Abstract
Effector proteins secreted by plant pathogens play important roles in promoting colonization. Blumeria effector candidate (BEC) 1019, a highly conserved metalloprotease of Blumeria graminis f. sp. hordei (Bgh), is essential for fungal haustorium formation, and silencing BEC1019 significantly reduces Bgh virulence. In this study, we found that BEC1019 homologs in B. graminis f. sp. tritici (Bgt) and Gaeumannomyces graminis var. tritici (Ggt) have complete sequence identity with those in Bgh, prompting us to investigate their functions. Transcript levels of BEC1019 were abundantly induced concomitant with haustorium formation in Bgt and necrosis development in Ggt-infected plants. BEC1019 overexpression considerably increased wheat susceptibility to Bgt and Ggt, whereas silencing this gene using host-induced gene silencing significantly enhanced wheat resistance to Bgt and Ggt, which was associated with hydrogen peroxide accumulation, cell death, and pathogenesis-related gene expression. Additionally, we found that the full and partial sequences of BEC1019 can trigger cell death in Nicotiana benthamiana leaves. These results indicate that Bgt and Ggt can utilize BEC1019 as a virulence effector to promote plant colonization, and thus these genes represent promising new targets in breeding wheat cultivars with broad-spectrum resistance.
Keywords: effector protein, Blumeria graminis f. sp. tritici, Gaeumannomyces graminis var. tritici, haustorium formation, necrosis development, cell death
1. Introduction
Wheat makes a substantial contribution to human calorie intake worldwide (Food and Agriculture Organization of the United Nations, http://www.fao.org/faostat/en), and plays a major role in ensuring global agricultural sustainability and food security [1]. The recent release of a high-quality, fully annotated and ordered bread wheat genome offers considerable promise for future developments in wheat cultivation [2]. However, recently published data indicate that global losses in wheat yield attributable to pathogenic fungi and pests account for approximately 21.5% of total losses, reaching up to 28.1% in food-deficit areas [3]. Although the planting of resistant cultivars and application of various fungicides have been the primary approaches adopted by farmers to stem the increasing incidence of plant fungal diseases, the latter is a potential source of environmental pollution [4]. Moreover, current agricultural practices typically involve the planting of single-genotype cereal crops over extensive areas, accelerating the selection of fungal strains that can overcome inherent crop genetic resistance [4]. There is, accordingly, a constant demand for innovative developments in sustainable agricultural and the introduction of novel resistance strategies in cereal crop breeding [4].
Based on their life histories, plant pathogens can be divided into those that kill the host and absorb nutrients from dead cells (necrotrophs) and those that require a living host to complete their life cycle (biotrophs) and those that have an initial biotrophic phase, then become necrotrophic (hemi-biotrophs) [5] and employ diverse strategies relating to host interaction. Plant defense responses consist of two layers designed to resist pathogen attack, namely, pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) and effector-triggered immunity (ETI) [6,7]. PAMPs, such as flagellin, chitin, and EF-Tu, are perceived by plant pattern recognition receptors (PRRs), thereby initiating a series of cellular signals, including the activation of a downstream mitogen-activated protein kinase cascade and induction of defense-related genes [6,8,9]. ETI, the second layer of plant defense, is activated by pathogen effectors, secreted by fungi, modulating host plant physiology to accommodate the fungi and provide the necessary nutrients [10,11]. Effectors are mostly recognized by nucleotide-binding site and leucine-rich repeat (NBS-LRR) proteins, encoded by host resistance (R) genes, involved in inhibiting pathogen colonization [10,11]. The direct and indirect interactions between effectors and R proteins lead to resistance, often accompanied by a hypersensitive response (HR) and local cell death that prevent pathogen colonization [8,12].
Take-all disease, caused by the soil-borne necrotrophic fungus Gaeumannomyces graminis var. tritici (Ggt), is an important root disease of cereals, capable of reducing grain quality and grain yield by 40–60% [13,14]. Upon infection, Ggt hyphae penetrate the root cortical cells and progress upward into the base of the stem, wherein they extract nutrients from the plant, causing premature death. The symptoms manifest as chocolate brown to black lesions on the infected roots and stem bases, premature ripening, and whiteheads [15]. Although breeding resistant wheat cultivars is considered the most promising and reliable measure to protect wheat from take-all, no effective resistant wheat varieties have been identified to date and traditional crop rotation is neither economically feasible nor practicable [16]. Recently, the use of beneficial microorganisms (e.g., Pseudomonas fluorescens, Bacillus subtilis, Bacillus velezensis, and Serratia proteamaculans) has been identified as an alternative, effective, and eco-friendly strategy for the control of take-all disease [17,18,19,20].
Blumeria graminis f. sp. tritici (Bgt), the causative agent of wheat powdery mildew, is an obligate biotrophic fungus and a major threat to common wheat (Triticum aestivum L.) production worldwide [21]. In a compatible interaction between wheat and Bgt, the fungal conidia germinate and penetrate the plant epidermal cell walls, and thereafter acquire nutrients by forming a specialized feeding structure, the haustorium, which is crucial for the subsistence of these obligate biotrophs [12,21]. Subsequently, a series of effector proteins are secreted into plant cells by the pathogen, which manipulate plant defense mechanisms and serve to promote colonization [22,23]. The interaction between barley and powdery mildew B. graminis f. sp. hordei (Bgh) is considered an ideal pathosystem in monocots for examining the influence of effectors on host genetic and molecular mechanisms [24,25]. Four avirulence (Avr) proteins AVRa1, AVRa13, AVRK1, and AVRA10, identified in Bgh are respectively recognized by the barley resistance proteins MLA1, MLA13, MLA10, and MLK1, which trigger HR in the host [24,25,26].
Proteomic and genomic studies have identified more than 500 Blumeria effector candidates (BECs) or candidate secreted effector proteins (CSEPs), for which homologous genes have been identified in Bgh and Bgt [27,28]. Although only a few BECs and CSEPs have been characterized, they have been shown to play vital roles in virulence. For example, the secreted Bgh effectors CSEP0105 and CSEP0162 significantly reduce the rate of fungal haustorium formation and interact with the heat shock proteins Hsp16.9 and Hsp17.5 [29]. Additionally, host-induced gene silencing (HIGS) of CSEP0055, which interacts with barley pathogenesis-related protein PR17c, reduces fungal aggressiveness [30]. Similarly, the ribonuclease-like effector candidate BEC1011 has been found to act as a suppressor of pathogen-induced host cell death [31]. Previous research has revealed that certain effectors are vital for haustorium development [32], and silencing of the Bgh effector BEC1019, which encodes a secreted metalloprotease, significantly reduces Bgh virulence in barley [33,34]. Additionally, BEC1019 is broadly conserved across a diverse range of organisms, including 37 plant pathogens (comprising major pathogens of rice, maize, and wheat), 15 animal pathogens, 4 insect pathogens, and 35 non-pathogens [33,34]. These observations, therefore, indicate that the conserved effector gene BEC1019 could potentially serve as a broad-spectrum target for the control of different pathogens.
The objectives of the present study were three-fold, namely, to (i) identify and align homologous genes in eight plant pathogens (including biotrophs, hemi-biotrophs, and necrotrophs) with the BEC1019 gene in Bgh, (ii) overexpress and silence BEC1019 in wheat to investigate its roles in the colonization of Bgt and Ggt, and (iii) determine whether hydrogen peroxide (H2O2) accumulation and cell death are involved in wheat disease resistance.
2. Results
2.1. BEC1019 is Broadly Conserved in Plant Fungal Pathogens
Bgt and Bgh are the two main formae speciales of B. graminis, and genome-wide analyses have indicated that almost 92% of the genes in Bgt and Bgh are homologous [27,28]. BEC1019 has been demonstrated to play key roles in the development of resistance to Bgh in barley [33,34]. Accordingly, we searched for a gene in Bgt homologous to the BEC1019 gene of Bgh. To identify the BEC1019 homolog in Bgt, primers designed based on Bgh BEC1019 and Bgt DNA were used to clone the candidate gene. The sequencing results showed that the nucleotide and protein sequences (without signal peptides) of Bgt BEC1019 are completely identical to those of Bgh BEC1019 (Figure 1). To analyze the conservation of BEC1019 in the diverse fungi, several major plant pathogens and one oomycete were selected to clone the homologs, including Puccinia striiformis f. sp. tritici, Pst (causes wheat stripe rust), Fusarium pseudograminearum, Fp (causes wheat crown rot disease), F. graminearum, Fg (causes wheat head blight and crown rot), Bipolaris sorokiniana, Bs (causes wheat root rot), Phytophthora infestans, Pi (causes Solanaceae plants late blight), and Verticillium dahliae, Vd (cause cotton and Solanaceae plants Verticillium wilt). Interestingly, BEC1019 homologs in biotrophic (Pst), hemi-biotrophic (Pi), and necrotrophic (Ggt, Fp, Fg, and Vd) pathogens are identical to those in Bgh (Accession No. AHZ59730.1) (Figure S1). The exception among the pathogens we examined was Bs, whose amino acid sequence showed 91.46% identity to that of Bgh (Figure S1).
Figure 1.
Nucleotide sequences of the homologous genes in Blumeria graminis f. sp. tritici (Bgt) and Gaeumannomyces graminis var. tritici (Ggt) were completely identical to the BEC1019 sequence in B. graminis f. sp. hordei (Bgh).
2.2. BEC1019 Is Highly Expressed during Haustorium Formation by Bgt and Concomitant with the Development of Necrotic Symptoms in Ggt
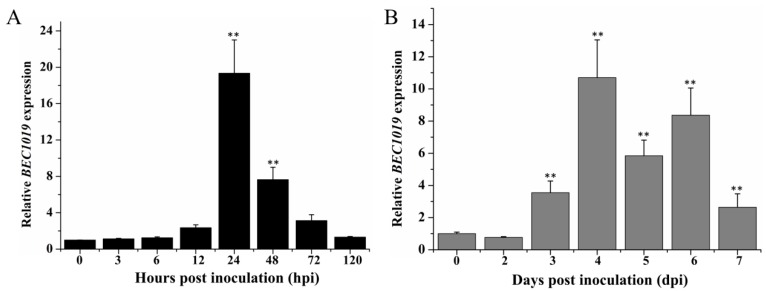
To investigate whether Bgt and Ggt employ the effector gene BEC1019 to facilitate host plant colonization and interfere with the different layers of wheat defenses induced during infection, we performed qRT-PCR to assess BEC1019 expression in Bgt-infected wheat leaves at different time points (0, 3, 6 12, 24, 48, 72, and 120 hpi) and in Ggt-infected wheat roots at different stages (0, 2, 3, 4, 5, 6, and 7 dpi). With regards to Bgt, the aforementioned time points represent the following stages of infection: non-germinated conidia (0 hpi), primary germ tube formation (3 hpi), appressorial germ tube formation (6 hpi), penetration (12 hpi), haustorium formation (24 hpi), secondary penetration (48 hpi), microcolony formation (72 hpi), and conidiophore formation (120 hpi) [29,35]. Using the elongation factor 1α (ef-1α) gene of Bgt as an internal reference gene, we found that the transcript level of BEC1019 was weakly induced during the first four stages of infection (0–12 hpi), whereas during haustorium formation (24 hpi), the abundance of BEC1019 increased by 19-fold compared with that at 0 hpi. Thereafter, BEC1019 expression gradually decreased with increasing Bgt colonization time, and at 120 hpi the BEC1019 transcript level had declined to pre-inoculation levels (Figure 2A).
Figure 2.
Relative expression patterns of BEC1019 at different stages of Blumeria graminis f. sp. tritici (Bgt) and Gaeumannomyces graminis var. tritici (Ggt) infection. (A) Wheat leaves inoculated with Bgt were harvested at 0, 3, 6, 12, 24, 48, 72, and 120 h post-infection (hpi). (B) Wheat roots inoculated with Ggt were harvested at 0, 2, 3, 4, 5, 6, and 7 days post-infection (dpi). The elongation factor 1α (ef-1α) gene of Bgt and 18S rRNA gene of Ggt were used as control genes for determinations of the relative expression of BEC1019. Bars indicate the means of three independent biological repetitions with standard errors. Double asterisks indicate significant differences relative to 0 hpi/dpi (p ≤ 0.01, according to t-tests).
The time points at which we monitored Ggt infection represent the following stages: un-inoculated (0 dpi), hyphal infection of the root surface (2 dpi), small (1 mm) necrotic lesions observed (3 dpi), 3-mm necrotic lesions present and hyphae observed in the root cortex cells (4 dpi), 6-mm necrotic lesions present and alteration of host tissue observed (5 dpi), larger necrotic lesions observed (6 dpi), and vascular tissue completely colonized by the pathogen (7 dpi) [18]. Using the 18S rRNA gene of Ggt as an internal reference gene, we observed that there was a marginal decrease in BEC1019 expression during the early stage of Ggt colonization (2 dpi). However, concomitant with necrosis development, BEC1019 was significantly up-regulated (3–6 dpi), peaking at 4 dpi. At this point, the root cortex cells had been colonized and BEC1019 levels were 11-fold higher than those of the control. Thereafter, BEC1019 transcript level showed a marginal decrease, concomitant with the complete colonization of vascular tissue (Figure 2B). Thus, our results indicate that Bgt BEC1019 is involved in haustorium formation, whereas that of Ggt is associated with necrotic symptom development.
2.3. Overexpression of BEC1019 Increases Wheat Susceptibility to Bgt and Ggt, whereas Silencing BEC1019 Enhances Resistance to both Biotrophic and Necrotrophic Pathogens
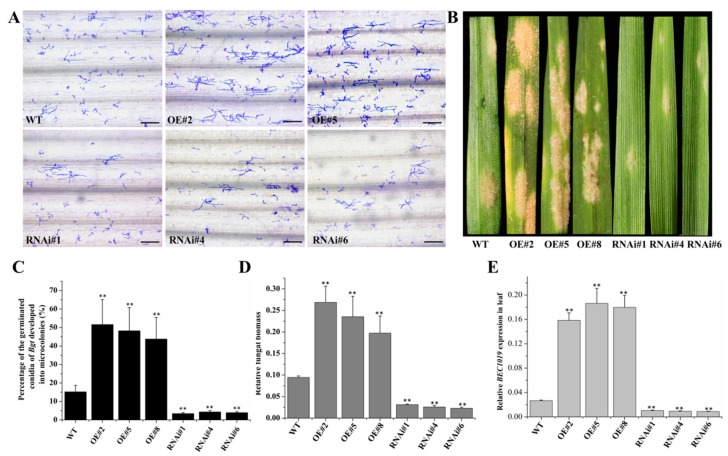
To assess the roles of BEC1019 in resistance development against wheat powdery mildew and take-all disease, transgenic plants harboring overexpression and RNAi vectors were inoculated with biotrophic (Bgt) and necrotrophic (Ggt) pathogens, respectively. The transgenic Aikang 58 wheat lines were initially inoculated with either water or fresh spores of Bgt, followed by microscopic observation at 60 hpi to calculate the percentages of germinated conidia that had developed into microcolonies. The results showed that the leaves of both overexpressing lines had a significantly larger number of Bgt microcolonies than did the leaves of control plants, whereas plants in which carrying BEC1019 RNAi vector had fewer microcolonies (Figure 3A,C). We assessed macroscopic disease symptoms at 5–6 days after Bgt infection and found that symptoms observed in the leaves of the overexpressing and RNAi transgenic plants were consistent with the findings of our microscopic analyses (Figure 3B). Additionally, quantification of fungal biomass in Bgt-infected transgenic plants indicated significantly enhanced Bgt fungal biomass in BEC1019-overexpressing plants, whereas there was a considerable decrease in fungal biomass in BEC1019-silenced wheat lines (Figure 3D). Compared with the control plants inoculated with Bgt, the levels of BEC1019/ef-1α expression were considerably higher in the leaves of overexpressing lines and lower in the leaves of knockdown plants, as determined by qRT-PCR (Figure 3E).
Figure 3.
Overexpression of BEC1019 in Aikang 58 wheat enhanced the susceptibility of plants to wheat powdery mildew Blumeria graminis f. sp. tritici (Bgt), whereas silencing BEC1019 increased resistance to Bgt. (A) Microscopic analysis revealed Bgt microcolony formation on the leaves of control plants and BEC1019-overexpressing and RNAi plants at 60 h post-inoculation (hpi). Scale bar = 25 µm. (B) Macroscopic phenotypes of Bgt infection on wild-type control plant leaves and BEC1019-overexpressing and RNAi plant leaves. (C) Percentages of germinated Bgt conidia on the BEC1019-overexpressing and RNAi plants and control plants at 60 hpi. (D) Quantification of Bgt fungal biomass in control wheat and BEC1019-overexpressing and RNAi plants. (E) Transcript levels of BEC1019 in Bgt-infected leaves of overexpressing and RNAi transgenic plants. Double asterisks indicate significant differences relative to control plants (p ≤ 0.01, according to t-tests).
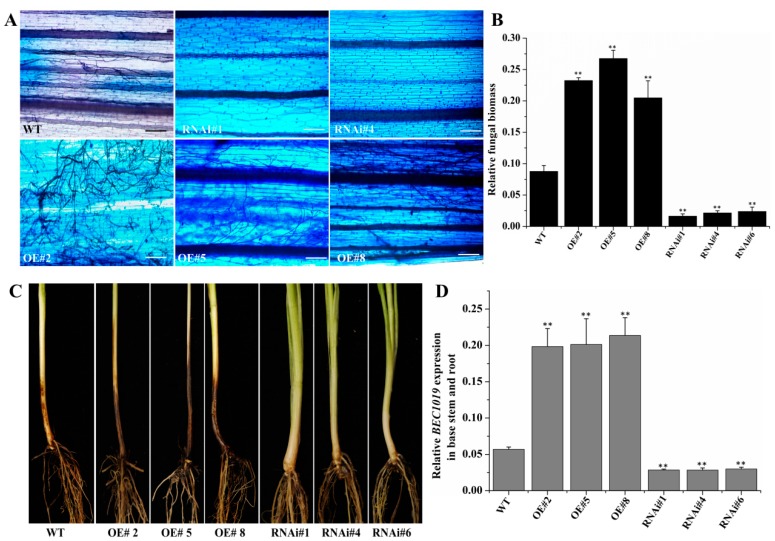
Interestingly, in response to Ggt infection, BEC1019-overexpressing and RNAi-silenced Zhoumai 26 wheat lines at 21 dpi exhibited a similar phenotype to that of Bgt-infected plants, and our micro- and macroscopic analyses indicated that up-regulation of BEC1019 significantly increased the susceptibility of wheat to Ggt compared with that of the wild-type control. In contrast, silencing BEC1019 considerably reduced the susceptibility of these plants (Figure 4A,C). Moreover, the relative biomass of Ggt was also increased in wheat lines overexpressing BEC1019 and reduced in BEC1019 RNAi lines (Figure 4B). Additionally, the levels of BEC1019/18S rRNA transcription in the base stem and root of Ggt-inoculated transgenic plants were similar to those in leaves inoculated with Bgt (Figure 4D). Semi-qRT-PCR results also revealed the presence of the ORF and partial sequence of BEC1019 in both overexpressing and RNAi transgenic lines (Figure S2).
Figure 4.
Overexpression of BEC1019 in Zhoumai 26 wheat increased susceptibility to the necrotrophic pathogen Gaeumannomyces graminis var. tritici (Ggt), whereas silencing BEC1019 decreased susceptibility to Ggt. (A) Macroscopic responses to Ggt infection on the stems and roots of BEC1019-overexpressing and RNAi lines and wild-type control plants. (B) Relative Ggt biomass of control wheat and BEC1019 overexpressing and RNAi plants. (C) Microscopic analysis showing the growth of Ggt hyphae on the stems of wild-type plants and BEC1019-overexpressing and RNAi plants after inoculation. Scale bar = 25 µm. (D) Transcript levels of BEC1019 in Ggt-infected base stems and roots of overexpressing and RNAi transgenic plants. Double asterisks indicate significant differences relative to the control plants (p ≤ 0.01, according to t-tests).
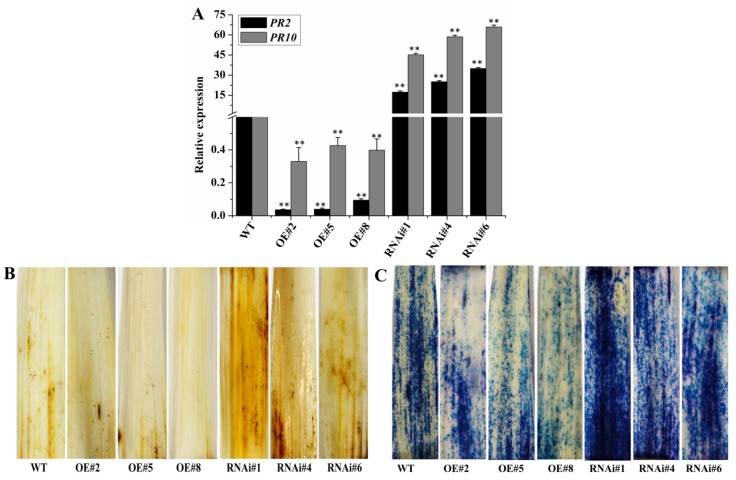
Compared with the control plants, expression of the wheat PR marker genes TaPR2 and TaPR10 in Bgt non-inoculated transgenic lines was significantly down-regulated in BEC1019-overexpressing wheat lines and up-regulated in BEC1019 RNAi lines (Figure 5A). Notably, in the silenced lines, the relative transcript levels of TaPR2 and TaPR10 were up-regulated by 17- to 66-fold compared with the controls (Figure 5A). To assess the effects of H2O2 accumulation and HR response in transgenic plants inoculated with Bgt, we performed DAB and trypan blue staining. At 3 dpi, DAB staining revealed a higher accumulation of H2O2 in BEC1019 RNAi lines than in those overexpressing BEC1019 and the controls (Figure 5B). Trypan blue analysis indicated a substantial increase in cell death in the BEC1019 RNAi lines, whereas the cell death observed in BEC1019-overexpressing plants was comparable to that detected in the wild-type control, all of which were inoculated with Bgt and sampled a 1 dpi (Figure 5C). These findings were consistent with the microscopic and macroscopic observations for Bgt.
Figure 5.
Defense-related traits identified in BEC1019-overexpressing and -silenced plants. (A) The results of qRT-PCR analyses showing the transcript levels of wheat defense marker genes TaPR2 and TaPR10 in BEC1019-overexpressing and RNAi plants. Double asterisks indicate significant differences relative to the control plants (p ≤ 0.01, according to t tests). (B) The leaves of wild-type wheat and BEC1019-overexpressing and RNAi plants were challenged with Blumeria graminis f. sp. tritici (Bgt) and sampled at 3 days post-infection (dpi) with DAB staining. (C) The leaves of wild-type wheat and BEC1019 transgenic plants were challenged with Bgt, sampled at 1 dpi, and used to observe cell death, which was detected by staining with trypan blue.
2.4. Transient Expression of BEC1019 Protein Triggers Cell Death in Nicotiana benthamiana
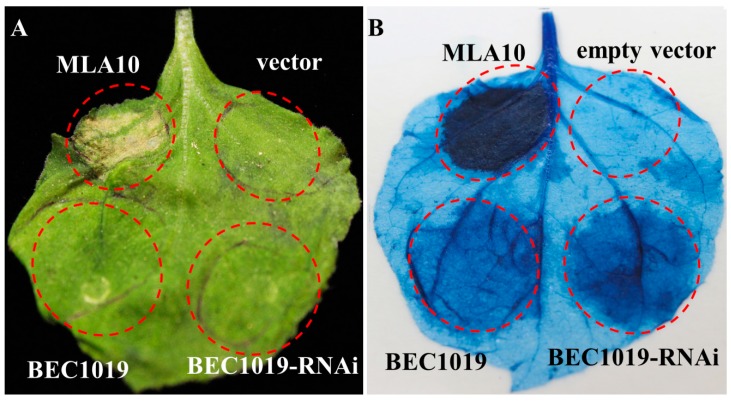
To determine whether the full-length and RNAi partial sequence of BEC1019 can trigger cell death, we performed Agrobacterium tumefaciens-mediated transient expression. The barley NLR immune receptor MLA10 was used as a positive control, the overexpression of which can induce a significant cell death phenotype in N. benthamiana leaves [36]. Accordingly, we observed that the transient expression of MLA10, CTAPi-GW-3HA-BEC1019, and CTAPi-GW-3HA-BEC1019-RNAi induced cell death (Figure 6), and MLA10 induced a typical cell death response at 5 days after agro-infiltration of the leaves. Cell death induced by the expression of full-length BEC1019 and RNAi segment 5 days after agro-infiltration was observed to be less pronounced than that caused by MLA10, indicating that BEC1019 might interact with certain disease resistance proteins to trigger cell death in N. benthamiana.
Figure 6.
Analysis of cell death triggered by BEC1019 in Nicotiana benthamiana. The barley resistance gene MLA10 (positive control) and full-length and RNAi partial sequences of BEC1019 were expressed via agro-infiltration in N. benthamiana (A), and cell death representing HR was visualized by trypan blue staining (B) at 5 days post-agro-infiltration. No HR induction was detected in the negative control (empty vector).
3. Discussion
The sequencing and annotation of the genomes of Bgh and Bgt in 2010 and 2013, respectively [27,28], provided valuable new genetic resources for research on powdery mildews. However, owing to difficulties in obtaining transgenic wheat plants, most studies on the interaction of powdery mildews and plants have been performed using barley and Bgh. Numerous Bgh-specific CSEPs and BECs are considered candidate effector genes and are predicted to be expressed mainly in haustoria. HIGS technology has considerably enhanced the functional verification of fungal genes implicated in the interactions between plants and pathogens [31], particularly Bgh [29,30,31,32,37] and other biotrophic fungal pathogens, such as Puccinia striiformis f. sp. tritici [38] and Fusarium graminearum [39]. Previous studies in which BEC1019 was silenced using HIGS have revealed the roles of this gene in Bgh colonization and haustorial development [33,34]. In the present study, we used HIGS to verify the roles played BEC1019, a conserved effector gene in Bgh, in Bgt and Ggt, which are, respectively, important obligate biotrophic and soil-borne necrotrophic fungi. The results showed that the BEC1019 homologs in Bgt and Ggt are identical to those in Bgh, and similarly enhance the colonization of these pathogens in wheat, which was consistent with previous research that conserved effector genes played the similar roles in different bacterial pathogens [40,41].
Previous studies have also found that homologs of the Bgh BEC1019 gene are present in the sequenced genomes of 96 other fungal pathogens [33,34]. Consistent with this finding, we identified BEC1019 homologous sequences in eight pathogens of cereal crops and Solanaceae species, including biotrophic, hemi-biotrophic, and necrotrophic fungi, sharing 100% sequence identity with Bgh BEC1019. The only exception among the pathogens we examined was Bipolaris sorokiniana, which showed certain differences in sequences at the 5′ and 3′ ends of BEC1019. However, given that we found that the homolog in the oomycete Phytophthora infestans (hemi-biotrophy) has 100% sequence identity with Bgh BEC1019, we cannot conclude that hemi-biotrophic pathogens have specific variants of this gene. Thus, in the future, homologous sequences in other hemi-biotrophic pathogens should be examined to gain a better understanding in this respect. It is speculated that BEC1019 might be an ancient gene that plays vital roles in the life cycles of fungi, and novel function, such as promoting the infection of plants, was acquired during the evolution of fungal pathogens.
Effector proteins are differentially expressed and assumed to be required at different stages of powdery mildew infection. Consistent with this notion, the Bgh effectors CSEP0081, CSEP0254, CSEP0055, CSEP0105, and CSEP0162 were only detected at low levels during the early stages of infection (0–12 hpi) [29,30,42,43]. However, from 24 hpi (haustorium formation), there was a substantial increase in effector transcript levels, with some (CSEP0105 and CSEP0254) showing peak expression at this stage, whereas the expression of other effectors (CSEP0081, CSEP0055, and CSEP0162) was further elevated at 48 hpi (secondary penetration). Our results indicated that BEC1019 expression in Bgt is similar to that of BEC1019, CSEP0105, and CSEP0254 in Bgh, with maximum expression coinciding with haustorium formation (24 hpi), thereby indicating that BEC1019 is probably involved in haustorium formation in Bgt.
To date, a few effector genes have been isolated from the biotrophic fungi Bgt, the first of which, AvrPm3a2/f2 (cloned in 2015), is abundantly expressed during haustorium formation and is specifically recognized by the wheat resistance genes Pm3a and Pm3f [43]. AvrPm2 encodes an RNase-like effector protein that belongs to a structurally conserved gene family [44], which includes the Bgh effector gene Avra13 (CSEP0372), and interacts with the barley resistance gene MLA13 [26]. Two additional well-characterized RNase-like effector genes in Bgh are BEC1011 [31] and BEC1054 [45], and all the Bgt and Bgh RNase-like effectors genes have been found to have similar structural homologies. To evade plant immune systems, the effectors AvrPm3a2/f2 and AvrPm2 have evolved under strong diversifying selection [43,44], which contrasts with the broadly conserved BEC1019. However, no similar effector genes have been identified in Ggt, and accordingly, the majority of studies on resistance to Ggt have focused on the overexpression of heterologous proteins in wheat to enhance resistance to this pathogen. For example, the overexpression of soybean GmPGIP3, an antimicrobial peptide found in potato, and the MYB gene of Thinopyrum intermedium in wheat have been shown to confer increased resistance to take-all disease caused by the colonization of Ggt [46,47,48].
In the present study, we found that overexpression of BEC1019 in wheat significantly promoted Bgt and Ggt virulence, thereby increasing the susceptibility of infected plants. Consistent with the role of BEC1019 in the Bgh infection of barley, silencing of this gene considerably reduced Bgt colonization in wheat epidermal cells and Ggt infection in wheat stems and roots, which is related to HR and H2O2 accumulation. Therefore, we conclude that BEC1019 is a highly conserved effector gene in fungi that is not only associated with biotrophic pathogen development but is also involved in necrotrophic fungal virulence. Accordingly, BEC1019 may serve as a common candidate effector gene that can be used to identify the proteins with which it interacts, thereby providing an alternative strategy for elucidating the mechanisms underlying resistance to BEC1019. Furthermore, this strategy could contribute to the breeding of cereal crops with long-lasting broad-spectrum resistance.
4. Materials and Methods
4.1. Plants and Fungal Materials
Wheat (Triticum aestivum L.) Aikang 58 and Zhoumai 26, provided by the Henan Institute of Science and Technology and the Zhoukou Academy of Agricultural Sciences, respectively, were used as transformation recipients in this study. Cultivars of Aikang 58 and Zhoumai 26 were susceptible to Bgt and Ggt, respectively. The naturally occurring biotrophic fungus B. graminis f. sp. tritici (Bgt) was isolated from material collected in a field in Zhoukou, Henan Province, China (33°62ʹN, 114°65ʹE), and maintained on Zhoumai 22, grown under a 16-h light (22 °C) and 8-h dark (20 °C) photoperiod with 60% atmospheric humidity.
Ggt LY3-21, the wheat hemi-biotroph Bipolaris sorokiniana (Bs), and the necrotrophic pathogens Fusarium pseudograminearum (Fp) and F. graminearum (Fg) were provided by the Henan Academy of Agricultural Science and cultured on potato dextrose agar at 22–25 °C. Puccinia striiformis f. sp. tritici (Pst), a gift from the Zhoukou Academy of Agricultural Sciences, was cultured on the susceptible wheat cultivar Mingxian 169 in a growth chamber under a 16-h light (16 °C) and 8-h dark (8 °C) photoperiod. The Solanaceae pathogens Phytophthora infestans (Pi) and Verticillium dahliae (Vd), provided by the Chinese Academy of Agricultural Sciences, were cultured on rye agar medium and Czapek–Dox medium, respectively.
4.2. Cloning of BEC1019 Homologous Genes
For DNA extraction, four to five leaves of Bgt-infected 7-day-old wheat, Pst-infected 12-day-old wheat and the other fungi cultivated on medium were ground to a powder in liquid nitrogen using a precooled mortar and pestle, according to the manufacturer’s instructions (SK8230; Shanghai Sangon Biological Engineering Corporation, Shanghai, China) of DNA extraction kit. The BEC1019 gene homologs were amplified using the BEC1019-F/R primer pair, designed based on the Bgh BEC1019 sequence. The polymerase chain reaction (PCR) program used for amplification was as follows: pre-denaturation at 94 °C for 5 min; followed by 30 cycles at 94 °C for 30 s, 58 °C for 45 s, and 72 °C for 1 min; with a final extension at 72 °C for 10 min. Corresponding amplicons were inserted into a pEASY-Blunt cloning vector (Beijing TransGen Biotech Corporation, Beijing, China) and recombinant plasmids were sequenced using the universal M13 primer.
4.3. Construction of BEC1019 Overexpression and RNAi Vectors
The BEC1019 sequence containing the full-length open reading frame (ORF) was amplified from Bgt DNA using the primer pair BEC1019-F1/R1, designed based on the BEC1019 sequence (GenBank accession no. KJ571201). To clone a 264-bp region (368–632 bp) of BEC1019, we designed the primer pair BEC1019-F2/R2 based on the sequence used in Barley stripe mosaic virus-induced gene silencing of Bgh [33,34], Gateway technology was used to construct overexpression and RNAi vectors. Initially, we inserted the complete and RNAi partial sequences of BEC1019 into a pDONR207 intermediate vector, generating pDONR-BEC1019 and pDONR-BEC1019-RNAi vectors, respectively. Recombining these into gateway destination vectors, CTAPi-GW-3HA and modified pCAMBIA2301 using LR clonase, generated the final vectors [49]. The primers used for amplification are listed in Table 1. The CTAPi-GW-3HA-BEC1019 and pCAMBIA2301-BEC1019-RNAi vectors were used to generate transgenic wheat lines, and BEC1019 transgenic T1 wheat was provided by the Plant Genetic Transformation Center of Henan Key Laboratory of Crop Molecular Breeding and Bioreactor. The CTAPi-GW-3HA-BEC1019 and CTAPi-GW-3HA-BEC1019-RNAi vectors were used to transiently transform in Nicotiana benthamiana leaves for the detection of cell death.
Table 1.
Primers names and sequences used in this study.
| Primer Name | Primer Sequence (5′-3′) | Primers Purpose |
|---|---|---|
| BEC1019-F | ATGCAGTCTGTATTGCTTTT | Homolog gene cloning of BEC1019 |
| BEC1019-R | CTAGACACAATGAACCTCGC | |
| BEC1019-F1 | GGGGACAGTTTGTACAAAAAAGCAGGCTTCATGCAGTCTGTATTGCTTTT | Construction of CTAPi-GW-3HA-BEC1019 vector |
| BEC1019-R1 | GGGGACCACTTTGTACAAGAAAGCTGGGTGACACAATGAACCTCGCCAT | |
| BEC1019-F2 | GGGGACAGTTTGTACAAAAAAGCAGGCTTCGTGATGACCCGGACAAAA | Construction of pCAMBIA2301-BEC1019-RNAi vector |
| BEC1019-R2 | GGGGACCACTTTGTACAAGAAAGCTGGGTAGGGCATCTTGGTAACCA | |
| BEC1019-F3 | GGGGACAGTTTGTACAAAAAAGCAGGCTTCGTGATGACCCGGACAAAA | Construction of CTAPi-GW-3HA-BEC1019-RNAi vector |
| BEC1019-R3 | GGGGACCACTTTGTACAAGAAAGCTGGGTAGGGCATCTTGGTAACCA | |
| BEC1019-F4 | TCATGTGGACATCGTCGGTC | q RT-PCR analysis of BEC1019 over-expression wheat lines |
| BEC1019-R4 | CACGCTGATGTCAAACGCAT | |
| BEC1019-F5 | AATGTGCAACCGAGAACCGA | q RT-PCR analysis of BEC1019 silencing wheat lines |
| BEC1019-R5 | TCCTCCAAAGGAAGCCGTTC | |
| TaPR2-F | CCGGCCATACTACCCGGC | q RT-PCR analysis for TaPR2 |
| TaPR2-R | ACACCTTGATGGCGCTGAGA | |
| TaPR10-F | ACGGAGCGGATGTGGAAG | q RT-PCR analysis for TaPR10 |
| TaPR10-R | GCCACCTGCGACTTGAGC | |
| TaActin-F | CCAGGTATCGCTGACCGTAT | Reference gene of wheat |
| TaActin-R | GCTGAGTGAGGCTAGGATGG | |
| Bgt-EF1a-F | GTCGGATTTAACCCCAAGGT | Reference gene of Bgt |
| Bgt-EF1a-R | TTTATCGGTAGGGCGACTTG | |
| Ggt-18S rRNA-F | CGAACTCGGTCGTTTAGAGG | Reference gene of Ggt |
| Ggt-18S rRNA-R | GGTATGTTCACAGGGGTTGG |
4.4. DNA and RNA Extraction and Quantitative Real-Time PCR
To evaluate the transcript levels of BEC1019 produced in response to Bgt and Ggt infection, wheat Aikang 58 leaves were sampled at 0, 3, 6, 12, 24, 48, 72, and 120 h post-infection (hpi) with Bgt and wheat Zhoumai 22 base stems and roots at 0, 2, 3, 4, 5, 6, and 7 days post-infection (dpi) with Ggt. To quantify fungal biomass, we used Bgt and Ggt DNA extracted from inoculated wheat leaves, using a DNeasy plant mini kit (QIAGEN, Dusseldorf, Germany). Total RNA used for the quantification of transcript levels was extracted from wheat leaves using TRIzol reagent (Life Technologies, Grand Island, NY, USA) according to the manufacturer’s instructions. The extracted RNA was treated with RNase-free DNase I (Takara Bio Inc., Shiga, Japan) to remove contaminant genomic DNA. First-strand complementary DNA (cDNA) was synthesized using a PrimeScript RT Perfect Real Time reagent kit (Takara Bio Inc., Shiga, Japan). The synthesized cDNAs were used as templates in the following PCRs. Quantitative reverse transcription PCR (qRT-PCR) was performed using the CFX96™ real-time PCR detection system (Bio-Rad, Hercules, CA, USA) with SYBR® Premix Ex Taq™ (Tli RNaseH Plus; Takara Bio Inc., Shiga, Japan). The PCR program used was as follows: 95 °C for 3 min, followed by 40 cycles at 95 °C for 10 s and 60 °C for 30 s. The ef-1α gene of Bgt (HM538432) and 18S rRNA gene of Ggt (FJ771002) were used for fungal quantification [50,51], and the TaActin (KC775780) gene was used as the wheat internal reference for normalization. The transcript levels of BEC1019 and the other genes were examined and fungal biomass were calculated using the 2−ΔΔCt method [52]. All assays for a particular gene were performed synchronously in triplicate under identical conditions, and all reactions had three biological replicates. The sequences of the primers used for the qRT-PCR are listed in Table 1.
4.5. Detection of H2O2 and Cell Death
H2O2 was detected histochemically using the 3,3-diaminobenzidine (DAB; Aladdin, Shanghai, China) staining method according to Thordal-Christensen et al. [53]. Leaves at 3 dpi with Bgt were stained with 1 mg/mL DAB dissolved in HCl-acidified (pH 3.8) distilled water for 6–8 h. Chlorophyll was extracted overnight in acetic acid:ethanol solution (3:1), and the leaves were stored in 50% glycerol. Leaves at 24 hpi with Bgt were stained with 0.4% trypan blue solution at 90 °C for 1 min. Chlorophyll was extracted in acetic acid:ethanol solution (3:1) at 37 °C, and the leaves were stored in 50% glycerol for subsequent observation [36].
N. benthamiana leaves were used to visualize cell death using the trypan blue staining method [36]. The CTAPi-GW-3HA-BEC1019 and CTAPi-GW-3HA-BEC1019-RNAi vectors were transformed into A. tumefaciens GV3101, and on reaching an OD600 of 0.2–0.5, the A. tumefaciens GV3101 cells carrying either of the two aforementioned vectors were infiltrated into N. benthamiana leaves. The leaves were sampled at 5 dpi and boiled for 5 min in trypan blue staining solution (20 mL lactic acid, 20 mL glycerol, 20 g phenol, and 20 mg trypan blue, dissolved in 20 mL distilled water), and subsequently de-stained in 2.5 g/mL chloral hydrate for 2–3 days.
4.6. Responses of Transgenic Wheat Plants to Bgt and Ggt
Overexpressing and RNAi transgenic Aikang 58 wheat leaves were inoculated with Bgt and the subsequently infected leaves were microscopically analyzed using previously described procedures [24], with minor modifications. Leaf segments were collected from three 1-week-old transgenic wheat lines and placed on a 1% agar plate containing 85 µM benzimidazole. These were incubated in a 20 °C climate chamber for more than 4 h and then inoculated with Bgt spores by gently spreading fresh conidia on the leaves at an appropriate density (~250 conidiospores per·cm2 of leaf). Bgt was allowed to develop on the leaves for 60 h, and then the leaf segments were fixed in ethanol/acetic acid (1:1, v/v), stained with Coomassie brilliant blue R250 in methanol (0.6%, w/v) for 10 s, rinsed in deionized water, and observed and enumerated under a BX61 upright microscope (Olympus, Tokyo, Japan). More than 1000 germinated spores on each leaf segment from each plant were observed. The phenotypes of the inoculated transgenic leaves were observed and photographed using a Canon EOS 600D camera (Canon Corp., Tokyo, Japan) at 7 dpi.
Ggt inoculation was based on the method described by Liu et al. [46]. Fresh plugs (4 × 4 cm2) containing Ggt colonies were placed on the surface of sterilized soil, and then 1-week-old seedlings of transgenic Zhoumai 26 were placed on top of the Ggt plugs and covered with 3 cm of soil. The plants were cultured in a climate box under a 16-h light (22 °C)/8-h dark (16 °C) regime at 60% relative humidity. For microscopic observations of Ggt fungal growth, the base sheaths of the seedlings were harvested at 21 dpi, fixed with 1:1 (v/v) ethanol/acetic acid for 24 h, stained with trypan blue for 6 h, and examined under a BX61 microscope.
4.7. Data Analysis
Data were statistically analyzed using an independent-sample t-test and one-way analysis of variance (ANOVA) followed by post hoc comparisons using the least significant difference (LSD) and Duncan tests (p ≤ 0.01). All analyses were performed using the statistical package for the social sciences (SPSS) version 17 following the instructions in the Survival Manual.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/18/4376/s1.
Author Contributions
Y.Z. (Yi Zhang), C.L., and T.G. conceived and designed the research; Y.Z. (Yi Zhang), Z.L., C.P., Y.D., Y.Z. (Yazhen Zhang), and P.T. performed the experiments; D.Y., K.X., J.Z., and X.L., provided fungal materials, reagents, and analytical tools; Y.Z. (Yi Zhang) analyzed the data, prepared figures, and wrote the paper; all authors reviewed the paper.
Funding
This research was financially supported by the National Natural Science Foundation of China (grant nos. 31571997, 31872129 and 31902030), Natural Science Foundation of Henan province (grant no. 182300410058), Foundation of Henan Science and Technology Committee (grant nos. 192102110001 and 192102110124), Foundation of Henan Educational Committee (grant no. 20A180034), and Training Program of Youth Backbone Teacher of Henan Province (grant no. 2017GGJS146).
Conflicts of Interest
The authors declare no conflict of interests. The reference 33 in the manuscript was retracted by the author in 2018 but the relevant experiments with our study were unaffected in their original study, which was mentioned in the reference 34 “A confounding effect of bacterial titer in a type III delivery-based assay of eukaryotic effector function”.
References
- 1.Godfray H.C., Beddington J.R., Crute I.R., Haddad L., Lawrence D., Muir J.F., Pretty J., Robinson S., Thomas S.M., Toulmin C. Food security: The challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 2.Appels R., Eversole K., Feuillet C., Keller B., Rogers J., Stein N., Pozniak C.J., Choulet F., Distelfeld A., Poland J., et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361:eaar7191. doi: 10.1126/science.aar7191. [DOI] [PubMed] [Google Scholar]
- 3.Savary S., Willocquet L., Pethybridge S.J., Esker P., McRoberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 4.Lo Presti L., Lanver D., Schweizer G., Tanaka S., Liang L., Tollot M., Zuccaro A., Reissmann S., Kahmann R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015;66:513–545. doi: 10.1146/annurev-arplant-043014-114623. [DOI] [PubMed] [Google Scholar]
- 5.Shen Q., Liu Y., Naqvi N.I. Fungal effectors at the crossroads of phytohormone signaling. Curr. Opin. Microbiol. 2018;46:1–6. doi: 10.1016/j.mib.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Macho A.P., Cyril Z. Plant PRRs and the activation of innate immune signaling. Mol. Cell. 2014;54:263–272. doi: 10.1016/j.molcel.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 8.Thomas B., Yang H.S. Innate immunity in plants: An arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–744. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J., Zhou J.M. Plant immunity triggered by microbial molecular signatures. Mol. Plant. 2010;3:783–793. doi: 10.1093/mp/ssq035. [DOI] [PubMed] [Google Scholar]
- 10.Jacob F., Vernaldi S., Maekawa T. Evolution and conservation of plant NLR functions. Front. Immuno. 2013;4:297. doi: 10.3389/fimmu.2013.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stella C., Maud B., Philippe M., Thomas K., Dodds P.N. A novel conserved mechanism for plant NLR protein pairs: The “integrated decoy” hypothesis. Front. Plant Sci. 2014;5:606. doi: 10.3389/fpls.2014.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stergiopoulos I., Wit P.J.G.M.D. Fungal effector proteins. Annu. Rev. Phytopathol. 2009;47:233. doi: 10.1146/annurev.phyto.112408.132637. [DOI] [PubMed] [Google Scholar]
- 13.Bithell S.L., McKay A.C., Butler R.C., Cromey M.G. Consecutive wheat sequences: Effects of contrasting growing seasons on concentrations of Gaeumannomyces graminis var. tritici DNA in soil and take-all disease across different cropping sequences. J. Agric. Sci. 2016;154:472–486. [Google Scholar]
- 14.Gutteridge R.J., Bateman G.L., Todd A.D. Variation in the effects of take-all disease on grain yield and quality of winter cereals in field experiments. Pest Manag. Sci. 2003;59:215–224. doi: 10.1002/ps.574. [DOI] [PubMed] [Google Scholar]
- 15.Guilleroux M., Osbourn A. Gene expression during infection of wheat roots by the ‘take-all’ fungus Gaeumannomyces graminis. Mol. Plant Pathol. 2004;5:203–216. doi: 10.1111/j.1364-3703.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu B., Huang L., Kang Z., Buchenauer H. Evaluation of endophytic bacterial strains as antagonists of take-all in wheat caused by Gaeumannomyces graminis var. tritici in greenhouse and field. J. Pest Sci. 2011;84:257–264. [Google Scholar]
- 17.Barret M., Frey-Klett P., Guillerm-Erckelboudt A.Y., Boutin M., Guernec G., Sarniguet A. Effect of wheat roots infected with the pathogenic fungus Gaeumannomyces graminis var. tritici on gene expression of the biocontrol bacterium Pseudomonas fluorescens Pf29Arp. Mol. Plant-Microbe Interact. 2009;22:1611–1623. doi: 10.1094/MPMI-22-12-1611. [DOI] [PubMed] [Google Scholar]
- 18.Kang X., Zhang W., Cai X., Zhu T., Xue Y., Liu C. Bacillus velezensis CC09: A potential ‘vaccine’ for controlling wheat diseases. Mol. Plant-Microbe Interact. 2018;31:623–632. doi: 10.1094/MPMI-09-17-0227-R. [DOI] [PubMed] [Google Scholar]
- 19.Wang M., Xing Y., Wang J., Xu Y., Wang G. The role of the chi1 gene from the endophytic bacteria Serratia proteamaculans 336x in the biological control of wheat take-all. Can. J. Microbiol. 2014;60:533–540. doi: 10.1139/cjm-2014-0212. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D.D., Guo X.J., Wang Y.J., Gao T.G., Zhu B.C. Novel screening strategy reveals a potent Bacillus antagonist capable of mitigating wheat take-all disease caused by Gaeumannomyces graminis var. tritici. Lett. Appl. Microbiol. 2017;65:512–519. doi: 10.1111/lam.12809. [DOI] [PubMed] [Google Scholar]
- 21.Panstruga R. Establishing compatibility between plants and obligate biotrophic pathogens. Curr. Opin. Plant Biol. 2003;6:320–326. doi: 10.1016/S1369-5266(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 22.Kamoun S. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu. Rev. Phytopathol. 2006;44:41–60. doi: 10.1146/annurev.phyto.44.070505.143436. [DOI] [PubMed] [Google Scholar]
- 23.Thomma B.P.H.J., Thorsten N., Joosten M.H.A.J. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell. 2011;23:4–15. doi: 10.1105/tpc.110.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian-Hua S., Yusuke S., Stefan M., Christoph B., Stéphane B., Beat K., Hikaru S., Bekir U., Somssich I.E., Paul S.L. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science. 2007;315:1098–1103. doi: 10.1126/science.1136372. [DOI] [PubMed] [Google Scholar]
- 25.Ridout C., Skamnioti P., Porritt O., Sacristan S., Jones J.D.G., Brown J. Multiple avirulence paralogues in cereal powdery mildew fungi may contribute to parasite fitness and defeat of plant resistance. Plant Cell. 2006;18:2402–2414. doi: 10.1105/tpc.106.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu X., Kracher B., Saur I.M.L., Bauer S., Ellwood S.R., Wise R., Yaeno T., Maekawa T., Schulze-Lefert P. Allelic barley MLA immune receptors recognize sequence-unrelated avirulence effectors of the powdery mildew pathogen. Proc. Natl. Acad. Sci. USA. 2016;113:E6486–E6495. doi: 10.1073/pnas.1612947113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spanu P.D., Abbott J.C., Amselem J., Burgis T.A., Soanes D.M., Stuber K., Ver Loren van Themaat E., Brown J.K., Butcher S.A., Gurr S.J. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science. 2010;330:1543–1546. doi: 10.1126/science.1194573. [DOI] [PubMed] [Google Scholar]
- 28.Wicker T., Oberhaensli S., Parlange F., Buchmann J.P., Shatalina M., Roffler S., Ben-David R., Dolezel J., Simkova H., Schulze-Lefert P. The wheat powdery mildew genome shows the unique evolution of an obligate biotroph. Nat. Genet. 2013;45:1092–1096. doi: 10.1038/ng.2704. [DOI] [PubMed] [Google Scholar]
- 29.Ali Abdurehim A., Carsten P., Torsten S.L., Mark K., JoRgensen H.J.L., Hans T.C. The barley powdery mildew candidate secreted effector protein CSEP0105 inhibits the chaperone activity of a small heat shock protein. Plant Physiol. 2015;168:321–333. doi: 10.1104/pp.15.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W.J., Pedersen C., Kwaaitaal M., Gregersen P.L., Mørch S.M., Hanisch S., Kristensen A., Fuglsang A.T., Collinge D.B., Thordal-Christensen H. Interaction of barley powdery mildew effector candidate CSEP0055 with the defence protein PR17c. Mol. Plant Pathol. 2012;13:1110–1119. doi: 10.1111/j.1364-3703.2012.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pliego C., Nowara D., Bonciani G., Gheorghe D.M., Xu R., Surana P., Whigham E., Nettleton D., Bogdanove A.J., Wise R.P. Host-induced gene silencing in barley powdery mildew reveals a class of ribonuclease-like effectors. Mol. Plant Microbe Interact. 2013;26:633–642. doi: 10.1094/MPMI-01-13-0005-R. [DOI] [PubMed] [Google Scholar]
- 32.Nowara D., Gay A., Lacomme C., Shaw J., Ridout C., Douchkov D., Hensel G., Kumlehn J., Schweizer P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell. 2010;22:3130–3141. doi: 10.1105/tpc.110.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whigham E., Qi S., Mistry D., Surana P., Xu R., Fuerst G., Pliego C., Bindschedler L.V., Spanu P.D., Dickerson J.A. Broadly conserved fungal effector BEC1019 suppresses host cell Death and enhances pathogen virulence in powdery mildew of barley (Hordeum vulgare L.) Mol. Plant Microbe Interact. 2015;28:968–983. doi: 10.1094/MPMI-02-15-0027-FI. [DOI] [PubMed] [Google Scholar]
- 34.Carter M.E., Bogdanove A.J., Roger I.W., Roger W.P. A confounding effect of bacterial titer in a type III delivery-based assay of eukaryotic effector function. Mol. Plant-Microbe Interact. 2018;31:1115–1116. doi: 10.1094/MPMI-05-18-0128-LE. [DOI] [PubMed] [Google Scholar]
- 35.Both M., Csukai M., Stumpf M.P., Spanu P.D. Gene expression profiles of Blumeria graminis indicate dynamic changes to primary metabolism during development of an obligate biotrophic pathogen. Plant Cell. 2005;17:2107–2122. doi: 10.1105/tpc.105.032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai S., Liu J., Chang C., Zhang L., Maekawa T., Wang Q., Xiao W., Liu Y., Chai J., Takken F.L. Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog. 2012;8:e1002752. doi: 10.1371/journal.ppat.1002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguilar G.B., Pedersen C., Thordal-Christensen H. Identification of eight effector candidate genes involved in early aggressiveness of the barley powdery mildew fungus. Plant Pathol. 2016;65:953–958. doi: 10.1111/ppa.12476. [DOI] [Google Scholar]
- 38.Qi T., Zhu X., Tan C., Liu P., Guo J., Kang Z. Host-induced gene silencing of an important pathogenicity factor PsCPK1 in Puccinia striiformis f. sp. tritici enhances resistance of wheat to stripe rust. Plant Biotechnol. J. 2018;16:797–807. doi: 10.1111/pbi.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng W., Song X.S., Li H.P., Cao L.H., Sun K., Qiu X.L., Xu Y.B., Yang P., Huang T., Zhang J.B. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 2015;13:1335–1345. doi: 10.1111/pbi.12352. [DOI] [PubMed] [Google Scholar]
- 40.Debroy S., Thilmony R., Kwack Y.B., Nomura K., He S.Y. A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proc. Natl. Acad. Sci. USA. 2004;101:9927–9932. doi: 10.1073/pnas.0401601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y.J., Lin N.C., Martin G.B. Two distinct Pseudomonas effector proteins interact with the pto kinase and activate plant immunity. Cell. 2002;109:589–598. doi: 10.1016/S0092-8674(02)00743-2. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed A.A., Pedersen C., Thordal-Christensen H. The barley powdery mildew effector candidates CSEP0081 and CSEP0254 promote fungal infection success. PLoS ONE. 2016;11:e0157586. doi: 10.1371/journal.pone.0157586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourras S., McNally K.E., Ben-David R., Parlange F., Roffler S., Praz C.R., Oberhaensli S., Menardo F., Stirnweis D., Frenkel Z. Multiple avirulence loci and allele-specific effector recognition control the Pm3 race-specific resistance of wheat to powdery mildew. Plant Cell. 2015;27:2991–3012. doi: 10.1105/tpc.15.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Praz C.R., Bourras S., Zeng F., Sanchez-Martin J., Menardo F., Xue M., Yang L., Roffler S., Boni R., Herren G. AvrPm2 encodes an RNase-like avirulence effector which is conserved in the two different specialized forms of wheat and rye powdery mildew fungus. New Phytol. 2017;213:1301–1314. doi: 10.1111/nph.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pennington H.G., Gheorghe D.M., Damerum A., Pliego C., Spanu P.D., Cramer R., Bindschedler L.V. Interactions between the powdery mildew effector BEC1054 and barley proteins identify candidate host targets. J. Proteome Res. 2016;15:826–839. doi: 10.1021/acs.jproteome.5b00732. [DOI] [PubMed] [Google Scholar]
- 46.Liu X., Yang L., Zhou X., Zhou M., Lu Y., Ma L., Ma H., Zhang Z. Transgenic wheat expressing Thinopyrum intermedium MYB transcription factor TiMYB2R-1 shows enhanced resistance to the take-all disease. J. Exp. Bot. 2013;64:2243–2253. doi: 10.1093/jxb/ert084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rong W., Qi L., Wang J., Du L., Xu H., Wang A., Zhang Z. Expression of a potato antimicrobial peptide SN1 increases resistance to take-all pathogen Gaeumannomyces graminis var. tritici in transgenic wheat. Funct. Integr. Genom. 2013;13:403–409. doi: 10.1007/s10142-013-0332-5. [DOI] [PubMed] [Google Scholar]
- 48.Wang A., Wei X., Rong W., Dang L., Du L.P., Qi L., Xu H.J., Shao Y., Zhang Z. GmPGIP3 enhanced resistance to both take-all and common root rot diseases in transgenic wheat. Funct. Integr. Genom. 2015;15:375–381. doi: 10.1007/s10142-014-0428-6. [DOI] [PubMed] [Google Scholar]
- 49.Liu J., Cheng X., Liu D., Xu W., Wise R., Shen Q.H. The miR9863 family regulates distinct Mla alleles in barley to attenuate NLR receptor-triggered disease resistance and cell-death signaling. PLoS Genet. 2014;10:e1004755. doi: 10.1371/journal.pgen.1004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker A.S., Bouguennec A., Confais J., Morgant G., Leroux P. Evidence of host-range expansion from new powdery mildew (Blumeria graminis) infections of triticale (×Triticosecale) in France. Plant Pathol. 2011;60:207–220. doi: 10.1111/j.1365-3059.2010.02379.x. [DOI] [Google Scholar]
- 51.Daval S., Lebreton L., Gazengel K., Boutin M., Guillerm-Erckelboudt A.Y., Sarniguet A. The biocontrol bacterium Pseudomonas fluorescens Pf29Arp strain affects the pathogenesis-related gene expression of the take-all fungus Gaeumannomyces graminis var. tritici on wheat roots. Mol. Plant Pathol. 2011;12:839–854. doi: 10.1111/j.1364-3703.2011.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 53.Thordal-Christensen H., Zhang Z., Wei Y., Collinge D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 2010;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.