Abstract
The Mediterranean diet is considered one of the most worldwide healthy dietary patterns thanks to a combination of foods rich mainly in antioxidants and anti-inflammatory nutrients. Many studies have demonstrated a strong and inverse relationship between a high level of Mediterranean diet adherence and some chronic diseases (such as cardiovascular diseases, diabetes, etc.) and cancer. Given its protective effects in reducing oxidative and inflammatory processes of cells and avoiding DNA damages, cell proliferation, and their survival, angiogenesis, inflammations and metastasis, the Mediterranean diet is considered a powerful and manageable method to fight cancer incidence. The aim of this narrative review was to determine the magnitude of interaction between the Mediterranean diet and more widespread types of cancer so as to give a first and useful overview on this relationship identifying, with a nutritional approach, those nutrients of Mediterranean diet able to reduce cancer incidence.
Keywords: Mediterranean diet, cancer incidence, cancer
1. Introduction
Cancer is the second leading cause of death globally: In 2018 alone, it was registered that about 9.6 million deaths were because of cancer in the ratio of one to six [1]. According to the World Cancer Research Fund and with reference to 2018, 18 million of new cases were diagnosed: Globally, a higher incidence of lung and breast cancer (incidence of 12.3% respectively) was registered, followed by a high incidence of colorectal cancer (10.6%), prostate cancer (7.5%), stomach cancer (6.1%) and liver cancer (5.0%) [2]. Although cancer develops with an homogeneous distribution between men (9.5 million of new diagnoses in 2018) and women (8.5 million of new cases in 2018), each type of neoplasia has a different incidence among male and female: In 2018, lung cancer (15.5%), prostate cancer (14.4%), and colorectal cancer (11.4%) affected men above all, while women have been mainly affected by breast cancer (25.4%), colorectal cancer (9.7%) and lung cancer (8.8%) [2].
Risk factors in cancer incidence are mainly linked with individual and environmental characteristics (heredity, particular exposure to noxious substances, or clinical conditions as hormone imbalance) or with peoples’ lifestyle: Physical activity, sedentary lifestyle and diet play a crucial role. Particularly, a high body mass index, a low intake of fruit and vegetables, the lack of physical activity, the use of alcohol and smoking represent the five and most important risk factors in the onset of several neoplasia, together with some chronic infections (e.g., due to Helicobacter pylori, Human papillomavirus, Hepatitis B, Hepatitis C and Epstein-Barr virus) [3,4,5,6] and with a lack of prevention, screening and treatment of cancer, typical shortages of developing countries especially [1].
Although smoking is the main factor risk, considered as the leading cause of 22% cancer death, the other factors, together with genetic and hereditary characteristics of each individual, contribute decidedly towards the transformation of healthy cells into cancer cells through a progression, more or less quickly able to change precancerous lesions in malignancies [7,8,9].
Diet, physical inactivity, sedentary lifestyle, and obesity (consequence of a healthy or non-healthy lifestyle) are, after smoking, the main risk factors in the onset of cancer: It is estimated that changes in alimentary habits can contribute to avoid cancer onset of 30–50% [10,11].
Dietary patterns based on regular intake of fruit, vegetables (especially garlic and cruciferous vegetables, as cabbages, broccoli, brussels sprout and wasabi) and by consequence the intake of aliments rich in selenium, folic acid, vitamins (B-12 or D), and antioxidants (e.g., carotenoids and lycopene) play a protective role in cancer onset so to reduce risk of breast cancer, colorectal cancer and prostate cancer of 60–70% and of lung cancer; 40–50% [10]. A high intake of products rich in fiber (e.g., whole grains) and a moderate intake of milk and dairy may reduce incidence of different types of cancer (e.g., colorectal cancer, lung cancer, stomach cancer, breast cancer, colorectal cancer, esophagus cancer and oral cancer) [2,12,13,14,15,16,17]. Vice versa, meat and animal products, rich in animal fats and oils and often cooked at high temperatures, may increase cancer incidence, especially for colorectal cancer, stomach cancer and prostate cancer [13,18,19,20,21,22,23].
In regards to alcohol intake and its positive or negative effects on health, there is no unanimity among studies: A moderate alcohol intake (until 30 g a day) could have a protective effect against the onset of kidney cancer, while an excessive intake surely is a risk factor in the onset of many cancers (oral cavity cancer, esophagus cancer, breast cancer, colorectal cancer, stomach cancer and liver cancer) [2].
Summarizing, from several studies emerged as the regular assumption of fruits and vegetables rich in fiber and vitamins, a low intake of meat, and a moderate intake of milk, dairy, and alcohol may be considered an optimal combination in the prevention of cancer. Combining these evidences with the healthier dietary patterns proposed as emerging medical prescription [24], the Mediterranean diet (MD) appears as the best diet pattern able to reflect many characteristics of an ideal healthy diet. It is considered one of the main dietary patterns able to give beneficial effects on longevity and to improve cardiovascular system functions so as to prevent many cardiac diseases or to contain their progression [25,26].
Given these assumptions, the main aim of this narrative review was to understand as Mediterranean diet may contribute to reduce cancer incidence analyzing the impact of MD on more kinds of neoplasia so to understand which areas of nutrition should be deepened to ensure better nutrition guidelines for global population and better rules of thumb in oncologic clinical practice. The novelty of this review is given by the evaluation of the MD impact only considered those studies which demonstrate a significant interaction between MD adherence and cancer. This choice was supported by two considerations. The first is inherent to the statistical results: The lack of significance means a lack of evidence which could be determined by a lack of the actual relationship or by a non-correct approach to the problem. For MD, this last issue is more evident given the complexity of the MD components due to the variety of foods. As emerged in some studies, there are two kinds of problems producing MD complexity: Several myths and misconceptions are associated with the traditional Mediterranean diet, and many difficulties are related with a correct distinction between Mediterranean foods and healthy, but non-Mediterranean foods [27]. The second reason of our work is related to the high heterogeneity [28] of results which does not allow to define a unique and determined relationship between MD and cancer.
2. Methodology
Through the analysis of several studies of the last 10 years (2009–2019) about the relationship between the Mediterranean diet and cancer incidence, excluding studies which, although methodologically correct, had not reported significant relationships, we marked out principal scientific facts with specific attention to the most widespread neoplasia, underlining for each of them the significant relationship and the main benefits of Mediterranean diet in the reduction of cellular mutation and in the slowdown of progression and spread of that determined pathology. This study started with a research on PubMed of a series of keywords related to Mediterranean diet and its interaction with some prevalent neoplasia (“Mediterranean diet”, “Mediterranean adherence”, “cancer”, “lung cancer”, “breast cancer”, “colorectal cancer”, “prostate cancer”, “bladder cancer”, “stomach cancer”, “endometrial cancer”, “cervical cancer”, “pancreatic cancer”, “bile tract cancer”). Types of cancer were selected in accordance with their percentage of incidence among worldwide population.
Afterwards, we considered only prospective or retrospective studies, excluding systematic reviews and meta-analyses. The last update of our dataset was performed in June 2019. Initially, we examined 220 articles; after the selection process (Figure 1), we identified 104 papers whose 53 characterized by a significant relationship between MD and cancer.
Figure 1.
Article selection process. Note: Papers with significant relationship were reported in bibliography together with the other articles considered to describe the whole topic.
3. Mediterranean Diet
The Mediterranean diet was recognized as “intangible cultural heritage of France and Italy, Greece, Spain and Morocco, respectively” by the United Nations Educational, Scientific and Cultural Organization (UNESCO) in 2010 [29], thereby to preserve the local biodiversity characteristics of Mediterranean countries and to promote a diet pattern able to give beneficial effects to human health. Considered for the first time by Ancel Keys and his colleagues as a diet poor in saturated lipids and able to protect cardiovascular system thanks to low cholesterol level in the blood [30], in the following years it was identified as a diet pattern composed by foods rich in high protective nutrients able to prevent from several diseases. Besides, given the protective role plays by MD on DNA damages, it was often investigated in the evaluation of general mortality as a potential and beneficial lifestyle in the increasing longevity in healthy people [30,31].
Mediterranean diet is characterized by a high intake of vegetables, legumes, fresh fruit, non-refined cereals, nuts, and olive oil (especially extra-virgin olive oil, i.e., obtained with a mechanical pressing and contained acidity rate lower than 0.8% (Regulations CEE n. 2568/91 and following updating [32]), by a moderate consumption of fish and dairy, by a low intake of red meats, and by a moderate use of ethanol, mainly red wine consumed during the main meals [26].
In Mediterranean countries, these foods are daily consumed, following precise and traditional habits: Olive oil is taken every day and jointly to main dishes (vegetables and legumes) so to improve their taste, fresh fruit is a form of dessert consumed at the end of the main meals or as a snack for mid-morning or in the afternoon, cheese is consumed with salad or stews and finally, red meat is the main dish of special occasion only (until 1970 in some areas of the Southern Italy or in Greece, meat intake consisted of annual consumption of one/two tame ungulate animals for each patriarchal family and of a moderate intake of poultry) [33,34,35,36].
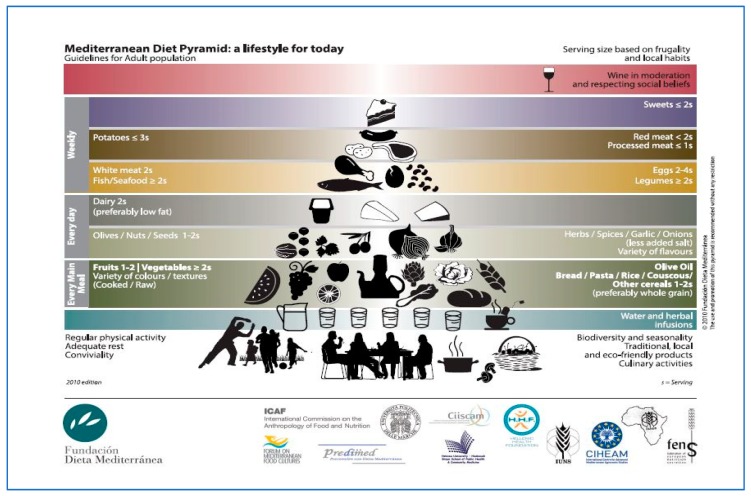
Given similar climatic and morphological features, distinctive traits of the whole Mediterranean area, the “classical” Mediterranean diet, as described above, has some specific peculiarities depending on the specificities of each country and even on the particularity within a certain area [36,37]. Therefore, for studying Mediterranean diet and its benefits on health, it has been necessary to identify common characteristics among different models of Mediterranean diet, so to creating an alimentary pyramid (Figure 2) in which main foods of each specific kind of Mediterranean diet and their intake frequency were reported, so to uniform different Mediterranean diet models with reference to both foods and their right intake quantity.
Figure 2.
Mediterranean diet pyramid. Source: Fundacion Dieta Mediterrànea.
Observing Figure 2, it is possible to note as the diet in its largest meaning, i.e., comprehensive of all specificities related to each Mediterranean area, is a diet pattern which promotes a high intake of wholegrain cereals, fruit and vegetable, a moderate consumption of dairy, poultry and fish and a low intake of red meat and sweets. Wine consumption is allowed, on condition that its use is moderate, while olive oil is recommended as fats substitute in each main meal [38]. Regular physical activity, adequate rest, conviviality, biodiversity and seasonality, traditional, local and eco-friendly products and culinary activities are an integral part of Mediterranean diet pyramid.
Mediterranean diet is often compared with some other healthy dietary patterns, as the Nordic diet, dietary approaches to stop hypertension (DASH), the Alternate Health Index (AHEI)-2010 or the vegetarian diet. All these types of diets have two kinds of characteristics: They are derived from precise guidelines and they are formed including or excluding specific foods. For example, the Nordic diet, one of the most studied healthy diet, is composed with healthy foods, as whole-grain cereals, fish, cabbage, rye bread, apples and pears, and root vegetables, but it is also characterized by unhealthy foods as margarine and sugar [28,39], so its impact on health is always evaluated with attention to a single food and not as an alimentary habits on the whole. The same considerations are possible about vegetarian diet: In this case, the choice to eliminate fish, meat and poultry consumption if on the one hand it improves body mass index, reducing obesity and overweight incidences, and reduces the amounts of saturated fatty acids, on the other hand it decreases levels of vitamin B12 and sex hormones, and it increases levels of homocysteine [40]. In both cases, the improvement in the disease’s prevention is linked with diet capacity to contrast or prevent some risk factors, as obesity, overweight or hormonal changes, but they do not provide a 360-degree protective and healthy effect.
On the contrary, the Mediterranean diet presents many advantages related with individual characteristics and exogenous values related with the preservation of traditions and habits and with the environmental protection [41]. The Mediterranean diet is characterized by positive, healthy and nutritional benefits as emerged by the large literature dedicated from 1960 to this dietary pattern; particularly the added value is due to a combination of healthy foods and their many nutritional benefits (high intake of vitamins and nutrients) with the diversity of foods (from vegetables and fruits to meat and fish, without alcohol ban), the respect of seasonality of each ingredient, the freshness of products so to maximize the content of protective nutrients and substances, and the use of all available foods, sweets included, in a context of frugality and moderation [42,43,44].
Since MD is not just a way of eating specific food, but it is a kind of philosophy involving all dimensions of an individual, the Mediterranean diet impacts on life habits changing individual approach to food and the use itself of food [44]. In fact, adhering to MD means learning a variety of food practices, food preparations skills, and culinary activities combining the pleasant tastes and smells of foods with the Mediterranean knowledge and traditions transmitted from generation to generation, living the meal time as a fundamental part of daily routine in which sociability, communication and conviviality engage a psychophysical dimension [43].
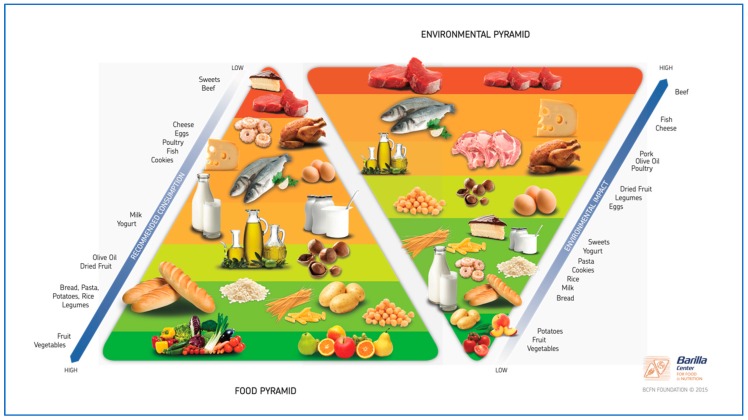
In terms of socio-economic and environmental factors, the choice to adopt a Mediterranean diet means to reduce the impact of food production on environment, reducing food processing loss, food wastage and packaging, energy consumption, transportation of foods, water consumption, and waste management, all environmental issues increased by a strong demand of meat and dairy products and of products no-linked with local and traditional habits. It derives that relationship between nutrition and environmental is inversely associated: More healthy, lower environmental impact [45], as emerged in the Double Pyramid proposed by Barilla Centre for Food and Nutrition in June 2009 (Figure 3). Environmental protection, although in the short run it does not seem linked with the onset of many disease, has a powerful effect in the long period: Factory farming or intensive cultivations have an enormous impact on environmental increasing greenhouse gas emission, exploitation of land and water sources, and pollution, among other reasons [42].
Figure 3.
Double Pyramid proposed by Barilla Centre for Food and Nutrition—Source: Barilla Center For Food and Nutrition (https://www.barillacfn.com/en/dissemination/double_pyramid/).
3.1. Mediterranean Diet: Open Issues
In accordance with definition of UNESCO, the Mediterranean diet is not a mere collection of some specific foods, but it is a way to promote social interaction and to increase sociocultural values thanks to the sharing of socialization moments and the respect for the territory and biodiversity, ensuring at the same time, the conservation and development of all those traditional activities typical of the Mediterranean basin, and the promotion of environmental conservation [29].
Nevertheless, increasing the adherence to MD is difficult because there are two fundamental aspects to taking in account: One is concerned with the economic accessibility of Mediterranean foods for all people [24,42,46] (it is estimated that world’s population will reach to nine billion people by 2050 [47]), and the second is linked with the transportation of Mediterranean foods in non-Mediterranean countries.
With attention to the first issue, the reasons to a low level of adherence are mainly linked with socio-economic factors and with an increase of prices of major food items involved in the MD pyramid. More specifically, people, especially disadvantaged people, are induced to choose less expensive eating patterns or to prefer energy-dense foods, i.e., those composed by refined grains, added sugar, and fats, because these foods are the lowest-cost option and the best source of energy [48]. Subjects reporting highest scores for the Western diet habits demonstrate (consciously or unconsciously) to protect the economic aspect rather than to enhance their diet quality, even if this behavior affects their health status (higher rates of obesity, diabetes, cardiovascular diseases, and some type of cancer are more common among lower socio-economical group than among better educated and more affluent people) [46,49,50].
The second issue is more complicated to solve. In fact, while socio-economic problems could be tackled with a major attention to public and global politics lowering the prices of some basic products as fruits and vegetables and improving their availability in the most citizen-attended places, the second issue regards a choice between sustainability and health. In fact, while reintroducing MD is easy in Mediterranean countries because it requires a change of individuals’ economic status, its transferability in non-Mediterranean countries, with all its aspects, traditional components included, is not a simply shifting of foods from an area to another, but it is a change in dietary habits [27].
3.2. Scores for Measuring the MD Adherence
Since the Mediterranean diet is given by a combination of several foods, it is impossible to determine which specific food produces a positive effect and contributes to fight certain pathologies. Therefore, over the years, it has been necessary to define new research methodologies (general description, dietetic pyramids, a priori or a posteriori scoring systems [51]) able to evaluate the whole diet pattern, tools that, although different in their estimate methods, aim to understand the level of adherence to MD of each individual involved in a specific study. Particularly, a priori and a posteriori scoring systems (a priori: Default score determined using nutritional data of each subject, e.g., the Mediterranean Diet Score (MDS) or the Italian Mediterranean Index; a posteriori: Score determined after a principal component analysis which defines diet pattern, e.g., alternate Mediterranean Diet (aMED) or Alternate Health Index (AHEI)) [52], used in many observational and prospective studies, aimed to determine, through an assessment of a MD score per individual, the incidence of each disease and the overall and disease-specific mortality rate. Thanks to these methodologies, they could state with absolute certainty that there is an inverse and significant correlation between MD and overall mortality rate (by 20–30%) and between MD and cardiovascular mortality (by 30–40%) [25,53,54,55,56,57].
All these methodologies, although they are statistically valid, do not consider that characteristic which, for who writes, is the main feature of the MD, i.e., that factor which differentiates this diet pattern from the whole kinds of healthy diets: Naturality. In fact, MD was not developed using healthy guidelines or recommendations, but it is part of the alimentary regimen of Mediterranean countries since Egyptian Pharaohs, and it was proposed as a healthy diet pattern in 1634 [52], becoming integral part of heritage of many generations. Therefore, MD is not a regimen scientifically imposed or a diet in its negative meaning, but it is a normal and traditional alimentary behavior which, jointed with a healthy lifestyle (e.g., physical activity and exposure to sunlight) may effectively reduce the onset of many diseases, without upsetting the individual’s approach to food, increasing indeed the sense of fulfilment and satisfaction.
In fact, although the MD development in the Southern Europe suffered in the last 20–25 years, a strong reduction because of a gradually abandon of Mediterranean alimentary traditions for alimentary habits rich in saturated fats and pre-packaged foods [58,59,60,61,62], the presence in those areas of typical Mediterranean products (as olive oil, tomatoes, vegetables, legumes, and dairy) continues to produce positive effects in the reduction of tumoral incidence in the population [63,64].
3.3. Beneficial Effects of Mediterranean Diet in the Cancer Prevention
The positive relationship (beneficial effects) between the Mediterranean diet and cancer is due to the high contents of antioxidants and anti-inflammatory nutrients contained in many foods of MD (legumes, fresh fruit or nuts, vegetables, fish, and olive oil, especially extra-virgin olive oil), which have a protective effect in the fighting cell degeneration and proliferation of cancer cells [65].
Paying attention to the relationship of specific foods and cancer, protective effects of the Mediterranean diet may be assigned to the high polyphenols concentration contained in olive oil, wine, and vegetables, all foods known for their antioxidant and anti-inflammatory capacity [66,67,68,69], and are rich in nutrients able to reduce proliferation of cancer cells, and to protect cell membrane from metastasis [67].
Besides, fruit and vegetables have a high quantity of carotenoids and vitamins, as vitamin C and E, folates and flavonoids, nutrients known for their antioxidant properties which allow the prevention of DNA damages [70,71]. Finally, omega-3, contained abundantly in fish, especially in sardines and mackerel (typical products of Mediterranean diet), and in nuts (almonds, walnuts, and pumpkin seeds) help to slow down cancer development affecting cell proliferation, their survival, angiogenesis, inflammations and metastasis [72]. A low consumption of meat contributes to moderate noxious effects due to high-temperature meat cooking, as well as to reduce animal fats intake [73].
Several studies demonstrated that a high adherence to MD is often associated with a lower risk of malignancies [7,9,15,69,74,75,76,77,78,79,80], although other studies showed a difficulty to identify a direct relationship between MD and cancer because of confounding factors or unclear definition of the MD score. The reason of these difficulties may be identified in the fact that it is impossible to establish a connection between MD adherence and cancer onset, this being determined by a cell degeneration produced by the influence of several factors; this impossibility is therefore due to a lack of a linear relationship between the two variables (MD adherence and cancer) [81] and to a presence of several variables, sometimes not directly perceptible, which modifies (increasing or reducing) the strength of the same relationship [82,83,84] or eliminates its linearity [85].
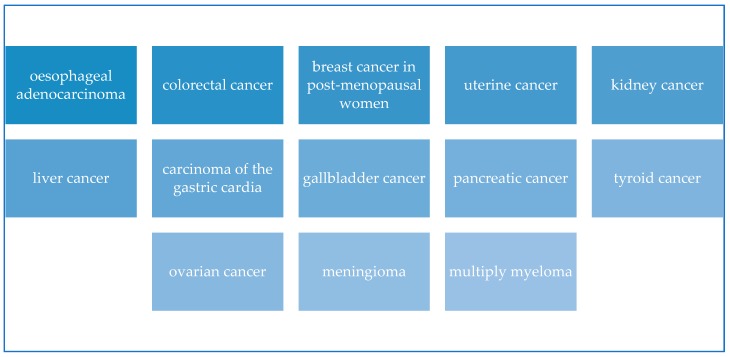
Diet and nutrition are two lifestyle factors having repercussion on the incidence of cancer, but the lack of significance of some studies and the lack of a direct relationship between nutrition and cancer reveal that other lifestyle factors (identified as confounding factors as physical inactivity and sedentary lifestyle) may contribute to the increasing cancer incidence worldwide. Managing properly diet, nutrition, and physical activity and reducing sedentary lifestyles could prevent more than half of cancers occurring today (Kerr 2017) thank to their contribution to reduce obesity which is a known risk factor for many malignancies (being overweight or obese increases the risk of incidence of at least 13 types of cancer (Figure 4)) [86]. Nevertheless, we underline that the Mediterranean diet is considered a form of lifestyle (as explicitly reported in Diet Pyramid in Figure 2) in which food and daily activities participate to realize the original Mediterranean diet. Therefore, we believe that the present methodology that provides for a clear separation between the Mediterranean diet and physical activity is not entirely correct. In fact, we imagine that this approach could be exactly the cause of the lack of confirmation regarding the association between physical activity, Mediterranean diet and cancer risk. In fact, the variety of neoplasia, etiologic differences among them, and the different impact of risk factors cause the lack of unanimity among researches because it was sometimes impossible to determine the cancer risk correlated directly with Mediterranean diet. Some studies demonstrated uniformly that there is a difference cancer incidence between Mediterranean and non-Mediterranean countries supposing that this difference is the result of the combination of many factors linked with diet, physical activity, quality of life and a higher exposure to sunlight [52]. But many biological questions could be behind this association. For this reason, in the following paragraph we provided a short explanation of the principal mechanisms about the relationship between Mediterranean foods and types of cancer.
Figure 4.
Cancers linked with obesity or overweight—Source information: Lauby-Secretan et al. (2016) [86].
3.4. Mechanisms between Mediterranean Diet Foods and Types of Cancers
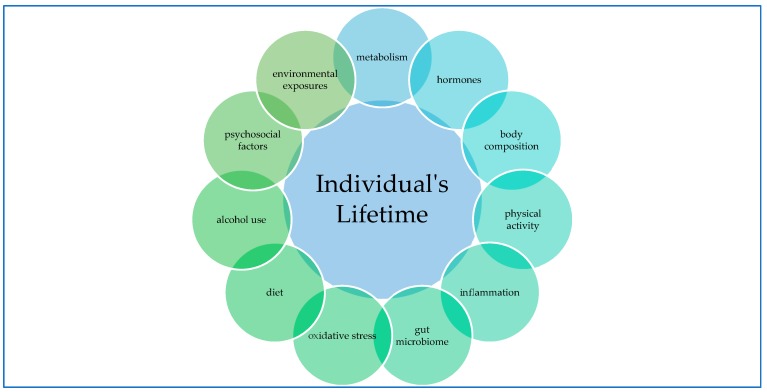
The biology of cancer is known to be heterogeneous and this variability is one of the issues which contributes to alter the relationship between dietary factors and cancer risk [87]. Diet is a part of an exposome that encompasses all of the exposure experienced by an individual over his or her lifetime (Figure 5). This means that mechanism involved in the cancer are so dynamic and intertwined that analyzing their effect is complex and that a cause–effect relationship between diet and cancer should be evaluated in the context of the individual’s exposome.
Figure 5.
Exposome—Characteristics extracted by Mayne et al. (2016).
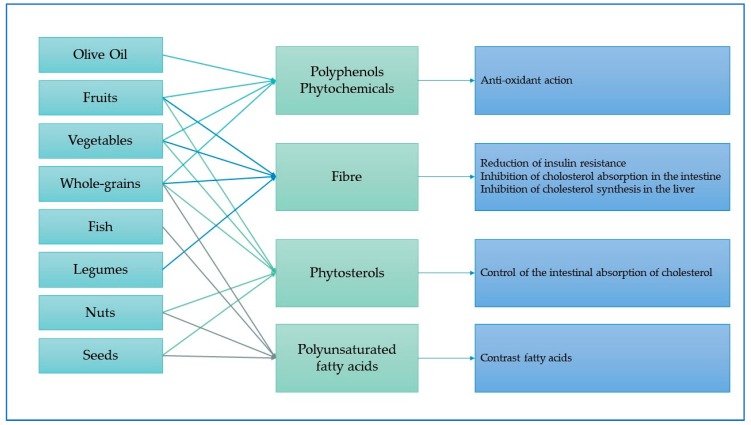
Determining the mechanism of interaction between Mediterranean foods and cancer requires an in-depth study of macro- and micro-elements contained in each food or produced by each of them in response of a certain cooking of method. In Figure 6, we reported main Mediterranean foods, their biochemical components and their consequent action. Overall, polyphenols and phytochemicals, contained in many Mediterranean foods, have a protective and antioxidant effect, even to balance fatty acids contained in some food of the Mediterranean diet, as olive oil. Fiber contained in whole grains, vegetables, legumes, and fruit, as well as increasing antioxidant vitamins and phytochemicals, reduce insulin resistance, inhibit cholesterol absorption in the intestine and cholesterol synthesis in the liver [24,88].
Figure 6.
Mechanism between Mediterranean diet components and beneficial effects—Source information: Lăcătușu et al. (2019).
Specifically, we noted that many Mediterranean foods contribute to reducing cancer risk with a series of mechanisms that reduce tumor cell growth (i.e., fish intake), anti-oxidative and anti-inflammatory effects (i.e., fruit, vegetables, and olive oil), increase chemoprotective effects (i.e., olive oil), and inhibit tumor development (i.e., dairy products) [89,90,91] (see Appendix A). Controversial results are emerged about red wine consumption, although recent researches demonstrated as wine micronutrients, such as polyphenols (resveratrol and quercetin, in particular) [83,84] could have positive effects against cancer. Observing a wholeness Mediterranean diet without regarding a single food and pathways leading to a favorable effect on various disease, cancer included, it emerged that this dietary pattern produces the following positive effects [92]:
-
-
lowering of lipid and modulating of their effects;
-
-
anti-inflammatory, anti-oxidative and anti-aggregating effects;
-
-
modulation of cancer-prone mediators (hormones or growth factors);
-
-
reduction, through the changes in amino acid content, of stimulation of hormones or other extra- and intracellular transmitting pathways involved in cancer;
-
-
changes in gut microbiota thanks to a positive and modified production of bacterial metabolites.
4. Impact of Mediterranean Diet on Cancer
4.1. Breast Cancer
As reported in the introduction, breast cancer is the neoplasia more widespread among women (less than 1% of men suffer from this cancer) so that in 2018 it affected 25% of women with a new diagnosis [2]. Breast cancer risk doubles every ten years until menopause, and the period after that the risk continues to increase but more slowly, though it remains high [93,94].
Environmental factors (e.g., pollution) and lifestyle affect sensibly the breast cancer onset: It was demonstrated a worrying increase of incidence among women in developing countries, historically less subjected to this neoplasia since they are less exposure to environmental and hormonal factors. It seems that this observed increase is due to the “Westernization” of their lifestyle which modifies their habits (e.g., use of alcohol or smoking) and their traditional diets favoring non-healthy diet patterns rich in saturated fats [95].
Mediterranean diets may be a protective factor in the decrease of breast cancer incidence thank to a regular intake of fiber, antioxidants, flavonoids included, vitamins and carotenoids, able to reduce estrogens and to increase level of sex-hormones and therefore to neutralize free radicals, to protect from DNA damages, and to reduce oxidative stress [96].
Several studies underlined the presence of an inverse relationship between high MD adherence and breast cancer incidence. Overall, it emerged that the incidence decreased of 6% in case of high MD adherence measured with arMED (adapted relative Mediterranean diet excluding alcohol) (HRarMEDhigh vs. arMEDlow = 0.94, 95% CI 0.88–1.00), and values of this decrease were slightly higher (7%) for post-menopause women (HRarMEDhigh vs. arMEDlow = 0.93, 95% CI: 0.87–0.99) and significantly higher (20%) in case of BC ER- (ER negative)/PR- (PR negative) HRarMEDhigh vs. arMEDlow = 0.80, 95% CI: 0.65–0.99) [97].
Modifying the determination method of MD adherence, i.e., considering aMED (Mediterranean diet score excluding alcohol) or MDS (Mediterranean diet score), as described by Trichopoulou [53], it emerged that incidence risk considerably decreased (40%) for post-menopause women (HRMD high vs. MD low = 0.60, 95% CI: 0.39–0.93) [94], and achieved 14% or 18% considering a medium or a high level of MD adherence and keeping a moderate alcohol intake (ORMDS=4-5 vs. MDS=0-3 = 0.86, 95% CI: 0.76–0.98 and ORMDS=6-9 vs. MDS=0-3 = 0.82, 95% CI: 0.71–0.95) [96]. See Table 1 for reviewed studies.
Table 1.
Summary of studies reviewed and relationships with statistical significance between MD score and breast cancer.
| Study | Study Characteristics | MD Adherence | Objective | Statistical Method | Results |
|---|---|---|---|---|---|
| Buckland [97] (2013) | Prospective Study Case-control Sample: 335,062 women Period: 1992 to 2000 Place: Europe |
arMED | Incidence of cancer | Cox proportional hazard regression model | HRarMEDhigh vs. arMEDlow = 0.94 (0.88–1.00) HRarMEDhigh vs. arMEDlow = 0.93 (0.87–0.99) HRarMEDhigh vs. arMEDlow = 0.80 (0.65–0.99) |
| Van den Brandt [94] (2017) | Prospective Study Case-control Sample: 62,573 women aged 55–69 years Period: 1986–2007 Place: Netherlands |
Mediterranean Diet Score | Incidence of cancer | Cox proportional hazard regression model | HRMD high vs. MD low = 0.60, 95% CI: 0.39–0.93 |
| Turati [96] (2018) | Prospective Study Case-control Sample: 6426 women Period: 1991–2008 Place: Italy and Switz |
Mediterranean Diet Score | Incidence of cancer | Logistic regression | ORMDS=4-5 vs. MDS=0-3 = 0.86 (0.76–0.98) ORMDS=6-9 vs. MDS=0-3 = 0.82 (0.71–0.95) ORMDS=4-5 vs. MDS=0-3 = 0.81, (0.71–0.91) |
Though many studies had demonstrated that there was a significant relationship between alcohol and breast cancer [98,99], a recent work showed that excluding completely alcohol from the diet pattern could contribute to increase benefits in case of medium adherence to MD (i.e., risk passes from 14% to 19%) (ORMDS=4-5 vs. MDS=0-3 = 0.81, 95% CI: 0.71–0.91), but it did not increase or reduce positive effects of MD in case of a high adherence to it (ORMDS=6-9 vs. MDS=0-3 = 0.81, 95% CI: 0.70–0.95) [96]. Vice versa, observing the impact of diet on breast cancer using a different diet pattern, it was noted that risk was three-times higher in case of no-healthy diets [100] and it approximately increased of seven-times for those who had preferred fried meat and had avoided stews or other dietic cooking methods [101].
4.2. Colorectal Cancer (CRC)
Diet in general and especially Mediterranean diet play a crucial role for colorectal cancer since healthy or non-healthy diet patterns are considered among the most important risk factors in the onset of this neoplasia [15,102]. In fact, a better quality of diet, whichever it is as long as healthy, is associated with a low CRC risk [14] independently from hereditary, traditional (e.g., belonging to a specific ethnic group) [103] or environmental factors. Important changes in the lifestyle (e.g., introduction of a regular physical activity and a healthy diet) and the exclusion of smoking could determine a reduction in CRC incidence of 70% [14].
It emerged that fiber, calcium and a regular intake of garlic may represent protective elements reducing CRC risk [2,17,104] while no-healthy diet patterns, e.g., those with a high intake of meat or alcohol, represent negative elements increasing CRC incidence [2,16,105,106,107].
A higher adherence to MD was able to reduce of about 30% and 45% CRC risk in men (ORQ4 vs. Q1 = 0.71, 95% CI: 0.55–0.92) and in women (ORQ4 vs. Q1 = 0.65, 95% CI: 0.40–0.77) respectively [108], and the percentage of risk significantly decreased also examining tumor sides (proximal colon ORQ4 vs. Q1 = 0.70, 95% CI: 0.51–0.97, distal colon ORQ4 vs. Q1 = 0.65, 95% CI: 0.48–0.89, and rectum ORQ4 vs. Q1 = 0.60, 95% CI: 0.45–0.81) [108].
On the contrary, a Western diet patterns, i.e., a “westernized” alimentary regimen characterized by a high consumption of dairy, meat, refined cereals, sweets, energy drink, and sauces [35,108], increased CRC risk for men (ORQ4 vs. Q1 = 1.45, 95% CI: 1.11–1.91) and women (ORQ4 vs. Q1 = 2.02, 95% CI: 1.44–2.84) respectively, and had a significant and negative impact in case of rectal cancer (ORQ4 vs. Q1 = 1.46, 95% CI: 1.05–2.01).
To understand how much a diet could impact on the CRC onset, it was noted that the lowest adherence to Western diet could reduce of 1/3 distal colon cancer and of 1/4 rectal cancer [108], since a low intake of sugared drink and read meat could decrease the risk to develop advanced polyps risk (ORsweety drinks = 0.56, 95% CI: 0.36–0.87; ORred meat = 0.63, 95% CI: 0.42–0.95) [13].
Vice versa, a high MD adherence could reduce of 1/5 and 1/4 distal colon and rectum cancer cases respectively [108], allowing a significant reduction of advanced polyps (ORMDS = 3-4 = 0.34 (0.17–0.65), ORMDS = 5-7 = 0.22 (0.11–0.43); ORMDS = 8-10 = 0.18 (0.07–0.47)) [13] and a decrease of CRC risk of 11% (OR = 0.89, 95% CI: 0.86–0.91) for each 1-point increase of MD score [109].
Using different adherence scores, as MMDS (Modified Mediterranean Diet Score), CSMMDS (Centre-Specific MMDS), and Italian Mediterranean Index, did not affect the predictive capacity of MD in the evaluation of the risk: An increase in the MD score, calculated with one of the just mentioned scores, could reduce CRC risk of 11%, 8% and 50% respectively even if confounding variables were considered [15,111].
The high protective capacity of MD was confirmed also in a recent Ratjen’s study (2017) in which they demonstrated as a high MD adherence (measured with MMDS) could reduce mortality rate in CRC patients (HRhighest quartile vs. lowest quartile = 0.48, 95% CI: 0.32–0.74) and, if sex or age variables were considered, it could be possible observing a reduction of mortality rate of 11% and 12% respectively for each 1-point increase of MMDS (HRhighest quartile vs. lowest quartile = 0.88, 95%CI: 0.81–0.96) [110]. See Table 2 for reviewed studies.
Table 2.
Summary of studies reviewed and relationships with statistical significance between MD score and colorectal cancer.
| Study | Study Characteristics | MD Adherence | Objective | Statistical Method | Results |
|---|---|---|---|---|---|
| Castello [108] (2018) | Multicase-control study Sample: 5138 Period: 2008–2013 Place: 11 Spanish provinces |
A posteriori score | Incidence of cancer | Logistic regression | Men: ORQ4 vs. Q1 = 0.71 (0.55–0.92) Women: ORQ4 vs. Q1 = 0.65, (0.40–0.77) Proximal colon: ORQ4 vs. Q1 = 0.70 (0.51–0.97) Distal colon: ORQ4 vs. Q1 = 0.65 (0.48–0.89) Rectum: ORQ4 vs. Q1 = 0.60, (0.45–0.81) |
| Fliss-Isakov [13] (2018) | Case-control study Sample: 783 patients Period: 2010–2015 Place: Israel |
A posteriori score | Incidence of cancer | Multivariate logistic regression | ORMDS = 3-4 = 0.34 (0.17–0.65), ORMDS = 5-7 = 0.22 (0.11–0.43); ORMDS = 8-10 = 0.18 (0.07–0.47) |
| Rosato [109] (2016) | Case-control study Sample: 10,549 patients Period: 1985–1991 Place: Milan (Italy) |
Mediterranean Diet Score | Incidence of cancer | Unconditional logistic regression | OR = 0.89, 95% CI: 0.86–0.91 (for each 1-point increase of MD) |
| Ratjen [110] (2017) | Prospective cohort study Sample: 1404 CRC patients Period: 2004–2007 Place: Northern Germany |
A posteriori score | Mortality rate in CRC patients | Cox proportional hazard regression model | HRhighest quartile vs. lowest quartile = 0.48 (0.32–0.74) HRhighest quartile vs. lowest quartile = 0.88 (0.81–0.96) (for each 1-point increase of MD) |
4.3. Prostate Cancer (PCa)
In accordance with Wilson and Giovannucci’s study (2012), lifestyle and diet pattern are the leading factors in the prevention of the most lethal prostate cancer cases [112]. For this kind of neoplasia, fats, especially animals derived fats and oils, dairy and calcium have a negative effect increasing the PCa incidence [18,19,20,113], while a high intake of fiber and vegetables, soya, legumes, green tea, and tomatoes, in other words an increase of folates, vitamins, especially vitamin C, and of nutrients as lycopene, have a protective effect on prostate, so reducing the cancer risk.
Studies conducted by Kenfield and Richard respectively, confirmed the presence of a strong relationship between diet pattern and prostate cancer risk. Both demonstrated as PCa was less widespread in Mediterranean area than in the Northern Europe ones [115,116]: A high MD adherence not only was inversely associated with a low incidence of prostate cancer, but also it was associated with lower cancer malignancy (44%, ORhigh score vs. low score = 0.66, 95% CI: 0.46–0.95) [114] and mortality rate for PCa (22%, HR = 0.78, 95% CI: 0.67–0.90) in patients without metastasis [115]. Overall, a high MD score was associated with a low likelihood of PCa (OR = 0.86, 95% CI: 0.77–0.96); thereby, PCa risk decreased until 78% in subjects with the highest MD scores, registering in particular a decrease of 14% for each one-point increase of MD score [85]. See Table 3 for reviewed studies.
Table 3.
Summary of studies reviewed and relationships with statistical significance between MD score and prostate cancer.
| Study | Study Characteristics | MD Adherence Measurement | Objective | Statistical Method | Results |
|---|---|---|---|---|---|
| Schneider [114] (2019) | Prospective study Sample: 2258 patients Period:2004–2009 Place: North Caroline, Louisiana (USA) |
Mediterranean Diet Score | Incidence of cancer | Multivariate logistic regression | ORhigh score vs. low score = 0.66 (0.46–0.95) |
| Kenfield [115] (2015) | Prospective study Sample: 47,867 men Period: 1986–2010 Place: USA |
Mediterranean Diet Score | Mortality rate in patients without metastasis | Cox proportional hazard regression model | HR = 0.78 (0.67–0.90) |
| Russo [85] (2018) | Case-control Sample: 356 patients Period: 2015–2016 Place: Catania (Italy) |
MEDILITE score | Incidence of cancer | Multivariate logistic regression | OR = 0.86 (0.77–0.96) (for each 1-point increase of MD score) |
4.4. Gastric Cancer (GC)
With reference to stomach cancer, although the predominant risk factor is the presence of Helicobacter pylori (responsible for 89% of GC cases) [117] and there is an inverse causality problem between risk factors related to diet and gastric cancer (causality determined by disease preclinical symptoms which modify alimentary habits) [118], in this case also it is not possible to exclude a predominant role of diet in the gastric cancer onset [119,120,121].
Observing more closely MD products and connecting them with GC, we may note as Mediterranean characteristics, which can contribute, as protective factors, to reduce GC risk, are mainly represented by a high intake of antioxidants, contained in fresh fruit and vegetables. In fact, antioxidants reduce oxidative DNA damages eliminating free radicals and taking part in many biological changes linked with all cancers (e.g., bioactivation of carcinogens, cell signaling, cell regulation circle, angiogenesis and inflammation), but in this specific neoplasia, a high intake of fresh fruit and vegetable may reduce the negative effect of Helicobacter pylori and its consequent damages [72].
Although there are many confounding factors in the onset of the GC cancer (as the just mentioned Helicobacter pylori), it was demonstrated as the adherence to a healthy lifestyle, in which the diet represents one of the fundamental elements, reduced significantly GC risk until 51% (HR 0.49, 95% CI 0.35, 0.70), with a higher incidence in case of cardia GC (HR 0.23, 95% CI 0.08, 0.68) than in case of non-cardia GC (HR 0.53, 95% CI 0.32, 0.87), so that 19% of patients would have avoided cancer onset in case of an improvement of their lifestyle (no smoking, moderate alcohol, healthy diet and normal BMI) [122]. A higher adherence to Mediterranean diet could reduce significantly GC incidence: Comparing subjects classified in the lowest category of MD adherence (0–3) with those in the following two categories (medium: 4–5 and high: ≥6) the percentage of risk decreased until 22% and 43% respectively, showing so a significant and inverse trend between MD and GC risk [69].
4.5. Bladder Cancer
549.393 new cases of bladder cancer have been diagnosed in 2018 with a global incidence of 3.2% and a stronger predominance among men (4.9%) than among women (1.5%) [2]. For this kind of cancer, diet plays an important role since many metabolites and pollutants (as arsenic contained in drinking tap water) of ingested foods are excreted through the urinary tract and therefore, they come into direct contact with the bladder mucosa [123,124]. Olive oil has a protective role in the reduction of risk of 38% and 50%, respectively, in case of a moderate (ORQ2 vs. Q1 = 0.62, 95% CI: 0.39–0.99) or a high (OR Q3 vs. Q1 = 0.47, 95% CI: 0.28–0.78) consumption of this typical Mediterranean product [125].
Analyzing globally the impact of MD on bladder cancer incidence, and also considering some confounding variables (such as smoking, obesity, sex, and age), they demonstrated that there was an inverse relationship between MD and bladder cancer risk with a reduction until 28% in case of a moderate adherence (ORMDS = 4-5 vs. MDS = 0-3 = 0.72, 95% CI: 0.54–0.98) and until 34% in case of a high adherence (ORMDS = 6-9 vs. MDS = 0-3 = 0.66, 95% CI: 0.47–0.93), i.e., they could observe a decrease of risk of 11% for each one-point increase in MD score [82].
Examining the effect of confounding factors, it emerged an influence of smoking habits on the impact of MD. This influence was slight and no-significant for non-heavy smokers (bladder cancer risk increases of 15% when it was compared with that reported by non-smokers), but it increased significantly for heavy smokers (more than 20 cigarettes/day). Combining the results, it emerged that the Mediterranean diet impact was stronger for non-smoker patients and when tumor invasiveness was classified as pT1-pT4 [82].
This means that MD benefits and its protective role are more evident if they are considered in a healthy lifestyle context aiming to avoid all risk factors which not only participate in the bladder cancer onset, but also, they could reduce positive effects of a healthy diet. To understand better how much a diet may reduce bladder cancer risk, two studies proved that greater adherence to the Mediterranean diet would have been able to avoid the onset of the disease in 12.6% [82] and 4% [126], respectively, of patients registered subsequently with bladder cancer.
4.6. Malignant Tumors of the Female Reproductive System
Ovarian cancer, vulva cancer, vagina cancer, cervical cancer and endometrial cancer are a series of tumors which affect the female reproductive system. While ovarian, vulva, and vagina cancer have a low incidence (1.7%, 0.3%, and 0.1% respectively in 2018) and their risk factors are related with elements not-linked with diet (as anticipated menarche, nulliparity, menopause after 55 years, smoking, and heredity), the etiology of cervical cancer and endometrial cancer could have a relevant correlation with the diet. More specifically, the onset of cervical cancer (the fourth most common cancer as well as the fourth cause of death in the world [127]), is strong correlated with the diet pattern: A regular consumption of fruit, vegetable and therefore of nutrients, as vitamins A, E, C, folates, carotenoids and minerals, may contribute to a reduction of cervical cancer risk [3] thanks to a protective role played by these nutrients to inhibit proliferation of cancerogenic cells and to prevent DNA damages [128,129].
Given the strong relationship between hrHPV (high-risk human papilloma virus) infection and cervical cancer [130], it was demonstrated that MD played a crucial role producing an indirect protective effect in the onset of these neoplasia. In fact, since there is a direct relationship between the Mediterranean diet and the slowdown of progression of hrHPV infection, the Mediterranean diet acts on the infection which, if it is reduced, does not contribute to increase cervical cancer risk. Therefore, a high adherence to MD was able to reduce of 60% the cervical cancer risk (adjORMDShigh vs. MDSlow = 0.40, 95% CI: 0.21–0.75), although this effect was mediate by a reduction of hrHPV. The same positive benefit was not observable in case of Western diet: In this case a greater adherence represented a risk factor in the onset of cervical cancer (adjORQ3 vs. Q1 = 1.77, 95% CI = 1.04–3.54 and adjORQ4 vs. Q1 = 1.97, 95%CI = 1.14–4.18) [3].
In regards to endometrial cancer (the fourth most common cancer in European women [131]), 382,069 new cases were observed in 2018 (5.3% of all women affected by cancer). The major factor in the onset of this cancer is identified in an unbalanced and/or prolonged exposure to endogenous or exogenous estrogens (situations happening in case of advanced menopause, nulliparity and in presence of polycystic ovary) [132], since the increase of estrogens, also consequent to some specific therapies, if it is not opposed by progesterone, may increase the mitotic activity of endometrial cells so producing unwanted DNA replications with a consequence rise of probability of mutation [133]. Given the strong correlation between being overweight or obesity and hormonal problems [134], it is possible to suppose an active role of the diet in the prevention on the endometrial cancer onset modifying estrogens production [135]. A regular consumption of vegetable and fruit had a protective effect in onset of endometrial cancer with a reduction of risk of 66% (adjOR5th quartile vs. 1st quartile: 0.34, 95% CI 0.17–0.68) and of 45% (adjOR5th quartile vs. 1st quartile: 0.55, 95% CI 0.28–1.06). A high adherence to MD could contribute to reduce risk of 43% (adjOR = 0.57, 95% CI: 0.39–0.86) and of 49% (adjOR = 0.51, 95% CI: 0.28–0.92) in case of moderate and high adherence respectively [4]. Analyzing incidence results considering confounding factors and MD score without reference to levels, it was also demonstrated as an increase of one-point in MD score could produce a decrease of risk of 16% and that this decrease was stronger in case of elderly women or in women no-taking of contraceptives or hormonal therapies [136].
4.7. Head-Neck Cancer (HNC)
Incidence of head-neck cancers—oral cavity tumors, oropharynx cancer, hypopharynx cancer, and pharynx cancer—registered a global incidence of 5.2% in 2018. The survival rate at five-years is 40–50% [137]. Although major risk factors are identified in smoking, excessive use of alcohol and in some infections, such as papillomavirus [138], diet plays a crucial role so that some epidemiological studies underlined as a protective effect can be determined by a high intake of fruit and vegetable, especially in case of oral cavity cancer [2,139]. For this reason, a high adherence to MD was inversely associated with a reduction of HNC risk of 20% in men (HR = 0.80; 95% CI: 0.64–1.01) and of 58% in women (HR = 0.42; 95% CI: 0.24–0.74) [138]. This evidence was stronger for oral cavity cancer or pharynx cancer for which the percentage of risk reduction achieved 80% in case of a high level of MD adherence (ORMDShigh vs. MDSlow = 0.20, 95% CI: 0.14–0.21), and became more evident for the young and ex-smokers [76].
In case of nasopharyngeal cancer, it was demonstrated as diet not only played a crucial role [140], but also it showed as a moderate adherence to MD score could reduce risk by 17% (ORMDS=5 vs. MDS≤4 = 0.83, 95% CI: 0.54–1.25) and a high adherence (MDS ≥ 6) could be able to reduce risk by 34% (ORMDS≥6 vs. MDS≤4 = 0.66, 95% CI: 0.44–0.99)), thus showing that an increase in adherence to the Mediterranean diet with a score above or equal to six would have been able to prevent 22% of the observed cases [64].
4.8. Biliary Tract Cancer (BTC), Pancreatic Tumors
Carcinoma of gall bladder and bile ducts are some of the biliary tract tumors. The first is one of the most malignant cancers because of the lateness of diagnosis, often in advanced phases, and the poor survival rate [141]. For these reasons, it is complex to determine incidence, although an intake of fresh fruit and vegetable together with a low consumption of prepacked foods rich in sodium, may represent a determinant factor in the onset of the disease. In fact, oxidative and inflammatory processes or other carcinogens effects could be decrease thank to an improvement of diet quality which surely influences the appearance of gallstones and reduces obesity, both considered risk factors in the onset of this kinds of cancers [142]. An increase of MD adherence, measured with mMED, was inversely associated with a reduction of risk of extra-hepatic BTC ((OR = 0.41 (0.25–0.67)) and gall bladder cancer (OR = 0.42 (0.23–0.79)) [142].
With attention to pancreatic cancer, considered the seventh worldwide cause of death [143], although it was registered as an higher adherence to MD was a protective factor against cancer onset (ORMDS≥6 vs. MDS=0-3 = 0.57, 95% CI: 0.34–0.95, ORMDS≥6 vs. MDS=0-3 = 0.51, 95% CI: 0.29–0.92; ORMDS≥6vsMDS=0-3 = 0.48, 95% CI: 0.35–067 values related to three different Italian centers) [75], we noted that there were not many significant correlations between healthy alimentary habits and the onset of pancreatic cancer. For this reason, some studies have been more concentrated to demonstrate how erroneous lifestyles and diet patterns (e.g., smoking, excessive alcohol assumption and low adherence to MD) increased the pancreatic cancer risk. In fact, there was a positive association between low intake of fruit and vegetable and high consumption of red meat and pancreatic risk [144]. At the same time, an excessive consumption of alcohol increased pancreatic risk until 60% and a low adherence to MD could determine a risk rise of 11.9% [145].
4.9. Lung Cancer
Lung cancer is the most leading cause of death in the westernized countries and its worldwide incidence is the greatest among the whole cancers (2.09 million of new cases in 2018). Given the strong relationship between smoking and lung cancer and between air pollution and lung cancer, it could seem that this kind of tumor is the least correlated with diet. Although there is a high complexity to identify a significant relationship between diet pattern and lung cancer incidence [5,100,146,147], two studies demonstrated that some healthy diet patterns (Healthy Eating Index-2010 (HEI-2010), Alternative Healthy Eating Index-2010 (AHEI-2010), alternate Mediterranean diet score (aMED), dietary approaches to stop hypertension (DASH)) were inversely associated with lung cancer (HEI-2010: HR = 0.83 (0.77–0.89), AHEI-2010: HR = 0.86 (0.80–0.92), aMED: HR = 0.85 (0.79–0.91), DASH: HR = 0.84 (0.78–0.90), MDS: HR7-9 vs. 0-3 = 0.64 (0.45–0.90)) [5,148]. Beneficial effects of MD were evident in case of heavy smokers: The MD score was inversely correlated with lung cancer in smokers, so that an increase of MD level adherence (medium or high) could determine a reduction of risk of either 62% (HR7-9 vs. 0-3 = 0.38 (0.19–0.75)) or 90% (HRaMED≥8 vs. aMED≤1 = 0.10 (0.01–0.77)) [148,149].
5. Conclusions
Examining jointly a series of significant studies, related to last 10 years, about the association between the Mediterranean diet and cancer risk or its incidence, it emerged that diet may represent a determinant factor in the cancer onset, especially when MD adherence is not an occasional or moderate diet pattern, but it is a regular and constant lifestyle. In our analysis of MD benefits, it emerged as diet is a protective factor against the cancer onset, especially when there is a high intake of olive oil, fresh fruit and vegetables, thanks to antioxidant and anti-inflammatory properties of these foods. Therefore, it is necessary to increase prospective studies in which it is evaluated how adherence to MD reduce cancer risk taking in account a series of exogenous variables as geographical areas (therefore their pollution), lifestyle, hereditary factors and origin of foods.
The lack of information about the quality of consumed alimentary products seems represent a limitation of research; if on the one hand the adherence to the MD is characterized by a high intake of some foods, the lack of information about the quality of the same foods (e.g., product treated or not-treated with chemical agents, antibiotics or hormones) could modify results reducing the benefits of MD; this means that the lack of significance in some studies could not be determined by an actual lack of relationship, but caused by the presence of noxious substances, which, in turn, are risk factors in the cancer onset, if not even the main reason for cell mutation. If this biological traceability of products could appear difficult in the case of fruit and vegetables, it becomes particularly complex for meat and, practically impossible, for fish (especially for sea fish). Although the Mediterranean diet recommends a low intake of meat, so that noxious products could have a low impact, it is necessary to underline as the total amount of hormones and antibiotics, ingested by fruit, vegetable, and meat, has an enormous noxious impact on human health (e.g., estradiol is class A1 carcinogens, progesterone, if ingested, causes damages to immune system until immunodepression, as well as it is class 2B carcinogens, androgen is class 2A carcinogens, antibiotics increases antibiotics resistance and altered intestinal flora raising cancer risk, especially for some kind of neoplasia as colorectal cancer) [150,151,152].
Many efforts have been made to reduce the use of hormones and antibiotics in agriculture and livestock farming (e.g., the promotion of organic farming (Reg. (CE) n° 834/2007), prohibition of hormones and antibiotics for auxin purpose in animals (96/22/EC, art. 11 del Reg CE n.1831/2003)), but it is necessary to evaluate in future studies the levels of adherence to the Mediterranean diet together with the origin of foods and their possible level of pollution.
In conclusion, from this narrative review it emerged clearly that the Mediterranean diet may contribute to the reduction of cancer onset in the worldwide population since it is characterized by a series of foods that, due to their antioxidant and anti-inflammatory properties, are able to prevent and counteract DNA damages and slow down the development of various forms of cancer, affecting negatively cell proliferation. Following this dietary pattern could be particularly complex both because of the difficult availability of the foods and because of the objective difficulty of satisfying the world demand for Mediterranean products ensuring, at the same time, the quantity and quality of the offered products. It is, therefore, necessary to invite governments and institutions to evaluate the cost-benefits of promoting education programs for proper nutrition in order to reduce cancer onset through prevention campaigns that educate a healthy lifestyle in which diet is one of the strengths, remembering that “The Mediterranean diet is a set of traditional practices, knowledge and skills passed on from generation to generation and providing a sense of belonging and continuity to the concerned communities” [29].
Appendix A
Table A1.
Elements linked with Mediterranean Food, effect of elements on cancer and cancer risk for each element.
| Typical Foods | Elements | Function | Cancer |
|---|---|---|---|
| Fruits & Vegetables | Antioxidants and micronutrients (carotenoids, vitamin C, vitamin E, selenium, dietary fiber, dithiolthiones, glucosinates, polyphenols, protease inhibitors, allium compounds, plant sterols, and limonene) | Anti-tumorigenic effect | Less risk of: -Epithelial cancer -Digestive tract cancer -Breast cancer -Female genital tract cancer -Urinary tract cancer |
| Fish | Long-chain omega-3 fatty acids docosahexaenoic acid and eicosapentaenoic acid | Reducing tumor cell growth Modulation of transcription factor activity and signal transduction Alteration of oestrogen metabolism |
Less risk of: -Liver cancer -Colorectal cancer |
| Heterocyclic amines and polycyclic aromatic hydrocarbons may be formed when fish is cooked on a grill or barbecue | Production of mutagenic chemicals | High risk of stomach cancer | |
| Olive oil | Polyphenols (oleuropein and hydroxytyrosol) | Antioxidant activity, anti-inflammatory and anti-mutagenic effects | Less risk of: -breast cancer -ovarian cancer -upper aero-digestive tract cancer -colorectal cancer |
| Oleic acid, poly unsaturated fatty acids (PUFA), low n-6 PUFA/n-3 PUFA ratio | Chemoprotective effect | ||
| Meat | Heterocyclic amines and polycyclic aromatic hydrocarbons formed when meat is cooked at high temperatures | Carcinogens | High risk of: -colorectal cancer -nasopharynx cancer -ung cancer -pancreatic cancer -bladder cancer -esophagus cancer (squamous cell carcinoma) -stomach (no-cardia) cancer |
| Haem iron, present in high level | Promotion of tumorigenesis by stimulating the endogenous formation of carcinogenic N-nitroso compounds | ||
| High-temperature cooking of red and processed meats may enhance production of advanced glycation endproducts (AGEs). | Produce several cancer-promoting effects | High risk of pancreatic cancer | |
| Consumption of meat may lead to insulin resistance and hyperinsulinemia, promoting growth of cancer cells | Promoting growth of cancer cells | ||
| Whole grains | Provide various nutrients: vitamin E, selenium, copper, zinc and bioactive non-nutrient compounds (lignans, phytoestrogens, and phenolic compounds), and dietary fiber | Anti-carcinogenic properties, as anti-oxidative activity Reduce insulin resistance |
Less risk of: -colorectum cancer -upper aero-digestive tract -stomach cancer -breast cancer -ovarian cancer -kidney cancer |
| Aflatoxin (mycotoxin produced by molds of the Aspergillus species) | High mutation load in TP3 | High risk of liver cancer | |
| Dairy Products | Calcium, lactic acid-producing bacteria, vitamin D, linoleic acids, lactoferrin, | Inhibit tumor development | Less risk of: -breast cancer (pre-menopausal and post-menopausal women) -colorectal cancer |
| High level of calcium | Downregulating the formation of the biologically active form of vitamin D → increasing cellular proliferation | Higher risk of prostate cancer | |
| Red Wine | Phytoalexin presents in grape skin | Antioxidant and cancer chemo preventive agent → inhibiting tumor initiation, promotion and progression | Controversial results about impact |
| Resveratrol and quercetin | Modulating cell cycle-regulating proteins Inducing apoptosis in multiple carcinoma cell lines Anti-inflammatory, growth → inhibiting activity and immunomodulation properties |
Information source: World Cancer Research Fund and Grosso et al. (2013).
Author Contributions
Conceptualization, M.C.M. and G.A.D.M.; methodology, M.C.M., F.S.; resources, M.C.M.; data curation, M.C.M.; Writing—Original draft preparation, M.C.M.; Writing—Review and editing, M.C.M., F.S.; visualization, M.C.M., F.S., C.R.; supervision, G.A.D.M., A.G.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.World Health Organization Cancer. [(accessed on 30 June 2019)]; Available online: www.who.int/news-room/fact-sheets/detail/cancer.
- 2.World Cancer Research Fund Worldwide Cancer Data. [(accessed on 30 June 2019)]; Available online: https://www.wcfr.org/dietandcancer/cancer-trend/worldwide-cancer-data.
- 3.Barchitta M., Maugeri A., Quattrocchi A., Agrifoglio O., Scalisi A., Agodi A. The Association of Dietary Patterns with High-Risk Human Papillomavirus Infection and Cervical Cancer: A Cross-Sectional Study in Italy. Nutrients. 2018;10:469. doi: 10.3390/nu10040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricceri F., Giraudo M.T., Fasanelli F., Milanese D., Sciannameo V., Fiorini L., Sacerdote C. Diet and Endometrial Cancer: A Focus on the Role of Fruit and Vegetable Intake, Mediterranean Diet and Dietary Inflammatory Index in the Endometrial Cancer Risk. BMC Cancer. 2017;17:757. doi: 10.1186/s12885-017-3754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodge A.M., Bassett J.K., Shivappa N., Hebert J.R., English D.R., Giles G.G., Severi G. Dietary Inflammatory Index, Mediterranean Diet Score, and Lung Cancer: A Prospective Study. Cancer Causes Control. 2016;27:907–917. doi: 10.1007/s10552-016-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodén S., Myte R., Wennberg M., Harlid S., Johansson I., Shivappa N., Hébert J.R., Van Guelpen B., Nilsson L.M. The Inflammatory Potential of Diet in Determining Cancer Risk; A Prospective Investigation of Two Dietary Pattern Scores. PLoS ONE. 2019;14:e0214551. doi: 10.1371/journal.pone.0214551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwingshackl L., Hoffmann G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis of Observational Studies. Cancer Med. 2015;4:1933–1947. doi: 10.1002/cam4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panunzio M., Caporizzi R., Cela E.P., Antoniciello A., Di Martino V., Ferguson L.R. Promotion of the Mediterranean Diet Incancer Long-Survivors by Means of the Med-Food Anticancer Program: A Pilot Study. Ann. Ig. 2019;31:45–51. doi: 10.7416/ai.2019.2258. [DOI] [PubMed] [Google Scholar]
- 9.Ndlovu T., van Jaarsveld F., Caleb O.J. French and Mediterranean-Style Diets: Contradictions, Misconceptions and Scientific Facts-A Review. Food Res. Int. 2019;116:840–858. doi: 10.1016/j.foodres.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson M.S. Nutrition and Cancer: A Review of the Evidence for an Anti-Cancer Diet. Nutr. J. 2004;3:19. doi: 10.1186/1475-2891-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vineis P., Wild C.P. Global Cancer Patterns: Causes and Prevention. Lancet. 2014;383:549–557. doi: 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- 12.Solans M., Castelló A., Benavente Y., Marcos-Gragera R., Amiano P., Gracia-Lavedan E., Costas L., Robles C., Gonzalez-Barca E., de la Banda E., et al. Adherence to the Western, Prudent, and Mediterranean Dietary Patterns and Chronic Lymphocytic Leukemia in the MCC-Spain Study. Haematologica. 2018;103:1881–1888. doi: 10.3324/haematol.2018.192526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fliss-Isakov N., Kariv R., Webb M., Ivancovsky D., Margalit D., Zelber-Sagi S. Mediterranean Dietary Components Are Inversely Associated with Advanced Colorectal Polyps: A Case-Control Study. World J. Gastroenterol. 2018;24:2617–2627. doi: 10.3748/wjg.v24.i24.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres Stone R.A., Waring M.E., Cutrona S.L., Kiefe C.I., Allison J., Doubeni C.A. The Association of Dietary Quality with Colorectal Cancer among Normal Weight, Overweight and Obese Men and Women: A Prospective Longitudinal Study in the USA. BMJ Open. 2017;7:e015619. doi: 10.1136/bmjopen-2016-015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bamia C., Lagiou P., Buckland G., Grioni S., Agnoli C., Taylor A.J., Dahm C.C., Overvad K., Olsen A., Tjønneland A., et al. Mediterranean Diet and Colorectal Cancer Risk: Results from a European Cohort. Eur. J. Epidemiol. 2013;28:317–328. doi: 10.1007/s10654-013-9795-x. [DOI] [PubMed] [Google Scholar]
- 16.Magalhães B., Bastos J., Lunet N. Dietary Patterns and Colorectal Cancer: A Case-Control Study from Portugal. Eur. J. Cancer Prev. 2011 doi: 10.1097/CEJ.0b013e328347220a. [DOI] [PubMed] [Google Scholar]
- 17.Song M., Garrett W.S., Chan A.T. Nutrients, Foods, and Colorectal Cancer Prevention. Gastroenterology. 2015 doi: 10.1053/j.gastro.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gann P.H. Risk Factors for Prostate Cancer. Rev. Urol. 2002;4:S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 19.Pelser C., Mondul A.M., Hollenbeck A.R., Park Y. Dietary Fat, Fatty Acids, and Risk of Prostate Cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol. Prev. Biomark. 2013;22:697–707. doi: 10.1158/1055-9965.EPI-12-1196-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aune D., Navarro Rosenblatt D.A., Chan D.S.M., Vieira A.R., Vieira R., Greenwood D.C., Vatten L.J., Norat T. Dairy Products, Calcium, and Prostate Cancer Risk: A Systematic Review and Meta-Analysis of Cohort Studies. Am. J. Clin. Nutr. 2014;101:87–117. doi: 10.3945/ajcn.113.067157. [DOI] [PubMed] [Google Scholar]
- 21.Cheung E., Wadhera P., Dorff T., Pinski J. Diet and Prostate Cancer Risk Reduction. Expert Rev. Anticancer Ther. 2008;8:43–50. doi: 10.1586/14737140.8.1.43. [DOI] [PubMed] [Google Scholar]
- 22.Hardin J., Cheng I., Witte J.S. Impact of Consumption of Vegetable, Fruit, Grain, and High Glycemic Index Foods on Aggressive Prostate Cancer Risk. Nutr. Cancer. 2011;63:860–872. doi: 10.1080/01635581.2011.582224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei M.Y., Giovannucci E.L. Lycopene, Tomato Products, and Prostate Cancer Incidence: A Review and Reassessment in the PSA Screening Era. J. Oncol. 2012 doi: 10.1155/2012/271063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lăcătușu C.M., Grigorescu E.D., Floria M., Onofriescu A., Mihai B.M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health. 2019;16:942. doi: 10.3390/ijerph16060942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckland G., González C.A., Agudo A., Vilardell M., Berenguer A., Amiano P., Ardanaz E., Arriola L., Barricarte A., Basterretxea M., et al. Adherence to the Mediterranean Diet and Risk of Coronary Heart Disease in the Spanish EPIC Cohort Study. Am. J. Epidemiol. 2009 doi: 10.1093/aje/kwp282. [DOI] [PubMed] [Google Scholar]
- 26.Trichopoulou A., Critselis E. Mediterranean Diet and Longevity. Eur. J. Cancer Prev. 2004;13:453–456. doi: 10.1097/00008469-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-González M.Á., Hershey M.S., Zazpe I., Trichopoulou A. Transferability of the Mediterranean Diet to Non-Mediterranean Countries. What Is and What Is Not the Mediterranean Diet. Nutrients. 2017;9:1226. doi: 10.3390/nu9111226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinu M., Pagliai G., Casini A., Sofi F. Mediterranean Diet and Multiple Health Outcomes: An Umbrella Review of Meta-Analyses of Observational Studies and Randomised Trials. Eur. J. Clin. Nutr. 2018;72:30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- 29.UNESCO . Representative List of the Intangible Cultural Heritage of Humanity Representative List of the Intangible Cultural Heritage of Humanity. UNESCO; Paris, France: 2013. [Google Scholar]
- 30.Keys A., Menotti A., Karvonen M.J., Aravanis C., Blackburn H., Buzina R., Djordjevic B.S., Dontas A.S., Fidanza F., Keys M.H., et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986;124:903–915. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Gonzalez M.A., Bes-Rastrollo M., Serra-Majem L., Lairon D., Estruch R., Trichopoulou A. Mediterranean Food Pattern and the Primary Prevention of Chronic Disease: Recent Developments. Nutr. Rev. 2009;67:S111–S116. doi: 10.1111/j.1753-4887.2009.00172.x. [DOI] [PubMed] [Google Scholar]
- 32.European Union Regolamento (CEE) [(accessed on 30 June 2019)]; Available online: http://data.europa.eu/eli/reg/1991/2568/2015-01-01.
- 33.Panagiotakos D.B., Pitsavos C., Chrysohoou C., Stefanadis C., Toutouzas P. Risk Stratification of Coronary Heart Disease in Greece: Final Results from the CARDIO2000 Epidemiological Study. Prev. Med. 2002 doi: 10.1006/pmed.2002.1108. [DOI] [PubMed] [Google Scholar]
- 34.Dilis V., Katsoulis M., Lagiou P., Trichopoulos D., Naska A., Trichopoulou A. Mediterranean Diet and CHD: The Greek European Prospective Investigation into Cancer and Nutrition Cohort. Br. J. Nutr. 2012;108:699–709. doi: 10.1017/S0007114512001821. [DOI] [PubMed] [Google Scholar]
- 35.Rizzello F., Spisni E., Giovanardi E., Imbesi V., Salice M., Alvisi P., Valerii M.C., Gionchetti P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients. 2019;11:1033. doi: 10.3390/nu11051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trichopoulou A., Lagiou P. Healthy Traditional Mediterranean Diet: An Expression of Culture, History, and Lifestyle. Nutr. Rev. 2009 doi: 10.1111/j.1753-4887.1997.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 37.Sofi F. The Mediterranean Diet Revisited: Evidence of Its Effectiveness Grows. Curr. Opin. Cardiol. 2009;24:442–446. doi: 10.1097/HCO.0b013e32832f056e. [DOI] [PubMed] [Google Scholar]
- 38.Prabhakaran D., Khandelwal S., Martínez-González M.A., Tong T.Y., Forouhi N.G., Trichopoulou A., Mozaffarian D., de Lorgeril M. Definitions and Potential Health Benefits of the Mediterranean Diet: Views from Experts around the World. BMC Med. 2014;12:112. doi: 10.1186/1741-7015-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pérez-Martínez P., Mikhailidis D.P., Athyros V.G., Bullo M., Couture P., Covas M.I., de Koning L., Delgado-Lista J., Díaz-López A., Drevon C.A., et al. Lifestyle Recommendations for the Prevention and Management of Metabolic Syndrome: An International Panel Recommendation. Nutr. Rev. 2017;75:307–326. doi: 10.1093/nutrit/nux014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilis W., Stec K., Zych M., Pilis A. Health Benefits and Risk Associated with Adopting a Vegetarian Diet. Rocz. Panstw. Zakl. Hig. 2014;65:9–14. [PubMed] [Google Scholar]
- 41.Dernini S., Berry E.M. Mediterranean Diet: From a Healthy Diet to a Sustainable Dietary Pattern. Front. Nutr. 2015;2:15. doi: 10.3389/fnut.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alsaffar A.A. Sustainable Diets: The Interaction between Food Industry, Nutrition, Health and the Environment. Food Sci. Technol. Int. 2016;22:102–111. doi: 10.1177/1082013215572029. [DOI] [PubMed] [Google Scholar]
- 43.Bach-Faig A., Berry E.M., Lairon D., Reguant J., Trichopoulou A., Dernini S., Medina F.X., Battino M., Belahsen R., Miranda G., et al. Mediterranean Diet Pyramid Today. Science and Cultural Updates. Public Health Nutr. 2011;14:2274–2284. doi: 10.1017/S1368980011002515. [DOI] [PubMed] [Google Scholar]
- 44.Bonaccio M., Iacoviello L., De Gaetano G. The Mediterranean Diet: The Reasons for a Success. Thromb. Res. 2012;129:401–404. doi: 10.1016/j.thromres.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Ciati R., Ruini L. Sustainable Diets and Biodiversity Directions and Solutions for Policy, Research and Action. Barilla Center for Food & Nutrition; Parma, ITALY: 2012. Double Pyramid: Healthy Food for People, Sustainable Food for the Planet. [Google Scholar]
- 46.Affret A., Severi G., Dow C., Rey G., Delpierre C., Boutron-Ruault M.C., Clavel-Chapelon F., Fagherazzi G. Socio-Economic Factors Associated with a Healthy Diet: Results from the E3N Study. Public Health Nutr. 2017;20:1574–1583. doi: 10.1017/S1368980017000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burlingame B., Dernini S. Sustainable Diets and Biodiversity: Directions and Solutions for Policy, Research and Action. [(accessed on 30 June 2019)]; Available online: http://www.fao.org/3/a-i3004e.pdf.
- 48.Drewnowski A., Specter S.E. Poverty and Obesity: The Role of Energy Density and Energy Costs. Am. J. Clin. Nutr. 2004;79:6–16. doi: 10.1093/ajcn/79.1.6. [DOI] [PubMed] [Google Scholar]
- 49.Darmon N., Drewnowski A. Does Social Class Predict Diet Quality? Am. J. Clin. Nutr. 2008;87:1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 50.Seconda L., Baudry J., Allès B., Hamza O., Boizot-Szantai C., Soler L.G., Galan P., Hercberg S., Lairon D., Kesse-Guyot E. Assessment of the Sustainability of the Mediterranean Diet Combined with Organic Food Consumption: An Individual Behaviour Approach. Nutrients. 2017;9:61. doi: 10.3390/nu9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis C., Bryan J., Hodgson J., Murphy K. Definition of the Mediterranean Diet: A Literature Review. Nutrients. 2015;7:9139–9153. doi: 10.3390/nu7115459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerber M., Hoffman R. The Mediterranean Diet: Health, Science and Society. Br. J. Nutr. 2015;113:S4–S10. doi: 10.1017/S0007114514003912. [DOI] [PubMed] [Google Scholar]
- 53.Trichopoulou A., Costacou T., Bamia C., Trichopoulos D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003 doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 54.Sofi F., Abbate R., Gensini G.F., Casini A. Accruing Evidence on Benefits of Adherence to the Mediterranean Diet on Health: An Updated Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2010 doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 55.Knoops K.T.B., De Groot L.C.P.G.M., Kromhout D., Perrin A.E., Moreiras-Varela O., Menotti A., Van Staveren W.A. Mediterranean Diet, Lifestyle Factors, and 10-Year Mortality in Elderly European Men and Women: The HALE Project. J. Am. Med. Assoc. 2004 doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 56.Buckland G., Agudo A., Travier N., María Huerta J., Cirera L., Tormo M.J., Navarro C., Dolores Chirlaque M., Moreno-Iribas C., Ardanaz E., et al. Adherence to the Mediterranean Diet Reduces Mortality in the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Br. J. Nutr. 2011 doi: 10.1017/S0007114511002078. [DOI] [PubMed] [Google Scholar]
- 57.Schwedhelm C., Boeing H., Hoffmann G., Aleksandrova K., Schwingshackl L. Effect of Diet on Mortality and Cancer Recurrence among Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Nutr. Rev. 2016;74:737–748. doi: 10.1093/nutrit/nuw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naska A., Trichopoulou A. Back to the Future: The Mediterranean Diet Paradigm. Nutr. Metab. Cardiovasc. Dis. 2014;24:216–219. doi: 10.1016/j.numecd.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 59.De Lorenzo A., Alberti A., Andreoli A., Iacopino L., Serrano P., Perriello G. Food Habits in a Southern Italian Town (Nicotera) in 1960 and 1996: Still a Reference Italian Mediterranean Diet? Diabetes Nutr. Metab. 2001;14:121–125. [PubMed] [Google Scholar]
- 60.Dernini S. The Erosion and the Renaissance of the Mediterranean Diet: A Sustainable Cultural Resource. Quad. Mediterrània. 2011;16:75–82. [Google Scholar]
- 61.Bonaccio M., Di Castelnuovo A., Costanzo S., Persichillo M., De Curtis A., Donati M.B., De Gaetano G., Iacoviello L. Adherence to the Traditional Mediterranean Diet and Mortality in Subjects with Diabetes. Prospective Results from the MOLI-SANI Study. Eur. J. Prev. Cardiol. 2016;23:400–407. doi: 10.1177/2047487315569409. [DOI] [PubMed] [Google Scholar]
- 62.Hauner H., Hauner D. The Impact of Nutrition on the Development and Prognosis of Breast Cancer. Breast Care. 2010;5:377–381. doi: 10.1159/000322648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russo G.I., Campisi D., Di Mauro M., Regis F., Reale G., Marranzano M., Ragusa R., Solinas T., Madonia M., Cimino S., et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules. 2017;22:2159. doi: 10.3390/molecules22122159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turati F., Bravi F., Polesel J., Bosetti C., Negri E., Garavello W., Taborelli M., Serraino D., Libra M., Montella M., et al. Adherence to the Mediterranean Diet and Nasopharyngeal Cancer Risk in Italy. Cancer Causes Control. 2017;28:89–95. doi: 10.1007/s10552-017-0850-x. [DOI] [PubMed] [Google Scholar]
- 65.Ciancarelli M.G., Massimo C., Amicis D., Ciancarelli I. Mediterranean Diet and Health Promotion: Evidence and Current Concerns. Med. Res. Arch. 2017 doi: 10.18103/mra.v5i7.1385. [DOI] [Google Scholar]
- 66.Machowetz A., Poulsen H.E., Gruendel S., Weimann A., Fitó M., Marrugat J., de la Torre R., Salonen J.T., Nyyssönen K., Mursu J., et al. Effect of Olive Oils on Biomarkers of Oxidative DNA Stress in Northern and Southern Europeans. FASEB J. 2007 doi: 10.1096/fj.06-6328com. [DOI] [PubMed] [Google Scholar]
- 67.Fitó M., Cladellas M., de la Torre R., Martí J., Muñoz D., Schröder H., Alcántara M., Pujadas-Bastardes M., Marrugat J., Ló-Sabater M.C., et al. Anti-Inflammatory Effect of Virgin Olive Oil in Stable Coronary Disease Patients: A Randomized, Crossover, Controlled Trial. Eur. J. Clin. Nutr. 2008 doi: 10.1038/sj.ejcn.1602724. [DOI] [PubMed] [Google Scholar]
- 68.Rossi M., Turati F., Lagiou P., Trichopoulos D., Augustin L.S., La Vecchia C., Trichopoulou A. Mediterranean Diet and Glycaemic Load in Relation to Incidence of Type 2 Diabetes: Results from the Greek Cohort of the Population-Based European Prospective Investigation into Cancer and Nutrition (EPIC) Diabetologia. 2013;56:2405–2413. doi: 10.1007/s00125-013-3013-y. [DOI] [PubMed] [Google Scholar]
- 69.Praud D., Bertuccio P., Bosetti C., Turati F., Ferraroni M., La Vecchia C. Adherence to the Mediterranean Diet and Gastric Cancer Risk in Italy. Int. J. Cancer. 2014;134:2935–2941. doi: 10.1002/ijc.28620. [DOI] [PubMed] [Google Scholar]
- 70.Pitsavos C., Panagiotakos D.B., Tzima N., Chrysohoou C., Economou M., Zampelas A., Stefanadis C. Adherence to the Mediterranean Diet Is Associated with Total Antioxidant Capacity in Healthy Adults: The ATTICA Study. Am. J. Clin. Nutr. 2005;82:694–699. doi: 10.1093/ajcn/82.3.694. [DOI] [PubMed] [Google Scholar]
- 71.Pérez-Jiménez J., Elena Díaz-Rubio M., Saura-Calixto F. Contribution of Macromolecular Antioxidants to Dietary Antioxidant Capacity: A Study in the Spanish Mediterranean Diet. Plant Foods Hum. Nutr. 2015 doi: 10.1007/s11130-015-0513-6. [DOI] [PubMed] [Google Scholar]
- 72.Castelló A., Boldo E., Pérez-Gómez B., Lope V., Altzibar J.M., Martín V., Castaño-Vinyals G., Guevara M., Dierssen-Sotos T., Tardón A., et al. Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Breast Cancer Risk: MCC-Spain Study. Maturitas. 2017;103:8–15. doi: 10.1016/j.maturitas.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 73.Li F., An S., Hou L., Chen P., Lei C., Tan W. Red and Processed Meat Intake and Risk of Bladder Cancer: A Meta-Analysis. Int. J. Clin. Exp. Med. 2014;7:2100. [PMC free article] [PubMed] [Google Scholar]
- 74.Giacosa A., Barale R., Bavaresco L., Gatenby P., Gerbi V., Janssens J., Johnston B., Kas K., La Vecchia C., Mainguet P., et al. Cancer Prevention in Europe. Eur. J. Cancer Prev. 2013;22:90–95. doi: 10.1097/CEJ.0b013e328354d2d7. [DOI] [PubMed] [Google Scholar]
- 75.Bosetti C., Turati F., Pont A.D., Ferraroni M., Polesel J., Negri E., Serraino D., Talamini R., La Vecchia C., Zeegers M.P. The Role of Mediterranean Diet on the Risk of Pancreatic Cancer. Br. J. Cancer. 2013;109:1360–1366. doi: 10.1038/bjc.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Filomeno M., Bosetti C., Garavello W., Levi F., Galeone C., Negri E., La Vecchia C. The Role of a Mediterranean Diet on the Risk of Oral and Pharyngeal Cancer. Br. J. Cancer. 2014;111:981–986. doi: 10.1038/bjc.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trichopoulou A., Lagiou P., Kuper H., Trichopoulos D. Cancer and Mediterranean Dietary Traditions. Cancer Epidemiol. Biomark. Prev. 2000;9:869–873. [PubMed] [Google Scholar]
- 78.Pelucchi C., Bosetti C., Rossi M., Negri E., La Vecchia C. Selected Aspects of Mediterranean Diet and Cancer Risk. Nutr. Cancer. 2009 doi: 10.1080/01635580903285007. [DOI] [PubMed] [Google Scholar]
- 79.Verberne L., Bach-Faig A., Buckland G., Serra-Majem L. Association between the Mediterranean Diet and Cancer Risk: A Review of Observational Studies. Nutr. Cancer. 2010 doi: 10.1080/01635581.2010.509834. [DOI] [PubMed] [Google Scholar]
- 80.Schwingshackl L., Hoffmann G. Does a Mediterranean-Type Diet Reduce Cancer Risk? Curr. Nutr. Rep. 2016;5:9–17. doi: 10.1007/s13668-015-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fasanelli F., Zugna D., Giraudo M.T., Krogh V., Grioni S., Panico S., Mattiello A., Masala G., Caini S., Tumino R., et al. Abdominal Adiposity Is Not a Mediator of the Protective Effect of Mediterranean Diet on Colorectal Cancer. Int. J. Cancer. 2017;140:2265–2271. doi: 10.1002/ijc.30653. [DOI] [PubMed] [Google Scholar]
- 82.Bravi F., Spei M.E., Polesel J., Di Maso M., Montella M., Ferraroni M., Serraino D., Libra M., Negri E., La Vecchia C., et al. Mediterranean Diet and Bladder Cancer Risk in Italy. Nutrients. 2018;10:1061. doi: 10.3390/nu10081061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kontou N., Psaltopoulou T., Soupos N., Polychronopoulos E., Linos A., Xinopoulos D., Panagiotakos D.B. The Role of Number of Meals, Coffee Intake, Salt and Type of Cookware on Colorectal Cancer Development in the Context of the Mediterranean Diet. Public Health Nutr. 2013;16:928–935. doi: 10.1017/S1368980012003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kontou N., Psaltopoulou T., Soupos N., Polychronopoulos E., Xinopoulos D., Linos A., Panagiotakos D.B. The Mediating Effect of Mediterranean Diet on the Relation between Smoking and Colorectal Cancer: A Case-Control Study. Eur. J. Public Health. 2013;23:742–746. doi: 10.1093/eurpub/cks109. [DOI] [PubMed] [Google Scholar]
- 85.Russo G.I., Solinas T., Urzì D., Privitera S., Campisi D., Cocci A., Carini M., Madonia M., Cimino S., Morgia G. Adherence to Mediterranean Diet and Prostate Cancer Risk in Sicily: Population-Based Case–Control Study. Int. J. Impot. Res. 2018 doi: 10.1038/s41443-018-0088-5. [DOI] [PubMed] [Google Scholar]
- 86.Lauby-Secretan B., Scoccianti C., Loomis D., Grosse Y., Bianchini F., Straif K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016 doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mayne S.T., Playdon M.C., Rock C.L. Diet, Nutrition, and Cancer: Past, Present and Future. Nat. Rev. Clin. Oncol. 2016;13:504–515. doi: 10.1038/nrclinonc.2016.24. [DOI] [PubMed] [Google Scholar]
- 88.Jacobs D.R., Gross M.D., Tapsell L.C. Food Synergy: An Operational Concept for Understanding Nutrition. Am. J. Clin. Nutr. 2009 doi: 10.3945/ajcn.2009.26736B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grosso G., Buscemi S., Galvano F., Mistretta A., Marventano S., La Vela V., Drago F., Gangi S., Basile F., Biondi A. Mediterranean Diet and Cancer: Epidemiological Evidence and Mechanism of Selected Aspects. BMC Surg. 2013;13:S14. doi: 10.1186/1471-2482-13-S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y., Li S., Meng X., Gan R.Y., Zhang J.J., Li H. Bin. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients. 2017;9:728. doi: 10.3390/nu9070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amor S., Châlons P., Aires V., Delmas D. Polyphenol Extracts from Red Wine and Grapevine: Potential Effects on Cancers. Diseases. 2018;6:106. doi: 10.3390/diseases6040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tosti V., Bertozzi B., Fontana L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Series A Biol. Sci. Med Sci. 2018 doi: 10.1093/gerona/glx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akram M., Iqbal M., Daniyal M., Khan A.U. Awareness and Current Knowledge of Breast Cancer. Biol. Res. 2017;50:33. doi: 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van den Brandt P.A., Schulpen M. Mediterranean Diet Adherence and Risk of Postmenopausal Breast Cancer: Results of a Cohort Study and Meta-Analysis. Int. J. Cancer. 2017;140:2220–2231. doi: 10.1002/ijc.30654. [DOI] [PubMed] [Google Scholar]
- 95.Tfayli A., Temraz S., Abou Mrad R., Shamseddine A. Breast Cancer in Low- and Middle-Income Countries: An Emerging and Challenging Epidemic. J. Oncol. 2010 doi: 10.1155/2010/490631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Turati F., Carioli G., Bravi F., Ferraroni M., Serraino D., Montella M., Giacosa A., Toffolutti F., Negri E., Levi F., et al. Mediterranean Diet and Breast Cancer Risk. Nutrients. 2018;10:326. doi: 10.3390/nu10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buckland G., Travier N., Cottet V., González C.A., Luján-Barroso L., Agudo A., Trichopoulou A., Lagiou P., Trichopoulos D., Peeters P.H., et al. Adherence to the Mediterranean Diet and Risk of Breast Cancer in the European Prospective Investigation into Cancer and Nutrition Cohort Study. Int. J. Cancer. 2013;132:2918–2927. doi: 10.1002/ijc.27958. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y., Nguyen N., Colditz G.A. Links between Alcohol Consumption and Breast Cancer: A Look at the Evidence. Women’s Health. 2015;11:65–77. doi: 10.2217/WHE.14.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shield K.D., Soerjomataram I., Rehm J. Alcohol Use and Breast Cancer: A Critical Review. Alcohol. Clin. Exp. Res. 2016;40:1166–1181. doi: 10.1111/acer.13071. [DOI] [PubMed] [Google Scholar]
- 100.Krusinska B., Hawrysz I., Wadolowska L., Slowinska M.A., Biernacki M., Czerwinska A., Golota J.J. Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study. Nutrients. 2018;10:470. doi: 10.3390/nu10040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toklu H., Nogay N.H. Effects of Dietary Habits and Sedentary Lifestyle on Breast Cancer among Women Attending the Oncology Day Treatment Center at a State University in Turkey. Niger. J. Clin. Pract. 2018;21:1576–1584. doi: 10.4103/njcp.njcp_238_18. [DOI] [PubMed] [Google Scholar]
- 102.Reedy J., Mitrou P.N., Krebs-Smith S.M., Wirfält E., Flood A., Kipnis V., Leitzmann M., Mouw T., Hollenbeck A., Schatzkin A., et al. Index-Based Dietary Patterns and Risk of Colorectal Cancer. Am. J. Epidemiol. 2008;168:38–48. doi: 10.1093/aje/kwn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park S.Y., Boushey C.J., Wilkens L.R., Haiman C.A., Le Marchand L. High-Quality Diets Associate With Reduced Risk of Colorectal Cancer: Analyses of Diet Quality Indexes in the Multiethnic Cohort. Gastroenterology. 2017;153:386–394. doi: 10.1053/j.gastro.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barrubés L., Babio N., Mena-Sánchez G., Toledo E., Ramírez-Sabio J.B., Estruch R., Ros E., Fitó M., Arós F., Fiol M., et al. Dairy Product Consumption and Risk of Colorectal Cancer in an Older Mediterranean Population at High Cardiovascular Risk. Int. J. Cancer. 2018;143:1356–1366. doi: 10.1002/ijc.31540. [DOI] [PubMed] [Google Scholar]
- 105.Aune D., Chan D.S.M., Vieira A.R., Navarro Rosenblatt D.A., Vieira R., Greenwood D.C., Kampman E., Norat T. Red and Processed Meat Intake and Risk of Colorectal Adenomas: A Systematic Review and Meta-Analysis of Epidemiological Studies. Cancer Causes Control. 2013 doi: 10.1007/s10552-012-0139-z. [DOI] [PubMed] [Google Scholar]
- 106.Cai S., Li Y., Ding Y., Chen K., Jin M. Alcohol Drinking and the Risk of Colorectal Cancer Death: A Meta-Analysis. Eur. J. Cancer Prev. 2014;23:532–539. doi: 10.1097/CEJ.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 107.Johnson I.T. The Cancer Risk Related to Meat and Meat Products. Br. Med. Bull. 2017;121:73–81. doi: 10.1093/bmb/ldw051. [DOI] [PubMed] [Google Scholar]
- 108.Castelló A., Amiano P., Fernández de Larrea N., Martín V., Alonso M.H., Castaño-Vinyals G., Pérez-Gómez B., Olmedo-Requena R., Guevara M., Fernandez-Tardon G., et al. Low Adherence to the Western and High Adherence to the Mediterranean Dietary Patterns Could Prevent Colorectal Cancer. Eur. J. Nutr. 2018;58:1–11. doi: 10.1007/s00394-018-1674-5. [DOI] [PubMed] [Google Scholar]
- 109.Rosato V., Guercio V., Bosetti C., Negri E., Serraino D., Giacosa A., Montella M., La Vecchia C., Tavani A. Mediterranean Diet and Colorectal Cancer Risk: A Pooled Analysis of Three Italian Case-Control Studies. Br. J. Cancer. 2016;115:862–865. doi: 10.1038/bjc.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ratjen I., Schafmayer C., di Giuseppe R., Waniek S., Plachta-Danielzik S., Koch M., Nöthlings U., Hampe J., Schlesinger S., Lieb W. Postdiagnostic Mediterranean and Healthy Nordic Dietary Patterns Are Inversely Associated with All-Cause Mortality in Long-Term Colorectal Cancer Survivors. J. Nutr. 2017;147:636–644. doi: 10.3945/jn.116.244129. [DOI] [PubMed] [Google Scholar]
- 111.Agnoli C., Grioni S., Sieri S., Palli D., Masala G., Sacerdote C., Vineis P., Tumino R., Giurdanella M.C., Pala V., et al. Italian Mediterranean Index and Risk of Colorectal Cancer in the Italian Section of the EPIC Cohort. Int. J. Cancer. 2013;132:1404–1411. doi: 10.1002/ijc.27740. [DOI] [PubMed] [Google Scholar]
- 112.Wilson K.M., Giovannucci E.L., Mucci L.A. Lifestyle and Dietary Factors in the Prevention of Lethal Prostate Cancer. Asian J. Androl. 2012 doi: 10.1038/aja.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Erdrich S., Bishop K.S., Karunasinghe N., Han D.Y., Ferguson L.R. A Pilot Study to Investigate If New Zealand Men with Prostate Cancer Benefit from a Mediterranean-Style Diet. PeerJ. 2015;3:e1080. doi: 10.7717/peerj.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schneider L., Su L.J., Arab L., Bensen J.T., Farnan L., Fontham E.T.H., Song L., Hussey J., Merchant A.T., Mohler J.L., et al. Dietary Patterns Based on the Mediterranean Diet and DASH Diet Are Inversely Associated with High Aggressive Prostate Cancer in PCaP. Ann. Epidemiol. 2019;29:16–22.e1. doi: 10.1016/j.annepidem.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 115.Kenfield S.A., DuPre N., Richman E.L., Stampfer M.J., Chan J.M., Giovannucci E.L. Mediterranean Diet and Prostate Cancer Risk and Mortality in the Health Professionals Follow-up Study. Eur. Urol. 2015;65:887–894. doi: 10.1016/j.eururo.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richard A., Faeh D., Rohrmann S., Braun J., Tarnutzer S., Bopp M. Italianity Is Associated with Lower Risk of Prostate Cancer Mortality in Switzerland. Cancer Causes Control. 2014;25:1523–1529. doi: 10.1007/s10552-014-0456-5. [DOI] [PubMed] [Google Scholar]
- 117.Plummer M., Franceschi S., Vignat J., Forman D., de Martel C. Global Burden of Gastric Cancer Attributable to Helicobacter Pylori. Int. J. Cancer. 2015 doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 118.Schulpen M., Peeters P.H., van den Brandt P.A. Mediterranean Diet Adherence and Risk of Pancreatic Cancer: A Pooled Analysis of Two Dutch Cohorts. Int. J. Cancer. 2019;144:1550–1560. doi: 10.1002/ijc.31872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Martel C., Forman D., Plummer M. Gastric Cancer. Epidemiology and Risk Factors. Gastroenterol. Clin. N. Am. 2013 doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Karimi P., Islami F., Anandasabapathy S., Freedman N.D., Kamangar F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomark. Prev. 2014 doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McKeown P.P., Logan K., McKinley M.C., Young I.S., Woodside J.V. Session 4: CVD, Diabetes and Cancer Evidence for the Use of the Mediterranean Diet in Patients with CHD. Proc. Nutr. Soc. 2010;69:45–60. doi: 10.1017/S0029665109991856. [DOI] [PubMed] [Google Scholar]
- 122.Buckland G., Travier N., Huerta J.M., Bueno-De-Mesquita H.B., Siersema P.D., Skeie G., Weiderpass E., Engeset D., Ericson U., Ohlsson B., et al. Healthy Lifestyle Index and Risk of Gastric Adenocarcinoma in the EPIC Cohort Study. Int. J. Cancer. 2015;137:598–606. doi: 10.1002/ijc.29411. [DOI] [PubMed] [Google Scholar]
- 123.Piyathilake C. Dietary Factors Associated with Bladder Cancer. Investig. Clin. Urol. 2016 doi: 10.4111/icu.2016.57.S1.S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pelucchi C., Bosetti C., Negri E., Malvezzi M., La Vecchia C. Mechanisms of Disease: The Epidemiology of Bladder Cancer. Nat. Clin. Pract. Urol. 2006 doi: 10.1038/ncpuro0510. [DOI] [PubMed] [Google Scholar]
- 125.Brinkman M.T., Buntinx F., Kellen E., Van Dongen M.C.J.M., Dagnelie P.C., Muls E., Zeegers M.P. Consumption of Animal Products, Olive Oil and Dietary Fat and Results from the Belgian Case-Control Study on Bladder Cancer Risk. Eur. J. Cancer. 2011;47:436–442. doi: 10.1016/j.ejca.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 126.Witlox W.J.A., van Osch F.H.M., Brinkman M., Jochems S., Goossens M.E., Weiderpass E., White E., van den Brandt P.A., Giles G.G., Milne R.L., et al. An Inverse Association between the Mediterranean Diet and Bladder Cancer Risk: A Pooled Analysis of 13 Cohort Studies. Eur. J. Nutr. 2019:1–10. doi: 10.1007/s00394-019-01907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Serrano B., Brotons M., Bosch F.X., Bruni L. Epidemiology and Burden of HPV-Related Disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018 doi: 10.1016/j.bpobgyn.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 128.García-Closas R., Castellsagué X., Bosch X., González C.A. The Role of Diet and Nutrition in Cervical Carcinogenesis: A Review of Recent Evidence. Int. J. Cancer. 2005 doi: 10.1002/ijc.21193. [DOI] [PubMed] [Google Scholar]
- 129.Field C.J., Johnson I.R., Schley P.D. Nutrients and Their Role in Host Resistance to Infection. J. Leukoc. Biol. 2002;71:16–32. [PubMed] [Google Scholar]
- 130.Li Y., Xu C. In: Human Papillomavirus-Related Cancers BT—Infectious Agents Associated Cancers: Epidemiology and Molecular Biology. Cai Q., Yuan Z., Lan K., editors. Springer; Singapore: 2017. pp. 23–34. [Google Scholar]
- 131.Ferlay J., Steliarova-Foucher E., Lortet-Tieulent J., Rosso S., Coebergh J.W.W., Comber H., Forman D., Bray F. Reprint of: Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries in 2012. Eur. J. Cancer. 2015 doi: 10.1016/j.ejca.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 132.Liang J., Shang Y. Estrogen and Cancer. Annu. Rev. Physiol. 2012 doi: 10.1146/annurev-physiol-030212-183708. [DOI] [PubMed] [Google Scholar]
- 133.Kaaks R., Lukanova A., Kurzer M.S. Obesity, Endogenous Hormones, and Endometrial Cancer Risk: A Synthetic Review. Cancer Epidemiol. Biomark. Prev. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 134.Onstad M.A., Schmandt R.E., Lu K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016;34:4225–4230. doi: 10.1200/JCO.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gaskins A.J., Mumford S.L., Zhang C., Wactawski-Wende J., Hovey K.M., Whitcomb B.W., Howards P.P., Perkins N.J., Yeung E., Schisterman E.F. Effect of Daily Fiber Intake on Reproductive Function: The BioCycle Study. Am. J. Clin. Nutr. 2009 doi: 10.3945/ajcn.2009.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Filomeno M., Bosetti C., Bidoli E., Levi F., Serraino D., Montella M., La Vecchia C., Tavani A. Mediterranean Diet and Risk of Endometrial Cancer: A Pooled Analysis of Three Italian Case-Control Studies. Br. J. Cancer. 2015;112:1816–1821. doi: 10.1038/bjc.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jemal A., Bray F., Ferlay J. Global Cancer Statistics: 2011. CA Cancer J. Clin. 2008 doi: 10.3322/caac.20107.Available. [DOI] [PubMed] [Google Scholar]
- 138.Li W.Q., Park Y., Wu J.W., Goldstein A.M., Taylor P.R., Hollenbeck A.R., Freedman N.D., Abnet C.C. Index-Based Dietary Patterns and Risk of Head and Neck Cancer in a Large Prospective Study. Am. J. Clin. Nutr. 2014;99:559–566. doi: 10.3945/ajcn.113.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garavello W., Lucenteforte E., Bosetti C., Talamini R., Levi F., Tavani A., Franceschi S., Negri E., Vecchia C.L. Diet Diversity and the Risk of Laryngeal Cancer: A Case-Control Study from Italy and Switzerland. Oral Oncol. 2009 doi: 10.1016/j.oraloncology.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 140.Chang E.T., Adami H.O. The Enigmatic Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol. Biomark. Prev. 2006 doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 141.Misra S., Chaturvedi A., Misra N.C., Sharma I.D. Carcinoma of the Gallbladder. Lancet. 2003;4:167–176. doi: 10.1016/S1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 142.Larsson S.C., Håkansson N., Wolk A. Healthy Dietary Patterns and Incidence of Biliary Tract and Gallbladder Cancer in a Prospective Study of Women and Men. Eur. J. Cancer. 2017;70:42–47. doi: 10.1016/j.ejca.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 143.Ferlay J., Soerjomataram I.I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D.D., Bray F. Cancer Incidence and Mortality Worldwide: IARC Cancer Base. Lyon Int. Agency Res. Cancer. 2015 doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 144.McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G., McCain R.S. Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. World J. Gastroenterol. 2018 doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rosato V., Polesel J., Bosetti C., Serraino D., Negri E., La Vecchia C. Population Attributable Risk for Pancreatic Cancer in Northern Italy. Pancreas. 2015;44:216–220. doi: 10.1097/MPA.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 146.Maisonneuve P., Shivappa N., Hébert J.R., Bellomi M., Rampinelli C., Bertolotti R., Spaggiari L., Palli D., Veronesi G., Gnagnarella P. Dietary Inflammatory Index and Risk of Lung Cancer and Other Respiratory Conditions among Heavy Smokers in the COSMOS Screening Study. Eur. J. Nutr. 2016;55:1069–1079. doi: 10.1007/s00394-015-0920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mitrou P.N., Kipnis V., Thiébaut A.C.M., Reedy J., Subar A.F., Wirfält E., Flood A., Mouw T., Hollenbeck A.R., Leitzmann M.F., et al. Mediterranean Dietary Pattern and Prediction of All-Cause Mortality in a US Population: Results from the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2007;167:2461–2468. doi: 10.1001/archinte.167.22.2461. [DOI] [PubMed] [Google Scholar]
- 148.Anic G.M., Park Y., Subar A.F., Schap T.E., Reedy J. Index-Based Dietary Patterns and Risk of Lung Cancer in the NIH-AARP Diet and Health Study. Eur. J. Clin. Nutr. 2016;70:123–129. doi: 10.1038/ejcn.2015.122. [DOI] [PubMed] [Google Scholar]
- 149.Gnagnarella P., Maisonneuve P., Bellomi M., Rampinelli C., Bertolotti R., Spaggiari L., Palli D., Veronesi G. Red Meat, Mediterranean Diet and Lung Cancer Risk among Heavy Smokers in the Cosmos Screening Study. Ann. Oncol. 2013;24:2606–2611. doi: 10.1093/annonc/mdt302. [DOI] [PubMed] [Google Scholar]
- 150.International Agency for Research on Cancer . Agents Classified by the IARC Monographs. International Agency for Research on Cancer; Lyon, France: 2019. [(accessed on 30 June 2019)]. pp. 1–124. Available online: https://monographs.iarc.fr/agents-classified-by-the-iarc/ [Google Scholar]
- 151.Jeong S.H., Kang D., Lim M.W., Kang C.S., Sung H.J. Risk Assessment of Growth Hormones and Antimicrobial Residues in Meat. Toxicol. Res. 2013 doi: 10.5487/TR.2010.26.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang H., Chang Y., Zheng Q., Zhang R., Hu C., Jia W. Altered Intestinal Microbiota Associated with Colorectal Cancer. Front. Med. 2019 doi: 10.1007/s11684-019-0695-7. [DOI] [PubMed] [Google Scholar]