Abstract
Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) have been proposed as universal signaling molecules in plant stress responses. There are a growing number of studies suggesting that hydrogen sulfide (H2S) or Reactive Sulfur Species (RSS) are also involved in plant abiotic as well as biotic stress responses. However, it is still a matter of debate as to how plants utilize those RSS in their signaling cascades. Here, we demonstrate that d-cysteine is a novel candidate for bridging our gap in understanding. In the genus of the tiny water-floating fern Azolla, a rapid root abscission occurs in response to a wide variety of environmental stimuli as well as chemical inducers. We tested five H2S chemical donors, Na2S, GYY4137, 5a, 8l, and 8o, and found that 5a showed a significant abscission activity. Root abscission also occurred with the polysulfides Na2S2, Na2S3, and Na2S4. Rapid root abscission comparable to other known chemical inducers was observed in the presence of d-cysteine, whereas l-cysteine showed no effect. We suggest that d-cysteine is a physiologically relevant substrate to induce root abscission in the water fern Azolla.
Keywords: abscission, Azolla, d-amino acid, d-cysteine, H2S, polysulfide, stress response
1. Introduction
Plants sense environmental conditions and transmit the environmental signals to regulate their growth and development. It is a long standing question as to how plants sense and transmit a variety of environmental signals without nervous systems [1]. Stomatal movement is one of the rapid responses of plants, both opening and closure movements finish within an hour [2]. Because of this advantage, the stomatal movement has been a model system to be investigated for many years to answer this question [3].
It has been revealed that hydrogen peroxide (H2O2) is involved in the stomatal closing response [4]. H2O2 belongs to Reactive Oxygen Species (ROS) which can be produced in abiotic as well as biotic stress conditions [5]. Later, the Reactive Nitrogen Species (RNS) nitric oxide (NO) was found to induce stomatal movements [6,7]. The potential for cross talk between ROS and RNS has become a subject of debate regarding the signaling mechanism in guard cells [8,9]. Early in this century, Reactive Sulfur Species (RSS) was hypothesized as the third group of redox-active molecular species that may be associated with oxidative stress [1,10,11]. In mammalian studies, there is an increasing number of reports suggesting regulatory functions of H2S or RSS in cellular signaling mechanisms [12]. Accordingly, it has recently been reported that H2S also influences stomatal movements [13]. To date, however, there seems to be contradictory results for the functions of H2S in stomatal movements [14].
Researchers may experience technical difficulties in conducting H2S experiments with land plants. In whole plant experiments, H2S has been delivered by fumigation of the gas [15], spraying of an NaHS solution [16,17], or inclusion of NaHS into a culture medium [18]. Pharmacological comparative studies may be difficult to conduct with those methods. To overcome such technical problems in plant H2S study, we suggest here the application of the water-floating fern Azolla, a good model plant to explore the physiological functions of chemical compounds [19,20,21].
Plants of the genus Azolla have been used for agriculture in East Asia as green manure [22]. Biological research on Azolla has a long history in botany [23], cell biology [24,25], and phytoremediation [26,27]. It has long been known that the roots of Azolla pinnata are deciduous [28]. In 1993, Kitoh and co-workers found that nitrite (NO2−) and volatile organic acids such as acetate or propionate contained in swine waste water cause root shedding in Azolla filiculoides [29]. Uheda and Kitoh experimentally reproduced the root shedding with a variety of inhibitors, such as the hemeprotein inhibitors sodium azide (NaN3) and sodium cyanide (NaCN), the uncouplers 2,4-dinitrophenol (DNP) and carbonyl cyanide m-chlorophenyhydrazone (CCCP), and the F0-F1 ATPase inhibitor N′,N′-dicyclohexylcarbodiimide (DCCD) [30]. The root shedding, caused by cell expansion in the abscission zone, was finished within an hour, a speed much faster than conventional abscission phenomena, which usually requires days. Since the cycloheximide treatment did not inhibit the root abscission induced by NaN3, they suggested that rapid root abscission does not need new protein synthesis, offering a novel type of plant abscission phenomenon [30].
Taking progress in NO studies into account, we speculated that NO production in cells treated with NO2−, NaN3, and DNP might have a role in the rapid abscission phenomenon. Thus, we reinvestigated their observations in terms of NO and RNS. In fact, A. pinnata was found to emit NO in the presence of NaN3 and NO2− [21]. Nitrite is now appreciated as an endogenous NO substrate for plants [31] as well as animals [32,33]. Moreover, H2O2 was found to exhibit a bimodal effect on rapid root abscission [34]. We have proposed a model involving an interplay between RNS and ROS in initiating free radical attack of polysaccharides in the apoplast to account for the rapid root abscission mechanism in Azolla plants [35]. Here, we reported the effects of novel H2S donors and polysulfides in induction of rapid root abscission that suggests RSS also have a role in the abscission mechanism.
2. Materials and Methods
Laboratory cultures of Azolla pinnata R, Br. were established from plants collected in April 2018 from a paddy field in Ogimi, Okinawa, Japan. The plants were thoroughly washed to remove attached mud and debris. The plants were then treated with a solution of 0.12% sodium hypochlorite and 0.01% Triton X-100 for 30 min followed by repeated washings in a large volume of distilled water and finally transferred into nutrient culture medium [20]. A. pinnata was cultured in a two-fifth strength cobalt-supplemented nitrogen-source-free Hoagland’s E-medium [20]. Medium pH was adjusted to 5.8 with potassium hydroxide (KOH). Plants were grown in a plant growth chamber (Type FLI-2000 H, Eyla, Tokyo, Japan) maintained at 27 °C, 16:8 h light:dark photoperiod and 50 μmol m−2 s−1 (at plant level) provided by fluorescent lamps (Type FL 40 SBR-A, NEC, Tokyo, Japan). For experiments, fronds were randomly selected from the culture stock and de-rooted manually using forceps. Rootless fronds were placed in the culture medium after rinsing in distilled water and transferred to fresh mediums every 7 days.
Abscission assays were carried out using roots of equal age (i.e., from fronds that had been de-rooted at the same time, 7 days prior to preforming the assay). Three to six fronds (with 20–30 roots) were suspended in a beaker containing 20 mL 10 mM Hepes-KOH (pH 7) or the culture medium (pH 5.8). The abscission test was carried out at room temperature (24 °C) under room light. The chemicals to be tested, the H2S chemical donors Na2S, GYY4137, 5a, 8l, and 8o (Dojindo Laboratories, Kumamoto, Japan) and the polysulfides Na2S2, Na2S3, Na2S4 (Dojindo Laboratories, Kumamoto, Japan) were subsequently supplied as concentrated stock solutions according to the instruction manuals. The total number of dropped roots following addition of the chemicals was recorded. The abscission response was quantified as the ratio of the detached to the initial number of roots.
H2S gas was measured with a handheld O2/H2S monitor (XOS-326, New Cosmos Electric, Osaka, Japan). The H2S that was released into the headspace (10 mL) of a beaker was monitored for 30 s. The H2S releasing activity of each solution was expressed as ppm/min.
3. Results
3.1. Effects of H2S Donors on the Root Abscission in Azolla
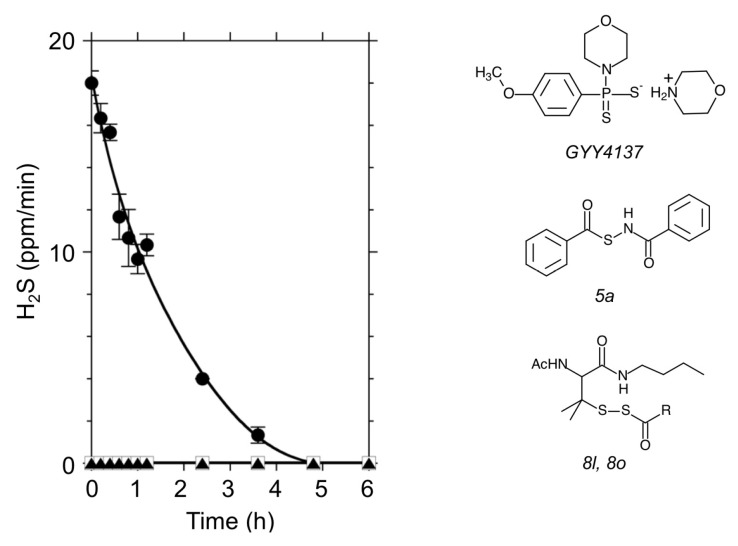
To investigate biological functions of H2S, the application of H2S donors would be the first choice for physiological experiments. Sodium hydrosulfide (NaHS) and sodium sulfide (Na2S) are inorganic compounds that release H2S by hydrolysis. Figure 1 shows a time course of H2S emission from a Na2S solution. After resolving Na2S into a Hepes-KOH buffer at pH 7.0, an abrupt release of H2S followed by its decay was observed (Figure 1). This rapid spontaneous reaction makes it difficult to obtain a stable H2S concentration for physiological purposes. To overcome this difficulty in application, the synthetic H2S donor GYY4137 has been used for stable delivery of H2S to cells in many studies. GYY4137 is a Lawesson’s reagent derivative that releases H2S via hydrolysis both in vivo and in vitro [36]. In 2011, Xian and co-workers discovered a series of N-(benzoylthio) benzamide derivatives that can be activated by thiols to release H2S [37,38]. The H2S donors 5a, 8l, and 8o are such new tools for exploring biological function of H2S. Under the conditions we used, unlike with Na2S, we did not measure detectable H2S release (ppm) into the air from either GYY4137 or 5a solutions (Figure 1).
Figure 1.
H2S gas release from chemical donors. Left panel shows time courses of H2S release from a solution containing 10 mM Hepes-KOH (pH 7.0). Abrupt production of H2S followed by its decay was observed with Na2S (black circle). Under the same conditions, H2S released into the air was negligible in GYY4137 (black triangle) and 5a (open square). The H2S donor concentration was 100 µM for each. Chemical structures of the H2S donors are illustrated on the right.
The water fern Azolla pinnata shows a characteristic rapid root abscission phenomenon in response to certain environmental stimuli. In laboratory experiments, a range of chemicals have been reported to be effective in abscising the roots, such as the uncouplers CCCP and DNP, the heme-binding NaN3 and KCN [21], the polyamines spermine and spermidine [20], the NO precursor NaNO2, and the NO donor spermine NONOate (SNN). Also, H2O2 appears to be involved in the abscission mechanism [34]. Since the rapid root abscission phenomenon is responsive to both the RNS NO and the ROS H2O2, it was logical to speculate that H2S or RSS might similarly exert an effect. To test this hypothesis, we compared the effects of five H2S donors: Na2S, GYY4137, 5a, 8l, and 8o on root abscission. Among the compounds tested, the H2S donor 5a was found to be effective in detaching the roots (Figure 2). Figure 2b demonstrates the root abscission induced by the H2S donor 5a at 200 µM. The detached end of a root abscised by the H2S donor 5a showed expanded cells within the abscission zone, which agrees with the morphological features of abscised roots as in previous reports [30,34,35].
Figure 2.
Photographs of root abscission in Azolla pinnata induced by the H2S donor 5a. (a) Photographs of a frond of A. pinnata. (b) Effect of 5a on the root abscission in 10 mM Hepes-KOH (pH 7.0). Photograph was taken 48 h after the addition of 5a. (−) In the absence of 5a (control), (+) in the presence of 200 µM 5a. (c) Expanded cells on the end of a detached root in an abscission zone. The white arrows indicate the expanded cells at end of the detached root.
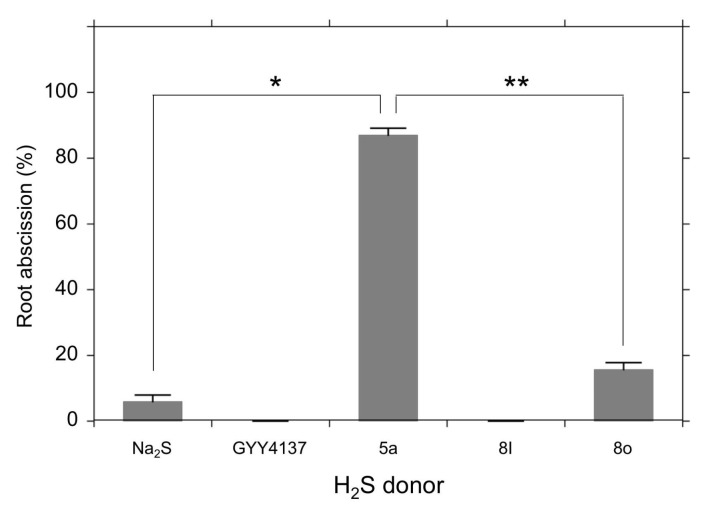
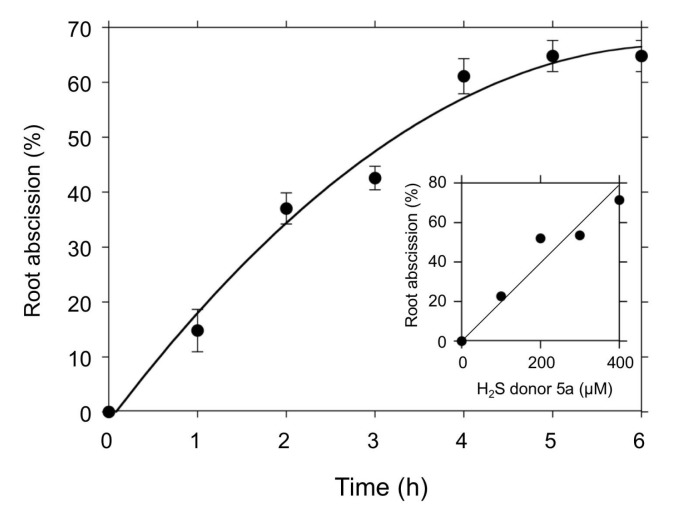
Figure 3 compares the effects of the H2S donors on the root abscission. With the same concentration (100 µM) and the same incubation time (24 h), the H2S donor 5a showed significantly higher root abscission among the five compounds. The effect of GYY4137 was negligible under the conditions used. The abscission induced by 5a was time dependent (Figure 4). With 100 µM 5a, the root abscission continued until 6 h and reached a plateau (Figure 4). Up to 400 µM, the effect of 5a showed concentration dependency (Figure 4, inset). Figure 3 and Figure 4 clearly demonstrate that the H2S donor 5a is a novel chemical compound that can induce the root abscission of Azolla.
Figure 3.
Effects of H2S donors on root abscission. Root abscission is represented as % of the detached roots incubated for 24 h with a 10 mM Hepes-KOH (pH 7.0) buffer containing the H2S donor (100 µM). The values are means ± SE (n = 3). Significant differences are indicated as * or ** (p < 0.001).
Figure 4.
Time course of the root abscission induced by the H2S donor 5a. The number of detached roots was counted every hour after the addition of the H2S donor 5a (100 µM) into a 10 mM Hepes-KOH (pH 7.0) buffer. Means ± SE (n = 3). Inset shows the 5a concentration dependence of the root abscission. The values are expressed as % of the detached roots after one-hour incubation.
3.2. Effects of Polysulfides on the Root Abscission
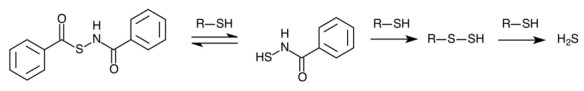
The H2S donors tested in this study released H2S by different mechanisms. Both Na2S and GYY4137 produce H2S by a spontaneous hydrolysis reaction. Although the final amount of H2S release may depend on pH, those types of H2S donors do not require specific conditions other than the presence of H2O. In contrast, the new types of H2S donors 5a, 8l, and 8o require the presence of thiols such as cysteine or reduced form of glutathione (GSH) [37]. In fact, no H2S emission from 5a in buffered solution was measured, as shown in Figure 1. The mechanisms for H2S release from 5a, 8l, and 8o are considered to be:
 |
(1) |
 |
(2) |
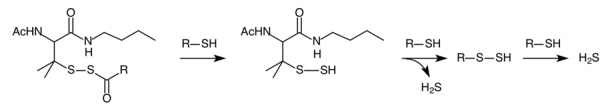
It is important to note that the H2S donor 5a (Equation (1)) as well as 8l and 8o (Equation (2)) decomposes with multiple steps. For each reaction, the presence of thiol (–SH) is required [37]. The difference in structure between 8l and 8o is the R moiety (8l: R = –CH3, 8o: R = –C(CH3)3). If the compound 5a, in fact, induced root abscission by its chemical reaction, the question arose as to whether the effect relied on H2S or on interactions with thiols within the cells. The minimal effect of the spontaneous H2S donor Na2S and GYY4137 on root abscission led us to check the actions of polysulfides.
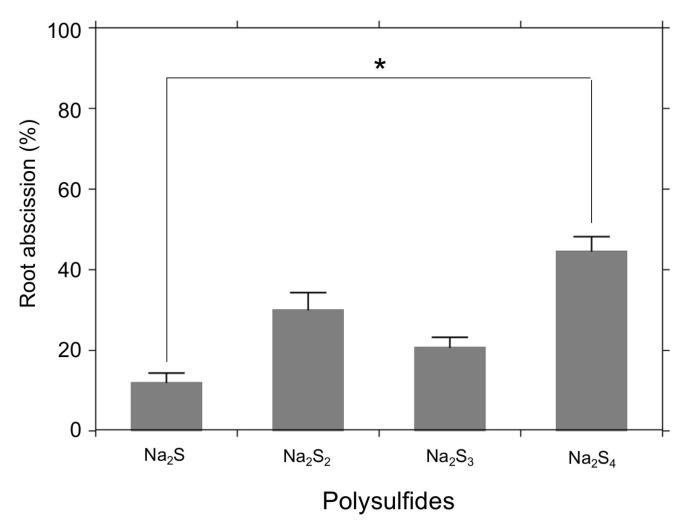
Figure 5 shows effects of sodium polysulfides (Na2Sn) on the root abscission. We compared sodium sulfide (Na2S), sodium disulfide (Na2S2), sodium trisulfide (Na2S3), and sodium tetrasulfide (Na2S4). All of these tested compounds showed some abscission-inducing effects, with the polysulfide Na2S4 being much more effective than Na2S in detaching the roots (Figure 5).
Figure 5.
Effects of polysulfides (Na2Sn) on root abscission. Root abscission is represented as % of the total number of the roots. Azolla was incubated for 24 h with a 10 mM Hepes-KOH (pH 7.0) buffer containing 500 µM polysulfide (Na2Sn). The values are means ± SE (n = 3). A significant difference is indicated as * (p < 0.05).
3.3. d-Cysteine-Induced Root Abscission
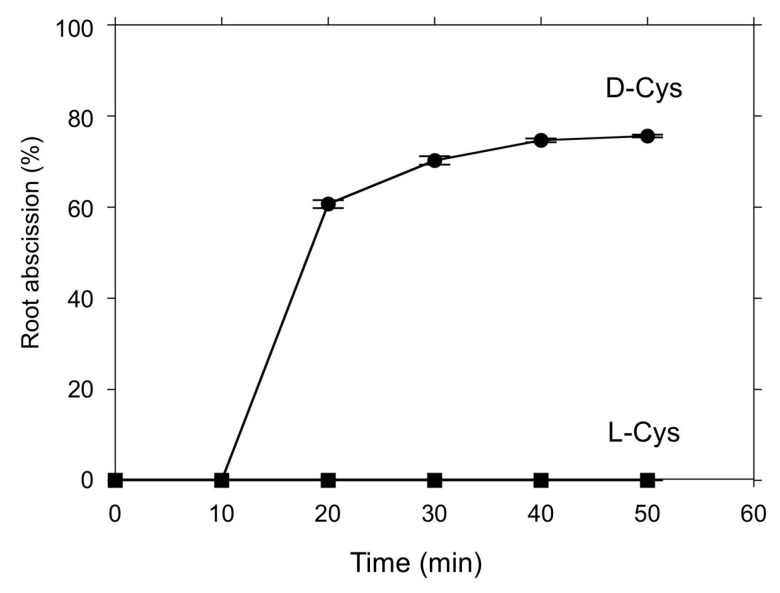
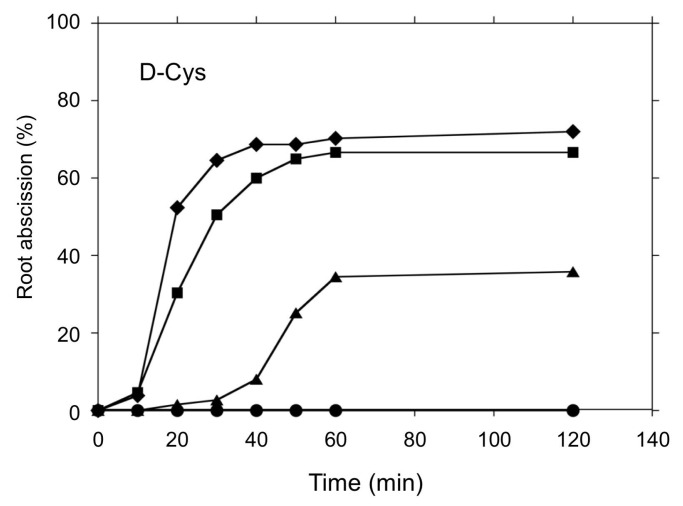
Although root abscission was induced by the H2S donor 5a (Figure 2 and Figure 3) and polysulfides (Figure 4), the abscission proceeded over the course of hours, much slower than for other previously reported chemical inducers [20,30,34]. There are increasing numbers of reports suggesting that l/d-cysteine could be an endogenous substrate for H2S synthesis in redox signaling mechanisms [39]. Thus, we tested the effects of l- and d-cysteine on root abscission in Azolla. Interestingly, d-cysteine, but not l-cysteine, was found to be efficient in inducing rapid root abscission (Figure 6). Unlike the case of the H2S donor 5a or polysulfides, the abscission finished less in than an hour, which is comparable to the effects of other chemical inducers. The effect of d-cysteine showed concentration dependence (Figure 7). The initial speed of the abscission increased with the d-cysteine concentration.
Figure 6.
Time course of the d-cysteine-induced root abscission in A. pinnata. 3 mM of either d-cysteine (d-Cys) or l-cysteine (l-Cys) was added into the culture medium (pH 5.8). Means ± SE (n = 5).
Figure 7.
Concentration dependence of d-cysteine on root abscission. d-cysteine at 0.65, 1.25, 2.5, and 5 mM (from the bottom to top) was added into the culture medium (pH 5.8). Means ± SE (n = 5).
4. Discussion
4.1. Application of Chemical H2S Donors for Inducing Root Abscission
To explore biological functions of H2S, physiological experiments with H2S chemical donors have been conducted both in plants [13] and mammals [40]. Since Na2S and NaHS are relatively cheap, these inorganic H2S donors can be applied for large-scale experiments using whole plants [18]. As shown in Figure 1, however, the H2S releasing activity of Na2S quickly decays such that it is virtually impossible to maintain a stable concentration within a physiological range. Moreover, the inorganic H2S donors are extremely moisture sensitive and they are easily oxidized in the presence of O2. To compensate for these technical difficulties, many synthetic new H2S donors have been developed. In plant science, GYY4137 has been employed to demonstrate physiological functions of H2S [13]. We expected that GYY4137 should induce root abscission, but found it does not have a significant abscission inducing activity even at mM concentrations (data not shown). The root abscission activity of Na2S was also weak. The novel H2S donors 5a, 8l, and 8o need cysteine or GSH for releasing H2S into an aqueous phase [38]. Because of this nature, we had not expected the abscission inducing activity of such thiol-activated type of H2S donors. However, the H2S donor 5a showed reasonably good abscission activity at sub mM concentrations (Figure 4).
Although recent studies have highlighted the “positive” regulatory functions of H2S, the molecule is yet cytotoxic and potentially inactivates metalloenzymes. The application of high concentrations of H2S from outside of the plants may disturb many enzymatic reactions or metabolisms that are required for the initiation of specific physiological events. We speculate that the structure of 5a bearing aromatic rings facilitates an efficient delivery of the compound to the target due to its lipophilic nature. The requirement of thiols (cysteine or GSH) could further localize the H2S production by the compound, thereby minimizing the negative impact of H2S. The difference in the effect between 8o and 8l could be also explained by difference in the polarity of the R moiety (Figure 1).
4.2. Effects of Polysulfides (Na2Sn) on the Root Abscission
Recent progress in mammalian H2S studies has strongly suggested that potential chemical entities regulating biological functions of RSS are polysulfur species (H2Sn), rather than H2S itself [40]. A growing number of reports have supported the participation of polysulfidation of cysteine thiols [41,42,43]. To test the involvement of polysulfidation, in this study we applied sodium polysulfides (Na2Sn) for the Azolla root abscission experiments. As shown in Figure 4, all polysulfides induced root abscission. The results may imply that polysulfidation of thiol(s) could be involved in the root abscission mechanism. In a good agreement with this aspect, we observed that S-methyl methane thiosulfonate (MMTS), which covalently sulfenylates the thiol of cysteine residues, inhibited nitrite-induced root abscission [34]. Moreover, excessive GSH exogenously added was reported to be suppressive to the nitrite-induced root abscission in A. pinnata [34]. It should be noted that the polysulfide effects required relatively higher concentrations and longer incubation time compared with the H2S donor 5a (Figure 3 and Figure 5). Since H2S emission (ppm) into the air was observed even in Na2S2, Na2S3, and Na2S4 solutions (data not shown), we suspect that effects of H2S might overlay the actions of the sodium polysulfides. Obviously, further confirmation is necessary to conclude the effects of Na2Sn have physiological relevance. Like with the H2S donor 5a, we may need to wait for new tools of synthetic polysulfides that can effectively mediate a local polysulfidation without spontaneous H2S production.
4.3. d-Cysteine is a Novel Inducer of Rapid Root Abscsision in Azolla
The present study has demonstrated that d-cysteine is a good inducer of rapid root abscission in A. pinnata (Figure 6 and Figure 7), a novel finding that provides an important clue to reveal the root abscission mechanism. d-amino acids had long been thought as a laboratory artifact. Only recently, their biological functions have come to be recognized [39]. In mammals, d-cysteine has been found to be the substrate for H2S synthesis catalyzed by 3-mercaptopyruvate sulfurtransferase (3MST) along with d-amino acid oxidase (DAO), namely, the 3MST/DAO pathway [44]. In plants, d-cysteine desulfhydrase (d-CDes) has been suggested to produce H2S [45]. The recently sequenced Azolla filiculoides genome encodes for a single d-CDes homolog [46]. d-cysteine may induce root abscission via a localized production of H2S that may react with an oxidized protein cysteine to form a hydropersulfide [35], or potentially by serving as a substrate for direct enzymatic formation of a persulfide on a cysteine thiol [40]. Interestingly, l-cysteine showed no abscission inducing activity (Figure 6). In Arabidopsis plants l-cysteine desulfhydrase activity has been shown to produce H2S in the plant cells [47]. It is of a great interest to speculate why Azolla does not respond to l-cysteine: is it either due to evolutional [48] or symbiotic [49] reasons?
5. Conclusions
Mammalian cells are surrounded by abundant hemeproteins such as blood hemoglobin and muscle myoglobin. Those proteins could maintain low local NO or H2S concentrations in the tissues, which is a necessary condition for NO or H2S acting as a signaling molecule [1]. In contrast, O2-evolving photosynthetic organisms, such as plants, produce H2O2, NO and H2S as the byproducts of the assimilation metabolisms under the light, particularly in stress conditions [39,50]. The lower hemeprotein content in plant tissue enables NO and H2S diffuse both in and out of the tissue, thereby permitting sensing of environmental conditions directly through those gases. Presumably, the ROS, RNS and RSS signals would be integrated at the functional thiol groups that are potentially modulated by those reactive molecular species in different forms [40]. In fact, the interplay among ROS, RNS, and RSS produce a variety of reactive products derived from thiols, such as nitrosothiol (SNO), thionitrous acid (HSNO), nitroxyl (HNO), nitropersulfide (SSNO−), and polysulfides H2Sn [40]. This study has, for the first time, demonstrated that d-cysteine is a strong inducer of rapid root abscission in A. pinnata. To reveal the molecular mechanism for the d-cysteine-induced root abscission, controlling the chemical redox reactions while monitoring those key molecules will be essential. As we experienced in NO studies [51], physiological experiments with gaseous H2S are difficult to handle, and it is sometimes hard to obtain good reproducibility. We suggest that Azolla is a good model system to explore RSS-mediated signaling mechanisms in plants because of its tiny size (advantageous for culture [29]), water floating nature (advantageous for pharmacological experiments [20,21]), and rapid response comparable to stomatal responses (advantageous for analysis [35]).
Acknowledgments
We are grateful to K. Kuroda for his technical support.
Author Contributions
Conceptualization, methodology, writing—original draft preparation, funding acquisition, H.Y.; investigation, M.P.O. and K.A.K.; editing, M.F.C.
Funding
This work was supported by JSPS KAKENHI Grant Number JP19K06339 to H.Y.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yamasaki H. The NO world for plants: Achieving balance in an open system. Plant Cell Environ. 2005;28:78–84. doi: 10.1111/j.1365-3040.2005.01297.x. [DOI] [Google Scholar]
- 2.Sakihama Y., Murakami S., Yamasaki H. Involvement of nitric oxide in the mechanism for stomatal opening in Vicia faba leaves. Biol. Plant. 2003;46:117–119. doi: 10.1023/A:1022378621030. [DOI] [Google Scholar]
- 3.Antoniou C., Savvides A., Christou A., Fotopoulos V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 2016;33:101–107. doi: 10.1016/j.pbi.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X., Zhang L., Dong F., Gao J., Galbraith D.W., Song C.-P. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001;126:1438–1448. doi: 10.1104/pp.126.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quan L.J., Zhang B., Shi W.W., Li H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008;50:2–18. doi: 10.1111/j.1744-7909.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- 6.Neill S.J., Desikan R., Clarke A., Hurst R.D., Hancock J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002;53:1237–1247. doi: 10.1093/jexbot/53.372.1237. [DOI] [PubMed] [Google Scholar]
- 7.Desikan R., Griffiths R., Hancock J., Neill S. A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desikan R., Cheung M.K., Bright J., Henson D., Hancock J.T., Neill S.J. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J. Exp. Bot. 2004;55:205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- 9.Ozgur R., Uzilday B., Iwata Y., Koizumi N., Turkan I. Interplay between the unfolded protein response and reactive oxygen species: A dynamic duo. J. Exp. Bot. 2018;69:3333–3345. doi: 10.1093/jxb/ery040. [DOI] [PubMed] [Google Scholar]
- 10.Giles G.I., Tasker K.M., Jacob C. Hypothesis: The role of reactive sulfur species in oxidative stress. Free Radic. Biol. Med. 2001;31:1279–1283. doi: 10.1016/S0891-5849(01)00710-9. [DOI] [PubMed] [Google Scholar]
- 11.Gruhlke M.C.H., Slusarenko A.J. The biology of reactive sulfur species (RSS) Plant Physiol. Biochem. 2012;59:98–107. doi: 10.1016/j.plaphy.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Li L., Rose P., Moore P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 13.Lisjak M., Srivastava N., Teklic T., Civale L., Lewandowski K., Wilson I., Wood M.E., Whiteman M., Hancock J.T. A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol. Biochem. 2010;48:931–935. doi: 10.1016/j.plaphy.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Lisjak M., Teklic T., Wilson I.D., Whiteman M., Hancock J.T. Hydrogen sulfide: Environmental factor or signalling molecule? Plant Cell Environ. 2013;36:1607–1616. doi: 10.1111/pce.12073. [DOI] [PubMed] [Google Scholar]
- 15.De Kok L., Bosma W., Maas F., Kuiper P. The effect of short-term H2S fumigation on water-soluble sulphydryl and glutathione levels in spinach. Plant Cell Environ. 1985;8:189–194. doi: 10.1111/1365-3040.ep11604605. [DOI] [Google Scholar]
- 16.Zhang H., Ye Y.-K., Wang S.-H., Luo J.-P., Tang J., Ma D.-F. Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regul. 2009;58:243–250. doi: 10.1007/s10725-009-9372-1. [DOI] [Google Scholar]
- 17.Min Y., Qin B.-P., Ping W., Li M.-L., Chen L.-L., Chen L.-T., Sun A.-Q., Wang Z.-L., Yin Y.-P. Foliar application of sodium hydrosulfide (NaHS), a hydrogen sulfide (H2S) donor, can protect seedlings against heat stress in wheat (Triticum aestivum L.) J. Integr. Agric. 2016;15:2745–2758. [Google Scholar]
- 18.Kaya C., Ashraf M., Akram N.A. Hydrogen sulfide regulates the levels of key metabolites and antioxidant defense system to counteract oxidative stress in pepper (Capsicum annuum L.) plants exposed to high zinc regime. Environ. Sci. Pollut. Res. 2018;25:1–7. doi: 10.1007/s11356-018-1510-8. [DOI] [PubMed] [Google Scholar]
- 19.Cohen M.F., Sakihama Y., Takagi Y.C., Ichiba T., Yamasaki H. Synergistic effect of deoxyanthocyanins from symbiotic fern Azolla spp. on hrmA gene induction in the cyanobacterium Nostoc punctiforme. Mol. Plant Microbe Interact. 2002;15:875–882. doi: 10.1094/MPMI.2002.15.9.875. [DOI] [PubMed] [Google Scholar]
- 20.Gurung S., Cohen M.F., Fukuto J., Yamasaki H. Polyamine-induced rapid root abscission in Azolla pinnata. J. Amino Acids. 2012;2012:493209. doi: 10.1155/2012/493209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurung S., Cohen M.F., Yamasaki H. Azide-dependent nitric oxide emission from the water fern Azolla pinnata. Russ. J. Plant Physiol. 2014;61:543–547. doi: 10.1134/S1021443714040086. [DOI] [Google Scholar]
- 22.Lumpkin T.A., Plucknett D.L. Azolla: Botany, physiology, and use as a green manure. Econ. Bot. 1980;34:111–153. doi: 10.1007/BF02858627. [DOI] [Google Scholar]
- 23.Leavitt R.G. The root-hairs, cap, and sheath of Azolla. Bot. Gaz. 1902;34:414–419. doi: 10.1086/328304. [DOI] [Google Scholar]
- 24.Gunning B., Hardham A., Hughes J. Evidence for initiation of microtubules in discrete regions of the cell cortex in Azolla root-tip cells, and an hypothesis on the development of cortical arrays of microtubules. Planta. 1978;143:161–179. doi: 10.1007/BF00387788. [DOI] [PubMed] [Google Scholar]
- 25.Gunning B., Hughes J., Hardham A. Formative and proliferative cell divisions, cell differentiation, and developmental changes in the meristem of Azolla roots. Planta. 1978;143:121–144. doi: 10.1007/BF00387786. [DOI] [PubMed] [Google Scholar]
- 26.Sela M., Tel-Or E., Fritz E., Huttermann A. Localization and toxic effects of cadmium, copper, and uranium in Azolla. Plant Physiol. 1988;88:30–36. doi: 10.1104/pp.88.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen M.F., Yamasaki H., Mazzola M. Bioremediation of soils by plant-microbe systems. Int. J. Green Energy. 2004;1:301–312. doi: 10.1081/GE-200033610. [DOI] [Google Scholar]
- 28.Rao H. The structure and life-history of Azolla pinnata R. Brown with remarks on the fossil history of the hydropterideæ. Proc. Plant Sci. 1935;2:175–201. [Google Scholar]
- 29.Kitoh S., Shiomi N., Uheda E. The growth and nitrogen fixation of Azolla filiculoides Lam. in polluted water. Aquat Bot. 1993;46:129–139. doi: 10.1016/0304-3770(93)90041-T. [DOI] [Google Scholar]
- 30.Uheda E., Kitoh S. Rapid shedding of roots from Azolla filiculoides plants in response to inhibitors of respiration. Plant Cell Physiol. 1994;35:37–43. [Google Scholar]
- 31.Yamasaki H., Sakihama Y., Takahashi S. An alternative pathway for nitric oxide production in plants: New features of an old enzyme. Trends Plant Sci. 1999;4:128–129. doi: 10.1016/S1360-1385(99)01393-X. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki H., Watanabe N.S., Fukuto J., Cohen M.F. Nitrite-dependent nitric oxide production pathway: Diversity of NO production systems. In: Tsukahara H., Kaneko K., editors. Studies on Pediatric Disorders, Oxidative Stress in Applied Basic Research and Clinical Practice. Springer; New York, NY, USA: 2014. pp. 35–54. [Google Scholar]
- 34.Cohen M.F., Gurung S., Birarda G., Holman H.Y.N., Yamasaki H. Bimodal effect of hydrogen peroxide and oxidative events in nitrite-induced rapid root abscission by the water fern Azolla pinnata. Front. Plant Sci. 2015;6:518. doi: 10.3389/fpls.2015.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen M.F., Gurung S., Fukuto J.M., Yamasaki H. Controlled free radical attack in the apoplast: A hypothesis for roles of O, N and S species in regulatory and polysaccharide cleavage events during rapid abscission by Azolla. Plant Sci. 2014;217:120–126. doi: 10.1016/j.plantsci.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W., Zhao Y., Baskar R., Tan C.-H., Moore P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137) Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y., Bhushan S., Yang C., Otsuka H., Stein J.D., Pacheco A., Peng B., Devarie-Baez N.O., Aguilar H.C., Lefer D.J. Controllable hydrogen sulfide donors and their activity against myocardial ischemia-reperfusion injury. ACS Chem. Biol. 2013;8:1283–1290. doi: 10.1021/cb400090d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y., Wang H., Xian M. Cysteine-activated hydrogen sulfide (H2S) donors. J. Am. Chem. Soc. 2010;133:15–17. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamasaki H., Cohen M.F. Biological consilience of hydrogen sulfide and nitric oxide in plants: Gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide. 2016;55:91–100. doi: 10.1016/j.niox.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Kimura H. Signaling by hydrogen sulfide (H2S) and polysulfides (H2Sn) in the central nervous system. Neurochem. Int. 2019;126:118–125. doi: 10.1016/j.neuint.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Takata T., Tsukuda A., Tsuchiya Y., Akaike T., Watanabe Y. The active-site cysteine residue of Ca2+/calmodulin-dependent protein kinase I is protected from irreversible modification via generation of polysulfidation. Nitric Oxide. 2019;86:68–75. doi: 10.1016/j.niox.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Ihara H., Kasamatsu S., Kitamura A., Nishimura A., Tsutsuki H., Ida T., Ishizaki K., Toyama T., Yoshida E., Hamid H.A. Exposure to electrophiles impairs reactive persulfide-dependent redox signaling in neuronal cells. Chem. Res. Toxicol. 2017;30:1673–1684. doi: 10.1021/acs.chemrestox.7b00120. [DOI] [PubMed] [Google Scholar]
- 43.Akaike T., Ida T., Wei F.-Y., Nishida M., Kumagai Y., Alam M.M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017;8:1177. doi: 10.1038/s41467-017-01311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibuya N., Koike S., Tanaka M., Ishigami-Yuasa M., Kimura Y., Ogasawara Y., Fukui K., Nagahara N., Kimura H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013;4:1366. doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 45.Papenbrock J., Riemenschneider A., Kamp A., Schulz-Vogt H.N., Schmidt A. Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants-from the field to the test tube and back. Plant Biol. 2007;9:582–588. doi: 10.1055/s-2007-965424. [DOI] [PubMed] [Google Scholar]
- 46.Azolla filiculoides Genome Encoding Azfi_s0002.g001593. [(accessed on 17 September 2019)]; Available online: https://www.fernbase.org/jbrowse_fernbase/?data=data%2Fjson%2FAzolla_asm_v1.1&loc=Azfi_s0002%3A9528035..9533485&tracks=DNA%2CGene%20models-high%20confidence&highlight=
- 47.Álvarez C., García I., Moreno I., Pérez-Pérez M.E., Crespo J.L., Romero L.C., Gotor C. Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell. 2012;24:4621–4634. doi: 10.1105/tpc.112.105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li F.-W., Brouwer P., Carretero-Paulet L., Cheng S., De Vries J., Delaux P.-M., Eily A., Koppers N., Kuo L.-Y., Li Z. Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nat. Plants. 2018;4:460. doi: 10.1038/s41477-018-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eily A.N., Pryer K.M., Li F.W. A first glimpse at genes important to the Azolla-Nostoc symbiosis. Symbiosis. 2019;78:149–162. doi: 10.1007/s13199-019-00599-2. [DOI] [Google Scholar]
- 50.Corpas F.J., Chaki M., Fernandez-Ocana A., Valderrama R., Palma J.M., Carreras A., Begara-Morales J.C., Airaki M., del Río L.A., Barroso J.B. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008;49:1711–1722. doi: 10.1093/pcp/pcn144. [DOI] [PubMed] [Google Scholar]
- 51.Yamasaki H., Watanabe N.S., Sakihama Y., Cohen M.F. An overview of methods in plant nitric oxide (NO) research: Why do we always need to use multiple methods? In: Gupta K.J., editor. Plant Nitric Oxide: Methods and Protocols. Springer; New York, NY, USA: 2016. pp. 1–14. [DOI] [PubMed] [Google Scholar]