Abstract
Background:
Klotho serum levels reflect nutritional state in adults including obesity and anorexia. The relationship between cord blood klotho levels at birth and parameters of growth including anthropometrics are not known.
Methods:
We evaluated the relationship between cord blood klotho, leptin and adipocyte hormones and infant, child and maternal anthropometrics and maternal depression in a cohort of 73 children. Non-parametric tests were used to assess differences between dichotomous and categorical predictors and klotho levels and Spearman’s rank coefficients were used to assess the relationship between klotho levels and continuous predictors. A multivariable log transformed linear regression model was used to test for independent predictors of serum klotho levels.
Results:
Mean klotho levels were 2864.9 ± 1409.7 (pg/mL) in cord blood and we found no relationship with infant sex, delivery specifics including gestational age or anthropometrics at birth. There was similarly no association between klotho levels at birth and future obesity at age 2. Leptin levels at birth were inversely associated with klotho levels in multivariable models after adjusting for other covariates (p < 0.01). Similarly, in multivariable models insulin levels were inversely correlated with klotho levels (p < 0.03). Leptin levels in our cohort of at-risk infants were more than 50% higher than other studies with neonates.
Conclusions:
We found no associations between weight or length at birth or obesity in early childhood and cord blood klotho levels. Cord blood klotho levels were inversely correlated with leptin and insulin levels at birth and should be further investigated to better understand the inter-relationship between this hormone and key regulators of growth and adiposity.
Keywords: growth, klotho, Latino, leptin, neonate
Introduction
Serum klotho levels are associated with nutritional status in studies showing reduced levels in anorexic and obese patients [1]. Studies have suggested that klotho is an important regulator of adiopocyte differentiation. In mice, eliminating klotho function resulted in lean mice with no fat tissue [2]. Similarly klotho knockout mice fed a high fat diet did not gain any weight compared with wild type mice who gradually gained body weight [2]. Klotho also has longevity properties and induces resistance to oxidative stress. In adult women, those with high levels of chronic stress had lower levels of klotho compared with those with lower stress burdens [3].
Klotho deficient mice have premature death and early symptoms that resemble aging [4]. In neonates, preterm infants have lower levels of klotho compared to infants born at term [5]. In part, this may be explained by body weight and length as klotho levels were also correlated with these parameters, although preterm infants are also known to suffer from a number of health deficits including increased risk for future metabolic diseases [5]. However, this same study did not find any association with glucose or insulin or insulin resistance as indicated by homeostatic model assessment-iinsulin resistance (HOMA-IR) levels [5].
In the current study, our aims were to investigate the relationship between cord blood klotho levels and stress exposures in pregnancy including maternal depressive symptoms, clinical depression and indices of growth including birth anthropometrics and weight and satiety hormones.
Materials and methods
In our previously described Latino, Eating and Diabetes cohort (LEAD) n = 97 of pregnant, self-identified Latina women, cord blood was obtained at delivery from a subset of women (n = 72) as were infant anthropometrics and hormones from cord blood as previously described [6, 7]. Briefly, serum leptin, insulin, insulin-like growth factor 1 (IGF1), peptide YY and ghrelin were assayed (Milliplex MAP Human Metabolic Hormone Magnetic Bead Panel [Millipore], Linco, St Charles, MO, USA). The minimum detectable concentration for leptin was 27 pg/mL, insulin 58 pg/mL, ghrelin 2 pg/mL and PYY 8 pg/mL. The intra-assay coefficient of variation (CV) and inter-assay CV for leptin, insulin and ghrelin and PYY were 3% and 4%; 3% and 6%; 2% and 8%; and 2% and 11%, respectively. Serum IGF-1 was assessed using the Milliplex MAP Human IGF-1 Single Plex. The minimum detectable concentration was 52 pg/mL and the intra-assay CV was 4% and the inter-assay was 7%. Women were assessed for prenatal depression using the Edinburgh Postnatal Depression Scale (EPDS) and the Center for Epidemiological Depression Scale (CESD) in pregnancy. Clinical depression was evaluated using the Mini International Neuropsychiatric Interview (MINI 5.0). Infant anthropometrics were collected at birth using standard digital scales and tape measures and were extracted from the medical record. Similarly, infant anthropmetrics (weight and length/height) were assessed at 6 months, 12 months and 2 years of age. Childhood obesity was defined as weight for length or body mass index (BMI) percentile ≥95th using standard Center for Disease Control growth curves [8]. All women provided written consent for their own and their children’s participation. Those women who did not provide cord blood at delivery either did not consent for that portion of the study or there were logistical and temporal issues associated with the delivery that prevented study staff from successfully obtaining cord blood. The study was approved by the Institutional Review Board of the University of California, San Francisco (Committee on Human Research).
Serum klotho levels were measured in cord blood using a solid-phase sandwich enzyme-linked immunosorbent assay (Immuno-Biological Laboratories, Takasaki, Japan) as previously described [9]. All samples were run in duplicate. The assay had an average intra-assay CV of approximately 3%–5%. Samples with a CV above 7% were repeated with 16 samples being re-run and the control sample in the second run being in close agreement with the first run.
Statistical analyses
Klotho values were assessed for normal distribution through graphing and tests for normality including the Shapiro-Wilk and Shapiro-Francia tests. As the data was not normally distribution, the Wilcoxon rank sum (Mann-Whitney) test was used to assess the different in klotho levels by dichotomous predictors and Kruksal-Wallis was used for categorical predictors. Spearman’s rank correlation coefficients (ρ) were used to assess the association between klotho levels and continuous predictors. p-Values <0.05 were determined to be statistically significant. Data are expressed as means plus or minus standard deviations and percentages. We log transformed the klotho values prior to conducting all regression analyses as the residuals of the regression analysis were not normally distributed. The natural log transformation converted the data into a normal distribution. Multivariable analyses included those variables that were significant at p < 0.05 in addition to those variables that previously demonstrated a plausible association with klotho levels in studies with neonates including gestational age and leptin levels. The exponentiated coefficients presented are the geometric ratio of the variable examined. Beta coefficients are presented and 95% confidence intervals (CLs) including p-values.
Results
The mean child cord blood klotho levels were 2864.9 ± 1409.7 pg/mL at birth in the 72 children tested (Table 1). We did not find any associations between maternal socio-demographics including education level and marital status and infant’s klotho levels at birth. Similarly, there were no associations between mother’s health status prior to pregnancy or in pregnancy and child outcomes including pre-pregnancy BMI category, preeclampsia or hypertension, excessive weight gain in pregnancy or maternal mental health in pregnancy including self-reported use of anti-depressants (Table 1).
Table 1:
Cord blood klotho, socio-demographics, and maternal and paternal characteristics (n = 73).
| Variable | Mean ± standard deviation or n/total (%) | Cord blood klotho mean ± standard deviation, pg/mL | ρ; p-Value |
|---|---|---|---|
| Klotho | 2864.9 ± 1409.7 | ||
| Socio-demographics | |||
| Maternal education | |||
| Less than high school | 56/72 (77.8) | 2996.7 ± 1479.5 | 0.19 |
| High school graduate or more | 16/72 (22.2) | 2509.1 ± 1059.2 | |
| Marital status | |||
| Single or divorced/separated/widowed | 12/73 (16.4) | 3260.6 ± 1800.4 | |
| Living with partner or married | 61/73 (83.6) | 2787.0 ± 1323.9 | 0.50 |
| Maternal health characteristics | |||
| Pre-pregnancy body mass index (BMI) | 26.9 ± 4.4 | 0.12; p = 0.31 | |
| Underweight or normal (below 24.9) | 27/69 (39.1) | 2611.9 ± 1054.2 | |
| Overweight (25.0–29.9) | 26/69 (37.7) | 3091.1 ± 1701.6 | |
| Obese (30.0 and above) | 16/69 (23.2) | 3017.8 ± 1539.5 | 0.47 |
| Preeclampsia/hypertension | |||
| No | 69/73 (94.5) | 2846.3 ± 1433.9 | |
| Yes | 4/73 (5.5) | 3185.0 ± 970.0 | 0.34 |
| Excess weight gain in pregnancy | |||
| No | 33/63 (52.4) | 2776.9 ± 1509.7 | |
| Yes | 44/63 (69.8) | 2773.6 ± 1349.0 | 0.74 |
| Depressive symptoms in pregnancy | |||
| No | 62/73 (84.9) | 2799.3 ± 1402.2 | |
| Yes | 11/73 (15.1) | 3234.4 ± 1461.8 | 0.20 |
| Clinical depression in pregnancy | |||
| No | 66/73 (90.4) | 2812.5 ± 1415.9 | |
| Yes | 7/73 (9.6) | 3358.8 ± 1346.6 | 0.16 |
| Anti-depressant use during pregnancy | |||
| No | 64/73 (87.7) | 2826.2 ± 1416.5 | |
| Yes | 9/73 (12.3) | 3140.1 ± 1409.4 | 0.51 |
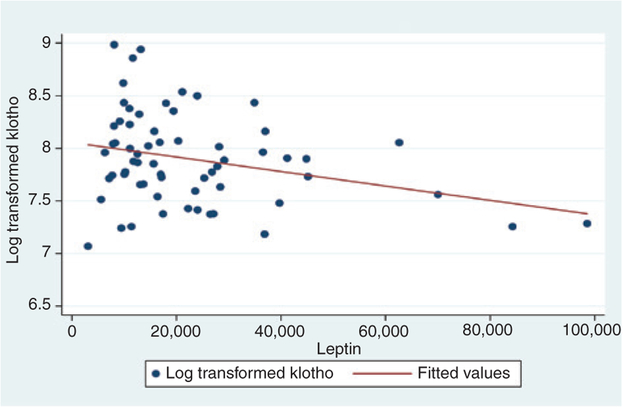
There were no differences in klotho levels by infant sex, gestational age or Apgar scores at 5 min (Table 2). We also did not find any association of klotho with birth anthropometrics including birth weight, macrosomia and birth weight percentile. Infants who were underweight (<2500 g) or preterm (<37 weeks) had lower klotho levels (1858.6 ± 674.1 vs. 2898.6 ± 1417.2 pg/mL) although the results trended towards statistical significant (p = 0.07) due to the small sample size (only n = 4 with low birth weight or preterm birth). Those with higher leptin had reduced klotho levels although the results were not statistically significant (R = −0.21; p = 0.10). Those in the highest tertile had klotho levels of 2353.7 ± 809.2 vs. 3359.2 ± 1769.6 pg/mL for those in the lowest tertile with the result trending towards statistical significance (p = 0.06; Table 2). Figure 1 displays log transformed klotho levels plotted in relation to leptin levels at birth with a superimposed regression line presenting predicted fitted values. A third of our cohort had leptin levels, which exceeded 27,000 pg/mL. Higher insulin levels were also inversely correlated with klotho levels but correlations were not statistically significant (R = −0.17; p = 0.16). We also did not find any association between klotho levels at birth at early childhood obesity at 6, 12 and 24 months of age (Table 2).
Table 2:
Cord blood klotho and child characteristics (n = 73).
| Variable | Population mean ± standard deviation or n/total | Cord blood klotho levels mean ± standard deviation, pg/mL | ρ; p-Value |
|---|---|---|---|
| Child birth characteristics | |||
| Gestational age (weeks) | 39.2 ± 1.3 | −0.01; p = 0.37 | |
| <40 | 49/72 (68.1) | 2792.4 ± 1204.6 | |
| ≥40 | 23/72 (31.9) | 2943.9 ± 1783.8 | 0.63 |
| Gestational age (weeks) tertiles | |||
| <39 | 17/72 (23.6) | 3212.8 ± 1351.4 | |
| 39–39.8 | 26/72 (36.1) | 2568.1 ± 962.2 | |
| >39.8 | 29/72 (40.3) | 2867.2 ± 1725.3 | 0.23 |
| Apgar score at 5 min | 8.9 ± 0.4 | ||
| <9 | 7/72 (9.7) | 2253.1 ± 680.5 | |
| ≥9 | 65/72 (90.3) | 2904.1 ± 1450.0 | 0.21 |
| Child sex | |||
| Female | 39/73 (53.4) | 2799.6 ± 1402.5 | |
| Male | 34/73 (46.6) | 2939.8 ± 1435.2 | 0.51 |
| Birth anthropometries | |||
| Birth weight, g | 3390.8 ± 469.8 | −0.07; p = 0.55 | |
| Macrosomic at birth (≥4000 g) | |||
| No | 67/72 (93.1) | 2858.6 ± 1442.7 | |
| Yes | 5/72 (6.9) | 2602.5 ± 764.8 | 0.97 |
| Underweight (<2500 g) or preterm (<37 weeks) | |||
| No | 68/72 (94.4) | 2898.6 ± 1417.2 | |
| Yes | 4/72 (5.6) | 1858.6 ± 674.1 | 0.07 |
| Birth weight percentile | 54.3 ± 24.9 | 0.04; p = 0.74 | |
| <50% | 36/72 (50.0) | 3036.8 ± 1713.2 | |
| ≥50% | 36/72 (50.0) | 2644.8 ± 993.1 | 0.79 |
| Birth weight for length below 5% | |||
| No | 64/72 (88.9) | 2902.3 ± 1473.5 | |
| Yes | 8/72 (11.1) | 2349.0 ± 387.2 | 0.39 |
| Head circumference percentile (tertiles) | 34.2 ± 1.3 | −0.05; p = 0.67 | |
| <27% | 23/72 (31.9) | 2819.6 ± 1495.5 | |
| 27%−50% | 27/72 (37.5) | 3034.1 ± 1576.6 | |
| >50% | 22/72 (30.6) | 2625.6 ± 1072.4 | 0.70 |
| Cord blood hormones | |||
| Cord blood glucose tertiles, mg/dL | 80.8 ± 2.9 | −0.01; p = 0.97 | |
| <72 | 18/56 (32.1) | 2879.5 ± 376.2 | |
| 72–87 | 19/56 (33.9) | 3079.9 ± 251.8 | |
| >87 | 19/56 (33.9) | 2773.9 ± 329.0 | 0.50 |
| Insulin-like growth factor (IGF-1) tertiles (pg/mL) | 29,841.8 ± 20,540.4 | 0.02; p = 0.87 | |
| <17,600 | 24/72 (33.3) | 2905.3 ± 1720.1 | |
| 17,600–35,120 | 24/72 (33.3) | 2863.1 ± 1342.0 | |
| >35,120 | 24/72 (33.3) | 2814.6 ± 1205.5 | 0.90 |
| Ghrelin tertiles (pg/mL) | 39.1 ± 22.3 | 0.15; p = 0.53 | |
| <22 | 6/20 (30.0) | 2372.4 ± 769.0 | |
| 22–45 | 7/20 (35.0) | 3093.0 ± 2189.9 | |
| >45 | 7/20 (35.0) | 2569.7 ± 816.4 | 0.88 |
| Insulin tertiles (pg/mL) | 968.4 ± 555.1 | −0.17; p = 0.16 | |
| <750 | 21/66 (31.8) | 3324.9 ± 1661.3 | |
| 750–1060 | 22/66 (33.3) | 2754.1 ± 1107.9 | |
| >1060 | 23/66 (34.8) | 2718.2 ± 1501.6 | 0.30 |
| Leptin tertiles (pg/mL) | 22,559.1 ± 18,368.4 | −0.21; p = 0.10 | |
| <11,200 | 21/63 (33.3) | 3359.2 ± 1769.6 | |
| 11,200–27,000 | 21/63 (33.3) | 3211.7 ± 1465.9 | |
| >27,000 | 21/63 (33.3) | 2353.7 ± 809.2 | 0.06 |
| PYY tertiles (pg/mL) | 324.6 ± 119.9 | 0.05; p = 0.67 | |
| <265 | 20/66 (30.3) | 2598.7 ± 1096.9 | |
| 265–350 | 23/66 (34.8) | 3290.9 ± 1743.1 | |
| >350 | 23/66 (34.8) | 2837.7 ± 1356.3 | 0.56 |
| Early childhood anthropometries | |||
| Obesity in early childhood | |||
| 6 months (yes) | 9/70 (12.9) | 2413.8 ± 700.1 | |
| No | 61/70 (87.1) | 2909.5 ± 1493.6 | 0.46 |
| 12 months (yes) | 9/64 (14.1) | 2679.8 ± 809.4 | |
| No | 55/64 (85.9) | 2932.7 ± 504.2 | 0.85 |
| 24 months (yes) | 6/61 (9.8) | 2908.6 ± 1115.6 | |
| No | 55/61 (90.2) | 2958.9 ± 1497.0 | 0.81 |
Figure 1:
Cord blood leptin in relation to klotho (log transformed).
In multivariable analysis, including gestational age, leptin levels and birth weight, leptin remained inversely correlated with klotho levels (beta coefficient 0.999992, 95% CI, 0.999986–1.126; p < 0.01). In multivariable analysis, there were no associations between klotho levels and the other variables in the model including gestational age (p = 0.84) and birth weight (g) (p = 0.26; results not shown). In a separate multivariable analysis, replacing leptin with insulin levels, insulin levels were also statistically significant and inversely associated with klotho levels (beta coeff = 0.99977, 95% CI 0.99956–0.99998; p = 0.03; results not shown).
Discussion
Mean klotho levels were 2864.9 ± 1409.7 (pg/mL) in our group of Latino neonates which is comparable with the two other studies that measured klotho from cord blood. In Ohata et al.’s [10] study of Japanese neonates (n = 23) cord serum measures of klotho were 3243 ± 1899 pg/mL (2011) and in a European study (n = 202), cord plasma klotho levels were 3004 ± 1353 pg/mL [11]. In the Japanese study, klotho levels were much higher in newborns than adults and infants at 4 days of life.
We found that klotho levels were inversely correlated with both leptin and insulin, reaching statistical significance in multivariable analysis for leptin and insulin. Previous studies have found a correlation between leptin levels and birth weight as well as other birth characteristics such as birth length and ponderal index [12]. Higher leptin levels have also been found in intrauterine growth restricted neonates and may be associated with subsequent development of insulin resistance [13]. Higher leptin or leptin resistance has also been noted to occur with obesity. In contrast with other studies, we did not find any association between birth weight and other birth characteristics such as gestational age and klotho levels [5].
It is possible that lower levels of klotho are associated with higher levels of leptin in normal weight neonates, who may already have metabolic disturbances and are at risk for future obesity. Although, the weight parameters for our cohort were largely in the normal range with the majority of children having normal birth weight and only 3.2% reporting low birth weight and 8.4% high birth weight, a high percentage had elevated leptin levels. The mean cord blood leptin in our cohort was 23.0 ± 18.4 ng/mL while 25.4% of the cohort had high leptin defined as leptin levels >90th percentile for neonates (>28 ng/mL) from the study by [14]. The mean leptin levels in our cohort were much higher (more than 50%) than the 15.7 ng/mL reported for Taiwanese infants [15] and also higher than the 13.97 ng/mL for large for gestational age infants in a US sample [16]. It is possible, at birth, that a subset of our infants already had leptin resistance, although the coexistence of elevated leptin levels and obesity has primarily been described in older children and adults [17].
The role of klotho in energy metabolism and weight gain is not well understood. Klotho is a protein that is mainly expressed in the kidneys with a smaller amount produced in adipose tissue [5], and regulates bone metabolism but may also be involved in insulin signaling. Our findings that klotho was inversely correlated with leptin and insulin differs from those findings of Siahandiou with postnatal infants at 14 and 28 days of life where there was no association between insulin or insulin resistance but with body size. Studies of adults with obesity and anorexia nervous found that nutritional deficiencies were associated with lower klotho levels, implying the potential role of klotho in nutritional homeostasis. It is possible that klotho impacts energy metabolism via its impact on leptin and appetite regulation; however future studies are needed to test this possibility.
Research funding:
National Institute of Diabetes and Digestive and Kidney Diseases, Funder Id: 10.13039/100000062, Grant Number: 097458 (to J.M.W). NINDS (R01 NS092918 to D.B.D.).
Footnotes
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- 1.Amitani M, Askawa A, Amitani H, Kaimoto K, Sameshima N, et al. Plasma klotho levels decrease in both anorexia nervosa and obesity. Nutrition 2013;29:1106–9. [DOI] [PubMed] [Google Scholar]
- 2.Ohnishi M, Kato S, Akiyoshi J, Atfi A, Razzaque MS. Dietary and genetic evidence for enhancing glucose metabolism and reducing obesity by inhibiting klotho functions. FASEB J 2011;25:2031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prather AA, Epel ES, Arenander J, Broestl L, Broestl L, et al. Longevity factor klotho and chronic psychological stress. Transl Psychiatry 2015;5:e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlatou MG, Remaley AT, Gold PW. Klotho: a humeral mediator in CSF and plasma that influences multiple complex disorders, including depression. Transl Psychiatry 2016;6:e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siahanidou T, Garatzioti M, Lazaropolou C, Kourlaba G, Papssotiriou I, et al. Plasma soluble alpha-klotho protein levels in premature and term neonates: correlation with growth and metabolic parameters. Eur J Endocrinol 2012;167: 433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wojcicki JM, Olveda R, Heyman MB, Elwan D, Lin J, et al. Cord blood telomere length in Latino infants: relation with maternal education and infant sex. J Perinatol 2016;36:235–41. [DOI] [PubMed] [Google Scholar]
- 7.Ville AP, Heyman MB, Medrano R, Wojcicki JM. Early antibiotic exposure and risk of childhood obesity in Latinos. Child Obes 2017;13:231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 2002;11:1–190. [PubMed] [Google Scholar]
- 9.Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, et al. Life extension factor klotho enhances cognition. Cell Rep 2014;7:1065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohata Y, Arahori H, Namba N, Kitaoka T, Hirai H, et al. Circulating levels of soluble alpha-Klotho are markedly elevated in human umbilical cord blood. J Clin Endocrinol Metab 2011;96:E943–7. [DOI] [PubMed] [Google Scholar]
- 11.Godang K, Froslie KF, Henriksen T, Isaksen GA, Voldner N, et al. Umbilical cord levels of sclerostin, placental weight, and birth weight are predictors of total bone mineral content in neonates. Eur J Endocrinol 2013;168:371–8. [DOI] [PubMed] [Google Scholar]
- 12.Karakosta P, Chatzi L, Plana E, Margioris A, Castanas E, et al. Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta-analysis. Pediatri Perinat Epidemiol 2011;25:150–63. [DOI] [PubMed] [Google Scholar]
- 13.Kyriakakou M, Malamitsi-Pucher A, Millitsi H, Boutsikou T, Margeli A, et al. Leptin and adiponectin concentrations in intrauterine growth restricted and appropriate for gestatational age fetuses, neonates and their mothers. Eur J Endocrinol 2008;158:343–8. [DOI] [PubMed] [Google Scholar]
- 14.Mouzaki A, Panagoulias I, Raptis G, Farri-Kostopoulou E. Cord blood leptin levels of healthy neonates are associated with IFN-gamma production by cord blood T-cells. PLoS One 2012;7:e40830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung WK, Lin SJ, Hwang YS, Wu CM, Wang YH, et al. Association of cord plasma leptin with birth size in term newborns. Pediatr Neonatol 2009;50:255–60. [DOI] [PubMed] [Google Scholar]
- 16.Christou H, Connors JM, Ziotopoulou M, Hatzidakis V, Papthanassoglou E, et al. Cord blood leptin and insulin-like growth factor levels are independent predictors of fetal growth. J Clin Endocrinol Metab 2001;86:935–8. [DOI] [PubMed] [Google Scholar]
- 17.Myers MB, Leibel RL, Seeley RJ, Schwatz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 2010;21:643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]