Abstract
A major area of growth for “nano-enabled” products has been the addition of nanoparticles (NPs) to surface coatings including paints, stains and sealants. Zinc oxide (ZnO) NPs, long used in sunscreens and sunblocks, have found growing use in surface coating formulations to increase their UV resistance, especially on outdoor products. In this work, ZnO NPs, marketed as an additive to paints and stains, were dispersed in Milli-Q water and a commercial deck stain. Resulting solutions were applied to either Micronized-Copper Azole (MCA) pressure treated lumber or a commercially available composite decking. A portion of coated surfaces were placed outdoors to undergo environmental weathering, while the remaining samples were stored indoors to function as experimental controls. Weathered and control treatments were subsequently sampled periodically for 6 months using a simulated dermal contact method developed by the Consumer Product Safety Commission (CPSC). The release of ZnO NPs, and their associated degradation products, was determined through sequential filtration, atomic spectroscopy, X-Ray Absorption Fine Structure Spectroscopy, and electron microscopy. Across all treatments, the percentage of applied zinc released through simulated dermal contact did not exceed 4%, although transformation and release of zinc highly dependent up dispersion medium. For MCA samples weathered outdoors, water-based applications released significantly more zinc than stain-based, 180 +/− 28, and 65 +/− 9 mg/m2 respectively. Moreover, results indicate that the number of contact events drives material release.
Keywords: Zinc, Nanomaterial, Exposure, XAFS, Nano-enabled, Surface coating
Introduction
Rapid developments in nanoparticle (NP) research have resulted in an increase in the number of “nano-enabled” products available for consumer purchase.(Foss Hansen et al., 2016; Mitrano et al., 2015) NPs have been added to a wide array of products to provide them with properties such as increased strength, reactivity, and durability. One product category experiencing the largest commercialization growth for “nano-enabled” products is surface coatings including paints, stains, and sealants.(Hanus and Harris, 2013; Keller et al., 2013; Teizer et al., 2012; van Broekhuizen et al., 2011) NPs used in surface coatings are an important area of study, as the nature of their application (i.e., at the product surface) drastically increases their environmental contact, potentially accelerating their transformation and release.(Clar et al., 2018) Few studies have examined the conditions that encourage transformation and release of NPs from such coatings. Previous research regarding the release of NPs from surface coatings focused on passive release conditions including leaching and façade runoff.(Al-Kattan et al., 2015; Al-Kattan et al., 2013; Kaegi et al., 2010; Kaegi et al., 2008; Shandilya et al., 2015; Zuin et al., 2013)
While these passive release studies clarified general patterns of NP release from surface coatings, they did not examine the potential release from physical contact, which may be the primary human exposure pathway depending on where the product is applied. Our recent work utilized a wipe technique to evaluate the release of copper from micronized copper pressure treated lumber,(Platten III et al., 2016) as well as cerium dioxide NPs from surface coatings(Clar et al., 2018) during simulated dermal contact. This method was adapted from the Consumer Product Safety Commission (CPSC) wipe method previously used to estimate the release of arsenic and chromium from pressure treated lumber.(Cobb, 2003; Thomas et al., 2005) This updated methodology allowed for the differentiation of particulate and ionic species released during contact, which may have dramatic consequences on the potential long-term health effects from product usage.
Zinc Oxide (ZnO) NPs have been utilized in a variety of applications including biomedical industries,(Kumar et al., 2015) textiles,(Petkova et al., 2016; Pulit-Prociak et al., 2016) and perhaps most notably in sunscreens for their UV protective properties.(Gulson et al., 2012; Jeon et al., 2016; Leite-Silva et al., 2016; Newman et al., 2009) These same UV protective properties desirable in sunscreens are being exploited in paints, stains, and sealants to increase the lifetime of outdoor surfaces by limiting the damage from extended UV exposure. In 2010, a materials flow analysis by Keller et. al. estimated that the global use of ZnO NPs was over 30,000 metric tons.(Keller et al., 2013) Furthermore, a survey conducted by Piccinno and coworkers found that while the vast majority of ZnO NPs would likely be used in cosmetics, roughly 30% would be used in nano-enabled paints.(Piccinno et al., 2012) While these figures are only estimates, they demonstrate the importance of understanding the release of ZnO from outdoor surface coatings and their potential environmental and human health impacts.
In this work, we examine the transformation and release of ZnO NPs from two coated surfaces, Micronized Copper-Azole (MCA) pressure treated lumber and a composite decking material, under reasonably intended use scenarios. Using a combination of our previously utilized wipe methodology(Clar et al., 2018) and simple batch leaching tests, we aim to understand the conditions that drive nanomaterial release in these systems. As the toxicity of ZnO NPs has been primarily linked to particle solubility and size,(Baek et al.; Franklin et al., 2007; Larner et al., 2012; Xiao et al., 2015) we attempt to not only track the total amount of zinc released during experimentation but differentiate between both particulate and ionic species.
Materials and Methods
Surface Specimens:
Two products were selected for ZnO coating, MCA lumber and a composite decking material. Materials were purchased in bulk from chain hardware retailors in Cincinnati, OH to minimize sample heterogeneity. Additional details on the MCA lumber and composite decking material can be found in our previous publications.(Clar et al., 2018; Platten III et al., 2016)
Nanoparticle Characterization:
ZnO NPs were obtained from BYK (Germany) as liquid dispersions (40 wt%) containing 20 nm particles stabilized with an organic capping agent. This product was selected based on its advertised use as a UV protective additive for water-based surface coatings including paints, stains, and sealants. Total zinc concentration was confirmed by ICP-OES (Thermo Fisher iCAP 6000 Series). Documentation provided by the company identified that methylisothiazolinone (MIT, 77 mg L−1), benzisothiazolinone (BIT, 77 mg L−1), and glycol ethers (≤ 0.2%) were present. The presence of a glycol ether stabilizing agent was confirmed with FTIR using a Bruker Vertex 80 (Bruker Corporation Billerica, Massachusetts). The hydrodynamic diameter (HDD) of the pristine material was determined using a ZetaSizer Nano Series (Malvern, Worcestershire, UK) after a 10:1 dilution in Milli-Q water (18.2 mΩ). HDD values were recorded using an average of three runs, each run consisting of 12 measurements. Primary particle size and morphology were investigated using a Scanning Transmission Electron Microscopy (STEM) using a JEOL JEM-201 OF equipped with a High Angle Annular Dark Field Detector (HAAFD; Peabody, MA) and Field Emission Scanning Electron Microscopy (FESEM) using a JEOL JSM-7600F (Tokyo, Japan). Finally, zinc speciation in pristine material was determined by X-Ray Photoelectron spectroscopy (XPS) using a PHI Quantera II (Physical Electronics, Chanhassen, MN) and X-Ray Absorbance Fine Structure (XAFS) Spectroscopy. Details on STEM, FESEM, and XPS sample preparation are presented in the Supporting Information.
Coating Procedure and Experimental Design:
Initial coating solutions were obtained by diluting 40g of the liquid dispersion, with 960g of either Milli-Q water or a commercially available wood stain (DEFY Stain for Hardwoods, Light Walnut, pH 8.3) before surface application. Zinc concentration in these solutions was approximately 20 g/L. The background concentrations of zinc in the wood stain were found to be 17 mg/L, which is negligible compared to the added ZnO. Concentrations of other major elements found in the wood stain are listed in Table S1. ZnO dispersed in wood stain was not applied to composite decking as it is not a realistic application of the product. ZnO in water test solutions were applied to both MCA lumber and composite decking as previously described.(Clar et al., 2018) Briefly, surface coatings were hand applied in two separate coats using a 2-inch roller and total applied volumes recorded. After application, each MCA subsample and composite decking subsample resulted in zinc coverage of approximately 295 mg and 187 mg, respectively. The increased coating application on MCA lumber over composite decking is due to the porous nature of the lumber accepting more fluid. All surfaces were air dried for between 48 and 72 h before use in wiping and leaching experiments. After drying, all coated surfaces were split into experimental and control groups. Experimental samples were placed outdoors at the U.S Environmental Protection Agency (EPA) Center Hill research facility in Cincinnati, OH to undergo extensive weathering through exposure to UV light, temperature variation, and precipitation. Details of weather conditions can be found in the Supporting Information (Table S2, Figure S1-S2). Control samples were stored indoors in constant darkness. Both outdoor samples and indoor controls were sampled periodically for 6 months using the CPSC wipe methodology described below.(Clar et al., 2018; Cobb, 2003; Platten III et al., 2016; Thomas et al., 2005)
In addition to pristine surfaces, a subset of MCA lumber was degraded in a UV light chamber (UVA 340 nm) for 1260 h before coating application. Details of the degradation process can be found in our previous publication.(Clar et al., 2018) After degradation, MCA lumber was coated with the appropriate ZnO dispersions and placed back in the unit for additional UV exposure and used in wiping and leaching experiments described below. Alternatively, artificially degraded boards placed in the UV light chamber were sampled weekly for 8 weeks.
Wiping Procedure:
Details of the CPSC wipe method has been previously reported.(Cobb, 2003; Platten III et al., 2016; Thomas et al., 2005) Briefly, polyester fabric cloths soaked in 2 mL of 0.9% NaCl solution were attached to the head of the CPSC wiping apparatus. (See Figure S2) The head of the apparatus (cloth attached) was dragged over a distance of 50 cm for an effective surface area of 450 cm.2 A wipe cycle consisted of moving the weight back and forth one time. Each sampling event consisted of 10 wipe cycles with the head of the apparatus rotated 90 degrees after five wipe cycles. After wipe sampling, polyester cloths were removed from the apparatus and placed in 50 mL HDPE tubes. Total metal extraction was achieved by adding 20mL of a 10% Nitric Acid and 20% hydrogen peroxide solution to each sample tube; sample tubes were then held in a water bath at 60 °C for 24 h. Solutions were diluted when necessary and metal concentrations determined by ICP-OES. All wipe analysis was performed on parallel surfaces in quadruplicate. Additional details of ICP-OES operation, and QA/QC samples are provided in the Supporting Information.
A fifth parallel wipe sample was processes separately to determine the size and form of particulate matter removed during simulated dermal contact. The cloth generated from this replicate was placed in 20 mL of Milli-Q water (pH ~6) and allowed to mix on a rotary shaker for a period of no less than 20 min. The resulting solution, containing large fragments of wood fibers and surface coatings was fractionated through a 0.45 μm Track-Etched Membrane filter (Whatman) and a 10 kDa (~3-5 nm) Amicon Ultrafiltration filter (Millipore, Billerica MA) and centrifuged at 4,500 g for 20 min using a fixed angle rotor. At each stage, a subsample of filtrate was acidified and processed by ICP-OES, 0.45 μm and 10kDA filters were reserved for imaging and zinc speciation analysis.
Leaching Procedure:
All leaching experiments were conducted as previously described.(Clar et al., 2018) Briefly, quadruplicate subsamples of coated surfaces (4 cm × 4 cm, “coupons”) were placed in 100 mL Milli-Q water adjusted to pH 4.2 with a synthetic precipitation leaching procedure (SPLP) solution. Samples were covered and mixed at 200 RPM continuously for 72 h using a magnetic stir bar. At no time did the pH of the leachate solution rise above 5.5 during the mixing period. After mixing, a 20-mL aliquot was removed. Reaming leachate was sequentially filtered through 0.45 μm and 10 kDa (~3-5 nm) membrane filters to collect any particulate material released during leaching. Subsamples of leachate were also collected at both filtration steps. Unfiltered, 0.45 μm and 10 kDa filtered liquids were acid digested and processed by ICP-OES to determine zinc concentration in each fraction. Solid filters and leached samples were reserved for either imaging or speciation analysis where appropriate.
Metal Speciation by X-Ray Absorption Fine Structure Spectroscopy (XAFS):
To track changes of zinc speciation throughout experimentation, XAFS spectra was collected on the initial ZnO material, an independent ZnO standard, filters and wood blocks produced by the wiping and leaching experiments. XAFS analyses were conducted at Sector 10BM (MRCAT)(Kropf et al., 2010) at the Advanced Photon Source at Argonne National Laboratory, U.S. Department of Energy, Argonne, IL. The incident beam was monochromatized by using a Si (111) fixed-exit, double-crystal monochromator. The beam size was focused to an area of 3000×500 μm. At least three scans were collected for each sample and standard to optimize signal/noise ratio and collected in transmission and florescence mode simultaneously. Data was collected at the Zn-K edge (9659 eV) after calibration using a Zn foil. Solid standards used for data analysis were diluted with polyvinyl pyrrolidine (PVP) when necessary and pressed in 13 mm diameter self-supported pellets and sealed in Kapton® tape before analysis. Liquid samples and standards were immobilized on sterile cotton swabs and sealed in Kapton® polyimide tape. XAFS data reduction, including averaging, background removal by spline fitting, normalization and derivatization followed standard methods using the IFEFFIT software package.(Ravel and Newville, 2005) Linear combination fitting (LCF) was conducted on normalized and first derivative spectra of the samples using the IFEFFET computer software package to quantify metal speciation. The fitting range was −20 to 60 eV relative to the Zn-K edge unless otherwise noted.
Results and Discussion
Nanoparticle Characterization:
ZnO NPs were obtained from the manufacturer as liquid dispersions in water with a listed particle size of 20 nm. A summary of the detailed characterization of these pristine particles is presented in Table 1. STEM analysis of the material is presented in Figure 1 and demonstrates the variety of particle morphologies present in the dispersion. While the particles are not heavily aggregated, the dispersion contains, rods, spheres, and hexagonal plates. Using the collected images, the average particle size was determined to be 28 +/− 23 nm through manual analysis of 270 particles. The large standard deviation is caused by the abundance of rod shape structures found in the sample, resulting in a calculated aspect ratio of approximately 2.3. Additionally, the effect of this varied morphology is evident in the HDD measurements of 164 +/− 2 nm. While the imaging analysis demonstrated the heterogeneous nature of the initial dispersion, both XPS and XAFS analysis confirm speciation of the particles as crystalline ZnO (Figure S3).
Table 1:
Summary of initial characterization of ZnO NPs used throughout this study. Particle size and aspect ratio estimates derived from STEM images are produced through measurement of 270 particles.
| Particle Size | |
|---|---|
| Manufacturer Listed | 20 nm |
| STEM | 28 +/− 22 nm |
| Hydrodynamic Diameter (HDD) | 164 +/− 2 nm |
| Aspect Ratio | 2.3 |
Figure 1:
HAADF TEM images of ZnO NPs used in this study. Particle Size was calculated as 28 +/− 22 nm by manual analysis of 270 particles.
Estimates of Dermal Transfer Using CPSC Methods:
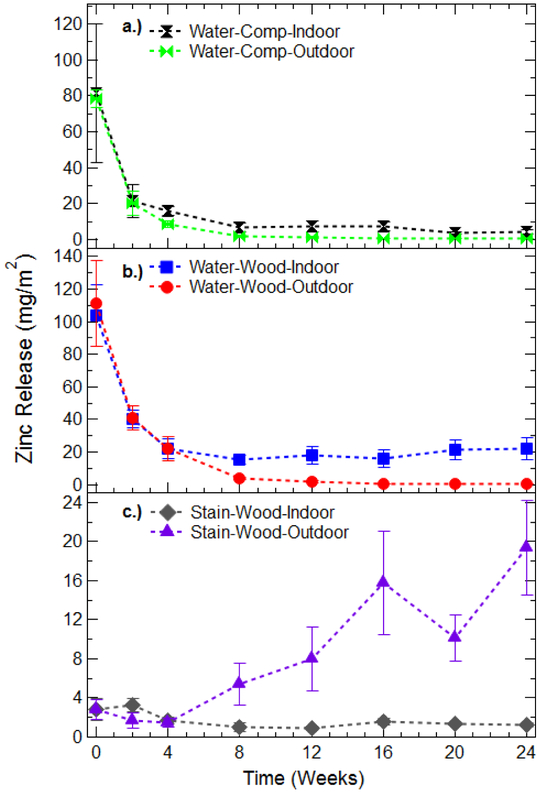
A summary of total zinc extracted from wipe cloths is shown in Figure 2. Release rates for each experimental condition are normalized for/to area wiped (450 cm2) during each sampling event. The results are also presented in terms of the total mass of Zn released throughout sampling (shown in Figure S4). Several interesting trends are apparent in the zinc release throughout the 6-month sampling period. With one exception (i.e., Stain-Wood-Outdoor), the largest zinc releases are found during the initial sampling stages, and total zinc release diminishes to a steady state after three to four sampling events. This behavior is similar to our previous studies examining cerium (as CeO2) release from treated wood products.(Clar et al., 2018) Additionally, large variability in the amount of released zinc is observed during the initial sampling events, and decreases throughout the experiment. This pattern is likely caused by both imperfections in the wood surface (knots, cracks) as well as slight deviations in surface coating thickness during application. Continued sampling events effectively “smooth” the surface and zinc release across experimental replicates converges. Figure 2 also highlights the differences in the total release of zinc based on the initial dispersion media. ZnO NPs dispersed in water and applied on composite surfaces resulted in an initial zinc release of approximately 80 mg Zn/m2 (Figure 2a), while the same formulation applied to MCA lumber surfaces results in an initial zinc release of approximately 105-112 mg Zn/m2 (Figure 2b). Clearly, dispersion of the initial ZnO NPs in wood stain before application dramatically reduces the initial Zn release (Figure 2c). When dispersed in wood stain and applied to MCA lumber, Zn release is below 5 mg/m2 initially, and remains below the release rate of water-based applications throughout the study duration. This result agrees with our previous studies using the same stain matrix in the dispersion of ceria NPs, and was attributed to the complex nature of the polymers and binders in the stain that allow for deeper penetration of the solution into the wood surface.(Clar et al., 2018)
Figure 2:
Release of zinc from coated boards. a.) Aqueous application on composite decking, b.) Aqueous application on MCA lumber, c.) Stain application on MCA lumber. Data for both weathered boards (Outdoor) and controls (Indoor) are an average of at least three replicates. Time Zero corresponds with the first wipe event after application of surface coatings and dry time of at least 48 h. Data has been normalized based on the area wiped per sampling event. Notice the difference in zinc release (y-axis) for each experiment treatment. The data presented represent the average of four replicate measurements and the error bars the standard deviation of the replicate values.
For the water-based applications (Figure 2a-b), there are minor differences between the Zn release characteristics from experimental outdoor samples and indoor controls. Indeed, for the first three sampling events of all water-based treatments, the zinc release data points are essentially the same. This suggests that the initial release of zinc is driven by the number of dermal contacts, and not the condition of the surface (i.e., pristine vs. weathered). After these initial sampling events a slight divergence between experimental samples and indoor controls develops, with indoor controls releasing more zinc than samples left outdoor. This difference is attributed to the nature of the open system in which outdoor experiments were completed. Rainfall events likely remove small portions of zinc from the surface, thereby decreasing the amount available for release in subsequent sampling events. The difference between zinc release from indoor and outdoor samples could potentially be used to estimate the total passive release of zinc to the environment throughout the 6-month weathering period. In the case of water-based applications, this difference was only 3.2 mg of zinc over the entire weathering period.
Contrary to the water-based application, stain-based application on MCA lumber surfaces showed interesting changes to zinc release after the first three sampling events (Figure 2c). Samples left to weather outdoors show an increase in zinc release throughout time compared to indoor controls. Initial zinc release from both indoor and outdoor boards is below 5 mg Zn/m2. After 8 weeks of outdoor exposure, zinc release increases to as high as 19 mg Zn/m2 for boards left outdoors, while indoor controls remain at steady state. Even though extended exposure to UV light, temperature variation, and precipitation appears to increase the release of zinc from stain dispersed samples, at no point does the release surpass the release from water-based applications, at least during the six-month time frame examined here (Figure S5).
To estimate the percentage of applied zinc removed from simulated dermal contact from all test surfaces, the total amount of zinc (in mg) released across the 6-month sampling period were totaled and divided by amounts of zinc initial applied to each subsample. Using this analysis, it becomes clear that while the initial contact events release the most zinc, the clear majority of applied material remains attached to the surface. Approximately 2.5% of the applied zinc was removed through simulated dermal contact on outdoor surfaces coated with the water-based formulation (MCA lumber and composite decking). The release rate drops to 1% when the ZnO was applied to MCA lumber using the stain-based formulation. While the stain-based application limits the amount of zinc initially released from the surface, continued sampling (i.e., past six months) could potentially result in larger zinc release (Figure 2c).
Estimates of Dermal Transfer from Pre-Weathered Boards:
Manufacturers of pressure treated lumber recommend that their products be allowed to weather for between 6 months and one year before customers apply a paint or stain to the surface. To investigate how the properties of the initial surface (i.e., aged vs. fresh) affect zinc release, a subset of MCA lumber was pre-weathered before surface coating. Pre-weathered boards were degraded identically to those in our previous work to control temperature and precipitation variability that would be present in outdoor weathering.(Clar et al., 2018) Pre-weathered boards were painted and sampled as previously discussed. Normalized zinc release data for these systems is shown in Figure 3 and Figure S6. Like fresh MCA lumber samples, the highest release of zinc from the pre-weathered boards takes place at the initial sampling events, degrading to a steady state over the course of 8 weeks. Again, the critical nature of dispersion medium is evident. Zinc release from water-based applications is as high as 40 mg/m2 whereas the highest release from stain-based application is 1.5 mg/m2.
Figure 3:

Release of zinc from MCA lumber that has undergone 1260 h of UV degradation before application of surface coatings. Data was collected in triplicate and has been normalized by the total area wiped during a sampling event. The data at Time Zero corresponds with the first wipe event after application of surface coatings and a dry time of at least 48 h. The data presented represent the average of four replicate measurements and the error bars the standard deviation of the replicate values.
Direct comparison between pre-weathered boards and boards coated as received is challenging due to differences in experimental design. Specifically, pre-weathered boards were stored in a UV light chamber and sampled weekly, while indoor control samples were kept in constant darkness over a period of 6-months. While the timescale for these experiments is very different, the amount of zinc removed from the surface through simulated dermal contact can be analyzed based on the number of wiping events that have taken place. This comparison is presented in Figure 4. When plotted in this manner, patterns of zinc release from pre-weathered and pristine boards are similar. For both water-based (Figure 4a) and stain-based (Figure 4b) applications there is minimal difference in zinc release from the third wiping event forward. The largest differences in release are evident during the first two sampling events, with the UV degraded samples releasing less zinc than the pristine controls. We have previously attributed this difference to the moisture loss that occurs in UV degraded boards over the 1260 h of exposure.(Clar et al., 2018) This moisture loss from the MCA lumber allows the water- and stain-based applications to penetrate more deeply into the wood, thereby reducing the amount available for dermal contact. A potential future study could confirm this hypothesis by repeating the experiments using kiln-dried MCA lumber with extremely low moisture content. Our data indicates that while the nature of the wood surface (i.e., pristine vs. degraded) may affect the initial release characteristics, the long-term release pattern will be governed by the number of surface contacts, the length of exposure, and the dispersion media used in application (Figure 2c).
Figure 4:
Release of zinc from pristine MCA lumber and UV degraded MCA lumber per wipe event. Data was collected in triplicate and has been normalized by the total area wiped during a sampling event. Notice the larger difference in zinc release (y-axis) for each experiment treatment. The data presented represent the average of four replicate measurements and the error bars the standard deviation of the replicate values.
Characterization of Zinc Released Through Simulated Dermal Contact:
Throughout experimentation, a relatively small amount of the initially applied zinc is removed through simulated dermal contact (2.5-3.6% for water-based, 0.2-1% for stain-based). However, the initial contact events immediately after application represent an acute exposure pathway to the ZnO NPs, especially for aqueous-based applications (Figures 2a-b). Therefore, it is critical to collect detailed information on the concentration of zinc released during these contacts, but also to understand the form and speciation of the material, due to the role these characteristics have on toxicity potential. As previously described, a single replicate from the wiping procedure was extracted with Milli-Q water and sequentially filtered. ICP-OES analysis of the resulting filtrate indicated that a substantial amount of zinc was retained on the 0.45 μm filters making them the most crucial for imaging and speciation analysis. The reserved filters were examined through FESEM with EDS capabilities to understand particle morphology associated with zinc release. Figure 5 is a Backscatter electron FESEM image of the 0.45 μm filter produced after the initial wiping event (Time Zero) for the Water/Wood/Outdoor formulation. While zinc is distributed throughout the wipe sample, it is also highly collocated with copper. The copper source in this sample is the MCA lumber itself, as previous work has shown it to be distributed throughout pristine samples.(Platten III et al., 2016) The colocation of copper and zinc, coupled with previous studies on the release of wood fragments released during simulated dermal contact on uncoated MCA lumber(Platten III et al., 2016), may indicate that when dispersed in water-based formulations, the zinc released during simulated dermal contact remains attached to wood fragments.
Figure 5:
BSE FESEM image of particulate matter retained on a 0.45μm filter after release and extraction using the CPSC estimate of dermal transfer test method. Sample is from a micronized copper pressure treated lumber coated with ZnO NPs dispersed in water after a 72-h drying period. Zinc is heavily collocated with copper as shown in the EDS Micrograph.
Similarly, the Water/Composite/Outdoor formulation samples had zinc distributed throughout the filter. Interestingly, continued sampling of the Water/Composite/Outdoor formulation samples resulted in small fragments of material ranging from roughly 2-25μm in size. EDS analysis identified these fragments as silicon and aluminum oxides, likely degradation products of the plastics capping agent used during the manufacturing process of the composite decking (Figure S7). Regarding the Stain/Wood/Outdoor treated lumber, acid digestion of the wipe cloths and ICP-OES analysis demonstrated increasing concentrations of zinc released with increased outdoor weathering (Figure 2c). Therefore, samples that had undergone extensive weathering were targeted for imaging analysis. Material extracted from the wipe cloths after 6-months of weathering produced a variety of particle sizes and morphologies (See Figure S8). EDS analysis of these aggregates indicated high levels of zinc, oxygen, silicon, iron, and copper. The presence of iron and silicon is not surprising as they are reported components of the commercial stain formulation. (Table S1). Similar to the cerium release studies, these results indicate that applied zinc oxide removed during simulated dermal contact, remains bound to both wood and stain fragments and is not found as “free” nanoparticles.(Clar et al., 2018)
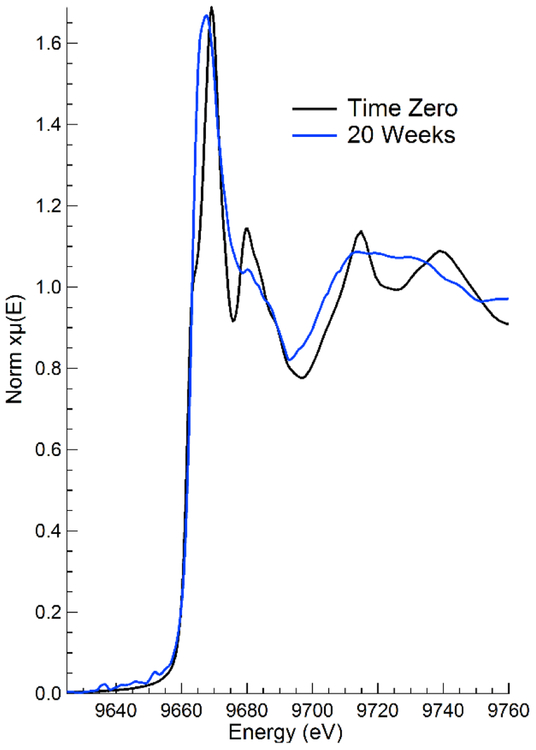
While electron microscopy images provide excellent information on the size and quantity of released aggregates, information on the species of zinc found during release is critical to assess its environmental and human health risks. The results of XAFS speciation analysis on the 0.45 μm filters produced during sequential filtration are presented in Table 2. Major differences in the analysis of normalized and first derivate of normalized spectra were not apparent, and first derivative fits were adopted herein. It is generally accepted that the inherent error in any LCF analysis is between 5-10%.(Gräfe et al., 2014) Due to the complex nature of the samples, zinc speciation was segmented into three main categories, Zn-Organic Complexes, Zn-Inorganic Complexes, and pristine ZnO as Nanobyk 3820. Additional details of speciation analysis, including reference spectra, and representative LCF results are presented in the Supporting Information (Figures S9 and S10). The resulting speciation analysis highlights the effect that dispersion medium (i.e., water, stain) plays in the degradation and transformation of the pristine ZnO throughout weathering. During the initial sampling events, the zinc released from Water/Wood/Outdoor Treatments is primarily ZnO. The low percentage of the Zn-Organic species likely captures the binding of the ZnO core to the organic capping agent used by the manufacturer during production. Continued sampling captures the dramatic transformation of the applied ZnO to other Zn-Inorganic complexes (i.e., ZnCl2) after only 8 weeks of outdoor weathering. Figure 6 clearly highlights the transformation of the initially applied ZnO to other Zn-Organic absorbed phases throughout the sampling period. The sharp features indicative of crystalline ZnO have been diminished or eliminated after 20 weeks of outdoor weathering.
Table 2:
Summary of zinc speciation extracted from CPSC wipe method cloths and coated wood surfaces as determined by LCF analysis of first derivate of normalized XAFS Spectra. Values in the table indicate relative percentage of each component contributing to the total observed signal. It is generally accepted that the inherent error in any LCF analysis is between 5-10%.(Gräfe et al., 2014) Samples marked N/A had low zinc concentrations with insufficient signal to noise ratios for reliable data analysis.
| Water – Composite – Outhoor Wipe | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Time Zero |
2 Weeks |
4 Weeks |
8 Weeks |
12 Weeks |
16 Weeks |
20 Weeks |
24 Weeks |
| ZnO | 93 | 18 | 0 | 0 | 0 | N/A | N/A | N/A |
| Zn-Inorganic | 0 | 50 | 62 | 74 | 42 | |||
| Zn-Organic | 7 | 31 | 38 | 26 | 58 | |||
| Water – Wood – Outhoor Wipe | ||||||||
| ZnO | 93 | 93 | 80 | 0 | 0 | 0 | 22 | N/A |
| Zn-Inorganic | 0 | 0 | 12 | 100 | 100 | 73 | 35 | |
| Zn-Organic | 7 | 7 | 8 | 0 | 0 | 27 | 43 | |
| Stain – Wood – Outhoor Wipe | ||||||||
| ZnO | 81 | 77 | 81 | 86 | 77 | 83 | 78 | N/A |
| Zn-Inorganic | 12 | 11 | 12 | 7 | 13 | 8 | 13 | |
| Zn-Organic | 7 | 11 | 7 | 7 | 10 | 9 | 10 | |
| Surface Samples (6-months) | ||||||||
| Species | Water/Wood/Indoor | Water/Wood/Outhoor | Stain/Wood/Indoor | Stain/Wood/Outhoor | ||||
| ZnO | 92 | 0 | 85 | 83 | ||||
| Zn-Fe2O3 | 0 | 0 | 15 | 14 | ||||
| Zn-Organic | 8 | 100 | 0 | 3 | ||||
Figure 6:
Normalized zinc XAFS data collected on 0.45μm filters collected from Water/Wood/Outdoor samples during simulated dermal contact experiments. Time Zero represents the first wipe event approximately 48 hours after coating application. The 20-week sample has undergone continuous outdoor weathering. Changes in spectra features highlight the degradation of pristine ZnO in the coating to amorphous absorbed zinc species.
This transformation is even more abrupt in the Water/Composite/Outdoor treatments, with the transformation of ZnO to both Zn-Organic and Zn-Inorganic complexes after only 4 weeks of outdoor weathering. While a portion of the ZnO character is recovered during subsequent sampling events of the Water/Wood/Outdoor treatment, the XAFS analysis demonstrates the transformation and degradation of the ZnO nanoparticles over a short time span. Alternatively, when dispersed in the stain, the character of the released zinc remains constant throughout the 6-month sampling period. The clear majority (>75%) of the released zinc remains as ZnO, while the Zn-Inorganic and Zn-Organic components capture interactions between the pristine ZnO and components of the stain formulation.
Speciation analysis of the 0.45 μm filters produced during the simulated dermal contact experiments provide valuable information on the nature of the zinc released. However, they are not an adequate representation of that material that remains on the coated surfaces after weathering and dermal contact, especially considering the clear majority of zinc applied remains on the wood surface. Therefore, subsamples from the MCA lumber coated boards were collected from the surface at the completion of the 6-month sampling period. The collected samples were ground and pressed into 13 mm self-supported pellets and analyzed for zinc speciation. LCF results for these samples are also presented in Table 2. The transformation of ZnO to Zn-Organic complexes is again apparent for the Water/Wood/Outdoor samples. The persistence of ZnO in the Water/Wood/Indoor samples is not unexpected since the samples remained covered indoors throughout the entire study. Similar to speciation data collected from 0.45 μm filters, zinc speciation in stain-based samples remained constant throughout the study. Zinc absorbed to iron oxides (Zn-Fe2O3) is representative of the interactions of zinc with the iron species present in the stain which are responsible for the light walnut color. The speciation analysis of the 0.45 μm filters produced from the simulated dermal contact, as well as the surface analysis, demonstrates the critical nature that dispersion medium plays in the transformation and release of ZnO NPs in these systems.
Zinc Release from Simulated Leaching:
Zinc concentrations found in unfiltered SPLP (pH 4.2) leachate, as well as sequentially filtered subsamples for each experimental condition, are presented in Figure 7. The total release of zinc varies based on the experimental treatment. Aqueous application on MCA lumber resulted in the highest zinc release, ranging between 30-28 mg/L, regardless of the filtration step. While the zinc leached from composite surfaces coated with this same aqueous-based formulation is lower at 6-9 mg/L, it is important to note that the composite material was coated with a much lower volume of the ZnO NP solution. In agreement with the data generated from simulated dermal contact, the stain-based application resulted in the lowest zinc release of any experimental treatment with concentrations of approximately 3 mg/L. Even though each treatment resulted in different total concentrations of zinc release, they all demonstrated similar behavior during sequential filtration. For both the aqueous- and stain-based applications on MCA lumber, over 90% of the zinc found in the unfiltered fraction was also recovered in the 10 kDa filtrate. This suggests that most zinc released during leaching is in an ionic form. The recovery rate in composite samples is similar, although with larger variability in the 10 kDa analysis. This result is reasonable as previous work has reported increased ZnO NP dissolution with decreasing pH.(Bian et al., 2011; Li et al., 2013) Based on the initial coverage of ZnO on each surface, the percentage of total zinc released varied between the surfaces and matrices. For water-based formulation, 48% of the applied ZnO was released as ionic zinc from MCA lumber, and 26% for composite decking. When applied to MCA lumber in a stain-based formulation, only 5.5% of the initially applied ZnO was released as ionic zinc.
Figure 7:

Results of Leaching Test performed on surface coupons that had been painted with different experimental treatments. Samples were mixed in SPLP (pH 4.2) solution for 72 h and then sequential filtered to determine size fractions of released material. Composite samples are coated only with ZnO dispersed in water as wood stain is not intended for application on composite surfaces. The lower-case letters indicate statistical differences in the mean concentration of Zn wiped from the surface over the six-month experiment as determined by a Fisher LSD means test (n= 4; α = 0.05). Identical letters indicate no statistical difference in the mean. The data presented represent the average of four replicate measurements and the error bars the standard deviation of the replicate values.
Zinc speciation was tracked before and after leaching by analyzing the surface of the coupons used during experimentation. LCF results for these samples are presented in Table 3. Once again, the dispersion media influences the stability of the ZnO after application. Aqueous-based application on MCA surfaces resulted in dramatic reduction of ZnO from 87% before leaching to 47% after a 72-h interaction period. Stain-based applications show a slight decrease in the ZnO character after leaching (77 to 71%), but is likely not meaningful considering the inherent error in LCF analysis. (Gräfe et al., 2014) Somewhat surprisingly, the zinc speciation shows virtually no change for composite decking samples before and after leaching. Additional leaching experiments using different water chemistry parameters may help to explain the results from the composite material. Regardless of the zinc species remaining on the leached samples, our results indicate that the majority of released zinc will be ionic in nature.
Table 3:
Summary of zinc speciation on wood blocks coated with ZnO NP dispersed in either Milli-Q water or wood stain before and after 72 hours of leaching in 100 mL of SPLP solution (pH 4.2). LCF results were derived from analysis of first derivative of normalized spectra using a variety of zinc standards. Values in the table indicate relative percentage of each component contributing to the total observed signal. It is generally accepted that the inherent error in any LCF analysis is between 5-10%.(Gräfe et al., 2014)
| Water/Wood | Stain/Wood | Water/Composite | ||||
|---|---|---|---|---|---|---|
| Species | Pristine | Leached | Pristine | Leached | Pristine | Leached |
| ZnO | 87 | 47 | 77 | 71 | 85 | 84 |
| Zn-Organic | 13 | 53 | 12 | 8 | 0 | 0 |
| Zn-Inorganic | 0 | 0 | 0 | 0 | 15 | 16 |
| Zn-Fe2O3 | - | - | 11 | 21 | - | - |
Zinc Exposure Assessment:
During simulated dermal contact studies, the largest release of zinc occurred during the initial contact events, with the aqueous-based applications demonstrating the largest release across all treatments. Throughout the 6-month study, the largest amount of zinc released from any single event was approximately 4.5 mg, while the total mass of zinc released in any treatment did not exceed 11 mg (Figures S4). The zinc totals collected herein are lower than doses found to have negative health impacts in model organisms. For example, Esmaeillou et al. found liver damage in Wistar rats during exposure to ZnO NPs, but only at a dose of 333 mg/kg/day.(Esmaeillou et al., 2013) Furthermore, XAFS analysis shows rapid degradation of the initial ZnO to other zinc complexes after only two months of outdoor weathering in water-based samples (Table 2). After this initial degradation, the potential exposure would likely be zinc ionic species and not the initially applied ZnO NPs. It appears that ingestion of ZnO NPs released from surface coatings through dermal contact may not constitute major a human health risk. However, additional research must be completed in similar systems to confirm these results.
Although extensive exposure from dermal contact and subsequent ingestion may not be a serious area of concern, a substantial amount of zinc was released from coated surfaces during extended contact with SPLP (pH 4.2) in leaching studies. Zinc concentrations in the leachate ranged from 31-3 mg/L depending on experimental treatment. Subsequent sequential filtration estimates indicated that most of the released zinc is in the ionic form. While this study did not directly evaluate the speciation of zinc passively released during outdoor weathering, resulting from leaching experiments suggested a heavy precipitation event immediately after coating application may stimulate the release of ZnO NPs and/or ionic zinc to surrounding water bodies through runoff. The ecotoxicity of ZnO NPs have been studied extensively and excellent reviews are available.(Ma et al., 2013) For comparison, negative biological effects have been reported in concentrations as low as 68 μg/L(Franklin et al., 2007) and closer to 1 mg/L(Xiao et al., 2015) depending on the aquatic test organism. Regardless of the zinc species present, the concentrations of zinc passively released leached from the coated surfaces in this study may create potentially toxic effects to aquatic organism during extended contact.
Implications and Future Research Directions
As more “nano-enabled” products become available for consumer purchase, there is an urgent need to understand the conditions under which these NPs may be released resulting in both environmental and human exposure. While screening techniques that track total concentrations are an excellent first step, detailed information regarding the nature and species of released material is critical for accurate risk assessment. This is especially important for nanoparticle-based products as preliminary research has demonstrated that the nanoparticles initially applied in these products will undergo degradation and transformation during their use. Specifically to this study, the nature of the dispersion solution during application dramatically affected release of ZnO NPs and their degradation products in both wiping and leaching experiments. Continued research needs to be directed toward the development of additional test methods to understand the release of NPs from consumer products. The results presented in this study emphasize the importance of temporal variability in understanding NP release. For example, early analysis (i.e., 1 month) of simulated dermal contact from stain applications indicated limited release. However, continued sampling showed the potential for increased ZnO exposure over time. Without these long-term studies, important information regarding NP transformation and release would be absent from any risk assessment strategy. Any method developed to understand NP release from consumer products must consider both the near and far field exposure scenarios. This study, as well as our previous work have highlighted the potential of the CPSC wipe methodology as a potential screening tool for NP release via dermal contact. Additional research should be conducted using products currently available on the market to understand current exposure scenarios.
Supplementary Material
Acknowledgements
Any opinions expressed in this paper are those of the authors(s) and do not, necessarily, reflect the official positions and policies of the U.S. EPA or the U.S. CPSC. Any mention of products or trade names does not constitute recommendation for use by the U.S. EPA or U.S. CPSC. Thanks to Jennifer Goetz for engineering support with the UV light chamber. Use of the Advanced Photon Source (APS), a U.S Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DEAC0206CH1357. MRCAT operations (Sector 10) are supported by the DOE and the MRCAT member institutions. This project was supported, in part, by an appointment in the Research Participation Program at the Office of the Research and Development (ORD), EPA administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the DOE and EPA.
Footnotes
Supporting Information
Additional text, two tables, and ten figures showing details of, metal analysis, nanoparticle characterization, XPS analysis, SEM images, model XAFS spectra and examples of LCF analysis. This material is available free of charge via the Internet.
References
- Al-Kattan A, Wichser A, Vonbank R, Brunner S, Ulrich A, Zuin S, et al. Characterization of materials released into water from paint containing nano-SiO2. Chemosphere 2015; 119: 1314–1321. [DOI] [PubMed] [Google Scholar]
- Al-Kattan A, Wichser A, Vonbank R, Brunner S, Ulrich A, Zuin S, et al. Release of TiO2 from paints containing pigment-TiO2 or nano-TiO2 by weathering. Environmental Science: Processes & Impacts 2013; 15: 2186–2193. [DOI] [PubMed] [Google Scholar]
- Baek S, Joo SH, Kumar N, Toborek M. Antibacterial effect and toxicity pathways of industrial and sunscreen ZnO nanoparticles on Escherichia coli. Journal of Environmental Chemical Engineering. [Google Scholar]
- Bian S-W, Mudunkotuwa IA, Rupasinghe T, Grassian VH. Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid. Langmuir 2011; 27: 6059–6068. [DOI] [PubMed] [Google Scholar]
- Clar JG, Platten WE, Baumann EJ, Remsen A, Harmon SM, Bennett-Stamper CL, et al. Dermal transfer and environmental release of CeO 2 nanoparticles used as UV inhibitors on outdoor surfaces: Implications for human and environmental health. Science of The Total Environment 2018; 613: 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb D. Cromated Copper Arsenic (CCA) CCA-treated Wood Analysis Memorandum from David Cobb to Patricia Bittner. U.S. Consumer Product Safety Commission, Washington, DC, 2003. [Google Scholar]
- Esmaeillou M, Moharamnejad M, Hsankhani R, Tehrani AA, Maadi H. Toxicity of ZnO nanoparticles in healthy adult mice. Environmental Toxicology and Pharmacology 2013; 35: 67–71. [DOI] [PubMed] [Google Scholar]
- Foss Hansen S, Heggelund LR, Revilla Besora P, Mackevica A, Boldrin A, Baun A. Nanoproducts - what is actually available to European consumers? Environmental Science: Nano 2016; 3: 169–180. [Google Scholar]
- Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a Freshwater Microalga (Pseudokirchneriella subcapitata): The Importance of Particle Solubility. Environmental Science & Technology 2007; 41: 8484–8490. [DOI] [PubMed] [Google Scholar]
- Gräfe M, Donner E, Collins RN, Lombi E. Speciation of metal(loid)s in environmental samples by X-ray absorption spectroscopy: A critical review. Analytica Chimica Acta 2014; 822: 1–22. [DOI] [PubMed] [Google Scholar]
- Gulson B, Wong H, Korsch M, Gomez L, Casey P, McCall M, et al. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Science of The Total Environment 2012; 420: 313–318. [DOI] [PubMed] [Google Scholar]
- Hanus MJ, Harris AT. Nanotechnology innovations for the construction industry. Progress in Materials Science 2013; 58: 1056–1102. [Google Scholar]
- Jeon S-k, Kim E-j, Lee J, Lee S. Potential risks of TiO2 and ZnO nanoparticles released from sunscreens into outdoor swimming pools. Journal of Hazardous Materials 2016; 317: 312–318. [DOI] [PubMed] [Google Scholar]
- Kaegi R, Sinnet B, Zuleeg S, Hagendorfer H, Mueller E, Vonbank R, et al. Release of silver nanoparticles from outdoor facades. Environmental Pollution 2010; 158: 2900–2905. [DOI] [PubMed] [Google Scholar]
- Kaegi R, Ulrich A, Sinnet B, Vonbank R, Wichser A, Zuleeg S, et al. Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environmental Pollution 2008; 156: 233–239. [DOI] [PubMed] [Google Scholar]
- Keller AA, McFerran S, Lazareva A, Suh S. Global life cycle releases of engineered nanomaterials. Journal of Nanoparticle Research 2013; 15: 1–17. [Google Scholar]
- Kropf AJ, Katsoudas J, Chattopadhyay S, Shibata T, Lang EA, Zyryanov VN, et al. The New MRCAT (Sector 10) Bending Magnet Beamline at the Advanced Photon Source. AIP Conference Proceedings 2010; 1234: 299–302. [Google Scholar]
- Kumar S, Ahlawat W, Kumar R, Dilbaghi N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosensors and Bioelectronics 2015; 70: 498–503. [DOI] [PubMed] [Google Scholar]
- Larner F, Dogra Y, Dybowska A, Fabrega J, Stolpe B, Bridgestock LJ, et al. Tracing Bioavailability of ZnO Nanoparticles Using Stable Isotope Labeling. Environmental Science & Technology 2012; 46: 12137–12145. [DOI] [PubMed] [Google Scholar]
- Leite-Silva VR, Sanchez WY, Studier H, Liu DC, Mohammed YH, Holmes AM, et al. Human skin penetration and local effects of topical nano zinc oxide after occlusion and barrier impairment. European Journal of Pharmaceutics and Biopharmaceutics 2016; 104: 140–147. [DOI] [PubMed] [Google Scholar]
- Li M, Lin D, Zhu L. Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli. Environmental Pollution 2013; 173: 97–102. [DOI] [PubMed] [Google Scholar]
- Ma H, Williams PL, Diamond SA. Ecotoxicity of manufactured ZnO nanoparticles – A review. Environmental Pollution 2013; 172: 76–85. [DOI] [PubMed] [Google Scholar]
- Mitrano DM, Motellier S, Clavaguera S, Nowack B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int 2015; 77: 132–147. [DOI] [PubMed] [Google Scholar]
- Newman MD, Stotland M, Ellis JI. The safety of nanosized particles in titanium dioxide– and zinc oxide– based sunscreens. Journal of the American Academy of Dermatology 2009; 61: 685–692. [DOI] [PubMed] [Google Scholar]
- Petkova P, Francesko A, Perelshtein I, Gedanken A, Tzanov T. Simultaneous sonochemical-enzymatic coating of medical textiles with antibacterial ZnO nanoparticles. Ultrasonics Sonochemistry 2016; 29: 244–250. [DOI] [PubMed] [Google Scholar]
- Piccinno F, Gottschalk F, Seeger S, Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. Journal of Nanoparticle Research 2012; 14: 1–11.22448125 [Google Scholar]
- Platten III WE, Sylvest N, Warren C, Arambewela M, Harmon S, Bradham K, et al. Estimating dermal transfer of copper particles from the surfaces of pressure-treated lumber and implications for exposure. Science of The Total Environment 2016; 548–549: 441–449. [DOI] [PubMed] [Google Scholar]
- Pulit-Prociak J, Chwastowski J, Kucharski A, Banach M. Functionalization of textiles with silver and zinc oxide nanoparticles. Applied Surface Science 2016; 385: 543–553. [Google Scholar]
- Ravel B, Newville M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat 2005; 12: 537–541. [DOI] [PubMed] [Google Scholar]
- Shandilya N, Le Bihan O, Bressot C, Morgeneyer M. Emission of Titanium Dioxide Nanoparticles from Building Materials to the Environment by Wear and Weather. Environmental Science & Technology 2015; 49: 2163–2170. [DOI] [PubMed] [Google Scholar]
- Teizer J, Venugopal M, Teizer W, Felkl J. Nanotechnology and Its Impact on Construction: Bridging the Gap between Researchers and Industry Professionals. Journal of Construction Engineering and Management 2012; 138: 594–604. [Google Scholar]
- Thomas TA, Levenson MS, Cobb DG, Midgett JD, Porter WK, Saltzman LE, et al. The Development of a Standard Hand Method and Correlated Surrogate Method for Sampling CCA (Pressure)-Treated Wood Surfaces for Chemical Residue. Journal of Children's Health 2005; 2: 181–196. [Google Scholar]
- van Broekhuizen P, van Broekhuizen F, Cornelissen R, Reijnders L. Use of nanomaterials in the European construction industry and some occupational health aspects thereof. Journal of Nanoparticle Research 2011; 13: 447–462. [Google Scholar]
- Xiao Y, Vijver MG, Chen G, Peijnenburg WJGM. Toxicity and Accumulation of Cu and ZnO Nanoparticles in Daphnia magna. Environmental Science & Technology 2015; 49: 4657–4664. [DOI] [PubMed] [Google Scholar]
- Zuin S, Gaiani M, Ferrari A, Golanski L. Leaching of nanoparticles from experimental water-borne paints under laboratory test conditions. Journal of Nanoparticle Research 2013; 16: 1–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.