Abstract
Background
Treatment of hepatitis C virus (HCV) with direct-acting antiviral (DAA) regimens has resulted in high rates of sustained virologic response (SVR). Treatment of vulnerable populations may be improved by incorporating an on-site intensive specialty pharmacy (ON-ISP).
Aims
To describe outcomes of HCV treatment at a safety-net hospital and proportion of subjects achieving SVR for those using the ON-ISP compared to an off-site pharmacy (OFF-SP).
Methods
A retrospective cohort study of 219 subjects treated for HCV with DAA at Boston Medical Center was conducted. Subject characteristics, virologic response, and pharmacy services used were recorded. We used multivariable logistic regression to test the association between ON-ISP and SVR after adjusting for covariates.
Results
SVR occurred in 71% of subjects by intention-to-treat (73% among ON-ISP users vs 57% among OFF-SP users) and 95% completing treatment per-protocol (96% among ON-ISP users vs 87% among OFF-SP users). Adjustment for age, sex, ethnicity, insurance, fibrosis, prior treatment, and MELD revealed an increased likelihood of SVR among users of ON-ISP: OR 6.0 (95% CI 1.18–31.0). No significant difference in treatment delay or adverse events was seen among users of either pharmacy type.
Conclusions
HCV treatment with DAA was well tolerated, but the rate of SVR was low (71%) compared to trials. This was due to loss to follow-up, as the per-protocol rate of SVR was much higher (95%). Use of ON-ISP was associated with an increase in SVR and may be valuable for improving care for vulnerable populations.
Keywords: Hepatitis C virus, Specialty pharmacy, Sustained virologic response, Safety-net hospital, Direct-acting antiviral, Clinical pharmacy
Introduction
Over the last several years, hepatitis C virus (HCV) treatment has shifted to better-tolerated interferon-free, direct-acting antiviral (DAA) regimens. Such regimens have demonstrated greater than 95% sustained virologic response (SVR) rates in clinical trials [1]. The success of DAA therapy has led to the widespread use in various patient populations. Recent studies demonstrate the effectiveness of DAA regimens against multiple HCV genotypes with most achieving SVR at a rate similar to that observed in prior clinical trials [2–12]. Whereas extensive data exist regarding the real-world effectiveness of DAA treatments [13–17], less is known about the effectiveness among more vulnerable populations including minority groups and patients of lower socioeconomic status [18].
Prior studies examining interferon-based treatments for chronic HCV demonstrated a decreased rate of SVR in black and Hispanic patients compared to non-Hispanic whites, partially due to issues with non-adherence and early discontinuation of treatment [12, 19–21]. DAA regimens offer simpler and shorter treatment schedules with minimal side effects which should improve treatment adherence and decrease early discontinuation [22]. However, numerous barriers, including low socioeconomic status, low health literacy and education, and substance use disorders continue to impact adherence and achievement of optimal treatment outcomes in vulnerable populations.
The use of multidisciplinary healthcare teams in the treatment of HCV allows for close clinical monitoring and ongoing evaluation to ensure compliance [23]. These healthcare teams include specialty pharmacies that provide targeted programs and services, including counseling for appropriate medication administration, education in managing adverse side effects, and coordination of insurance benefits. These services minimize delays in initiation of treatment and may improve HCV treatment outcomes [23]. In prior studies, use of specialty pharmacies improved adherence to both two and three drug regimens; however, little is known about the impact of specialty pharmacy use on SVR rate with DAA regimens [23, 24].
Thus, we aimed to examine the association between use of an on-site intensive specialty pharmacy program (ON-ISP) and SVR rate for a real-world cohort of HCV-infected patients at an urban safety-net hospital with a diverse patient population including many patients of minority background and lower socioeconomic status. The primary outcome of interest was the rate of SVR in patients enrolling in ON-ISP versus off-site specialty pharmacy care (OFF-ISP). We hypothesized that subjects who utilized the ON-ISP would have a higher rate of SVR compared to those who participated in the OFF-ISP after adjusting for potential confounding variables. Secondary outcomes of interest included comparisons across the two groups of the interval between prescription and initiation of treatment (i.e., “time to treatment”), the rate of adverse events, the need for dose reductions or early termination of treatment, and treatment adherence.
Methods
Study Sample
This was an observational, intent-to-treat cohort analysis of HCV-infected patients receiving treatment with DAA at Boston Medical Center, Boston, MA. Data for this study were obtained from our prescription database for HCV and included all subjects given prescriptions for HCV treatment. Additional data were abstracted from the medical record, including subject demographics, laboratory values, pharmacy information, and clinical history by two co-investigators (AT, RS).
Eligible subjects included all adults 18 years of age or older infected with HCV and treated with DAA regimens with or without ribavirin and with a recommended duration from 8 to 24 weeks between August 2014 and September 2015. Liver transplant recipients or those enrolled in clinical trials for HCV therapy were excluded. DAA were prescribed by four board-certified gastroenterologists and two non-physician providers. The regimen prescribed, timing of follow-up visits, and laboratory testing were at the discretion of the treating provider(s) as all treatments were administered in routine practice. This study protocol was reviewed and approved by the Boston Medical Center Institutional Review Board.
Pharmacy Program
All subjects received medications from a specialty pharmacy supplier. The pharmacy programs were defined as ON-ISP or OFF-SP. All subjects received care from an ambulatory care clinical pharmacy specialist (TZ) and certified pharmacy technician or liaison (ML). This care included assistance with insurance approval paperwork from the liaison, review of all anti-HCV orders for appropriateness, discussion with outside providers for adjustment of medications with drug–drug interactions, medication reconciliation, and HCV treatment education from the pharmacist.
Prior to beginning treatment, all subjects were explained the HCV visit schedule and treatment protocol by the prescribing provider. At a minimum, all subjects were seen for a pre-treatment visit, an end-of-treatment visit, and a 12-week post-treatment visit by the prescribing provider. Regular telephone and clinic follow-up visits were also scheduled with the pharmacist approximately once monthly. These visits included routine laboratory studies and assessment of any potential adverse medication reactions and adherence.
When permitted by the insurance provider, subjects were offered use of the ON-ISP. Nearly, all subjects (184/185) elected to use the ON-ISP when offered. The additional ON-ISP services included prescription refill monitoring, direct communication with other medical providers in the clinic to discuss any potential medication issues, and the choice to pick-up medications in the clinic or have them delivered to a subject’s home directly. The remainder of the subjects were required to utilize an OFF-SP by their insurance carriers, which did not provide these added services.
Covariates and Baseline Measurements
Subject demographics and other historical information were identified at the time of treatment initiation (Table 1), including age, sex, race, liver fibrosis stage (F0–F4), MELD score, and history of prior treatment for HCV infection with interferon, ribavirin, and DAA therapy. The presence of cirrhosis was determined by histology, imaging, or elastography within 4 weeks of starting treatment. A total of 18.7% (41/219) of subjects had a liver biopsy. Decompensated liver disease was defined as any history of variceal hemorrhage, ascites, or hepatic encephalopathy. Laboratory values recorded (alanine aminotransferase, aspartate aminotransferase, creatinine, albumin, platelets, and HCV RNA) were the most recent values within three months preceding the start of treatment. Stage of hepatic fibrosis was defined using vibration-controlled transient elastography (Fibroscan©, Echosens, Paris, France) according to published fibrosis stage liver stiffness thresholds [25] or via liver biopsy.
Table 1.
Cohort demographics, by specialty pharmacy type
| Characteristic | On-site intensive specialty pharmacy (n = 184) | Off-site specialty pharmacy (n = 35) |
|---|---|---|
| Age, y-median (IQR) | 59 (53–62) | 57 (52.5–61) |
| Women, n (%) | 57 (31.0) | 8 (22.9) |
| Ethnicity, n (%) | ||
| Black | 79 (42.9) | 15 (42.9) |
| White | 56 (30.4) | 14 (40.0) |
| Hispanic | 34 (18.5) | 1 (2.9) |
| Asian | 7 (3.8) | 2 (5.7) |
| Other/unknown | 8 (4.3) | 3 (8.6) |
| Insurance, n (%) | ||
| Private | 50 (27.2) | 21 (60.0) |
| Medicaid | 63 (34.2) | 9 (25.7) |
| Medicare | 27 (14.7) | 3 (8.6) |
| Medicare and medicaid | 33 (17.9) | 2 (5.7) |
| Free care | 15 (8.2) | 0 (0.0) |
| Genotype, n (%) | ||
| 1 | 168 (91.3) | 33 (94.3) |
| 2 | 4 (2.2) | 1 (2.9) |
| 3 | 5 (2.7) | 0 |
| 4 | 5 (2.7) | 0 |
| 5 | 0 | 0 |
| 6 | 2 (1.1) | 1 (2.9) |
| Cirrhosis, n (%) | 93 (50.5) | 21 (60.0) |
| Decompensated cirrhosis | 28/86 (32.6) | 6/21 (28.6) |
| MELD-median (IQR) | 7 (6–7.9) | 7 (6–7.5) |
| Prior treatment, n (%) | ||
| Treatment-naive | 125/181 (69.1) | 18 (51.4) |
| Non-responder | 21/181 (11.6) | 6 (17.1) |
| Relapsed | 20/181 (11.0) | 2 (5.7) |
| Stopped due to adverse effects | 15/181 (8.3) | 7 (20.0) |
| PPI use (any dose), n (%) | 50 (27.2) | 8 (22.9) |
| Opioid maintenance therapy, n (%) | 30 (16.3%) | 4 (11.4%) |
IQR interquartile range, MELD model for end-stage liver disease, PPI proton-pump inhibitor
Treatment Outcome
The primary study outcome was the prevalence of SVR in the two groups (ON-ISP vs OFF-SP). SVR was defined as an undetectable HCV RNA on all tests after the end of treatment including at least one test 12 weeks or longer after the end of treatment. Subjects were considered not to have achieved SVR if HCV RNA was detectable on or after treatment, if treatment was not completed due to non-adherence or adverse effects and HCV RNA was detectable, or if the subject died during treatment or within 12 weeks of completion. Subjects with an undetectable HCV RNA at the end of treatment who did not return for repeat HCV RNA testing 12 weeks or more after the end of treatment visit were excluded from the primary SVR analysis. Secondary study outcomes included time to initiation of treatment, the number of reported adverse events, and the need for reduction in dose or early termination of treatment.
Treatment Adherence and Adverse Events
For the ON-ISP group, treatment adherence and adverse events were routinely assessed and documented during phone or clinic visits with pharmacy staff. This included subject self-report and pharmacy refill monitoring, as clinically indicated during treatment. Treatment adherence and adverse events among subjects using OFF-SP were assessed by review of any available telephone encounters, visit notes, fulfillment of on-treatment laboratories, or end-of-treatment visit notes.
Statistical Analysis
Medians and proportions were used to describe the study sample and treatment outcomes. Multivariable logistic regression models were used to assess association of specialty pharmacy type (ON-ISP vs OFF-SP) with SVR after adjusting for age, sex, race, prior HCV treatment, fibrosis stage, and MELD score. In a sensitivity analysis, we considered previously excluded subjects with an undetectable HCV RNA at the end of treatment who did not return for repeat HCV RNA testing 12 weeks or more after the end-of-treatment visit as having achieved an SVR, and we repeated the multivariable model. Univariate comparisons using Fisher’s exact test or the Mann–Whitney U-test were used for categorical variables and continuous variables, respectively. Data were analyzed using SAS software (Version 9.3, SAS Institute Inc., Cary, NC); all significance testing was two-sided based on α = 0.05.
Results
Study Sample Characteristics
A total of 219 subjects with HCV were treated with DAA within the study period (Table 1). The majority of subjects in the cohort were non-white (66%) and a minority of subjects had private insurance (31%), while the remainder had various combinations of Medicare and Medicaid-sponsored coverage. Subjects were predominantly infected with genotype 1 HCV (92%) and treatment-naïve (67%). Treatment-experienced subjects had previously received regimens including interferon, ribavirin, boceprevir, or telaprevir. Approximately half had cirrhosis with a median MELD score of 7 (IQR 6–8), and 30% of cirrhotic subjects had some form of decompensation. Most subjects were treated with ledipasvir/sofosbuvir (90%) with only a small minority of any treatment regimens requiring ribavirin (19%). Most subjects utilized the ON-ISP (84%), with the remainder (16%) using OFF-SP (p < 0.0001).
Use of ON-ISP was higher among Hispanic subjects than among non-Hispanic whites (p = 0.03), those with publicly funded compared to private insurance (p < 0.0001), and those with no prior HCV treatment experience (p = 0.05). No statistical difference between groups (ON-ISP vs OFF-SP) was observed in the prevalence of cirrhosis, decompensated cirrhosis, use of proton-pump inhibitors (PPI), HMG-CoA reductase inhibitors, opioids, or regimens containing ribavirin.
Completion of Planned Visits
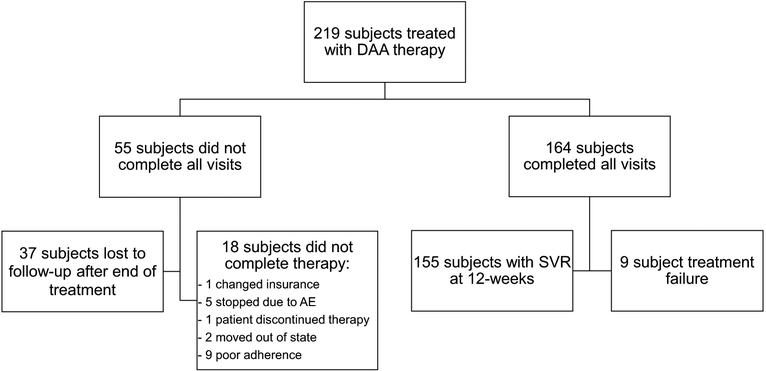
A total of 164 subjects completed the study protocol including the SVR 12-week post-treatment visit whereas 55 subjects did not complete all planned visits (Fig. 1). Of the 55 subjects that did not complete all planned visits, 37 subjects completed treatment and had undetectable HCV RNA at the end-of-treatment visit but did not return for the 12-week post-treatment visit or have measurement of HCV RNA after completing therapy and were considered lost to follow-up. All of these subjects remained engaged with care in the safety-net hospital system. Study characteristics for those lost to follow-up compared to those not lost to follow-up are shown in Table 2. Overall 8/37 subjects (21.6%) in the lost to follow-up group used the OFF-SP, whereas 27/182 subjects (14.8%) in the non-lost to follow-up group used the OFF-SP; however, this difference was not statistically significant. Eighteen subjects did not complete treatment for a variety of reasons as summarized in the Fig. 1.
Fig. 1.
Study flow chart
Table 2.
Cohort demographics for those lost to follow-up after the end-of-treatment visit compared to those not lost to follow-up
| Characteristic | Lost to follow-up after end-of-treatment visit (n = 37) | Not lost to follow-up (n=182) | p value |
|---|---|---|---|
| Age, y-median (IQR) | 56 (52–60) | 59 (53–62) | NS |
| Women, n (%) | 8 (21.6) | 57 (31.3) | NS |
| Ethnicity, n (%) | NS | ||
| Black | 20 (54.1) | 74 (40.7) | |
| White | 10 (27.0) | 60 (33.0) | |
| Hispanic | 4 (10.8) | 31 (17.0) | |
| Asian | 0 (0) | 9 (5.0) | |
| Other/unknown | 3 (8.1) | 3 (1.65) | |
| Insurance, n (%) | NS | ||
| Private | 10 (27.0) | 61 (32.8) | |
| Medicaid | 12 (32.4) | 60 (32.3) | |
| Medicare | 6 (16.2) | 24 (12.9) | |
| Medicare and medicaid | 6 (16.2) | 29 (15.6) | |
| Free care | 3 (8.1) | 12 (6.5) | |
| Genotype, n (%) | NS | ||
| 1 | 33 (89.2) | 167 (92.3) | |
| 2 | 0 | 5 (2.8) | |
| 3 | 3 (8.1) | 2 (1.1) | |
| 4 | 1 (2.7) | 4 (2.2) | |
| 5 | 0 | 0 | |
| 6 | 0 | 3 (1.7) | |
| Cirrhosis, n (%) | 17 (46.0) | 97 (54.0) | NS |
| Decompensated cirrhosis | 12/17 (70.6) | 61/97 (62.9) | NS |
| MELD-median (IQR) | 6.5 (6.4–7) | 6.8 (6.4–8.3) | NS |
| Prior treatment, n (%) | NS | ||
| Treatment-naive | 20/36 (55.6) | 124/179 (69.3) | |
| Non-responder | 5/36 (13.9) | 22/179 (12.3) | |
| Relapsed | 6/36 (16.7) | 16/179 (8.9) | |
| Stopped due to adverse effects | 5/36 (13.9) | 17/179 (9.5) | |
| PPI use (any dose), n (%) | 7 (18.9) | 50 (27.5) | NS |
| OFF-SP, n (%) | 8 (21.6) | 27 (14.8) | NS |
IQR interquartile range, MELD model for end-stage liver disease, PPI proton-pump inhibitor
Achievement of SVR
Overall, SVR was achieved and documented in 71% (155/219) of the entire study population based on intention-to-treat. The rate of SVR was 73% (135/184) among ON-ISP users and 57% (20/35) of OFF-SP users. Upon expanding the definition of responders to include the 37 subjects with undetectable HCV RNA at the end of treatment but were lost to follow-up prior to documentation of true 12-week SVR, the percentage of responders increased to 88% (192/219). Among the subjects completing treatment and returning for post-treatment follow-up per-protocol, 95% (155/164) achieved SVR (Fig. 1). Using the per-protocol analysis, 96% (135/141) of ON-ISP users achieved SVR compared to 87% (20/23) of OFF-SP users.
Multivariable Model for the Association Between Specialty Pharmacy Type and SVR
In the multivariable logistic regression model, subjects using the ON-ISP had a 5.8 × increased odds of SVR (OR: 6.0 (95% CI 1.18–31.0, p = 0.03) compared to those using the OFF-SP, after adjusting for age, sex, ethnicity, insurance type, cirrhosis, history of prior treatment, and MELD score. In a sensitivity analysis using the more inclusive definition of response above, based on undetectable HCV at end-of-treatment, similar results were observed.
Time to Treatment
No difference was observed between the median number of days between prescription of DAA and initiation of treatment in the ON-ISP group compared to the OFF-SP group (31 vs 35 days). There was significant variability in time to treatment in both groups ranging from 2 to 408 days.
Adverse Events
There were no between-group differences in the overall incidence of adverse events reported (52%) or in the overall incidence of complications attributed to adverse events (3.7%). These were generally mild in severity; only 5 subjects discontinued treatment due to an adverse event. The most common adverse events were fatigue (40.2%) and headache (23.7%). Severe adverse events were infrequent and included renal failure (1 subject), decompensated cirrhosis (1.9% of subjects with cirrhosis), and anemia (3.2%). The only observed death during the study period occurred in the subject with acute renal failure. This occurred after completion of treatment and SVR, in the setting of sepsis, and was felt to be unrelated to treatment. Most adverse events did not require intervention. Observed responses to adverse events included emergency department visits (0.9%), hospital admissions (1.8%), or dose reductions (2.3%). Use of ON-ISP was not associated with the incidence of adverse effects or discontinuation of treatment compared to use of the OFF-SP.
Treatment Adherence
Treatment adherence rates were high in users of both pharmacy types: 92% of subjects using the ON-ISP reported adherence to treatment, as did 87% of those using OFF-SP (Table 3). Of the 18 subjects who did not complete therapy, five discontinued therapy because of adverse side effects including depression, severe headache, gastrointestinal distress, generalized fatigue, and renal failure secondary to HCV-associated fibrillary glomerulonephritis. The remaining 13 subjects did not complete therapy for a variety of reasons as discussed in the Fig. 1.
Table 3.
Outcomes of HCV treatment among patients using on-site intensive specialty pharmacy versus off-site specialty pharmacy services
| Characteristic | On-site intensive specialty pharmacy (n = 184) | Off-site specialty pharmacy (n = 35) |
|---|---|---|
| Treatment, n (%) | ||
| Ledipasvir/sofosbuvir | 145 (78.8) | 26 (74.3) |
| Ledipasvir/sofosbuvir + ribavirin | 17 (9.2) | 7 (20.0) |
| Simeprevir/sofosbuvir | 7 (3.8) | 0 |
| Sofosbuvir + ribavirin | 9 (4.9) | 1 (2.9) |
| Sofosbuvir + ribavirin + IFN | 1 (0.1) | 0 |
| Ombitasvir/paritaprevir/ritonavir/dasabuvir + ribavirin | 5 (2.7) | 1 (2.9) |
| Treatment length, n (%) | ||
| 8 weeks | 29 (15.8) | 3 (8.6) |
| 12 weeks | 128 (69.6) | 32 (91.4) |
| 24 weeks | 27 (14.7) | 0 |
| Time to treatment, days-median (IQR) | 31 (18–49) | 35 (28–65.5) |
| SVR, n (%) (based on intention-to-treat) | 135 (73) | 20 (57) |
| SVR, n (%) (based on those completing all follow- up testing per-protocol) | 135/141 (96) | 20/23 (87) |
| Adverse event, n (%) | 94 (51.1) | 19 (54.3) |
| Dose reductions, n (%) | 5 (2.7) | 0 |
| Early termination, n (%) | 4 (2.2) | 1 (2.9) |
IQR interquartile range, SVR sustained virologic response, IFN interferon
Discussion
In this retrospective analysis of DAA-based treatment for HCV at an urban safety-net hospital, our findings were threefold. First, the observed rate of SVR was 71% (155/219) based on intention-to-treat. Among those who completed treatment and had follow-up laboratory testing per-protocol, 95% (155/164) had SVR. Second, after adjustment for potential confounding variables, the rate of SVR was higher among subjects using the ON-ISP compared to the OFF-SP. Third, treatment was generally well tolerated in both groups of subjects. Although adverse events were common (52%), most were minor, with only 9/219 subjects requiring any change in management due to an adverse event.
The present study demonstrates the effectiveness of HCV treatment in an urban, safety-net hospital population. This cohort achieved SVR at a lower rate (71%) than that previously reported in clinical trials of DAA-based treatment for chronic HCV infection. The higher rate of SVR (95%) observed among subjects who completed treatment and returned for follow-up laboratory testing is more comparable to that reported in the setting of clinical trials, likely in less diverse, more affluent populations with more clinical resources available [3]. This may suggest that the reduced overall rate of SVR observed is attributable to difficulties in clinical implementation, rather than biological differences. Indeed, most of the subjects who did not achieve SVR were lost to follow-up after completing therapy and achieving a non-detectable HCV viral load at the end of treatment, so most, if not all, of these subjects likely did achieve SVR. A recent study in a safety-net population demonstrated high rates of treatment success that was comparable to those in resource-rich settings using a multidisciplinary care model that relied heavily on pharmacy assistance [18]. Additionally, another study compared rates of SVR in patients treated with interferon-based regimens who used a specialty pharmacy program versus standard retail pharmacy. Although no difference in the rate of SVR was found between users of a specialty pharmacy program and a standard retail pharmacy, non-white subjects receiving specialty pharmacy care did have an increased rate of SVR compared to white subjects [26]. This is consistent with our experience caring for a largely non-white patient population. Compared to prior, interferon-based regimens, there may also be a greater potential benefit of intensive pharmacy care in DAA regimens where there is a more vital need for daily adherence [27].
Our model of intensive specialty pharmacy care is a patient-centered approach with frequent contact, education, and reminders which may improve adherence. The intensive specialty pharmacy program fosters collaboration between prescribers, clinical pharmacists, and pharmacy liaisons to quickly assess the severity and relevance of potential adverse events. Given the high observed frequency of symptomatic adverse events, rapid review by pharmacists and coordination with prescribers may lead to interventions such as patient reassurance, dose reductions, or changes in other medications, as alternatives to early treatment discontinuation or patient non-adherence. This may be especially true in underserved populations with the lower health literacy [23, 28]. Despite these efforts and the use of relatively well-tolerated and short-duration DAA regimens, we observed a high rate of loss to follow-up or self-discontinuation of therapy (21%). This suggests there is room for improvement in the delivery of care and perhaps in the selection of patients for treatment with these costly medications, particularly with the potential risk of resistance if treatment is not complete. Also, individual insurance plans did not all cover the ON-ISP, so this type of program may not be available to all individuals. Additional work is needed to understand how an ON-ISP can be best integrated into the multidisciplinary care of patients with HCV.
The primary strength of this study is the inclusion of all patients receiving DAA treatment for HCV at our center, a racially and socioeconomically diverse population that reflects that of many safety-net medical centers. By studying all patients treated in routine clinical practice, our findings may be more generalizable than those of clinical trials. We studied patients with all stages of liver disease, from minimal fibrosis to decompensated cirrhosis to provide results applicable to a broad spectrum of patients.
The current study has several limitations. It was a retrospective analysis based on the medical record, with medication adherence self-reported, and pharmacy dispensing records accessed for confirmation only when there was a concern for non-compliance, which may lead to an overestimation of adherence. Because a relatively small number of subjects used the OFF-SP, we had limited power to detect a difference compared to the ON-ISP group and we observed limited precision of our estimate of the true effect of the ON-ISP. Benefits of the ON-ISP may have included closer monitoring, experienced staff accustomed to working with patients with lower health literacy, availability of mailing prescriptions, and subject comfort with using a familiar pharmacy, all of which may have improved adherence to treatment and, therefore, achieving SVR. It was beyond the scope of our study to determine what specific intervention used by the on-site intensive pharmacy program may have been most beneficial to patients. Future efforts should include larger, prospective study of intensive multidisciplinary models in the treatment of HCV-infected patients. Qualitative studies are also needed to understand more about the factors that may be most beneficial to patients.
This study is one of the first to demonstrate the practical effectiveness of DAA regimens in a diverse, urban patient population where HCV is prevalent. SVR was achieved in 95% of subjects completing treatment of HCV with DAA regimens and returning for follow-up testing and in 71% of all subjects enrolled. The observed rate of SVR based on intention-to-treat is lower than that reported in clinical trials, reflecting the difficulties of implementation in a vulnerable patient population. The use of an on-site, intensive specialty pharmacy was associated with a significant increase in the rate of SVR after adjustment for potentially confounding subject characteristics. Clinical pharmacists with advanced training in HCV management can provide extensive patient education, support patient adherence, and enhance continuity of care. Use of an on-site, intensive specialty pharmacy program may be a relatively simple method to improve the rate of SVR, which is particularly important given the high clinical cost of HCV treatment failure and high financial cost of the treatment itself.
Funding
Dr. Long is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases K23 DK113252 and the Boston University School of Medicine Department of Medicine Career Investment Award. Dr. Fricker is supported in part by the National Center for Advancing Translational Sciences and National Institutes of Health (BU-CTSI Grant Number 1UL1TR001430). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- DAA
Direct-acting antiviral
- HCV
Hepatitis C virus
- OFF-SP
Off-site specialty pharmacy
- ON-ISP
On-site intensive specialty pharmacy
- SVR
Sustained virologic response
Footnotes
Conflict of interest There are no known conflicts of interest for any author.
References
- 1.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. [DOI] [PubMed] [Google Scholar]
- 2.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64:405–414. [DOI] [PubMed] [Google Scholar]
- 3.Berden FA, de Knegt RJ, Blokzijl H, et al. Limited generalizability of registration trials in hepatitis C: a nationwide cohort study. PLoS One. 2016;11:e0161821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamorro-de-Vega E, Gimenez-Manzorro A, Rodriguez-Gonzalez CG, et al. Effectiveness and safety of ombitasvir-paritaprevir/ritonavir and dasabuvir with or without ribavirin for HCV genotype 1 infection for 12 weeks under routine clinical practice. Ann Pharmacother. 2016;50:901–908. [DOI] [PubMed] [Google Scholar]
- 5.Flisiak R, Janczewska E, Wawrzynowicz-Syczewska M, et al. Real-world effectiveness and safety of ombitasvir/paritaprevir/ritonavir ± dasabuvir ± ribavirin in hepatitis C: AMBER study. Aliment Pharmacol Ther. 2016;44:946–956. [DOI] [PubMed] [Google Scholar]
- 6.Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the veterans affairs national health care system. Gastroenterology. 2016;151:457–471.e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terrault NA, Zeuzem S, Di Bisceglie AM, et al. Effectiveness of ledipasvir–sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology. 2016;151:1131–1140.e1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welzel TM, Nelson DR, Morelli G, et al. Effectiveness and safety of sofosbuvir plus ribavirin for the treatment of HCV genotype 2 infection: results of the real-world, clinical practice HCV-TARGET study. Gut. 2017;66:1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welzel TM, Petersen J, Herzer K, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65:1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong RJ, Nguyen MT, Trinh HN, et al. Community-based real-world treatment outcomes of sofosbuvir/ledipasvir in Asians with chronic hepatitis C virus genotype 6 in the United States. J Viral Hepat. 2017;24:17–21. [DOI] [PubMed] [Google Scholar]
- 11.Yee BE, Nguyen NH, Jin M, Lutchman G, Lim JK, Nguyen MH. Lower response to simeprevir and sofosbuvir in HCV genotype 1 in routine practice compared with clinical trials. BMJ Open Gastroenterol. 2016;3:e000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younossi ZM, Park H, Gordon SC, et al. Real-world outcomes of ledipasvir/sofosbuvir in treatment-naive patients with hepatitis C. Am J Manag Care. 2016;22:SP205–SP211. [PubMed] [Google Scholar]
- 13.Alam I, Brown K, Donovan C, et al. Real-world effectiveness of simeprevir-containing regimens among patients with chronic hepatitis C virus: the SONET study. Open Forum Infect Dis. 2017;4:ofw258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buggisch P, Vermehren J, Mauss S, et al. Real-world effectiveness of 8-week treatment with ledipasvir/sofosbuvir in chronic hepatitis C. J Hepatol. 2018;68:663–671. [DOI] [PubMed] [Google Scholar]
- 15.Tapper EB, Bacon BR, Curry MP, et al. Real-world effectiveness for 12 weeks of ledipasvir–sofosbuvir for genotype 1 hepatitis C: the Trio health study. J Viral Hepat. 2017;24:22–27. [DOI] [PubMed] [Google Scholar]
- 16.Wedemeyer H, Craxi A, Zuckerman E, et al. Real-world effectiveness of ombitasvir/paritaprevir/ritonavir ± dasabuvir ± ribavirin in patients with hepatitis C virus genotype 1 or 4 infection: a meta-analysis. J Viral Hepat. 2017;24:936–943. [DOI] [PubMed] [Google Scholar]
- 17.Wehmeyer MH, Ingiliz P, Christensen S, et al. Real-world effectiveness of sofosbuvir-based treatment regimens for chronic hepatitis C genotype 3 infection: results from the multicenter German hepatitis C cohort (GECCO-03). J Med Virol. 2018;90:304–312. [DOI] [PubMed] [Google Scholar]
- 18.Yek C, de la Flor C, Marshall J, et al. Effectiveness of direct-acting antiviral therapy for hepatitis C in difficult-to-treat patients in a safety-net health system: a retrospective cohort study. BMC Med. 2017;15:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feuerstadt P, Bunim AL, Garcia H, et al. Effectiveness of hepatitis c treatment with pegylated interferon and ribavirin in urban minority patients. Hepatology. 2010;51:1137–1143. [DOI] [PubMed] [Google Scholar]
- 20.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon Alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. [DOI] [PubMed] [Google Scholar]
- 21.Singal AG, Dharia TD, Malet PF, Alqahtani S, Zhang S, Cuthbert JA. Long-term benefit of hepatitis C therapy in a safety net hospital system: a cross-sectional study with median 5-year follow-up. BMJ Open. 2013;3:e003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agency for Healthcare R, Quality. Interventions to improve patient adherence to hepatitis c treatment: comparative effectiveness. Comp Eff Rev. 2012;20:1–16. [PubMed] [Google Scholar]
- 23.Henderson RR, Visaria J, Bridges GG, Dorholt M, Levin RJ, Frazee SG. Impact of specialty pharmacy on telaprevir-containing 3-drug hepatitis C regimen persistence. J Managed Care Spec Pharm. 2014;20:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieveld FI, van Vlerken LG, Siersema PD, van Erpecum KJ. Patient adherence to antiviral treatment for chronic hepatitis B and C: a systematic review. Ann Hepatol. 2013;12:380–391. [PubMed] [Google Scholar]
- 25.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. [DOI] [PubMed] [Google Scholar]
- 26.Cohen SM, Kwasny MJ, Ahn J. Use of specialty care versus standard retail pharmacies for treatment of hepatitis C. Ann Pharmacother. 2009;43:202–209. [DOI] [PubMed] [Google Scholar]
- 27.Younossi ZM, Stepanova M, Henry L, Nader F, Younossi Y, Hunt S. Adherence to treatment of chronic hepatitis C: from interferon containing regimens to interferon and ribavirin free regimens. Medicine. 2016;95:e4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demian MN, Shapiro RJ, Thornton WL. An observational study of health literacy and medication adherence in adult kidney transplant recipients. Clin Kidney J. 2016;9:858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]