Abstract
Stone cell content is an important factor affecting pear fruit flavor. Lignin, a major component of pear stone cells, hinders the quality and value of commercial fruit. The completion of the Chinese white pear (Pyrus bretschneideri) genome sequence provides an opportunity to perform integrative analysis of the genes encoding the eleven protein families (i.e., PAL, C4H, 4CL, HCT, C3H, CSE, CCoAOMT, CCR, F5H, COMT, and CAD) in the phenylpropanoid pathway. Here, a systematic study based on expression patterns and phylogenetic analyses was performed to identify the members of each gene family potentially involved in the lignification in the Chinese white pear. The phylogenetic analysis suggested that 35 P. bretschneideri genes belong to bona fide lignification clade members. Compared to other plants, some multigene families are expanded by tandem gene duplication, such as HCT, C3H, COMT, and CCR. RNA sequencing was used to study the expression patterns of the genes in different tissues, including leaf, petal, bud, sepal, ovary, stem, and fruit. Eighteen genes presented a high expression in fruit, indicating that these genes may be involved in the biosynthesis of lignin in pear fruit. Similarly to what has been observed for Populus trichocarpa, a bimolecular fluorescence complementation (BiFC) experiment indicated that P. bretschneideri C3H and C4H might also interact with each other to regulate monolignol biosynthesis in P. bretschneideri, ultimately affecting the stone cell content in pear fruits. The identification of the major genes involved in lignin biosynthesis in pear fruits provides the basis for the development of strategies to improve fruit quality.
Keywords: lignin toolbox, expression, pear, phylogenetic analysis
1. Introduction
Lignin, an aromatic heteropolymer, is one of the major structural substances produced during the secondary thickening of plant cell walls [1,2,3,4]. The role of lignin is to provide impermeability and rigidity to plant cell walls and to protect plant tissues from pathogens. Lignin consists of different phenylpropanoids, mainly the monolignols p-coumaryl, coniferyl, and sinapyl alcohols, which differ in their degree of methoxylation [1,5]. When these monolignols are incorporated into lignin, they are referred as to p-hydroxyphenyl (H), syringyl (S) and guaiacyl (G) units, respectively [1,5]. Previous studies have shown that there are great differences in the lignin composition and content within and between species, among organs and/or tissues of the same plant, in response to environmental conditions experienced by plants, and in different developmental processes in plants [3,6,7].
Generally, the major bottleneck of the wood industry was reported to be the resistance of lignin to degradation [8,9,10]. The corrosive and expensive chemical treatments are necessary to overcome that resistance during pulp and paper manufacturing processes [9,10]. Recent studies have provided evidence that lignin is a crucial factor in determining fruit final mouthfeel, which is the most important trait of the fruit [11,12,13]. Therefore, the lignin biosynthetic pathway was always the hot point in the studies of fruits species [11,12,13]. Compared to previous studies, the lignin biosynthetic pathway has been identified to be more complex, which has been engineered several times in recent decades [1,14,15,16]. Meanwhile, several new pathways, such as CSE (caffeoyl shikimate esterase), have been discovered [17,18,19,20]. For example, together with 4CL, CSE can hydrolyze caffeoyl shikimate into caffeoyl-CoA, which bypasses the second reaction carried out by HCT. Remarkably, the previously published manuscripts showed that CSE may be important for lignification in only a few species [17,19,20]; it is not known whether this catalytic step is essential for some groups of plants—grasses and other monocots, for example. Altogether, there are eleven gene families involved in the synthesis of monolignols, namely the PAL (phenylalanine ammonia-lyase), C4H (cinnamate 4-hydroxylase), 4CL (4-coumarate:CoA ligase), HCT (shikimate O-hydroxycinnamoyl-transferase), C3H (p-coumarate 3-hydroxylase), CSE (caffeoyl shikimate esterase), CCoAOMT (caffeoyl CoA 3-O-methyltransferase), CCR (cinnamoyl CoA reductase), F5H (ferulate 5-hydroxylase), COMT (caffeate/5-hydroxyferulate O-methyl-transferase), and CAD (cinnamoyl CoA reductase) gene families. Though these genes encoding enzymes in lignin biosynthesis have been identified and characterized in some plant species, such as Arabidopsis thaliana, Populus trichocarpa, and Eucalyptus grandis [3,21], little is known about the lignin biosynthetic pathway in the economically important crop P. bretschneideri. The Chinese white pear (P. bretschneideri) is a native species of China, belonging to the subfamily Pomoideae of the family Rosaceae [22], and is widely cultivated in Asia. Previous studies have shown that the content of stone cells in pear fruit is one of the determinants of fruit quality [11,12,23]. Lignin is the main component of stone cells. Until recently, only a few lignin biosynthetic genes from the Chinese white pear have been cloned [11,12,23]. In recent years, researchers studying P. bretschneideri have focused on the identification and characterization of different families of transcription factor families and their corresponding functions [13,24,25], whereas a systematic analysis of monolignol biosynthesis has not been performed. In order to further analyze the monolignol biosynthesis pathway in P. bretschneideri, a comprehensive genome-wide analysis of lignin biosynthetic genes was carried out, as reported in the present study. By comparative phylogenetic analysis with other plants, eleven P. bretschneideri gene families and 35 genes belonging to bona fide clades were identified. Subsequent RNA sequencing data suggested that 18 genes located on the bona fide clades may participate in lignin biosynthesis of pear fruit.
2. Materials and Methods
2.1. In Silico Identification of P. bretschneideri Phenylpropanoid/Monolignol-Pathway Genes
We collected and analyzed the A. thaliana protein sequences for each of eleven gene families of the monolignol-pathway and downloaded the TAIR database (http://www.arabidopsis.org/). Subsequently, these A. thaliana protein function domains were examined using the PFAM (http://pfam.xfam.org/) [26] and InterProScan (http://www.ebi.ac.uk/interpro/) databases [27], as shown in Table 1. Then, we used the HMM model and BLASTp searches to predict the P. bretschneideri phenylpropanoid/monolignol-pathway genes by scanning the P. bretschneideri genome database with HMMER v3.2.1 software [28]. To further define the bona fide clades in P. bretschneideri (i.e., clades with homologs of genes that have been experimentally confirmed to have participated in lignification), we collected sequences of the investigated bona fide enzymes that contain true enzymatic biological/activity function according to previously published manuscripts [3,29].
Table 1.
Identification of lignin biosynthesis genes.
| Enzymes | Domains | E-Value |
|---|---|---|
| PAL * | PF00221/TIGR01226 | 1.00 × 10−30 |
| C4H | PTHR19383:SF33 | 1.00 × 10−30 |
| 4CL | PTHR11968:SF43 | 1.00 × 10−30 |
| HCT | PF02458 | 1.00 × 10−30 |
| C3H | PTHR19383:SF44 | 1.00 × 10−30 |
| CSE | PF03552 | 1.00 × 10−30 |
| CCOAMT | PTHR10509 | 1.00 × 10−30 |
| CCR ** | PTHR10366:SF9 | 1.00 × 10−24 |
| F5H | PTHR19383:SF46 | 1.00 × 10−30 |
| COMT *** | PIRSF005739 | 1.00 × 10−8 |
| CAD | PTHR11695:SF38 | 1.00 × 10−30 |
* According to previously published articles [30], the E value cutoff of 1.00 × 10−24 for CCR (cinnamoyl CoA reductase) gene identification was used. ** The identification of PAL (phenylalanine ammonia-lyase) genes involved two domains. *** According to previously published articles [30], we used E value cutoff of 1.00 × 10−8 for COMT gene identification.
2.2. Phylogenetic and Synteny Analyses
Similar to what have been reported by Carocha et al. (2015) [3], we obtained the sequences of bona fide enzymes from Medicago truncatula, Nicotiana tabacum, Petroselinum crispum, P. trichocarpa, V. vinifera, A. thaliana, E. grandis, and O. sativa verified to contain true biological function/enzymatic activity to determine the bona fide clades in P. bretschneideri (i.e., clades with homologs of genes that have been experimentally confirmed to be participated in lignification). MAFFT software was used to align the protein sequence, and alignments were improved manually [31]. The trees were built and computed in IQ-tree using the maximum likelihood method based on the best model as implemented in IQ-tree software [32]. Bootstrap-supported consensus trees were inferred from 1000 replicates, and the phylogenetic trees were reviewed using iTOL and Figtree software [33]. The GFF3 annotation, CDS, and protein file in P. bretschneideri were obtained from the GigaDB database. The Microsyn software and MCScanX pipeline were used to identify the synteny relationship of monolignol-pathway gene families in P. bretschneideri with E-value 10−5 [34,35].
2.3. Expression Analysis of P. bretschneideri Phenylpropanoid/Monolignol-Pathway Genes
RNA sequencing data [36] for eight tissues from P. bretschneideri individuals were obtained from the public NCBI database. The accession numbers and sample details for above the RNA sequencing data are presented in the availability of data and materials section. The FASTX-toolkit software was used to remove low-quality base-calls (Q < 20) of raw reads. The absolute transcript abundance values obtained for the phenylpropanoid/monolignol-pathway genes were computed from FPKM values obtained with Hisat2 and Stringtie software [37], as described by Carocha et al. (2015) [3].
2.4. BiFC Assays
The coding sequences of PbC3H1, PbC4H1, and PbC4H3 were amplified by PCR with specific primers (Table S1). To generate the BiFC (bimolecular fluorescence complementation) constructs, the coding region of PbC3H1—excluding its stop codon—was sub-cloned into pUC-SPYNE, and the coding regions of PbC4H1 and PbC4H3—excluding their stop codons—were sub-cloned into the pUC-SPYCE vector by the GenRec Assembly Master Mix Kit (General Biosystems, Chuzhou, China). An equal OD of PbC3H1-SPYNE together with PbC4H1-SPYCE and PbC4H3-SPYCE were injected into 5-week-old Nicotiana benthamiana leaves separately for Agrobacterium-mediated transient expression [38]. For microscopic observation, a laser confocal microscope (Zeiss LSM700, Germany) was used to capture the fluorescence signals of the reconstituted YFP of the lower epidermal cells of leaves cut four days after injection according to previously published manuscripts [24].
2.5. Availability of Data and Materials
Pear Petal_1, Accession: SRR8119898; Pear Petal_2, Accession: SRR8119899; Pear Petal_3, Accession: SRR8119905; Pear Sepal_1, Accession: SRR8119889; Pear Sepal_2, Accession: SRR8119902; Pear Sepal_3, Accession: SRR8119903; Pear Ovary_1, Accession: SRR8119904; Pear Ovary_2, Accession: SRR8119891; Pear Ovary_3, Accession: SRR8119895; Pear stem_1, Accession: SRR8119890; Pear stem_2, Accession: SRR8119892; Pear stem_3, Accession: SRR8119907; Pear bud_1, Accession: SRR8119893; Pear bud_2, Accession: SRR8119894; Pear bud_3, Accession: SRR8119906; Pear Fruit1, Accession: SRR8119900; Pear Fruit2, Accession: SRR8119901; Pear Leaves1, Accession: SRR8119896; Pear Leaves2, Accession: SRR8119897.
3. Results and Discussion
3.1. PAL
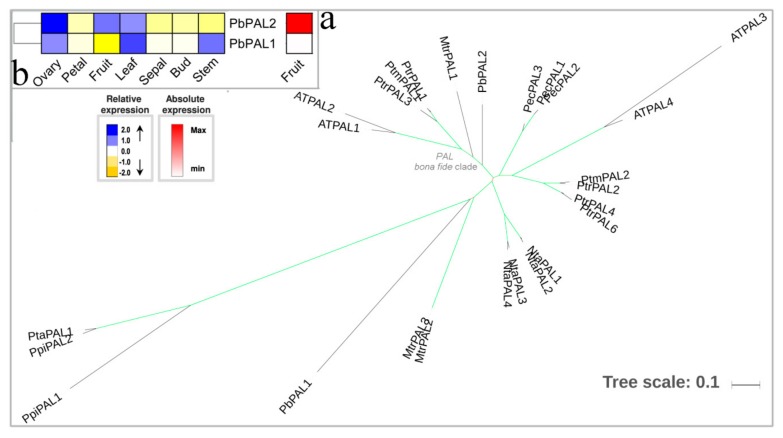
PAL (EC: 4.3.1.5) is the first enzyme in the phenylpropanoid/monolignol-pathway that catalyzes phenylalanine deamination to cinnamic acid. Cinnamic acid is a common intermediate for the formation of plant-specific phenylpropane derivatives, a process which has been well established in many plants, including A. thaliana and Populus trichocarpa [39,40,41]. The ML tree was constructed, and then the bona fide PAL enzymes were highlighted (Figure 1a, Table S2). In comparison to Medicago truncatula, Nicotiana tabacum, Petroselinum crispum, and A. thaliana, where PAL is encoded by three or four genes, the P. bretschneideri only contained two PAL family members. Among them, only one PbPAL2 protein were clustered in the PAL bona fide clade, and the remaining PbPAL1 was positioned outside, which exhibited a closer evolutionary relationship with gymnosperm PAL family members. The PbPAL1 gene presented strong expression in leaf and stem with weak expression in fruit, while the PbPAL2 gene showed high expression in ovary and fruit (Figure 1b). Additionally, the PbPAL2 gene was close to AtPAL1 and AtPAL2 in terms of its evolutionary relationship and has been reported to be mainly involved in the phenylpropanoid pathway [42,43]. By combining evolutionary relationships with expression patterns, PbPAL2 was the most likely gene involved in the P. bretschneideri phenylpropanoid/monolignol-pathway.
Figure 1.
Phylogenetic tree and expression profiles of the PAL bona fide clade. (a) ML tree built with PAL bona fide enzymes from several species. (b) The expression profiles P. bretschneideri PAL bona fide genes. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbPAL genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4. The tree scale is the number of amino acid substitution per site.
3.2. The Hydroxylation Steps
C3H (EC: 1.14.14.1), C4H (EC: 1.14.11.11), and F5H (EC: 1.14.13), which belong to the cytochrome P450 family, are mainly involved in catalyzing the hydroxylation steps of the monolignol pathway [44].
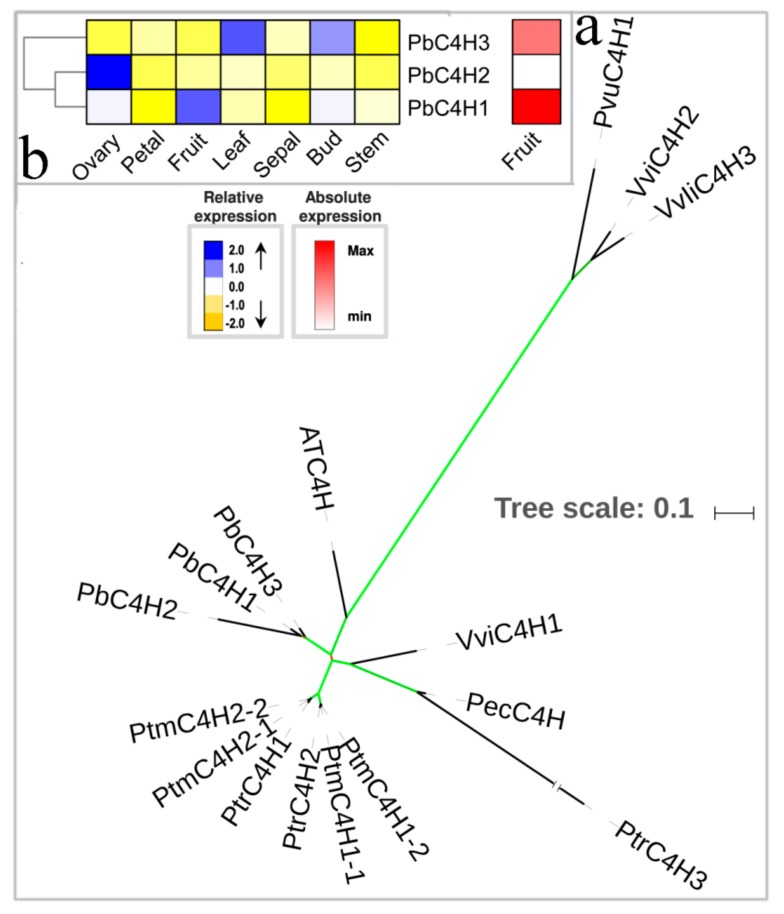
The hydroxylation of coumaric acid to p-coumaric acid is catalyzed by C4H, the second enzyme of the phenylpropane pathway [45]. Thus far, only a single C4H gene has been identified in A. thaliana. Though multiple family members are identified in other plant species, they generally do not exceed four members. For example, P. trichocarpa has three C4H genes, and O. sativa contains four members. The P. bretschneideri genome contains three C4H genes encoding PbC4H1, PbC4H2, and PbC4H3 (Figure 2a, Table S2). Previously published manuscripts have confirmed that members of Class I are mainly involved in lignin biosynthesis [2,46]. In the present study, three members (PbC4H1, PbC4H2, and PbC4H3) were identified as Class I. PbC4H2 was strongly expressed in the ovary, showing weak expression in other six tissues, including petal, leaf, sepal, bud, stem, and fruit. Both PbC4H1 and PbC4H3 showed high expression in fruit, but the PbC4H3 gene preferentially expressed in leaf and bud. Remarkably, we also noted that the expression of PbC4H1 was, in fruit, higher than that of PbC4H3 (three-fold higher) (Figure 2b). Taken together, our data indicate that PbC4H1 was the main C4H gene that contributed to the lignin biosynthesis of pear fruit; however, it is also very likely that the role of PbC4H3 was not very prominent.
Figure 2.
Phylogenetic tree and expression profiles of the C4H bona fide clade. (a) ML tree built with C4H bona fide enzymes from several species. (b) The expression profiles P. bretschneideri C4H bona fide genes. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbC4H genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4. The tree scale is the number of amino acid substitution per site.
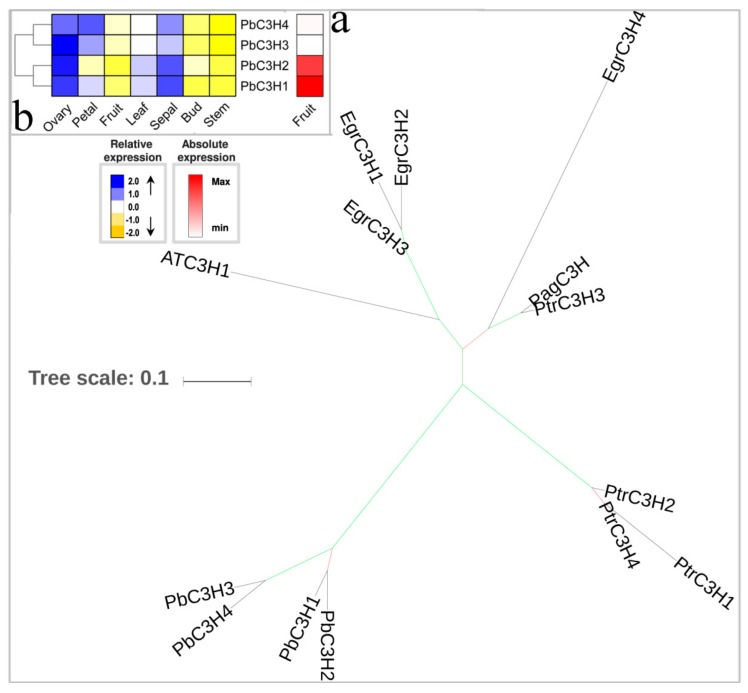
3-hydroxylation of 4-coumaroyl-shikimate is mainly is mainly catalyzed by C3H [47]. A. thaliana exhibited lignin depletion in meta-hydroxylated G and S units after knocking down C3H [48,49]. The C3H catalyzed reaction is irreversible, mainly towards G and S lignin [49]. Though A. thaliana and Oryza sativa have single copy C3H genes [21], the P. bretschneideri genome contained four members. PbC3H1/PbC3H2 and PbC3H3/PbC3H4 were located in chromosome 8 and chromosome 15 (Table S2), respectively, and have been shown to be expanded by lineage-specific tandem duplications, which is consistent with this gene family in P. trichocarpa and E. grandis. The expression patterns of these four C3H genes were very similar and were highly expressed in the ovary and sepal and weakly expressed in bud and stem, indicating functional redundancy occurred after tandem duplication. Additionally, we also noticed that PbC3H1 and PbC3H2 were highly expressed in fruit compared to other two genes (2.5-fold higher) (Figure 3b). Taken together, our data indicate that both PbC3H1 and PbC3H2 might contribute to lignin biosynthesis of pear fruit.
Figure 3.
Phylogenetic tree and expression profiles of the C3H bona fide clade. (a) ML tree built with C3H bona fide enzymes from several species. (b) The expression profiles P. bretschneideri C3H bona fide genes. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbC3H genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4. The tree scale is the number of amino acid substitution per site.
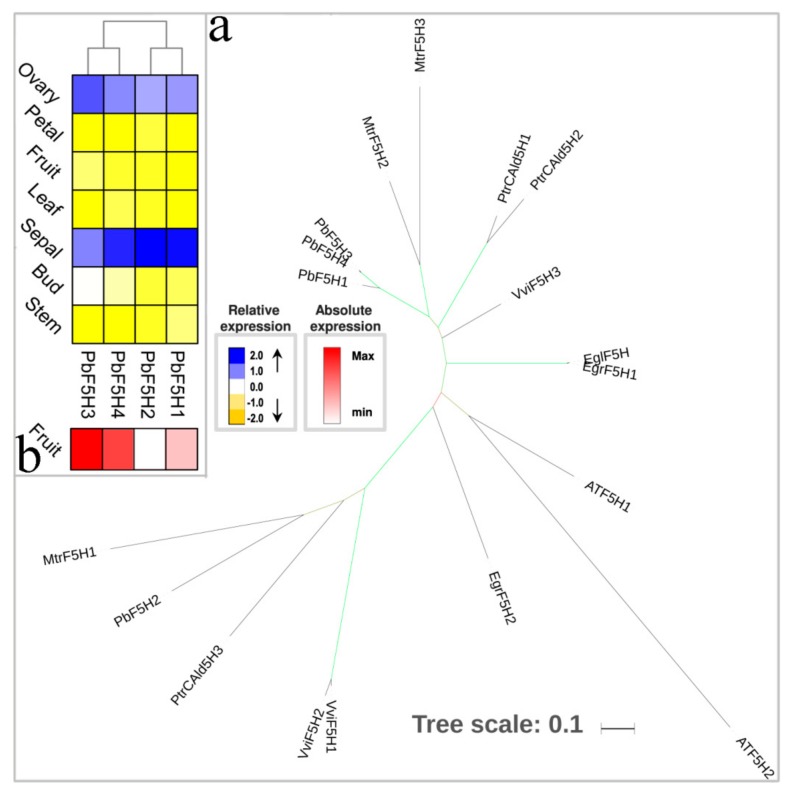
F5H contributes to the pathway leading to sinapyl alcohol and, finally, to S lignin [14,50,51]. In A. thaliana, two F5H paralogs (AtF5H1 and AtF5H2) were identified, and AtF5H1 had been proved to have contributed to lignification [52,53]. Additionally, the overexpression of F5H showed a substantially higher proportion of S units than normal: Up to c. 84% in Nicotiana tabacum, up to 93.5% in P. trichocarpa, and as high as 92% in AtF5H1-up-regulated A. thaliana [52,54,55]. The P. bretschneideri genome harbors four F5H genes encoding PbF5H1, PbF5H2, PbF5H3, and PbF5H4 (Figure 4a). PbF5H1 and PbF5H2 were located in chromosome 2 and chromosome 10, respectively. Both PbF5H3 and PbF5H4 were placed in same chromosome (Chr 15) (Table S2). Previous studies have shown that the G lignin is greater than S lignin in pear fruit [11,12], which may be related to the low expression of PbF5H genes in pear fruit. To further identify the PbF5H genes that might be involved in lignin synthesis in pear fruits, we performed the absolute expression level of PbF5H genes in pear fruits. Compared to PbF5H2 gene, PbF5H1, PbF5H3 and PbF5H4 were relatively highly expressed in fruit. These three genes are therefore probably involved in lignin biosynthesis of pear fruit.
Figure 4.
Phylogenetic tree and expression profiles of the F5H bona fide clade. (a) ML tree built with F5H bona fide enzymes from several species. (b) The expression profiles P. bretschneideri F5H bona fide genes. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbF5H genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4. The tree scale is the number of amino acid substitution per site.
3.3. 4CL
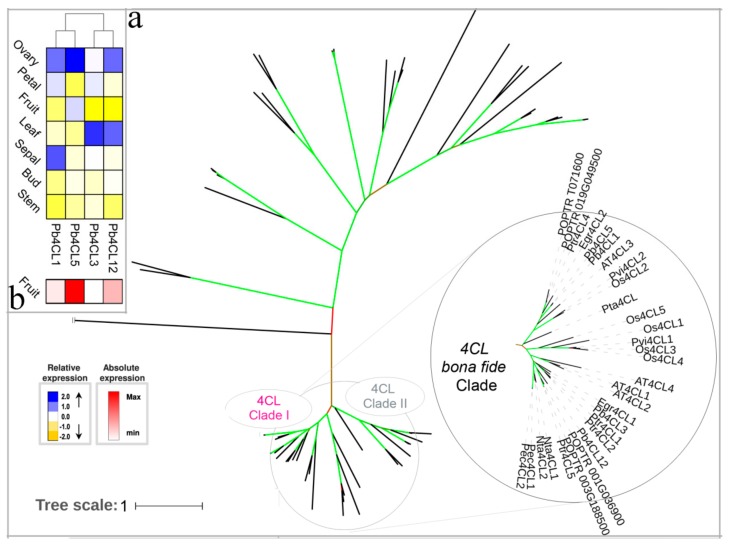
4CL is located at the center of the pathway and is a key step in the metabolism of phenylpropanoid [56]. Many 4CL members have been identified and biochemically characterized in A. thaliana and P. trichocarpa [56,57]. The 4CL gene family contain the ACS (acyl: CoA synthetase) superfamily, allowing for discrimination of the 4CL bona fide clade, clustered into two clades based on previously published manuscripts [21,58]. Unlike A. thaliana, E. grandis, and P. trichocarpa, P. bretschneideri has two 4CL genes (Pb4CL1 and Pb4CL5) in clade II related to soluble phenolic and flavonoid biosynthesis (Figure 5a). The P. bretschneideri genome had two members (Pb4CL3 and Pb4CL12) in clade I, which is consistent with the dicots with two-to-four members in this clade. The Pb4CL3 and Pb4CL12 genes were highly expressed in the leaf, followed by the ovary. Compared to the Pb4CL3 gene, Pb4CL12 was highly expressed in fruit (Figure 5b). By combining the roles of a clade I members in lignin biosynthesis and expression analysis, the strong expression of Pb4CL12 in fruits may involve the lignin biosynthesis of pear fruit.
Figure 5.
Phylogenetic tree and expression profiles of the 4CL bona fide clade. (a) ML tree built with 4CL bona fide enzymes from several species. (b) The expression profiles P. bretschneideri 4CL bona fide genes. Subclade containing the bona fide genes is highlighted. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of Pb4CL genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4, Figure S1. The tree scale is the number of amino acid substitution per site.
3.4. HCT
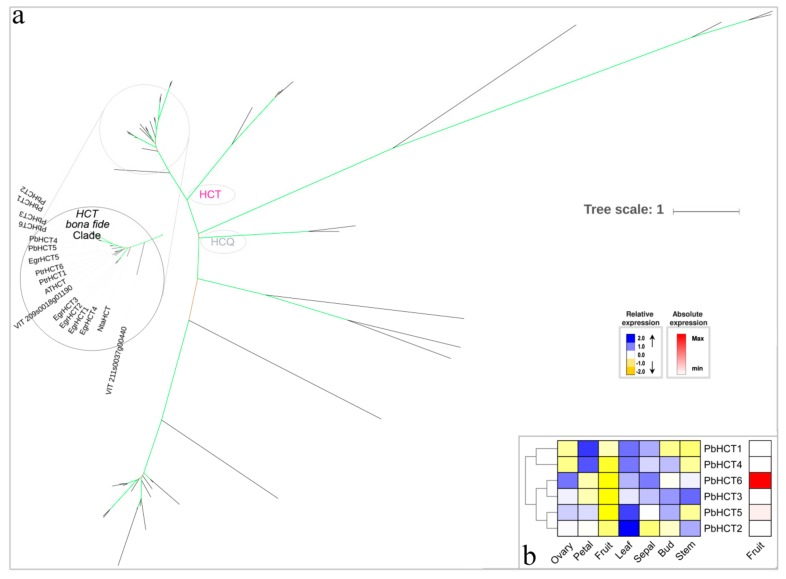
HCT can catalyze C3H to produce substrates for the 3-hydroxylation of hydroxy cinnamyl phenolic rings, with the reactions both immediately preceding and following the insertion into monolignol precursors [59,60]. With caffeyl-CoA and p-coumaroyl-CoA as the preferred substrates, HCT is capable of transferring an acyl group to the acceptor compound shikimic acid, which ultimately produces p-coumaroyl shikimate [59,60]. Subsequently, we found that six PbHCT genes were identified corresponding to the bona fide HCT (Figure 6a). These PbHCT genes exhibited a lineage-specific expansion as compared with the other dicots, such as A. thaliana and M. truncatula, which only contained one HCT gene each; P. trichocarpa had two genes. PbHCT1/PbHCT2 and PbHCT5/PbHCT6 were in a tandem arrangement of chromosome 4 and chromosome 17, respectively, suggesting that these gene pairs have undergone tandem gene duplication events (Table S2). Both PbHCT1/PbHCT2 and PbHCT5/PbHCT6 exhibited a very distinct profile; for instance, PbHCT1 was highly expressed in the petal, and PbHCT2 was preferentially expressed leaves, indicating that functional divergence occurred after tandem duplication events (Figure 6b) [22]. Additionally, PbHCT6 was highly expressed in fruit compared to other genes (40-fold higher), so this gene was probably involved in lignin biosynthesis of pear fruit.
Figure 6.
Phylogenetic tree and expression profiles of the HCT bona fide clade. (a) ML tree built with HCT bona fide enzymes from several species. (b) The expression profiles P. bretschneideri HCT bona fide genes. Subclade containing the bona fide genes is highlighted. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbHCT genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4, Figure S2. The tree scale is the number of amino acid substitution per site.
3.5. CSE
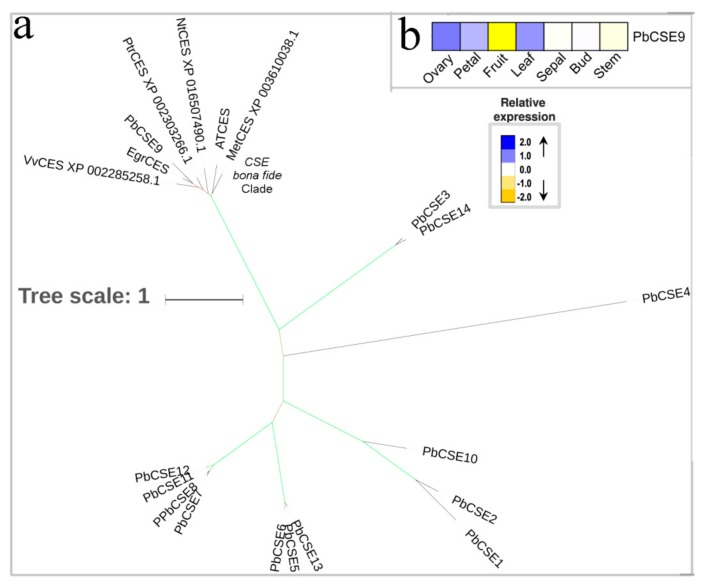
The CSE enzyme has been identified to be involved in the A. thaliana lignin biosynthetic pathway. Its main role is to catalyze the produced caffeic acid [17]. Compared with wild-type A. thaliana, the CSE mutant plants showed a reduced lignin content and an increased relative proportion of p-hydroxyphenyl in the lignin polymer [17]. CSE can form an alternative pathway to caffeyl-CoA with 4CL [17]. In the present study, we obtained its orthologous CSE genes from the NCBI database by using the ATCSE gene that has been previously characterized. Ha et al. (2016) have shown that MetCSE plays an essential role for lignification in M. truncatula [19]. Subsequently, we generated the CSE phylogenetic tree and found that the PbCSE9 gene belongs to the bona fide CSE enzymes (Figure 7a). The expression pattern of PbCSE9 suggested that it expressed in all seven tissues tested (Figure 7b) and preferentially and strongly expressed in the ovary and leaf but weakly expressed in the fruit. Remarkably, the PbCSE9 has a closer evolutionary relationship to the E. grandis, M. truncatula, A. thaliana, and the V. vinifera CSE genes (Figure 7a). Recent studies have shown that the CSE genes of E. grandis, M. truncatula and A. thaliana are involved in lignification [17,19,20]. PbCSE9 is therefore probably involved in lignin biosynthesis of pear fruit.
Figure 7.
Phylogenetic tree and expression profiles of the CSE bona fide clade. (a) ML tree built with CSE bona fide enzymes from several species. (b) The expression profiles P. bretschneideri CSE bona fide genes. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbCSE genes in different tissues are indicated in Table S3. The tree scale is the number of amino acid substitution per site.
3.6. The methylation Steps
S-adenosyl-L-Met-dependent O-methyltransferase (OMT) is widely present in plants and acts on Phe-derived substrates during the production of plant secondary compounds other than lignin. CCoAOMT and COMT are members of the OMT family, and they play an important role in the methylation step of the monolignol pathway [61].
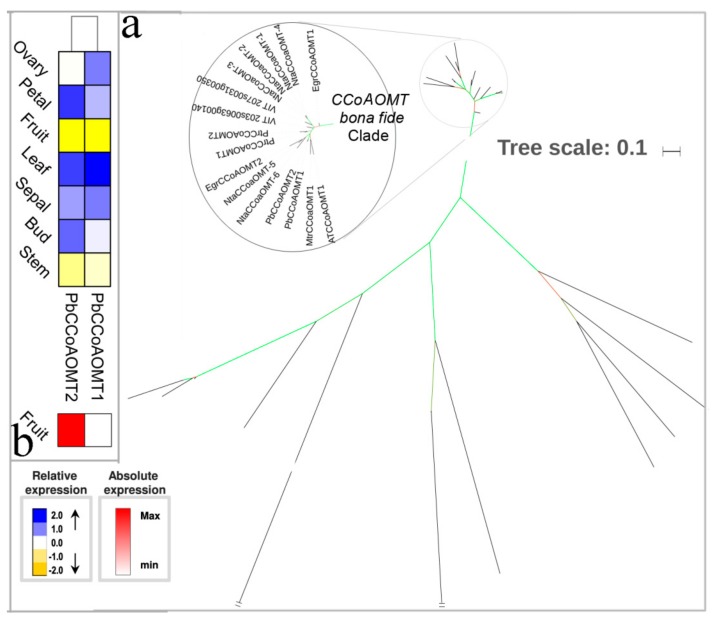
CCoAOMT may mainly act on the conversion of caffeyl-CoA to feruloyl-CoA in vitro, while in vivo it mainly catalyzes the SAM-dependent methylation of the phenolic hydroxyl group in positions three and five [14,62]. In many plants, functional studies of CCoAOMT have shown that they play an important role in the synthesis of G units. In the present study, we identified two PbCCoAOMT genes belong to the bona fide CCoAOMT (Figure 8a), the same number as in P. trichocarpa and E. grandis. PbCCoAOMT1 was placed on the chromosome 17, while PbCCoAOMT2 was placed on Scaffold (Table S2). The expression of PbCCoAOMT1 was strongly expressed in the petal, and PbCCoAOMT2 were preferentially and strongly expressed in the leaf. We also noted that PbCCoAOMT2 showed a six-fold higher expression relative to PbCCoAOMT1 in fruit (Figure 8b). Therefore, PbCCoAOMT2 is the most likely candidate involved in lignin biosynthesis of pear fruit.
Figure 8.
Phylogenetic tree and expression profiles of the CCoAOMT bona fide clade. (a) ML tree built with CCoAOMT bona fide enzymes from several species. (b) The expression profiles P. bretschneideri CCoAOMT bona fide genes. Subclade containing the bona fide genes is highlighted. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbCCoAOMT genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4, Figure S3. The tree scale is the number of amino acid substitution per site.
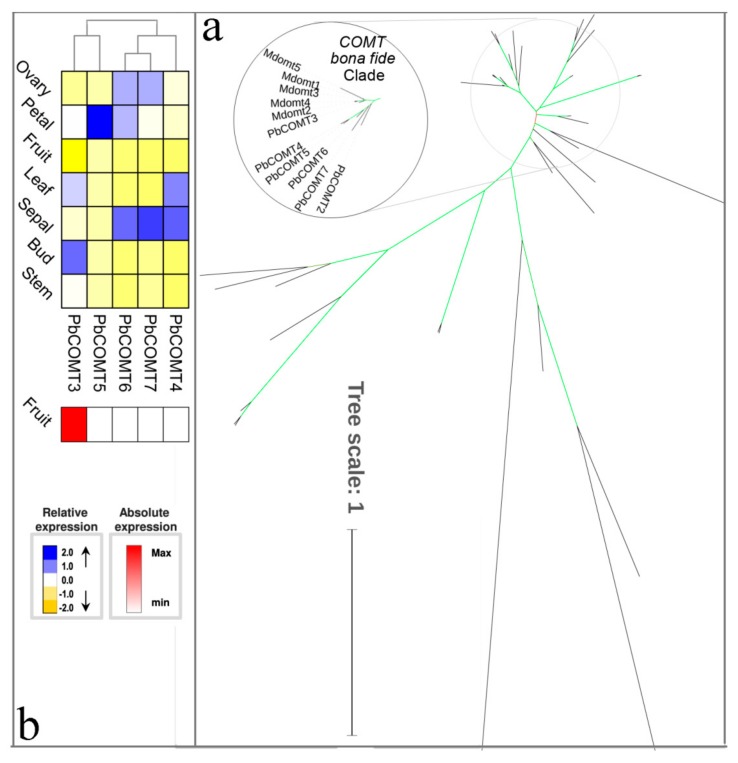
COMT was initially described in angiosperms as a bifunctional enzyme methylating 5-hydroxyferulic acid and caffeic acid [14,62]. Recently, many COMT genes have been isolated and functionally studied in some plants, such as P. trichocarpa and A. thaliana. The transgenic studies suggested that the COMT gene was primary involved in S lignin biosynthesis in vitro [63]. In the present study, we identified five PbCOMT genes belonging to bona fide COMT. Compared to other bona fide clades with fewer COMT members (Figure 9a), the P. bretschneideri COMT gene contains five members that were derived from a different duplication mechanism. PbCOMT4, PbCOMT5, PbCOMT6, and PbCOMT7 were all placed on a 20-Kb genomic region of chromosome 7 (Table S2), indicating that these genes were derived from tandem duplication events followed by functional divergence. PbCOMT4, PbCOMT6, and PbCOMT7 showed similar expression patterns that were very different from that of PbCOMT5, presenting higher expression in sepal and low expression in the stem (Figure 9b). Additionally, the expression patterns of these PbCOMT genes revealed that PbCOMT3 was expressed in the seven tissues examined, and the expression peaked in the bud and leaf. We also note that the amino acid residues described in this study related to the catalytic activity and chemical interactions of the COMT enzyme are completely conserved in PbCOMT3. Compared to the other COMT genes, PbCOMT3 was also highly expressed in fruit (50-fold higher), so this gene was the most likely candidate for lignin biosynthesis of pear fruit.
Figure 9.
Phylogenetic tree and expression profiles of the COMT bona fide clade. (a) ML tree built with COMT bona fide enzymes from several species. (b) The expression profiles P. bretschneideri COMT bona fide genes. Subclade containing the bona fide genes is highlighted. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbCOMT genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4, Figure S4. The tree scale is the number of amino acid substitution per site.
3.7. The Two Last Reductive Steps
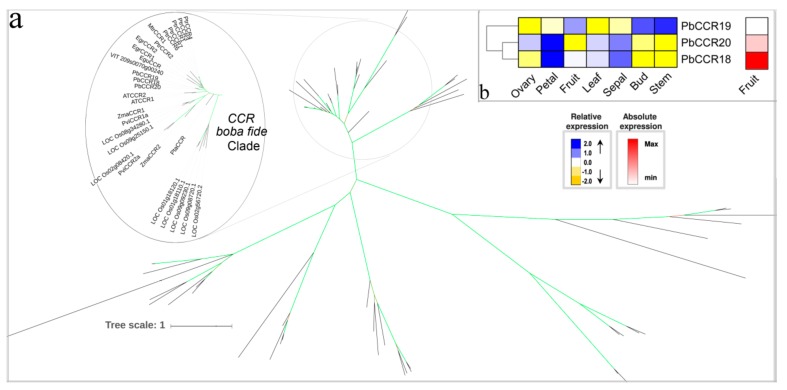
CCR catalyzes the reduction of cinnamoyl:CoA esters to their corresponding cinnamaldehydes, which is the first committed step of the monolignol biosynthesis. Purified enzymes from a number of species including A. thaliana, E. grandis, and P. trichocarpa were presented to be active towards sinapoyl-CoA, 5-hydroxyferuloyl-CoA, 4-coumaroyl-CoA, feruloyl-CoA, and caffeoyl-CoA [64,65]. Barakat et al. (2011) revealed and highlighted the bona fide CCR family by studying the CCR superfamily in land plants [66]. In the present study, we identified three PbCCR genes, and they all contained the CCR signature (NWYCY: Essential for its enzymatic activity) [67], including PbCCR18, PbCCR19, and PbCCR20 (Figure 10a). In all clades, AtCCR2 is mainly involved in defense mechanisms and respond biotic or abiotic stress, and its expression was poorly expressed during development; however, the AtCCR1 gene plays an important role in developmental lignin biosynthesis [65]. Indeed, PbCCR18, PbCCR19, and PbCCR20 were located on the same chromosome 17 derived from tandem duplication events followed by functional divergence. Both PbCCR18 and PbCCR20 showed similar expression patterns that that were very different from that of PbCCR19, presenting higher expression in the petal, followed by the sepal, and, finally, a low expression in the stem (Figure 10b). Additionally, PbCCR18 was identified to be preferentially and highly expressed (15-fold higher) in fruit than other three genes—this being in accordance with a role for lignin biosynthesis of pear fruit.
Figure 10.
Phylogenetic tree and expression profiles of the CCR bona fide clade. (a) ML tree built with CCR bona fide enzymes from several species. (b) The expression profiles P. bretschneideri CCR bona fide genes. Subclade containing the bona fide genes is highlighted. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbCCR genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4, Figure S5. The tree scale is the number of amino acid substitution per site.
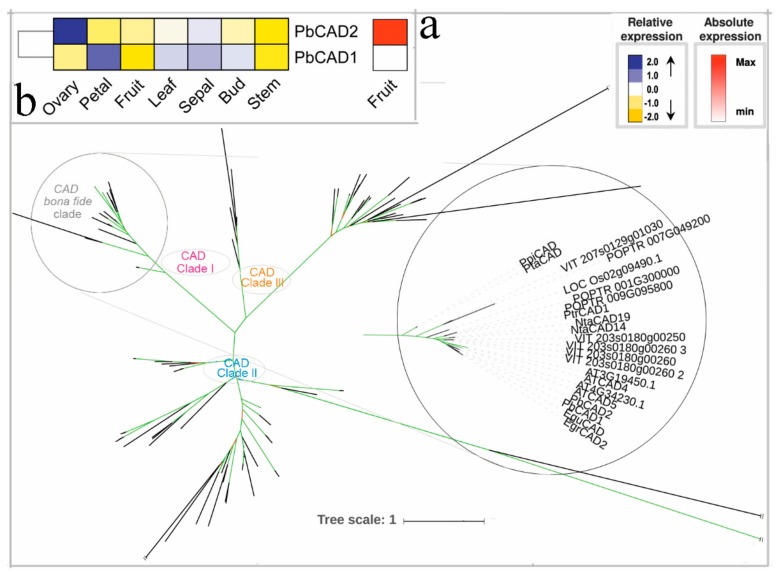
CAD is involved in the final step in the biosynthesis of monoligenol, which is capable of reducing cinnamaldehyde to alcohol. CAD and CAD-like genes have been identified in many plants, and they constitute large gene families such as A. thaliana, E. grandis, and P. trichocarpa [2,3,21]. Subsequent functional characterization confirms that different CAD gene family members have low homology and different affinities for various substrates and may have various physiological effects [68]. Previous studies have shown that the CAD gene family can be divided into three subfamilies during evolution, and there are certain differences between their evolutionary relationships and expression patterns [2,69,70,71]. As shown in Figure 11, clade I contains all bona fide CAD genes. Remarkably, in this clade I, V. vinifera, N. tabacum, E. grandis, and A. thaliana were represented by two CAD genes. P. trichocarpa (PtrCAD1) was the exception, with only one CAD gene involved in monolignol biosynthesis (Figure 11). In the present study, we found that PbCAD1 and PbCAD2 were placed on chromosomes 10 and chromosomes 14, respectively, a placement resulted from a segmental duplication events (Table S2). These two PbCAD genes show divergent expression patterns. PbCAD2 highly expressed in the ovary, and PbCAD1 preferentially expressed in the petal (Figure 11b). Remarkably, PbCAD2 showed eight-fold higher expression than PbCAD1 in fruit, indicating this gene was the most likely putative for lignin biosynthesis of pear fruit.
Figure 11.
Phylogenetic tree and expression profiles of the CAD bona fide clade. (a) ML tree built with CAD bona fide enzymes from several species. (b) The expression profiles P. bretschneideri CAD bona fide genes. Subclade containing the bona fide genes is highlighted. The green color of the tree branches suggests strong node support (bootstrap support ≥ 95% and SH-aLRT ≥ 75%). The FPKM values of PbCAD genes in different tissues are indicated in Table S3. The gene accession number is indicated in Table S4, Figure S6. The tree scale is the number of amino acid substitution per site.
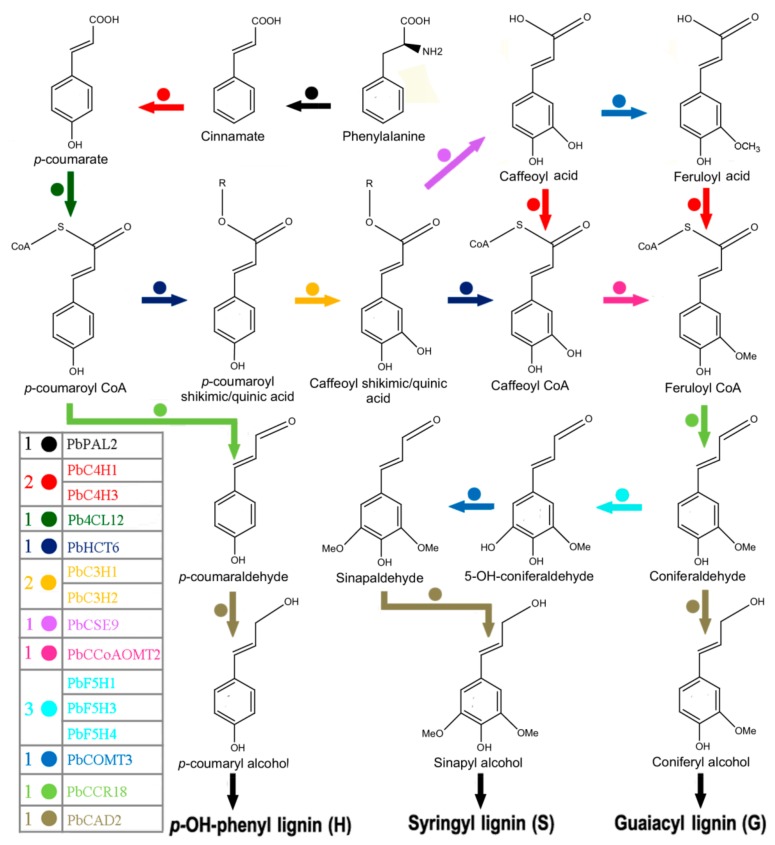
3.8. Hypothetical Pathways Involved in the Biosynthesis of Lignin in Pear Fruit
Through a close combination of evolutionary analysis and expression patterns, we identified thirty-five bona fide clade members of eleven gene families in the P. bretschneideri genome, and then highlighted 15 as the most likely major genes involved in lignin synthesis of pear fruit. The core fruit lignification toolbox comprises PbPAL2, PbC4H1, PbC4H3, PbF5H1, PbF5H3, PbF5H4, Pb4CL12, PbHCT6, PbCSE9, PbCCoAOMT2, PbCOMT3, PbCCR20, and PbCAD2 (Figure 12). To further obtain the expression profile of preferentially expressed and/or strongly expressed genes in pear fruit, we observed a unique expression pattern by using RNA sequencing data. In addition, we also noticed that some other genes are expressed in these eight tissues, such as Pb4CL1, Pb4CL5, PbCOMT6, PbCOMT7, and PbHCT4. Considering their expression patterns, we can speculate that these genes may be involved in the biosynthesis of other phenylpropanoid compounds other than the lignin metabolic pathway.
Figure 12.
Hypothetical pathways involved in the biosynthesis of lignin in pear fruit. The biosynthetic pathway was adapted from previous findings [3,14,17,72]. The 15 P. bretschneideri genes encoding enzymes located in the bona fide clades constitute the core group of lignin synthesis in pear fruit.
3.9. BiFC Assays
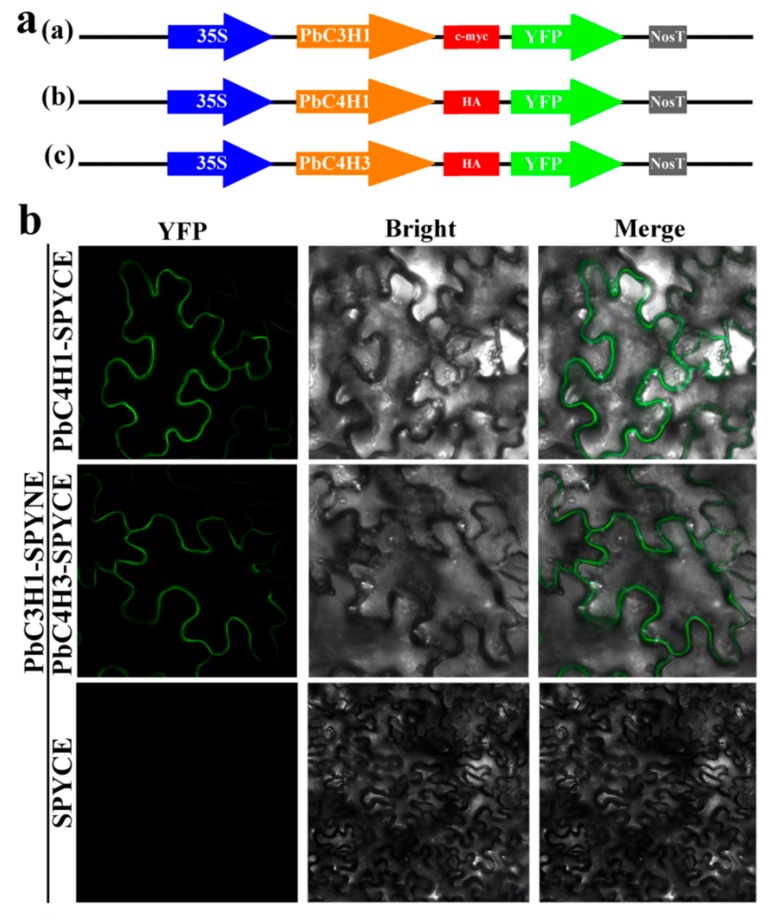
In the previous studies, Chen et al. (2011) found that P. trichocarpa C3H3 (PtrC3H3) interacted with PtrC4H1 and/or PtrC4H2, causing a significant increase in their catalytic activity and efficiency [73]. They may form heterodimers involved in monolignol biosynthesis [73]. To further understand such a molecular mechanisms in P. bretschneideri, we used the BiFC to explore interactions between the selected P. bretschneideri C3H and C4H genes. In the present study, PbC3H1 was fused to the N-terminal fragment of YFP, while PbC4H1 and PbC4H3 were fused to the C-terminal fragment of YFP. A complete fluorescent YFP complex will form and be detected when the two tested proteins bind to each other. In the present study, we observed YFP fluorescence on the membrane when PbC3H1-YFPN was injected with PbC4H1-YFPC or PbC4H3-YFPC, but not with YFPC (Figure 13). Our data suggested that P. bretschneideri C3H and C4H might also interact with each other to regulate monolignol biosynthesis in P. bretschneideri, ultimately affecting stone cell content in pear fruits.
Figure 13.
In vivo BiFC analysis of interaction between PbC3H and PbC4H co-expressed in N. benthamiana leaf cells. The coding regions of PbC3H and PbC4H were fused to the N-terminal and C-terminal halves of YFP, respectively. (a): Schematic representation of the 35S: PbC3H1-YFP, 35S: PbC4H1-YFP, and 35S: PbC4H3-YFP fusion constructs used for transient expression. (b): A laser confocal microscope (Zeiss LSM700, Germany) was used to capture the fluorescence signals of the reconstituted YFP of the lower epidermal cells of leaves cut four days injection.
4. Conclusions
Stone cells, mainly composed of lignin, are important factors affecting the quality of pear fruit. In the present study, a systematic analysis of the evolutionary relationships and expression patterns of the lignin biosynthesis gene families in P. bretschneideri genome was carried out. Subsequently, we highlighted the evolutionary histories of these lignin biosynthesis gene families and those members who may be involved in the lignin synthesis of pear fruit. Our research provides a solid foundation for future functional studies and molecular breeding to improve pear fruit quality.
Acknowledgments
We extend our thanks to the reviewers and editors for their careful reading and helpful comments on this manuscript. This study was supported by the Science Research Project of Chaohu University (No. XLY-201801).
Supplementary Materials
The following are available online at https://www.mdpi.com/2218-273X/9/9/504/s1, Figure S1: Unrooted protein phylogenetic tree of the 4CL multigene family. Subclade containing the bona fide genes is highlighted. Green and red represent high and low bootstrap values respectively. Figure S2: Unrooted protein phylogenetic tree of the HCT/HCQ multigene family. Subclade containing the bona fide genes is highlighted. Green and red represent high and low bootstrap values respectively. Figure S3: Unrooted protein phylogenetic tree of the CCoAOMT multigene family. Subclade containing the bona fide genes is highlighted. Green and red represent high and low bootstrap values respectively. Figure S4: Unrooted protein phylogenetic tree of the COMT gene family. Subclade containing the bona fide genes is highlighted. Green and red represent high and low bootstrap values respectively. Figure S5: Unrooted protein phylogenetic tree of the CCR multigene family. Subclade containing the bona fide genes is highlighted. Green and red represent high and low bootstrap values respectively. Figure S6: Unrooted protein phylogenetic tree of the CAD multigene family. Subclade containing the bona fide genes is highlighted. Green and red represent high and low bootstrap values respectively. Table S1: Primers used in this study; Table S2: Identification of lignin toolbox in pear fruit; Table S3: The expression profiles of P. bretschneideri lignin toolbox bona fide genes; Table S4: Full-length protein sequences used to assemble the phylogenetic trees.
Author Contributions
Y.C. and L.J. designed and performed the experiments; X.L., Y.C., and L.J. analyzed the data; Y.C. and L.J. contributed reagents/materials/analysis tools; Y.C. wrote the paper. All authors reviewed and approved the final submission.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Vanholme R., Demedts B., Morreel K., Ralph J., Boerjan W. Lignin biosynthesis and structure. Plant Physiol. 2010;153:895–905. doi: 10.1104/pp.110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raes J., Rohde A., Christensen J.H., De Peer Y.V., Boerjan W. Genome-wide characterization of the lignification toolbox in arabidopsis. Plant Physiol. 2003;133:1051–1071. doi: 10.1104/pp.103.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carocha V., Soler M., Hefer C., Cassan-Wang H., Fevereiro P., Myburg A.A., Paiva J.A.P., Grima-Pettenati J. Genome-wide analysis of the lignin toolbox of eucalyptus grandis. New Phytol. 2015;206:1297–1313. doi: 10.1111/nph.13313. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q., Luo L., Zheng L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018;19:335. doi: 10.3390/ijms19020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ralph J., Lapierre C., Marita J.M., Kim H., Lu F., Hatfield R.D., Ralph S.A., Chapple C., Franke R., Hemm M.R. Elucidation of new structures in lignins of cad- and comt-deficient plants by nmr. Phytochemistry. 2001;57:993–1003. doi: 10.1016/S0031-9422(01)00109-1. [DOI] [PubMed] [Google Scholar]
- 6.Muthamilarasan M., Khan Y., Jaishankar J., Shweta S., Lata C., Prasad M. Integrative analysis and expression profiling of secondary cell wall genes in c4 biofuel model setaria italica reveals targets for lignocellulose bioengineering. Front. Plant Sci. 2015;6:965. doi: 10.3389/fpls.2015.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhuiyan N.H., Selvaraj G., Wei Y., King J.L. Role of lignification in plant defense. Plant Signal. Behav. 2009;4:158–159. doi: 10.4161/psb.4.2.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plomion C., Leprovost G., Stokes A. Wood formation in trees. Plant Physiol. 2001;127:1513–1523. doi: 10.1104/pp.010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudet A., Kajita S., Grimapettenati J., Goffner D. Lignins and lignocellulosics: A better control of synthesis for new and improved uses. Trend. Plant Sci. 2003;8:576–581. doi: 10.1016/j.tplants.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Grimapettenati J., Goffner D. Lignin genetic engineering revisited. Plant Sci. 1999;145:51–65. doi: 10.1016/S0168-9452(99)00051-5. [DOI] [Google Scholar]
- 11.Jin Q., Yan C., Qiu J., Zhang N., Lin Y., Cai Y. Structural characterization and deposition of stone cell lignin in dangshan su pear. Sci. Hortic. 2013;155:123–130. doi: 10.1016/j.scienta.2013.03.020. [DOI] [Google Scholar]
- 12.Cai Y., Li G., Nie J., Lin Y., Nie F., Zhang J., Xu Y. Study of the structure and biosynthetic pathway of lignin in stone cells of pear. Sci. Hortic. 2010;125:374–379. doi: 10.1016/j.scienta.2010.04.029. [DOI] [Google Scholar]
- 13.Cao Y., Han Y., Li D., Lin Y., Cai Y. Myb transcription factors in chinese pear (pyrus bretschneideri rehd.): Genome-wide identification, classification, and expression profiling during fruit development. Front. Plant Sci. 2016;7:577. doi: 10.3389/fpls.2016.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys J.M., Chapple C. Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 2002;5:224–229. doi: 10.1016/S1369-5266(02)00257-1. [DOI] [PubMed] [Google Scholar]
- 15.Baucher M., Halpin C., Petit-Conil M., Boerjan W. Lignin: Genetic engineering and impact on pulping. Crit. Rev. Biochem. Mol. Bio. 2003;38:305–350. doi: 10.1080/10409230391036757. [DOI] [PubMed] [Google Scholar]
- 16.Ralph J., Lundquist K., Brunow G., Lu F., Kim H., Schatz P.F., Marita J.M., Hatfield R.D., Ralph S.A., Christensen J.H. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids. Phytochem. Rev. 2004;3:29–60. doi: 10.1023/B:PHYT.0000047809.65444.a4. [DOI] [Google Scholar]
- 17.Vanholme R., Cesarino I., Rataj K., Xiao Y., Sundin L., Goeminne G., Kim H., Cross J., Morreel K., Araujo P. Caffeoyl shikimate esterase (cse) is an enzyme in the lignin biosynthetic pathway in arabidopsis. Science. 2013;341:1103–1106. doi: 10.1126/science.1241602. [DOI] [PubMed] [Google Scholar]
- 18.Adams Z., Ehlting J., Edwards R. The regulatory role of shikimate in plant phenylalanine metabolism. J. Theor. Bio. 2019;462:158–170. doi: 10.1016/j.jtbi.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Ha C.M., Escamillatrevino L., Yarce J.C.S., Kim H., Ralph J., Chen F., Dixon R.A. An essential role of caffeoyl shikimate esterase in monolignol biosynthesis in medicago truncatula. Plant J. 2016;86:363–375. doi: 10.1111/tpj.13177. [DOI] [PubMed] [Google Scholar]
- 20.Saleme M.D.L.S., Cesarino I., Vargas L., Kim H., Vanholme R., Goeminne G., Van Acker R., Fonseca F., Pallidis A., Voorend W. Silencing caffeoyl shikimate esterase affects lignification and improves saccharification in poplar. Plant Physiol. 2017;175:1040–1057. doi: 10.1104/pp.17.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamberger B., Ellis M., Friedmann M., de Azevedo Souza C., Barbazuk B., Douglas C.J. Genome-wide analyses of phenylpropanoid-related genes in populus trichocarpa, arabidopsis thaliana, and oryza sativa: The populus lignin toolbox and conservation and diversification of angiosperm gene families this article is one of a selection of papers published in the special issue on poplar research in canada. Botany. 2007;85:1182–1201. [Google Scholar]
- 22.Wu J., Wang Z., Shi Z., Zhang S., Ming R., Zhu S., Khan M.A., Tao S., Korban S.S., Wang H. The genome of the pear (pyrus bretschneideri rehd.) Genome Res. 2013;23:396–408. doi: 10.1101/gr.144311.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y., Han Y., Meng D., Li D., Jin Q., Lin Y., Cai Y. Structural, evolutionary, and functional analysis of the class iii peroxidase gene family in chinese pear (pyrus bretschneideri) Front. Plant Sci. 2016;7:1874. doi: 10.3389/fpls.2016.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y., Han Y., Meng D., Li D., Jiao C., Jin Q., Lin Y., Cai Y. B-box genes: Genome-wide identification, evolution and their contribution to pollen growth in pear (pyrus bretschneideri rehd.) BMC Plant Biol. 2017;17:156. doi: 10.1186/s12870-017-1105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y.P., Han Y., Jin Q., Lin Y., Cai Y. Comparative genomic analysis of the grf genes in chinese pear (pyrus bretschneideri rehd), poplar (populous), grape (vitis vinifera), arabidopsis and rice (oryza sativa) Front. Plant Sci. 2016;7:1750. doi: 10.3389/fpls.2016.01750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Punta M., Coggill P.C., Eberhardt R.Y., Mistry J., Tate J., Boursnell C., Pang N., Forslund K., Ceric G., Clements J. The pfam protein families database. Nucleic Acids Res. 2011;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zdobnov E.M., Apweiler R. Interproscan—An integration platform for the signature-recognition methods in interpro. Bioinformatics. 2001;17:847–848. doi: 10.1093/bioinformatics/17.9.847. [DOI] [PubMed] [Google Scholar]
- 28.Mistry J., Finn R.D., Eddy S.R., Bateman A., Punta M. Challenges in homology search: Hmmer3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013;41:e121. doi: 10.1093/nar/gkt263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soler M., Camargo E.L.O., Carocha V., Cassan-Wang H., San Clemente H., Savelli B., Hefer C.A., Paiva J.A.P., Myburg A.A., Grima-Pettenati J. The eucalyptus grandis r2r3-myb transcription factor family: Evidence for woody growth-related evolution and function. New Phytol. 2015;206:1364–1377. doi: 10.1111/nph.13039. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z., Zhang D., Hu J., Zhou X., Ye X., Reichel K.L., Stewart N.R., Syrenne R.D., Yang X., Gao P., et al. Comparative genome analysis of lignin biosynthesis gene families across the plant kingdom. BMC Bioinformatics. 2009;10:S3. doi: 10.1186/1471-2105-10-S11-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katoh K., Standley D.M. Mafft multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Bio. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. Iq-tree: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Bio. Evol. 2014;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letunic I., Bork P. Interactive tree of life (itol) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Tang H., DeBarry J.D., Tan X., Li J., Wang X., Lee T.-h., Jin H., Marler B., Guo H. Mcscanx: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai B., Yang X., Tuskan G.A., Cheng Z.-M. Microsyn: A user friendly tool for detection of microsynteny in a gene family. BMC Bioinformatics. 2011;12:79. doi: 10.1186/1471-2105-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q., Qiao X., Yin H., Zhou Y., Dong H., Qi K., Li L., Zhang S. Unbiased subgenome evolution following a recent whole-genome duplication in pear (pyrus bretschneideri rehd.) Hortic. Res. 2019;6:34. doi: 10.1038/s41438-018-0110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pertea M., Kim D., Pertea G.M., Leek J.T., Salzberg S.L. Transcript-level expression analysis of rna-seq experiments with hisat, stringtie and ballgown. Nat. Protoc. 2016;11:1650. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 2010;40:428–438. doi: 10.1111/j.1365-313X.2004.02219.x. [DOI] [PubMed] [Google Scholar]
- 39.Liang X., Dron M., Cramer C.L., Dixon R.A., Lamb C.J. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J. Bio. Chem. 1989;264:14486–14492. [PubMed] [Google Scholar]
- 40.Subramaniam R., Reinold S., Douglas C.J. Structure, inheritance, and expression of hybrid poplar (populus trichocarpa × populus deltoides) phenylalanine ammonia-lyase genes. Plant Physiol. 1993;102:71–83. doi: 10.1104/pp.102.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kao Y., Harding S.A., Tsai C. Differential expression of two distinct phenylalanine ammonia-lyase genes in condensed tannin-accumulating and lignifying cells of quaking aspen. Plant Physiol. 2002;130:796–807. doi: 10.1104/pp.006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohde A., Morreel K., Ralph J., Goeminne G., Hostyn V., De Rycke R., Kushnir S., Van Doorsselaere J., Joseleau J., Vuylsteke M. Molecular phenotyping of the pal1 and pal2 mutants of arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell. 2004;16:2749–2771. doi: 10.1105/tpc.104.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J., Gu M., Lai Z., Fan B., Shi K., Zhou Y., Yu J., Chen Z. Functional analysis of the arabidopsis pal gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010;153:1526–1538. doi: 10.1104/pp.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehlting J., Hamberger B., Millionrousseau R., Werckreichhart D. Cytochromes p450 in phenolic metabolism. Phytochem. Rev. 2006;5:239–270. doi: 10.1007/s11101-006-9025-1. [DOI] [Google Scholar]
- 45.Ro D.K., Douglas C.J. Reconstitution of the entry point of plant phenylpropanoid metabolism in yeast (saccharomyces cerevisiae): Implications for control of metabolic flux into the phenylpropanoid pathway. J. Bio. Chem. 2004;279:2600–2607. doi: 10.1074/jbc.M309951200. [DOI] [PubMed] [Google Scholar]
- 46.Costa M.A., Collins R.E., Anterola A.M., Cochrane F.C., Davin L.B., Lewis N.G. An in silico assessment of gene function and organization of the phenylpropanoid pathway metabolic networks in arabidopsis thaliana and limitations thereof. Phytochemistry. 2003;64:1097–1112. doi: 10.1016/S0031-9422(03)00517-X. [DOI] [PubMed] [Google Scholar]
- 47.Schoch G.A., Goepfert S., Morant M., Hehn A., Meyer D., Ullmann P., Werckreichhart D. Cyp98a3 from arabidopsis thaliana is a 3′-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J. Bio. Chem. 2001;276:36566–36574. doi: 10.1074/jbc.M104047200. [DOI] [PubMed] [Google Scholar]
- 48.Franke R., Humphreys J.M., Hemm M.R., Denault J.W., Ruegger M.O., Cusumano J.C., Chapple C. The arabidopsis ref8 gene encodes the 3-hydroxylase of phenylpropanoid metabolism. Plant J. 2002;30:33–45. doi: 10.1046/j.1365-313X.2002.01266.x. [DOI] [PubMed] [Google Scholar]
- 49.Abdulrazzak N., Pollet B., Ehlting J., Larsen K., Asnaghi C., Ronseau S., Proux C., Erhardt M., Seltzer V., Renou J. A coumaroyl-ester-3-hydroxylase insertion mutant reveals the existence of nonredundant meta -hydroxylation pathways and essential roles for phenolic precursors in cell expansion and plant growth. Plant Physiol. 2005;140:30–48. doi: 10.1104/pp.105.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chapple C.C.S., Vogt T., Ellis B.E., Somerville C. An arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell. 1992;4:1413–1424. doi: 10.1105/tpc.4.11.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osakabe K., Tsao C.C., Li L., Popko J.L., Umezawa T., Carraway D.T., Smeltzer R.H., Joshi C.P., Chiang V.L. Coniferyl aldehyde 5-hydroxylation and methylation direct syringyl lignin biosynthesis in angiosperms. Proc. Natl. Acad. Sci. USA. 1999;96:8955–8960. doi: 10.1073/pnas.96.16.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer K., Shirley A.M., Cusumano J.C., Belllelong D.A., Chapple C. Lignin monomer composition is determined by the expression of a cytochrome p450-dependent monooxygenase in arabidopsis. Proc. Natl. Acad. Sci. USA. 1998;95:6619–6623. doi: 10.1073/pnas.95.12.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marita J.M., Ralph J., Hatfield R.D., Chapple C. Nmr characterization of lignins in arabidopsis altered in the activity of ferulate 5-hydroxylase. Proc. Natl. Acad. Sci. USA. 1999;96:12328–12332. doi: 10.1073/pnas.96.22.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franke R., Mcmichael C.M., Meyer K., Shirley A.M., Cusumano J.C., Chapple C. Modified lignin in tobacco and poplar plants over-expressing the arabidopsis gene encoding ferulate 5-hydroxylase. Plant J. 2000;22:223–234. doi: 10.1046/j.1365-313x.2000.00727.x. [DOI] [PubMed] [Google Scholar]
- 55.Stewart J.J., Akiyama T., Chapple C., Ralph J., Mansfield S.D. The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar1. Plant Physiol. 2009;150:621–635. doi: 10.1104/pp.109.137059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehlting J., Shin J.J.K., Douglas C.J. Identification of 4-coumarate:Coenzyme a ligase (4cl) substrate recognition domains. Plant J. 2001;27:455–465. doi: 10.1046/j.1365-313X.2001.01122.x. [DOI] [PubMed] [Google Scholar]
- 57.Ehlting J., Buttner D., Wang Q., Douglas C.J., Somssich I.E., Kombrink E. Three 4-coumarate:Coenzyme a ligases in arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. Plant J. 1999;19:9–20. doi: 10.1046/j.1365-313X.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 58.De Azevedo Souza C., Barbazuk B., Ralph S.G., Bohlmann J., Hamberger B., Douglas C.J. Genome-wide analysis of a land plant-specific acyl: Coenzymea synthetase (acs) gene family in arabidopsis, poplar, rice and physcomitrella. New Phytol. 2008;179:987–1003. doi: 10.1111/j.1469-8137.2008.02534.x. [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann L., Maury S., Martz F., Geoffroy P., Legrand M. Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J. Bio. Chem. 2003;278:95–103. doi: 10.1074/jbc.M209362200. [DOI] [PubMed] [Google Scholar]
- 60.Hoffmann L., Besseau S., Geoffroy P., Ritzenthaler C., Meyer D., Lapierre C., Pollet B., Legrand M. Silencing of hydroxycinnamoyl-coenzyme a shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant Cell. 2004;16:1446–1465. doi: 10.1105/tpc.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye Z.H., Varner J.E. Differential expression of two o-methyltransferases in lignin biosynthesis in zinnia elegans. Plant Physiol. 1995;108:459–467. doi: 10.1104/pp.108.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boerjan W., Ralph J., Baucher M. Lignin biosynthesis. Ann. Rev. Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 63.Jouanin L., Goujon T., de Nadaï V., Martin M.-T., Mila I., Vallet C., Pollet B., Yoshinaga A., Chabbert B., Petit-Conil M., et al. Lignification in transgenic poplars with extremely reduced caffeic acid O-methyltransferase activity. Plant Physiol. 2000;123:1363–1374. doi: 10.1104/pp.123.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarni F., Grand C., Boudet A. Purification and properties of cinnamoyl-coa reductase and cinnamyl alcohol dehydrogenase from poplar stems (populus x euramericana) FEBS J. 1984;139:259–265. doi: 10.1111/j.1432-1033.1984.tb08002.x. [DOI] [PubMed] [Google Scholar]
- 65.Lauvergeat V., Lacomme C., Lacombe E., Lasserre E., Roby D., Grimapettenati J. Two cinnamoyl-coa reductase (ccr) genes from arabidopsis thaliana are differentially expressed during development and in response to infection with pathogenic bacteria. Phytochemistry. 2001;57:1187–1195. doi: 10.1016/S0031-9422(01)00053-X. [DOI] [PubMed] [Google Scholar]
- 66.Barakat A., Yassin N.B.M., Park J.S., Choi A., Herr J., Carlson J.E. Comparative and phylogenomic analyses of cinnamoyl-coa reductase and cinnamoyl-coa-reductase-like gene family in land plants. Plant Sci. 2011;181:249–257. doi: 10.1016/j.plantsci.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 67.Lacombe E., Hawkins S., Van Doorsselaere J., Piquemal J., Goffner D., Poeydomenge O., Boudet A., Grimapettenati J. Cinnamoyl coa reductase, the first committed enzyme of the lignin branch biosynthetic pathway: Cloning, expression and phylogenetic relationships. Plant J. 1997;11:429–441. doi: 10.1046/j.1365-313X.1997.11030429.x. [DOI] [PubMed] [Google Scholar]
- 68.Sibout R., Eudes A., Mouille G., Pollet B., Lapierre C., Jouanin L., Seguin A. Cinnamyl alcohol dehydrogenase-c and -d are the primary genes involved in lignin biosynthesis in the floral stem of arabidopsis. Plant Cell. 2005;17:2059–2076. doi: 10.1105/tpc.105.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo D., Ran J., Wang X. Evolution of the cinnamyl/sinapyl alcohol dehydrogenase (cad/sad) gene family: The emergence of real lignin is associated with the origin of bona fide cad. J. Mol. Evol. 2010;71:202–218. doi: 10.1007/s00239-010-9378-3. [DOI] [PubMed] [Google Scholar]
- 70.Barakat A., Bagniewskazadworna A., Choi A., Plakkat U., Diloreto D.S., Yellanki P., Carlson J.E. The cinnamyl alcohol dehydrogenase gene family in populus: Phylogeny, organization, and expression. BMC Plant Bio. 2009;9:26. doi: 10.1186/1471-2229-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sibout R., Eudes A., Pollet B., Goujon T., Mila I., Granier F., Seguin A., Lapierre C., Jouanin L. Expression pattern of two paralogs encoding cinnamyl alcohol dehydrogenases in arabidopsis. Isolation and characterization of the corresponding mutants. Plant Physiol. 2003;132:848–860. doi: 10.1104/pp.103.021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barros J., Serk H., Granlund I., Pesquet E. The cell biology of lignification in higher plants. Ann. Bot. 2015;115:1053–1074. doi: 10.1093/aob/mcv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen H., Li Q., Shuford C.M., Liu J., Muddiman D.C., Sederoff R.R., Chiang V.L. Membrane protein complexes catalyze both 4- and 3-hydroxylation of cinnamic acid derivatives in monolignol biosynthesis. Proc. Natl. Acad. Sci. USA. 2011;108:21253–21258. doi: 10.1073/pnas.1116416109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Pear Petal_1, Accession: SRR8119898; Pear Petal_2, Accession: SRR8119899; Pear Petal_3, Accession: SRR8119905; Pear Sepal_1, Accession: SRR8119889; Pear Sepal_2, Accession: SRR8119902; Pear Sepal_3, Accession: SRR8119903; Pear Ovary_1, Accession: SRR8119904; Pear Ovary_2, Accession: SRR8119891; Pear Ovary_3, Accession: SRR8119895; Pear stem_1, Accession: SRR8119890; Pear stem_2, Accession: SRR8119892; Pear stem_3, Accession: SRR8119907; Pear bud_1, Accession: SRR8119893; Pear bud_2, Accession: SRR8119894; Pear bud_3, Accession: SRR8119906; Pear Fruit1, Accession: SRR8119900; Pear Fruit2, Accession: SRR8119901; Pear Leaves1, Accession: SRR8119896; Pear Leaves2, Accession: SRR8119897.