Abstract
Purpose:
Utilization of stereotactic body radiation therapy (SBRT) for treatment of localized prostate cancer is increasing. Guidelines and payers variably support the use of prostate SBRT. We therefore sought to systematically analyze biochemical recurrence-free survival (bRFS), physician-reported toxicity, and patient-reported outcomes after prostate SBRT.
Methods and Materials:
A systematic search leveraging Medline via PubMed and EMBASE for original articles published between January 1990 and January 2018 was performed. This was supplemented by abstracts with sufficient extractable data from January 2013 to March 2018. All prospective series assessing curative-intent prostate SBRT for localized prostate cancer reporting bRFS, physician-reported toxicity, and patient-reported quality of life with a minimum of 1-year follow-up were included. The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Meta-analyses were performed with random-effect modeling. Extent of heterogeneity between studies was determined by the I2 and Cochran’s Q tests. Meta-regression was performed using Hartung—Knapp methods.
Results:
Thirty-eight unique prospective series were identified comprising 6116 patients. Median follow-up was 39 months across all patients (range, 12-115 months). Ninety-two percent, 78%, and 38% of studies included low, intermediate, and high-risk patients. Overall, 5- and 7-year bRFS rates were 95.3% (95% confidence interval [CI], 91.3%-97.5%) and 93.7% (95% CI, 91.4%-95.5%), respectively. Estimated late grade ≥3 genitourinary and gastrointestinal toxicity rates were 2.0% (95% CI, 1.4%-2.8%) and 1.1% (95% CI, 0.6%-2.0%), respectively. By 2 years post-SBRT, Expanded Prostate Cancer Index Composite urinary and bowel domain scores returned to baseline. Increasing dose of SBRT was associated with improved biochemical control (P = .018) but worse late grade ≥3 GU toxicity (P = .014).
Conclusions:
Prostate SBRT has substantial prospective evidence supporting its use, with favorable tumor control, patient-reported quality of life, and levels of toxicity demonstrated. SBRT has sufficient evidence to be supported as a standard treatment option for localized prostate cancer while ongoing trials assess its potential superiority.
Summary
Stereotactic body radiation therapy (SBRT) is increasingly being utilized in the treatment of localized prostate cancer. We performed a systematic review and meta-analysis of over 6000 men treated with prostate SBRT on prospective studies, assessing biochemical disease control, patient-reported quality of life, and acute and late treatment-related toxicity. We demonstrate that prostate SBRT provides excellent disease control, favorable patient-reported quality of life and results in minimal serious acute or late toxicity.
Introduction
Prostate cancer is the most common cancer diagnosed in men in the United States, and as such, it contributes greatly to the national health care expenditure on cancer care.1,2 External beam radiation therapy is an effective curative treatment option for men with localized prostate cancer and has traditionally been delivered with small daily doses of radiation therapy over 8 to 9 weeks. A fundamental reason that radiation therapy was historically delivered in small doses over many fractions was the inability to spatially spare normal tissues adjacent to the high-dose target volume. Normal tissues generally are better able to tolerate smaller doses of radiation delivered over many weeks, and thus conventional fractionation results in a therapeutic window whereby toxicity to normal tissue is acceptable while still providing tumor control. A serious drawback to this treatment approach is that the high number of fractions increases health care costs compared with shorter treatment durations and also creates burdens and logistical challenges for patients.3
Prostate cancer is also radiobiologically unique in that it has a low alpha-to-beta ratio, which suggests that the therapeutic ratio should favor hypofractionation (larger doses per fraction with fewer total fractions).4 Fortunately, imaging and treatment technologies have markedly improved over the past 3 decades and now allow the ability to substantially reduce doses delivered to the rectum and bladder when treating prostate cancer. This has provided the opportunity to study varying degrees of hypofractionated treatment regimens and has inspired numerous clinical trials assessing the optimal dose per fraction when treating prostate cancer with radiation therapy.5–12 These trials have demonstrated that moderate hypofractionation (eg, 20 treatments) has comparable efficacy and toxicity data to the conventional ≥37 treatments of radiation therapy.5–12 Stereotactic body radiation therapy (SBRT) represents an extreme form of hypofractionation in which treatment is usually delivered in 4-7 fractions. Development and optimization of this ultrahypofractionation technique over the last 20 years has resulted in incorporation of SBRT into routine clinical practice, and SBRT is now a standard-of-care treatment option for many tumors of the lung, brain, spine, liver, and pancreas.
In prostate cancer, there have been multiple phase 1, 2, and even phase 3 trials assessing SBRT, and these demonstrate a similar toxicity profile and noninferior disease control compared with conventionally fractionated radiation therapy.13–47 Despite this recent surge of data to support SBRT, select organizations have yet to update their guidelines to support the adoption of SBRT in the treatment of prostate cancer. For example, the American Society for Radiation Oncology, American Urological Association, and American Society of Clinical Oncology released their guidelines for the treatment of prostate cancer with hypo-fractionated radiation therapy and stated there is “low” quality of evidence for SBRT for intermediate and high-risk prostate cancer based on what they determined to be a paucity of prospective data,48 although they do acknowledge that SBRT may be offered for men with intermediate risk disease, with a preference for treatment as part of a clinical trial or multi-institutional registry. This inspired our group to conduct an independent systematic review and meta-analysis of all prospective studies performed and published or presented to date to comprehensively assess outcomes after prostate cancer SBRT including tumor control, toxicity, and patient-reported quality of life (QOL).
Methods and Materials
Search strategy and study selection
This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines.49 A systematic literature search was performed using the MEDLINE (via PubMed) and EMBASE electronic databases and supplemented with review of national meeting abstracts. Prostate SBRT was defined as treatment delivered over less than 10 treatments with greater than or equal to 5 Gy per fraction. Search terms included “prostate” and “cancer” and “radiation or radiotherapy” and “stereotactic or hypofractionated.” Only findings published in English between January 1990 and January 2018 were considered for inclusion from the electronic database search, and abstracts from scientific meetings were limited to those published in English from January 2010 through March 2018. Studies were only included if they clearly stated they were prospective. In addition, only studies including at least 20 patients with average follow-up greater than or equal to 12 months and reporting on biochemical recurrence-free survival (bRFS), acute or late toxicity, or QOL were considered. The Preferred Reporting Items for Systematic Review and Meta-Analyses flow diagram is shown in Figure E1 (available at https://doi.org/10.1016/j.ijrobp.2019.03.51) with records from each step independently reviewed by W.C.J and D.E.S.
Data extraction
W.C.J and D.E.S independently extracted data from the 38 identified studies.13–35,37–47,50–68 Variables extracted included study and patient-level characteristics, such as study type, phase of trial, sample size, dose per fraction, fraction number, duration of follow-up, age, clinical tumor stage, baseline prostate-specific antigen, National Comprehensive Cancer Network (NCCN)69 risk group of enrolled patients, receipt of androgen-deprivation therapy (ADT), bRFS, physician-reported toxicity, and patient-reported QOL, if reported. Individual patient data was not used. Outcomes for each endpoint were extracted, and the scales used for QOL and toxicity reporting were collected.
Endpoint definitions
Primary endpoints of the study included 5-year overall bRFS, physician-reported acute and late grade ≥3 toxicity for both genitourinary (GU) and gastrointestinal (GI) domains, and patient-reported QOL using the Expanded Prostate Cancer Index Composite (EPIC-26)70 for urinary, bowel, and sexual domain subscales.70 Secondary analyses were preplanned and included a meta-regression of the impact of covariables on bRFS and late toxicity.
Sensitivity analyses were performed for late toxicity. Because late toxicity is a time-dependent event occurring beyond 3 months post-SBRT, we performed analyses, including all studies and also restricted to studies with a minimum of 5-year median follow-up.
Statistical analysis and reporting risk of bias
For meta-analyses, both a random-effects model and a fixed-effects model were generated. The extent of heterogeneity was significant, and as such, a random-effects model was chosen and reported for all meta-analyses. Extent of heterogeneity between studies was assessed with Cochran’s Q test and an I2 test. Study weighting was based on the inverse of the variance rather than sample size because the inverse variance serves to minimize the variance of the combined effect. Meta-regression was performed using Hartung—Knapp methods to determine factors associated with bRFS and late toxicity. Covariables used in the model for bRFS included NCCN risk group, percent of cohort using ADT, and prescribed dose (expressed as biologically equivalent dose [BED] using an alpha-to-beta ratio of 2.5). Only BED was used in the late GI and GU toxicity models.
Publication bias was assessed using studies that reported 5-year bRFS and those reporting late grade ≥3 GI and GU toxicity with funnel plots and Egger’s tests. To estimate the adjusted outcome rate when correcting for publication bias, the Duval and Tweedie trim-and-fill method was used. To generate modeled bRFS curves, random-effects modeling was performed to estimate bRFS at 1, 2, 3, 5, 7, and 10 years post-SBRT. All statistical analyses were performed by using Comprehensive Meta-Analysis v3 software (Biostat, Englewood, NJ). All P values were 2-tailed with significance set at P = .05.
Results
A total of 2265 unique studies were identified using our search strategy. After screening, 158 full-text articles and abstracts were reviewed for eligibility. Thirty-eight studies met eligibility criteria for quantitative analysis, composed of 6116 patients (Table 1, Fig. E1, Table E1, available at https://doi.org/10.1016/j.ijrobp.2019.03.051). Twenty-two studies were clinical trials, of which 17 were phase 2 or 3 trials composed of 2174 patients. All but 2 studies had at least 1 associated published manuscript. The median follow-up was 39 months across all patients (range, 12-115 months), and 1386 patients on 8 studies had a median follow-up of at least 60 months. Ninety-two percent, 78%, and 38% of studies enrolled low, intermediate, and high-risk patients, respectively. Intermediate-risk disease was the most common risk group represented in studies (n = 2901). Gleason score was extractable for 4,971 patients (81%); of those, 55%, 42%, and 3% had Gleason scores of 6, 7, and 8-10, respectively. Fifteen percent of patients (n = 654) received ADT with SBRT. The most common dose per fraction was 7.25 Gy (range, 5-10 Gy), and the median fraction number was 5 (range, 4-9). Twenty-nine studies reported the timing of treatment fractions with 72% delivering treatment on consecutive days or every other day and 7% limiting fractions to 2 per week. In the remaining 21% of studies, at least half of the patients received once-weekly treatments.
Table 1.
Summary trial and patient characteristics
| Trial level n (%) or median (range) | Patient level* n (%) or mean (range) | |
|---|---|---|
| Trials and prospective series (n) | 38 | 6116 |
| Phase 1 | 1 | 45 |
| Phase 1/2 | 4 | 245 |
| Phase 2 | 15 | 1,536 |
| Phase 3 | 2 | 638 |
| Prospective | 14 | 1,472 |
| Registry | 2 | 2,180 |
| Patients (n) | 6116 | 6116 |
| Fractions | 5 (4-9) | 5 (4-9) |
| Dose per fraction | 7.25 Gy (5-10 Gy) | 7.4 Gy (5-10 Gy) |
| Average follow-up† | 30 mo (12-115 mo) | 39 mo (12-115 mo) |
| Average age‡ | 68 y (63-77 y) | 68 y (63-77 y) |
| Clinical stage, n (%)§ | ||
| T1 | 28 (88) | 67% |
| T2 | 28 (88) | 31% |
| T3 | 6 (19) | 2% |
| T4 | 1 (3) | 0% |
| Pre-SBRT PSA∥ | 6.8 ng/mL (4.7-72 ng/mL) | 7.5 ng/mL (4.7-72 ng/mL) |
| NCCN risk group (V4.2018) | ||
| Low | 34 (92) | 2745 (45) |
| Intermediate | 29 (78) | 2901 (47) |
| High | 15 (38) | 470 (8) |
| ADT receipt with SBRT¶ | 19 trials | 654 (15%) |
| Number reporting bRFS | 33 (87) | 5778 (95) |
| Number reporting acute or late toxicity | 37 (97) | 6044 (99) |
| Number reporting HRQOL | 25 (66) | 3973 (65) |
| Number using EPIC-26 | 16 (42) | 3293 (54) |
| Number reporting AUA IPSS | 13 (34) | 2399 (39) |
Abbreviations: ADT = androgen-deprivation therapy; AUA = American Urological Association; bRFS = biochemical recurrence-free survival; EPIC = expanded prostate cancer index composite; HRQOL = health-related quality of life; IPSS = International Prostate Symptom Score; NCCN = National Comprehensive Cancer Network; PSA = prostate-specific antigen; SBRT = stereotactic body radiation therapy.
Mean values weighted by number of patients per trial.
Thirty-eight series report average or median follow-up.
Thirty-four series report average age.
Thirty-two series report clinical T stage.
Thirty series report PSA.
Thirty-three series report whether or not ADT was used with SBRT.
Biochemical control
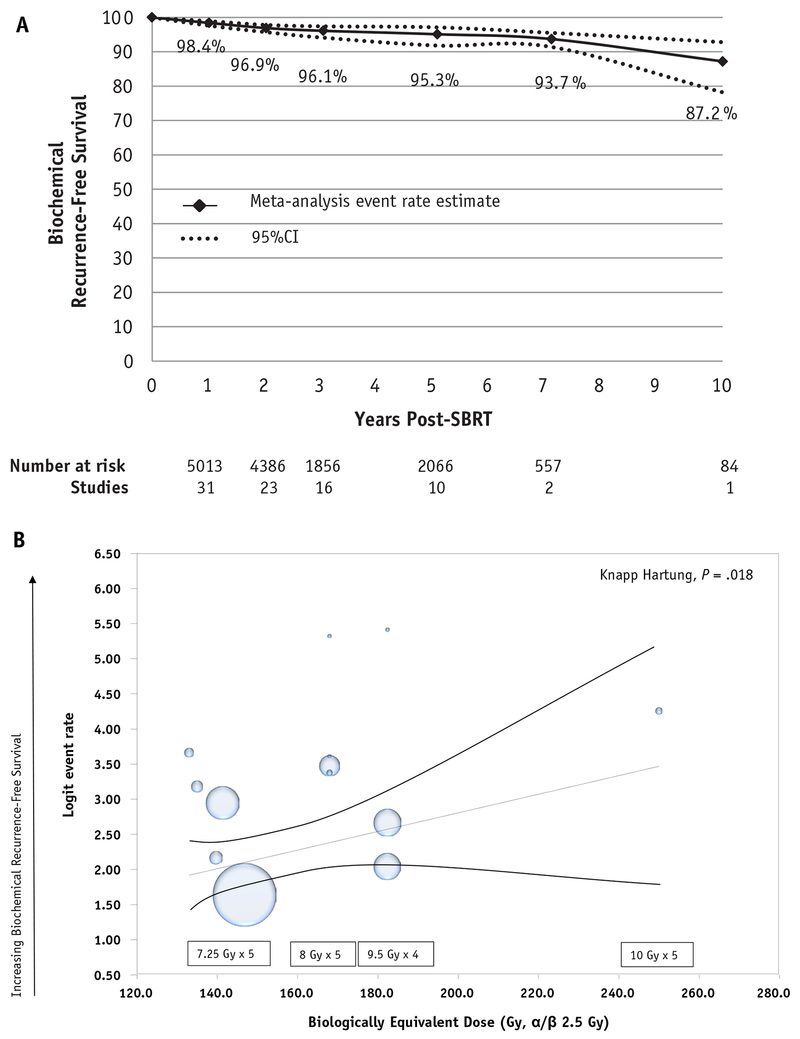
A total of 33 studies (87%) reported data on biochemical control, accounting for 95% (n = 5778) of patients in our meta-analysis. As data for bRFS were not manually extracted from Kaplan—Meier curves, reported bRFS time points were, in general, consistent with and not in excess of reported median follow-up durations for each study. Of the 14 studies (n = 2343) reporting 5-year bRFS on meta-analysis using a random-effects model the overall 5-year bRFS was 95.3% (95% CI, 91.3%-97.5%, I2 87.96, Q value 74.9, P < .001) (Fig. 1, Fig. 2A). On meta-regression it was found that essentially all variance in outcome was accounted for by the percent of patients with low-risk disease per trial and dose of SBRT used. In a metaregression model including dose (BED2.5 Gy), percent of cohort receiving ADT, and NCCN risk group, increasing dose (Fig. 2B, x axis) was significantly associated with improved bRFS (Fig. 2B, y axis) (P = .018). A part of a sensitivity analysis of our model also assessed the impact of the increasing percent of low-risk disease in a metaregression, and this too was associated with improved bRFS (P = .018), further supporting the overall validity of our model. ADT use was not significantly associated (P = .91) with bRFS.
Fig. 1.
Meta-analysis using random effects model of 5-year biochemical recurrence-free survival.
Fig. 2.
Biochemical recurrence-free survival on overall cohort over time. (A) Meta-analysis of biochemical recurrence-free survival at 1, 2, 3, 5, 7, and 10 years post—stereotactic body radiation therapy. Time points are connected for illustrative purposes. (B) Meta-regression assessing the impact of the increasing dose (represented as biologically equivalent dose with an alpha-to-beta ratio of 2.5) on 5-year biochemical recurrence-free survival.
Many of the included studies enrolled more than 1 NCCN risk group. However, not all studies provided biochemical control estimates by risk group. Studies that enrolled high-risk patients rarely separately reported bRFS outcomes by risk group, precluding reliable reporting of just the high-risk subset of patients. Of studies that reported rates by risk group, the 5-year bRFS for low and intermediate-risk disease were 96.7% (95% CI, 95.2%-97.8%) and 92.1% (89.2%-94.3%), respectively (Fig. E3A, Fig. E3B, available at https://doi.org/10.1016/j.ijrobp.2019.03.051).
Physician-reported toxicity
Acute toxicity
A total of 37 studies (97%) reported data on acute or late toxicity that contained 99% (n = 6044) of patients in our meta-analysis. Acute grade ≥3 toxicity was very rare (≤1% combined GU and GI grade ≥3 toxicity). Acute grade 3 GU toxicity occurred in 0.5% of patients, with no grade 4 events (Table E2, available at https://doi.org/10.1016/j.ijrobp.2019.03.051). Acute grade 3 GI toxicity occurred in 0.06% of patients and grade 4 in 0.03%.
Late toxicity
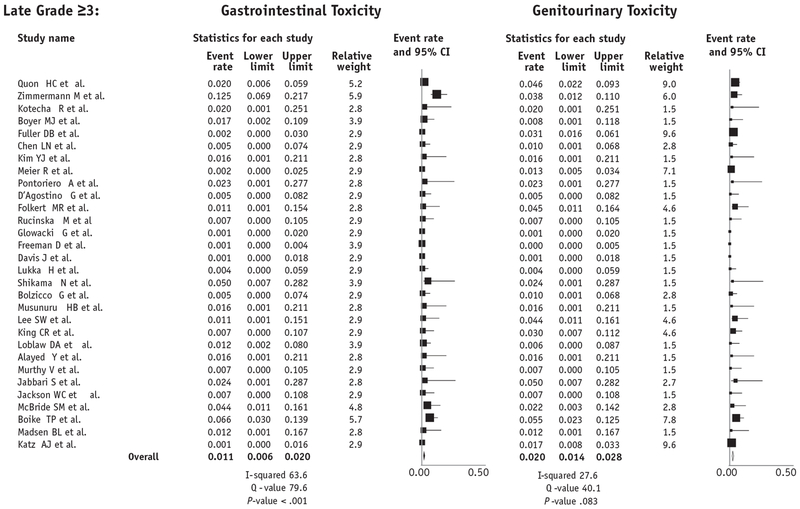
On meta-analysis using random effects modeling, late grade ≥3 GU toxicity rates are estimated to be 2.0% (95% CI, 1.4%-2.8%, I2 27.6, Q value 40.1, P = .083), and late grade ≥3 GI toxicity rates were estimated to be 1.1% (95% CI, 0.6-2.0%, I2 63.6, Q value 79.6, P < .001) (Fig. 3). As a sensitivity analysis, when restricting to studies that had only a median follow-up of at least 5 years (n = 7 studies, 1284 patients), estimated late grade ≥3 GU toxicity rates were similar (2.2% vs 2.0%), as were late grade ≥3 GI toxicities (0.8% vs 1.1%).
Fig. 3.
Late meta-analysis using random effects model of late grade ≥3 gastrointestinal and genitourinary toxicity after prostate stereotactic body radiation therapy.
A meta-regression of late toxicity and dose was performed for both late grade ≥3 GU and GI toxicity. Thirty studies comprised of 5127 patients were included in this analysis. There was a significant association with increasing dose (BED2.5) (Fig. 4A, x axis) and late grade ≥3 GU toxicity (Fig. 4A, y axis) (P = .014). In contrast, there was no significant association with dose (BED2.5) (Fig. 4B, x axis) and late grade ≥3 GI toxicity (Fig. 4B, y axis) (P = .56).
Fig. 4.
Meta-regression modeling of the impact increasing stereotactic body radiation therapy dose on (A) late genitourinary toxicity and (B) late gastrointestinal toxicity.
A summary of the frequency of all acute and late grade 2, 3, and 4 GU and GI toxicities can be found in Table E2 (available at https://doi.org/10.1016/j.ijrobp.2019.03.051).
Patient-reported QOL
A total of 25 studies (66%) reported data on patient-reported QOL containing 65% of patients in our meta-analysis (n = 3973). EPIC-2670 was assessed in 3293 patients, and the American Urology Association’s International Prostate Symptom Score71 was used by 2399 patients (Table 1). Given significant heterogeneity of reporting International Prostate Symptom Scores, it did not permit a reliable quantitative synthesis. EPIC-26 information was readily extractable for urinary, bowel, and sexual domain scales, and data were pooled (Fig. 5). Expanded Prostate Cancer Index Composite urinary and bowel scores returned to baseline by 2 years post-treatment (P = .90 and .09, respectively) and remained nonsignificantly different to scores 5 years post-SBRT (P = .50 and .80, respectively). As expected, sexual domain scores gradually decreased over time, not reaching statistical significance until 3 years post-SBRT (P = .01).
Fig. 5.
Expanded Prostate Cancer Index Composite patient-reported quality of life changes after stereotactic body radiation therapy on urinary, bowel, and sexual quality of life from baseline to 5 years post—stereotactic body radiation therapy.
Publication bias
There was significant publication bias found when analyzing studies reporting 5-year bRFS and late grade ≥3 GU and GI toxicity (Fig. E4A–E4C, available at https://doi.org/10.1016/j.ijrobp.2019.03.051). A funnel plot of the studies used to calculate 5-year bRFS demonstrated asymmetry that was confirmed with Egger’s regression test (P < .001), indicating the presence of publication bias. When adjusting for this bias by using the trim-and-fill method, the originally observed 95.3% bRFS rate decreased to 93.1% (95% CI, 89.1%-95.7%). Similar results were found for late grade ≥3 GU toxicity (P < .001, adjusted rate of 2.7% [95% CI, 1.8% to 4.1%]) and late grade ≥3 GI toxicity (P < .001, adjusted rate of 3.0% [95% CI, 1.7% to 5.4%]).
Discussion
We herein demonstrate that there is considerable evidence that prostate SBRT is an effective treatment for localized prostate cancer, with a very favorable toxicity profile that has minimal impact on long-term urinary and bowel QOL. Moreover, to date, no randomized study has demonstrated that altering radiotherapeutic dose, fractionation, or target volume affects prostate cancer—specific or overall survival. Thus, toxicity, QOL, patient convenience, and cost become increasingly important when determining the optimal treatment method for patients with localized prostate cancer, and our findings support NCCN guidelines and Medicare policies, which recognize prostate SBRT as a standard of care treatment option for many men with localized prostate cancer.
For men electing to pursue radiation therapy, the historically standard 8 to 9 weeks of conventional external beam radiation therapy can be inconvenient and necessitates use of substantial resources. This in part motivated numerous randomized noninferiority trials to test the use of 4 to 6 weeks of moderate hypofractionation compared with the conventional 8 to 9 weeks of radiation therapy. The largest of these trials, the Conventional Versus Hypofractionated High-Dose Intensity Modulated Radiation Therapy for Prostate Cancer (CHHiP) trial (n = 3126), demonstrated 5 years post-treatment that 4 weeks of radiation therapy was noninferior regarding biochemical recurrence and toxicity compared with 8 weeks of radiation therapy.10 This is now recommended as a standard of care per NCCN guidelines.69 More recently, results from the HYPO-RT-PC trial (n = 1200) were presented comparing a 7-fraction SBRT regimen to conventional radiation therapy.44 It demonstrated that at 5 years post-treatment, SBRT was noninferior regarding biochemical recurrence and late toxicity.44 Thus, there is now level 1 evidence with similar duration follow-up supporting the noninferiority of both moderate hypofractionation and SBRT compared with conventional dose-escalated radiation therapy, although we eagerly await long-term results from the HYPO-RT-PC trial. The biochemical control rates from our analysis, which included results from the HYPO-RT-PC trial, compare favorably with outcomes from the CHHiP trial. Five-year biochemical failure—free survival for men with low and intermediate-risk disease on the CHHiP trial were 96.6% and 90.2%, respectively, for men treated with 60 Gy in 20 fractions, compared with 96.7% and 92.1% in the present analysis.
Our study is the first meta-analysis to comprehensively include all prospective studies involving prostate SBRT and, importantly, to include the landmark HYPO-RT-PC trial and other recently reported and published trials. Our systematic review and meta-analysis findings should be compared and contrasted with the guideline statement from the American Society for Radiation Oncology, American Urological Association, and American Society of Clinical Oncology,48 which performed their initial data search multiple years ago in a rapidly changing field. These guidelines state that there is “moderate” quality evidence for prostate SBRT for low-risk disease and “low” quality evidence for the use of prostate SBRT in men with intermediate-risk disease. Although they state that SBRT may be offered to men with intermediate-risk disease, they encourage that this be done in the setting of a clinical trial or multi-institutional registry. Our study demonstrated that 78% of prospective studies included intermediate-risk men, nearly 50% had Gleason 7 disease, and intermediate risk-disease had more patients represented than low-risk in prospective SBRT studies. Given that there is randomized phase 3 noninferiority evidence for patients with intermediate and high-risk prostate cancer for prostate SBRT, our findings suggest that there is stronger quality evidence to support SBRT for intermediate-risk disease than there is for low-risk disease. This is further strengthened by the recent release of acute toxicity data from the Prostate Advances in Comparative Evidence B () randomized trial in men with intermediate-risk disease demonstrating numerically lower, but not significantly different, acute toxicity for SBRT compared with conventional RT. In addition, most moderate hypofractionation studies have limited enrollment of high-risk patients or use of pelvic nodal radiation therapy, similar to the available evidence for SBRT. Yet, moderate hypofractionation is endorsed by NCCN across all risk groups, including clinical nodepositive disease, whereas SBRT is “acceptable in practices with appropriate technology, physics, and clinical expertise” for men with very low to favorable intermediate-risk prostate cancer.69
Our toxicity results should also be compared with other radiation therapy modalities that are used for intermediate or high-risk disease, such as combination brachytherapy. Nearly all of the prospective trials using combination brachytherapy with sufficient follow-up report combined grade ≥3 GU and GI toxicity of 10% to 30%.72–74 Our study demonstrated that both GI and GU grade ≥3 toxicity from prostate SBRT were less than 2.5%, which also compares favorably to reported toxicity rates after treatment with external beam alone.10 This was nicely demonstrated in a recent pooled study by Kishan et al demonstrating that SBRT had one of the lowest associated toxicity profiles of any radiation therapy—based modalities assessed in prospective studies.75 Even this rate was largely driven by select high-dose SBRT trials using doses as high as 10 Gy x 5 fractions.17 Our meta-regression for late grade ≥3 GU toxicity confirmed that there was a significant increase in severe GU toxicity with increasing dose. It is important to note that dose escalation beyond 7.25-8 Gy x 5 fractions may be safely achievable, given that ≥5 mm margins were used in multiple SBRT studies, magnetic resonance imaging was not mandated to be used on all studies, and almost none used rectal spacers. Thus, our reported toxicity estimates for both GU and GI may further be improved by adoption of available technologies shown to reduce treatment-related morbidity. Although increasing prescribed dose was not significantly associated with increasing late severe GI toxicity, it is important to note that >95% of the data to inform this analysis were in dose ranges well below 10 Gy x 5, and extreme SBRT dose escalation has reported unacceptably high late GI morbidity.17
Although we believe our data supports SBRT as a standard of care treatment option for men with localized prostate cancer, there is insufficient evidence to imply that it should be the primary standard of care for localized prostate cancer. This would require either a superiority or noninferiority trial demonstrating that SBRT is in fact superior to other fractionation schemas regarding tumor control or toxicity and QOL. National Research Group GU005 () is a superiority trial that aims to investigate if prostate SBRT is superior to moderate hypofractionation regarding QOL using the EPIC-26. Secondary outcomes relate to investigating if prostate SBRT is also superior regarding disease-free survival. The noninvasive and convenient nature of SBRT, with associated low toxicity and favorable tumor control rates, has also led to an exciting international trial, the Prostate Advances in Comparative Evidence A trial.76 This trial is randomizing men with intermediate-risk disease to SBRT versus radical prostatectomy. Given the lower urinary incontinence side effects and improved erectile function associated with conventional radiation therapy compared with surgery found in the Prostate Testing for Cancer and Treatment trial77 and the low rectal toxicity reported on trials to date with prostate SBRT, it will be interesting to see the QOL comparisons of prostate SBRT with surgery.
The analyses presented have limitations. First, our analysis was a study-level meta-analysis. Patient-level characteristics were extracted, and only studies reporting the same outcome at the same time point were pooled, which is an inherent limitation. Interestingly, our data is consistent with a smaller individual-patient study of patients with low and intermediate-risk disease demonstrating very comparable toxicity rates.78 Second, biochemical control analyses were limited in that most studies including high-risk patients did not separately report outcomes by risk group. Also, although ADT was used in 15% of patients, there was not enough available information that could be extracted to determine the impact of ADT in a quantitative manner, nor was ADT duration always specified. In addition, pelvic nodal radiation therapy was rarely used, and its benefit in the context of prostate SBRT is unknown, similar to its unclear benefit with moderate hypofractionated or conventional radiation therapy. Third, late toxicity is a time-dependent outcome, and there was heterogeneous follow-up. Thus, sensitivity analyses were conducted for late toxicity to determine if follow-up time could have affected the results. Importantly, the rates were essentially identical for late toxicity when only including studies with a median follow-up of ≥5 years. Notably, we did find significant publication bias for both late GU and GI toxicity. However, statistically adjusting for this bias, toxicity rate estimates increased by only 1% to 2%. Last, the number of patients reporting on QOL decreased over time, which could affect the reliability of our estimates at later time points. However, given the minimal acute and late toxicity observed, we hypothesize that our QOL findings are likely representative for the majority of men treated with prostate SBRT.
Conclusions
Prostate SBRT has substantial evidence to support its use for treatment of localized prostate cancer, particularly in men with intermediate-risk disease. Phase 1 to 3 trials consistently report excellent tumor control and patient-reported QOL with very low acute and late toxicity. Our findings support that SBRT could be considered a standard radiotherapeutic strategy for localized prostate cancer while ongoing trials assess its potential superiority to other treatment methods.
Supplementary Material
Acknowledgments
The authors thank the Prostate Cancer Foundation (D.E.S.) and the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence (D.E.S., P50CA186786) for their support. This research was partially supported by National Institutes of Health grant CA 083654 (H.E.H.).
Disclosures: Varian Medical Systems, Inc (A.U.K.); Myriad Genetics research funding and consulting and GenomeDx research funding (T.M.M.); consultation for Ferring, Astellas consulting and research funding, Janssen consulting and research funding, Nanobiotix consulting, Dendreon consulting, Blue Earth consulting, GenomeDX consulting, Bayer consulting, and equity from Augmenix, Inc (P.L.N.); Augmenix, Inc research funding (N.D.); F.Y.F. is the cofounder of Bayer, Sanofi, Clovis, and PFS Genomics, Inc; and Z.S.Z. is on the external advisory board for Scripps Proton Therapy Center and is a paid consultant for EMD Serono.
Footnotes
Note—An online CME test for this article can be taken at https://academy.astro.org.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2019.03.051.
References
- 1.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol 2011;29:1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodges JC, Lotan Y, Boike TP, Benton R, Barrier A, Timmerman RD. Cost-effectiveness analysis of stereotactic body radiation therapy versus intensity-modulated radiation therapy: An emerging initial radiation treatment option for organ-confined prostate cancer. J Oncol Pract 2012;8:e31s–e37s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelius IR, Bentzen SM. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: Bad news, good news, or no news? Int J Radiat Oncol Biol Phys 2013;85:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royce TJ, Lee DH, Keum N, et al. Conventional versus hypofractionated radiation therapy for localized prostate cancer: A meta-analysis of randomized noninferiority trials [e-pub ahead of print]. Eur Urol Focus 2019. 10.1016/j.euf.2017.10.011 Accessed May 2018. [DOI] [PubMed]
- 6.Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): Final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2016; 17:1061–1069. [DOI] [PubMed] [Google Scholar]
- 7.Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol 2013;31:3860–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated dose-escalated intensity modulated radiation therapy versus conventionally fractionated intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2016;96:S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: Final results of a phase III randomized trial. J Clin Oncol 2017;35:1891–1897. [DOI] [PubMed] [Google Scholar]
- 10.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016;17:1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol 2017;35:1884–1890. [DOI] [PubMed] [Google Scholar]
- 12.Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol 2016;34:2325–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson WC, Dess RT, Litzenberg DW, et al. A multi-institutional phase 2 trial of prostate stereotactic body radiation therapy (SBRT) using continuous real-time evaluation of prostate motion with patient-reported quality of life. Pract Radiat Oncol 2018;8:40–47. [DOI] [PubMed] [Google Scholar]
- 14.Boyer MJ, Papagikos MA, Kiteley R, Vujaskovic Z, Wu J, Lee WR. Toxicity and quality of life report of a phase II study of stereotactic body radiotherapy (SBRT) for low and intermediate risk prostate cancer. Radiat Oncol 2017;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannan R, Tumati V, Xie XJ, et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer-Results from a multi-institutional clinical trial. Eur J Cancer 2016;59:142–151. [DOI] [PubMed] [Google Scholar]
- 16.Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol 2011;29:2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DW, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014; 89:509–517. [DOI] [PubMed] [Google Scholar]
- 18.Macias VA, Blanco ML, Barrera I, Garcia R. A phase II study of stereotactic body radiation therapy for low-intermediate-high-risk prostate cancer using helical tomotherapy: Dose-volumetric parameters predicting early toxicity. Front Oncol 2014;4:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantz C A phase II trial of stereotactic ablative body radiotherapy for low-risk prostate cancer using a non-robotic linear accelerator and real-time target tracking: Report of toxicity, quality of life, and disease control outcomes with 5-year minimum follow-up. Front Oncol 2014; 4:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alayed Y, Cheung P, Pang G, et al. Dose escalation for prostate stereotactic ablative radiotherapy (SABR): Late outcomes from two prospective clinical trials. Radiother Oncol 2018;127:213–218. [DOI] [PubMed] [Google Scholar]
- 21.Loblaw A, Cheung P, D’Alimonte L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: Toxicity, biochemical, and pathological outcomes. Radiother Oncol 2013;107:153–158. [DOI] [PubMed] [Google Scholar]
- 22.Helou J, D’Alimonte L, Quon H, et al. Stereotactic ablative radiotherapy in the treatment of low and intermediate risk prostate cancer: Is there an optimal dose? Radiother Oncol 2017;123:478–482. [DOI] [PubMed] [Google Scholar]
- 23.Tang CI, Loblaw DA, Cheung P, et al. Phase I/II study of a five-fraction hypofractionated accelerated radiotherapy treatment for low-risk localised prostate cancer: Early results of pHART3. Clin Oncol (R Coll Radiol) 2008;20:729–737. [DOI] [PubMed] [Google Scholar]
- 24.Quon HC, Musunuru HB, Cheung P, et al. Dose-escalated stereotactic body radiation therapy for prostate cancer: Quality-of-life comparison of two prospective trials. Front Oncol 2016;6:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elias E, Helou J, Zhang L, et al. Dosimetric and patient correlates of quality of life after prostate stereotactic ablative radiotherapy. Radiother Oncol 2014;112:83–88. [DOI] [PubMed] [Google Scholar]
- 26.Quon HC, Ong A, Cheung P, et al. Once-weekly versus every-other-day stereotactic body radiotherapy in patients with prostate cancer (PATRIOT): A phase 2 randomized trial. Radiother Oncol 2018;127: 206–212. [DOI] [PubMed] [Google Scholar]
- 27.D’Agostino G, Franzese C, De Rose F, et al. High-quality linac-based stereotactic body radiation therapy with flattening filter free beams and volumetric modulated arc therapy for low-intermediate risk prostate cancer. A mono-institutional experience with 90 patients. Clin Oncol 2016;28:e173–e178. [DOI] [PubMed] [Google Scholar]
- 28.Scorsetti M, Alongi F, Clerici E, et al. Stereotactic body radiotherapy with flattening filter-free beams for prostate cancer: Assessment of patient-reported quality of life. J Cancer Res Clin Oncol 2014;140: 1795–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alongi F, Cozzi L, Arcangeli S, et al. Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: Preliminary report of a phase II study. Radiat Oncol 2013; 8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King CR, Brooks JD, Gill H, Presti JC Jr. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877–882. [DOI] [PubMed] [Google Scholar]
- 31.King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti JC Jr. Stereotactic body radiotherapy for localized prostate cancer: Interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 2009;73:1043–1048. [DOI] [PubMed] [Google Scholar]
- 32.Wiegner EA, King CR. Sexual function after stereotactic body radiotherapy for prostate cancer: Results of a prospective clinical trial. Int J Radiat Oncol Biol Phys 2010;78:442–448. [DOI] [PubMed] [Google Scholar]
- 33.McBride SM, Wong DS, Dombrowski JJ, et al. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma: Preliminary results of a multi-institutional phase 1 feasibility trial. Cancer 2012;118:3681–3690. [DOI] [PubMed] [Google Scholar]
- 34.Madsen BL, Hsi RA, Pham HT, Fowler JF, Esagui L, Corman J. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: First clinical trial results. Int J Radiat Oncol Biol Phys 2007;67:1099–1105. [DOI] [PubMed] [Google Scholar]
- 35.Gomez CL, Xu X, Qi XS, et al. Dosimetric parameters predict short-term quality-of-life outcomes for patients receiving stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol 2015;5: 257–262. [DOI] [PubMed] [Google Scholar]
- 36.Kim YJ, Cho KH, Pyo HR, et al. A phase II study of hypofractionated proton therapy for prostate cancer. Acta Oncol 2013;52: 477–485. [DOI] [PubMed] [Google Scholar]
- 37.Fuller DB, Naitoh J, Mardirossian G. Virtual HDR CyberKnife SBRT for localized prostatic carcinoma: 5-year disease-free survival and toxicity observations. Front Oncol 2014;4:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuller DB, Kane BL, Medbery CA, et al. 5-year outcomes from a prospective multi-institutional trial of heterogeneous dosing stereotactic body radiotherapy (SBRT) for low- and intermediate-risk prostate cancer. J Clin Oncol 2017;35:35. [DOI] [PubMed] [Google Scholar]
- 39.Vargas CE, Hartsell WF, Dunn M, et al. Hypofractionated versus standard fractionated proton-beam therapy for low-risk prostate cancer: Interim results of a randomized trial PCG GU 002. Am J Clin Oncol 2018;41:115–120. [DOI] [PubMed] [Google Scholar]
- 40.Vargas CE, Hartsell WF, Dunn M, et al. Image-guided hypofractionated proton beam therapy for low-risk prostate cancer: Analysis of quality of life and toxicity, PCG GU 002. Rep Pract Oncol Radiother 2016;21:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmermann M, Taussky D, Menkarios C, et al. Prospective phase II trial of once-weekly hypofractionated radiation therapy for low-risk adenocarcinoma of the prostate: Late toxicities and outcomes. Clin Oncol (R Coll Radiol) 2016;28:386–392. [DOI] [PubMed] [Google Scholar]
- 42.Menkarios C, Vigneault É, Brochet N, et al. Toxicity report of once weekly radiation therapy for low-risk prostate adenocarcinoma: Preliminary results of a phase I/II trial. Radiat Oncol 2011;6:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lukka H, Stephanie P, Bruner D, et al. Patient-reported outcomes in NRG oncology/RTOG 0938, a randomized phase 2 study evaluating 2 ultrahypofractionated regimens (UHRs) for prostate cancer. Int J Radiat Oncol Biol Phys 2016;94:2. [Google Scholar]
- 44.Widmark A, Gunnlaugsson A, Beckman L, et al. OC-0599: Ultra-hypofractionation for prostate cancer: Outcome from the Scandinavian phase 3 HYPO-RT-PC trial. Radiother Oncol 2018;127:S314. [Google Scholar]
- 45.Musunuru HB, D’Alimonte L, Davidson M, et al. Phase I/II study of stereotactic ablative radiotherapy including regional lymph node irradiation in patients with high-risk prostate cancer (SATURN): Early toxicity and quality of life. Int J Radiat Oncol Biol Phys 2018;102: 1438–1447; Accessed May 2018. [DOI] [PubMed] [Google Scholar]
- 46.Folkert MR, Zelefsky MJ, Hannan R, et al. Multi-institutional phase 2 trial of high-dose stereotactic body radiation therapy with temporary hydrogel spacer for low- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;99:1319–1320. [Google Scholar]
- 47.Meier RM, Bloch DA, Cotrutz C, et al. Multicenter trial of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer: Survival and toxicity endpoints. Int J Radiat Oncol Biol Phys 2018; 102:296–303. [DOI] [PubMed] [Google Scholar]
- 48.Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: An ASTRO, ASCO, and AUA evidence-based guideline. J Clin Oncol 2018;36:3411–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aluwini S, van Rooij P, Hoogeman M, et al. CyberKnife stereotactic radiotherapy as monotherapy for low- to intermediate-stage prostate cancer: Early experience, feasibility, and tolerance. J Endourol 2010; 24:865–869. [DOI] [PubMed] [Google Scholar]
- 51.Aluwini S, van Rooij P, Hoogeman M, Kirkels W, Kolkman-Deurloo I-K, Bangma C. Stereotactic body radiotherapy with a focal boost to the MRI-visible tumor as monotherapy for low- and intermediate-risk prostate cancer: Early results. Radiat Oncol 2013;8: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolzicco G, Favretto MS, Satariano N, Scremin E, Tambone C, Tasca A. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: The Georgetown University experience. Radiat Oncol 2013;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis J, Sharma S, Shumway R, et al. Stereotactic body radiotherapy for clinically localized prostate cancer: Toxicity and biochemical disease-free outcomes from a multi-institutional patient registry. Cureus 2015;7:e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freeman D, Dickerson G, Perman M. Multi-institutional registry for prostate cancer radiosurgery: A prospective observational clinical trial. Front Oncol 2014;4:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glowacki G, Majewski W, Wojcieszek P, Grabinska K, Wozniak G, Miszczyk L. Ultrahypofractionated CyberKnifeTM based stereotactic radiotherapy versus conventional radiotherapy in patients with prostate cancer - acute toxicity evaluation in two phase II prospective studies. Neoplasma 2017;64:599–604. [DOI] [PubMed] [Google Scholar]
- 57.Jabbari S, Weinberg VK, Kaprealian T, et al. Stereotactic body radiotherapy as monotherapy or post-external beam radiotherapy boost for prostate cancer: Technique, early toxicity, and PSA response. Int J Radiat Oncol Biol Phys 2012;82:228–234. [DOI] [PubMed] [Google Scholar]
- 58.Katz AJ, Kang J. Stereotactic body radiotherapy as treatment for organ confined low- and intermediate-risk prostate carcinoma, a 7-year study. Front Oncol 2014;4:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katz AJ, Kang J. Quality of life and toxicity after SBRT for organ-confined prostate cancer, a 7-year study. Front Oncol 2014;4:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kotecha R, Djemil T, Tendulkar RD, et al. Dose-escalated stereotactic body radiation therapy for patients with intermediate- and high-risk prostate cancer: Initial dosimetry analysis and patient outcomes. Int J Radiat Oncol Biol Phys 2016;95:960–964. [DOI] [PubMed] [Google Scholar]
- 61.Lee SW, Jang HS, Lee JH, Kim SH, Yoon SC. Stereotactic body radiation therapy for prostate cancer patients with old age or medical comorbidity: A 5-year follow-up of an investigational study. Medicine (Baltimore) 2014;93:e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miszczyk L, Namysl Kaletka A, Napieralska A, et al. CyberKnife radioablation of prostate cancer - preliminary results for 400 patients. Asian Pac J Cancer Prev 2017;18:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murthy V, Gupta M, Mulye G, et al. Early results of extreme hypofractionation using stereotactic body radiation therapy for high-risk, very high-risk and node-positive prostate cancer. Clin Oncol (R Coll Radiol) 2018;30:442–447. [DOI] [PubMed] [Google Scholar]
- 64.Pontoriero A, Iati G, Mondello S, et al. High-dose robotic stereotactic body radiotherapy in the treatment of patients with prostate cancer: Preliminary results in 26 patients. Technol Cancer Res Treat 2016;15: 179–185. [DOI] [PubMed] [Google Scholar]
- 65.Rucinska M, Kieszkowska-Grudny A, Nawrocki S. SHARP hypofractionated stereotactic radiotherapy is well tolerated in prostate cancer: Toxicity and quality of life assessment. Strahlenther Onkol 2016;192:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shikama N, Kumazaki Y, Miyazawa K, Nihei K, Hashimoto S, Tsukamoto N. Rectal toxicity after extremely hypofractionated radiotherapy using a non-isocentric robotic radiosurgery system for early stage prostate cancer. World J Oncol 2016;7:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tambas M, Agaoglu F, Iribas A, et al. Conventionally fractionationed volumetric arc therapy versus hypofractionated stereotactic body radiotherapy: Quality of life, side effects, and prostate-specific antigen kinetics in localized prostate cancer. Value Health Reg Issues 2016;10: 91–99. [DOI] [PubMed] [Google Scholar]
- 68.Tree AC, Ostler P, Hoskin P, et al. Prostate stereotactic body radiotherapy-first UK experience. Clin Oncol (R Coll Radiol) 2014;26: 757–761. [DOI] [PubMed] [Google Scholar]
- 69.NCCN. National Comprehensive Cancer Network clinical practice guidelines in oncology. Prostate cancer. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf Accessed May 8, 2019
- 70.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899–905. [DOI] [PubMed] [Google Scholar]
- 71.Barry MJ, Fowler FJ Jr., O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549–1557; discussion 1564. [DOI] [PubMed] [Google Scholar]
- 72.Rodda S, Tyldesley S, Morris WJ, et al. ASCENDE-RT: An analysis of treatment-related morbidity for a randomized trial comparing a low dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:286–295. [DOI] [PubMed] [Google Scholar]
- 73.Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol 2005;23: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 74.Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol 2012;103:217–222. [DOI] [PubMed] [Google Scholar]
- 75.Kishan AU, Dang A, Katz AJ, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open 2019;2:e188006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morrison K, Tree A, Khoo V, As NJV, TMG OBotP. The PACE trial: International randomised study of laparoscopic prostatectomy vs. stereotactic body radiotherapy (SBRT) and standard radiotherapy vs. SBRT for early stage organ-confined prostate cancer. J Clin Oncol 2018;36:TPS153; TPS153. [Google Scholar]
- 77.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kishan AU, Katz AJ, Mantz C, et al. Long-term outcomes of stereotactic body radiotherapy for low- and intermediate-risk prostate adenocarcinoma: A multi-institutional consortium study. J Clin Oncol 2018;36:84; 84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.