Abstract
Background:
While over half of stroke survivors recover the ability to walk without assistance, deficits persist in the performance of walking adaptations necessary for safe mobility in the home and community. One such adaptation is the ability to walk or step backward. Post-stroke rehabilitation rarely includes assessment of backward walking (BW) and BW deficits have not been quantified in post-stroke community ambulators.
Objective:
To quantify spatiotemporal and kinematic BW characteristics in post-stroke community ambulators and compare their performance to healthy older adults.
Methods:
Individuals post-stroke (n=15, 60.1±12.9 years, forward speed: 1.13±0.23 m/s) and healthy adults (n=12, 61.2±16.2 years, forward speed: 1.40±0.13 m/s) performed forward walking (FW) and BW during a single session. Step characteristics and peak lower extremity joint angles were extracted using 3D motion analysis and analyzed with mixed-method ANOVAs (group, walking condition).
Results:
The stroke group demonstrated greater reductions in speed, step length and cadence and a greater increase in double-support time during BW compared to FW (p<.01). Compared to FW, the post-stroke group demonstrated greater reductions in hip extension and knee flexion during BW (p<.05). The control group demonstrated decreased plantarflexion and increased dorsiflexion during BW, but these increases were attenuated in the post-stroke group (p<.05).
Conclusions:
Assessment of BW can unmask post-stroke walking impairments not detected during typical FW. BW impairments may contribute to the difficulties reported by adults post-stroke when navigating the home and community settings. Therefore, BW should be assessed when determining readiness for home and community ambulation.
Keywords: backward walking, stroke rehabilitation, walking adaptability
Introduction
Although post-stroke rehabilitation emphasizes recovery of walking function, nearly 75% of individuals report difficulty walking in the community and 50% of adults post-stroke living at home experience falls.1,2 The inability to walk safely in the home and community limits participation in life roles, engagement in physical activity, and may be associated with feelings of isolation and depression.3 Walking in the community, as well as in the home, requires walking adaptability—the ability to modify the stepping pattern to meet task goals and environmental demands.4,5 Walking adaptations include the negotiation of obstacles and uneven terrain as well as changes in speed and direction. After stroke, rehabilitation efforts place little emphasis on walking adaptability and instead focus on forward steady-state walking (i.e. walking uninterrupted in a straight line on a flat surface).6 Given the mobility challenges experienced by adults post-stroke, greater attention to walking adaptability is warranted.4,7
Backward walking (BW) is a walking adaptation that has received little attention until recently in the post-stroke literature.8–12 While continuous BW may rarely be needed, BW for short distances is critical for safe negotiation of tight spaces or a crowded room, when opening a door, or when backing up to sit down. Although many features of BW are similar to forward walking (FW),13 the task demands and neural control of BW are generally different than those for FW. For instance, BW alters visual flow, requires greater muscle activity and has higher metabolic costs.14,15 Compared to FW, BW also requires greater activation of the sensorimotor cortices.16 Similarly, it is likely that BW involves increased activation of the prefrontal cortex, as previously shown during other walking adaptability tasks, reflecting increased attentional demands.17,18 Despite the differing demands of BW, healthy controls are able to easily perform this adaptability task. Specifically, healthy adults generally reverse their stepping pattern such that BW is very similar to time-reversed FW with only minor changes in gait characteristics and limb kinematics.13
Given the importance of BW, it is critical to know if this pattern reversal from FW to BW can also be performed post-stroke. A single prior study demonstrated individuals with moderate to severe FW impairment after stroke demonstrate even greater gait deficits during BW.10 More critical to our understanding of community mobility is the determination of how those with relatively well-recovered FW (i.e. based on the achievement of a post-stroke community ambulation) perform BW as these individuals are more likely to face task demands necessitating BW. In addition, the ability to walk backward may be a useful measure of mobility function and fall risk in adults post-stroke as found in older adults.19 Recent studies also suggest that BW may have particular value in the rehabilitation of adults with neurological injuries.9,11,12,20 Therefore, despite the potential value of BW in post-stroke gait assessment and rehabilitation, BW characteristics have not been investigated in post-stroke community ambulators.
The purpose of this study was to investigate BW in adults post-stroke. Since healthy adults perform BW using a strategy that is largely a time-reversal of FW characteristics, we compared the changes in walking mechanics (from FW to BW) in post-stroke community ambulators to those in healthy adults. We hypothesized that, relative to the controls, the post-stroke community ambulators would demonstrate impairments in BW as evidenced by significantly slower speeds, decreased peak joint angles and altered spatiotemporal step characteristics.
Methods
Participants
Fifteen post-stroke community ambulators were included (Table 1). All post-stroke participants were part of a larger parent study on assessment of walking adaptability. Recruitment occurred through an approved research recruitment registry and through local health care providers and researchers. Inclusion criteria for the post-stroke community ambulators were a single stroke primarily affecting motor function on one side of the body, age greater than 18 years, the ability to walk (forward walking) at least 10 meters independently or with supervision using a cane or an orthotic device, and the ability to follow verbal directions for task completion. Exclusion criteria included the need for physical assistance from another person or the use of a walker, another neurological condition in addition to stroke, more than one stroke, and severe visual impairments affecting walking. For this study, participants were included only if they walked without assistive and orthotic devices and demonstrated a self-selected FW speed greater than 0.8 m/s, the threshold proposed by Perry et al. as predictive of “unlimited” community ambulation status after stroke.21 In addition, 12 healthy individuals (age: 61.2 years, SD 16.1 years; 5 females) were recruited and served as controls. The Institutional Review Boards of the collaborating universities approved the study and all participants provided informed consent. The STROBE guidelines were used to ensure the reporting of this observational study.
Table 1.
Characteristics of the post-stroke participants.
| Gender | Months since stroke | Type | Side affected | Stroke location | Age | FW gait speed (m/s) |
|---|---|---|---|---|---|---|
| Male | 18 | Ischemic | Right | Left internal capsule and corona radiata | 55 | 0.81 |
| Female | 7 | Ischemic | Left | Right frontal lobe | 60 | 0.87 |
| Female | 12 | Ischemic | Left | Unknown | 64 | 0.80 |
| Male | 8 | Ischemic | Left | Right near brainstem | 74 | 1.22 |
| Male | 23 | Hemorrhagic | Left | Unknown | 65 | 0.91 |
| Male | 80 | Ischemic | Right | Unknown | 37 | 1.17 |
| Male | 10 | Ischemic | Left | Right lentiform nucleus/corona radiata regions | 78 | 0.96 |
| Male | 11 | Ischemic | Left | Right thalamus | 65 | 1.31 |
| Male | 15 | Ischemic | Left | Right putamen | 72 | 1.48 |
| Male | 6 | Ischemic | Left | Unknown | 61 | 1.13 |
| Female | 7 | Ischemic | Right | Left middle cerebral artery | 54 | 1.43 |
| Male | 38 | Ischemic | Right | Basilar artery | 67 | 1.36 |
| Female | 5 | Ischemic | Left-sensory, Right-motor | Right cerebellum near brainstem | 30 | 1.15 |
| Female | 123 | Ischemic | Right | Unknown | 54 | 1.31 |
| Male | 34 | Ischemic | Right | Right cerebellar peduncle | 66 | 1.00 |
| Post-stroke group average: 60.1±12.9* | ||||||
| Control group average: 61.2±16.2 | ||||||
Group averages not significantly different (p=.855)
Data collection
The participants performed the study procedures during a single visit to a research motion analysis center. All testing was led by a licensed research physical therapist who was assisted by trained research staff. For both FW and BW, participants walked along a straight path over an unobstructed 10-meter course. Participants were guarded by a staff member to help ensure safety. The participants were given standard instructions to walk at their comfortable pace. A 12-camera motion capture system (VICON, Los Angeles, CA, USA) was used to record three-dimensional kinematics. A modified Helen Hayes marker set with 66 reflective markers was used to define body segments. Kinematic data were sampled at 100 Hz while participants performed at least two trials of both forward and backward walking over 10 meters. All trials were completed at a self-selected speed and no orthotic or assistive devices were used. Since BW is a relatively novel task for individuals post-stroke, participants performed up to three trials of BW prior to data collection to ensure familiarity with the task.
Data analysis
Kinematic data were low-pass filtered using a 4th order Butterworth filter with cutoff frequency of 7 Hz. Spatiotemporal gait characteristics and lower extremity joint angles were calculated using a 15-segment experimental model in Visual 3D (C-Motion Inc., Germantown, MD, USA). Gait initiation and termination steps were removed from the analysis. The examined spatiotemporal gait characteristics were gait speed (meters/second), step length (meters), cadence (steps/minute) and the percent of the gait cycle spent in double limb support. To compare FW and BW kinematics, the average peak joint angles in the sagittal plane were calculated for the hip, knee and ankle across all the steps and for all participants.
Statistical analysis
Paired t-tests were first conducted to determine any differences between the left and right step lengths and left and right joint angles in controls. Since no significant differences were detected, the right-sided measures were used for all subsequent analyses. For the post-stroke individuals, data from the paretic leg was used. To determine any differences between the groups in FW performance, independent t-tests were completed for spatiotemporal data and average peak angles. To test the study hypothesis, a mixed method ANOVA was performed with the factors of group (healthy control, stroke) and walking condition (FW, BW). The interaction effect was evaluated to determine if there were differences between groups in their walking adaptation from forward to backward. The threshold for statistical significance was set to α<0.05. All data were analyzed with the Statistical Package for the Social Sciences (SPSS) 24 statistical software.
Results
Spatiotemporal gait characteristics
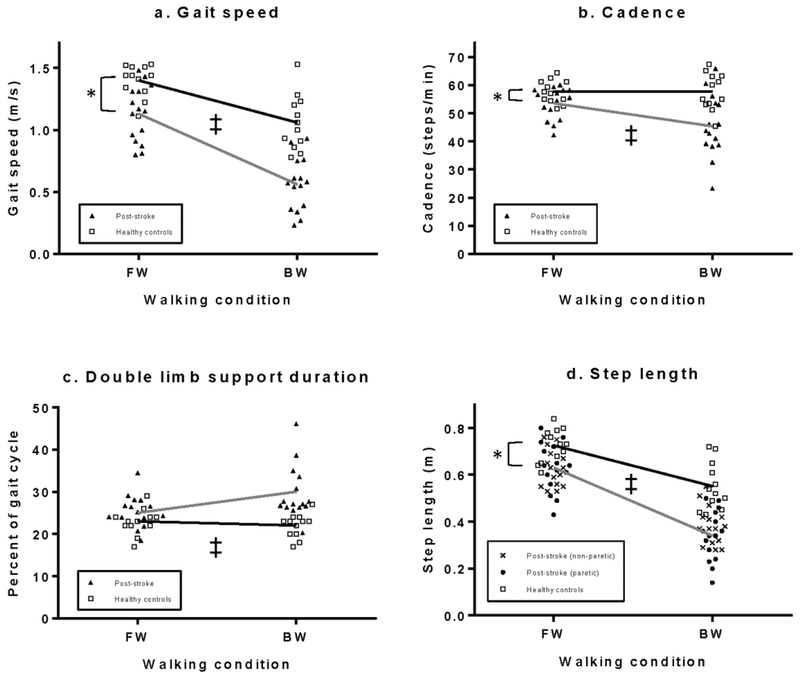
During FW, post-stroke community ambulators had a significantly slower gait speed (p=.001) with a smaller step length (p=.013) and slower cadence (p=.044) as compared to the healthy controls (Fig. 1a, b, d). Percentage of gait cycle in double limb support was not significantly different between groups (p=.078, Fig. 1c).
Fig. 1.
a-d. Spatiotemporal gait characteristics during FW and BW. The groups demonstrated significant differences in gait speed, cadence and step length during FW (*). Interactions effects (‡) were found for all four characteristics. Post-stroke community ambulators (gray lines) demonstrated a greater decline in speed, cadence and step length than healthy controls (black line), and the groups demonstrated opposite trends in double limb support percentage. For completeness, the non-paretic step lengths are included in the figure but were not part of the statistical analysis.
Analysis of the changes in spatiotemporal characteristics from FW to BW revealed significant interaction effects for all four spatiotemporal gait characteristics (Table 2, Fig. 1a–d). Relative to FW, the post-stroke community ambulators had greater reductions in speed, step length, and cadence during BW (p=.002, p=.003 and p=.007, respectively). For the change in double limb support from FW to BW, healthy controls demonstrated a slight decrease in double limb support when walking backward while post-stroke community ambulators showed an increase (p=.005).
Table 2.
Interaction effects (group by direction of walking) to assess group differences in adaptation to BW. Mean difference of each variable was calculated as the BW minus FW value.
| Gait measure | Healthy control mean difference (SD) | Post-stroke mean difference (SD) | ANOVA interaction term test statistic | ANOVA interaction term p-value |
|---|---|---|---|---|
| Speed (m/s) | −0.34 (0.18) | −0.57 (0.16) | F(1,25)=12.552 | .002 |
| Step length (m) | −0.17 (0.05) | −0.29 (0.11) | F(1,25)=10.814 | .003 |
| Cadence (steps/min) | 0.10 (7.10) | −8.15 (7.28) | F(1,25)=8.763 | .007 |
| DLS percentage | −0.92 (3.55) | 4.25 (4.96) | F(1,25)=9.370 | .005 |
| Hip flexion | 1.46 (4.64) | −1.32 (5.52) | F(1,25)=1.954 | .174 |
| Hip extension | −12.24 (3.60) | −16.57 (6.47) | F(1,25)=4.286 | .049 |
| Knee flexion | −8.16 (5.66) | −4.55 (4.89) | F(1,25)=11.445 | .002 |
| Knee extension | 7.54 (4.15) | 15.37 (5.38) | F(1,25)=2.841 | .104 |
| Dorsiflexion | 4.89 (4.38) | 1.60 (3.83) | F(1,25)=4.338 | .048 |
| Plantarflexion | −8.82 (7.61) | −1.98 (6.23) | F(1,25)=6.609 | .016 |
Kinematics
During FW, average peak hip flexion and extension were not significantly different between the control and post-stroke groups (p=.823 and p=.754, respectively; Fig. 2a, 3a, b). Average peak knee flexion was significantly less (p=.013) for the post-stroke community ambulators during FW and the healthy controls demonstrated significantly less FW peak knee extension (i.e., greater hyperextension; p=.041; Fig. 2b, 3c, d). Average peak ankle plantarflexion during FW was significantly less in the post-stroke community ambulators (p=.002) while there was no difference in ankle dorsiflexion (p=.678; Fig 2c, 3e, f).
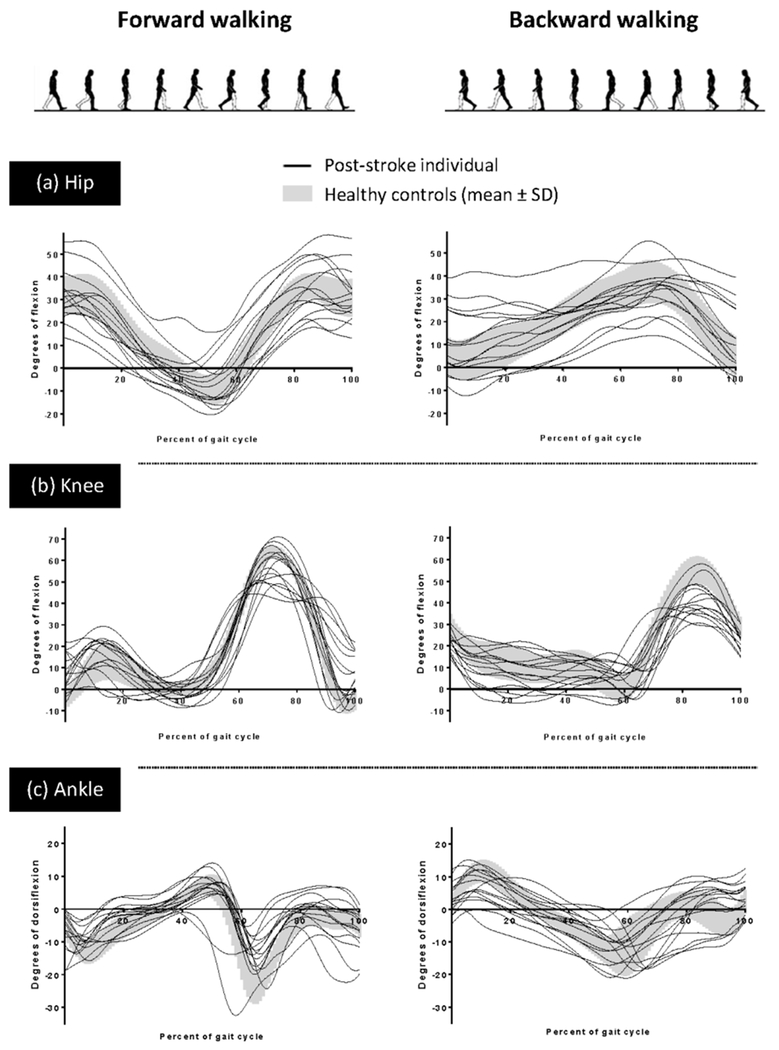
Fig. 2.
Average sagittal plane joint angles across the gait cycle for each post-stroke community ambulator (paretic limb) and the average and standard deviation of the healthy control group for the (a) hip, (b) knee, and (c) ankle during FW and BW.
Fig. 3.
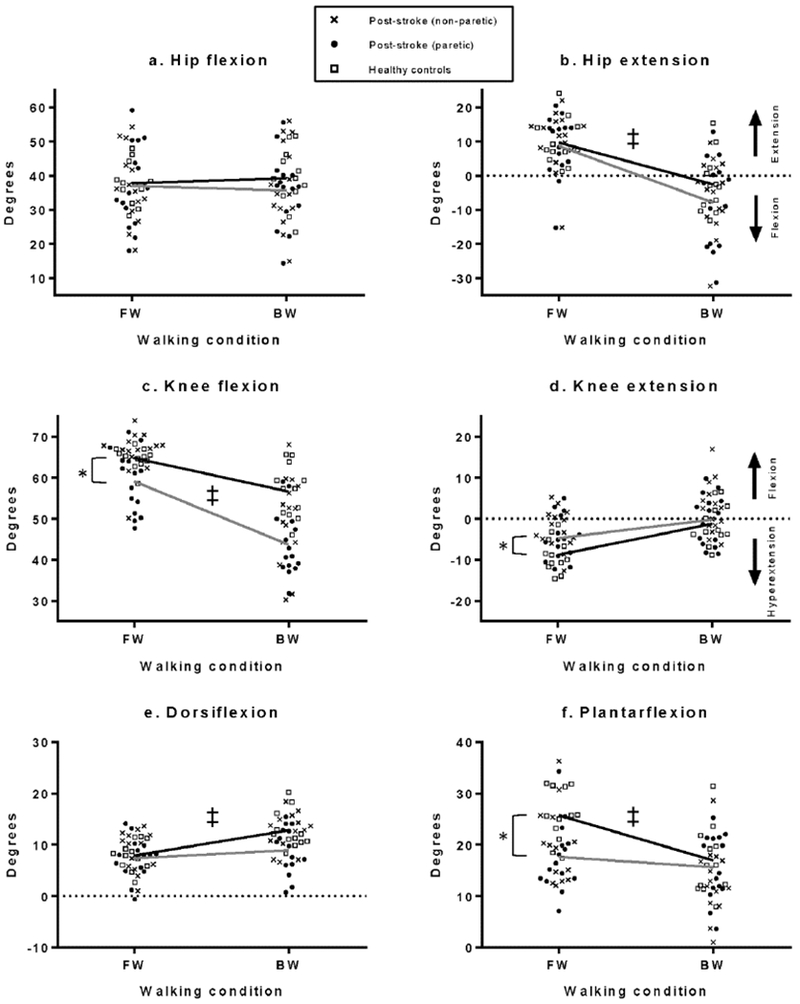
a-f. Average peak sagittal plane joint angles during FW and BW. There were significant group differences during FW in average peak knee flexion, knee extension and ankle plantarflexion (*). Interaction effects (‡) were found for average peak hip extension, knee flexion, dorsiflexion and plantarflexion. During BW, the post-stroke community ambulators (gray lines) demonstrated a greater decrease in hip extension and knee flexion, a smaller increase in dorsiflexion and a smaller decrease in plantarflexion compared to the healthy controls (black lines).
The analysis of the kinematic changes from FW to BW revealed significant interaction effects at the hip, knee and ankle (Table 2; Fig. 3e, f). Post-stroke community ambulators demonstrated a significantly greater reduction in peak hip extension during BW relative to FW performance (p=.049; Fig. 3b). The groups did not significantly differ in the change in peak hip flexion (p=.174). The reduction in peak knee flexion from FW to BW was significantly greater in post-stroke community ambulators (p=.002). The small change in peak knee extension was not significant between groups (p=.104). At the ankle, post-stroke community ambulators demonstrated a significantly smaller reduction in plantarflexion and a smaller increase in dorsiflexion during BW (p=.016 and p=.048, respectively; Fig. 3e, f).
Discussion
The observed changes in walking function post-stroke suggest that assessment of BW can unmask walking impairments not detected during typical FW. Specifically, post-stroke community ambulators have more pronounced kinematic and spatiotemporal impairments during BW than during FW. Backward walking is a critical walking adaptation necessary for safe mobility in the home and community. More specifically, during BW the post-stroke group demonstrated greater reductions in speed and step length, as well as impairments in lower extremity kinematics when compared to controls. Since the post-stroke individuals met a common clinically-defined threshold for ability to engage in community ambulation based on a steady-state FW speed,21 these findings highlight the importance of evaluating walking adaptations such as BW when determining readiness for community ambulation.
Several kinematic findings support that BW was more impaired than forward walking for the stroke participants. The post-stroke group demonstrated normal hip extension (average= 8.69 degrees, SD 9.24 degrees) during FW, but failed to achieve even a neutral hip position (average peak hip extension= −7.88 degrees, SD 12.72 degrees) when walking backward. Generating hip extension during a step backward may be especially difficult for individuals post-stroke. BW hip extension requires a pattern of muscle activity that is not used during FW: open-chain contraction of the hip extensors with concurrent activation of knee flexors.13,22 Furthermore, this pattern necessitates muscle activation outside of the stereotypical (i.e. synergistic) movement patterns found post-stroke.23 Therefore, the post-stroke individuals may have used compensatory motions such as pelvic elevation with retraction to step backward.
Other mild kinematic impairments found in FW were amplified during BW. Peak knee flexion was further reduced in BW, interfering with shortening the limb in swing and clearing the foot. Indeed, we noted some individuals who did not clear the foot during BW swing and instead slid their foot backward on the ground, suggesting that these individuals may have had more severe BW impairment. Prolonging a flat foot positon or sliding it during swing may also be a strategy to increase stability. Nevertheless, a lack of foot clearance during BW may place individuals at risk for tripping.
BW impairment in the post-stroke group was also demonstrated through the differences in step characteristics. Compared to healthy controls, the reductions (changes from FW to BW) in speed, step length and cadence were greater in the post-stroke group. Additionally, double limb support percentage increased during BW only in the post-stroke group. These altered BW step characteristics are similar to those demonstrated by other individuals with mobility impairments, such as the elderly and those with Parkinson disease, when walking backward.24,25 Furthermore, the changes in step characteristics are consistent with a cautious gait pattern, which most often presents when the individual perceives a threat to their stability.26 During BW, a cautious gait pattern may be adopted due to a fear of falling and the interaction of post-stroke sensorimotor impairments with the unique requirements of BW. For instance, impairments in proprioception after stroke may force reliance on visual input to maintain balance and control the placement of the paretic limb, but BW reduces the visual input.27 Additionally, it is well established that reduced and delayed muscle activity after stroke impairs the ability to quickly and appropriately adapt FW step kinematics.28 It is likely that these motor impairments will also impact BW and possibly be exacerbated given the increased difficulty of the task of BW.
Deficits in BW may also present after stroke because of difficulty recruiting the necessary increase in cortical activity. BW requires greater activation of the sensorimotor cortices, a region that may be have been damaged by the stroke.16 Furthermore, walking adaptability tasks require more attention, as demonstrated by increases in prefrontal cortical activity.17,29 It is well-established that performing walking tasks with high attentional requirements result in a decrement in performance.30 Furthermore, it is likely that the high attentional demands of BW for individuals after stroke would make it difficult to attend to other cognitive or motor dual tasks that will be frequently encountered simultaneously when ambulating in the community.
Currently, the degree of post-stroke walking recovery is commonly based on assessments of FW performance such as the 10 meter or 6 minute walk tests.21,31 Yet, individuals who are classified as community ambulators still have difficulty accessing the community environment and experience an increased rate of falls.1,2 Given the unique demands of BW and differential capacity of these post-stroke community ambulators to perform FW versus BW, assessment of BW could provide insight into recovery and capacity for community ambulation. Additionally, assessment of BW step characteristics in older adults has been found to be more sensitive to differentiating fallers from non-fallers, as compared to assessment of FW characteristics, and may have similar discriminating capability post-stroke.19
Although this study provides evidence of walking adaptability impairments post-stroke, we recognize some limitations should be considered. First, we did not control speed between groups during FW and BW. While speed differences impact stepping characteristics, we reasoned that it was important to capture the participant’s comfortable walking speed and represent their potential performance in the community. Second, up to three practice trials of BW were allowed before recording but some participants performed fewer practice trials. The purpose of the practice trials was to ensure participant familiarity with the task without inducing fatigue. Third, our analysis of the kinematics focused on the sagittal plane, therefore limiting insights regarding other movement strategies. The study was also limited to individuals who walked without an assistive device at a speed greater than 0.8 m/s. However, since this is one of the first studies to describe BW after stroke, we focused on the individuals who demonstrated a high level of FW recovery and would likely be able to perform walking adaptability tasks. Finally, this study included individuals with sub-acute or chronic stroke. Future studies should consider individuals with slower FW speeds, separate the analysis of participants with subacute versus chronic stroke, and consider additional measures such as analysis of muscle activation to examine underlying mechanisms of BW impairment in individuals post-stroke.
In current rehabilitation practice, the capacity for community ambulation is often predicted from FW performance. Despite this, these post-stroke community ambulators demonstrated deficits in BW step characteristics and kinematics that were more pronounced than those demonstrated during FW. Consistent with previous research, these findings suggest achievement of a threshold FW speed does not necessarily predict the capacity for performance of community ambulation.31,32 Furthermore, the impaired step characteristics and kinematics evident in the adults post-stroke suggest that performance of BW in a community or home setting would be limited for these individuals and may contribute to unsafe mobility. Direct assessment of walking adaptability is therefore warranted when determining capacity for community mobility.
STROBE Statement—Checklist of items that should be included in reports of cross-sectional studies
| Item No | Recommendation | Page # | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 1 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 2-3 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 3 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 4 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 4-5 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants | 4 |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 6-7 |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 5 |
| Bias | 9 | Describe any efforts to address potential sources of bias | N/A |
| Study size | 10 | Explain how the study size was arrived at | 4 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 5 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 5 |
| (b) Describe any methods used to examine subgroups and interactions | 5 | ||
| (c) Explain how missing data were addressed | N/A | ||
| *No missing data | |||
| (d) If applicable, describe analytical methods taking account of sampling strategy | N/A | ||
| (e) Describe any sensitivity analyses | N/A | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 4 |
| (b) Give reasons for non-participation at each stage | N/A | ||
| (c) Consider use of a flow diagram | N/A | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 15 |
| (b) Indicate number of participants with missing data for each variable of interest *No missing data | N/A | ||
| Outcome data | 15* | Report numbers of outcome events or summary measures | 16 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 16 |
| (b) Report category boundaries when continuous variables were categorized | N/A | ||
| *No categorical data | |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | N/A | ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | 5-6 |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 6-7 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 11 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 7-11 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 11 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 11-12 |
Give information separately for exposed and unexposed groups.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org.
Acknowledgements:
This work was supported by the Brooks-PHHP Research Collaboration, the Brooks Rehabilitation Collaborative Research Fund, University of North Florida, NIH National Center for Medical Rehabilitation Research T32 Neuromuscular Plasticity Training Program grant (T32HDO43730), NIH/NICHD K12 Rehabilitation Research Career Development Program (HDO55929) and a Promotion of Doctoral Studies (PODS) – Level I Scholarship from the Foundation for Physical Therapy. The funding sources had no involvement in the study design, data analysis, writing of the manuscript or decision to submit for publication. There are no conflicts of interest.
References
- 1.Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Archives of physical medicine and rehabilitation 2002;83:165–70. [DOI] [PubMed] [Google Scholar]
- 2.Robinson CA, Shumway-Cook A, Ciol MA, Kartin D. Participation in community walking following stroke: subjective versus objective measures and the impact of personal factors. Physical therapy 2011;91:1865–76. [DOI] [PubMed] [Google Scholar]
- 3.Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Archives of physical medicine and rehabilitation 2002;83:1035–42. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian CK, Clark DJ, Fox EJ. Walking adaptability after a stroke and its assessment in clinical settings. Stroke research and treatment 2014;2014:591013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patla AE, Shumway-Cook A. Dimensions of mobility: defining the complexity and difficulty associated with community mobility. J Aging Phys Activ 1999;7:7–19. [Google Scholar]
- 6.Latham NK, Jette DU, Slavin M, et al. Physical therapy during stroke rehabilitation for people with different walking abilities. Archives of physical medicine and rehabilitation 2005;86:S41–S50. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins KA, Clark DJ, Balasubramanian CK, Fox EJ. Walking on uneven terrain in healthy adults and the implications for people after stroke. NeuroRehabilitation 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim CY, Lee JS, Kim HD. Comparison of the Effect of Lateral and Backward Walking Training on Walking Function in Patients with Poststroke Hemiplegia: A Pilot Randomized Controlled Trial. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists 2017;96:61–7. [DOI] [PubMed] [Google Scholar]
- 9.Kim K, Lee S, Lee K. Effects of Progressive Body Weight Support Treadmill Forward and Backward Walking Training on Stroke Patients’ Affected Side Lower Extremity’s Walking Ability. Journal of physical therapy science 2014;26:1923–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makino M, Takami A, Oda A. Comparison of forward walking and backward walking in stroke hemiplegia patients focusing on the paretic side. Journal of physical therapy science 2017;29:187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose DK, DeMark L, Fox EJ, Clark DJ, Wludyka P. A Backward Walking Training Program to Improve Balance and Mobility in Acute Stroke: A Pilot Randomized Controlled Trial. Journal of neurologic physical therapy : JNPT 2018;42:12–21. [DOI] [PubMed] [Google Scholar]
- 12.Yang YR, Yen JG, Wang RY, Yen LL, Lieu FK. Gait outcomes after additional backward walking training in patients with stroke: a randomized controlled trial. Clinical rehabilitation 2005;19:264–73. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Kim J, Son J, Kim Y. Kinematic and kinetic analysis during forward and backward walking. Gait & posture 2013;38:674–8. [DOI] [PubMed] [Google Scholar]
- 14.Chaloupka EC, Kang J, Mastrangelo MA, Donnelly MS. Cardiorespiratory and metabolic responses during forward and backward walking. The Journal of orthopaedic and sports physical therapy 1997;25:302–6. [DOI] [PubMed] [Google Scholar]
- 15.Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. Journal of neurophysiology 1998;80:1868–85. [DOI] [PubMed] [Google Scholar]
- 16.Kurz MJ, Wilson TW, Arpin DJ. Stride-time variability and sensorimotor cortical activation during walking. NeuroImage 2012;59:1602–7. [DOI] [PubMed] [Google Scholar]
- 17.Clark DJ, Rose DK, Ring SA, Porges EC. Utilization of central nervous system resources for preparation and performance of complex walking tasks in older adults. Frontiers in aging neuroscience 2014;6:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. NeuroImage 2015;112:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritz NE, Worstell AM, Kloos AD, Siles AB, White SE, Kegelmeyer DA. Backward walking measures are sensitive to age-related changes in mobility and balance. Gait & posture 2013;37:593–7. [DOI] [PubMed] [Google Scholar]
- 20.Foster H, DeMark L, Spigel PM, Rose DK, Fox EJ. The effects of backward walking training on balance and mobility in an individual with chronic incomplete spinal cord injury: A case report. Physiotherapy theory and practice 2016;32:536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke; a journal of cerebral circulation 1995;26:982–9. [DOI] [PubMed] [Google Scholar]
- 22.Jansen K, De Groote F, Massaad F, Meyns P, Duysens J, Jonkers I. Similar muscles contribute to horizontal and vertical acceleration of center of mass in forward and backward walking: implications for neural control. Journal of neurophysiology 2012;107:3385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neckel N, Pelliccio M, Nichols D, Hidler J. Quantification of functional weakness and abnormal synergy patterns in the lower limb of individuals with chronic stroke. Journal of neuroengineering and rehabilitation 2006;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elboim-Gabyzon M, Rotchild S. Spatial and temporal gait characteristics of elderly individuals during backward and forward walking with shoes and barefoot. Gait & posture 2017;52:363–6. [DOI] [PubMed] [Google Scholar]
- 25.Hackney ME, Earhart GM. Backward walking in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 2009;24:218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delbaere K, Sturnieks DL, Crombez G, Lord SR. Concern about falls elicits changes in gait parameters in conditions of postural threat in older people. The journals of gerontology Series A, Biological sciences and medical sciences 2009;64:237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonan IV, Colle FM, Guichard JP, et al. Reliance on visual information after stroke. Part I: Balance on dynamic posturography. Archives of physical medicine and rehabilitation 2004;85:268–73. [DOI] [PubMed] [Google Scholar]
- 28.van Swigchem R, van Duijnhoven HJ, den Boer J, Geurts AC, Weerdesteyn V. Deficits in motor response to avoid sudden obstacles during gait in functional walkers poststroke. Neurorehabilitation and neural repair 2013;27:230–9. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins KA, Fox EJ, Daly JJ, et al. Prefrontal over-activation during walking in people with mobility deficits: Interpretation and functional implications. Human movement science 2018;59:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plummer P, Eskes G, Wallace S, et al. Cognitive-motor interference during functional mobility after stroke: state of the science and implications for future research. Archives of physical medicine and rehabilitation 2013;94:2565–74 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulk GD, He Y, Boyne P, Dunning K. Predicting Home and Community Walking Activity Poststroke. Stroke; a journal of cerebral circulation 2017;48:406–11. [DOI] [PubMed] [Google Scholar]
- 32.Lord SE, Rochester L. Measurement of community ambulation after stroke: current status and future developments. Stroke; a journal of cerebral circulation 2005;36:1457–61. [DOI] [PubMed] [Google Scholar]