Abstract
A summary of the First Signature Series Event, “Advancements in Cellular Therapies and Regenerative Medicine for Digestive Diseases,” held on May 3, 2017, in London, United Kingdom, is presented. Twelve speakers from three continents covered major topics in the areas of cellular therapy and regenerative medicine applied to liver and gastrointestinal medicine as well as to diabetes mellitus. Highlights from their presentations, together with an overview of the global impact of digestive diseases and a proposal for a shared online collection and data-monitoring platform tool, are included in this proceedings. Although growing evidence demonstrate the feasibility and safety of exploiting cell-based technologies for the treatment of digestive diseases, regulatory and methodological obstacles will need to be overcome before the successful implementation in the clinic of these novel attractive therapeutic strategies.
Keywords: digestive diseases, immune tolerance, mesenchymal stromal cells, tissue regeneration
Introduction
The Signature Series Event “Advancements in Cellular Therapies and Regenerative Medicine for Digestive Diseases” was held as a pre-meeting of the 25th International Society for Cellular Therapy annual congress in London, United Kingdom, May 3, 2017. This was the first workshop organized under the auspices of the Society that was fully dedicated to the application of stem cell and tissue engineering technologies to digestive diseases. The symposium convened opinion leaders from three continents and seven countries, with a common interest in developing cell therapy platforms and regenerative medicine (RM) technologies for clinical application in liver diseases and diabetes, as well as illnesses affecting the digestive tract. The event represented an opportunity to share knowledge and experience and promote understanding of the supporting technologies and potential target populations, with the overarching goal of enabling the clinical implementation of promising cell- and RM-based therapies. This article succinctly reports the topics that were discussed and the debate generated as a result.
Background
Prof. Carolina Ciacci: the global impact of digestive diseases
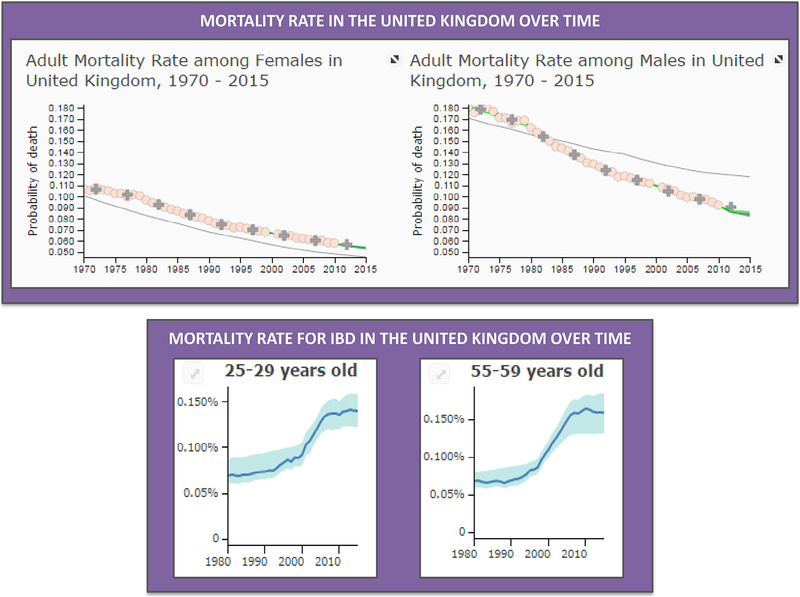
The Signature Series Event was opened by Professor Ciacci, who contextualized the global impact of digestive diseases. According to http://vizhub.healthdata.org, in the past 25 years, human life expectancy has increased by more than 5 years world-wide, mainly due to a significant decrease of mortality for cardiovascular diseases and cancer (Figure 1, upper panel). By contrast, improvement of management of digestive diseases, including colorectal cancer, accounts for an average increase of life expectancy from 1990 to 2015 of only 0.1 year. In addition, the prevalence of a number of disorders affecting the digestive system, mostly those characterized by a chronic inflammatory process, has undergone a significant increase not only because a growing number of patients are now correctly diagnosed but also by virtue of a real spread in the general population [1]. Among the illnesses showing an increased frequency are inflammatory bowel diseases, including Crohn disease (CD) and ulcerative colitis [2], and other autoimmune conditions affecting the intestine, such as celiac disease [3]; the liver, such as autoimmune hepatitis [4]; and the pancreas, with autoimmune pancreatitis [5] and type I diabetes [6]. These disorders are expected to reach epidemic levels in the near future and, as their peak of incidence is in the young adult age-group with consequent impairment of patient’s work productivity, the social and economic impact may be disastrous. Moreover, recent pooled data demonstrate that the mortality rate of diseases such as inflammatory bowel disease is on the rise [7] (Figure 1, lower panel). Specifically, Jess et al. found that from 1982 to 2010, mortality was increased by approximately 10% in patients with ulcerative colitis and by 50% among those suffering from CD compared with the general population [7]. Altogether, this information reveals that currently available therapeutic options are unsatisfactory and that, sadly, a definitive cure is still on the too-distant horizon. The complex, and in most cases, still unclear, interplay among genetic, epigenetic and environmental factors that underlies and triggers the majority of these illnesses represents the biggest challenge on the way to a definitive cure [8]. At the same time, vis-à-vis inadequate treatment tools, common sense suggests that this apparently inextricable complexity may not be resolved with a “magic bullet” like a pill or an intravenous drug; rather, alternative cellular or RM-based therapeutic tools leveraging the ability of cells to repair and regenerate dysfunctional tissues with the ultimate goal of restoring function should be tested. Notwithstanding the therapeutic potential, as of October 31, 2017, among all the clinical trials registered on the National Institutes of Health Clinical Trial Database (https://clinicaltrials.gov), only a small proportion was aimed at applying mesenchymal stromal cells (MSCs) and RM in digestive diseases. Specifically, those using MSCs included 55 for liver diseases, 77 for gastrointestinal diseases, 4 for pancreas diseases and 58 for diabetes, whereas those applying RM were 6 for liver diseases, 11 for gastrointestinal diseases, 1 for pancreas diseases and 15 for diabetes. Therefore, the aim of this workshop was to bring together the leading experts in clinical applications of MSCs and RM in digestive diseases to overcome this view by sharing ideas and methodologies and identifying a number of key challenges, the solution for which will accelerate the advent of cellular therapy and tissue engineering as a bedside reality. A synopsis of the presentations followed by a compilation of current key questions and recommendations follows. The full program of the meeting and list of participants is available in the online supplementary data for this article.
Figure 1.
Mortality rate in the United Kingdom over time. During the past 45 years, we witnessed a significant decrease of mortality, as showed by the data from both female and male adult populations in the United Kingdom (upper panel). By contrast, in the past 30 years, the mortality rate in inflammatory bowel disease (IBD) has risen (lower panel). Data are from http://www.healthdata.org/gbd.
Session I: liver diseases
In the liver, a number of triggers, such as obesity, alcohol, viruses, drugs and chemicals, result in cirrhosis [9], one of the most prevalent conditions worldwide [1]. Currently, liver transplantation is the only therapeutic option for end-stage liver disease, although application is limited by inadequate organ supply, high morbidity and cost. As a consequence of the progressively increasing gap between available organs and patients in need for a new liver, the mortality rate for patients on the waiting list has recently achieved a dramatic 20%, which represents a urgent call for action [10]. In this scenario, new treatment strategies aimed at either replacing dysfunctional livers or preventing progression of chronic liver disease toward its end stage are eagerly awaited.
Prof. Michael Ott: pluripotent stem cells for liver disease
Prof. Ott addressed the role that pluripotent stem cells may have as cell source to treat liver diseases. The need for reliable therapeutic alternatives to orthotropic liver transplantation has driven global efforts to establish cell therapy procedures able to provide stable and reliable sources of functional hepatocytes or of hepatocyte-like cells to be used for repopulation of damaged liver parenchyma. Direct induction of hepatocytes from fibroblasts holds potential as a strategy for RM. Capitalizing on the original studies from Yamanaka’s group on the generation of induced pluripotent stem cells (iPSCs) from somatic cells [11], recent studies have shown lineage reprogramming of human fibroblasts into hepatocytes by ectopic expression of transcription factors FOXA3, HNF1A and HNF4A or the combination of HNF1A, HNF4A and HNF6 together with the maturation factors ATF5, PROX1 and CEBPA [12, 13]. In London, Prof. Ott presented land-mark results from a series of elegant experiments performed in collaboration with Dr. Sharma showing in vivo generation of induced hepatocytes using transcription factor induction and genetic fate tracing in mouse models of chronic liver disease [14]. Transcription factors essential for critical hepatocyte function(s), such as albumin secretion and CYP3A activity, were identified using a loss of function screen in hepatocytes. In response to persistent inflammatory injury large numbers of stellate cells undergo “activation” to pro-fibrogenic myofibroblasts that accumulate in the liver and promote fibrosis. To demonstrate the potential therapeutic effects of direct reprogramming in chronic liver disease, selected transcription factors FOXA3, GATA4, HNF1A and HNF4A required for maintenance of the hepatocyte phenotype were co-overexpressed using a polycistronic lentiviral vector. By day 14, induced hepatocytes had acquired an epithelial-like morphology, a transcriptional profile consistent, albeit not identical, to primary hepatocytes and functional characteristics fundamental to hepatocytes such as cytochrome P450 (CYP1A2 and 3A) activity and the ability store glycogen, uptake low-density lipoprotein and to secrete albumin. To demonstrate that myofibroblasts could be forced to differentiate into induced hepatocytes in vivo, the group developed a method of labeling induced hepatocytes derived from non-parenchymal cells by using the Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mouse. The ROSAmT/mG is a cell membrane-targeted, two-color fluorescent Cre-reporter allele. Prior to Cre-recombination, cell membrane–localized tdTomato fluorescence expression is widespread in cells/tissues. Cre-recombinase expressing cells (and future cell lineages derived from these cells) have cell membrane–localized enhanced green fluorescent protein (EGFP) fluorescence expression replacing the red expression. Adult mT/mG mice were injected with an adeno-associated virus expressing Cre-recombinase under the transcriptional control of the liver-specific transthyretin promoter. As a result, membranous EGFP fluorescence in hepatocytes and tdTomato membranous fluorescence in non-parenchymal cells of the liver were expressed 4 weeks after AAV-transthyretin-Cre injection. To demonstrate the potential for in situ hepatocyte regeneration, extensive liver fibrosis was induced by carbon tetrachloride treatment twice a week from day 30 to 90. Forced ectopic expression of the four transcription factors to induce hepatocyte generation was performed at day 97. To maximize transduction of the four transcription factors essential for induced hepatocyte generation in vivo, an adenovirus vector overexpressing the four transcription factors of interest with modified tropism was developed. By coupling viral knobs with a peptide fragment of the nerve growth factor, the adenovirus vector was directed with specificity and high-affinity for the p75 neurotrophin receptor present on hepatic stellate cells and myofibroblasts [15]. Costaining of EGFP with cell-specific markers confirmed preferential transduction of approximately 30% of stellate cells in normal and 20% of myofibroblasts in carbon tetrachloride–induced fibrotic livers of BALB/c mice. The percentage of in vivo generated induced hepatocytes among the total hepatocyte population ranged from 0.2% to 1.2% following viral transduction, whereas control mice did not show any reprogrammed cells. Importantly, when the adenovirus vector was injected into uninjured mice, no induced hepatocytes were detected. Induced hepatocytes recovered from livers showed stable reprograming as determined by absence of exogenous transcription factors, normal proliferative capacity after partial hepatectomy and chromosomal integrity. Functional improvement was evidenced by the ability of recovered induced hepatocytes to secrete albumin, synthesize urea and store glycogen, as well as by the presence of glycerides and lipids and by cytochrome activity. Moreover, in vivo reprograming of myofibroblasts to induced hepatocytes resulted in significant reduction of collagen and hydroxyproline levels, indicating decreased liver fibrosis. This observation highlights the possible collateral benefit of using myofibroblasts as target cells for direct reprograming. Direct reprogramming of hepatic myofibroblasts may not only provide induced hepatocytes to repopulate the damaged liver parenchyma and ultimately restore function but, by reducing the number of myofibroblasts or/and by extinguishing the pro-fibrotic triggers, may also contribute to further reversing fibrosis [16].
Dr. Vincenzo Cardinale: innovative clinical-grade cryopreservation and grafting strategies fasten the translation of an effective biliary tree stem cell therapy
Dr. Vincenzo Cardinale presented data from clinical studies that his group at Sapienza University of Rome (Italy) is conducting to prove efficacy of innovative clinical cryopreservation technology and grafting strategies that are expected to fasten the translation of an effective biliary tree stem cell therapy. Although several sources of stem cells including hepatic stem cells, biliary tree stem cells, MSCs, adipose-derived stem cells, umbilical cord cells, amniotic fluid–derived epithelial cells, embryonic stem cells (ESCs) and iPSCs have been investigated for their potential as therapy for chronic liver failure, homing of therapeutic cells to the liver remains a challenge [17]. Although the injection into the hepatic artery showed the greatest percent of engraftment [18], injection into the liver parenchyma (~10–20%) and the portal vein (<5%) was still consistent with poor engraftment and significant ectopic cell distribution to the vascular beds of other tissues suggesting a high risk of ectopic liver formation [19]. Given that hyaluronic acid is selectively and specifically cleared by the liver and that it has been implicated in various aspects of stem cell therapy optimization [20], Cardinale’s team hypothesized that it would not only enhance engraftment but also improve the applicability of human biliary tree stem cells to treat liver cirrhosis [21]. Interestingly, hyaluronic acid coating of human biliary tree stem cells markedly improved viability, colony formation, and population doubling in primary cultures and resulted in higher expression of integrins that are key players and mediators of cell attachment to the extracellular matrix. When hyaluronic acid–coated biliary tree stem cells were transplanted via the spleen into the liver of immunocompromised mice, the engraftment efficiency increased from 3% of uncoated cells to 11%. Notably, hyaluronic acid–coated human biliary tree stem cell transplantation in mice resulted in a 10-fold increase of human albumin gene expression in the liver and in a 2-fold increase of human albumin serum levels with respect to uncoated cells. Moreover, when other organs were sectioned and stained to track the cells in question, only minimal ectopic cell distribution was detected. Furthermore, because one of the major limitations of cellular therapies is their need for long-term storage, researchers are devising strategies to make this possible. To meet this critical aspect of liver cellular therapies and optimize their sourcing, Cardinale’s group has developed a cryopreservation protocol consisting of a stepwise use of serum-free Kubota’s medium supplemented with 10% dimethyl sulfoxide, 15% human serum albumin and 0.1% hyaluronans [22]. When freshly isolated biliary tree stem cells were cultured in vitro and compared with their cryopreserved counterparts, no differences were noted in terms of self-replication, stemness traits and multi-potency. Just like freshly isolated cells, cryopreserved cells were able to differentiate into functional hepatocytes, cholangiocytes or pancreatic islets and to yield similar capacity to secrete albumin and glucose-inducible insulin. This technology may be expanded to multiple cell types and promises to facilitate the establishment of cell banks with obvious logistic advantages.
Dr. Debashis Haldar: MSCs for inflammatory liver disease
Dr. Haldar presented a review of the use of MSCs to treat inflammatory liver diseases. Initial observations that bone marrow cells may contribute to hepatic repair and regeneration [23–25] were followed by studies in animal models of chronic liver injury showing variable therapeutic effects [26–28]. Although it is well known that MSCs home to areas of acute inflammation, reduce inflammatory damage and oxidative stress and even contribute to differentiated epithelium, the impact on prevention of induction and/or progression of fibrosis remains controversial [29]. Along with the anti-inflammatory action of MSCs and various lines of evidence suggesting that they may directly exert antifibrotic effects [30], conflicting data paradoxically point to a possible pro-fibrotic role [29]. For instance, MSCs may reduce the in vitro production of profibrotic factors and influence macrophages polarization, but in animal models of chronic liver disease, diametrically opposed results have supported both an anti-fibrotic role and an increase in markers of fibrosis such as collagen deposition. In fact, in an elegant series of gender-mismatched bone marrow reconstitution studies, Russo et al. showed that bone marrow cells contributed to myofibroblast generation and collagen expression [31]. By using bone marrow cells with a β-gal reporter under the control of a α2(I)collagen enhancer, this group demonstrated the presence of β-gal–expressing cells around areas of scarring in the liver after 12 weeks of carbon tetrachloride injury, and in situ hybridization confirmed collagen expression in bone marrow–derived cells. To determine whether the bone marrow–derived cells’ functionally contributed to increased fibrosis in this model, irradiated mice received bone marrow–derived cells from Col-1a1rr mice, which have a mutated collagenase-resistant collagen, and upon injury, wild-type mice developed extensive pericellular fibrosis similar to that seen in Col-1a1rr mice. To determine which cells within the bone marrow were responsible, this group performed new gender-mismatched reconstitution experiments, showing that marrow stromal cells, not hematopoietic stem cells, were responsible for bone marrow–derived myofibroblasts in the liver. The same group also published a case series of male patients who had undergone liver transplantations from female donors and who had gone on to require another transplant for cirrhosis [32]. Explanted liver tissues were analyzed forY chromosome by in situ hybridization together with markers for hepatic stellate cells and myofibroblasts, revealing that 14–45% of myofibroblast were indeed of recipient origin. In contrast to these reports implicating bone marrow stromal cells in fibrosis, Miyata et al. provided evidence of a dominant role for hematopoietic stem cells [33], and although other groups have not been able to replicate a role for bone marrow–derived stem/stromal cells in fibrosis [34], an important contribution of hepatic stellate cells to fibrosis [35] has emerged. Significant heterogeneity in experimental designs, that is, approaches to inducing chronic liver disease, timing, route of administration and animal models have precluded an in-depth understanding of potential MSC-mediated mechanisms of action in this condition. Despite mixed results, more than 10 clinical trials between 2007 and 2014 using either autologous [36] or allogenic [37] MSCs suggested that they are at least safe in chronic liver disease [38,39]. The end points of the studies were to evaluate the safety and efficacy of bone marrow and umbilical cord MSC transplantation. The cells were mostly infused intravenously, although two studies reported infusions via the hepatic artery [39,40] and one in the spleen [41]. Moreover, there was great variation in both the number of cells infused per patient and the frequency of injections among the trials. The results of the studies seemed promising in terms of improvement of liver function and a model for an end-stage liver disease score. This score is based on objective variables (international normalized ratio and serum creatinine, bilirubin and sodium concentration) and has been validated as a predictor of survival among patients with advanced liver disease [42]. However, for most of the studies discussed by Dr. Haldar, data regarding evaluation of liver histology after cell transplantation is lacking; most studies were underpowered to detect significant differences, controls were either lacking or inadequate and the follow-up period was too short. One of the primary challenges remains the inability to track and monitor the transplanted cells and the absence of standardized transplantation protocols. Thus, standardizing and harmonizing protocols that fully agree on the timing of cell injection following the stage of liver fibrosis, number (perhaps type) of cells and administration route would significantly allow for meaningful advances in the field to be achieved. Overall, a large body of literature suggests that MSCs may exert a potent anti-inflammatory effect in the setting of liver diseases, that when exploited in a timely fashion following acute liver inflammation, may reduce progression to fibrosis. The need for well-controlled randomized clinical trials in this area is paramount to address key questions regarding the potential applicability of this technology as a widely accepted strategy for the management of acute liver disease.
Dr. Basak Uygun: bioengineering the liver—future prospects
Dr. Uygun presented the state-of-the-art of liver bioengineering, starting by emphasizing that a major issue in manufacturing hepatic grafts for transplantation purposes is the difficulty of producing a three-dimensional architecture that mimics the in vivo environment. At Harvard Medical School, her group [43–45] is using native extracellular matrix–based scaffolds to bioengineering liver grafts. In fact, native extracellular matrix represents a biochemically, geometrically and spatially ideal platform for organ bioengineering and regeneration because it is biocompatible, has both basic components (proteins and polysaccharides) [46], retains an intact and patent vasculature that, when implanted in vivo, sustains physiologic blood pressure and is able to drive differentiation of progenitor cells into an organ-specific phenotype [47]. When cells are seeded within an intact extracellular matrix scaffold and are allowed to mature into bioreactors, cells attach to the scaffold, proliferate and show signs of active metabolism and effective function [46]. For these reasons, organ-derived scaffolds have become the scaffolding material of choice in the bioengineering of complex organs [48]. By adapting the perfusion de-cellularization technique to liver and introducing perfusion re-cellularization to innovate the process of cell seeding of the extracellular matrix graft, Uygun et al. were able to preserve both the liver-specific extracellular matrix and three-dimensional architecture [43]. Most importantly, the de-cellularized liver matrix retained the underlying matrix of the vascular network, which can be readily connected to the circulation, facilitating rapid oxygen and nutrient delivery after transplantation. Retention of vascular structures allowed for transplantation of partially recellularized liver graft in rats, and post-transplantation analysis demonstrated preservation of hepatocyte structure and function with minimal signs of ischemic damage. Preliminary re-cellularization with endothelial cells also indicated attachment and viability within the re-cellularized matrix in vitro. For translation, the major challenge besides the regeneration of the parenchymal compartment—that, as of now, not only has never been reported but its feasibility has been critically questioned [49]—is the scaling up to human-compatible models. In that respect, Mazza et al. recently reported on the complete de-cellularization of whole human liver and lobes to form an extracellular matrix scaffold with a preserved architecture [50]. Decellularized human liver cubic scaffolds were repopulated for up to 21 days using different human cell lines of hepatic stellate cells, hepatocellular carcinoma and hepatoblastoma, with excellent viability, motility and proliferation, and remodeling of the extracellular matrix. The key contribution of Mazza and co-workers is the development of a novel decellularization protocol based on high g-force oscillation, leading to the successful removal of immunogenic cellular materials, while maintaining the extracellular matrix protein composition and three-dimensional architecture. This allowed the entire human liver to be de-cellularized instead of the wedge sections of normal human liver tissue obtained by surgical resections for the development of biological human liver extracellular matrix scaffolds.
Session II: intestinal diseases
Regarding the intestine, almost all information available on cell therapies refers to CD, given that ulcerative colitis has a rescue and definitive therapeutic option with proctocolectomy for refractory cases. The goal in the therapeutic management of CD patients relies on the induction and maintenance of remission while achieving mucosal healing, with the ultimate effect of preserving intestinal function [51]. Currently accepted therapies are based on the use of immunomodulators or cytokine-directed immune-neutralizing antibodies to modify the natural history of the illness and therefore ameliorating the overall outcome [52]. Nevertheless, a high proportion of treated patients either does not respond or does not tolerate these molecules, fostering interest toward alternative treatment approaches, in particular cellular therapies based on the use of MSCs.
Dr. StefaniaVetrano: MSCs—the potential to regenerate and restore tolerance in immune-mediated intestinal diseases
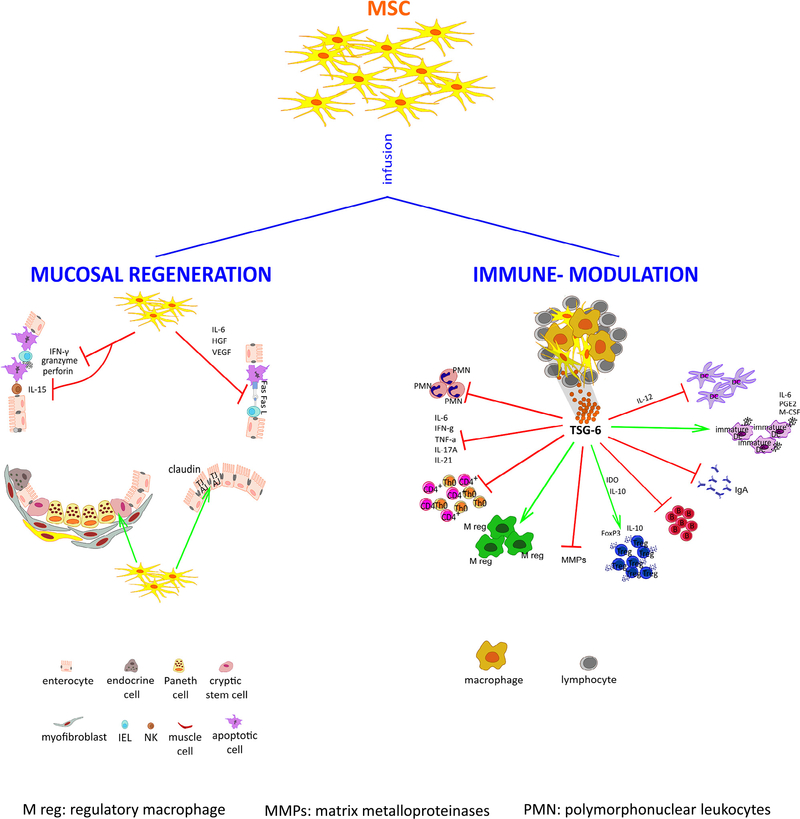
Dr. Vetrano provided evidence of the potential of MSCs to induce regeneration of the intestinal epithelium and restore self-tolerance in immune-mediated intestinal diseases (Figure 2). After successful preliminary experiments in mouse models of colitis showing the striking ability of MSCs to prevent and improve tissue damage [53, 54], a number of open-label phase 1–2 studies were conducted to test the use of autologous or allogeneic systemic infusions of bone marrow- and placenta-derived MSCs for the treatment-resistant CD [55–58]. The results showed this therapeutic approach to be feasible and safe, as well as significantly effective, with disease remission achieved in half the patients in a follow-up ranging from 6 weeks to 24 months. Notably, the best outcome was obtained when serial infusions were performed [58], thus opening up the question of the half-life or at least the duration of the therapeutic effects of MSCs in this clinical setting, a crucial point in establishing the right schedule of infusions. Importantly, although initially it was believed that MSCs would exert their effects locally after engraftment in the damaged dysfunctional tissue [59], it has become clear that homing in the gastrointestinal district is not a sine qua non condition because MSCs actually exert their function through the release of paracrine factors [60], consistent with data from other health science fields [61–64]. Vetrano’s group has recently observed that although an intraperitoneal injection of bone marrow–derived MSCs ameliorated the mucosal features and overall outcome in an experimental model of colitis [65], <1% of injected MSCs reached the inflamed colon. Indeed, most of the MSCs remained at the site of injection, forming aggregates together with immune cells (i.e., macrophages and T and B lymphocytes), thus promoting the secretion of immunoregulatory molecules. Interestingly, neither therapeutic effect nor MSC aggregates were observed when the cells were administered intravenously, thus raising the importance of the route of administration of MSCs to exert their beneficial effects. The tumor necrosis factor-α-stimulated gene-6, an anti-inflammatory protein, was found highly increased in sera of mice treated with MSCs within 72 h from the intraperitoneal injection compared with placebo controls, which correlated with the amelioration of colitis. Moreover, similar to MSCs, recombinant tumor necrosis factor-α–stimulated gene-6 treatment improved survival rate by reducing both systemic and mucosal levels of inflammatory mediators, neutrophil infiltration and metalloproteinase activity and enhancing expansion of macrophages and T cells with a regulatory phenotype. These findings strongly correlate with other reports demonstrating that MSCs modulate inflammatory response both promoting a switch from a T-helper 1 cytotoxic response to a less detrimental T-helper 2 one [66] and recruiting transforming growth factor-β and interleukin-10 producing regulatory T cells in the inflamed gut [67], while promoting mucosal healing by stimulating angiogenesis [68]. The evidence that MSCs lacking tumor necrosis factor-α–stimulated gene-6 fail to exert any therapeutic effect in experimental colitis further corroborates the hypothesis that this molecule is crucial for MSC activities and can be considered a predictive biomarker to assess the response to MSC-based therapy in the treatment of CD. It has also emerged that MSCs’ paracrine functions could be largely mediated by their extracellular vesicles. These are membrane bodies that comprise microvesicles and exosomes [69] containing full bioactive molecules—namely, proteins, lipids, mRNA, microRNAs and nuclei acids [70]. After receptor-mediated attachment or internalization, target cells eventually undergo a wide variety of epigenetic reprogramming and phenotypic changes [71], which may be exploited for therapeutic purposes. In this regard, the role of extracellular vesicles as conveyors of immune and regenerative capabilities has already been demonstrated in several in vitro [72] and in vivo experimental models [73–75]. Extracellular vesicles have a series of advantages over their cellular counterpart because they are more stable; induce stronger and reproducible signaling; are easier to sterilize, store and deliver; and carry no risk of aneuploidy or immune rejection upon allogeneic administration. MSC-derived extracellular vesicles may therefore be regarded as an alternative, cell-free therapy for chronic inflammatory conditions [71, 76].
Figure 2.
Role of mesenchymal stem/stromal cells in mucosal regeneration and immune-modulation. Once injected in vivo, mesenchymal stem/stromal cells are able to rescue enterocytes from apoptosis by blocking both cytolytic mechanisms, that is, Fas-Fas ligand cognate interaction and perforin-granzyme secretion, inhibit IL-15 secretion and function; preserve the epithelial barrier by reassembling claudins, the apical-most proteins of the tight junctions; and protect crypt stem cells (left side). Moreover, mesenchymal stem/stromal cells form aggregates with lymphocytes and release TSG-6, a potent anti-inflammatory soluble factor, that reduces the levels of proinflammatory mediators, such as TNF-α, IFN-γ, IL-6, IL-17A and IL-21; inhibit the recruitment of neutrophils; block the MMP activity and immunoglobulin secretion, while promoting the expansion of T cells (mainly the FoxP3+) and macrophages with immunosuppressive and regulatory activities. In addition, they cause an abortive maturation of dendritic cells, thus preventing efficient antigen presentation to T lymphocytes (right side). Red lines indicate an inhibitory effect; green lines indicate a stimulatory effect. AJ, adherens junctions; B, B cell; DC, dendritic cell; FasL, Fas ligand; Fox, transcription factor Forkhead box; HGF, hepatocyte growth factor; IDO, indoleamine 2,3-dioxygenase; IEL, intraepithelial lymphocyte; IFN, interferon; Ig, immunoglobulin; IL, interleukin; M-CSF, macrophage-colony stimulating factor; M reg, regulatory macrophage; MMPs, matrix metalloproteinases; MSC, mesenchymal stem/stromal cell; NK, natural killer cell; PGE2, prostaglandin E2; PMN, polymorphonuclear leukocytes;Th,T helper;Treg, regulatory T cell;TGF, transforming growth factor;TNF, tumor necrosis factor; TJ, tight junctions; TSG-6, tumor necrosis factor (TNF) stimulates gene-6; VEGF, vascular endothelial growth factor. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Prof. Daniel C. Baumgart: fistulizing crohn disease—can stem cells successfully take on the challenge?
Prof. Baumgart reported the results of a randomized, double-blind, parallel-group, placebo-controlled study conducted in 49 hospitals of seven European countries and Israel from July 6, 2012, to July 27, 2015, in which expanded allogeneic adipose-derived MSCs (Cx601) were used to treat perianal fistulas complicating CD [77]. These lesions affect up to 28% of patients during the first 2 decades after diagnosis, particularly those with colonic disease and rectal involvement [78], and because they are frequently complex and penetrate into neighboring organs [79], they tend to cause severe morbidity [80], thus dramatically impairing patients’ quality of life. Unfortunately, the treatment armamentarium remains inadequate. In fact, conventional medical treatment strategies (i.e., antibiotics and immunomodulators) are ineffective, and lesions tend to recur in more than two-thirds of patients [78,80]. Overall, only a few patients achieve long-term remission [80], and debilitating surgeries with diverting stomas or proctocolectomy are often the last resort after failure of or intolerability to medical treatment. In this scenario, the need for alternative effective and noninvasive therapies is urgent. Following promising phase 1 and 2 studies (see Table I), 212 patients suffering from CD and draining complex perianal fistulas who did not respond to conventional or biological treatments were recruited and randomly assigned to receive either a single local injection of placebo or of an industrial preparation of adipose-derived MSCs (Cx601) [77]. Cell therapy was more effective than placebo in achieving combined remission (defined as closure of all treated draining external openings, and absence of collections >2 cm of the treated perianal fistulas, confirmed by magnetic resonance imaging), because 50% of MSC-treated patients achieved remission versus 34% in the placebo group at week 24, within a shorter period of time (6.7 versus 14 weeks). Moreover, MSC-treated patients experienced a lower complication rate as demonstrated by the fact that only 17% of these patients versus 29% of those enrolled in the placebo group reported treatment-related adverse events (i.e., anal abscess and proctalgia). Other than safety and efficacy, it is worth emphasizing that the cell preparation studied offers a ready-to-use cellular suspension that may overcome one of the major hurdles commonly encountered in cellular therapy: the lack of standardization in the manufacturing of the cells of interest and the need for a Good Manufacturing Protocol–certified cell factory. However, although the sustainability of the MSC effect at 6–12 months (see Table I) appears higher than that observed with the biological agents that represent the first-line treatment for this condition [90], if follow-up is extended to 2 or 5 years [91,92], the rate of fistula recurrence increases progressively, highlighting the need to repeat the treatment over time.
Table I.
Mesenchymal stromal cell therapy in fistulizing Crohn disease.
| Study (year) | Number of patients (F/M) Age (years) | Disease duration (years) | Study Phase | Source | Number of injections | Fistula closure total/partial | Follow-up | Relapse |
|---|---|---|---|---|---|---|---|---|
| Garcia-Olmo (2009) [81] | 14 (NA) NA (range 33–62) |
NA | Phase 2 | Autologous adipose-tissue | 2 | Yes | 12 months | Yes |
| Ciccocioppo (2011) [82] | 10 (3/7) Median 33 (range 16–59) |
Median 9,5 (range 2–21) | Phase 1–2 | Autologous bone marrow | 4 | Yes | 12 months | Not |
| de la Portilla (2013) [83] | 24 (13/11) Mean 36.0 (SD 9.0) |
>12 months | Phase 1–2 | Allogeneic adipose-tissue | 2 | Yes | 6 months | Yes |
| Lee (2013) [84] | 43 (13/30) Median 26 (range 21–32) |
Median 5,5 (range 1–8) | Phase 1 | Autologous adipose-tissue | 2 | Yes | 12 months | Not |
| Cho (2013) [85] | 10 (6/4) Mean 26.5 (SD 6.0) |
NA | Phase 1 | Autologous adipose-tissue | 1 | Yes | 8 months | Not |
| Molendijck (2015) [86] | 21 (9/12) Median 29 (range 24–41) |
Median 5 (range 0.5–10) | Phase 2 | Allogeneic bone marrow | 1 | Yes | 6 months | Not |
| Park (2015) [87] | 6 (2/4) Mean 32 |
Range 1–12 | Phase 1 | Allogeneic adipose-tissue | 1 | Yes 3/3 | 8 months | Yes |
| Garcia-Arranz (2016) [88] | 10 (10) Mean 35 (range 31–55) |
Mean 11.6 (range 1–23) | Phase l–2a | Allogeneic adipose-tissue | 1–2 | Yes (Recto-vaginal) | 12 months | Yes |
| Dietz (2017) [89] | 12 (6/6) Mean 54 (range 18–58) |
Mean 6.5 (Range 2–17) | Phase 1 | Autologous adipose-tissue + Bioabsorbable matrix | 1 | Yes 10/2 | 6 months | Not |
NA, not available; F/M, female/male.
Prof. Paolo De Coppi: bioengineering the gut—future prospects of regenerative medicine
Prof. De Coppi updated us on the work done by his team in the field of upper gastrointestinal bioengineering, the overarching goal of which is to provide alternative therapies for esophageal atresia. This is a relatively rare congenital defect that affects 1 in 2,500–4,000 births [93] and comprises a variety of congenital anatomic defects that are caused by an abnormal embryological development. Anatomically, the most frequent defect is a congenital obstruction of the esophagus with interruption of the continuity of the esophageal wall, with or without a concomitant tracheoesophageal fistula. The treatment depends on the severity of the condition. In approximately 90% of cases, primary anastomosis of the esophagus can be done, but in the most severe cases, in which an extremely long gap exists, treatment consists of esophageal replacement by gastric pull-up or transposition of a colonic or jejunum segment. Unfortunately, these procedures are burdened by high morbidity and both the prognosis and quality of life of affected patients remain largely inferior to the general population. In this setting, an ad hoc bioengineered esophageal segment manufactured from the patient’s own cells may potentially offer a valuable therapeutic alternative. Notably, previous experience with upper airway bioengineering [94,95] has shown feasibility of such an approach, even if safety and long-term efficacy remain to be proven [96]. Work from De Coppi’s team at University College of London (United Kingdom) has shown that hollow organs like the gastrointestinal tract can be successfully decellularized to obtain acellular extracellular matrix scaffolds that may be used as template for the regeneration and bioengineering of the organ of interest [97]. Capitalizing on this successful approach and understanding of the molecular embryology underlying foregut development and the defects associated with esophageal atresia, his group is using a combinational approach in which acellular extracellular matrix scaffolds obtained from the pig are seeded with patientderived cells. Investigations have progressed quite successfully to the point that the team has announced that the first clinical trial may start in 2018 after approval from the Medicines and Healthcare Products Regulatory Agency (http://www.gosh.nhs.uk/research-and-innovation/nihr-great-ormond-street-brc/brc-news/researchers-announce-oesophagus-regeneration-trial).
Session III: endocrine pancreas and type 1 diabetes
Type 1 diabetes represents a formidable platform for the application of cellular therapies aiming to replenish the β-cell compartment or modulate the immune system to either arrest the disease or prevent its recurrence after whole pancreas or islet transplantation. The symposium focused on the first topic. In general, the management of diabetes mellitus consists of a combination of medical (based on oral antidiabetic agents and insulin injections) and behavioral (dietary restrictions and regular physical activity) approaches. However, although exogenous insulin therapy is effective at preventing acute metabolic decompensation and is lifesaving, less than 40% of type 1 diabetic patients achieve recommended therapeutic goals, and therefore a large number of diabetic patients, especially those who do not use insulin-pump devices [98], are inadequately controlled and destined eventually to develop one or more end-stage organ complications during their lifetime. In this scenario, β-cell replacement is the only therapy at present that reliably establishes a long-term stable euglycemic state; however, its applicability in the form of either whole pancreas or islet transplantation has been limited by critical hurdles such as a shortage of usable pancreases, need for anti-rejection therapy, cost and associated morbidity [99,100]. Moreover, although islet transplantation may appear more appealing due to its lower cost and morbidity compared with whole pancreas transplantation, so far it has not replicated the same outstanding results as whole pancreas transplantation [101–104]. Thus, transplantation currently represents the standard of care in β-cell replacement and should be offered to patients with a low surgical risk, whereas type 1 diabetic patients with a high surgical risk should undergo islet transplantation [104]. Yet at a recent Opinion Leaders Meeting of The International Pancreas and Islet Transplant Association (IPITA), in conjunction with the Transplantation Society (TTS) on the Future of β-Cell Replacement, it was felt that islet transplantation will become the preferred therapeutic procedure for β-cell replacement because of the metabolic efficiency and the superior safety profile of the islet in relation to whole pancreas transplantation [105]. For islet transplantation to replace pancreas transplantation, the most important future improvements need to be made in isolation techniques so that single infusions with consistently high islet yields become the norm, as well as in delivery methods so that islets can engraft and be viable long term in supportive niches, as well as in the identification of potentially inexhaustible sources of islets across species and in camouflaging techniques to prevent allorecognition.
Prof. Lorenzo Piemonti: state of the art of cell therapy for type 1 diabetes
Prof. Piemonti gave the introductory lecture with which the state of the art of cellular therapies as they are applied to type 1 diabetes [106] was illustrated, starting with the revival of xenotransplantation [107]. The use of xeno islets represents an older alternative that has gained renewed interested due to novel recent perspectives [107]. Porcine islets are a valuable source of transplantable islets because of their physiological affinity to their human counterparts, their theoretical abundance and, importantly, because pigs can be genetically modified for making their islets more suitable for the transplantation in humans [108]. In fact, the deletion of the galactose-α1,3-galactose gene, a saccharide expressed on cells of lower mammals but not on cells of humans or monkeys and against which humans have natural preformed antibodies, as well as the (recently reported) inactivation of porcine endogenous retroviruses enabled by CRISPR-Cas9 technology [109], bear potential to rule out the two major hurdles of islet xenotransplantation, namely the risks of an hyperacute immunologic rejection and zoonosis that so far have been limited to nonhuman species. Moreover, neonatal pig islets are also being microencapsulated to prevent allorecognition and escape host immune surveillance, and translation into humans has been performed, although with unclear results (reviewed in Pellegrini et al.) [106]. Currently, however, the most significant advances and the greatest promise come from the stem cell field, which is offering opportunities for the cell therapy of single-cell disorders such as type 1 diabetes. In fact, various groups have shown that human ESCs and iPSCs are able to generate pancreatic progenitors and/or functional β-cells in vitro (reviewed in Pellegrini et al.) [106]. In particular, the optimization of in vitro strategies to differentiate human ESCs into mature insulin-secreting cells has made considerable progress and recently led to the first clinical trial of stem cell treatment for this condition. In addition, the discovery that it is possible to derive human iPSC from somatic cells has shown that it may be possible to derive a sufficient number of patient-specific cells through the reprogramming and differentiation of patients’ own cells, suggesting that in the future, this technology may allow immunosuppression-free transplantation. In the case of both ESC- and iPSC-derived β cells, cells are phenotypically and physiologically similar to β cells and can treat diabetic mice after implantation. Moreover, the stem cell approach may synergize well with other developing innovations such as the generation of immune isolating and retrievable devices, which is fundamental to allow cell therapy without immunosuppression and to overcome safety concerns.
Dr. M. Cristina Nostro: generation of pancreatic progenitors from human pluripotent stem cells
Dr. Cristina Nostro addressed a major hurdle that the field is encountering. In fact, despite that it is possible to engineer human pluripotent stem cells to generate pancreatic progenitor cells that may be used as source of functional β cells (and other mature pancreatic cells) when transplanted into mice, the efficiency of pancreatic progenitor cell generation in vitro is highly variable (ranging between 6% and 80%) and cell-dependent, thus negatively affecting reproducibility and validation of in vitro and in vivo studies, and consequently translation to the clinic. Therefore, increasing purity of pancreatic progenitor cells is crucial to producing mature cell types for clinical applications. Dr. Nostro’s group in Toronto recently described the use of a proteomics approach to phenotypically characterize pancreatic progenitor cells obtained from human pluripotent stem cells and thus allow sorting of these cells from non-pancreatic progenitor cell populations during differentiation [110]. Specifically, the pancreatic secretory granule membrane major glycoprotein2, which is co-expressed with NKX6–1 and PTF1A in human developing pancreata, was identified as critical pancreatic progenitor cell-specific surface marker. Strikingly, these glycoprotein2+ cells obtained from human pluripotent stem cells were able to generate β-like cells (C-PEPTIDE+/NKX6–1+) more efficiently compared with glycoprotein2− and unsorted populations, highlighting the potential therapeutic applications of this marker.
Dr. Alice Tomei: conformal coating to improve islet encapsulation technology
Islet encapsulation has been designed to immuno-isolate transplanted cells and prevent direct immune attack against alloantigens, thus allowing immunosuppression-free islet transplantation. By this technique, islets are incorporated within biocompatible capsules that allow nutrient diffusion and glucose-stimulated insulin secretion while providing immuno-isolation from allo- and auto-reactive cells. Clinical trials have proven the safety but not the long-term efficacy of islet encapsulation [111]. However, the majority of trials have been conducted with capsules of uniform 500–1000 m diameter, despite islets having a variable size between 50 and 350 m and with the peritoneal cavity as the transplant site. Therefore, the reasons for clinical failure of those traditional encapsulation protocols can be associated with the large capsule size, which impairs diffusion of nutrients to the encapsulated islets, causes delays in glucose-stimulated insulin secretion and limits the choice of the transplant site. In fact, it is known that cells can only survive within a 300-m distance from the source of nutrients [111]. If we consider that islet size ranges between 50 and 350 m and that capsules may measure up to 1000 m, larger islets are prone to central ischemia and delayed glucose-stimulated insulin secretion, and the larger the capsules, the worse these effects will be. To minimize the distance between the islets and the source of nutrients—namely, the host capillary bed—naked isolated islets can be transplanted in confined and vascularized sites, where they can be rapidly revascularized reaching the optimal configuration that eliminates the risk of central hypoxia [112]. Conversely, due to their large volume, islets encapsulated in traditional large capsules cannot be transplanted in confined sites at curative doses [113]. In this regard, Tomei’s group has recently developed an encapsulation technology that allows “wrapping” each individual islet with a uniformly thin (≈15 μm) layer of biomaterial, generating capsules that “conform” to the size and shape of the islet [114]. By reducing the diffusion distance of 10-fold, this conformal coating allows increased nutrient transport. By reducing the overall graft volume more than 100-fold, conformal coating also makes possible islet transplantation in well-vascularized confined sites without the need to use the intraperitoneal cavity as site of injection, further maximizing nutrient transport. Importantly, contrary to islets in traditional microcapsules, conformal coated islets display no delay in glucose-stimulated insulin secretion [114,115]. Tomei’s group demonstrated long-term euglycemia after transplantation of fully MHC-mismatched conformal coated grafts in diabetic mice without immunosuppression using polyethylene glycol hydrogels [115], and they were able to show that their conformal coating platform is suitable for use with essentially unlimited insulin-secreting cell sources derived from stem cells (unpublished results). Moreover, because macrophage activation and suboptimal oxygen tension at the graft site right after transplantation are responsible for loss of a portion conformal coated islets in the first few days after transplantation, nanomedicine approaches that target innate immune cell activation [116] and increase local oxygen concentrations were successfully integrated within the conformal coating system (unpublished results).
Conclusions
Dr. Catherine Klersy: proposal of an online platform for collection and monitoring of data from clinical trials exploring the use of MSCs in digestive diseases
The final presentation from Dr. Klersy was a call for an online-platform for collection and monitoring of data from clinical trials exploring the use of MSCs in digestive diseases. The expectation is that developing a shared platform for collection and monitoring of cell therapy–related trial data would facilitate the identification of the design and outcome measures needed to speed up the translation of this enormous potential to patients’ care. Therefore, she proposed establishing a new online registry by using RedCap (Research Electronic Data Capture), a novel workflow methodology and software tool that expedites the electronic collection of research data from a single- or multi-site clinical research study that was developed at the Vanderbilt University (Nashville, Tennessee, USA) [117]. The software supports a secure web-based application for developing fully functional case report forms and surveys. In particular, through RedCap it is possible to implement: (a) full user authentication (log-on/password) to restrict users to study functions; (b) an intuitive interface for validated data entry; (c) real-time data validation and integrity checks for ensuring data quality; (d) de-identification options to be applied to data exports to remove fields that contain notes and other information that could identify patients; (e) centralized, secure storage of research data with backups (f) automated export procedures for seamless data downloads to common statistical packages and (g) procedures for importing data from external sources [117]. All data collected would be entered into a local regulation-compliant data management system provided by the Service of Biometry & Clinical Trial Center of the I.R.C.C.S. Policlinico San Matteo Foundation (Pavia, Italy).
Acknowledgments
The authors thank both the International Society for Cellular Therapy and both the Sponsors of the First Signature Series Event, “Advancements in Cellular Therapies and Regenerative Medicine for Digestive Diseases,” May 3, 2017, London, United Kingdom: TiGenix (Parque Tecnologico de Madrid, Spain) and Sino-Biocan (Biotechnology Co., Ltd., Dongcheng, Beijing, China).
Footnotes
Disclosure of interest: The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- [1].Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12:720–7. [DOI] [PubMed] [Google Scholar]
- [3].Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 2010;42:587–95. [DOI] [PubMed] [Google Scholar]
- [4].Gronbaek L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol 2014;60:612–17. [DOI] [PubMed] [Google Scholar]
- [5].Schneider A, Michaely H, Weiss C, Hirth M, Rückert F, Wilhelm TJ, et al. Prevalence and incidence of autoimmune pancreatitis in the population living in the Southwest of Germany. Digestion 2017;96:187–98. [DOI] [PubMed] [Google Scholar]
- [6].Mayer-Davis EJ, Dabelea D, Lawrence JM. Incidence trends of Type 1 and Type 2 diabetes among youths, 2002–2012. N Engl J Med 2017;377:301. doi: 10.1056/NEJMc1706291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jess T, Frisch M, Simonsen J. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clin Gastroenterol Hepatol 2013;11:43–8. [DOI] [PubMed] [Google Scholar]
- [8].Torres J, Burisch J, Riddle M, Dubinsky M, Colombel JF. Preclinical disease and preventive strategies in IBD: perspectives, challenges and opportunities. Gut 2016;65: 1061–9. [DOI] [PubMed] [Google Scholar]
- [9].Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008; 371:838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Santivasi WL, Strand JJ, Mueller PS, Beckman TJ. The organ transplant imperative. Mayo Clin Proc 2017;92:940–6. [DOI] [PubMed] [Google Scholar]
- [11].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- [12].Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 2014;14:370–84. [DOI] [PubMed] [Google Scholar]
- [13].Du Y, Wang J, Jia J, Song N, Xiang C, Xu J, et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell 2014;14:394–403. [DOI] [PubMed] [Google Scholar]
- [14].Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, et al. Direct reprogramming of hepatic myofibroblasts into hepatocytes in vivo attenuates liver fibrosis. Cell Stem Cell 2016;18:797–808. [DOI] [PubMed] [Google Scholar]
- [15].Reetz J, Genz B, Meier C, Kowtharapu BS, Timm F, Vollmar B, et al. Development of adenoviral delivery systems to target hepatic stellate cells in vivo. PLoS ONE 2013;8:e67091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Puche JE, Lee YA, Jiao J, Aloman C, Fiel MI, Munoz U, et al. A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. Hepatology 2013;57:339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lanzoni G, Oikawa T, Wang Y, Cui CB, Carpino G, Cardinale V, et al. Concise review: clinical programs of stem cell therapies for liver and pancreas. Stem Cells 2013;31:2047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, et al. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant 2010;19:409–18. [DOI] [PubMed] [Google Scholar]
- [19].Puppi J, Strom SC, Hughes RD, Bansal S, Castell JV, Dagher I, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant 2012;21:1–10. [DOI] [PubMed] [Google Scholar]
- [20].Turner RA, Wauthier E, Lozoya O, McClelland R, Bowsher JE, Barbier C, et al. Successful transplantation of human hepatic stem cells with restricted localization to liver using hyaluronan grafts. Hepatology 2013;57:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nevi L, Carpino G, Costantini D, Cardinale V, Riccioni O, Di Matteo S, et al. Hyaluronan coating improves liver engraftment of transplanted human biliary tree stem/progenitor cells. Stem Cell ResTher 2017;8:68. doi: 10.1186/s13287-017-0492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nevi L, Cardinale V, Carpino G, Costantini D, Di Matteo S, Cantafora A, et al. Cryopreservation protocol for human biliary tree stem/progenitors, hepatic and pancreatic precursors. Sci Rep 2017;7:6080. doi: 10.1038/s41598-017-05858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science 1999;284:1168–70. [DOI] [PubMed] [Google Scholar]
- [24].Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 2000;31:235–40. [DOI] [PubMed] [Google Scholar]
- [25].Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000;6:1229–34. [DOI] [PubMed] [Google Scholar]
- [26].Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology 2004;40:1304–11. [DOI] [PubMed] [Google Scholar]
- [27].Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, et al. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol 2005;33:108–19. [DOI] [PubMed] [Google Scholar]
- [28].Quintanilha LF, Mannheimer EG, Carvalho AB, Paredes BD, Dias JV, Almeida AS, et al. Bone marrow cell transplant does not prevent or reverse murine liver cirrhosis. Cell Transplant 2008;17:943–53. [DOI] [PubMed] [Google Scholar]
- [29].Haldar D, Henderson NC, Hirschfield G, Newsome PN. Mesenchymal stromal cells and liver fibrosis: a complicated relationship. FASEB J 2016;30:3905–28. [DOI] [PubMed] [Google Scholar]
- [30].Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation 2004;78:83–8. [DOI] [PubMed] [Google Scholar]
- [31].Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 2006;130:1807–21. [DOI] [PubMed] [Google Scholar]
- [32].Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology 2004;126:955–63. [DOI] [PubMed] [Google Scholar]
- [33].Miyata E, Masuya M, Yoshida S, Nakamura S, Kato K, Sugimoto Y, et al. Hematopoietic origin of hepatic stellate cells in the adult liver. Blood 2008;111:2427–35. [DOI] [PubMed] [Google Scholar]
- [34].Higashiyama R, Moro T, Nakao S, Mikami K, Fukumitsu H, Ueda Y, et al. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology 2009;137:1459–66e1. doi: 10.1053/j.gastro.2009.07.006. [DOI] [PubMed] [Google Scholar]
- [35].Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moore JK, Stutchfield BM, Forbes SJ. Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Aliment Pharmacol Ther 2014;39:673–85. [DOI] [PubMed] [Google Scholar]
- [37].Huebert RC, Rakela J. Cellular therapy for liver disease. Mayo Clin Proc 2014;89:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].King A, Barton D, Beard HA, Than N, Moore J, Corbett C, et al. REpeated AutoLogous Infusions of STem cells In Cirrhosis (REALISTIC): a multicentre, phase II, open-label, randomised controlled trial of repeated autologous infusions of granulocyte colony-stimulating factor (GCSF) mobilised CD133+ bone marrow stem cells in patients with cirrhosis. A study protocol for a randomised controlled trial. BMJ Open 2015;5:e007700. doi: 10.1136/bmjopen-2015-007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int 2014;34:33–41. [DOI] [PubMed] [Google Scholar]
- [40].Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology 2011;54:820–8. [DOI] [PubMed] [Google Scholar]
- [41].Amin MA, Sabry D, Rashed LA, Aref WM, el Ghobary MA, Farhan MS, et al. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. ClinTransplant 2013;27:607–12. [DOI] [PubMed] [Google Scholar]
- [42].Kamath PS, Kim WR, Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology 2007;45:797–805. [DOI] [PubMed] [Google Scholar]
- [43].Uygun BE, Izamis M-L, Jaramillo M, Chen Y, Price G, Ozer S, et al. Discarded livers find a new life: engineered liver grafts using hepatocytes recovered from marginal livers. Artif Organs 2017;41:579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Geerts S, Ozer S, Jaramillo M, Yarmush ML, Uygun BE. Nondestructive methods for monitoring cell removal during rat liver decellularization. Tissue Eng Part C Meth 2016; 22:671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bruinsma BG, Kim Y, Berendsen TA, Ozer S, Yarmush ML, Uygun BE. Layer-by-layer heparinization of decellularized liver matrices to reduce thrombogenicity of recellularized liver grafts. J Clin Transl Res 2015;1–9. doi: 10.18053/jctres.201501.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 2011;13:27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Orlando G, Soker S, Stratta RJ. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Ann Surg 2013;258:221–32. [DOI] [PubMed] [Google Scholar]
- [48].Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet 2012;379:943–52. [DOI] [PubMed] [Google Scholar]
- [49].Remuzzi A, Figliuzzi M, Bonandrini B, Silvani S, Azzollini N, Nossa R, et al. Experimental evaluation of kidney regeneration by organ scaffold recellularization. Sci Rep 2017;7:43502. doi: 10.1038/srep43502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mazza G, Al-Akkad W, Telese A, Longato L, Urbani L, Robinson B, et al. Rapid production of human liver scaffolds for functional tissue engineering by high shear stress oscillation-decellularization. Sci Rep 2017;7:5534. doi: 10.1038/s41598-017-05134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- [52].Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- [53].Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009;136:978–89. [DOI] [PubMed] [Google Scholar]
- [54].Jung WY, Kang JH, Kim KG, Kim HS, Jang BI, Park YH, et al. Human adipose-derived stem cells attenuate inflammatory bowel disease in IL-10 knockout mice. Tissue Cell 2015;47:86–93. [DOI] [PubMed] [Google Scholar]
- [55].Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut 2010;59:1662–9. [DOI] [PubMed] [Google Scholar]
- [56].Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, et al. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut 2012;61:468–9. [DOI] [PubMed] [Google Scholar]
- [57].Mayer L, Pandak WM, Melmed GY, Hanauer SB, Johnson K, Payne D, et al. Safety and tolerability of humanplacenta-derived cells (PDA001) in treatment-resistant crohn’s disease: a phase 1 study. Inflamm Bowel Dis 2013;19:754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol 2014;12:64–71. [DOI] [PubMed] [Google Scholar]
- [59].Wagner B, Henschler R. Fate of intravenously injected mesenchymal stem cells and significance for clinical application. Adv Biochem Eng Biotechnol 2013;130:19–37. [DOI] [PubMed] [Google Scholar]
- [60].Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J Cell Mol Med 2010;14:2190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Santeramo I, Herrera Perez Z, Illera A, Taylor A, Kenny S, Murray P, et al. Human kidney-derived cells ameliorate acute kidney injury without engrafting into renal tissue. Stem Cells Transl Med 2017;6:1373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med 2017;doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X, et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta 2017;1863:2085–92. [DOI] [PubMed] [Google Scholar]
- [64].Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev 2017;26:617–31. [DOI] [PubMed] [Google Scholar]
- [65].Sala E, Genua M, Petti L, Anselmo A, Arena V, Cibella J, et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology 2015;149:163–76. [DOI] [PubMed] [Google Scholar]
- [66].Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther 2011;2:34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 2008;36:309–18. [DOI] [PubMed] [Google Scholar]
- [68].Manieri NA, Mack MR, Himmelrich MD, Worthley DL, Hanson EM, Eckmann L, et al. Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. J Clin Invest 2015;125:3606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol 2015;77:13–27. [DOI] [PubMed] [Google Scholar]
- [71].Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 2013;13:1637–53. [DOI] [PubMed] [Google Scholar]
- [72].Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol 2014;5:556. doi: 10.3389/fimmu.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 2009;20:1053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012;126:2601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010;4:214–22. [DOI] [PubMed] [Google Scholar]
- [76].Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 2015;23:812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Panes J, Garcia-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet 2016;388:1281–90. [DOI] [PubMed] [Google Scholar]
- [78].Bell SJ, Williams AB, Wiesel P, Wilkinson K, Cohen RC, Kamm MA. The clinical course of fistulating Crohn’s disease. Alim Pharmacol Ther 2003;17:1145–51. [DOI] [PubMed] [Google Scholar]
- [79].Eglinton TW, Barclay ML, Gearry RB, Frizelle FA. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum 2012;55:773–7. [DOI] [PubMed] [Google Scholar]
- [80].Molendijk I, Nuij VJ, van der Meulen-de Jong AE, van der Woude CJ. Disappointing durable remission rates in complex Crohn’s disease fistula. Inflamm Bowel Dis 2014;20:2022–8. [DOI] [PubMed] [Google Scholar]
- [81].Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 2009;52:79–86. [DOI] [PubMed] [Google Scholar]
- [82].Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 2011;60:788–98. [DOI] [PubMed] [Google Scholar]
- [83].de la Portilla F, Alba F, García-Olmo D, Herrerías JM, Gonzalez FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis 2013;28: 313–23. [DOI] [PubMed] [Google Scholar]
- [84].Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim DS, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells 2013;31:2575–81. [DOI] [PubMed] [Google Scholar]
- [85].Cho YB, Lee WY, Park KJ, Kim M, Yoo H-W, Yu CS. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant 2013;22:279–85. [DOI] [PubMed] [Google Scholar]
- [86].Molendijck I, Bonsing BA, Roelofs H, Peeters KCMJ, Wasser MNJM, Dijkstra G, et al. Allogeneic bone marrow-serived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology 2015;149:918–27. [DOI] [PubMed] [Google Scholar]
- [87].Park KJ, Ryoo S-B, Kim JS, Kim TI, Baik SH, Kim HJ, et al. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: a pilot clinical trial. Colorectal Dis 2015;18:468–76. [DOI] [PubMed] [Google Scholar]
- [88].Garcia-Arranz M, Herreros MD, Gonzalez-Gomez C, de la Quintana P, Guadalajara H, Georgiev-Hristov T, et al. Treatment of Crohn’s-related rectovaginal fistula with allogeneic expanded-adipose derived stem cells: a phase I–IIa clinical trial. Stem Cell Transl Med 2016;5:1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dietz AB, Dozois EJ, Fletcher JG, Butler GW, Radel D, Lightner AL, et al. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology 2017;153:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut 2014;63:1381–92. [DOI] [PubMed] [Google Scholar]
- [91].Cho YB, Park KJ, Yoon SN, Song KH, Kim DS, Jung SH, et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med 2015;4:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ciccocioppo R, Gallia A, Sgarella A, Kruzliak P, Gobbi PG, Corazza GR. Long-term follow-up of Crohn disease fistulas after local injections of bone marrow–derived mesenchymal stem cells. Mayo Clin Proc 2015;90:747–55. [DOI] [PubMed] [Google Scholar]
- [93].Sfeir R, Michaud L, Salleron J, Gottrand F. Epidemiology of esophageal atresia. Dis Esophagus 2013;26:354–5. [DOI] [PubMed] [Google Scholar]
- [94].Gonfiotti A, Jaus MO, Barale D, Baiguera S, Comin C, Lavorini F, et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet 2014; 383:238–44. [DOI] [PubMed] [Google Scholar]
- [95].Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 2012;380:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Vogel G. Trachea transplants test the limits. Science 2013;340:266–8. [DOI] [PubMed] [Google Scholar]
- [97].Totonelli G, Maghsoudlou P, Garriboli M, Riegler J, Orlando G, Burns AJ, et al. A rat decellularized small bowel scaffold that preserves villus-crypt architetcure for intestinal regeneration. Biomaterials 2012;33:3401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA 2017;318:1358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Orlando G, Stratta RJ, Light J. Pancreas transplantation for type 2 diabetes mellitus. Curr Opin Organ Transplant 2011;16:110–15. [DOI] [PubMed] [Google Scholar]
- [100].Maffi P, Secchi A. Clinical results of islet transplantation. Pharmacol Res 2015;98:86–91. [DOI] [PubMed] [Google Scholar]
- [101].Lablanche S, Borot S, Wojtusciszyn A, Bayle F, Tetaz R, Badet L, et al. Five-year metabolic, functional, and safety results of patients with type 1 diabetes transplanted with allogenic islets within the Swiss-French GRAGIL network. Diabetes Care 2015;38:1714–22. [DOI] [PubMed] [Google Scholar]
- [102].Gruessner AC, Gruessner RW. Long-term outcome after pancreas transplantation: a registry analysis. Curr Opin Organ Transplant 2016;21:377–85. [DOI] [PubMed] [Google Scholar]
- [103].Gruessner RW, Gruessner AC. Pancreas after islet transplantation: a first report of the International Pancreas Transplant Registry. Am J Transplant 2016;16:688–93. [DOI] [PubMed] [Google Scholar]
- [104].Balamurugan AN, Naziruddin B, Lockridge A, Tiwari M, Loganathan G, Takita M, et al. Islet product characteristics and factors related to successful human islet transplantation from the Collaborative Islet Transplant Registry (CITR) 1999–2010. Am J Transplant 2014;14:2595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bartlett ST, Markmann JF, Johnson P, Korsgren O, Hering BJ, Scharp D, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016;100(Suppl. 2):S1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pellegrini S, Cantarelli E, Sordi V, Nano R, Piemonti L. The state of the art of islet transplantation and cell therapy in type 1 diabetes. Acta Diabetol 2016;53:683–91. [DOI] [PubMed] [Google Scholar]
- [107].Cowan PJ, Tector AJ. The resurgence of xenotransplantation. Am J Transplant 2017;17:2531–6. [DOI] [PubMed] [Google Scholar]
- [108].Klymiuk N, Aigner B, Brem G, Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev 2010;77:209–21. [DOI] [PubMed] [Google Scholar]
- [109].Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 2017;357:1303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Cogger KF, Sinha A, Sarangi F, McGaugh EC, Saunders D, Dorrell C, et al. Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors. Nat Commun 2017;8:331. doi: 10.1038/s41467-017-00561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Tomei AA, Villa C, Ricordi A. Development of an encapsulated stem cell-based therapy for diabetes. Exp Opin Biol Ther 2015;15:1321–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Najjar M, Manzoli V, Abreu M, Villa C, Martino MM, Molano RD, et al. Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol Bioeng 2015;112: 1916–26. [DOI] [PubMed] [Google Scholar]
- [113].Villa C, Manzoli V, Abreu M, Verheyen CA, Seskin N, Najjar M, et al. Effects of composition of alginate-polyethylene glycol microcapsules and transplant site on encapsulated islet graft outcomes in mice. Transplantation 2017;101:1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci USA 2014;111:10514–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Manzoli V, Villa C, Bayer AL, Morales L, Molano RD, Torrente Y, et al. Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol. Am J Transplant 2017;doi: 10.1111/ajt.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Dane KY, Nembrini C, Tomei AA, Eby JK, O’Neil CP, Velluto D, et al. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J Control Release 2011;156:154–60. [DOI] [PubMed] [Google Scholar]
- [117].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]