Abstract
Persistent research over the past few decades has clearly established that the insulin-like family of growth factors, which is composed of insulin and insulin-like growth factors 1 (IGF1) and 2 (IGF2), plays essential roles in sexual development and reproduction of both males and females. Within the male and female reproductive organs, ligands of the family act in an autocrine/paracrine manner, in order to guide different aspects of gonadogenesis, sex determination, sex-specific development or reproductive performance. Although our knowledge has greatly improved over the last years, there are still several facets that remain to be deciphered. In this review, we first briefly outline the principles of sexual development and insulin/IGF signaling, and then present our current knowledge, both in rodents and humans, about the involvement of insulin/IGFs in sexual development and reproductive functions. We conclude by highlighting some interesting remarks and delineating certain unanswered questions that need to be addressed in future studies.
Keywords: INS/IGF signaling, sexual development, mammalian reproduction, steroidogenesis, Sertoli cell development
1. Introduction
From an evolutionary point of view, metabolism, growth and reproduction are tightly connected. Indeed, fertility reaches its full potential after completion of growth and puberty, and depends on the individual’s metabolic status (for a review, see [1]). Indicative of these intricate connections is the fact that excessive leanness or obesity in both men and women are associated with reproductive dysfunctions and hypogonadotropic hypogonadism [1].
The interconnection of metabolism, growth and reproduction is a direct result of common regulatory networks and signaling pathways [2]. In particular, the insulin-like family of growth factors (henceforth referred to as the insulin/IGF system) plays a pivotal role in the regulation of cell metabolism, growth, proliferation, differentiation and survival, and affects nearly every organ [3]; its constituents are important players in a network of biochemical events that link metabolic pathways, mitogenic processes and reproductive functions.
Remarkably, the insulin/IGF system is characterized by a high level of complexity due to the presence of multiple ligands, receptors and signaling pathways. In addition, the bioactivities of insulin, IGF1 and IGF2 depend on the concerted effects of a number of factors, including nutritional status, developmental stage, ligand biosynthesis, interactions with other hormonal systems, regulation of ligand bioavailability by IGFBPs (IGF binding proteins) and others. Dysregulation of the insulin/IGF axis has major pathological implications, ranging from metabolic disorders to growth deficits, cancer development and reproductive disorders. Importantly, although it has long been known that the reproductive capacity of an individual is regulated by the hypothalamic–pituitary–gonadal (HPG) axis [4], accumulating evidence has gradually revealed that the activity of local gonadal factors such as IGFs is also of prime importance for reproductive performance.
In this review, we focus our attention on the insulin/IGF system, whose extensive study has demonstrated its key roles in mediating several processes of adrenogonadal development and functions in mammals. First, we briefly describe the components of the insulin/IGF system; next, we provide a short overview of gonadogenesis, sex determination, and sex-specific development in males and females; then, we present gene expression and biological data that reveal the roles played by insulin/IGF signaling during sexual development and in mediating reproductive functions. In the final section, we conclude by outlining several questions that remain to be answered, as well as directions that need to be followed in future studies.
2. The Insulin/IGF System
The insulin/IGF system is composed of three members, including insulin and insulin-like growth factors 1 (IGF1) and 2 (IGF2) (Figure 1). IGFs are small, single-chain, mitogenic polypeptides that are structurally similar to proinsulin [5]. Locally produced IGFs are mainly involved in autocrine/paracrine activities, whereas circulating pancreas-produced insulin and liver-produced IGF1 mediate endocrine activities [6]. The bioavailability of IGF1 and IGF2 is regulated by a family of six high-affinity binding proteins (IGFBP1-6), which sequester IGFs or act as an IGF “reservoir” by increasing their half-life and allowing for their gradual release [7].
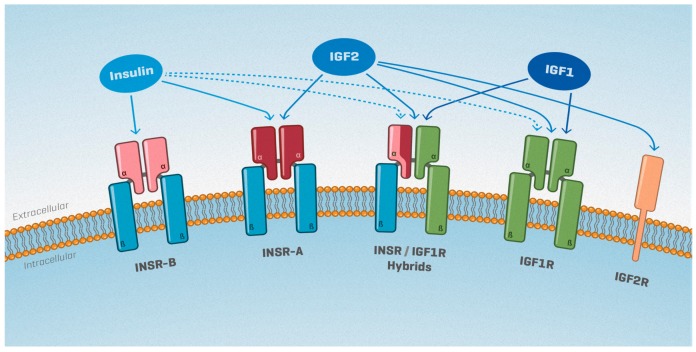
Figure 1.
The insulin/insulin-like growth factors (IGF) system: Ligand–receptor binding affinities. Insulin receptor (INSR) and insulin-like growth factor receptor (IGF1R) receptors are each composed of two αβ dimers. InsR has two splice variants, whose respective proteins (INSR-A and INSR-B) differ in their extracellular α subunit. INSR αβ dimers can bind to IGF1R αβ dimers, forming INSR/IGFR hybrid receptors. The M6P/IGF2R receptor is unrelated to INSR and IGF1R and lacks intrinsic tyrosine kinase activity. The relative binding affinities of each receptor to insulin, IGF1 and IGF2 are indicated by solid arrows (high affinity) or broken arrows (low affinity).
IGFs and insulin exert their physiological effects by activating two tyrosine kinase receptors, namely the type-1 insulin-like growth factor receptor (IGF1R) and the insulin receptor (INSR) (for a review, see [8]) (Figure 1). Each receptor is a heterotetrameric glycoprotein composed of two ligand-binding extracellular α subunits and two transmembrane β subunits. In addition, two alternative splicing isoforms of INSR exist: the fetal INSR-A isoform, which lacks exon 11 and mediates mitogenic effects [9], and the classical INSR-B isoform, which predominantly signals insulin’s metabolic activities. Of note, cells that co-express INSR and IGF1R can also form hybrid receptors composed of the α and β subunits of INSR-A or INSR-B bound to the α and β subunits of IGF1R (Figure 1).
The affinity of INSR and IGF1R towards each IGF differs significantly (Figure 1). Insulin binds with the greatest affinity to both INSR isoforms, but not to the hybrid INSR/IGF1R receptors [9,10]. IGF1 mediates its mitogenic effects primarily through IGF1R, but can also bind IGF1R/INSR-A and IGF1R/INSR-B hybrid receptors [11,12,13,14,15]. Finally, IGF2 signals through IGF1R, but has also been found to bind to the fetal ISNR-A with an affinity close to that of insulin [9]. IGF2 can also bind to the INSR-A/IGF1R hybrid receptor, as well as to the cation-independent mannose-6-phosphate/IGF2 receptor (IGF2R). It should be noted, though, that the M6P/IGF2R is unrelated to INSR and IGF1R, lacks intrinsic tyrosine kinase activity, and is thought to serve as a mechanism for clearing circulating IGF2 [16].
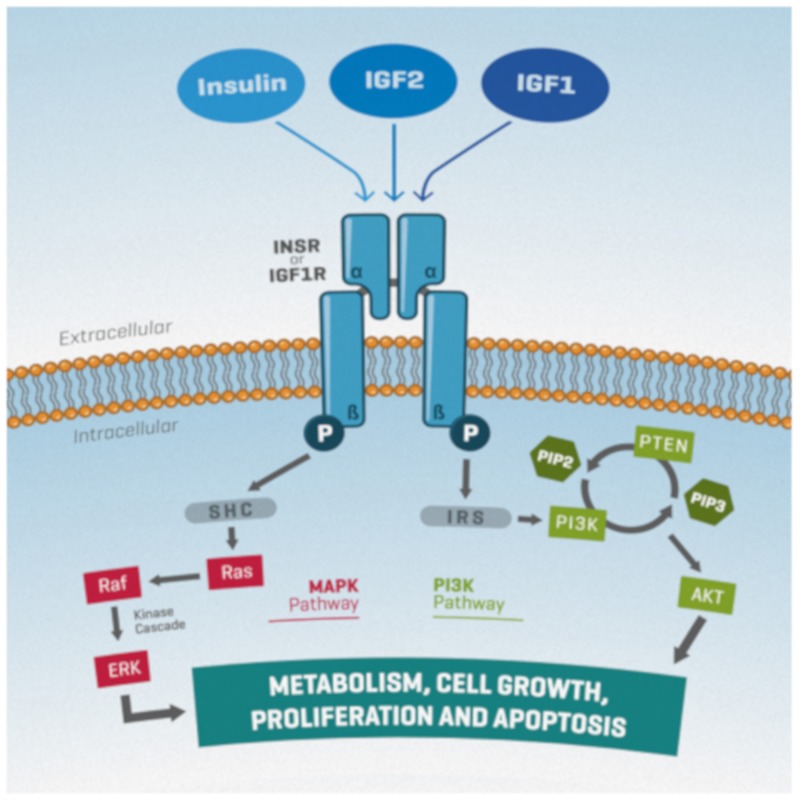
Upon ligand binding, phosphorylated receptors recruit IRS (insulin receptor substrate) proteins or SHC (Src homology domain-containing) proteins, leading to activation of two main pathways, the phosphatidylinositol-3 kinase (PI3K)/PTEN/AKT and the ERK/MAPK, both of which are associated with proliferation, differentiation, metabolism, and survival [17] (Figure 2). However, evidence has pointed towards the involvement of additional pathways, such as the JAK-STAT [18].
Figure 2.
A simplified schematic representation of insulin/IGF signaling. Ligand binding to their receptors leads to autophosphorylation of the β subunits. Subsequent recruitment and phosphorylation of IRS (insulin receptor substrate) or SHC (Src homology domain-containing) proteins leads to the activation of the MAPK (Ras/Raf/ERK) or PI3K (PI3K/PTEN/AKT) signaling pathways. AKT activation is modulated positively and negatively by PI3K and PTEN, respectively, through PIP3 or PIP2 production. Both pathways are associated with cell proliferation, differentiation, metabolism, and survival. Arrows indicate signal transduction.
Although several physiological roles of the insulin/IGF system have been revealed over the years, our understanding is still somewhat limited due to the system’s particular complexity. In addition to the multiplicity of ligands, receptors, IGF binding proteins, IGFBP proteases; the variability in ligand binding affinities for their receptors; and the variety of signaling pathways activated downstream of receptor activation, further complexity is added due to the fact that INSR and IGF1R can translocate to the nucleus and function as transcription factors [19].
3. Genital Ridge Formation, Sex Determination and Sex-Specific Gonadal Development
In mammals, the establishment of functional testes and ovaries involves three sequential processes: formation of the bipotential gonadal primordium, followed by sex determination and sex-dimorphic differentiation of the gonads. (Importantly, this review is not intended to provide exhaustive information on gonadal development and sex determination; hence, we would like to refer the readers to a number of relevant reviews e.g., [20,21]).
In mice, the gonadal primordium arises as a thickening of the epithelium along the coelomic surface of the mesonephros around embryonic day 10.0 (E10.0). At this stage, the gonadal primordium is composed mostly of primordial germ cells (PGCs) and Nr5a1-expressing somatic cell precursors [22]. These multipotent somatic progenitors give rise to the supporting and steroidogenic lineages: Sertoli (SC) and Leydig (LC) cells in the testis, granulosa and theca cells in the ovary.
The first sex dimorphic event, or sex determination, coincides with the commitment of a subset of somatic progenitors to the supporting fate. Mutually antagonistic male and female programs are initiated around E11.5: An Sry/Sox9/Fgf9 cascade is triggered in XY individuals [23,24,25], whereas an Rspo1/Wnt4/Ctnnb1/Foxl2 network drives ovarian fate in XX embryos [26]. As a result, somatic precursors adopt a supporting lineage fate and differentiate into pre-SCs in males and pre-granulosa cells in females. Once established, supporting cells direct the sex differentiation of other lineages such as the steroidogenic and germ cells.
Fetal LCs (FLCs) of the testis first appear around E12.5 [27] and are thought to differentiate from several sources, including interstitial cells derived from the coelomic epithelium and migrating cells derived from the mesonephros [28]. FLCs are responsible for masculinizing the male urogenital system through androgen production. However, after birth, they are gradually replaced by adult LCs (ALCs) [27], which produce the testosterone responsible for spermatogenesis, maintenance of secondary sex characteristics and fertility. In females, theca cells become distinguishable after birth upon receiving granulosa cell-derived signals [29], and produce androgens that are further converted into estrogens by the granulosa cells.
Germ cell sex determination is defined by the timing of meiotic entry. XX PGCs differentiate around E12.5–13.5 as meiotic oocytes that arrest at prophase I, whereas XY PGCs develop as mitotically quiescent prospermatogonia around E14.5–16.5 (for comprehensive reviews, see [30,31]). In both sexes, gametogenesis is completed during adulthood. Spermatogenesis, the process through which diploid spermatogonia differentiate into haploid spermatozoa, is a continuous process occurring throughout life; oocyte maturation, on the other hand, occurs at each estrous cycle and oogenesis is fully completed only upon fertilization.
Although gametogenesis during adulthood is under the control of the HPG axis, the somatic environment of the gonad also has a major impact on gamete production. For instance, SCs are in direct physical association with all types of germ cells (GCs) and provide them with structural support and a preserved environment that is tightly regulated by the blood-testis barrier; they also assist their movement towards the seminiferous epithelium lumen and sustain their development through their secretory products (for comprehensive reviews, see [32,33]). Importantly, an individual SC can only support a finite number of GCs; hence, the final testis size, the number of GCs and the adult sperm output are directly linked to the total number of SCs [34]. Similarly, in females, oocytes are enclosed together with granulosa and theca cells in follicles, the functional unit of the ovary; they are in physical contact with granulosa cells, which regulate their growth and maturation [35].
4. Expression of Insulin/IGF Family Members in the Gonads of Mice and Humans
IGF1 is synthesized by most, if not all, tissues in the body, where it exerts autocrine or paracrine effects [36]. IGF1 is also produced by the liver and then secreted as an endocrine hormone into the bloodstream. Regulation of hepatic IGF1 production is complex and involves mainly the function of growth hormone (GH). IGF2 is also expressed in numerous tissues, including the liver, but its hepatic production is not regulated by GH. Importantly, the IGF2/Igf2 gene displays genomic imprinting: in humans, as well as other mammals like rodents, the paternally inherited IGF2/Igf2 allele is expressed, while the maternal allele is transcriptionally silent [37]. This monoallelic expression results from differential DNA methylation of the two alleles, ensuring correct gene dosage, otherwise leading to several types of growth disorders [37,38]. Sex-specific methylation imprints are initially established during embryonic life, in developing oocytes and prospermatogonia [39]. They are transmitted to the next generation, maintained in somatic cells of the embryo, while erased in PGCs. In mice, Igf2 is expressed mainly during embryonic development and ceases a few weeks after birth [40]. Thus, IGF2 exerts its proliferative and anti-apoptotic actions only during embryonic and fetal stages, while postnatal growth is IGF2-independent. In humans, however, a different pattern is observed, since both IGF1 and IGF2 remain expressed throughout life and have been reported to act as important modulators of muscle growth and differentiation [41].
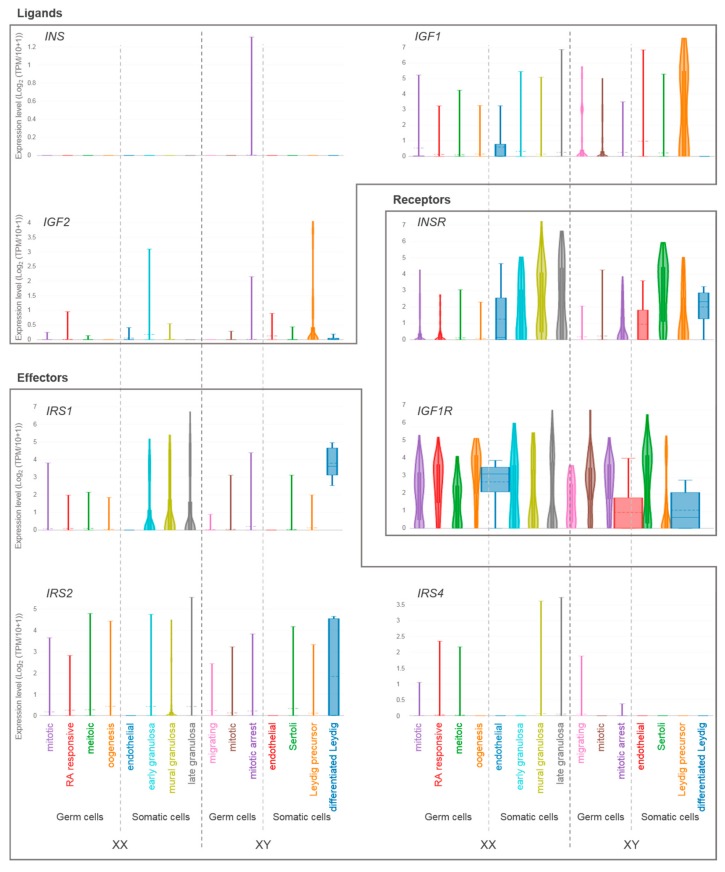
Our group recently provided a detailed expression profile of insulin/IGF system members in murine developing testes using single-cell RNA sequencing [42] (Figure 3 and Table 1): Igf1 and Igf2 transcripts were found in all cell types, with notably higher expression levels in interstitial progenitors. In contrast, Insr transcripts were found exclusively in LCs, whereas Igf1r was expressed in all cell types. The gene coding for the effector protein Irs2 was found to be expressed at higher levels in immature SCs, whereas Irs1, Ins1, and Ins2 transcripts were not detected or minimally expressed. Overall, these data suggest that local testis-secreted IGFs exert their paracrine effects on SCs and LCs.
Figure 3.
Single-cell gene expression levels of insulin/IGF system members in developing human ovaries and testes. Violin plots showing relative expression levels of genes coding for ligands (INS, IGF1, IGF2), receptors (INSR, IGFR) and effector proteins (IRS1, IRS2, IRS4) in human fetal gonads (data from Li et al., [46], adapted from The ReproGenomics Viewer [47,48]). Somatic and germ cell clusters for each sex are indicated on the x-axis. Expression levels are indicated on the y-axis. TPM, transcripts per million; RA, retinoic acid.
Table 1.
Summary of insulin/IGF system expression pattern in developing testis, mouse models and human phenotypes related to sexual development and reproduction.
| Ins2/INS | Igf1/IGF1 | Igf2/IGF2 | Insr/INSR | Igf1r/IGF1R | Irs1/IRS1 | Irs2/IRS2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Mouse XY | Gene expression 1 | Interstitial progenitors | - | +++ | +++ | - | +++ | - | + |
| Leydig cells | - | + | + | + | +++ | - | + | ||
| Sertoli cells | - | - | - | - | +++ | - | +++ | ||
| Germ cells | - | - | + | - | +++ | - | + | ||
| Endothelial cells | - | + | +++ | - | +++ | - | - | ||
| Phenotypes | - Insulin-injected and HFD mouse models: reduced steroidogenic enzyme gene expression and steroidogenesis [49] | - Constitutive KO: steroidogenic failure, PLC markers upregulation, reduced testis size and sperm count [50,51] | - Constitutive KO: no testicular defects [52] | - Constitutive double KO: reduced proliferation rates of somatic progenitors, male-to-female sex reversal [53,54] | - Constitutive KO: no testicular defects [44] | - Constitutive KO: reduced testis size and sperm count [44] | |||
| - LC specific-KO: adult Leydig cells (ALCs) maturation defects, cell-autonomous steroidogenic failure, non-cell autonomous PLCs (progenitor Leydig cells) enrichment, reduced testis size and sperm count. Fetal LCs (FLCs) not affected [55] | |||||||||
| - LC specific-KI: age-dependent germ cell degeneration [56] | - Sertoli (SC) specific-KO: reduced follicle stimulating hormone (FSH)-dependant SC proliferation, testis size and sperm count [43] acting through IGF/PTEN/PI3K pathway [42] | ||||||||
| - GC specific-KO: no testicular defects [43] | |||||||||
| Human XY | Gene expression 1 | Interstitial progenitors | - | +++ | + | +++ | +++ | - | - |
| Leydig cells | - | - | - | +++ | +++ | +++ | +++ | ||
| Sertoli cells | - | - | - | +++ | +++ | - | - | ||
| Germ cells | - | + | - | + | +++ | - | - | ||
| Endothelial cells | - | - | - | +++ | +++ | - | - | ||
| Conditions | - Homozygous mutation: reproductive system not affected [57,58,59,60] | - Paternally inherited IGF2 mutation: ambiguous genitalia, penoscrotal hypospadia, unilateral cryptorchidism, hypogonadism [61] | |||||||
| - Positive correlation between IGF1 and testicular volume [62] | |||||||||
| - IGF1 administration to Laron syndrome patients: increased testis size [62] | |||||||||
After birth, Igf1 and Igf2 are both robustly expressed in GCs, markedly in spermatogonia [43], while all IRS proteins are found in the adult mouse testis [44]. Notably, although insulin is primarily produced by pancreatic β-cells, it has recently been shown that the testis also produces insulin; interestingly though, it is pancreatic, and not testicular, insulin that regulates the male HPG axis and is thus essential for mouse male fertility [45].
In human developing gonads, INSR is preferentially expressed in somatic cells in both sexes, whereas IGF1R is ubiquitously expressed (Figure 3 and Table 1, data from [46]). IRS1 and IRS2 are highly expressed in LCs in males, and only IRS1 is expressed in females, specifically in granulosa cells. IRS3, IRS4, and INS are minimally expressed or not detected. Interestingly, IGF1 and IGF2 are preferentially expressed in PLCs (progenitor Leydig cells), suggesting that, similar to what we observed in XY mice, locally PLC-produced IGFs exert paracrine actions on surrounding somatic cells.
5. Biological Effects of the Insulin/IGF System in Sexual Development and Reproduction
5.1. Differential Contribution of IGF Ligands to Sexual Development and Reproduction
The fact that several members of the insulin/IGF system play significant roles in sexual development has been firmly established over the last decades, mainly through the use of in vivo mouse models (summarized in Table 1).
Originally, it was shown that mice with a targeted disruption of the Igf1 gene exhibit intrauterine growth retardation and postnatal growth failure, ultimately leading to dwarfism, with a ~70% reduction in body weight [50]. Interestingly, both male and female Igf1 mutant mice are infertile with reduced libido: Males have small testes and reduced sperm count due to defects in androgen synthesis by LCs, while mutant females fail to ovulate despite hCG stimulation, possess an infantile uterus and exhibit hypoplasia of the myometrium. In men, homozygous mutations of the IGFI gene result in similar phenotypic features, except for the fact that their reproductive system is not affected [57,58,59,60]. However, IGF1 or GH administration to boys/men with non-GH deficient (GHD) short stature, congenital isolated GHD or GH resistance improves reproductive parameters such as testicular volume and sperm production (reviewed in [62]). This suggests that although IGF1 mutations have so far been shown to be compatible with normal testicular development and function, exogenous IGF1 administration is able to positively affect testicular parameters.
Contrary to IGF1, no essential roles in male sexual development have been revealed for IGF2. Deletion of Igf2 results in placental insufficiency [52,63], intrauterine growth restriction [64,65] and reduced fetal weight, but these abnormalities are not accompanied by any testicular defects.
The role of insulin in testis development has been more difficult to decipher. There have been a few publications suggesting a possible link between insulin and testis development in humans and pigs [66,67], but for the time being, this link has not been firmly confirmed. Nonetheless, it has been shown that overexpression of INS in LCs reduces the number of germ cells and gradually leads to infertility in mice [56], and that insulin induces the expression of DAX1 (officially NR0B1) in LCs, which in turn inhibits testicular steroidogenesis both in vivo and in vitro [49].
Nevertheless, it should be kept in mind that, because of the redundancy of the IGF ligands, a potential role of IGF2 and insulin in gonadal development and function may be masked by the presence of IGF1.
5.2. The Insulin/IGF System and Sex Determination
The multiplicity of interactions and the large range of affinity between IGFs and their cognate receptors, either as homodimers or as hybrid heterodimers, implies that IGF signaling must include both IGF1R and INSR-mediated transduction. In 2003, Nef et al. [53] generated triple knockout mice for Insr, Igf1r, and Insrr (insulin receptor-related receptor, an orphan receptor of the insulin receptor gene family [68]). This study showed that insulin/IGF signaling is absolutely essential for sex determination and testis differentiation in mice, since triple knockout embryos display a complete male-to-female sex reversal, characterized by reduced expression of Sry and Sox9 [53].
Later on, a similar phenotype was recapitulated in mice that were mutant only for Insr and Igf1r: double mutant embryos show reduced proliferation rates of somatic progenitor cells in both XX and XY gonads, prior to sex determination, due to a reduction in Nr5a1 expression. As a result, mutant mice display complete agenesis of the adrenal gland and absence of testis development [54]. Notably, a delay in ovarian differentiation and germ cell entry into meiosis is also observed in these mice, suggesting that, regardless of the genetic sex, gonads lacking insulin/IGF signaling remain in an undifferentiated state, with no clear activation of either testicular or ovarian genetic programs for several days. In fact, prior to sex determination, insulin/IGF signaling orchestrates a complex and dynamic transcriptional program in the bipotential somatic precursors, which, when prematurely altered, results in adrenal specification and gonadal development failures.
Overall, these results highlight the essential role played by the insulin/IGF signaling pathway in mediating different aspects of adrenogonadal development, such as adrenal specification, testicular differentiation and ovarian development. Importantly, they have shed light on a crucial, but so far underestimated, signaling pathway underlying sex determination.
Such studies, though, are somewhat limited due to the fact that the constitutive invalidation of Insr or Igf1r in mice leads to perinatal lethality [53,54]. More recently, the use of animal models with cell-specific disruptions has allowed a detailed investigation of the roles played by individual insulin/IGF signaling members in mediating adrenal, testicular and ovarian development and function (these will be analyzed in subsequent sections).
5.3. The Role of the Insulin/IGF System in Sertoli and Granulosa Cells
In both sexes, gamete production critically relies on the supporting lineage. Indeed, as mentioned earlier, the physical association of supporting cells with GCs provides the latter with structural support, as well as with an appropriate somatic environment that regulates spermatogenesis and folliculogenesis—and, hence, fertility.
The biological actions of IGFs on SCs and granulosa cells have been extensively studied both in vitro and in vivo (see below). In vitro, IGFs have been found to be crucial for FSH-mediated events in supporting cells of both sexes. In particular, IGF1/IGF1R function is essential for FSH-mediated AKT activation, subsequent steroidogenic enzyme expression and estradiol production in human and rodent granulosa cells [69]. In a similar manner, IGF1 and FSH synergistically activate AKT/PI3K in immature rat SCs [70]. In this system, IGF1 inhibits FSH-mediated steroidogenic enzyme expression and estrogen production [71]. Thus, although IGF1 triggers the activation of the same signaling molecules in both Sertoli and granulosa cells, this results in opposite biological responses in regard to estrogen production, thereby highlighting once again the complexity of IGF signaling.
The use of animal models with cell-specific gene disruptions has been a tremendous contribution to the deciphering of insulin/IGF activity with respect to spermatogenesis and folliculogenesis. Recently, our studies with SC-specific knockout mouse models for the Insr and/or Igf1r genes provided in vivo evidence that the insulin/IGF receptor family plays a major role in regulating immature SC proliferation, maturation and, ultimately, daily sperm production [43]. Adult testes of mice lacking both Insr and Igf1r in SCs (SC-Insr;Igf1r) display a 75% reduction in testis size and daily sperm production, as a result of a reduced proliferation rate of immature SCs during the late fetal and early neonatal testicular period. On the other hand, testes lacking only Insr or Igf1r in Sertoli cells display a weight reduction of 14% and 35% respectively, compared to control littermates. The fact that concomitant ablation of both receptors results in a much more severe reduction in testis weight implies the existence of significant redundancy, but also suggests that INSR and IGF1R act in a synergistic manner to regulate SC number and testis size. In fact, taking into account both the ligand binding affinities and the relative contribution of INSR and IGF1R to the phenotype, we propose that IGF2 exerts the major role in promoting SC proliferation, since, as previously mentioned, IGF2 signals through both the IGF1R and the fetal ISNR-A isoform [9,14]. Moreover, when compared to the influence of known regulators of neonatal SC proliferation such as FSH, androgens or thyroid hormones, our data indicate that, in fact, IGFs are proportionally the major regulators of SC number and testis size in mammals. Indeed, mice lacking the FSH receptor specifically in SCs show a 55–60% reduction in testis weight and SC number [72] (whereas SC-Insr;Igf1r mice show 75%). Importantly though, an investigation of the potential interactions between FSH and the insulin/IGF pathway has shown that insulin/IGF signaling is actually necessary to mediate the proliferative effects of FSH on immature SCs [43].
As mentioned in Section 2, upon IGF binding, phosphorylated receptors recruit IRS proteins or SHC proteins, leading to activation of two main pathways, the PI3K/PTEN/AKT and the ERK/MAPK [17]. The relevance of IRS proteins for SC development and sperm production in vivo has been revealed through the constitutive deletion of the genes coding for these proteins: Irs2 knockout mice (but not Irs1 mutants) show a 45% testis weight reduction, associated with fewer SCs, GCs, and epididymal sperm, whereas LC number and testosterone production are not affected [44]. Crucially, this study has revealed the importance of IGF/IRS2 signaling in mediating SC development, testis size and subsequent sperm production; however, the cell-autonomous effects of IGFs could not be analyzed in this particular mouse model. In addition, although several reports have indicated that, in vitro, IGF1 signals through the PI3K pathway in supporting cells, the mechanism underlying the in vivo effects of insulin/IGF signaling in promoting SC proliferation, testis size and sperm production was only recently elucidated. More precisely, the generation of mice with a SC-specific deletion of Pten (phosphatase and tensin homolog), a negative regulator of the PI3K/AKT pathway, has allowed researchers to decipher the IGF-dependent intracellular events promoting SC proliferation. Although ablation of Pten alone appears to be dispensable for SC proliferation and spermatogenesis, inactivation of Pten in the absence of Insr and Igf1r rescues SC proliferation rate during late fetal development, as well as subsequent testis size and sperm production [42]. These findings suggest that, in vivo, IGFs promote the proliferation of immature SCs through the IGF/PTEN/PI3K pathway.
In females, Zhou and colleagues have shown that the in vivo inhibition of IGF1R activity or expression prevents the FSH-mediated granulosa cell functions, such as expression of steroidogenic genes and estradiol production [69]. More precisely, they have found that IGF1R signaling is necessary for FSH-induced activation of AKT, as well as for the subsequent differentiation of human cumulus granulosa cells to the mural/preovulatory stage [73]. In fact, there exists an intricate interplay between IGF2 and FSH during granulosa cell differentiation: FSH is a potent enhancer of IGF2 expression in human granulosa cells and, in its turn, IGF2 activation of IGF1R and AKT is necessary for FSH to stimulate CYP19A1 expression and proliferation of granulosa cells [74]. Overall, these findings suggest the existence of a positive loop interaction between FSH and IGF2 that is critical for human granulosa cell proliferation and differentiation.
In 2017, the same research group provided the ultimate proof of a crosstalk between IGF and FSH signaling within the ovaries: the authors generated a conditional mouse knockout model in which Igf1r is specifically ablated in ovarian granulosa cells, and found that Igf1r expression is essential for steroidogenesis, follicle survival and fertility in females. Their study revealed that the ovaries of such mutant mice are smaller than those of control littermates, contain no antral follicles even after gonadotropin stimulation, and are therefore infertile [75]. More specifically, the authors found that the knockdown in Igf1r expression causes a loss of responsiveness to FSH in preantral follicles, and thereby concluded that IGF1R is indeed critical for FSH-induced differentiation of granulosa cells in vivo.
Overall, a plethora of studies performed during the last few decades has thoroughly characterized the relevance of insulin/IGF signaling in the development of supporting cells and reproduction on the whole. In both sexes, IGFs signal through the PI3K/PTEN/AKT pathway and are required for FSH-induced cellular processes. However, the biological responses upon FSH/IGF signaling differ between the two sexes: Differentiation and survival are triggered in granulosa cells, whereas proliferation and maturation are promoted in SCs. As a consequence, suppression of insulin/IGF signaling in granulosa cells leads to infertility, whereas suppression in SCs leads to males which, although able to produce offspring, show a dramatic reduction in their final pool of SCs.
5.4. The Role of the Insulin/IGF System in Steroidogenic Lineages: FLCs, ALCs and Adrenal Glands
Androgen production and fertility in sexually mature males are dependent on the postnatal differentiation of ALCs. ALCs originate from spindle-shaped progenitor Leydig cells (PLCs) around postnatal days 7–10 (P7–P10) [76,77]. These PLCs differentiate into round immature LCs between P10–20, then increase in size and number, and eventually mature into ALCs with enhanced steroidogenic capacity [76,77].
Testicular levels of IGF1 increase after P7 and peak at P24, coinciding with the beginning of pubertal rise in testosterone secretion and the timing of ALC differentiation and maturation [51]. In fact, IGF1 has been shown to act as a critical factor in the establishment of a normal number of LCs, their maturation and their steroidogenic capacity [50,78,79], both in vivo and in vitro. More recently, an in vivo deletion of Insr and Igf1r in steroidogenic cells showed that insulin/IGF signaling is required for adrenocortical development, as well for ALC maturation and steroidogenic function, but that it is dispensable for fetal LCs function [55]. As a result, mutant males show a 55% mortality rate due to adrenal defects, while the surviving males display quantitative alterations of sperm production, along with a failure to produce offspring because of an absence of mating behavior. Interestingly, cell-lineage tracing in this mouse model has allowed the discrimination between cell-autonomous and non-autonomous effects of insulin/IGF signaling in steroidogenic cells. Cell-autonomous steroidogenic failure in differentiated LCs was accompanied by non-cell autonomous PLC enrichment, suggesting the existence of a crosstalk within the steroidogenic lineage between cells at different steps of differentiation. Of note, the up-regulation of PLC markers observed in Igf1 null mice could also be the result of an indirect PLC stimulation, rather than an arrest in the differentiation, as originally proposed by the authors [78].
It is worth noting here that the adrenal and testicular phenotypes resulting from Insr and Igf1r deletion in steroidogenic cells are similar to the dysgenesis observed in mice lacking the steroidogenic factor 1 gene (Sf1, officially Nr5a1) in this cell type [80]. Similar phenotypes between the two mouse models are also observed in the constitutive knockouts of these genes [54,81]. These evidence point toward a possible cooperation between NR5A1 and insulin/IGF signaling during several steps of adrenogonadal development. In fact, such direct cooperation has been shown in vitro in rat LCs [82]. In addition, the Sf1-mediated form of adrenal hypoplasia congenital syndrome in humans is characterized by primary adrenal failure and gonadal dysgenesis [83]. It is thus possible that insulin/IGF signaling is a conserved major regulator of steroidogenic lineage development and function, although a more thorough investigation is needed.
5.5. Cell-Autonomous Insulin/IGF Signaling Does Not Play an Essential Role in Germ Cell Development and Gamete Production
In vitro studies in several species have demonstrated a role for both insulin and IGF1 in oocyte maturation and early embryo development. For instance, the addition of IGF1 to the maturation medium of oocytes accelerates meiotic progression and increases the number of bovine blastocysts [84]. Similarly, the presence of insulin in bovine oocyte maturation medium leads to accelerated meiotic progression [85], but also exerts mitogenic and anti-apoptotic activities [86]. On the contrary, however, specific ablation of Insr, Igf1r or both receptors in mouse oocytes during follicle/oocyte growth and meiotic progression does not affect female fertility: Mutant females exhibit normal estrous cyclicity, oocyte development and maturation, parturition frequency and litter size [87], indicating that, at least in rodents, oocyte insulin/IGF signaling is dispensable for oocyte and follicular development, maturation, as well as early embryonic development.
Similarly, it has been shown that when either Insr, Igf1r, or both, are inactivated specifically in mouse male germ cells, spermatogenesis remains unaffected and adult individuals are fertile, with normal testis size, seminiferous epithelium histology, sperm production and reproductive functions [43].
This lack of phenotype in both male and female GC-specific mutants, along with the fact that other cell-specific mutants do show gonadal development and reproductive alterations, indicate that, at least in mice, IGFs may act primarily on somatic rather than germ cells.
6. Concluding Remarks and Future Perspectives
Over the last few decades, detailed and extensive studies have firmly established the essential role played by the insulin/IGF family in mammalian sexual development and reproduction. Mainly through the use of conditional knockout mouse models, several obstacles have been overcome and have led to significant findings. For instance, it is now clear that IGFs are absolutely essential for testis differentiation and function: more specifically, both receptors are necessary for testis specification [54]; for establishing SC number, testis size, and sperm output [43]; but also for LC development and steroidogenic function [55]. It is now also clear that insulin/IGF signaling plays an important role in the female reproductive function: IGF1 is essential for ovulation to occur normally [50], while granulosa cell-produced Igf1r is pivotal for steroidogenesis and follicle survival [75].
Obviously, although tremendous progress has been achieved over the years, there are several additional issues that need to be clarified:
First of all, the relative contribution of endocrine, paracrine and autocrine effects of IGFs in the testis and the ovary is still unclear. It is possible that some of the observed phenotypes result from a combination of such effects. Also, the eventual outcome of IGF activity on gonadal development and function could be further modulated by IGFBPs. Indeed, circulating and tissue resident IGFs can bind with differential affinity to the six IGFBPs, whose expression is cell type specific [7]. These interactions have been proposed to regulate IGF bioactivity both positively and negatively, depending on the tissue context [7]. Therefore—and given the importance of the insulin/IGF system for gonadal function—IGFBPs may represent another layer of regulation for sexual development and reproduction. It should be noted here that, although no gonadal phenotype has been reported in Igfbp null mice [88,89,90,91], dynamic IGFBP expression during folliculogenesis has been observed in humans, thereby suggesting a relevant role for these genes in female fertility [90].
Second, although numerous knockout animal models have been created, we still do not have an in vivo conditional model for every member of the insulin/IGF family in each cell type of the testis and the ovary. Such a “collection” of animals would most certainly provide us with a more complete understanding of the precise intratesticular and intraovarian roles played by insulin/IGF family members.
Third, the exact mechanism through which insulin/IGF signaling functions in the male and female reproductive organs is still not fully understood. Very few molecular mechanisms have been proposed in order to explain the phenotypes observed in the animal models that have been described in this review. For instance, the reduced testis size observed in SC-Insr;Igf1r male mice might be attributed to the reduction of Nr5a1 expression: Baba et al. have shown that suppression of Nr5a1 by siRNA reduces the production of ATP and NADPH, but also lowers the expression of genes involved in glucose metabolism [91]. One might hence postulate that disruption of the Nr5a1 gene in vivo could lead to insufficient production of ATP and NADPH through impaired activation of glucose metabolism, and thereby result in dysgenesis of steroidogenic tissues [91]. Similar molecular explanations for other knockout phenotypes are needed in order to fully comprehend how insulin/IGF signaling functions in testes and ovaries.
Lastly, another aspect that requires further investigation is whether insulin/IGF signaling acts in coordination—or in antagonism—with other signaling pathways within the reproductive organs. For example, since both IGF1R and FSHR activate AKT signaling [92], and since studies in male as well as in female mice [43,75] have revealed that insulin/IGF signaling is actually crucial for FSH in order to mediate its proliferative and differentiation effects in Sertoli and granulosa cells, respectively, it has been proposed that a crosstalk between the two pathways exists. Unraveling the precise “mechanistic” details of this crosstalk would be of great importance in the context of human infertility treatment [62]. Therefore, a careful evaluation of all signaling pathways that are active during sexual development and reproduction is much needed, as it will undoubtedly offer interesting new perspectives in reproductive biology.
Fortunately, novel technologies that have been developed over the past few years currently provide researchers in the field with extremely powerful tools. The possibility of individual cell profiling at the transcriptome, proteome—or even epigenome—level will certainly prove to be an instrument of outstanding significance: knowing the exact molecular profile of each cell type in the testis and the ovary, at different stages of development, will help us comprehend in depth the mechanisms that control sexual development. From a medical perspective, such knowledge will offer important insight with regards to human infertility treatment. Moreover, if performed in parallel in multiple species, such analyses could yield significant information about the evolutionary history of reproductive function.
Acknowledgments
The authors apologize to colleagues whose work has been omitted due to space constraints. The authors would like to thank Valentin Durand Graphic Design for help with artwork.
Abbreviations
| AKT | Protein kinase B |
| ALC | Adult Leydig cell |
| ATP | Adenosine triphosphate |
| CTNNB1 | Catenin (cadherin associated protein), beta 1 |
| CYP19A1 | Cytochrome P450, family 19, subfamily a, polypeptide 1 |
| E | Embryonic day |
| ERK | Extracellular signal-regulated kinase |
| FGF9 | Fibroblast growth factor 9 |
| FLC | Fetal Leydig cell |
| FOXL2 | Forkhead box L2 |
| FSH | Follicle stimulating hormone |
| HCG | Human chorionic gonadotropin |
| HPG | Hypothalamic–pituitary–gonadal |
| GC | Germ cell |
| GH | Growth hormone |
| IGFs | Insulin-like growth factors |
| IGF1R | Insulin-like growth factor 1 receptor |
| IGF2R | Insulin-like growth factor 2 receptor |
| IGFBP | Insulin-like growth factor binding protein |
| INS | Insulin |
| INSR | Insulin receptor |
| INSR-A | Insulin receptor isoform A |
| INSR-B | Insulin receptor isoform B |
| IRR | Insulin receptor-related receptor |
| IRS | Insulin receptor substrate |
| JAK | Janus-family tyrosine kinase |
| LC | Leydig cell |
| M6P/IGF2R | Mannose-6-phosphate/IGF2 receptor |
| MAPK | Mitogen-activated protein kinase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NR0B1 | Nuclear receptor subfamily 0 group B member 1 (also known as DAX1) |
| NR5A1 | Nuclear receptor subfamily 5, group A, member 1 (also known as Sf1) |
| P | Postnatal day |
| PGC | Primordial germ cell |
| PI3K | Phosphatidylinositol-3 kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PLC | Progenitor Leydig cell |
| PTEN | Phosphatase and tensin homolog deleted on chromosome 10 |
| RA | Retinoic acid |
| RAF | Rapidly accelerated fibrosarcoma |
| RSPO1 | R-spondin 1 |
| SC | Sertoli cell |
| SHC | Src homology domain-containing |
| siRNA | Small interfering RNA |
| SOX9 | SRY (sex determining region Y)-box 9 |
| SRY | Sex determining region of chromosome Y |
| STAT | Signal transducer and activator of transcription |
| TPM | Transcripts per million |
| WNT4 | Wingless-type MMTV integration site family, member 4 |
Author Contributions
Writing—original draft preparation, Y.N., M.D.P., S.N.; writing—review and editing, Y.N., M.D.P., S.N.
Funding
Research in the Nef laboratory is funded by grants from the Swiss National Science Foundation (grants 31003A_173070 and 51PHI0-141994) and by the Département de l’Instruction Publique of the State of Geneva.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Michalakis K., Mintziori G., Kaprara A., Tarlatzis B.C., Goulis D.G. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism. 2013;62:457–478. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Hansen M., Flatt T., Aguilaniu H. Reproduction, fat metabolism, and life span: What is the connection? Cell Metab. 2013;17:10–19. doi: 10.1016/j.cmet.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efstratiadis A. Genetics of mouse growth. Int. J. Dev. Biol. 1998;42:955–976. [PubMed] [Google Scholar]
- 4.Kaprara A., Huhtaniemi I.T. The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism. 2018;86:3–17. doi: 10.1016/j.metabol.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Agrogiannis G.D., Sifakis S., Patsouris E.S., Konstantinidou A.E. Insulin-like growth factors in embryonic and fetal growth and skeletal development (Review) Mol. Med. Rep. 2014;10:579–584. doi: 10.3892/mmr.2014.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Roith D., Bondy C., Yakar S., Liu J.-L., Butler A. The Somatomedin Hypothesis: 2001. Endocr. Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 7.Lewitt M.S., Boyd G.W. The Role of Insulin-Like Growth Factors and Insulin-Like Growth Factor-Binding Proteins in the Nervous System. Biochem. Insights. 2019;12:1178626419842176. doi: 10.1177/1178626419842176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher E.J., LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol. Metab. 2010;21:610–618. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frasca F., Pandini G., Scalia P., Sciacca L., Mineo R., Costantino A., Goldfine I.D., Belfiore A., Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell. Biol. 1999;19:3278–3288. doi: 10.1128/MCB.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boone D.N., Lee A.V. Targeting the insulin-like growth factor receptor: Developing biomarkers from gene expression profiling. Crit. Rev. Oncog. 2012;17:161–173. doi: 10.1615/CritRevOncog.v17.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker J., Liu J.P., Robertson E.J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. doi: 10.1016/S0092-8674(05)80085-6. [DOI] [PubMed] [Google Scholar]
- 12.Dinchuk J.E., Cao C., Huang F., Reeves K.A., Wang J., Myers F., Cantor G.H., Zhou X., Attar R.M., Gottardis M., et al. Insulin receptor (IR) pathway hyperactivity in IGF-IR null cells and suppression of downstream growth signaling using the dual IGF-IR/IR inhibitor, BMS-754807. Endocrinology. 2010;151:4123–4132. doi: 10.1210/en.2010-0032. [DOI] [PubMed] [Google Scholar]
- 13.Liu J.P., Baker J., Perkins A.S., Robertson E.J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. doi: 10.1016/S0092-8674(05)80084-4. [DOI] [PubMed] [Google Scholar]
- 14.Louvi A., Accili D., Efstratiadis A. Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev. Biol. 1997;189:33–48. doi: 10.1006/dbio.1997.8666. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Pelzer A.M., Kiang D.T., Yee D. Down-regulation of Type I Insulin-like Growth Factor Receptor Increases Sensitivity of Breast Cancer Cells to Insulin. Cancer Res. 2007;67:391–397. doi: 10.1158/0008-5472.CAN-06-1712. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh P., Dahms N.M., Kornfeld S. Mannose 6-phosphate receptors: New twists in the tale. Nat. Rev. Mol. Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 17.Simpson A., Petnga W., Macaulay V.M., Weyer-Czernilofsky U., Bogenrieder T. Insulin-Like Growth Factor (IGF) Pathway Targeting in Cancer: Role of the IGF Axis and Opportunities for Future Combination Studies. Target. Oncol. 2017;12:571–597. doi: 10.1007/s11523-017-0514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Himpe E., Kooijman R. Insulin-like growth factor-I receptor signal transduction and the Janus Kinase/Signal Transducer and Activator of Transcription (JAK-STAT) pathway. BioFactors. 2009;35:76–81. doi: 10.1002/biof.20. [DOI] [PubMed] [Google Scholar]
- 19.Sarfstein R., Werner H. Minireview: Nuclear insulin and insulin-like growth factor-1 receptors: A novel paradigm in signal transduction. Endocrinology. 2013;154:1672–1679. doi: 10.1210/en.2012-2165. [DOI] [PubMed] [Google Scholar]
- 20.Nef S., Stévant I., Greenfield A. Characterizing the bipotential mammalian gonad. Curr. Top. Dev. Biol. 2019;134:167–194. doi: 10.1016/bs.ctdb.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Stévant I., Nef S. Genetic Control of Gonadal Sex Determination and Development. Trends Genet. 2019;35:346–358. doi: 10.1016/j.tig.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Morohashi K. The ontogenesis of the steroidogenic tissues. Genes Cells. 1997;2:95–106. doi: 10.1046/j.1365-2443.1997.1060304.x. [DOI] [PubMed] [Google Scholar]
- 23.Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 24.Hacker A., Capel B., Goodfellow P., Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- 25.Koopman P., Münsterberg A., Capel B., Vivian N., Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348:450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- 26.Chassot A.A., Gregoire E.P., Magliano M., Lavery R., Chaboissier M.C. Genetics of Ovarian Differentiation: Rspo1, a Major Player. Sex. Dev. 2008;2:219–227. doi: 10.1159/000152038. [DOI] [PubMed] [Google Scholar]
- 27.Potter S.J., Kumar D.L., Defalco T. Origin and Differentiation of Androgen- Producing Cells in the Gonads. Chin. Phys. 2016;58:101–134. doi: 10.1007/978-3-319-31973-5_5. [DOI] [PubMed] [Google Scholar]
- 28.Shima Y., Morohashi K. Leydig progenitor cells in fetal testis. Mol. Cell. Endocrinol. 2017;445:55–64. doi: 10.1016/j.mce.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Rotgers E., Jørgensen A., Yao H.H.-C. At the Crossroads of Fate—Somatic Cell Lineage Specification in the Fetal Gonad. Endocr. Rev. 2018;39:739–759. doi: 10.1210/er.2018-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrimson K.S., Hogarth C.A. Germ Cell Commitment to Oogenic Versus Spermatogenic Pathway: The Role of Retinoic Acid. Results Probl. Cell Differ. 2016;58:135–166. doi: 10.1007/978-3-319-31973-5_6. [DOI] [PubMed] [Google Scholar]
- 31.Spiller C., Bowles J. Sexually dimorphic germ cell identity in mammals. Curr. Top. Dev. Biol. 2019;134:253–288. doi: 10.1016/bs.ctdb.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Jégou B. The Sertoli cell. Baillieres. Clin. Endocrinol. Metab. 1992;6:273–311. doi: 10.1016/S0950-351X(05)80151-X. [DOI] [PubMed] [Google Scholar]
- 33.Mruk D.D., Cheng C.Y. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 34.Rebourcet D., Darbey A., Monteiro A., Soffientini U., Tsai Y.T., Handel I., Pitetti J.-L., Nef S., Smith L.B., O’Shaughnessy P.J. Sertoli Cell Number Defines and Predicts Germ and Leydig Cell Population Sizes in the Adult Mouse Testis. Endocrinology. 2017;158:2955–2969. doi: 10.1210/en.2017-00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsueh A.J.W., Kawamura K., Cheng Y., Fauser B.C.J.M. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015;36:1–24. doi: 10.1210/er.2014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupont J., Holzenberger M. Biology of insulin-like growth factors in development. Birth Defects Res. Part C Embryo Today Rev. 2003;69:257–271. doi: 10.1002/bdrc.10022. [DOI] [PubMed] [Google Scholar]
- 37.DeChiara T.M., Robertson E.J., Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-X. [DOI] [PubMed] [Google Scholar]
- 38.Peters J. The role of genomic imprinting in biology and disease: An expanding view. Nat. Rev. Genet. 2014;15:517–530. doi: 10.1038/nrg3766. [DOI] [PubMed] [Google Scholar]
- 39.Mann J.R. Imprinting in the Germ Line. STEM CELLS. 2001;19:287–294. doi: 10.1634/stemcells.19-4-287. [DOI] [PubMed] [Google Scholar]
- 40.Lui J.C., Finkielstain G.P., Barnes K.M., Baron J. An imprinted gene network that controls mammalian somatic growth is down-regulated during postnatal growth deceleration in multiple organs. Am. J. Physiol. Integr. Comp. Physiol. 2008;295:R189–R196. doi: 10.1152/ajpregu.00182.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren H., Yin P., Duan C. IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J. Cell Biol. 2008;182:979–991. doi: 10.1083/jcb.200712110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neirijnck Y., Kühne F., Mayère C., Pavlova E., Sararols P., Foti M., Atanassova N., Nef S. Tumor Suppressor PTEN Regulates Negatively Sertoli Cell Proliferation, Testis Size, and Sperm Production In Vivo. Endocrinology. 2019;160:387–398. doi: 10.1210/en.2018-00892. [DOI] [PubMed] [Google Scholar]
- 43.Pitetti J.-L., Calvel P., Zimmermann C., Conne B., Papaioannou M.D., Aubry F., Cederroth C.R., Urner F., Fumel B., Crausaz M., et al. An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol. Endocrinol. 2013;27:814–827. doi: 10.1210/me.2012-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffeth R.J., Carretero J., Burks D.J. Insulin receptor substrate 2 is required for testicular development. PLoS ONE. 2013;8:e62103. doi: 10.1371/journal.pone.0062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoeller E.L., Albanna G., Frolova A.I., Moley K.H. Insulin rescues impaired spermatogenesis via the hypothalamic–pituitary–gonadal axis in Akita diabetic mice and restores male fertility. Diabetes. 2012;61:1869–1878. doi: 10.2337/db11-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L., Dong J., Yan L., Yong J., Liu X., Hu Y., Fan X., Wu X., Guo H., Wang X., et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell. 2017;20:858–873.e4. doi: 10.1016/j.stem.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Darde T.A., Sallou O., Becker E., Evrard B., Monjeaud C., Le Bras Y., Jégou B., Collin O., Rolland A.D., Chalmel F. The ReproGenomics Viewer: An integrative cross-species toolbox for the reproductive science community. Nucleic Acids Res. 2015;43:W109–W116. doi: 10.1093/nar/gkv345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darde T.A., Lecluze E., Lardenois A., Stévant I., Alary N., Tüttelmann F., Collin O., Nef S., Jégou B., Rolland A.D., et al. The ReproGenomics Viewer: A multi-omics and cross-species resource compatible with single-cell studies for the reproductive science community. Bioinformatics. 2019 doi: 10.1093/bioinformatics/btz047. [DOI] [PubMed] [Google Scholar]
- 49.Ahn S.W., Gang G.-T., Kim Y.D., Ahn R.-S., Harris R.A., Lee C.-H., Choi H.-S. Insulin directly regulates steroidogenesis via induction of the orphan nuclear receptor DAX-1 in testicular Leydig cells. J. Biol. Chem. 2013;288:15937–15946. doi: 10.1074/jbc.M113.451773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker J., Hardy M.P., Zhou J., Bondy C., Lupu F., Bellvé A.R., Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- 51.Wang G.-M., O’Shaughnessy P.J., Chubb C., Robaire B., Hardy M.P. Effects of Insulin-Like Growth Factor I on Steroidogenic Enzyme Expression Levels in Mouse Leydig Cells. Endocrinology. 2003;144:5058–5064. doi: 10.1210/en.2003-0563. [DOI] [PubMed] [Google Scholar]
- 52.DeChiara T.M., Efstratiadis A., Robertson E.J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 53.Nef S., Verma-Kurvari S., Merenmies J., Vassalli J.-D., Efstratiadis A., Accili D., Parada L.F. Testis determination requires insulin receptor family function in mice. Nature. 2003;426:291–295. doi: 10.1038/nature02059. [DOI] [PubMed] [Google Scholar]
- 54.Pitetti J.-L., Calvel P., Romero Y., Conne B., Truong V., Papaioannou M.D., Schaad O., Docquier M., Herrera P.L., Wilhelm D., et al. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet. 2013;9:e1003160. doi: 10.1371/journal.pgen.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neirijnck Y., Calvel P., Kilcoyne K.R., Kühne F., Stévant I., Griffeth R.J., Pitetti J.-L., Andric S.A., Hu M.-C., Pralong F., et al. Insulin and IGF1 receptors are essential for the development and steroidogenic function of adult Leydig cells. FASEB J. 2018;32:3321–3335. doi: 10.1096/fj.201700769RR. [DOI] [PubMed] [Google Scholar]
- 56.Shirneshan K., Binder S., Böhm D., Wolf S., Sancken U., Meinhardt A., Schmid M., Engel W., Adham I.M. Directed overexpression of insulin in Leydig cells causes a progressive loss of germ cells. Mol. Cell. Endocrinol. 2008;295:79–86. doi: 10.1016/j.mce.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Woods K.A., Camacho-Hübner C., Savage M.O., Clark A.J.L. Intrauterine Growth Retardation and Postnatal Growth Failure Associated with Deletion of the Insulin-Like Growth Factor I Gene. N. Engl. J. Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 58.Bonapace G., Concolino D. A novel mutation in a patient with insulin-like growth factor 1 (IGF1) deficiency. J. Medical Genetics. 2003;40:913–917. doi: 10.1136/jmg.40.12.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walenkamp M.J.E., Karperien M., Pereira A.M., Hilhorst-Hofstee Y., Van Doorn J., Chen J.W., Mohan S., Denley A., Forbes B., Van Duyvenvoorde H.A., et al. Homozygous and Heterozygous Expression of a Novel Insulin-Like Growth Factor-I Mutation. J. Clin. Endocrinol. Metab. 2005;90:2855–2864. doi: 10.1210/jc.2004-1254. [DOI] [PubMed] [Google Scholar]
- 60.Netchine I., Azzi S., Houang M., Seurin D., Perin L., Ricort J.-M., Daubas C., Legay C., Mester J., Herich R., et al. Partial Primary Deficiency of Insulin-Like Growth Factor (IGF)-I Activity Associated with IGF1 Mutation Demonstrates Its Critical Role in Growth and Brain Development. J. Clin. Endocrinol. Metab. 2009;94:3913–3921. doi: 10.1210/jc.2009-0452. [DOI] [PubMed] [Google Scholar]
- 61.Begemann M., Zirn B., Santen G., Wirthgen E., Soellner L., Büttel H.-M., Schweizer R., Van Workum W., Binder G., Eggermann T. Paternally Inherited IGF2 Mutation and Growth Restriction. N. Engl. J. Med. 2015;373:349–356. doi: 10.1056/NEJMoa1415227. [DOI] [PubMed] [Google Scholar]
- 62.Cannarella R., Condorelli R.A., La Vignera S., Calogero A.E. Effects of the insulin-like growth factor system on testicular differentiation and function: A review of the literature. Andrology. 2018;6:3–9. doi: 10.1111/andr.12444. [DOI] [PubMed] [Google Scholar]
- 63.Constância M., Hemberger M., Hughes J., Dean W., Ferguson-Smith A., Fundele R., Stewart F., Kelsey G., Fowden A., Sibley C., et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 64.Randhawa R., Cohen P. The role of the insulin-like growth factor system in prenatal growth. Mol. Genet. Metab. 2005;86:84–90. doi: 10.1016/j.ymgme.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 65.Chernausek S.D. Update: Consequences of Abnormal Fetal Growth. J. Clin. Endocrinol. Metab. 2012;97:689–695. doi: 10.1210/jc.2011-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aquila S., Gentile M., Middea E., Catalano S., Andò S. Autocrine Regulation of Insulin Secretion in Human Ejaculated Spermatozoa. Endocrinology. 2005;146:552–557. doi: 10.1210/en.2004-1252. [DOI] [PubMed] [Google Scholar]
- 67.Carpino A., Rago V., Guido C., Casaburi I., Aquila S. Insulin and IR-beta in pig spermatozoa: A role of the hormone in the acquisition of fertilizing ability. Int. J. Androl. 2010;33:554–562. doi: 10.1111/j.1365-2605.2009.00971.x. [DOI] [PubMed] [Google Scholar]
- 68.Dissen G.A., Garcia-Rudaz C., Tapia V., Parada L.F., Hsu S.-Y.T., Ojeda S.R. Expression of the Insulin Receptor-Related Receptor Is Induced by the Preovulatory Surge of Luteinizing Hormone in Thecal-Interstitial Cells of the Rat Ovary. Endocrinology. 2006;147:155–165. doi: 10.1210/en.2005-0386. [DOI] [PubMed] [Google Scholar]
- 69.Zhou P., Baumgarten S.C., Wu Y., Bennett J., Winston N., Hirshfeld-Cytron J., Stocco C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol. Endocrinol. 2013;27:511–523. doi: 10.1210/me.2012-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan S.A., Ndjountche L., Pratchard L., Spicer L.J., Davis J.S. Follicle-Stimulating Hormone Amplifies Insulin-Like Growth Factor I-Mediated Activation of AKT/Protein Kinase B Signaling in Immature Rat Sertoli Cells. Endocrinology. 2002;143:2259–2267. doi: 10.1210/endo.143.6.8838. [DOI] [PubMed] [Google Scholar]
- 71.Rappaport M.S., Smith E.P. Insulin-Like Growth Factor I Inhibits Aromatization Induced by Follicle-Stimulating Hormone in Rat Sertoli Cell Culture1. Biol. Reprod. 1996;54:446–452. doi: 10.1095/biolreprod54.2.446. [DOI] [PubMed] [Google Scholar]
- 72.Abel M.H., Baker P.J., Charlton H.M., Monteiro A., Verhoeven G., De Gendt K., Guillou F., O’Shaughnessy P.J. Spermatogenesis and Sertoli Cell Activity in Mice Lacking Sertoli Cell Receptors for Follicle-Stimulating Hormone and Androgen. Endocrinology. 2008;149:3279–3285. doi: 10.1210/en.2008-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baumgarten S.C., Convissar S.M., Fierro M.A., Winston N.J., Scoccia B., Stocco C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells. J. Clin. Endocrinol. Metab. 2014;99:2995–3004. doi: 10.1210/jc.2014-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baumgarten S.C., Convissar S.M., Zamah A.M., Fierro M.A., Winston N.J., Scoccia B., Stocco C. FSH Regulates IGF-2 Expression in Human Granulosa Cells in an AKT-Dependent Manner. J. Clin. Endocrinol. Metab. 2015;100:E1046–E1055. doi: 10.1210/jc.2015-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baumgarten S.C., Armouti M., Ko C., Stocco C. IGF1R Expression in Ovarian Granulosa Cells Is Essential for Steroidogenesis, Follicle Survival, and Fertility in Female Mice. Endocrinology. 2017;158:2309–2318. doi: 10.1210/en.2017-00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Shaughnessy P.J., Morris I.D., Huhtaniemi I., Baker P.J., Abel M.H. Role of androgen and gonadotrophins in the development and function of the Sertoli cells and Leydig cells: Data from mutant and genetically modified mice. Mol. Cell. Endocrinol. 2009;306:2–8. doi: 10.1016/j.mce.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Wang G., Hardy M.P. Development of Leydig Cells in the Insulin-Like Growth Factor-I (IGF-I) Knockout Mouse: Effects of IGF-I Replacement and Gonadotropic Stimulation1. Biol. Reprod. 2004;70:632–639. doi: 10.1095/biolreprod.103.022590. [DOI] [PubMed] [Google Scholar]
- 78.Hu G.-X., Lin H., Chen G.-R., Chen B.-B., Lian Q.-Q., Hardy D.O., Zirkin B.R., Ge R.-S. Deletion of the Igf1 gene: Suppressive effects on adult Leydig cell development. J. Androl. 2010;31:379–387. doi: 10.2164/jandrol.109.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manna P.R., Chandrala S.P., King S.R., Jo Y., Counis R., Huhtaniemi I.T., Stocco D.M. Molecular mechanisms of insulin-like growth factor-I mediated regulation of the steroidogenic acute regulatory protein in mouse leydig cells. Mol. Endocrinol. 2006;20:362–378. doi: 10.1210/me.2004-0526. [DOI] [PubMed] [Google Scholar]
- 80.Buaas F.W., Gardiner J.R., Clayton S., Val P., Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012;139:4561–4570. doi: 10.1242/dev.087247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo X., Ikeda Y., Parker K.L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 82.Sirianni R., Chimento A., Malivindi R., Mazzitelli I., Andò S., Pezzi V. Insulin-like growth factor-I, regulating aromatase expression through steroidogenic factor 1, supports estrogen-dependent tumor Leydig cell proliferation. Cancer Res. 2007;67:8368–8377. doi: 10.1158/0008-5472.CAN-06-4064. [DOI] [PubMed] [Google Scholar]
- 83.Fujieda K., Tajima T. Molecular Basis of Adrenal Insufficiency. Pediatr. Res. 2005;57:62R–69R. doi: 10.1203/01.PDR.0000159568.31749.4D. [DOI] [PubMed] [Google Scholar]
- 84.Sakaguchi M., Dominko T., Yamauchi N., Leibfried-Rutledge M.L., Nagai T., First N.L. Possible mechanism for acceleration of meiotic progression of bovine follicular oocytes by growth factors in vitro. Reproduction. 2002;123:135–142. doi: 10.1530/rep.0.1230135. [DOI] [PubMed] [Google Scholar]
- 85.Stefanello J.R., Barreta M.H., Porciuncula P.M., Arruda J.N., Oliveira J.F., Oliveira M.A., Gonçalves P.B. Effect of angiotensin II with follicle cells and insulin-like growth factor-I or insulin on bovine oocyte maturation and embryo development. Theriogenology. 2006;66:2068–2076. doi: 10.1016/j.theriogenology.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 86.Augustin R., Pocar P., Wrenzycki C., Niemann H., Fischer B. Mitogenic and anti-apoptotic activity of insulin on bovine embryos produced in vitro. Reproduction. 2003;126:91–99. doi: 10.1530/rep.0.1260091. [DOI] [PubMed] [Google Scholar]
- 87.Pitetti J.L., Torre D., Conne B., Papaioannou M.D., Cederroth C.R., Xuan S., Kahn R., Parada L.F., Vassalli J.D., Efstratiadis A., et al. Insulin Receptor and IGF1R Are Not Required for Oocyte Growth, Differentiation, and Maturation in Mice. Sex. Dev. 2009;3:264–272. doi: 10.1159/000252813. [DOI] [PubMed] [Google Scholar]
- 88.Ning Y., Schuller A.G.P., Conover C.A., Pintar J.E. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol. Endocrinol. 2008;22:1213–1225. doi: 10.1210/me.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ning Y., Hoang B., Schuller A.G.P., Cominski T.P., Hsu M.-S., Wood T.L., Pintar J.E. Delayed Mammary Gland Involution in Mice with Mutation of the Insulin-Like Growth Factor Binding Protein 5 Gene. Endocrinology. 2007;148:2138–2147. doi: 10.1210/en.2006-0041. [DOI] [PubMed] [Google Scholar]
- 90.Bøtkjær J.A., Pors S.E., Petersen T.S., Kristensen S.G., Jeppesen J.V., Oxvig C., Andersen C.Y. Transcription profile of the insulin-like growth factor signaling pathway during human ovarian follicular development. J. Assist. Reprod. Genet. 2019;36:889–903. doi: 10.1007/s10815-019-01432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baba T., Otake H., Sato T., Miyabayashi K., Shishido Y., Wang C.-Y., Shima Y., Kimura H., Yagi M., Ishihara Y., et al. Glycolytic genes are targets of the nuclear receptor Ad4BP/SF-1. Nat. Commun. 2014;5:3634. doi: 10.1038/ncomms4634. [DOI] [PubMed] [Google Scholar]
- 92.Walker W.H., Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]