Abstract
Over the last few decades, chitosan has become a good candidate for tissue engineering applications. Derived from chitin, chitosan is a unique natural polysaccharide with outstanding properties in line with excellent biodegradability, biocompatibility, and antimicrobial activity. Due to the presence of free amine groups in its backbone chain, chitosan could be further chemically modified to possess additional functional properties useful for the development of different biomaterials in regenerative medicine. In the current review, we will highlight the progress made in the development of chitosan-containing bioscaffolds, such as gels, sponges, films, and fibers, and their possible applications in tissue repair and regeneration, as well as the use of chitosan as a component for drug delivery applications.
Keywords: chitosan, biomaterials, tissue engineering, regenerative medicine, bone, cartilage
1. Introduction
Development of biomaterials is an active research field with the purpose of designing scaffolds for the regeneration of tissues and organs damaged by disease or injuries. Defining and designing appropriate material for tissue engineering is a critical step in tissue engineering and regenerative medicine [1]. In the past few decades, significant attention has been given to natural polymers because of their biocompatibility and structural similarity to the extracellular matrix components. Abundant availability and unique biological activity of each natural polymer makes them a matching candidate for the development of novel natural or/and semi-synthetic materials closely resembling the natural structure and functionality of tissues required for successful regeneration. Starch, collagen, alginate, cellulose, hyaluronic acid, chitin, and chitosan (CS), are attractive natural polymers suitable for tissue regeneration. CS is a linear natural carbohydrate biopolymer derived from chitin with a structural similarity to glycosaminoglycans of the extracellular matrix (ECM) implicated in cell–cell adhesion [2]. The hydrophilic structure of CS promotes cell adhesion, proliferation, and differentiation of different types of cells and the polycationic nature of CS at a mildly acidic condition allows immobilization of negatively charged enzymes, proteins, and DNA for gene delivery [3,4]. CS for tissue engineering and regenerative medicine could be designed in various forms, such as hydrogels, sponges, fibers, sheets, films, and other structures [5].
2. Structure and Physico-Chemical Properties
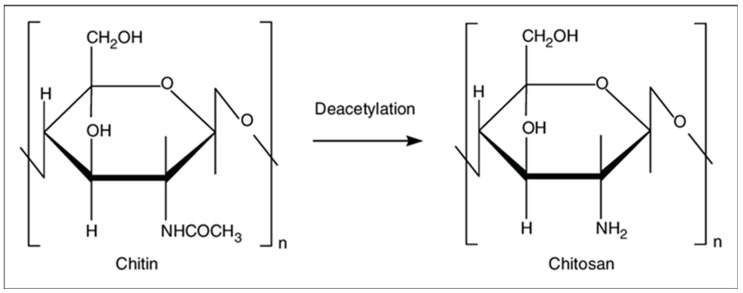
Chitin is the second most abundant natural polymer [6] and consists of 2-acetamido-2-deoxy-β-d-glucose through a β (1→4) linkage and is extracted from the shells of marine crustaceans, insects, or fungi. Chitin is insoluble in water and most organic solvents, and therefore its use in biomaterials fabrication is limited. CS is a linear polysaccharide derived from partial deacetylation of chitin, as shown in Figure 1. It is a copolymer of randomly located (1→4)-2-acetamido-2-deoxy-β-d-glucan (N-acetyl d-glucosamine) and (1→4)-2-amino-2-deoxy-β-d-glucan (d-glucosamine) units. The number of amino groups as a ratio between d-glucosamine to the sum of d-glucosamine and N-acetyl d-glucosamine is indicated as a deacetylation degree (DD) and should be at least 60% for CS. The deacetylation of chitin is conducted by chemical hydrolysis (alkaline conditions) [7] or by enzymatic hydrolysis (chitin deacetylase) [8]. CS is soluble in dilute organic acids such as acetic acid [9], as well as diluted hydrochloric acid, and further modification of CS is accessible due to the availability of amino groups [6]. The fungal source of CS is preferred at the industrial scale because of its narrower molecular mass distribution, all-year-round availability, more controlled and scalable production, and less immunogenicity in comparison to a seafood source, which could cause allergies and limit biomedical application [7].
Figure 1.
Chitin and chitosan structure.
The physical properties of CS depend on several factors, such as the molecular weight, DD, and purity of the product [10]. CS solubility is pH dependent [11] and it is soluble in diluted acids achieved by protonation of the amino groups of the d-glucosamine residues [12]. Availability of protonated amino groups enables CS to form complexes with metal ions [13,14], natural or synthetic anionic (poly(acrylic acid)) polymers [15], lipids, proteins, and DNA. CS-based scaffolds can be chemically cross-linked by glutaraldehyde, oxidized dextran or other oxidized carbohydrates, 1,1,3,3-tetramethoxypropan, and genipin [15,16,17]. It is important to note that CS is a unique semi-natural positively charged polysaccharide at acidic conditions [18]. This property is used to develop CS-based polyelectrolytes for the preparation of films via a layer-by-layer deposition technique [15]. The amino groups of CS could react with aldehyde groups through reductive amination [9]. Hydroxyl groups along a CS chain enables etherification and esterification [19]. In addition, CS possesses important properties, such as high biocompatibility, biodegradability, antibacterial activity, non-antigenicity, and high adsorption properties that make CS a good candidate for tissue engineering and other biomedical applications [8].
3. Chitosan in Tissue Engineering and Regenerative Medicine
3.1. Chitosan for Wound Healing
Skin regeneration is a complex process that consists of four overlapping phases—hemostasis, inflammation, proliferation, and tissue remodeling [20]. In other words, skin regeneration is a dynamic process involving blood elements, extracellular components, soluble factors, and cells [21]. Therefore, the treatment of skin lesions requires dressing that not only ensures physical protection of the wound but also enhances the healing, provides antimicrobial protection, and reduces scar formation [22].
CS has very strong hemostatic activity which is not dependent on host coagulation pathway [23] but depends on CS’s molecular weight and DD [24,25]. The number of amine groups has a direct effect on blood coagulation, where moderate DD (68.36%) causes the formation of a mesh-like structure within CS, thus facilitating interaction with blood components, whereas higher DD results in stronger hydrogen bonds within CS causing the formation of a crystalline structure with limited ability to interact with red blood cells [24,25,26,27]. Higher molecular weight could further increase the procoagulation effect due to higher interaction between polyelectrolytes [28,29]. There are several CS containing hemostatic products available on the market and approved by the Food and Drug Administration of the United States (FDA), such as Celox®, HemCon®, Axiostat®, Chitoflex®, and Chitoseal® [30].
In addition to the hemostatic effect of CS, it was shown that CS affects all stages of healing in various ways. It was shown that CS induces migration of neutrophils [31], and neutrophil-like HL60 cells secrete IL-8, a potent neutrophil chemokine, in response to CS in direct correlation with the level of N-acetylation [32]. CS has an immunomodulatory effect which is important for the wound healing process and depends on DD [33]. It was shown that micro- and nano-sized CS particles induce inflammasome formation by macrophages [33,34,35,36]. In contrast, macro-sized CS scaffolds inhibit the release of IL-1β and thus the formation of inflammasomes in mouse and human macrophages in vitro [37], making the use of macro-sized CS scaffolds rational when excessive inflammation is present. Moreover, CS also affects the expression of growth factors by increasing TGF-β1 expression in the early post-injury phase [38] and decreasing it in later stages by binding to anionic growth factors [39]. High DD CS stimulates proliferation of dermal fibroblasts allowing fibrous tissue formation and re-epithelialization [40,41]. The polyelectrolyte complex-based cryogel of CS-gelatin-oxidized dextran (Ox.D) and different CS-oxidized dextran compositions showed elastic modulus in the range 2.7–14.3 + 0.4 kPa. The proliferation rate for cell culture of fibroblasts on CS-Ox.D-gelatin (1:1:1) increased significantly compared to the other CS compositions with Ox.D due to internal porosity of pore walls [15,16]. CS containing scaffolds for wound healing could be made as 2D (films and fibers) and 3D (gels and sponges) with the properties required for wound management [42]. The antimicrobial effect of CS could be enhanced by the addition of antimicrobial agents. In a recent study, a complex CS-cordycepin hydrogel with increased antimicrobial activity was developed without the addition of any cross-linking agents via a freeze-drying method where negatively charged cordycepin adhered to positively charged CS chains [43]. In another study, textile polyethylene terephthalate composed of layer-by-layer coated CS was loaded with chlorhexidine and the mechanical stability of the composite was increased by thermal post-treatment which also increased the duration of chlorhexidine release up to 7 weeks [44]. CS alone or in complex with other natural polymers is also used as a part of asymmetric membranes, usually in an underlying layer that is in contact with the damaged skin [45]. Addition of nanoparticles (NPs) into hydrogels is another strategy used in biomaterial preparation [13]. Shah and colleagues developed triple-component nanocomposite film that contained CS-silver-sericin and was loaded with moxifloxacin. The obtained films possess not only high antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA) strains (clinical isolates) but also support wound healing in a rat model, similar to commercial wound dressings [46]. Most of the CS composite films containing collagen have intrinsic properties to induce healing, but the drawback is an allergic reaction to non-human collagen and therefore other safe substitutes are in demand. For example, human keratin-CS membrane with improved mechanical properties produced by the UV-crosslinking method shows potential as a wound dressing [47]. CS-chondroitin sulfate-based polyelectrolyte complex shows an efficient antimicrobial effect and cytocompatibility suitable for wound healing applications [48]. Furthermore, positively charged CS containing biomaterials could be loaded with growth factors and cytokines to improve their performance in the wound healing process. In a recent study, CS NPs prepared through ionotropic gelation with tripolyphosphate [49] were loaded with granulocyte-macrophage colony-stimulating factor (GM-CSF) as a part of a nanocrystalline cellulose–hyaluronic acid composite prepared by a freeze-drying method [50]. Loading efficiency of GM-CSF was as high as 97.4 ± 1.68% with sustained release of ~100% over 48 h and in vivo experiments have shown that composites loaded with encapsulated GM-CSF in CS NPs induce greater wound closure compared to the composite alone [50]. Polycaprolactone nanofibers loaded with CS NPs containing GM-CSF also showed accelerated wound closure [51]. Modification of CS with peptides also promotes wound closure, for example, CS hydrogels made from Ser-Ile-Lys-Val-Ala-Val-chitosan macromers [52] when applied in vivo induces collagen expression, angiogenesis, expression of TGF-β1, and inhibits the expression of TNF-α, IL-1β, and IL-6 mRNA in a mouse skin wound model [53]. CS could be further modified to increase affinity for the growth factors. For example, developed heparin-like polysaccharide (2-N, 6-O-sulfated CS) has a high affinity to the vascular endothelial growth factor in comparison to heparin due its higher sulfonation degree [54,55].
3.2. Bone and Cartilage Regeneration
During the development of biomaterials for bone and cartilage regeneration, it is necessary to not only create a scaffold that is biocompatible and biodegradable, but also contains suitable mechanical properties with interconnected pores [15] that supports the differentiation status of cells, as well as the differentiation of stem cells into osteocytes and chondrocytes [56]. It is sometimes not possible to prepare a biomaterial with these desired properties using only one polymer. Therefore, composite or hybrid materials are created where a supportive scaffold could be added to comply with the necessary mechanical properties [57]. CS is used to create biomaterials for the regeneration of hard tissues such as bone and cartilage. In a hydrated state, CS scaffolds lack mechanical stability and therefore require extra modifications [58]. CS induces apatite deposition [59,60,61] and this phenomenon of the polymer has been used to enhance biomineralization of composite materials because CS favors calcium/phosphate ion accumulation and enhances the biomineralization potential of poly(ethylene glycol) diacrylate/CS-based hydrogel [52].
3.2.1. Bone
CS mechanical properties are usually increased by the addition of hydroxyapatite due to its biological similarity to bone inorganic component [62]. In addition to hydroxyapatite, other composites, such as nano-zirconia/CS, nano-calcium zirconate/CS, and strontium-modified CS/montmorillonite composites with comparable mechanical properties were designed [63,64]. It was shown that MC3T3-E1 pre-osteoblastic cells when cultured on a CS-graft-polycaprolactone copolymer surface, in comparison to a tissue culture-treated polystyrene surface, show significantly higher alkaline phosphatase activity, deposition of calcium, and ECM synthesis [65]. For example, the addition of hydroxyapatite or bioglass to the matrix led to a compressive strength increase compared to CS alone. The polycationic nature of CS provides the possibility of designing polyelectrolyte complexes with polyanionic polymers to improve the mechanical properties of composite scaffolds [15,66]. In one study, CS/chondroitin/nano-bioglass-based polyelectrolyte composite material was developed with improved bioactivity, such as accumulation of apatite and increased expression of type-1 collagen by MG63 osteoblast-like cells in vitro and with osteointegration of the scaffold in vivo [67]. CS possesses active biomineralization properties and these could be further increased by introducing other polymers such as fucoidan [17,68] and bioglass [69].
Freeze-dried CS/gelatin scaffolds crosslinked with either glutaraldehyde or genipin support bone regeneration in vivo in mice inducing ECM production with minimal inflammatory reactions [70]. Thermosensitive hydrogel based on CS and beta-glycerophosphate was developed, however, it presented some biocompatibility issues due to an increased amount of substances required for gelation at body temperature. Recently, it was shown that the addition of TEMPO-oxidized cellulose nanofiber induced faster gelation and increased porosity with improved biocompatibility in vitro and in vivo in comparison to CS [71]. CS could be layered on top of metal (e.g., titanium) implants to increase osteointegration [72,73]. Composite materials based on polypyrrole/CS was synthesized through in situ electrochemical polymerization in oxalic acid medium and coated on 316L SS implants showing biocompatibility and protection against corrosion [74]. Recently, CS has been utilized in 3D printing for various tissue engineering applications [75]. CS-hydroxyapatite hydrogels were produced by a thermal cross-linking reaction using glycerol phosphate disodium salt and successfully printed on an extruder-based bioprinter. As a result, cells seeded on the printed scaffold increased osteogenic markers expression in comparison to 3D printed alginate and alginate-hydroxyapatite scaffolds [76].
3.2.2. Cartilage
Regeneration of cartilage damaged by injury, disease (osteoarthritis), and degeneration as a result of aging is an important task in modern orthopedics. The approaches used to regenerate cartilage are microfracture, mosaicplasty, autologous chondrocyte, and biomaterial implantation [77]. An important limitation is the absence of blood vessels in the cartilage tissue, thus, the task of creating a biomaterial capable of stimulating the regeneration of cartilage under avascular conditions is the main goal of tissue engineering [78].
Designed biomaterials created for cartilage regeneration should be able to support cell proliferation and differentiation. Therefore, the use of cells and 3D scaffold together is a practical approach in tissue engineering [79,80]. The microstructural architecture, physicochemical, and biochemical properties of the scaffold should be able to provide a temporary template for cells and support ECM synthesis required for the formation of cartilage tissue [81]. This means that scaffolds, in addition to their biocompatibility and biodegradability, should be porous with interconnected pores [79]. Three-dimensional scaffolds, such as hydrogels, fibrous materials, and foams/sponges, are common scaffolds used in cartilage regeneration research [81]. Usually, scaffolds include cells (differentiated chondrocytes and stem cells) and bioactive molecules (peptides, growth factors, and cytokines). Hydrogels could offer high water content and support chondrogenesis potential, implantation without open surgery, and in situ scaffold formation. The low mechanical properties of hydrogels (E ≈ 200 kPa) [77] can be overcome with the use of solid supporters which improve the mechanical stability of the hydrogel [82].
CS as a natural material with a structural similarity to sulfated glycosaminoglycans provides a compatible microenvironment for chondrocyte proliferation, ECM synthesis, and chondrogenesis [78,80,83,84,85]. It was also demonstrated that chondrocytes cultured in CS-alginate beads reduce the expression of inflammatory cytokines (IL-6 and IL-8) and increase cartilage matrix components (hyaluronan and aggrecan) synthesis in vitro, in comparison to alginate beads alone [86]. CS derivative carboxymethyl-CS in a dose-dependent manner reduced the inflammatory profile of primary rat chondrocytes by reducing iNOS expression and upregulating the anti-inflammatory cytokine IL-10 in vitro [87]. In another study, the addition of hyaluronic acid-CS NPs to a pellet co-culture of the human infrapatellar fat pad (IPFP)-derived mesenchymal stem cells (MSCs) with osteoarthritic chondrocytes increased chondrogenic differentiation [88]. Human IPFP-MSCs seeded on 3D-printed CS scaffolds in chondrogenic media containing TGF-β3 and BMP-6 attach, proliferate, and differentiate into chondrocyte-like cells modulating the formation of cartilaginous tissue in vitro [89].
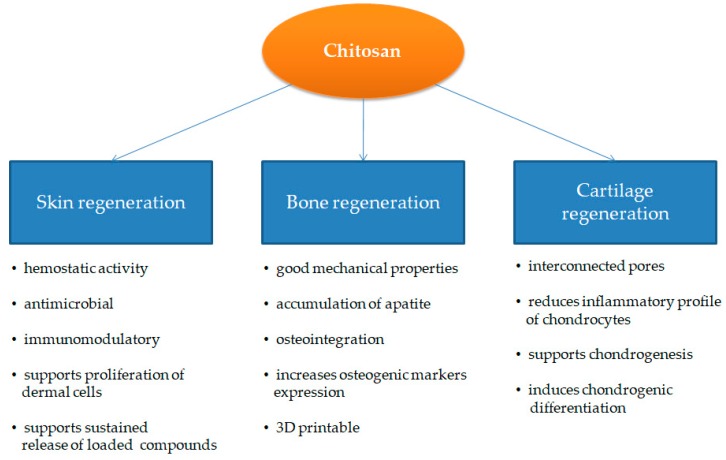
CS also interacts with collagen via electrostatic interactions between abundant amino groups and sulfo groups [90], and freeze-dried type 2 collagen-CS hybrid scaffold possesses improved stiffness in comparison to single component scaffolds, with a good porous structure resembling cartilage [91]. Moreover, type II collagen-CS scaffolds were also combined in the bi-layered scaffold with poly(lactic-co-glycolic acid) (PLGA) to further increase the mechanical and functional properties of biocomposites for cartilage regeneration [92]. CS-silk fibroin blends have also shown potential in cartilage regeneration [93,94]. One study found that bovine chondrocytes seeded on CS fibers made by an electrospinning method with a diameter of 300 nm have a 2-fold higher ratio of collagen II/collagen I in comparison to cells cultured on sponge-like scaffolds [95]. It is also important to note that a new type of supermacroporous scaffold made by a cryogelation method (cryogel) is gaining attention [13,14,15,16]. Supermacroporous (85–100 µm pore diameter) CS-agarose-gelatin scaffolds made by a cryogelation method (cryogel) possess good mechanical properties with an affable compression modulus of approximately 44 kPa of 5% cryogel at 15% deformation [96]. In vivo experiments for the repair of subchondral cartilage defects in female New Zealand white rabbits using CS-agarose-gelatin cryogels have shown the formation of hyaline cartilage without any hypertrophy markers by the fourth week post-implantation [97]. It is important to note that CS films induce human bone marrow MSCs to differentiate into chondrocyte-like spheroids in vitro via mTOR/S6K activation [98]. The main advantages of CS for skin, bone, and cartilage regeneration are highlighted in Figure 2.
Figure 2.
Main properties of chitosan (CS) used for skin, bone, and cartilage regeneration.
3.3. Chitosan for Drug Delivery
As a natural component, CS presents itself as an interesting substance for drug delivery applications. It is biodegradable and susceptible to degradation by lysozyme produced by mucosal tissue [99] and chitinase produced by intestinal flora [100]. CS solubility increases under acidic conditions which is useful for oral delivery of the drug. However, low solubility under physiological pH possesses some limitations. Due to its mucoadhesive nature [101], CS has been used as a vehicle to deliver drugs to nasal [102], ocular [103], buccal [104], and pulmonary tissues [105]. For drug delivery purposes, CS is used in the form of nano/microparticles which is synthesized by emulsion, coacervation/precipitation, ionic gelation, reverse micellar methods, etc. [9]. The problem of solubility of CS under physiological conditions, which is required for efficient delivery of drugs, is usually solved by chemical modification of CS and includes quaternization, alkylation, acetylation, carboxymethylation, CS/polyol salt combinations, synthesis of N-trimethyl CS, generation of sugar-bearing CS, conjugation with polyethylene oxide, generation of glycol-CS, etc. [9,106]. For the encapsulation of hydrophobic substances, amphiphilic CS derivatives were synthesized [107]. CS moiety is modified with a long chain alkyl group with hydrophobic function, and the addition of hydrophilic groups, such as succinyl, to the amino group enables CS derivative to form micelles in aqueous media [108]. Micelle-forming N-succinyl-N′-octyl CS (SOC), N-octyl-N-trimethyl CS, and N-octyl-O-sulfate have been studied to deliver doxorubicin, hydroxycamptothecin (10-HCPT), and paclitaxel for tumor-targeted therapy with increased encapsulation [107].
CS NPs produced by an emulsion method is also used for the delivery of proteins and peptides [109]. Its high loading efficiency and sustained release of proteins in CS particles have been reported. However, it includes sequential cross-linking with tripolyphosphate, glutaraldehyde, and genipin, which could affect the biological activity of loaded proteins [110]. The emulsion method’s limitation could be prevented with the use of coacervation/precipitation, ionic gelation, polyelectrolyte formation, spray drying, and supercritical fluid drying methods [111]. CS microspheres loaded with recombinant human interleukin-2 have been prepared by a coacervation/precipitation method without the use of cross-linking agents [112]. The polycationic nature of CS is used to prepare polyelectrolyte complexes which spontaneously form upon mixing. For example, heparin is widely used with CS polyelectrolyte complex due to its ability to bind growth factors and cytokines [113,114,115,116,117]. In a recent study, CS-heparin NPs were used for the delivery of siRNA against vascular endothelial growth factor in human retinal epithelial cells (ARPE-19) with a 2-fold higher transfection efficiency in comparison to carrying plasmid DNA alone [118]. In addition to gene delivery, CS could be modified to deliver growth factors and cytokines [119]. The addition of a sulfate group to CS mimics heparin and heparan sulfate and retains its intrinsic antimicrobial properties [120]. Sulfated CS is able to bind fibroblast growth factor-2 [121] and bone morphogenetic protein-2 [122] and protects them from proteolytic cleavage [123]. Moreover, it was shown that sulfated CS binds to the proteins better than heparin [124].
CS as a non-viral gene delivery system has also been explored [9,125]. CS was used as a non-viral delivery system for plasmid transfection in 1995 [126]. The polycationic nature of CS interacts not only with negatively charged nucleic acid molecules forming a polyelectrolyte complex [127,128], but also with negatively charged cellular membranes, which results in increased uptake efficiency [129]. Nowadays, CS is used to deliver siRNA [130] and miRNA [131,132]. A widely used method for the preparation of CS for gene delivery is ionic gelation [133,134] and coacervation [135,136]. Recent application of CS and its derivatives for drug delivery is summarized in Table 1.
Table 1.
Chitosan and its derivatives for drug delivery. NPs: nanoparticles.
| CS/Derivatives | Type/Delivery System | Application | Ref. |
|---|---|---|---|
| N-succinyl-N′-octyl chitosan (SOC) | Self-assembled polymeric micelles | Controlled anticancer drug release | [108,137] |
| Tumor targeted therapy | [138,139,140] | ||
| Biomedical optical imaging | [141] | ||
| N-octyl-N-trimethyl chitosan | Self-assembled polymeric micelles | Controlled anticancer drug release | [142] |
| Tumor targeted therapy | [143] | ||
| N-octyl-O-sulfate chitosan | Self-assembled polymeric micelles | Absorption enhancement of anticancer drug | [144,145] |
| Tumor targeted therapy | [146,147,148] | ||
| Increasing stability of drug loaded liposomes | [149] | ||
| 2-[phenylhydrazine (or hydrazine)-thiosemicarbazone]-chitosan | Powder | Pharmaceutical and food industries | [150] |
| (Ser-Ile-Lys-Val-Ala-Val) peptide-modified chitosan | Hydrogel | Skin substitutes for wound closure in mice | [53,151] |
| Galactosylated chitosan (GC) | NPs | Tumor targeted therapy | [152,153,154,155] |
| siRNA delivery | [156,157] | ||
| N-palmitoyl chitosan (NPCS) | MPs and micelles | Tumor targeted therapy | [158,159] |
| O-palmitoyl chitosan (OPC) | Liposomes | Intestinal drug delivery | [160] |
| Hydroxyapatite/CS | NPs | Drug delivery | [161,162,163,164] |
| CS loaded with antioxidant NPs | Hydrogel | Drug release | [165] |
| PEGylated CS | NPs | Tumor targeted therapy | [166,167,168] |
| Chitosan-based vaccine | Polyelectrolyte, NPs | Intranasal CS-DNA vaccine | [169,170] |
4. Conclusions
CS, as a natural polymer, is actively used in tissue engineering and regenerative medicine as a biomaterial alone, as well as in combination with other polymers. In addition to its suitable mechanical physico-chemical properties, CS has a natural ability to stimulate tissue regeneration. Active research is underway in improving CS-containing scaffolds for wound healing, bone, and cartilage regeneration. In addition to this, CS-containing polymers are being actively studied for the delivery of drugs for targeted tumor therapy and nucleic acid delivery in genetic engineering applications. Further research on the preparation of CS-containing scaffolds via 3D printing and cryogelation methods will facilitate the application of CS in biomedicine. CS, as a part of any material, could introduce valuable properties such as antimicrobial activity, mucoadhesiveness, and biocompatibility, which are in demand for biomedical use. We believe that further research on CS and the search for new variations in its use with other polymers will reveal even greater prospects and properties of this unique polymer in biomedical applications.
Funding
A.S. is supported by a grant from the Ministry of Education and Science of the Republic of Kazakhstan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Oryan A., Alidadi S., Moshiri A., Maffulli N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014;9:18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez-Vázquez M., Vega-Ruiz B., Ramos-Zúñiga R., Saldaña-Koppel D.A., Quiñones-Olvera L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015;2015:1–15. doi: 10.1155/2015/821279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoven V.P., Tangpasuthadol V., Angkitpaiboon Y., Vallapa N., Kiatkamjornwong S. Surface-charged chitosan: Preparation and protein adsorption. Carbohydr. Polym. 2007;68:44–53. doi: 10.1016/j.carbpol.2006.07.008. [DOI] [Google Scholar]
- 4.Saranya N., Moorthi A., Saravanan S., Devi M.P., Selvamurugan N. Chitosan and its derivatives for gene delivery. Int. J. Biol. Macromol. 2011;48:234–238. doi: 10.1016/j.ijbiomac.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesan J., Anil S., Kim S.K., Shim M.S. Chitosan as a vehicle for growth factor delivery: Various preparations and their applications in bone tissue regeneration. Int. J. Biol. Macromol. 2017;104:1383–1397. doi: 10.1016/j.ijbiomac.2017.01.072. [DOI] [PubMed] [Google Scholar]
- 6.Tharanathan R.N., Kittur F.S. Chitin—The Undisputed Biomolecule of Great Potential. Crit. Rev. Food Sci. Nutr. 2003;43:61–87. doi: 10.1080/10408690390826455. [DOI] [PubMed] [Google Scholar]
- 7.Ghormade V., Pathan E.K., Deshpande M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017;104:1415–1421. doi: 10.1016/j.ijbiomac.2017.01.112. [DOI] [PubMed] [Google Scholar]
- 8.Grifoll-Romero L., Pascual S., Aragunde H., Biarnés X., Planas A. Chitin Deacetylases: Structures, Specificities, and Biotech Applications. Polymers. 2018;10:352. doi: 10.3390/polym10040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahsan S.M., Thomas M., Reddy K.K., Sooraparaju S.G., Asthana A., Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018;110:97–109. doi: 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- 10.Balau L., Lisa G., Popa M., Tura V., Melnig V. Physico-chemical properties of Chitosan films. Open Chem. 2004;2:638–647. doi: 10.2478/BF02482727. [DOI] [Google Scholar]
- 11.Fan M., Hu Q., Shen K. Preparation and structure of chitosan soluble in wide pH range. Carbohydr. Polym. 2009;78:66–71. doi: 10.1016/j.carbpol.2009.03.031. [DOI] [Google Scholar]
- 12.Roberts G.A.F. Chitin Chemistry. Macmillan Education; London, UK: 1992. Solubility and Solution Behaviour of Chitin and Chitosan; pp. 274–329. [Google Scholar]
- 13.Berillo D., Mattiasson B., Kirsebom H. Cryogelation of chitosan using noble-metal ions: In situ formation of nanoparticles. Biomacromolecules. 2014;15:2246–2255. doi: 10.1021/bm5003834. [DOI] [PubMed] [Google Scholar]
- 14.Berillo D., Cundy A. 3D-macroporous chitosan-based scaffolds with in situ formed Pd and Pt nanoparticles for nitrophenol reduction. Carbohydr. Polym. 2018;192:166–175. doi: 10.1016/j.carbpol.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Berillo D., Elowsson L., Kirsebom H. Oxidized Dextran as Crosslinker for Chitosan Cryogel Scaffolds and Formation of Polyelectrolyte Complexes between Chitosan and Gelatin. Macromol. Biosci. 2012;12:1090–1099. doi: 10.1002/mabi.201200023. [DOI] [PubMed] [Google Scholar]
- 16.Akilbekova D., Shaimerdenova M., Adilov S., Berillo D. Biocompatible scaffolds based on natural polymers for regenerative medicine. Int. J. Biol. Macromol. 2018;114:324–333. doi: 10.1016/j.ijbiomac.2018.03.116. [DOI] [PubMed] [Google Scholar]
- 17.Lu H.-T., Lu T.-W., Chen C.-H., Lu K.-Y., Mi F.-L. Development of nanocomposite scaffolds based on biomineralization of N,O-carboxymethyl chitosan/fucoidan conjugates for bone tissue engineering. Int. J. Biol. Macromol. 2018;120:2335–2345. doi: 10.1016/j.ijbiomac.2018.08.179. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen-Nygaard J., Strand S., Vårum K., Draget K., Nordgård C., Nilsen-Nygaard J., Strand S.P., Vårum K.M., Draget K.I., Nordgård C.T. Chitosan: Gels and Interfacial Properties. Polymers. 2015;7:552–579. doi: 10.3390/polym7030552. [DOI] [Google Scholar]
- 19.Badwan A., Rashid I., Omari M., Darras F. Chitin and Chitosan as Direct Compression Excipients in Pharmaceutical Applications. Mar. Drugs. 2015;13:1519–1547. doi: 10.3390/md13031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rousselle P., Montmasson M., Garnier C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019;75:12–26. doi: 10.1016/j.matbio.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Clark R. Principles of Tissue Engineering. Academic Press; San Diego, CA, USA: 1997. Wound repair: Lessons for tissue engineering. [Google Scholar]
- 22.Abdelrahman T., Newton H. Wound dressings: Principles and practice. Surgery. 2011;29:491–495. doi: 10.1016/j.mpsur.2011.06.007. [DOI] [Google Scholar]
- 23.Khan M.A., Mujahid M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019;124:138–147. doi: 10.1016/j.ijbiomac.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 24.Hu Z., Lu S., Cheng Y., Kong S., Li S., Li C., Yang L. Investigation of the Effects of Molecular Parameters on the Hemostatic Properties of Chitosan. Molecules. 2018;23:3147. doi: 10.3390/molecules23123147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J., Tian F., Wang Z., Wang Q., Zeng Y.-J., Chen S.-Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008;84:131–137. doi: 10.1002/jbm.b.30853. [DOI] [PubMed] [Google Scholar]
- 26.Guo X., Sun T., Zhong R., Ma L., You C., Tian M., Li H., Wang C. Effects of Chitosan Oligosaccharides on Human Blood Components. Front. Pharmacol. 2018;9:1412. doi: 10.3389/fphar.2018.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X., Zhang X., Zhou J., Li L. An investigation of chitosan and its derivatives on red blood cell agglutination. RSC Adv. 2017;7:12247–12254. doi: 10.1039/C6RA27417J. [DOI] [Google Scholar]
- 28.Klokkevold P.R., Fukayama H., Sung E.C., Bertolami C.N. The effect of chitosan (poly-N-acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J. Oral Maxillofac. Surg. 1999;57:49–52. doi: 10.1016/S0278-2391(99)90632-8. [DOI] [PubMed] [Google Scholar]
- 29.Hattori H., Ishihara M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of chitosan. Biomed. Mater. 2015;10:015014. doi: 10.1088/1748-6041/10/1/015014. [DOI] [PubMed] [Google Scholar]
- 30.Hu Z., Zhang D.-Y., Lu S.-T., Li P.-W., Li S.-D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Mar. Drugs. 2018;16:273. doi: 10.3390/md16080273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueno H., Yamada H., Tanaka I., Kaba N., Matsuura M., Okumura M., Kadosawa T., Fujinaga T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 1999;20:1407–1414. doi: 10.1016/S0142-9612(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 32.Park C.J., Gabrielson N.P., Pack D.W., Jamison R.D., Wagoner Johnson A.J. The effect of chitosan on the migration of neutrophil-like HL60 cells, mediated by IL-8. Biomaterials. 2009;30:436–444. doi: 10.1016/j.biomaterials.2008.09.060. [DOI] [PubMed] [Google Scholar]
- 33.Bueter C.L., Lee C.K., Rathinam V.A.K., Healy G.J., Taron C.H., Specht C.A., Levitz S.M. Chitosan but Not Chitin Activates the Inflammasome by a Mechanism Dependent upon Phagocytosis. J. Biol. Chem. 2011;286:35447–35455. doi: 10.1074/jbc.M111.274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bueter C.L., Lee C.K., Wang J.P., Ostroff G.R., Specht C.A., Levitz S.M. Spectrum and Mechanisms of Inflammasome Activation by Chitosan. J. Immunol. 2014;192:5943–5951. doi: 10.4049/jimmunol.1301695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gudmundsdottir S., Lieder R., Sigurjonsson O.E., Petersen P.H. Chitosan leads to downregulation of YKL-40 and inflammasome activation in human macrophages. J. Biomed. Mater. Res. Part A. 2015;103:2778–2785. doi: 10.1002/jbm.a.35417. [DOI] [PubMed] [Google Scholar]
- 36.Fong D., Grégoire-Gélinas P., Cheng A.P., Mezheritsky T., Lavertu M., Sato S., Hoemann C.D. Lysosomal rupture induced by structurally distinct chitosans either promotes a type 1 IFN response or activates the inflammasome in macrophages. Biomaterials. 2017;129:127–138. doi: 10.1016/j.biomaterials.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Vasconcelos D.P., de Torre-Minguela C., Gomez A.I., Águas A.P., Barbosa M.A., Pelegrín P., Barbosa J.N. 3D chitosan scaffolds impair NLRP3 inflammasome response in macrophages. Acta Biomater. 2019;91:123–134. doi: 10.1016/j.actbio.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 38.Baxter R.M., Dai T., Kimball J., Wang E., Hamblin M.R., Wiesmann W.P., McCarthy S.J., Baker S.M. Chitosan dressing promotes healing in third degree burns in mice: Gene expression analysis shows biphasic effects for rapid tissue regeneration and decreased fibrotic signaling. J. Biomed. Mater. Res. A. 2013;101:340–348. doi: 10.1002/jbm.a.34328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai C.-W., Chiang I.-N., Wang J.-H., Young T.-H. Chitosan delaying human fibroblast senescence through downregulation of TGF-β signaling pathway. Artif. Cells Nanomed. Biotechnol. 2017;46:1–12. doi: 10.1080/21691401.2017.1394873. [DOI] [PubMed] [Google Scholar]
- 40.Howling G.I., Dettmar P.W., Goddard P.A., Hampson F.C., Dornish M., Wood E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials. 2001;22:2959–2966. doi: 10.1016/S0142-9612(01)00042-4. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton V., Yuan Y., Rigney D.A., Puckett A.D., Ong J.L., Yang Y., Elder S.H., Bumgardner J.D. Characterization of chitosan films and effects on fibroblast cell attachment and proliferation. J. Mater. Sci. Mater. Med. 2006;17:1373–1381. doi: 10.1007/s10856-006-0613-9. [DOI] [PubMed] [Google Scholar]
- 42.Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013;49:780–792. doi: 10.1016/j.eurpolymj.2012.12.009. [DOI] [Google Scholar]
- 43.Song R., Zheng J., Liu Y., Tan Y., Yang Z., Song X., Yang S., Fan R., Zhang Y., Wang Y. A natural cordycepin/chitosan complex hydrogel with outstanding self-healable and wound healing properties. Int. J. Biol. Macromol. 2019;134:91–99. doi: 10.1016/j.ijbiomac.2019.04.195. [DOI] [PubMed] [Google Scholar]
- 44.Aubert-Viard F., Mogrovejo-Valdivia A., Tabary N., Maton M., Chai F., Neut C., Martel B., Blanchemain N. Evaluation of antibacterial textile covered by layer-by-layer coating and loaded with chlorhexidine for wound dressing application. Mater. Sci. Eng. C. 2019;100:554–563. doi: 10.1016/j.msec.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 45.Alves P., Santos M., Mendes S., Miguel P.S., de Sá D.K., Cabral S.D.C., Correia J.I., Ferreira P., Alves P., Santos M., et al. Photocrosslinkable Nanofibrous Asymmetric Membrane Designed for Wound Dressing. Polymers. 2019;11:653. doi: 10.3390/polym11040653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah A., Ali Buabeid M., Arafa E.-S.A., Hussain I., Li L., Murtaza G. The wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films loaded with moxifloxacin. Int. J. Pharm. 2019;564:22–38. doi: 10.1016/j.ijpharm.2019.04.046. [DOI] [PubMed] [Google Scholar]
- 47.Lin C.-W., Chen Y.-K., Lu M., Lou K.-L., Yu J. Photo-Crosslinked Keratin/Chitosan Membranes as Potential Wound Dressing Materials. Polymers. 2018;10:987. doi: 10.3390/polym10090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma S., Swetha K.L., Roy A. Chitosan-Chondroitin sulfate based polyelectrolyte complex for effective management of chronic wounds. Int. J. Biol. Macromol. 2019;132:97–108. doi: 10.1016/j.ijbiomac.2019.03.186. [DOI] [PubMed] [Google Scholar]
- 49.Koukaras E.N., Papadimitriou S.A., Bikiaris D.N., Froudakis G.E. Insight on the Formation of Chitosan Nanoparticles through Ionotropic Gelation with Tripolyphosphate. Mol. Pharm. 2012;9:2856–2862. doi: 10.1021/mp300162j. [DOI] [PubMed] [Google Scholar]
- 50.Karimi Dehkordi N., Minaiyan M., Talebi A., Akbari V., Taheri A. Nanocrystalline cellulose–hyaluronic acid composite enriched with GM-CSF loaded chitosan nanoparticles for enhanced wound healing. Biomed. Mater. 2019;14:035003. doi: 10.1088/1748-605X/ab026c. [DOI] [PubMed] [Google Scholar]
- 51.Tanha S., Rafiee-Tehrani M., Abdollahi M., Vakilian S., Esmaili Z., Naraghi Z.S., Seyedjafari E., Javar H.A. G-CSF loaded nanofiber/nanoparticle composite coated with collagen promotes wound healing in vivo. J. Biomed. Mater. Res. Part A. 2017;105:2830–2842. doi: 10.1002/jbm.a.36135. [DOI] [PubMed] [Google Scholar]
- 52.Chen X., Zhang M., Chen S., Wang X., Tian Z., Chen Y., Xu P., Zhang L., Zhang L., Zhang L. Peptide-Modified Chitosan Hydrogels Accelerate Skin Wound Healing by Promoting Fibroblast Proliferation, Migration, and Secretion. Cell Transplant. 2017;26:1331–1340. doi: 10.1177/0963689717721216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X., Fu W., Cao X., Jiang H., Che X., Xu X., Ma B., Zhang J. Peptide SIKVAV-modified chitosan hydrogels promote skin wound healing by accelerating angiogenesis and regulating cytokine secretion. Am. J. Transl. Res. 2018;10:4258–4268. [PMC free article] [PubMed] [Google Scholar]
- 54.Yu Y., Chen R., Sun Y., Pan Y., Tang W., Zhang S., Cao L., Yuan Y., Wang J., Liu C. Manipulation of VEGF-induced angiogenesis by 2-N, 6-O-sulfated chitosan. Acta Biomater. 2018;71:510–521. doi: 10.1016/j.actbio.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 55.Wang C., Yu Y., Chen H., Zhang S., Wang J., Liu C. Construction of cytokine reservoirs based on sulfated chitosan hydrogels for the capturing of VEGF in situ. J. Mater. Chem. B. 2019;7:1882–1892. doi: 10.1039/C8TB02895H. [DOI] [PubMed] [Google Scholar]
- 56.Titorencu I., Albu M., Nemecz M., Jinga V. Natural Polymer-Cell Bioconstructs for Bone Tissue Engineering. Curr. Stem Cell Res. Ther. 2016;12:165–174. doi: 10.2174/1574888X10666151102105659. [DOI] [PubMed] [Google Scholar]
- 57.Saravanan S., Leena R.S., Selvamurugan N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016;93:1354–1365. doi: 10.1016/j.ijbiomac.2016.01.112. [DOI] [PubMed] [Google Scholar]
- 58.Deepthi S., Venkatesan J., Kim S.-K., Bumgardner J.D., Jayakumar R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016;93:1338–1353. doi: 10.1016/j.ijbiomac.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 59.He L.-H., Yao L., Xue R., Sun J., Song R. In-situ mineralization of chitosan/calcium phosphate composite and the effect of solvent on the structure. Front. Mater. Sci. 2011;5:282–292. doi: 10.1007/s11706-011-0140-6. [DOI] [Google Scholar]
- 60.Tuzlakoglu K., Reis R.L. Formation of bone-like apatite layer on chitosan fiber mesh scaffolds by a biomimetic spraying process. J. Mater. Sci. Mater. Med. 2007;18:1279–1286. doi: 10.1007/s10856-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 61.Leonor I.B., Baran E.T., Kawashita M., Reis R.L., Kokubo T., Nakamura T. Growth of a bonelike apatite on chitosan microparticles after a calcium silicate treatment. Acta Biomater. 2008;4:1349–1359. doi: 10.1016/j.actbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Elkholy S., Yahia S., Awad M., Elmessiery M. In vivo evaluation of β-CS/n-HA with different physical properties as a new bone graft material. Clin. Implant Dent. Relat. Res. 2018;20:416–423. doi: 10.1111/cid.12599. [DOI] [PubMed] [Google Scholar]
- 63.Gaihre B., Jayasuriya A.C. Comparative investigation of porous nano-hydroxyapaptite/chitosan, nano-zirconia/chitosan and novel nano-calcium zirconate/chitosan composite scaffolds for their potential applications in bone regeneration. Mater. Sci. Eng. C. 2018;91:330–339. doi: 10.1016/j.msec.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koç Demir A., Elçin A.E., Elçin Y.M. Strontium-modified chitosan/montmorillonite composites as bone tissue engineering scaffold. Mater. Sci. Eng. C. 2018;89:8–14. doi: 10.1016/j.msec.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Georgopoulou A., Kaliva M., Vamvakaki M., Chatzinikolaidou M. Osteogenic Potential of Pre-Osteoblastic Cells on a Chitosan-graft-Polycaprolactone Copolymer. Materials. 2018;11:490. doi: 10.3390/ma11040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Habibovic P., Barralet J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011;7:3013–3026. doi: 10.1016/j.actbio.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 67.Singh B.N., Veeresh V., Mallick S.P., Jain Y., Sinha S., Rastogi A., Srivastava P. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019;133:817–830. doi: 10.1016/j.ijbiomac.2019.04.107. [DOI] [PubMed] [Google Scholar]
- 68.Lu H.-T., Lu T.-W., Chen C.-H., Mi F.-L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019;128:973–984. doi: 10.1016/j.ijbiomac.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Keller L., Regiel-Futyra A., Gimeno M., Eap S., Mendoza G., Andreu V., Wagner Q., Kyzioł A., Sebastian V., Stochel G., et al. Chitosan-based nanocomposites for the repair of bone defects. Nanomed. Nanotechnol. Biol. Med. 2017;13:2231–2240. doi: 10.1016/j.nano.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Georgopoulou A., Papadogiannis F., Batsali A., Marakis J., Alpantaki K., Eliopoulos A.G., Pontikoglou C., Chatzinikolaidou M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci. Mater. Med. 2018;29:59. doi: 10.1007/s10856-018-6064-2. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen T.H.M., Abueva C., Van Ho H., Lee S.-Y., Lee B.-T. In vitro and in vivo acute response towards injectable thermosensitive chitosan/TEMPO-oxidized cellulose nanofiber hydrogel. Carbohydr. Polym. 2018;180:246–255. doi: 10.1016/j.carbpol.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L., Wu K., Song W., Xu H., An R., Zhao L., Liu B., Zhang Y. Chitosan/siCkip-1 biofunctionalized titanium implant for improved osseointegration in the osteoporotic condition. Sci. Rep. 2015;5:10860. doi: 10.1038/srep10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dwivedi P., Narvi S.S., Tewari R.P. Application of polymer nanocomposites in the nanomedicine landscape: Envisaging strategies to combat implant associated infections. J. Appl. Biomater. Funct. Mater. 2013;11:129–142. doi: 10.5301/JABFM.2013.11544. [DOI] [PubMed] [Google Scholar]
- 74.Kumar A.M., Suresh B., Das S., Obot I.B., Adesina A.Y., Ramakrishna S. Promising bio-composites of polypyrrole and chitosan: Surface protective and in vitro biocompatibility performance on 316L SS implants. Carbohydr. Polym. 2017;173:121–130. doi: 10.1016/j.carbpol.2017.05.083. [DOI] [PubMed] [Google Scholar]
- 75.Liu J., Sun L., Xu W., Wang Q., Yu S., Sun J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019;207:297–316. doi: 10.1016/j.carbpol.2018.11.077. [DOI] [PubMed] [Google Scholar]
- 76.Demirtaş T.T., Irmak G., Gümüşderelioğlu M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication. 2017;9:035003. doi: 10.1088/1758-5090/aa7b1d. [DOI] [PubMed] [Google Scholar]
- 77.Liao I.-C., Moutos F.T., Estes B.T., Zhao X., Guilak F. Composite Three-Dimensional Woven Scaffolds with Interpenetrating Network Hydrogels to Create Functional Synthetic Articular Cartilage. Adv. Funct. Mater. 2013;23:5833–5839. doi: 10.1002/adfm.201300483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nettles D.L., Elder S.H., Gilbert J.A. Potential Use of Chitosan as a Cell Scaffold Material for Cartilage Tissue Engineering. Tissue Eng. 2002;8:1009–1016. doi: 10.1089/107632702320934100. [DOI] [PubMed] [Google Scholar]
- 79.Freedman B.R., Mooney D.J. Biomaterials to Mimic and Heal Connective Tissues. Adv. Mater. 2019;31:1806695. doi: 10.1002/adma.201806695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin R., Moreira Teixeira L.S., Dijkstra P.J., Karperien M., van Blitterswijk C.A., Zhong Z.Y., Feijen J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2544–2551. doi: 10.1016/j.biomaterials.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 81.Liu M., Zeng X., Ma C., Yi H., Ali Z., Mou X., Li S., Deng Y., He N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang H., Zhang X., Hu X., Dai L., Zhu J., Man Z., Chen H., Zhou C., Ao Y. Directing chondrogenic differentiation of mesenchymal stem cells with a solid-supported chitosan thermogel for cartilage tissue engineering. Biomed. Mater. 2014;9:035008. doi: 10.1088/1748-6041/9/3/035008. [DOI] [PubMed] [Google Scholar]
- 83.Kuo C.-Y., Chen C.-H., Hsiao C.-Y., Chen J.-P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015;117:722–730. doi: 10.1016/j.carbpol.2014.10.056. [DOI] [PubMed] [Google Scholar]
- 84.VandeVord P.J., Matthew H.W.T., DeSilva S.P., Mayton L., Wu B., Wooley P.H. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002;59:585–590. doi: 10.1002/jbm.1270. [DOI] [PubMed] [Google Scholar]
- 85.Choi B., Kim S., Lin B., Wu B.M., Lee M. Cartilaginous Extracellular Matrix-Modified Chitosan Hydrogels for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces. 2014;6:20110–20121. doi: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- 86.Oprenyeszk F., Sanchez C., Dubuc J.-E., Maquet V., Henrist C., Compère P., Henrotin Y. Chitosan enriched three-dimensional matrix reduces inflammatory and catabolic mediators production by human chondrocytes. PLoS ONE. 2015;10:e0128362. doi: 10.1371/journal.pone.0128362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kong Y., Zhang Y., Zhao X., Wang G., Liu Q. Carboxymethyl-chitosan attenuates inducible nitric oxide synthase and promotes interleukin-10 production in rat chondrocytes. Exp. Ther. Med. 2017;14:5641–5646. doi: 10.3892/etm.2017.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang S., Song X., Li T., Xiao J., Chen Y., Gong X., Zeng W., Yang L., Chen C. Pellet coculture of osteoarthritic chondrocytes and infrapatellar fat pad-derived mesenchymal stem cells with chitosan/hyaluronic acid nanoparticles promotes chondrogenic differentiation. Stem Cell Res. Ther. 2017;8:264. doi: 10.1186/s13287-017-0719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ye K., Felimban R., Traianedes K., Moulton S.E., Wallace G.G., Chung J., Quigley A., Choong P.F.M., Myers D.E. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PLoS ONE. 2014;9:e99410. doi: 10.1371/journal.pone.0099410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sionkowska A., Wisniewski M., Skopinska J., Kennedy C.J., Wess T.J. Molecular interactions in collagen and chitosan blends. Biomaterials. 2004;25:795–801. doi: 10.1016/S0142-9612(03)00595-7. [DOI] [PubMed] [Google Scholar]
- 91.Haaparanta A.-M., Järvinen E., Cengiz I.F., Ellä V., Kokkonen H.T., Kiviranta I., Kellomäki M. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2014;25:1129–1136. doi: 10.1007/s10856-013-5129-5. [DOI] [PubMed] [Google Scholar]
- 92.Su J.-Y., Chen S.-H., Chen Y.-P., Chen W.-C. Evaluation of Magnetic Nanoparticle-Labeled Chondrocytes Cultivated on a Type II Collagen-Chitosan/Poly(Lactic-co-Glycolic) Acid Biphasic Scaffold. Int. J. Mol. Sci. 2017;18:87. doi: 10.3390/ijms18010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhardwaj N., Nguyen Q.T., Chen A.C., Kaplan D.L., Sah R.L., Kundu S.C. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials. 2011;32:5773–5781. doi: 10.1016/j.biomaterials.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu J., Fang Q., Yu X., Wan Y., Xiao B. Chitosan-Based Nanofibrous Membrane Unit with Gradient Compositional and Structural Features for Mimicking Calcified Layer in Osteochondral Matrix. Int. J. Mol. Sci. 2018;19:2330. doi: 10.3390/ijms19082330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noriega S.E., Hasanova G.I., Schneider M.J., Larsen G.F., Subramanian A. Effect of fiber diameter on the spreading, proliferation and differentiation of chondrocytes on electrospun chitosan matrices. Cells Tissues Organs. 2012;195:207–221. doi: 10.1159/000325144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bhat S., Tripathi A., Kumar A. Supermacroprous chitosan–agarose–gelatin cryogels: In vitro characterization and in vivo assessment for cartilage tissue engineering. J. R. Soc. Interface. 2011;8:540–554. doi: 10.1098/rsif.2010.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gupta A., Bhat S., Jagdale P.R., Chaudhari B.P., Lidgren L., Gupta K.C., Kumar A. Evaluation of three-dimensional chitosan-agarose-gelatin cryogel scaffold for the repair of subchondral cartilage defects: An in vivo study in a rabbit model. Tissue Eng. Part A. 2014;20:3101–3111. doi: 10.1089/ten.tea.2013.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu T.-J., Chiu F.-Y., Chiu H.-Y., Chang M.-C., Hung S.-C. Chondrogenic Differentiation of Mesenchymal Stem Cells in Three-Dimensional Chitosan Film Culture. Cell Transplant. 2017;26:417–427. doi: 10.3727/096368916X693464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vårum K.M., Holme H.K., Izume M., Stokke B.T., Smidsrød O. Determination of enzymatic hydrolysis specificity of partially N-acetylated chitosans. Biochim. Biophys. Acta. 1996;1291:5–15. doi: 10.1016/0304-4165(96)00038-4. [DOI] [PubMed] [Google Scholar]
- 100.Rathore A.S., Gupta R.D. Chitinases from Bacteria to Human: Properties, Applications, and Future Perspectives. Enzyme Res. 2015;2015:791907. doi: 10.1155/2015/791907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sogias I.A., Williams A.C., Khutoryanskiy V.V. Why is Chitosan Mucoadhesive? Biomacromolecules. 2008;9:1837–1842. doi: 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
- 102.Casettari L., Illum L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release. 2014;190:189–200. doi: 10.1016/j.jconrel.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 103.Irimia T., Dinu-Pîrvu C.-E., Ghica M.V., Lupuleasa D., Muntean D.-L., Udeanu D.I., Popa L. Chitosan-Based in Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs. 2018;16:373. doi: 10.3390/md16100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Batista P., Castro P., Madureira A.R., Sarmento B., Pintado M. Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals. 2019;12:32. doi: 10.3390/ph12010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ortiz M., Jornada D.S., Pohlmann A.R., Guterres S.S. Development of Novel Chitosan Microcapsules for Pulmonary Delivery of Dapsone: Characterization, Aerosol Performance, and In Vivo Toxicity Evaluation. AAPS PharmSciTech. 2015;16:1033–1040. doi: 10.1208/s12249-015-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baranwal A., Kumar A., Priyadharshini A., Oggu G.S., Bhatnagar I., Srivastava A., Chandra P. Chitosan: An undisputed bio-fabrication material for tissue engineering and bio-sensing applications. Int. J. Biol. Macromol. 2018;110:110–123. doi: 10.1016/j.ijbiomac.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 107.Ahmed S., Ali A., Sheikh J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018;116:849–862. doi: 10.1016/j.ijbiomac.2018.04.176. [DOI] [PubMed] [Google Scholar]
- 108.Xu X., Li L., Zhou J., Lu S., Yang J., Yin X., Ren J. Preparation and characterization of N-succinyl-N′-octyl chitosan micelles as doxorubicin carriers for effective anti-tumor activity. Colloids Surf. B Biointerfaces. 2007;55:222–228. doi: 10.1016/j.colsurfb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 109.Mohammed M.A., Syeda J.T.M., Wasan K.M., Wasan E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics. 2017;9:53. doi: 10.3390/pharmaceutics9040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen K.-Y., Zeng S.-Y. Fabrication of Quaternized Chitosan Nanoparticles Using Tripolyphosphate/Genipin Dual Cross-Linkers as a Protein Delivery System. Polymers. 2018;10:1226. doi: 10.3390/polym10111226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Divya K., Jisha M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018;16:101–112. doi: 10.1007/s10311-017-0670-y. [DOI] [Google Scholar]
- 112.Özbaş-Turan S., Akbuǧa J., Aral C. Controlled Release of Interleukin-2 from Chitosan Microspheres. J. Pharm. Sci. 2002;91:1245–1251. doi: 10.1002/jps.10122. [DOI] [PubMed] [Google Scholar]
- 113.Samorezov J.E., Alsberg E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv. Drug Deliv. Rev. 2015;84:45–67. doi: 10.1016/j.addr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin C., Romero R., Sorokina L.V., Ballinger K.R., Place L.W., Kipper M.J., Khetani S.R. A polyelectrolyte multilayer platform for investigating growth factor delivery modes in human liver cultures. J. Biomed. Mater. Res. A. 2018;106:971–984. doi: 10.1002/jbm.a.36293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tan Q., Tang H., Hu J., Hu Y., Zhou X., Tao Y., Wu Z. Controlled release of chitosan/heparin nanoparticle-delivered VEGF enhances regeneration of decellularized tissue-engineered scaffolds. Int. J. Nanomed. 2011;6:929–942. doi: 10.2147/IJN.S18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liang Y., Kiick K.L. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014;10:1588–1600. doi: 10.1016/j.actbio.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mansurov N., Chen W.C.W., Awada H., Huard J., Wang Y., Saparov A. A controlled release system for simultaneous delivery of three human perivascular stem cell-derived factors for tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2018;12:e1164–e1172. doi: 10.1002/term.2451. [DOI] [PubMed] [Google Scholar]
- 118.Pilipenko I., Korzhikov-Vlakh V., Sharoyko V., Zhang N., Schäfer-Korting M., Rühl E., Zoschke C., Tennikova T. pH-Sensitive Chitosan–Heparin Nanoparticles for Effective Delivery of Genetic Drugs into Epithelial Cells. Pharmaceutics. 2019;11:317. doi: 10.3390/pharmaceutics11070317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zeng K., Groth T., Zhang K. Recent Advances in Artificially Sulfated Polysaccharides for Applications in Cell Growth and Differentiation, Drug Delivery, and Tissue Engineering. ChemBioChem. 2019;20:737–746. doi: 10.1002/cbic.201800569. [DOI] [PubMed] [Google Scholar]
- 120.Zhong Z., Li P., Xing R., Liu S. Antimicrobial activity of hydroxylbenzenesulfonailides derivatives of chitosan, chitosan sulfates and carboxymethyl chitosan. Int. J. Biol. Macromol. 2009;45:163–168. doi: 10.1016/j.ijbiomac.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 121.Ho Y.-C., Wu S.-J., Mi F.-L., Chiu Y.-L., Yu S.-H., Panda N., Sung H.-W. Thiol-Modified Chitosan Sulfate Nanoparticles for Protection and Release of Basic Fibroblast Growth Factor. Bioconj. Chem. 2010;21:28–38. doi: 10.1021/bc900208t. [DOI] [PubMed] [Google Scholar]
- 122.Peschel D., Zhang K., Fischer S., Groth T. Modulation of osteogenic activity of BMP-2 by cellulose and chitosan derivatives. Acta Biomater. 2012;8:183–193. doi: 10.1016/j.actbio.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 123.Weltrowski A., da Silva Almeida M.-L., Peschel D., Zhang K., Fischer S., Groth T. Mitogenic Activity of Sulfated Chitosan and Cellulose Derivatives is Related to Protection of FGF-2 from Proteolytic Cleavage. Macromol. Biosci. 2012;12:740–750. doi: 10.1002/mabi.201100518. [DOI] [PubMed] [Google Scholar]
- 124.Farrugia B.L., Mi Y., Kim H.N., Whitelock J.M., Baker S.M., Wiesmann W.P., Li Z., Maitz P., Lord M.S. Chitosan-Based Heparan Sulfate Mimetics Promote Epidermal Formation in a Human Organotypic Skin Model. Adv. Funct. Mater. 2018;28:1802818. doi: 10.1002/adfm.201802818. [DOI] [Google Scholar]
- 125.Santos-Carballal B., Fernández Fernández E., Goycoolea F., Santos-Carballal B., Fernández Fernández E., Goycoolea F.M. Chitosan in Non-Viral Gene Delivery: Role of Structure, Characterization Methods, and Insights in Cancer and Rare Diseases Therapies. Polymers. 2018;10:444. doi: 10.3390/polym10040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mumper R., Wang J., Claspell J., Rolland A. Novel polymeric condensing carriers for gene delivery. Proc. Control. Release Soc. 1995;22:178–179. [Google Scholar]
- 127.Jayakumar R., Chennazhi K.P., Muzzarelli R.A.A., Tamura H., Nair S.V., Selvamurugan N. Chitosan conjugated DNA nanoparticles in gene therapy. Carbohydr. Polym. 2010;79:1–8. doi: 10.1016/j.carbpol.2009.08.026. [DOI] [Google Scholar]
- 128.MacLaughlin F.C., Mumper R.J., Wang J., Tagliaferri J.M., Gill I., Hinchcliffe M., Rolland A.P. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J. Control. Release. 1998;56:259–272. doi: 10.1016/S0168-3659(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 129.Venkatesh S., Smith T.J. Chitosan-membrane interactions and their probable role in chitosan-mediated transfection. Biotechnol. Appl. Biochem. 1998;27:265–267. [PubMed] [Google Scholar]
- 130.Vauthier C., Zandanel C., Ramon A.L. Chitosan-based nanoparticles for in vivo delivery of interfering agents including siRNA. Curr. Opin. Colloid Interface Sci. 2013;18:406–418. doi: 10.1016/j.cocis.2013.06.005. [DOI] [Google Scholar]
- 131.Cryan S.-A., McKiernan P., Cunningham C.M., Greene C. Targeting miRNA-based medicines to cystic fibrosis airway epithelial cells using nanotechnology. Int. J. Nanomed. 2013;8:3907. doi: 10.2147/IJN.S47551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ban E., Kwon T.-H., Kim A. Delivery of therapeutic miRNA using polymer-based formulation. Drug Deliv. Transl. Res. 2019 doi: 10.1007/s13346-019-00645-y. [DOI] [PubMed] [Google Scholar]
- 133.Csaba N., Köping-Höggård M., Alonso M.J. Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int. J. Pharm. 2009;382:205–214. doi: 10.1016/j.ijpharm.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 134.Csaba N., Köping-Höggård M., Fernandez-Megia E., Novoa-Carballal R., Riguera R., Alonso M.J. Ionically crosslinked chitosan nanoparticles as gene delivery systems: Effect of PEGylation degree on in vitro and in vivo gene transfer. J. Biomed. Nanotechnol. 2009;5:162–171. doi: 10.1166/jbn.2009.1017. [DOI] [PubMed] [Google Scholar]
- 135.Mao H.Q., Roy K., Troung-Le V.L., Janes K.A., Lin K.Y., Wang Y., August J.T., Leong K.W. Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. J. Control. Release. 2001;70:399–421. doi: 10.1016/S0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 136.Corsi K., Chellat F., Yahia L., Fernandes J.C. Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles. Biomaterials. 2003;24:1255–1264. doi: 10.1016/S0142-9612(02)00507-0. [DOI] [PubMed] [Google Scholar]
- 137.Zhu H., Cao J., Cui S., Qian Z., Gu Y. Enhanced Tumor Targeting and Antitumor Efficacy via Hydroxycamptothecin-Encapsulated Folate-Modified N-Succinyl-N′-Octyl Chitosan Micelles. J. Pharm. Sci. 2013;102:1318–1332. doi: 10.1002/jps.23470. [DOI] [PubMed] [Google Scholar]
- 138.Gu Y., Li S., Feng S., Ding L., Liu Y., Zhu Q., Qian Z. Nanomedicine engulfed by macrophages for targeted tumor therapy. Int. J. Nanomed. 2016;11:4107–4124. doi: 10.2147/IJN.S110146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tu Z., Ma Y., Akers W., Achilefu S., Gu Y. Therapeutic effect of the treatment for colorectal cancer with adenoviral vectors mediated estrogen receptor β gene therapy combined with thermotherapy. J. Cancer Res. Clin. Oncol. 2014;140:623–632. doi: 10.1007/s00432-014-1611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Woraphatphadung T., Sajomsang W., Rojanarata T., Ngawhirunpat T., Tonglairoum P., Opanasopit P. Development of Chitosan-Based pH-Sensitive Polymeric Micelles Containing Curcumin for Colon-Targeted Drug Delivery. AAPS PharmSciTech. 2018;19:991–1000. doi: 10.1208/s12249-017-0906-y. [DOI] [PubMed] [Google Scholar]
- 141.Deng D., Qu L., Zhang J., Ma Y., Gu Y. Quaternary Zn–Ag–In–Se Quantum Dots for Biomedical Optical Imaging of RGD-Modified Micelles. ACS Appl. Mater. Interfaces. 2013;5:10858–10865. doi: 10.1021/am403050s. [DOI] [PubMed] [Google Scholar]
- 142.Zhang C., Ding Y., Yu L., Ping Q. Polymeric micelle systems of hydroxycamptothecin based on amphiphilic N-alkyl-N-trimethyl chitosan derivatives. Colloids Surf. B Biointerfaces. 2007;55:192–199. doi: 10.1016/j.colsurfb.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 143.Zhang F., Fei J., Sun M., Ping Q. Heparin modification enhances the delivery and tumor targeting of paclitaxel-loaded N-octyl-N -trimethyl chitosan micelles. Int. J. Pharm. 2016;511:390–402. doi: 10.1016/j.ijpharm.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 144.Mo R., Jin X., Li N., Ju C., Sun M., Zhang C., Ping Q. The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles. Biomaterials. 2011;32:4609–4620. doi: 10.1016/j.biomaterials.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 145.Mo R., Xiao Y., Sun M., Zhang C., Ping Q. Enhancing effect of N-octyl-O-sulfate chitosan on etoposide absorption. Int. J. Pharm. 2011;409:38–45. doi: 10.1016/j.ijpharm.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 146.Jin X., Mo R., Ding Y., Zheng W., Zhang C. Paclitaxel-Loaded N-Octyl-O-sulfate Chitosan Micelles for Superior Cancer Therapeutic Efficacy and Overcoming Drug Resistance. Mol. Pharm. 2014;11:145–157. doi: 10.1021/mp400340k. [DOI] [PubMed] [Google Scholar]
- 147.Ju C., Sun J., Zi P., Jin X., Zhang C. Thermosensitive Micelles–Hydrogel Hybrid System Based on Poloxamer 407 for Localized Delivery of Paclitaxel. J. Pharm. Sci. 2013;102:2707–2717. doi: 10.1002/jps.23649. [DOI] [PubMed] [Google Scholar]
- 148.Qu G., Yao Z., Zhang C., Wu X., Ping Q. PEG conjugated N-octyl-O-sulfate chitosan micelles for delivery of paclitaxel: In vitro characterization and in vivo evaluation. Eur. J. Pharm. Sci. 2009;37:98–105. doi: 10.1016/j.ejps.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 149.Qu G., Wu X., Yin L., Zhang C. N-octyl-O-sulfate chitosan-modified liposomes for delivery of docetaxel: Preparation, characterization, and pharmacokinetics. Biomed. Pharmacother. 2012;66:46–51. doi: 10.1016/j.biopha.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 150.Zhong Z., Zhong Z., Xing R., Li P., Mo G. The preparation and antioxidant activity of 2-[phenylhydrazine (or hydrazine)-thiosemicarbazone]-chitosan. Int. J. Biol. Macromol. 2010;47:93–97. doi: 10.1016/j.ijbiomac.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 151.Chen X., Cao X., Jiang H., Che X., Xu X., Ma B., Zhang J., Huang T. SIKVAV-Modified Chitosan Hydrogel as a Skin Substitutes for Wound Closure in Mice. Molecules. 2018;23:2611. doi: 10.3390/molecules23102611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu W., Wang F., Zhu Y., Li X., Liu X., Pang J., Pan W. Galactosylated Chitosan-Functionalized Mesoporous Silica Nanoparticle Loading by Calcium Leucovorin for Colon Cancer Cell-Targeted Drug Delivery. Molecules. 2018;23:3082. doi: 10.3390/molecules23123082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ning Q., Liu Y.-F., Ye P.-J., Gao P., Li Z.-P., Tang S.-Y., He D.-X., Tang S.-S., Wei H., Yu C.-Y. Delivery of Liver-Specific miRNA-122 Using a Targeted Macromolecular Prodrug toward Synergistic Therapy for Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces. 2019;11:10578–10588. doi: 10.1021/acsami.9b00634. [DOI] [PubMed] [Google Scholar]
- 154.Liu W., Zhu Y., Wang F., Li X., Liu X., Pang J., Pan W. Galactosylated chitosan-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. R. Soc. Open Sci. 2018;5:181027. doi: 10.1098/rsos.181027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yen H., Young Y., Tsai T., Cheng K., Chen X., Chen Y., Chen C., Young J., Hong P. Positively charged gold nanoparticles capped with folate quaternary chitosan: Synthesis, cytotoxicity, and uptake by cancer cells. Carbohydr. Polym. 2018;183:140–150. doi: 10.1016/j.carbpol.2017.11.096. [DOI] [PubMed] [Google Scholar]
- 156.Huang Y., Guo J., Gui S. Orally targeted galactosylated chitosan poly(lactic-co-glycolic acid) nanoparticles loaded with TNF-a siRNA provide a novel strategy for the experimental treatment of ulcerative colitis. Eur. J. Pharm. Sci. 2018;125:232–243. doi: 10.1016/j.ejps.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 157.Xiao B., Chen Q., Zhang Z., Wang L., Kang Y., Denning T., Merlin D. TNFα gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J. Control. Release. 2018;287:235–246. doi: 10.1016/j.jconrel.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bai M.-Y., Tang S.-L., Chuang M.-H., Wang T.-Y., Hong P. Evaluation of Chitosan Derivative Microparticles Encapsulating Superparamagnetic Iron Oxide and Doxorubicin as a pH-Sensitive Delivery Carrier in Hepatic Carcinoma Treatment: An in vitro Comparison Study. Front. Pharmacol. 2018;9:1025. doi: 10.3389/fphar.2018.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bin J., Xiao Y., Lin Z.T., Deng Y.L., Chen Y., Le D.E., Bin J., Li M., Liao Y., Liu Y., et al. High molecular weight chitosan derivative polymeric micelles encapsulating superparamagnetic iron oxide for tumor-targeted magnetic resonance imaging. Int. J. Nanomed. 2015;10:1155. doi: 10.2147/IJN.S70022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zariwala M.G., Bendre H., Markiv A., Farnaud S., Renshaw D., Taylor K.M., Somavarapu S. Hydrophobically modified chitosan nanoliposomes for intestinal drug delivery. Int. J. Nanomed. 2018;13:5837–5848. doi: 10.2147/IJN.S166901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chiesa E., Dorati R., Conti B., Modena T., Cova E., Meloni F., Genta I. Hyaluronic Acid-Decorated Chitosan Nanoparticles for CD44-Targeted Delivery of Everolimus. Int. J. Mol. Sci. 2018;19:2310. doi: 10.3390/ijms19082310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Song Q., Jia J., Niu X., Zheng C., Zhao H., Sun L., Zhang H., Wang L., Zhang Z., Zhang Y. An oral drug delivery system with programmed drug release and imaging properties for orthotopic colon cancer therapy. Nanoscale. 2019 doi: 10.1039/C9NR03802G. [DOI] [PubMed] [Google Scholar]
- 163.Feiz M.S., Meshkini A. Targeted delivery of adenosine 5′-triphosphate using chitosan-coated mesoporous hydroxyapatite: A theranostic pH-sensitive nanoplatform with enhanced anti-cancer effect. Int. J. Biol. Macromol. 2019;129:1090–1102. doi: 10.1016/j.ijbiomac.2018.08.158. [DOI] [PubMed] [Google Scholar]
- 164.Kim G.H., Won J.E., Byeon Y., Kim M.G., Wi T.I., Lee J.M., Park Y.-Y., Lee J.-W., Kang T.H., Jung I.D., et al. Selective delivery of PLXDC1 small interfering RNA to endothelial cells for anti-angiogenesis tumor therapy using CD44-targeted chitosan nanoparticles for epithelial ovarian cancer. Drug Deliv. 2018;25:1394–1402. doi: 10.1080/10717544.2018.1480672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Iglesias N., Galbis E., Díaz-Blanco M.J., Lucas R., Benito E., de-Paz M.-V. Nanostructured Chitosan-Based Biomaterials for Sustained and Colon-Specific Resveratrol Release. Int. J. Mol. Sci. 2019;20:398. doi: 10.3390/ijms20020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen J., Yang X., Huang L., Lai H., Gan C., Luo X. Development of dual-drug-loaded stealth nanocarriers for targeted and synergistic anti-lung cancer efficacy. Drug Deliv. 2018;25:1932–1942. doi: 10.1080/10717544.2018.1477856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xie P., Du P., Li J., Liu P. Stimuli-responsive hybrid cluster bombs of PEGylated chitosan encapsulated DOX-loaded superparamagnetic nanoparticles enabling tumor-specific disassembly for on-demand drug delivery and enhanced MR imaging. Carbohydr. Polym. 2019;205:377–384. doi: 10.1016/j.carbpol.2018.10.076. [DOI] [PubMed] [Google Scholar]
- 168.Wang K., Zhuang J., Liu Y., Xu M., Zhuang J., Chen Z., Wei Y., Zhang Y. PEGylated chitosan nanoparticles with embedded bismuth sulfide for dual-wavelength fluorescent imaging and photothermal therapy. Carbohydr. Polym. 2018;184:445–452. doi: 10.1016/j.carbpol.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 169.Zhao H., Yang J., Qian Q., Wu M., Li M., Xu W. Mesenteric CD103+DCs Initiate Switched Coxsackievirus B3 VP1-Specific IgA Response to Intranasal Chitosan-DNA Vaccine Through Secreting BAFF/IL-6 and Promoting Th17/Tfh Differentiation. Front. Immunol. 2018;9:2986. doi: 10.3389/fimmu.2018.02986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sinani G., Sessevmez M., Gök M.K., Özgümüş S., Alpar H.O., Cevher E. Modified chitosan-based nanoadjuvants enhance immunogenicity of protein antigens after mucosal vaccination. Int. J. Pharm. 2019;569:118592. doi: 10.1016/j.ijpharm.2019.118592. [DOI] [PubMed] [Google Scholar]