Abstract
Purpose of Review
Nonalcoholic fatty liver disease (NAFLD), the most prevalent cause of chronic liver disease worldwide, is strongly associated with obesity and insulin resistance.
Recent Findings
Significant weight loss can improve NAFLD and nonalcoholic steatohepatitis (NASH). Diet and exercise that result in a sustained body weight reduction of 7–10% can improve liver fat content, NASH, and fibrosis. Vitamin E can be considered in patients with biopsy-proven NASH without diabetes, though caution must be used in those with prostate cancer. Pioglitazone improves liver histology, including fibrosis, and can be considered in patients with or without diabetes. Glucagon-like peptide-1 (GLP-1) antagonists may be beneficial in NASH, but more studies are needed before they can be recommended. Bariatric surgery, with resultant weight loss, can result in improvement in liver fat and inflammation.
Summary
NAFLD treatment includes diet and exercise with a target 7–10% weight reduction. Treatment goals include improvements in liver fat content, liver inflammation, and fibrosis.
Keywords: Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, Obesity, Diabetes, Body mass index, Magnetic resonance elastography, NAFLD activity score, Intrahepatic triglycerides, Bariatric surgery
Introduction
Nonalcoholic fatty liver disease, a chronic liver disease characterized by excess fat deposition in the liver in the absence of secondary causes, is strongly associated with obesity and the metabolic syndrome. Estimates of the prevalence of nonalcoholic fatty liver disease (NAFLD) ranges from 25% in the general population [1] to well over 50% in persons with diabetes [2]. NAFLD refers to a spectrum of liver disease and can range from liver fat infiltration with minimal inflammation, known as simple steatosis, to nonalcoholic steatohepatitis (NASH), which is marked by inflammatory cell infiltrate with hepatocyte ballooning with or without fibrosis, to end stage liver disease or cirrhosis. Liver fibrosis ranges in severity from stage 1, with mild fibrosis, to stage 4, which is characterized by bridging fibrosis. Approximately 25–30% of individuals with NAFLD have NASH [3], and individuals with NASH, particularly those with liver fibrosis, can progress to cirrhosis and hepatocellular carcinoma [4, 5]. However, beyond liver-related morbidity and mortality, individuals with NASH have worse atherogenic dyslipidemia and insulin resistance, which results in an increased risk of cardiovascular disease and cardiovascular-related mortality [6].
Therapies for NAFLD and NASH are needed to combat these important and highly prevalent conditions. Fortunately, NAFLD and NASH are reversible, as is liver fibrosis, especially before cirrhosis is well-established. The cornerstone of therapy is weight loss, as a reduction in body mass index (BMI) of 5% is associated with a 25% relative reduction in liver fat as measured by magnetic resonance imaging (MRI) [7]. In this review, we will summarize biomarkers used to diagnose and monitor individuals with NAFLD. We will also discuss various treatment strategies, including dietary interventions, exercise, and pharmacologic options, as well as the effectiveness of bariatric surgery.
Determining NAFLD and NASH
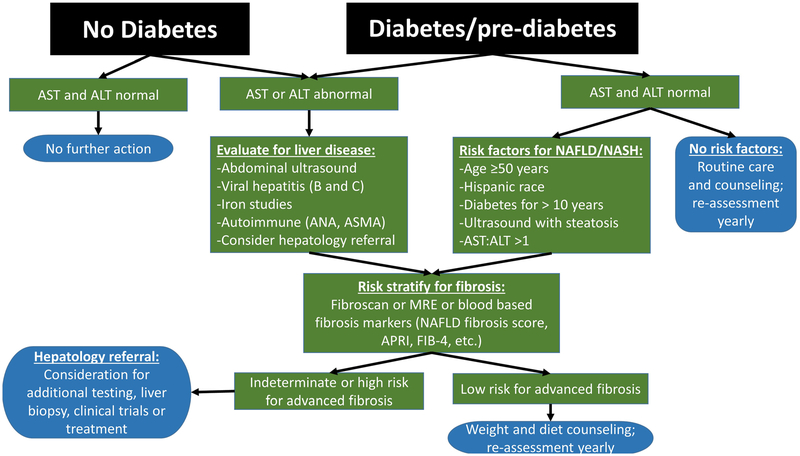
There are several mechanisms for identifying individuals with NAFLD and NASH (Table 1). Liver biopsy is the gold standard for diagnosis; however, it is invasive and costly, so it is not optimal and it is not practical to use on a large scale. Assessing change or improvement in NAFLD and NASH requires that parameters be assessed serially. Various proxies can be used to identify NAFLD, as outlined in Table 1, including traditional imaging techniques—ultrasound, computed tomography (CT), or MRI—or more specialized imaging tests including ultrasound-based transient elastography (FibroScan) or magnetic resonance spectroscopy (MRS), magnetic resonance protein density fat fraction, or magnetic resonance elastography (MRE). Blood-based biomarkers, such as elevated aspartate or alanine aminotransferase levels (AST or ALT), and/or gamma-glutamyltransferase (GGT), or diagnostic models, such as the NAFLD Fibrosis Score, which incorporates multiple blood-based and clinical parameters, are also useful, though have lower sensitivity and specificity compared with imaging-based tools. NASH by definition requires the presence of inflammation on liver histology and currently cannot be reliably diagnosed without the use of liver biopsy to identify inflammation. Pathologically, NAFLD is frequently staged by the NAFLD activity score (NAS), which grades steatosis (0–3), lobular inflammation (0–3), and liver cell injury (0–2); a NAS greater ≥ 5 is consistent with NASH. A practical algorithm for identifying patients in the clinic with NASH and at risk for fibrosis is proposed in Fig. 1.
Table 1.
Methods to diagnosis advanced fibrosis (stage 3 or greater) in NAFLD
| Components | PPV | NPV | |
|---|---|---|---|
| Liver biopsy | Liver tissue | Gold standard (but sampling error exists) | |
| FibroScan (transient elastography) [8] | Ultrasound shear wave velocity | 90 | 80 |
| MR elastography [9] | MR-based wave propagation in tissue | 94 | 95 |
| FIB-4 index [10] | Age | 61 | 92 |
| AST, ALT | |||
| Platelets | |||
| APR1 [10] | AST | 47 | 91 |
| Platelets | |||
| FibroTest (FibroSure) [10] | Age | 62 | 89 |
| Sex | |||
| Bilirubin | |||
| GGT | |||
| Apolipoprotein A1 | |||
| Alpha-2-macroglobulin | |||
| Haptoglobin | |||
| NAFLD fibrosis score [11] | Age | 82 | 88 |
| BMI | |||
| Diabetes | |||
| AST/ALT | |||
| Platelets | |||
| Albumin | |||
| Hepascore [10] | Age | 57 | 92 |
| Sex | |||
| Bilirubin | |||
| GGT | |||
| Hyaluronic Acid | |||
| Alpha-2-macroglobulin | |||
| BARD [10] | BMI | 37 | 87 |
| Diabetes | |||
| AST/ALT | |||
PPV positive predictive value, NPV negative predictive value, MR magnetic resonance, FIB-4 fibrosis-4, AST aspartate aminotransferase, ALT alanine aminotransferase, APRI aspartate aminotransferase to platelet ratio index, GGT gamma-glutamyl transferase, BMI body mass index
Fig. 1.
Treatment algorithm for nonalcoholic fatty liver disease. This treatment algorithm shows a practical approach to be used clinically to identify patients at highest risk for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and fibrosis. AST, aspartate aminotransferase; ALT, alanine aminotransferase; ANA, anti-nuclear antibody, ASMA, anti-smooth muscle antibody; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; MRE, magnetic resonance elastography; APRI, AST to platelet ratio index; FIB-4, fibrosis-4 index for liver fibrosis
Dietary Interventions
Dietary modifications are a key part of the initial approach to any patient with NAFLD or NASH. A 52-week lifestyle intervention consisting of a 750 kcal/day calorie deficit diet was tested in 261 individuals with NASH and paired liver biopsies to measure the impact of a hypocaloric diet on histologic features of NASH [12]. In this study, subjects with ≥ 5% weight loss had a greater reduction in NAS compared with those with < 5% weight loss. Subjects with ≥ 10% weight loss all had reductions in NAS, while 90% had resolution of NASH and 45% showed regression of fibrosis; of note, only 10% of participants achieved this degree of weight loss [12]. A systematic review of 23 studies involving lifestyle interventions for NAFLD demonstrated similar results. Across studies, participants who achieved an average weight reduction of 4–10% consistently demonstrated improvements in measures of liver fat or serum transaminase levels [13].
A number of studies have investigated the effect of unsaturated fatty acids on the progression of NAFLD. Polyunsaturated fatty acids (PUFA) have been postulated to mitigate NAFLD and NASH via several mechanisms: activation of hepatic peroxisome proliferator-activated receptor alpha, promoting fatty acid oxidation [14]; downregulation of sterol regulatory element binding protein-1, reducing lipogenesis [15]; and decreasing production of inflammatory mediators including, IL-6 and tumor necrosis factor [16, 17]. A meta-analysis investigating the effects of omega-3 PUFA on NAFLD suggested that omega-3 PUFA supplementation may decrease liver fat based on imaging and histologic parameters [18]. Several randomized controlled trials, however, demonstrated conflicting results. A 2018 meta-analysis of 18 studies by Yan et al., involving 1424 patients, found that omega-3 PUFA supplementation improved liver fat content on imaging as well as liver biochemical tests, glucose, and insulin resistance [19]. In a year-long randomized, placebo-controlled trial of omega-3 PUFA for NAFLD in which 34 subjects completed the study, there was no significant difference between groups with regard to the primary endpoint of NAS reduction [20], though the omega-3 PUFA group showed a significant decrease in liver fat on MRI [20]. Similarly, a multicenter, randomized, placebo-controlled trial of ethyleicosapentanoic acid for NASH failed to demonstrate any histologic improvement after 12 months of treatment [21]. These findings were confirmed in another similar study [22]. Overall, despite some evidence of liver fat improvement on imaging, without histologic improvement, these trials demonstrate a lack of sufficient evidence to recommend PUFA supplementation in patients with NAFLD.
Carbohydrate intake may be associated with NAFLD progression. A cross-sectional analysis in the Framingham Heart Study demonstrated a positive association between sugar-sweetened beverage consumption and liver fat as measured by CT after adjusting for multiple covariates [23]. Additionally, high fructose consumption has been associated with increased histologic markers of hepatic fibrosis [24]; however, in a systematic review and meta-analysis of controlled feeding trials, isocaloric exchange of fructose for other carbohydrates did not induce NAFLD [25]. Several studies have investigated the effect of low-carbohydrate diets on NAFLD, with inconclusive results. A randomized controlled trial of 170 individuals with obesity compared the effect of a hypocaloric low-carbohydrate diet versus a low-fat diet on weight loss and intrahepatic triglyceride (IHTG) content by MRS. After 6 months, there was no difference in weight loss or IHTG content between groups [26]. In a trial that randomized 56 subjects with biopsy-proven NAFLD to low-fat diet plus exercise, low-carbohydrate diet plus exercise, and exercise alone, NAS decreased in all groups without significant differences between diets [27]. Overall, based on these studies, it is uncertain whether improvement in liver fat is related to weight loss or reduction in specific macronutrients; further head-to-head comparison trials are needed.
High-protein diets are associated with improved weight maintenance [28] and may be beneficial in NAFLD. A prospective study examined changes in liver fat and inflammation in 37 subjects assigned to isocaloric plant-based or animal-based high-protein diets. Within 6 weeks, both diet groups demonstrated a 36–48% reduction in liver fat as measured by MRS [29]. Of note, these reductions were independent of changes in body weight and both groups showed decreased markers of systemic inflammation and insulin resistance [29]. Another prospective observational study examined the effect of a hypocaloric high-fiber, high-protein diet on hepatic fat content as measured by transient elastography in 60 patients with hepatic steatosis [30]. Results demonstrated a significant reduction liver fat content, as measured by the controlled attenuation parameter. Of note, liver stiffness, a surrogate marker of liver fibrosis, GGT, and serum lipids also significantly decreased [30]. Additional studies are needed to evaluate the long-term effects of high-protein diets on NAFLD.
Over the past several years, emerging evidence suggests the Mediterranean diet, which is rich in monounsaturated fatty acids and PUFA as well as aromatic compounds such as polyphenols [16], may be beneficial in NAFLD. Clinical practice guidelines for the management of NAFLD, supported by European liver and diabetes societies, named the Mediterranean diet as the diet of choice in individuals with NAFLD [31]. In a Spanish cross-sectional study of 82 subjects with biopsy-proven NAFLD, higher adherence to the Mediterranean diet was associated with a lower likelihood of NASH [32]; a similar study in Greece showed a 36% lower likelihood of NASH in patients who were adherent to the Mediterranean diet [33]. Longitudinal studies have shown comparable results. A randomized controlled crossover trial comparing the Mediterranean diet with a low-fat diet in 12 subjects with biopsy-proven NAFLD showed improved insulin sensitivity and reduced hepatic steatosis on MRS after 6 weeks in the Mediterranean diet group [34]. In another randomized study of 98 subjects, individuals randomized to the Mediterranean diet for 6 months had reduced hepatic steatosis on ultrasound compared with control [35•]. Though these results are encouraging, additional long-term randomized trials with larger sample sizes are needed to confirm the beneficial effects of the Mediterranean diet on NAFLD. Further studies demonstrating histologic improvement in NAFLD would also strengthen the argument the Mediterranean diet as a treatment for NAFLD.
Taken together, the available evidence suggests that weight loss of at least 5% can improve NAFLD, with a weight loss of 7–10% having some impact on NASH and fibrosis. Specific diets such as carbohydrate restriction and the Mediterranean diet may be efficient methods of reducing caloric intake and have shown promising results in observational studies and small randomized trials. Larger studies demonstrating benefits of these specific diets on NAFLD and liver-related outcomes are needed.
Physical Activity and Exercise Interventions
Physical activity and exercise are also important in the treatment of NAFLD. Exercise decreases hepatic free fatty acid uptake and decreases IHTG [36]. Aerobic exercise activates lipolysis via upregulating uncoupling protein 1 and peroxisome proliferator-activated receptor-gamma pathways to improve liver fat [37]. Also, exercise may alter adipokine levels. Specifically, resistance training will result in an increase in type II muscle fibers which alters myokine levels and activates glucose transporter 4, which may improve insulin sensitivity. Additional mechanisms have also been proposed [37].
Different types of exercise (aerobic vs resistance vs interval training) and doses of exercise (frequency, duration, intensity) may have varying impacts on improving liver fat and fibrosis, so investigations have made an effort to determine what constitutes sufficient and optimal exercise in order to reduce liver fat content, reverse NASH, or improve fibrosis. Current American Association for the Study of Liver Diseases guidelines note that exercise on its own could improve hepatic steatosis but the effects on NASH are less clear [38]. The impact of exercise on NAFLD is difficult to study as most studies have both dietary and exercise components. A recent meta-analysis showed that, even in the absence of weight loss, exercise leads to a 20–30% relative risk reduction in IHTG, though the absolute risk reduction was relatively small [39].
The majority of studies on the type of exercise effective at reducing liver fat have involved aerobic exercise rather than resistance or interval training. In one investigation, 82 patients with ultrasound-defined NAFLD were randomized for 3 months of resistance training or stretching exercises. Those in the resistance training group experienced a significantly greater improvement in ultrasound-defined liver fat [40]. A systematic review which included 13 aerobic and 4 resistance protocols for NAFLD showed both types of exercise required similar durations (median 45 min, 3 times per week, for 12 weeks) but the resistance protocols required less energy consumption and less maximal oxygen consumption to achieve liver fat improvement (as measured by imaging or liver biopsy) [41]. Both aerobic exercise and resistance exercise improved liver fat, though resistance training alone was generally less effective than aerobic exercise in some studies [42].
An alternative type of exercise is known as high-intensity interval training (HIIT), in which bursts of high-intensity exercise are followed by brief recovery periods. In a study from the UK, 23 subjects were randomized to either HIITor control for 12 weeks. Those in the HIIT group had a significant decrement in liver fat, as measured by MRS, as well as improvement in AST [43]. However, a meta-analysis which included 12 randomized controlled studies showed that HIIT may be less beneficial compared with a moderate-to-high volume of continuous moderate exercise.
Little is known regarding the optimal frequency, duration, or intensity of physical activity to improve NAFLD. Participants in the Framingham Heart Study who participated in ≥ 150 min/week of moderate-to-vigorous physical activity had less CT-defined hepatic steatosis, after accounting for body mass index [44]. Another study noted a possible dose response with the most improvement in liver fat and markers of inflammation with ≥ 250 min per week of moderate-tovigorous physical activity [45], though this has not been shown in all studies [46]. Also, it is not clear if more vigorous activity yields additional benefits over moderate activity for improving hepatic steatosis. There was no difference in hepatic fat reduction in a randomized study of 48 subjects with NAFLD treated with aerobic exercise programs including low-to-moderate intensity low- or high-volume activity, vigorous intensity low-volume activity, or placebo [46]. A randomized controlled study based in China showed that a group randomized to vigorous exercise did not have significantly greater reductions in MRS-based IHTG, compared with those who were randomized to moderate exercise over a 12-month period [47]. Whereas many studies have a relatively short defined intervention period, which may be less realistic in a real-world setting, a large Korean-based observational study showed that even modest amounts of exercise, such as 1–2 times weekly for at least 10 min, were associated with improvement of ultrasound-defined NAFLD and a reduced incidence of NAFLD over a 5-year period [48•].
In summary, even modest amounts of moderate exercise, of different types, whether aerobic or resistance training, can improve liver fat and should be part of the therapy for NAFLD. More studies are needed to determine the optimal dose of exercise to improve NAFLD. Additionally, more studies are needed to determine if exercise is effective for treatment of NASH and fibrosis as the vast majority of studies have not investigated the effect of exercise on more advanced NAFLD phenotypes.
Pharmacologic Therapy
Currently, pharmacologic based-therapies for NAFLD are limited, though this is an area of active investigation with many ongoing clinical trials. Weight loss can improve NAFLD, NASH, and fibrosis, but medications that improve weight loss such as phentermine and topiramate have not been studied with regard to their effect on NAFLD specifically. Vitamin E, an anti-inflammatory medication, has been shown to improve histologic features of NASH compared with placebo in a randomized study of individuals with NAFLD and without diabetes [49••]; these findings were confirmed in a recent meta-analysis which also showed improvements in histologic features of NASH, but not fibrosis [50]. Vitamin E is recommended for patients with biopsy-proven NASH without diabetes or decompensated cirrhosis [51]. However, given the concerns of high-dose Vitamin E increasing all-cause mortality [52] and the risk of prostate cancer [53], Vitamin E is only used after an informed discussion with patients and its use for NASH is limited in the setting of a personal or family history of prostate cancer. Pioglitazone, when used in patients without diabetes in a randomized controlled trial, did not improve NASH but improved AST and ALT elevations, as well as steatosis and lobular inflammation [49••].
Some studies have investigated the effect of antidiabetic drugs on NAFLD among individuals with concomitant diabetes. Pioglitazone, used for 18 months in individuals with diabetes in conjunction with a hypocaloric diet, has been shown to reduce NAS and mean fibrosis score significantly more than placebo [54]. Pioglitazone should be considered as treatment in patients with biopsy-proven NASH, regardless of diabetes status [51]. However, pioglitazone can cause weight gain [55] and increased heart failure events [56], so should be used with caution. GLP-1 agonists (i.e., liraglutide) have been of particular interest recently, though data are limited. A randomized controlled trial including individuals with diabetes and ultrasound-defined NAFLD showed that those treated with liraglutide for 24 weeks had the largest reduction in intrahepatic fat on ultrasound, compared with those treated with metformin or a sulfonylurea [57•]. Additional studies using histologic endpoints are needed before GLP-1 agonists can be recommended for treatment of NAFLD.
Finally, statins have also been proposed as a therapy that may improve NAFLD, given that the use of statins is associated with a lower risk of progression of fibrosis in patients with other etiologies of liver disease [58]. While statins may be indicated for many patients with NAFLD due to the comorbidities of metabolic syndrome—including hypercholesterolemia—clinicians may be concerned to prescribe them due to concern for hepatotoxicity. A 2015 prospective cohort study followed 20 patients with biopsy-proven NASH who were given rosuvastatin and lifestyle advice [59]. Despite no change in BMI, the authors found resolution of NASH upon repeat liver biopsy in 19 of the patients, indicating a potential beneficial effect of statin therapy. However, a 2017 post hoc analysis of a trial involving 101 patients with biopsy-proven NASH found no histologic improvement for the patients who were started on a statin (84% were on simvastatin, 11% on rosuvastatin) during the 36 months of the study [60]. Overall, studies of statins in NAFLD have been small and over relatively short time periods. However, despite these mixed results, no studies have demonstrated that statins are harmful in patients with NAFLD or NASH so statins remain an important therapy in patients with hyperlipidemia and cardiovascular disease.
Surgical Intervention
Whereas weight loss through lifestyle interventions remains the mainstay of NAFLD therapy [61], the effects of weight loss surgery, including sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), and adjustable gastric banding (AGB), on NAFLD have been described in a number of studies. In a large 2014 study, Caiazzo et al. followed 1236 patients who had undergone RYGB or AGB [62]. Those who underwent RYGB had a 26% weight loss compared with the AGB group with a 21% weight loss. Though NAFLD improved with both surgeries, there was greater improvement in the patients who underwent RYGB, despite greater baseline BMI and more severe NAFLD, compared with the AGB group at 1 and 5 years. A prospective observational study of 52 participants considered improvements in liver enzymes, serum high density lipoprotein (HDL) levels, and ultrasound findings of liver fat before and 1 year after laparoscopic SG [63]. One year after laparoscopic SG, 90% of individuals with baseline NAFLD achieved NAFLD resolution on follow-up ultrasound and the follow-up reduction in liver fat correlated with improvement in HDL cholesterol. A 2017 randomized controlled trial by Kalinowski et al. compared SG with RYGB, with the original aim of considering effects on glycemic control [64]. A secondary analysis of the same data used intraoperative liver biopsies to identify those patients with histological NAFLD and NASH at baseline [65]. Seventy-two patients were included, with 36 randomized to each procedure. Those that underwent SG but not RYGB showed significant improvements in AST, ALT, and GGT at 12 months, which may indicate a greater benefit on liver fat and/or inflammation for SG, though follow-up histology was not obtained. Additional studies are needed to determine the optimal surgical strategies to improve NAFLD phenotypes and to investigate the cost-effectiveness.
Conclusion
Future directions in the investigation of therapies for NAFLD must include larger, well-designed studies that carefully consider co-variables such as change in BMI and weight loss. Additionally, strategies must be translated to real-world settings beyond the contained setting of clinical trials. Without noninvasive tools to diagnose and monitor NASH, progress is limited. Fortunately, noninvasive techniques to diagnosis and monitor liver fibrosis are available, though more studies are needed to define their use and determine if improvements in ultrasound-based or MRI-based fibrosis assessments correlate with clinical outcomes. Pharmacologic therapies that improve NAFLD throughout the spectrum of disease are needed.
Grant Support
Dr. Long is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases K23 DK113252, the Boston University School of Medicine Department of Medicine Career Investment Award, and the Boston University Clinical Translational Science Institute UL1 TR001430.
Michelle T. Long has received research funding from Echosens Corporation.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- GLP-1
Glucagon-like peptide-1
- BMI
Body mass index
- MRI
Magnetic resonance imaging
- CT
Computed tomography
- MRS
Magnetic resonance spectroscopy
- MRE
Magnetic resonance elastography
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- GGT
Gamma-glutamyltransferase
- NAS
NAFLD activity score
- PUFA
Polyunsaturated fatty acids
- IHTG
Intrahepatic triglycerides
- HIIT
High-intensity interval training
- SG
Sleeve gastrectomy
- RYGB
Roux-en-Y gastric bypass
- AGB
Adjustable gastric banding
Footnotes
Conflict of Interest Katherine T. Brunner declares that she has no conflict of interest.
Cameron J. Henneberg declares that he has no conflict of interest.
Robert M. Wilechansky declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100:2231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313:2263–73. [DOI] [PubMed] [Google Scholar]
- 4.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155:443–457.e17. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149: 389–397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–53. [DOI] [PubMed] [Google Scholar]
- 7.Patel NS, Doycheva I, Peterson MR, Hooker J, Kisselva T, Schnabl B, et al. Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2015;13:561–568.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foucher J. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, George J, Bugianesi E, Rossi E, Boer WBD, van der Poorten D, et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26:1536–43. [DOI] [PubMed] [Google Scholar]
- 11.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- 12.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378.e5 quiz e14–15. [DOI] [PubMed] [Google Scholar]
- 13.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–66. [DOI] [PubMed] [Google Scholar]
- 14.Arendt BM, Comelli EM, Ma DWL, Lou W, Teterina A, Kim T, et al. Altered hepatic gene expression in nonalcoholic fatty liver disease is associated with lower hepatic n-3 and n-6 polyunsaturated fatty acids. Hepatology. 2015;61:1565–78. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi Y, Yahagi N, Izumida Y, Nishi M, Kubota M, Teraoka Y, et al. Polyunsaturated fatty acids selectively suppress sterol regulatory element-binding protein-1 through proteolytic processing and autoloop regulatory circuit. J Biol Chem. 2010;285:11681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24:2083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int. 2017;37:936–49. [DOI] [PubMed] [Google Scholar]
- 18.Parker HM, Johnson NA, Burdon CA, Cohn JS, O’Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56: 944–51. [DOI] [PubMed] [Google Scholar]
- 19.Yan J-H, Guan B-J, Gao H-Y, Peng X-E. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97:e12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol. 2015;62:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M, EPE-A Study Group. No significant effects of ethyleicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377–384.e1. [DOI] [PubMed] [Google Scholar]
- 22.Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the WELCOME* study. Hepatology. 2014;60:1211–21. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Fox CS, Jacques PF, Speliotes EK, Hoffmann U, Smith CE, et al. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol. 2015;63:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatol Baltim Md. 2010;51:1961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haufe S, Engeli S, Kast P, Böhnke J, Utz W, Haas V, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatol Baltim Md. 2011;53:1504–14. [DOI] [PubMed] [Google Scholar]
- 27.Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Ther Adv Gastroenterol. 2013;6:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen TM, Dalskov S-M, van Baak M, Jebb SA, Papadaki A, Pfeiffer AFH, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markova M, Pivovarova O, Hornemann S, Sucher S, Frahnow T, Wegner K, et al. Isocaloric diets high in animal or plant protein reduce liver fat and inflammation in individuals with type 2 diabetes. Gastroenterology. 2017;152:571–585.e8. [DOI] [PubMed] [Google Scholar]
- 30.Arslanow A, Teutsch M, Walle H, Grünhage F, Lammert F, Stokes CS. Short-term hypocaloric high-fiber and high-protein diet improves hepatic steatosis assessed by controlled attenuation parameter. Clin Transl Gastroenterol. 2016;7:e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of nonalcoholic fatty liver disease. Obes Facts. 2016;9:65–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aller R, Izaola O, de la Fuente B, De Luis Román DA. Mediterranean diet is associated with liver histology in patients with non alcoholic fatty liver disease. Nutr Hosp. 2015;32:2518–24. [DOI] [PubMed] [Google Scholar]
- 33.Kontogianni MD, Tileli N, Margariti A, Georgoulis M, Deutsch M, Tiniakos D, et al. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutrition. 2014;33:678–83. [DOI] [PubMed] [Google Scholar]
- 34.Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–43. [DOI] [PubMed] [Google Scholar]
- 35.•.Misciagna G, Del Pilar DM, Caramia DV, Bonfiglio C, Franco I, Noviello MR, et al. Effect of a low glycemic index Mediterranean diet on non-alcoholic fatty liver disease. A randomized controlled clinici trial. J Nutr Health Aging. 2017;21:404–12 [DOI] [PubMed] [Google Scholar]; This randomized study showed that those randomized to the Mediterranean diet for 6 months had reduced hepatic steatosis on ultrasound compared with the control group.
- 36.Marchesini G, Petta S, Grave RD. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology. 2016;63:2032–43. [DOI] [PubMed] [Google Scholar]
- 37.Guo R, Liong EC, So KF, Fung M-L, Tipoe GL. Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in nonalcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2015;14:139–44. [DOI] [PubMed] [Google Scholar]
- 38.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 39.Exercise and NAFLD: is it worth the effort? - Schweitzer - 2017. - Hepatology - Wiley Online Library [Internet]. [cited 2019 Feb 7];Available from: https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.29356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zelber-Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol: WJG. 2014;20:4382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. Hepatol. 2017;66:142–52. [DOI] [PubMed] [Google Scholar]
- 42.Keating SE, Hackett DA, Parker HM, Way KL, O’Connor HT, Sainsbury A, et al. Effect of resistance training on liver fat and visceral adiposity in adults with obesity: a randomized controlled trial. Hepatol Res Off J Jpn Soc Hepatol. 2017;47:622–31. [DOI] [PubMed] [Google Scholar]
- 43.Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci. 2015;129: 1097–105. [DOI] [PubMed] [Google Scholar]
- 44.Long MT, Pedley A, Massaro JM, Hoffmann U, Esliger DW, Vasan RS, et al. Hepatic steatosis is associated with lower levels of physical activity measured via accelerometry. Obes Silver Spring Md. 2015;23:1259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh S, Shida T, Yamagishi K, Tanaka K, So R, Tsujimoto T, et al. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology. 2015;61:1205–15. [DOI] [PubMed] [Google Scholar]
- 46.Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63:174–82. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H-J, He J, Pan L-L, Ma Z-M, Han C-K, Chen C-S, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: a randomized clinical trial. JAMA Intern Med. 2016;176:1074–82. [DOI] [PubMed] [Google Scholar]
- 48.•.Sung K-C, Ryu S, Lee J-Y, Kim J-Y, Wild SH, Byrne CD. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J Hepatol. 2016;65:791–7 [DOI] [PubMed] [Google Scholar]; This large Korean-based observational study showed that even modest amounts of exercise, as examined over a 5-year follow-up period, were associated with improvement in and reduced incidence of NAFLD.
- 49.••.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85 [DOI] [PMC free article] [PubMed] [Google Scholar]; Landmark trial in nondiabetic patients showing that Vitamin E treatment improved NASH. Pioglitazone improved AST and ALT elevations, steatosis, and lobular inflammation, but not NASH.
- 50.Said A, Akhter A. Meta-analysis of randomized controlled trials of pharmacologic agents in non-alcoholic steatohepatitis. Ann Hepatol 2017;16:0–0. [DOI] [PubMed] [Google Scholar]
- 51.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases: hepatology, Vol. XX, no. X, 2017. Hepatology. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 52.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37. [DOI] [PubMed] [Google Scholar]
- 53.Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305–15. [DOI] [PubMed] [Google Scholar]
- 55.Aghamohammadzadeh N, Niafar M, Dalir Abdolahinia E, Najafipour F, Mohamadzadeh Gharebaghi S, Adabi K, et al. The effect of pioglitazone on weight, lipid profile and liver enzymes in type 2 diabetic patients. Ther Adv Endocrinol Metab. 2015;6:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erdmann E, Charbonnel B, Wilcox RG, Skene AM, Massi-Benedetti M, Yates J, et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08). Diabetes Care. 2007;30:2773–8. [DOI] [PubMed] [Google Scholar]
- 57.•.Feng W, Gao C, Bi Y, Wu M, Li P, Shen S, et al. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease: 比较格列齐特、利拉 鲁肽和二甲双胍对糖尿病合并非酒精性脂肪肝的影响的随机研究. J Diabetes. 2017;9:800–9 [DOI] [PubMed] [Google Scholar]; This randomized trial comparing several diabetes medications over 24 weeks show that those treated with liraglutide had the largest reduction in intrahepatic fat on ultrasound, compared with those treated with metformin or glicazide.
- 58.Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kargiotis K. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J Gastroenterol. 2015;21:7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Hecht J, Tio F, et al. Liver safety of statins in prediabetes or T2DM and nonalcoholic steatohepatitis: post hoc analysis of a randomized trial. J Clin Endocrinol Metab. 2017;102:2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwenger KJ. Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caiazzo R, Lassailly G, Leteurtre E, Baud G, Verkindt H, Raverdy V, et al. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg. 2014;260:893–9. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz-Tovar J, Alsina ME, Alpera MR, OBELCHE Group. Improvement of nonalcoholic fatty liver disease in morbidly obese patients after sleeve gastrectomy: association of ultrasonographic findings with lipid profile and liver enzymes. Acta Chir Belg. 2017;117:363–9. [DOI] [PubMed] [Google Scholar]
- 64.Kalinowski P, Paluszkiewicz R, Wróblewski T, Remiszewski P, Grodzicki M, Bartoszewicz Z, et al. Ghrelin, leptin, and glycemic control after sleeve gastrectomy versus Roux-en-Y gastric bypass—results of a randomized clinical trial. Surg Obes Relat Dis. 2017;13:181–8. [DOI] [PubMed] [Google Scholar]
- 65.Kalinowski P, Paluszkiewicz R, Ziarkiewicz-Wróblewska B, Wróblewski T, Remiszewski P, Grodzicki M, et al. Liver function in patients with nonalcoholic fatty liver disease randomized to Rouxen-Y gastric bypass versus sleeve gastrectomy: a secondary analysis of a randomized clinical trial. Ann Surg. 2017;266:738–45. [DOI] [PubMed] [Google Scholar]