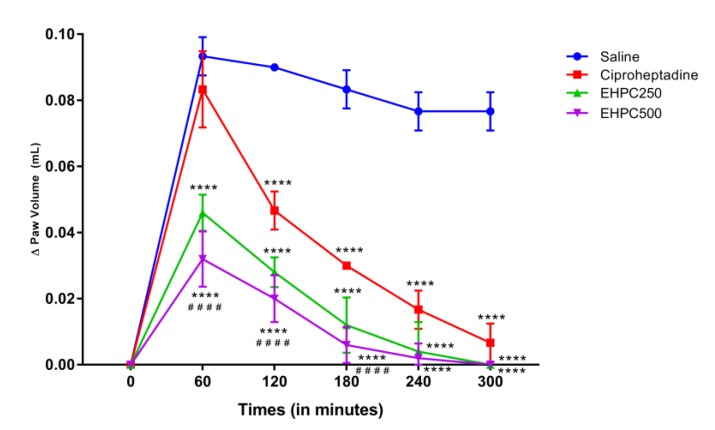
EHPC treatment at both doses was statistically different from the saline group at all-time points, as shown in Figure 3. The reduction in paw edema in the EHPC 250 mg/kg group from 1 to 5 h varied between 52% and 100% compared to the saline group; and in the EHPC 500 mg/kg treated group the reduction in paw edema ranged from 66% to 100% over this same time period compared to the saline group. Animals treated with both doses of EHPC also showed greater efficiency in reducing edema than the group treated with standard drug cyproheptadine. EHPC groups 250 and 500 mg/kg significantly better than the drug on 1 to 3 h after induction (Figure 3). In the first hour of evaluation, the 500 mg/kg EHPC was 84% more efficient in reducing edema than cyproheptadine (Figure 3). Thus, demonstrating that the EHPC interferes more powerfully than the drug, acting mainly in the first hours of induction of the inflammatory process.
Figure 3.
Dextran–Induced Paw Edema induced by subplantar administration of 1% dextran in mice treated orally with 0.9% NaCl, ciproheptadine 10 mg/kg, EHP 250 mg/kg and EHP500 mg/kg. **** p < 0.0001 vs. CTRL; #### p < 0.0001 vs. ciproheptadine (ANOVA; Tukey).
2.4. In Vivo Anti–Nociceptive Activity
2.4.1. Acetic Acid Writhing Test
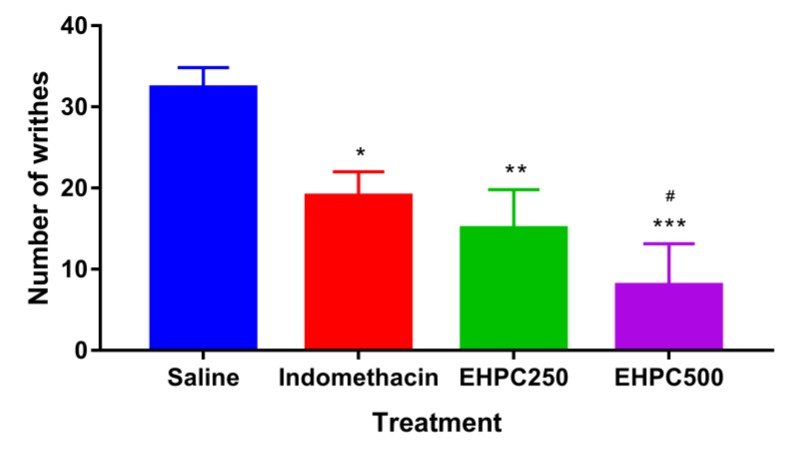
Using orally administered EHPC extract at concentrations of 250 and 500 mg/kg, the number of abdominal contortions produced by intraperitoneal administration of 0.8% acetic acid solution in animals was significantly reduced by 54% (p < 0.01) and 76% (p < 0.001), respectively, compared with saline. There was no statistical difference between the decrease in the number of writhing between the indomethacin and EHPC250 groups, but we found that pollen extract at a concentration of 500 mg/mL was more efficient than indomethacin, causing 58% less abdominal writhing (p < 0.05). The results of writhing test are showed in Figure 4.
Figure 4.
Writhing induced by the intraperitoneal administration of 0.8% acetic acid (10 mL/kg) in oral mice treated with 0.9% NaCl, indomethacin 10 mg/kg, EHPC 250 and 500 mg/kg. * p < 0.05; ** p < 0.01; *** p < 0.001 vs. NaCl; # p < 0.05 vs Indomethacin (ANOVA; Tukey).
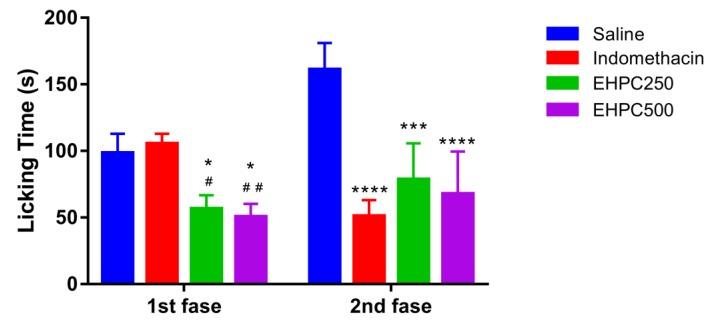
2.4.2. Formalin Test
In the neurogenic phases (0–5 min) formalin-induced pain test, treatments using EHPC orally administered at concentrations of 250 and 500 mg/kg, decreases the time that the animals passed licking/biting the induced paw in 42% and 47%, respectively, generating statistically significant results when compared to the saline group (both p < 0.05). Compared with indomethacin, the extract also showed a significant reduction, where 250 mg/kg reduced 46% more than indomethacin (p < 0.05) and 500 mg/kg reduced 52% more than the standard drug (p < 0.005). In the inflammatory phases (15–30 min), the extract in 250 and 500 mg/kg doses reduces the inflammation levels in 52% (p < 0.01) and 59% (p < 0,0001), respectively, in comparison with saline. Indomethacin reduces the inflammation on 67%, statistically differing from saline. Also, the anti-inflammatory effect of pollen extracts was not statistically significant different from indomethacin (Figure 5).
Figure 5.
Formalin test induced by subplantar administration of 2.5% formalin in mice treated orally with 0.9% NaCl, indomethacin 10 mg/kg, EHPC 250 and 500 mg/kg. * p < 0.05; *** p < 0.01; **** p < 0.001 vs. NaCl; # p < 0.05; ## p < 0.005 vs Indomethacin (ANOVA; Tukey).
2.5. LC-ESI-IT-MS/MS Analysis
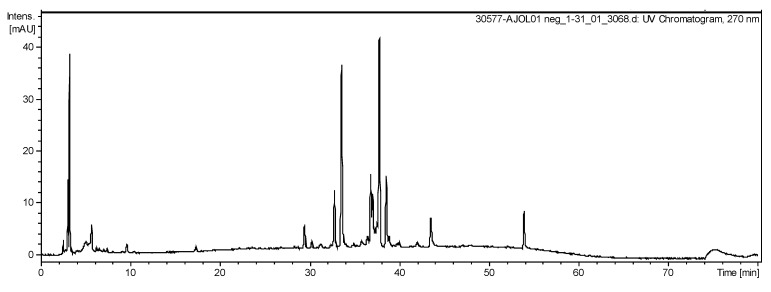
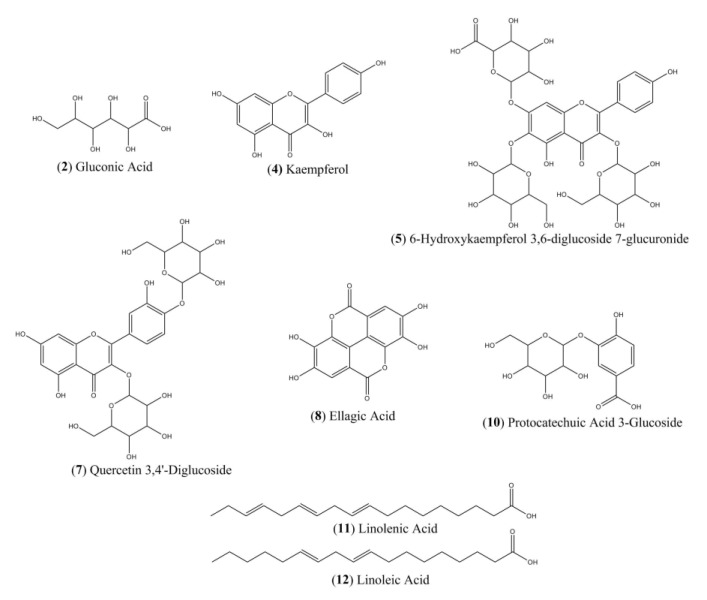
The HPLC chromatogram showed a number of peaks corresponding EHPC to organic acids and phenolic compounds (Figure 6). Table 2 summarizes the molecular weight, molecular ion [M-H]− and major product ions obtained by LC-MS/MS for 10 EHPC fragmentation peaks. The compounds (Figure 7) were identified by comparing their fragmentation profiles with the compounds described in the literature data.
Figure 6.
HPLC fingerprint (270 nm) of the hydroethanolic pollen extract collected by M. fasciculata from Chapadinha—MA.
Table 2.
Identification of compounds by LC-ESI-IT-MS/MS, in negative mode, of the hydroethanolic pollen extract collected by M. fasciculata from Chapadinha - MA.
| Nº | Time Retention (min) | [M-H]− | MSn Ion m/z (−) | Tentative Identification |
|---|---|---|---|---|
| 1 | 2.9 | 539 | 195 | gluconic acid derivate |
| 2 | 3.0 | 195 | 177; 129 | gluconic acid |
| 3 | 29.4 | 571 | 285 | kaempeferol derivative |
| 4 | 29.5 | 285 | 255 | kaempferol |
| 5 | 30,1 | 801 | 539; 285 | 6-hydroxykaempferol 3,6-diglucoside 7-glucuronide |
| 6 | 33.6 | 603 | 301 | ellagic acid dimer |
| 7 | 33.6 | 625 | 301 | quercetin 3,4’-diglucoside |
| 8 | 33.6 | 301 | - | ellagic acid |
| 9 | 36.8 | 1345 | 672; 522; 372 | NI |
| 10 | 36.9 | 315 | 299; 153 | protocatechuic acid 3-glucoside |
| 11 | 39.2 | 277 | 233; 179 | linolenic acid |
| 12 | 42.3 | 279 | 261 | linoleic acid |
NI—not identified.
Figure 7.
Chemical structures of the compounds identified by LC-ESI-IT-MS/MS in the hydroethanolic pollen extract collected by M. fasciculata from Chapadinha—MA.
2.6. Molecular Docking
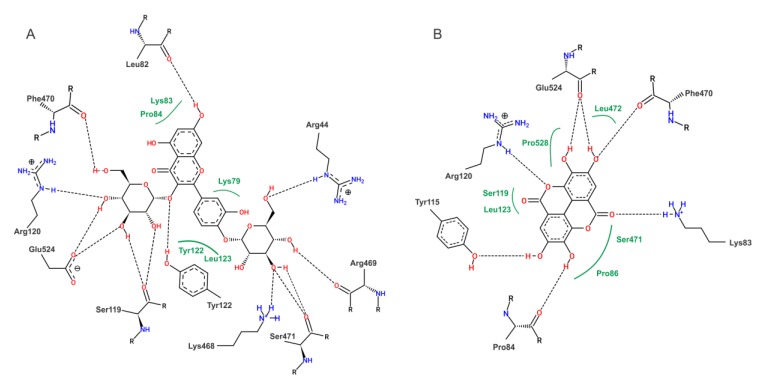
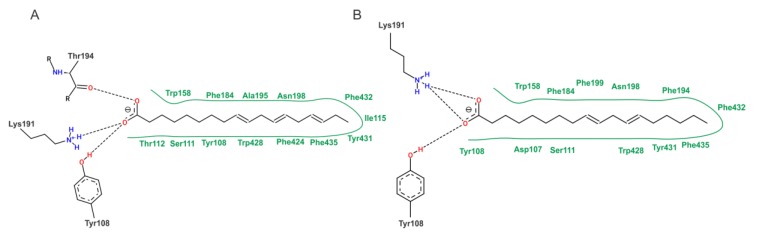
To our molecular docking analysis, we used all compounds identified by HPLC-MS/MS on EHPC hydroethanolic extract. On general, the phenolic compounds showed highest affinity parameters on COX-2. The quercetin 3,4’-diglucoside and ellagic acid were the compounds that showed the best energy affinity parameters with COX-2, with binding free energy values of -8.13 and -7.60 kcal/mol and 1.11 and 2.68 µM inhibition constant, respectively (Table 3). In to the compounds present in the extract, the molecular docking of commercial NSAID Meloxicam was also performed. The results of meloxicam were close to quercetin 3,4’-diglucoside and ellagic acid parameters. The interactions performed by quercetin 3,4’-diglucoside and ellagic acid with the amino acid residues of the active site of COX-2 are shown in Figure 8.
Table 3.
Free-binding energies (ΔGbind in kcal/mol) and inhibition constant (Ki, in µM) obtained by molecular docking of the compounds identified in the EHPC hydroethanolic extract with the COX-2 structure and H1 histamine receptor.
| COX-2 | Histamine H1 Receptor | ||||
|---|---|---|---|---|---|
| Ligand | ΔGbind (kcal/mol) | Ki (μM) | Ligand | ΔGbind (kcal/mol) | Ki (μM) |
| Quercetin 3,4’-diglucoside | −8.13 | 1.11 | Linolenic acid | −9.15 | 0.18 |
| Ellagic acid | −7.60 | 2.68 | Linoleic acid | −8.72 | 0.40 |
| Kaempferol | −7.44 | 3.54 | Kaempferol | −8.32 | 0.80 |
| 6-Hydroxykaempferol 3,6-diglucoside 7-glucuronide | −7.07 | 6.57 | Protocatechuic acid 3-glucoside | −7.49 | 3.25 |
| Protocatechuic acid 3-glucoside | −6.91 | 8.59 | Ellagic acid | −6.51 | 23.74 |
| Linolenic acid | −6.62 | 13.99 | Quercetin 3,4’-diglucoside | −2.31 | 201 |
| Linoleic acid | −5.96 | 42.49 | 6-Hydroxykaempferol 3,6-diglucoside 7-glucuronide | −1.18 | 1370 |
| Gluconic acid | −4.51 | 491.05 | Gluconic acid | 0.77 | 2719 |
| Meloxicam | −8.63 | 0.49 | Doxepin | −10.36 | 0.02 |
Figure 8.
2D representation of the interactions of COX-2 residues with quercetin 3, 4’-diglucoside (A) and ellagic acid (B). Dashed lines—represent hydrogen bonds; green lines—van der Waals interactions.
Regarding the type H1 histamine receptor, the compounds that presented the best electronic affinity parameters were linolenic and linoleic acids, being binding free energy values were −9.15 and −8.72 kcal/mol, respectively (Table 3). In addition, we perform the redocking of the Doxepin, ligand found in the original structure of H1 histamine receptor, using the same conditions as the other ligands to validate our protocol. The value of the free binding energy was −10.06 kcal/mol and the root mean square deviation (RMSD) between the predicted docking conformation and the observed X-ray crystal structure was 0.21 Å. Values below 2 Å indicate that the docking protocol is valid. The interactions performed by linolenic and linoleic acids with the amino acid residues of the active site of H1 histamine receptor are shown in Figure 9.
Figure 9.
2D representation of the interactions of H1 histamine receptor residues with linolenic (A) and linoleic acids (B). Dashed line—represent hydrogen bonds; green lines—van der Waals interactions.
3. Discussion
Besides all extracts evaluated, the EHPC was the extract that show most efficient antioxidant activity. Our DPPH• IC50 is lower compared to Apis mellifera bee pollen extract that show DPPH• IC50 ranging 810 to 4690 µg/mL [22]. Also better to the DPPH• IC50 found using the bee pollen extract from Portugal [23] and the bee pollen extract from Turkey [24]. However, samples of the M. fasciculata geopropolis extract showed much better DPPH• IC50 than those found in the EHPC [16,17]. The presence of higher levels of phenolic and flavonoid compounds found in geopropolis may justify this difference. Our FRAP and ABTS•+ results of EHPC is according with previous studies using pollen extract [25,26]. Extracts with antioxidant properties have metabolites that have free radical scavenging properties and inhibit enzymes xanthine oxidase and lipoxygenase [27]. The interaction of these natural antioxidants with reactive oxygen species involved in the evolution of the inflammatory process has therefore encouraged several studies on the effects of these antioxidants on the formation of pro-inflammatory eicosanoids derived from COX arachidonic acid metabolism. Thus, according to our results, the EHPC has encouraging antioxidant activity, evidenced in the results of DPPH• radical sequestration and iron reducing potential (FRAP) and ABTS•+ results
COX is a key enzyme for inflammatory mediators’ biosynthesis, as it acts in the metabolization of arachidonic acid, leading to the production of prostaglandins, prostacyclin’s and thromboxane’s. In addition to pathological processes such as inflammation, various physiological processes are also involved. COX isoforms are classified as COX-1—with constitutive role, inducible COX-2 - linked to inflammatory processes, in addition to COX-3, which is a variant of COX-1 [28,29,30]. In the present study, all pollen extracts collected by Melipona fasciculata, when evaluated on in vitro inhibition assay, were capable of producing an inhibitory effect on COX-1 and COX-2, but the best results were expressed by the EHPC, which inhibited 100% to COX-2, and only 27% to COX-1 at a concentration of 10 µg/mL, thus being the extract with the highest affinity and most effective in inhibiting COX-2. Similar results were also reported in a study with Cistus spp. ethanolic bee pollen extract, which was also more selective for COX-2, however doses of 10 µg/mL reduced by 50% [31], and in our study, equivalent doses of EHPC reduced 100%, showing that our pollen extract was more efficient. These data indicate that the anti-inflammatory mechanism of action of the EHPC involves COX-2 inhibitory activity and consequent reduction of prostaglandin synthesis. Studies by Lee [32] also show that the anti-inflammatory effect of bee pollen is due to COX-2 inhibition through gene suppression.
Our investigations of the anti-inflammatory effect of EHPC showed that the 500 mg/kg dose significantly reduced carrageenan-induced paw edema from 1 h to 5 h, with reductions ranging from 52% to 100% of edema. The dose of 250 mg/kg also reduced the edema, however only differed statistically from the saline group from 3h, and by 5h had reached 100% reduction. These results prove the anti-inflammatory action of EHPC, since the carrageenan-induced paw edema model induces a powerful two-stage inflammation, the first occurs within 1h of carrageenan administration and cytoplasmic enzymes, histamine, are released and serotonin from mast cells. In the second phase (1–6 h) there is an increase in prostaglandin production by COX-2 activation and NO release and continuity between phases is provided by kinins [33,34]. The results of the present study also allow us to state that the EHPC interferes with both phases of inflammation. As is also the case with the bee pollen ethanolic extract from Cistus spp. (100 and 300 mg/kg) which significantly reduced carrageenan-induced edema in 1, 3, 4 and 5 h after induction [31]. Based in our results, a possible hypothesis from extract activity is that the EHPC that may act also as H1 histamine receptor antagonist and act on the COX-2 inhibition, thus impossibility the progression of the inflammatory process.
The anti-inflammatory effect of EHPC was also demonstrated in the dextran-induced paw edema model, in which the 250 and 500 mg/kg dose of EHPC reduced the edema from 1 to 5 h after evaluation. This model is characterized by increased vascular permeability due to mast cell degranulation with histamine and serotonin release [35]. That way, a possible explanation for the anti-edematogenic effect of EHPC could probably be due to a blocking effect on the synthesis, release or activation of vasoactive amines (histamine and 5-HT), causing possible vasoconstrictor activity. As proposed by Vasconcelos [36] by evaluating the effect of Erythrina velutina hydroethanolic extracts, which was also able to significantly inhibit dextran-induced paw edema at all times, however reaching a maximum of 51.3% inhibition of 400 mg/kg, while our extract (EHPC) has reduced by up to 100% at a dose of 250 mg/kg.
In this study, the antinociceptive activity of the EHPC was evaluated using the acetic acid-induced writhing test and the formalin test in mice. With the test of abdominal writhing induced by acetic acid, it was found that the extract was able to reduce the number of writhes by up to 76% at the dose of 500 mg/kg, being more effective than indomethacin. Acetic acid-induced abdominal writhing testing is a sensitive method for assessing peripheral action analgesics. Acetic acid induces the analgesic effect indirectly via endogenous mediators present in inflammatory processes such as bradykinin, serotonin, histamine, substance P and prostaglandins, which stimulate peripheral nociceptive neurons that respond to anti-inflammatory drugs [37,38]. Our hypothesis in the present study is that the antinociceptive action of the extract occurs through the peripheral reduction in prostaglandin synthesis, caused by the inhibition of cyclooxygenases as suggested by Abdulmalik et al. [39] when verifying the antinociceptive effect with the same pain model in the Ficus iteophylla leaves extract.
Studies conducted with propolis ethanolic extract also reported similar antinociceptive results, with a reduction in the number of writhing at doses of 100, 200 and 400 mg/kg by 21.73%, 38.96% and 40.68%, respectively [40]. However, EHPC results observed in the present study were considerably higher.
The present study also indicated that the hydroethanolic pollen extract collected by M. fasciculata has analgesic properties not only on the peripheral but also on the central nervous system. Since in the formalin test the EHPC 250 and 500 mg/kg decreases the time animals spent licking/biting the induced paw by 42% and 47%, respectively, in the first phase, called the neurogenic phase, or early phase goes from zero to five minutes, which is the result of direct nociceptor stimulation and reflects central pain [41]. In the second phase, EHPC (250 and 500 mg/kg) also reduced inflammation levels by 52% and 59% compared with saline. This phase, which lasts from 15 to 30 min is called the inflammatory phase, and is characterized by local inflammation with release of inflammatory mediators and hyperalgesia [42].
Thus, the action of the extract in the first phase, reducing paw licking time is typical of an action demonstrated by centrally acting drugs (opioids) such as morphine, which also produces effects in the second phase [43]. However, the effect of the extract in the second phase may reflect not only the central action of the extract, but also a peripheral action, by inhibiting biosynthesis of mediators responsible for inflammation, such as inhibition of cyclooxygenase and consequently prostaglandins, such as It was also suggested by Moniruzzaman et al. [43] who reported that Adenanthera pavonin ethanolic leaf extract inhibited nociceptive responses in both phases of the formalin test. Choi [44] also found that the Pinus spp., pollen ethanolic extract (100 and 200 mg/kg, orraly) produced a significant inhibition of both phases of the formalin pain test in mice, and the author suggest that the different polyphenols found in pine pollen could be responsible for antinociceptive and anti-inflammatory activity’s.
In the chemical analysis, we found phenolic, flavonoid, and fatty acids in the EHPC. Phenolic and flavonoid compounds are commonly reported with various biological activities, among which we highlight anti-inflammatory activity, which is usually associated with the ability of these compounds to modulate cellular components that participate in the mechanism of inflammation, such as pro-cytokines. such as TNF-α and IL-1, and inhibition of the activity of enzymes involved in the arachidonic acid pathway such as cyclooxygenase and lipoxygenase [45,46].
According our results from anti-inflammatory and antinociceptive activities from EHPC that suggests the H1 histamine receptor and COX-2 were a possible pathway of extract action, we also performed the molecular docking of the compounds identified in the EHPC against these targets.
Our results suggest that the fatty acids linolenic and linoleic acid are the compounds that have the best interaction parameters with the histamine H1 receptor. Linolenic acid was able to inhibit histamine release in RBL-2H3 cells [47]. Both fatty acids were also effective in preventing histamine release by rat peritoneal mast cells [48]. Also reported that the linoleic acid did not have an effect on lipid peroxidation and no show radical scavenging activity, indicating that this molecule no have antioxidant activity [49]. Regarding to COX-2, the quercetin 3,4’-diglucoside and ellagic acid was the compounds that have the best interaction parameters. Few papers report the biological activity from quercetin 3,4’-diglucoside, but this compound was shows potential antioxidant activity (including DPPH• and FRAP) [50,51] anti-urolithics, anti-urease pathogenesis, and anti-gout activity [51] anti-platelet aggregation [50], was inhibitor of α-amylase, α-glucosidase, acethyl and butyrylcholinesterase, anti-diabetic and could inhibit the proliferation of cancer cell lines [52]. The ellagic acid were described with a cornucopia of activities like antioxidant, anti-hepatotoxic, anti-steatosic, anti-cholestatic, anti-fibrogenic, anti-hepatocarcinogenic and antiviral properties [53]. The anti-inflammatory activity from ellagic acid were also reported, and the commonly attributed to reduction of NO, IL-1β, TNF-α, COX-2 and NF-κB [54]. These findings reinforce our hypothesis, built on the data from the present study, that these molecules have the potential to be the target of research for new drugs with anti-inflammatory and antinoniceptive activity.
4. Materials and Methods
4.1. Obtaining Pollen and Preparing Extracts
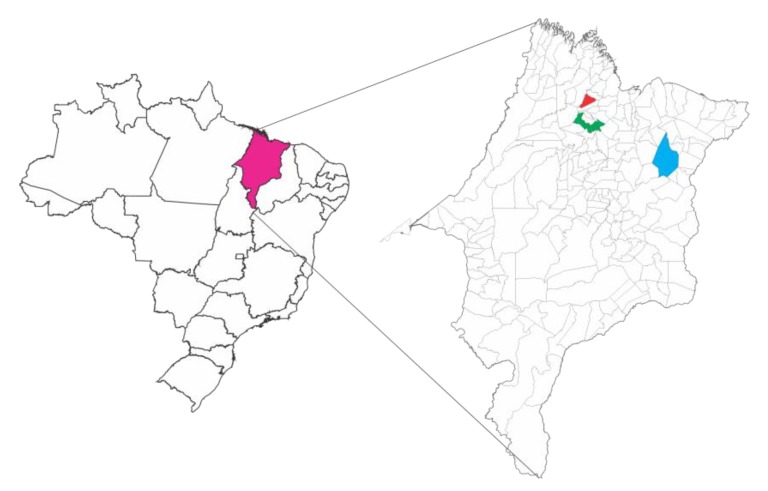
The samples of pollen collected by stinglees bee M. fasciculata was obtained in the municipality of Chapadinha (Cerrado, Brazilian savannah, Brazil), Palmeirândia and Viana (Baixada Maranhense, flooded fields area, Brazil) (Figure 10) in the state of Maranhão, Brazil, being taken directly from the beehives in the stingless beehives of these cities. After collection, pollen samples were identified, placed in a sterile container and kept refrigerated at 4 °C until use. The present research is registered on National System of Genetic Heritage Management and Associated Traditional Knowledge (SISGEN) under code AD841D2.
Figure 10.
Localization of Maranhão State (in magenta) and the cities of Chapadinha (in blue), Palmeirândia (on red) and Viana (on green).
The pollen samples were individually extracted by maceration with 70% ethanol/water (70:30, v/v) with a solid to solvent ratio of 1 to 5 (m/v) for 72 h, with solvent renewal every 24 h. The resulting product from the three extractions was combined, filtered and concentrated in a rotary evaporator under vacuum at 40 °C, thus obtaining the pollen hydroethanolic extracts, which were coded for EHPC (Chapadinha sample), EHPP 1 to 4 (Palmeirândia samples) and EHPV 1 to 3 (Viana samples) and kept refrigerated until their use.
4.2. Determination of Total Phenolic Content (TPC) in Hydroethanolic Pollen Extracts
The total polyphenol contents in the extracts were determined using Folin–Ciocalteau reagent and 20% sodium carbonate (NaCO3). The reaction mixture was kept in the dark for 2h at room temperature and absorbance was measured at 760 nm using Lambda 35 UV–Vis spectrophotometer (Perkin Elmer, Inc., Waltham, MA, USA). The TPC was calculated from a gallic acid calibration curve (2,5–40.0 μg/mL) and expressed as gallic acid equivalent (%). Analyses were performed in triplicate and the mean value was calculated for each sample [16].
4.3. Determinations of Total Flavonoid Concentration (TFC) in Hydroethanolic Pollen Extracts
For total flavonoid concentration we used photocolorimetric method with 5% methanolic aluminum chloride solution (AlCl3). The mixture was kept in the dark for 30 min at room temperature and absorbance was measured at 425 nm in UV–Vis Lambda 35 spectrophotometer (Perkin Elmer, Inc., Waltham, MA, USA). The concentration was calculated from the calibration curve constructed with standard quercetin solution (Merck, Darmstadt, Germany) (1–30.00 μg/mL) and expressed as quercetin equivalent (%). The analyses were performed in triplicate [55].
4.4. Determination of Antioxidant Activity
4.4.1. DPPH• Radical Scavenging Activity
The antioxidant activity of hydroethanolic pollen extracts was evaluated by using the DPPH• free radical scavenging assay as described by Brand–Willians et al., [56] with modifications from Dutra et al. [16]. The samples pollen extracts were diluted on methanol at different concentrations (30–480 µg/mL) and added to a methanol solution of DPPH• (40.0 μg/mL). After 30 min of reaction at room temperature in the dark, the absorbance of each solution was read at 517 nm in a Lambda 35 UV−Vis spectrophotometer (Perkin–Elmer, Inc., USA). Methanol was used as the control, and DPPH• solution was used as the blank. The percent inhibition was calculated using the formula
DPPH• scavenging activity (%)
| 100 − [(Asample − Ablank) × 100/Acontrol] | (1) |
where Asample = absorbance of the sample after 30 min of reaction, Ablank = absorbance of the blank, and Acontrol = absorbance of the control.
The percentage of scavenging activity was plotted against the sample concentration to obtain the IC50, defined as the concentration of sample necessary to cause 50% inhibition. All experiments were done in triplicate.
4.4.2. Ferric Reducing Antioxidant Power Assay (FRAP)
This protocol was used to determine the antioxidant activity based on iron reduction using the FRAP assay. FRAP measures the ferric–reducing ability of a sample in acid medium (pH 3.6), forming an intense blue color as the ferric tripyridyltriazine (Fe3+−TPTZ) complex is reduced to the ferrous (Fe2+) form. The test was performed according Benzie et al. [57] with modifications from Dutra et al. [16]. The samples of pollen extracts were diluted on methanol at different concentrations (12,5–200 μg/mL). The absorbance of the reaction mixture was read at 593 nm in a Lambda 35 UV−vis spectrophotometer (Perkin–Elmer, Inc., USA) using FRAP solution as a blank. The results were expressed as millimoles of Fe2+ per gram of sample. All experiments were done in triplicate.
4.4.3. ABTS•+ Assay
The ABTS solution was prepared in water and potassium persulfate and kept in the dark room for 16 h before testing for the complete oxidation of ABTS•+ and the generation of the highly stable chromophore cation radical 2,2′–azino–bis(3 ethylbenzothiazoline–6–sulfonic acid) (ABTS•+) [58] with [59] modifications. The ABTS•+ solution was diluted with 70% ethanol/water (70:30, v/v) until the absorbance at 734 nm reached 0.7 ± 0.02. Readings were performed by reacting 1000–20 μg/mL of pollen extracts with the ABTS•+ solution. All studies were performed at least in triplicate monitoring the decrease in absorbance for 6 min; results reported corresponded to the % of remaining chromophores compared to conditions in the absence of antioxidants. The IC50 values were determined to each sample, using the formula:
| Scavenging ability (%) = (1 − Asample/Ablank) × 100 | (2) |
4.5. COX Inhibition Assay
The assay was performed according to the manufacturer’s recommendations (COX Colorimetric Inhibitor Screening—Cayman Chemical®, Ann Arbor, Michigan, USA). 96-well microplates were initially identified, and inhibition tests were performed in triplicate for each concentration tested (2, 10 and 50 µg/mL) of pollen extract and for the reaction controls: BW—control without enzyme; A—with enzyme only, without inhibitor. The 3 “BW” wells received 160 µL Tris–HCl buffer, 10 µL HEME and 10 µL solvent–70% ethanol/water (70:30, v/v), used to dilute samples. The wells “A1–A3” received 150 µL Tris–HCl buffer, 10 µL HEME, 10 µL enzyme (COX–1 or COX–2) and 10 µL solvent 70% ethanol/water (70:30, v/v). Wells with pollen extract received 150 µL of Tris–HCl buffer, 10 µL of HEME, 10 µL of enzyme (COX–1 or COX–2) and 10 µL of solvent–diluted 70% ethanol/water (70:30, v/v) pollen extract at concentrations 2, 10 and 50 µg/mL. After mixing all of these reagents in each well, the plate was gently shaken for a few seconds, followed by a 5 min incubation period. Subsequently, 20 µL of the colorimetric substrate solution was added to each well of the plate and then 20 µL of arachidonic acid, substrate of the COX–catalyzed enzyme reaction, was added to each well. The plates were shaken again and incubated at 25 °C for a further two minutes and then read at 590 nm.
4.6. Animals
The present study used 80 adult male Mus musculus mice, Swiss strain, with weights ranging from 25 to 35 g, which were procured from the Central Vivarium (Biotério Central) of Federal University of Maranhão (UFMA), São Luis, Brazil. Animals were placed in polyethylene boxes (n = 5 per box) and provided with free access to food and water in an environment with controlled temperature and 12/12 h light/dark cycle at 22 °C. This study was carried out in accordance with the recommendations of IASP Guidelines for the Use of Animals in Research and according with National Council for Animal Experimentation Control–CONCEA. The experimental protocols were approved in 16 January 2017 by the UFMA Ethics in Animal Use Committee (CEUA), ruling no. 64/2016, protocol no. 23115.016655/2016–83.
4.7. Anti–Inflammatory Activity
4.7.1. Carrageenan–Induced Paw Edema Test
Mice were randomized to groups (n = 5) treated oral with vehicle (saline) (10 mL/kg), pollen extract (250 and 500 mg/kg) or indomethacin (10 mg/kg). After 60 min of treatment, paw edema was induced by subplantar administration of 50 µL of 1% carrageenan into the right paw of the animal. The paw volume of the animal was measured by the digital plethysmometer (Ugo Basile Model, Verese, Italy) during 0, 1, 2, 3, 4 and 5 h after induction [60,61]. The edema value was also obtained by the difference between the right paw volume in the respective hour comparing with the basal volume, being expressed as the variation of the paw volume (mL) over time.
4.7.2. Dextran–Induced Paw Edema Test
The test was used to evaluate pharmacological activity from subplantar administration of 1% dextran. Mice were randomized to groups (n = 5) orally treated with vehicle (saline) (10 mL/kg), pollen extract (250 and 500 mg/kg) or Ciproheptadine 10 mg/kg. After 60 min of treatment, paw edema was induced by subplantar administration of 50 µL of 1% dextran into the right paw of the animal. The paw volume of the animal was measured by the digital plethysmometer (Ugo Basile Model, Verese, Italy) during 0, 1, 2, 3, 4 and 5 h after induction [35]. The edema value was also obtained by the difference between the right paw volume in the respective hour comparing with the basal volume, being expressed as the variation of the paw volume (mL) over time.
4.8. Anti–Nociceptive Activity
4.8.1. Acetic Acid Writhing Test
The mice (n = 5) were treated orally with pollen extract (250 and 500 mg/kg), indomethacin (10 mg/kg) or vehicle (saline) (10 mL/kg) one hour before intraperitoneal (ip) administration of the acetic acid solution at 0.8% (10 mL/kg). The number of writhes was counted for each animal over a period of 20 min after administration of the acetic acid solution. Results were expressed as the average of the cumulative number of writhes [61,62].
4.8.2. Formalin Test
The mice (n = 5) were treated orally with pollen extract (250 and 500 mg/kg), indomethacin (10 mg/kg) or vehicle (saline) (10 mL/kg) one hour before the subplantar injection of 20 μL of 2.5% formalin in the right paw. The nociceptive response, characterized by paw licking or biting, was observed during the first 5 min to assess neurogenic mechanisms and then from minutes 15 to 30 to assess inflammatory mechanisms [61,63].
4.9. HPLC– ESI–MS/MS Analysis
The pollen extract was analyzed by HPLC (LC–20AD Shimadzu, Kyoto, JP) and a Phenomenex Luna C–18 (250 × 4.6 mm-5 µm) column at 25 °C was used. The mobile phases consisted of ultrapure water containing 0.1% formic acid (A) and methanol (B). The following linear gradient was applied: 0 min, 5% B; 1−60 min, 5−100% B; 60−70 min, 100% B at flow of 1 mL/min. The LC was coupled to a mass spectrometer (Amazon Speed ETD, Bruker, Massachusetts, USA) equipped with electrospray ionization (ESI) and an ion–trap (IT) type analyzer in negative mode, under the following conditions: 4.5 kV capillary voltage, capillary temperature 325 °C, entrainment gas (N2) flow 12 L/min, nitrogen nebulizer pressure at 27 psi. The acquisition range was m/z 100–1000, with two or more events.
4.10. Computational Study
4.10.1. Predictive Models and Theoretical Calculations
The compounds identified in the pollen extract from Chapadinha had their geometric, electronic and vibrational properties optimized using the Gaussian program 09 [64]. The GaussView 5.0.8 [65] was used to obtain 3D structural models. Geometric optimization calculations were performed according to the Functional Density Theory (DFT) method, combining the functional hybrid B3LYP and the set of bases 6–31 ++ G (d, p).
4.10.2. Molecular Docking
All docking procedures utilized Autodock 4.2 package [66,67]. The structure of cyclooxygenase 2 (COX-2) (PDB ID 1DDX), H1-type histamine receptor (PDB ID 3RZE) and ligands were prepared for docking simulations with AutoDock Tools, version 1.5.6 [68]. Macromolecules had too many ligands and artifacts removed from their original files preserving only macromolecules. Docking methodology described in literature were used [69] with modifications [61,70]. Gasteiger partial charges were calculated after addition of all hydrogens. Non–polar hydrogens from COX–2, H1 histamine receptor and pollen extract compounds were subsequently merged. The dimensions of the cubic box in the X–, Y– and Z–axes were 70 Å × 70 Å × 700 Å, respectively, with a spacing of 0.375 Å between grid points. The grid box was centered on the oxygen atom of Arg120 residue from COX-2 structure and in the oxygen atom of Trp428 residue H1 receptor structure and Lamarckian genetic algorithm (LGA) was chosen to search for the best conformations, with 100 runs for each compound. Initial coordinates of COX–2 or H1 receptor and pollen extracts secondary metabolites interactions were chosen using the criterion of lowest docking conformation of cluster with lowest energy combined with visual inspection.
4.11. Statistical Analysis
Statistical analyzes between experimental groups were performed by analysis of variance (ANOVA) followed by Tukey test. The results that presented probability of occurrence of null hypothesis lower than 5% (p < 0.05) were considered statistically significant. Statistical analysis was performed using Graphpad Prima® 7 software.
5. Conclusions
The hydroethanolic pollen extract collected by M. fasciculata shows great content of polyphenols, flavonoids, and antioxidant activity. The selected extract, also shows high inhibitory activity against COX-2, being this isoform inhibited 68% more that COX-1. The selected extract as well shows highly anti-inflammatory and antinociceptive activity. The in-silico results, in concordance to in vivo results, suggests that this activity can be due to action of the extracts compounds on histamine release inhibition and prostaglandins synthesis inhibition. Thus, we demonstrated for the first time these activities from the pollen collected by M. fasciculata with results higher compared to products to others bees, including Apis mellifera, and this evidence the potential use of stingless bee products in the development for new therapeutic agents.
Acknowledgments
We would like thanks to the National Center of High Performance Processing (CENAPAD-UFC) of the Federal University of Ceará for the availability of the computational resources used in the in silico tests, also thanks’ to Fundação de Amparo a Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA) for financial support and scholarship CCV and thanks to José Roberto Brito de Freitas for providing the Chapadinha pollen samples and to Vinicyus Teles Chagas and Antonio Marcus de Andrade Paes for support in ABTS analysis.
Abbreviations
| LPS | lipopolysaccharide |
| NO | Nitric oxide |
| TNF-α | tumor necrosis fator α |
| IL | Interleukin |
| DPPH• | 2,2-difenil-1-picrilhidrazil |
| FRAP | Ferric reducing antioxidant power |
| ABTS | 2,2′-azinobis-3-ethylbenzotiazoline-6-sulfonic acid |
| EHPP | Hydroethanolic pollen extract from Palmeirândia–MA, Brazil |
| EHPV | Hydroethanolic pollen extract from Viana–MA, Brazil |
| EHPC | Hydroethanolic pollen extract from Chapadinha–MA, Brazil |
| IC50 | Concentration of sample necessary to cause 50% inhibition |
| COX | Cyclooxygenase |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Concentration |
| NI | Not identified |
| HPLC | High performance liquid chromatography |
| LC-ESI-IT-MS/MS | liquid chromatography coupled to mass spectrometer with electrospray ionization and ion trap analyzer |
| RMSD | root mean square deviation |
| LGA | Lamarckian genetic algorithm |
| DFT | Density Functional Theory |
| UFMA | Federal University of Maranhão |
| CONCEA | National Council for Animal Experimentation Control |
| CEUA | Ethics in Animal Use Committee |
| AlCl3 | Aluminum chloride |
| NaCO3 | Sodium carbonate |
| 5-HT | 5-hydroxytryptamine |
Author Contributions
Conceptualization, A.J.O.L., M.d.S.d.S.C. and M.N.d.S.R.; methodology, A.J.O.L., C.C.V., F.A.N.P., R.H.M.S, P.F.d.S.Q., C.V.F., C.Q.d.R. and M.d.S.d.S.C.; formal analysis, A.J.O.L., C.C.V., C.Q.d.R., J.B.S.G., M.d.S.d.S.C. and M.N.d.S.R.; investigation, A.J.O.L., C.C.V., F.A.N.P., R.H.M.S., P.F.d.S.Q., C.V.F., C.Q.d.R. and M.d.S.d.S.C. writing—original draft preparation, A.J.O.L., C.C.V., writing—review and editing, A.J.O.L., C.C.V., M.d.S.d.S.C., C.Q.d.R., R.M.R., S.T.d.J.R.M.L. and M.N.d.S.R. supervision, M.d.S.d.S.C., and M.N.S.R., project administration, A.J.O.L. and M.d.S.d.S.C., funding acquisition, M.d.S.d.S.C. and M.N.d.S.R.
Funding
This research was funded by Fundação de Amparo a Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA), grant number CADPROD-5197/17.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Kibble M., Saarinen N., Tang J., Wennerberg K., Makela S., Aittokallio T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 2015;32:1249–1266. doi: 10.1039/C5NP00005J. [DOI] [PubMed] [Google Scholar]
- 2.Mastinu A., Bonini S.A., Rungratanawanich W., Aria F., Marziano M., Maccarinelli G., Abate G., Premoli M., Memo M., Uberti D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients. 2019;11:728. doi: 10.3390/nu11040728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonini S.A., Premoli M., Tambaro S., Kumar A., Maccarinelli G., Memo M., Mastinu A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018;227:300–315. doi: 10.1016/j.jep.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A., Premoli M., Aria F., Bonini S.A., Maccarinelli G., Gianoncelli A., Memo M., Mastinu A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta. 2019;249:1681–1694. doi: 10.1007/s00425-019-03138-x. [DOI] [PubMed] [Google Scholar]
- 5.Rao P.V., Krishnan K.T., Salleh N., Gan S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016;26:657–664. doi: 10.1016/j.bjp.2016.01.012. [DOI] [Google Scholar]
- 6.Gabriele M., Parri E., Felicioli A., Sagona S., Pozzo L., Biondi C., Domenici V., Pucci L. Phytochemical composition and antioxidant activity of Tuscan bee pollen of different botanic origins. Ital. J. Food Sci. 2015;27:248–259. [Google Scholar]
- 7.Komosinska-Vassev K., Olczyk P., Kazmierczak J., Mencner L., Olczyk K. Bee pollen: Chemical composition and therapeutic application. Evid. Based Complement. Altern. Med. 2015;2015:297425. doi: 10.1155/2015/297425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang K., Wu D., Ye X., Liu D., Chen J., Sun P. Characterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 2013;61:708–718. doi: 10.1021/jf304056b. [DOI] [PubMed] [Google Scholar]
- 9.Fatrcova-Sramkova K., Nozkova J., Kacaniova M., Mariassyova M., Rovna K., Stricik M. Antioxidant and antimicrobial properties of monofloral bee pollen. J. Environ. Sci. Health Part B. 2013;48:133–138. doi: 10.1080/03601234.2013.727664. [DOI] [PubMed] [Google Scholar]
- 10.Denisow B., Denisow-Pietrzyk M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016;96:4303–4309. doi: 10.1002/jsfa.7729. [DOI] [PubMed] [Google Scholar]
- 11.Sattler J.A.G., de Melo I.L.P., Granato D., Araújo E., da Silva de Freitas A., Barth O.M., Sattler A., de Almeida-Muradian L.B. Impact of origin on bioactive compounds and nutritional composition of bee pollen from southern Brazil: A screening study. Food Res. Int. 2015;77:82–91. doi: 10.1016/j.foodres.2015.09.013. [DOI] [Google Scholar]
- 12.Villas-Bôas J. Manual Tecnológico de Aproveitamento Integral dos Produtos das Nativas Sem Ferrão. 2nd ed. Instituto Sociedade, População e Natureza (ISPN); Brasília, Brazil: 2018. [Google Scholar]
- 13.Lee K.-H., Kim A.-J., Choi E.-M. Antioxidant and antiinflammatory activity of pine pollen extract in vitro. Phytother. Res. 2009;23:41–48. doi: 10.1002/ptr.2525. [DOI] [PubMed] [Google Scholar]
- 14.Kanashiro A., Souza J.G., Kabeya L.M., Azzolini A.E.C.S., Lucisano-Valim Y.M. Elastase release by stimulated neutrophils inhibited by flavonoids: Importance of the catechol group. Z. Naturforsch. C. 2007;62:357–361. doi: 10.1515/znc-2007-5-607. [DOI] [PubMed] [Google Scholar]
- 15.Sahin H., Aliyazicioglu R., Yildiz O., Kolayli S., Innocenti A., Supuran C.T. Honey, pollen, and propolis extracts show potent inhibitory activity against the zinc metalloenzyme carbonic anhydrase. J. Enzyme Inhib. Med. Chem. 2011;26:440–444. doi: 10.3109/14756366.2010.503610. [DOI] [PubMed] [Google Scholar]
- 16.Dutra R.P., Nascimento F.R.F., Guerra R.N.M., De Barros Abreu B.V., Cunha M.S., Batista M.C.A., Ribeiro M.N.S., Torres L.M.B. Phenolic acids, hydrolyzable tannins, and antioxidant activity of geopropolis from the stingless bee melipona fasciculata smith. J. Agric. Food Chem. 2014;62:2549–2557. doi: 10.1021/jf404875v. [DOI] [PubMed] [Google Scholar]
- 17.Batista M.C.A., Abreu B.V., De B., Dutra R.P., Cunha M.S., Amaral F.M.M.D., Torres L.M.B., Ribeiro M.N., De S. Chemical composition and antioxidant activity of geopropolis produced by Melipona fasciculata (Meliponinae) in flooded fields and cerrado areas of MaranhÃ\poundso State, northeastern Brazil. Acta Amaz. 2016;46:315–322. doi: 10.1590/1809-4392201600034. [DOI] [Google Scholar]
- 18.Dutra R.P., Bezerra J.L., Silva M.C.P., Batista M.C.A., Patrício F.J.B., Nascimento F.R.F., Ribeiro M.N.S., Guerra R.N.M. Antileishmanial activity and chemical composition from Brazilian geopropolis produced by stingless bee Melipona fasciculata. Rev. Bras. Farmacogn. 2019;29:287–293. doi: 10.1016/j.bjp.2019.02.009. [DOI] [Google Scholar]
- 19.Liberio S.A., Pereira A.J.O.L.A., Dutra R.P., Reis A.S., Araujo M.J.A.M., Mattar N.S., Silva L.A., Ribeiro M.N.S., Nascimento F.R.F., Guerra R.N.M., et al. Antimicrobial activity against oral pathogens and immunomodulatory effects and toxicity of geopropolis produced by the stingless bee Melipona fasciculata Smith. BMC Complement. Altern. Med. 2011;11:108. doi: 10.1186/1472-6882-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha M.S. Ph.D. Thesis. Universidade Federal do Maranhão; São Luís, MA, Brazil: 2017. Composição Química e Atividade Antitumoral da Geoprópolis de Melipona fasciculata Smith. [Google Scholar]
- 21.Batista M.C.A. Ph.D. Thesis. Universidade Federal do Maranhão; São Luís, MA, Brazil: 2016. Bioprospecção Anti-Helmíntica de Geoprópolis de Melipona fasciculata Smith em Testes In Vitro com Ovos e Larvas de Haemochus Contortus de Pequenos Ruminantes. [Google Scholar]
- 22.Carpes S.T., Mourão G.B., De Alencar S.M., Masson M.L. Chemical composition and free radical scavenging activity of Apis mellifera bee pollen from Southern Brazil. Braz. J. Food Technol. 2009;12:220–229. doi: 10.4260/BJFT2009800900016. [DOI] [Google Scholar]
- 23.Morais M., Moreira L., Feas X., Estevinho L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011;49:1096–1101. doi: 10.1016/j.fct.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Akin M., Nalbantoglu S., Saki N. Total phenols, antioxidant potential and tyrosinase inhibitory activity of honeybee collected pollen from Turkey. Res. J. Biotechnol. 2013;8:15–18. [Google Scholar]
- 25.LeBlanc B.W., Davis O.K., Boue S., DeLucca A., Deeby T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009;115:1299–1305. doi: 10.1016/j.foodchem.2009.01.055. [DOI] [Google Scholar]
- 26.Mărghitaş L.A., Stanciu O.G., Dezmirean D.S., Bobiş O., Popescu O., Bogdanov S., Campos M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009;115:878–883. doi: 10.1016/j.foodchem.2009.01.014. [DOI] [Google Scholar]
- 27.de Pascual-Teresa S., Johnston K.L., DuPont M.S., O’Leary K.A., Needs P.W., Morgan L.M., Clifford M.N., Bao Y., Williamson G. Quercetin Metabolites Downregulate Cyclooxygenase-2 Transcription in Human Lymphocytes Ex Vivo but Not In Vivo. J. Nutr. 2004;134:552–557. doi: 10.1093/jn/134.3.552. [DOI] [PubMed] [Google Scholar]
- 28.Chandrasekharan N.V., Dai H., Roos K.L.T., Evanson N.K., Tomsik J., Elton T.S., Simmons D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho W.A., Carvalho R.D.S., Rios-Santos F. Analgésicos inibidores específicos da ciclooxigenase-2: Avanços terapêuticos. Rev. Bras. Anestesiol. 2004;54:448–464. doi: 10.1590/S0034-70942004000300017. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X., Cao J., Zhong L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn. Schmiedeberg’s Arch. Pharmacol. 2009;379:581–586. doi: 10.1007/s00210-009-0399-7. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama H., Sakamoto T., Araki Y., Hara H. Anti-inflammatory effect of bee pollen ethanol extract from Cistus sp. of Spanish on carrageenan-induced rat hind paw edema. BMC Complement. Altern. Med. 2010;10:30. doi: 10.1186/1472-6882-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J.-H. Intracellular Antioxidant Activity and Inhibition of Bee Pollens on the Production of Inflammatory Mediators (P06-081-19) Curr. Dev. Nutr. 2019;3:596. doi: 10.1093/cdn/nzz031.P06-081-19. [DOI] [Google Scholar]
- 33.Vinegar R., Schreiber W., Hugo R. Biphasic development of carrageenin edema in rats. J. Pharmacol. Exp. Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- 34.Vinegar R., Truax J.F., Selph J.L., Johnston P.R., Venable A.J.O.L., McKenzie K.K. Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed. Proc. 1987;46:118–126. [PubMed] [Google Scholar]
- 35.Lo T.N., Almeida A.P., Beaven M.A. Dextran and carrageenan evoke different inflammatory responses in rat with respect to composition of infiltrates and effect of indomethacin. J. Pharmacol. Exp. Ther. 1982;221:261–267. [PubMed] [Google Scholar]
- 36.Vasconcelos S.M.M., Sales G.T.M., Lima N., Lobato R.D.F.G., Macêdo S.D., Barbosa-Filho J.M., Leal L.K.A.M., Fonteles M.M.F., Sousa F.C.F., Oliveira J.L., et al. Anti-inflammatory activities of the hydroalcoholic extracts from Erythrina velutina and E. mulungu in mice. Rev. Bras. Farmacogn. 2011;21:1155–1158. doi: 10.1590/S0102-695X2011005000134. [DOI] [Google Scholar]
- 37.Arslan R., Bektas N., Ozturk Y. Antinociceptive activity of methanol extract of fruits of Capparis ovata in mice. J. Ethnopharmacol. 2010;131:28–32. doi: 10.1016/j.jep.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 38.Bahamonde S.M.A., Flores M.L., CÃ\textthreesuperiorrdoba O.L., Taira C.A., Gorzalczany S. Antinociceptive and anti-inflammatory activities of an aqueous extract of Chiliotrichum diffusum. Rev. Bras. Farmacogn. 2013;23:699–705. doi: 10.1590/S0102-695X2013005000051. [DOI] [Google Scholar]
- 39.Abdulmalik I.A., Sule M.I., Musa A.M., Yaro A.H., Abdullahi M.I., Abdulkadir M.F., Yusuf H. Evaluation of analgesic and anti-inflammatory effects of ethanol extract of Ficus iteophylla leaves in rodents. Afr. J. Tradit. Complement. Altern. Med. AJTCAM. 2011;8:462–466. doi: 10.4314/ajtcam.v8i4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parandin R., Daroogari S. Anti-Inflammatory and Antinociceptive Activities of the Ethanolic Extract of Propolis in Male Mice and Rats. Zahedan J. Res. Med. Sci. 2019;21 doi: 10.5812/zjrms.84150. [DOI] [Google Scholar]
- 41.Sulaiman M.R., Hussain M.K., Zakaria Z.A., Somchit M.N., Moin S., Mohamad A.S., Israf D.A. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia. 2008;79:557–561. doi: 10.1016/j.fitote.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Milano J., Oliveira S.M., Rossato M.F., Sauzem P.D., Machado P., Beck P., Zanatta N., Martins M.A.P., Mello C.F., Rubin M.A., et al. Antinociceptive effect of novel trihalomethyl-substituted pyrazoline methyl esters in formalin and hot-plate tests in mice. Eur. J. Pharmacol. 2008;581:86–96. doi: 10.1016/j.ejphar.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 43.Moniruzzaman M., Khatun A., Imam M.Z. Evaluation of Antinociceptive Activity of Ethanol Extract of Leaves of Adenanthera pavonina. Evid. Based. Complement. Altern. Med. 2015;2015:412497. doi: 10.1155/2015/412497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi E.-M. Antinociceptive and antiinflammatory activities of pine (Pinus densiflora) pollen extract. Phytother. Res. 2007;21:471–475. doi: 10.1002/ptr.2103. [DOI] [PubMed] [Google Scholar]
- 45.Ambriz-Pérez D.L., Leyva-López N., Gutierrez-Grijalva E.P., Heredia J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016;2:1131412. [Google Scholar]
- 46.Złotek U., Gawlik-Dziki U., Dziki D., Świeca M., Nowak R., Martinez E. Influence of Drying Temperature on Phenolic Acids Composition and Antioxidant Activity of Sprouts and Leaves of White and Red Quinoa. J. Chem. 2019 doi: 10.1155/2019/7125169. [DOI] [Google Scholar]
- 47.Kawasaki M., Toyoda M., Teshima R., Sawada J., Saito Y. Effect of alpha-linolenic acid on the metabolism of omega-3 and omega-6 polyunsaturated fatty acids and histamine release in RBL-2H3 cells. Biol. Pharm. Bull. 1994;17:1321–1325. doi: 10.1248/bpb.17.1321. [DOI] [PubMed] [Google Scholar]
- 48.Tasaka K., Akagi M., Miyoshi K., Mio M., Makino T. Anti-allergic constituents in the culture medium of Ganoderma lucidum. (I). Inhibitory effect of oleic acid on histamine release. Agents Actions. 1988;23:153–156. doi: 10.1007/BF02142526. [DOI] [PubMed] [Google Scholar]
- 49.Fagali N., Catala A. Antioxidant activity of conjugated linoleic acid isomers, linoleic acid and its methyl ester determined by photoemission and DPPH techniques. Biophys. Chem. 2008;137:56–62. doi: 10.1016/j.bpc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Ko E.Y., Nile S.H., Jung Y.-S., Keum Y.S. Antioxidant and antiplatelet potential of different methanol fractions and flavonols extracted from onion (Allium cepa L.) 3 Biotech. 2018;8:155. doi: 10.1007/s13205-018-1184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nile S.H., Nile A.S., Keum Y.S., Sharma K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chem. 2017;235:119–126. doi: 10.1016/j.foodchem.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 52.Nile A., Nile S.H., Kim D.H., Keum Y.S., Seok P.G., Sharma K. Valorization of onion solid waste and their flavonols for assessment of cytotoxicity, enzyme inhibitory and antioxidant activities. Food Chem. Toxicol. 2018;119:281–289. doi: 10.1016/j.fct.2018.02.056. [DOI] [PubMed] [Google Scholar]
- 53.García-Niño W.R., Zazueta C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015;97:84–103. doi: 10.1016/j.phrs.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 54.El-Shitany N.A., El-Bastawissy E.A., El-desoky K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int. Immunopharmacol. 2014;19:290–299. doi: 10.1016/j.intimp.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Dutra R.P., Nogueira A.M.C., Marques R.R.D.O., Costa M.C.P., Ribeiro M.N.S. Avaliação farmacognóstica de geoprópolis de Melipona fasciculata Smith da Baixada maranhense, Brasil. Braz. J. Pharmacogn. 2008;18:557–562. doi: 10.1590/S0102-695X2008000400010. [DOI] [Google Scholar]
- 56.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 57.Benzie I.F., Strain J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxid. Power”: The Frap Assay. Anal. Biochem. 1996;76:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 58.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay Roberta. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 59.Chagas V.T., de Sousa Coelho R.M.R., Gaspar R.S., da Silva S.A., Mastrogiovanni M., de Jesus Mendonça C., de Sousa Ribeiro M.N., de Andrade Paes A.M., Trostchansky A. Corrigendum to “Protective Effects of a Polyphenol-Rich Extract from Syzygium cumini (L.) Skeels Leaf on Oxidative Stress-Induced Diabetic Rats”. Oxid. Med. Cell. Longev. 2019;2019:5785798. doi: 10.1155/2019/5785798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 61.Silva R.H.M., Lima N.D., Lopes A.J.O., Vasconcelos C.C., de Mesquita J.W.C., de Mesquita L.S.S., Lima F.C.V.M., Ribeiro M.N.S., Ramos R.M., Cartagenes M.D.S.S., et al. Antinociceptive Activity of Borreria verticillata: In vivo and in silico Studies. Front. Pharmacol. 2017;8:283. doi: 10.3389/fphar.2017.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koster R., Anderson M., De Beer E.J. Acetic Acid-Induced Analgesic Screening. Fed. Proc. 1959;18:418–420. [Google Scholar]
- 63.Hunskaar S., Hole K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 64.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., et al. Gaussian 09. Gaussian, Inc.; Wallingford, CT, USA: 2016. [Google Scholar]
- 65.Dennington R., Keith T.A., Millam J.M. GaussView5. Gaussian, Inc.; Wallingford, CT, USA: 2016. [Google Scholar]
- 66.Goodsell D.S., Morris G.M., Olson A.J. Automated docking of flexible ligands: Applications of AutoDock. J. Mol. Recognit. 1996;9:1–5. doi: 10.1002/(SICI)1099-1352(199601)9:1<1::AID-JMR241>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 67.Morris G.M., Huey R., Olson A.J. Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinform. 2008;8:8–14. doi: 10.1002/0471250953.bi0814s24. [DOI] [PubMed] [Google Scholar]
- 68.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- 70.Calado G.P., Lopes A.J.O., Costa Junior L.M., Lima F., Silva L.A., Pereira W.S., Amaral F.M., Garcia J.B., Cartagenes M.S., Nascimento F.R. Chenopodium ambrosioides L. Reduces Synovial Inflammation and Pain in Experimental Osteoarthritis. PLoS ONE. 2015;10:e0141886. doi: 10.1371/journal.pone.0141886. [DOI] [PMC free article] [PubMed] [Google Scholar]