Abstract
The cannabinoid receptor 1 (CBR1) is involved in a variety of physiological pathways and has long been considered a golden target for therapeutic manipulation. A large body of evidence in both animal and human studies suggests that CB1R antagonism is highly effective for the treatment of obesity, metabolic disorders and drug addiction. However, the first-in-class CB1R antagonist/inverse agonist, rimonabant, though demonstrating effectiveness for obesity treatment and smoking cessation, displays serious psychiatric side effects, including anxiety, depression and even suicidal ideation, resulting in its eventual withdrawal from the European market. Several strategies are currently being pursued to circumvent the mechanisms leading to these side effects by developing neutral antagonists, peripherally restricted ligands, and allosteric modulators. In this review, we describe the progress in the development of therapeutics targeting the cannabinoid receptor 1 in the last two decades.
Keywords: CB1 receptor, psychiatric side effects, neutral antagonists, peripherally restricted antagonists, allosteric modulators, therapeutics development
1. INTRODUCTION
The medicinal and psychoactive properties of marijuana have been known for centuries. The last few decades witnessed the discovery and cloning of two class A Rhodopsin-like G protein-coupled receptors, named cannabinoid 1 and 2 receptors (CB1R and CB2R), that mediate the biological effects of the main psychoactive constituent of Cannabis sativa, (−)-delta 9-tetrahydrocannabinol (Δ9-THC). While found ubiquitously throughout the whole body, CB1R is preferentially localized at central and peripheral nerve terminals, mediating inhibition of neurotransmitter release [1]. CB2R is located primarily on blood and immune cells, modulating inflammatory responses [2]. These two receptors, together with their endogenous ligands and functional proteins involved in their synthesis, transport, and activation, make up the endocannabinoid system. The most well-known endogenous ligands, also named endocannabinoids, are anandamide and 2-arachidonoylglycerol (2-AG). These endocannabinoids are synthesized on-demand from cell membrane arachidonic acid derivatives. They exert their effects for a short period before being removed from their site of action by degradation by fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL) [3].
Being one of the most abundant G protein-coupled receptors (GPCRs) found in mammalian brain, CB1R has received considerable attention for its role in many physiological processes including pain regulation, learning and memory, appetite and food intake, lipogenesis, and cravings [4–6]. A plethora of selective and non-selective CB1R agonists and antagonists have been developed, some of which are widely used as research tools such as the agonists CP55,940, WIN55,212–2, and AM251 [7–9]. However, no synthetic cannabinoids are currently in the clinics. The only FDA-approved dosage formulations of phytocannabinoids contain cannabidiol (CBD) and Δ9-THC (or its synthetic analogue nabilone), and are used in clinics for the treatment of seizures, pain relief, appetite stimulation, and suppression of nausea and vomiting.
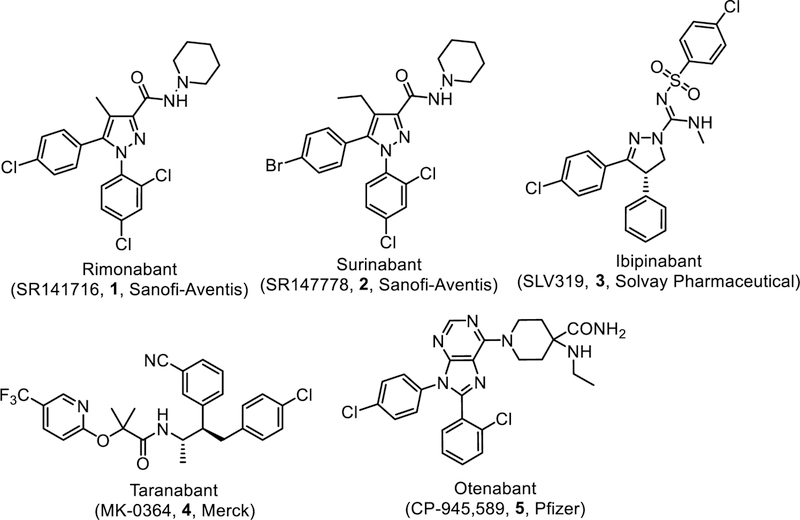
The observation that endocannabinoid are overactive in obese humans [10–12] and obese animals in both genetic and diet-induced obesity [13–14] has spurred research effort to develop CB1R antagonists for obesity treatment. After initial disappointing results with modifications of the structures of ∆9-THC and the non-steroidal anti-inflammatory drug pravadoline, the first therapeutic agent to emerge from decades of cannabinoid research is the CB1R antagonist/inverse agonist rimonabant (SR141716, Acomplia®, Sanofi-Aventis). In 2006, rimonabant was licensed in United Kingdom for the treatment of obesity and related metabolic risk factors in nondiabetic and diabetic overweight and obese patients [15–18]. Rimonabant showed improvement in glycemic control and lipid profile in type 2 diabetic patients [19], as well as loss of visceral and hepatic fat in abdominally obese patients [20]. It also demonstrated positive effects for smoking cessation [21–22]. Unfortunately, it was subsequently withdrawn from the European market in 2008 due to occurrence of psychiatric adverse effects such as depression, anxiety, and even suicidal ideation. Other concerns noted with CB1R antagonists/inverse agonists included increased incidence of headache, irritability, insomnia, dizziness, nausea, vomiting, malaise and pruritus [23]. Prompted by the risk of these untoward side effects, the pharmaceutical industry terminated ongoing clinical trials of other brain-penetrant CB1R antagonists such as the longer acting second generation surinabant (SR147778, Sanofi-Aventis), ibipinabant (SLV319, Solvay Pharmaceutical), taranabant (MK-0364, Merck), and otenabant (CP-945,598, Pfizer) (Fig. 1).
Fig. (1).
CB1R antagonists/inverse agonists that were advanced to clinical trials.
Given the current lack of effective treatment for obesity and drug addiction, and the important physiologic role CB1R plays as one of the most abundant GPCRs in the CNS, novel strategies have been explored to eliminate or mitigate the central psychiatric adverse effects of the CB1R signaling pathway but retain the pivotal therapeutic benefits of CB1R inhibitory mechanism. These strategies include the development of CB1 receptor neutral antagonists, peripherally restricted antagonists, and allosteric modulators.
Neutral antagonists: The CB1R is known to possess constitutive activity that is crucial for a variety of cellular processes to maintain homeostasis [24]. As a result, inverse agonism at the CB1R reducing or blocking this constitutive activity could lead to long-lasting effects on CB1R function that might have clinical ramifications [25]. Neutral antagonists that antagonize endocannabinoids but do not change the basal CB1R activity are expected to be devoid of the potential downside consequences of inverse agonism at CB1R, thus having therapeutic potential in pathological conditions due to excessive receptor activation [26].
Peripherally restricted antagonists: There is increasing evidence in both animal and human studies supporting the premise that the effects of CB1R antagonists in weight loss and metabolic diseases can be produced at peripheral sites and through cell-signaling mechanisms without requiring involvement of the CNS-receptor mediated actions [15,17,27–28]. Therefore, peripherally restricted CB1 receptor antagonists that have no blood-brain barrier (BBB) penetration and limited CNS exposure are expected to retain certain therapeutic efficacy in the treatment of obesity and diabetes without the liability of CNS side effects.
Allosteric modulators: GPCR allosteric modulators modulate the activities of orthosteric agonists by binding to the allosteric binding site(s) which is/are topologically distinct from and often less highly conserved than the orthosteric binding site. They exert biological effects only in the presence of orthosteric ligands. Therefore, GPCR allosteric modulators may offer greater subtype selectivity, selective spatial and temporal signaling, and effect “ceiling” (thus lower risk of over dosing). Importantly, the CB1R allosteric modulators disclosed so far appear to have reduced inverse agonism [29–30]. Further, since the endocannabinoids are transiently released on demand and removed from their sites of action by cellular uptake, CB1 allosteric modulators may not have a long-lasting effect compared to orthosteric ligands. These signaling properties suggest that allosteric modulators may have inherent advantages in therapeutic applications involving CB1R.
In this review, we will summarize the ongoing efforts in these three approaches to target CB1R signaling pathways in a more selective and specific manner to minimize the psychiatric side effects. Each class of compounds will be discussed in the chronological order of their disclosure.
2. NEUTRAL ANTAGONISTS
It has been established that the endocannabinoid system exists in a tonically active state in most tissues examined [31–32]. At a basal state, a constitutively active receptor exists in an equilibrium between active state and inactive state and probably some intermediate states [33]. Upon binding, an agonist shifts the equilibrium favoring the active state, triggering downstream physiological effects, whereas an inverse agonist shifts the equilibrium from the basal state to the inactive state. Thus CB1R basal functional activity could play an important role in maintaining cellular homeostasis [24]. As a result, reduction of basal levels of the CB1R active state, in the absence of decreased neurotransmitter release, could lead to pharmacological ramifications including untoward side effects. As endocannabinoids are found to be elevated in pathological conditions, a neutral antagonist which blocks signaling tone, but does not reduce the basal level of active state receptor, could be a safer therapeutic option than those produced through receptor blockade and inactivation [26].
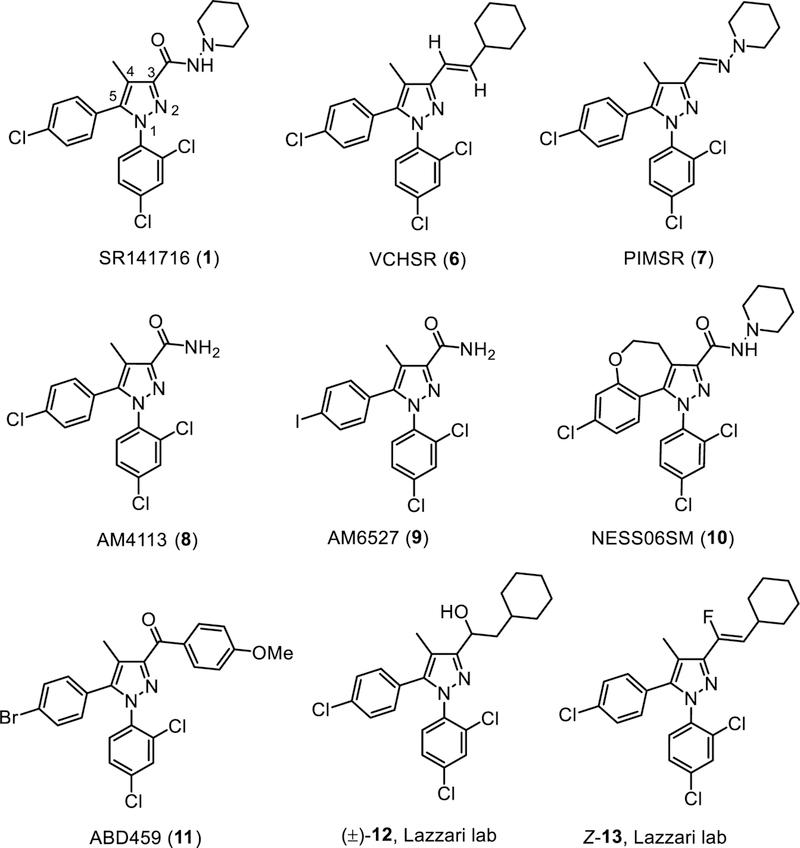
Though withdrawn from the market, SR141716 still remains a valuable research tool and extensively used as the prototypic selective CB1R inverse agonists. The pyrazole scaffold of SR141716 has been widely used as the starting point for side chain modifications in newer generations of CB1R antagonists as depicted in Fig. 2. Other non-pyrazole-based neutral antagonists are shown in Fig. 3.
Fig. (2).
Structures of pyrazole-based CB1R neutral antagonists.
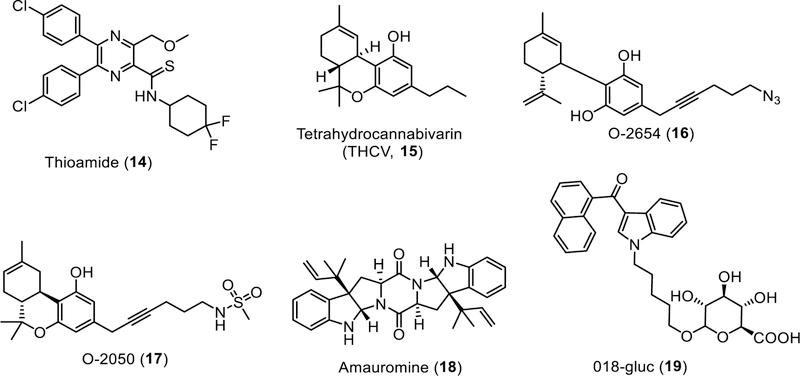
Fig. (3).
Structures of non-pyrazole-based CB1R neutral antagonists.
2.1. Pyrazole derivatives
VCHSR
VCHSR is one of the first reported neutral antagonists. It was designed from molecular modeling studies which demonstrated the critical role of the residue Lys1923.28 in the inverse agonism of SR141716 by preferential binding to the carboxamide group in the inactive CB1R state [34–35]. The carbonyl oxygen was therefore removed by modification of the amide to an ethenyl moiety to eliminate hydrogen bonding capability at the C3 position but preserve the carboxamide trans geometry.
VCHSR attenuated WIN55,212-induced hCB1R-mediated inhibition of calcium current in rat superior cervical ganglion neurons but had no effect when administered alone up to 10 µM [34]. When administered on the first postnatal day in mice, VCHSR inhibited milk ingestion and growth, similar to SR141716 albeit the effects were milder [36].
PIMSR
PIMSR is another pyrazole derivative which was designed computationally to stabilize both the active and inactive states of CB1R to afford neutral antagonism [37]. The C3-carboxamide moiety of the pyrazole scaffold was substituted by an imine group in PIMSR to remove interaction with the Lys1923.28 residue. Similar to VCHSR, PIMSR had no effect in the calcium flux functional assay alone, unlike SR141716 which exhibited an increase in calcium flux [37].
The CNS-penetrant PIMSR was shown to be devoid of dysphoric effects in electrical brain stimulation reward studies [38]. This encouraging result prompted the studies of its metabolic effects in rodents. PIMSR was found to reduce weight, food intake, adiposity, and alcohol-induced hepatic steatosis following an acute administration of a high level of ethanol. In addition, PIMSR improved glycemic control and lipid homeostasis in obese mice caused by a high-fat diet. Though PIMSR partially prevented alcohol-induced alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactic acid dehydrogenase (LDH) in the binge alcoholic hepatic steatosis model, it was found to elevate ALT and liver weight which are markers of liver injury with chronic administration in the obese mice model. The elevation of ALT level by PIMSR treatment was proposed to be unlikely due to the CB1R neutral antagonism as other neutral antagonists, such as AM6545, did not induce liver injury [38].
AM4113
First reported in 2007 [39], AM4113 is one of the most extensively characterized CB1R neutral antagonists and widely used as a pharmacological tool to study the CB1R signaling pathways. AM4113 is an analogue of SR141716 without the piperidinyl group ring at the C3-carboxamide. In competitive [3H]CP55,940 binding assays, AM4113 had a Ki value of 0.80 ± 0.44 nM and exhibited a 100-fold selectivity for CB1R over CB2R [40]. AM4113 had no effect on the forskolin-stimulated cAMP accumulation in CB1R-transfected HEK-293 cells up to 10 µM concentration [40].
As expected with CB1 receptor antagonism, AM4113 attenuated the effects of the agonist AM411 in the cannabinoid tetrad test (locomotion, analgesia, catalepsy, and hypothermia) [24,39], and blocked hypothermia induced by CP55,940 [40]. AM4113 also reduced food intake and weight gain in rats [39–40], which was confirmed to be CB1R-mediated using CB1R knockout rats [41]. Finally, AM4113 demonstrated no effect on conditioned gaping or vomiting in rats [40] and ferrets [42].
AM4113 also displayed positive profiles as a promising potential for the treatment for abuse of opioids, nicotine, marijuana, and binge alcohol drinking in numerous behavioral studies. Like AM251, pretreatment of AM4113 attenuated the aversive affective properties of naloxone-precipitated morphine withdrawal in rats [43]. AM4113 significantly attenuated nicotine taking behavior, motivation for nicotine, cue-, priming- and stress-induced reinstatement of nicotine-seeking behavior in rats. In mice, AM4113 suppressed ethanol consumption and preference without any significant effects on body weight, ambulatory activities, and preference for taste such as saccharin and quinine [44]. AM4113 could substitute for rimonabant in the rimonabant-induced conditioned suppression of saccharin drinking [45]. In nonhuman primate studies, both AM4113 and SR141716 surmountably antagonized the discriminative stimulus effects of the CB1R full agonist AM4054 [46]. In squirrel monkeys, both AM4113 and SR141716 attenuated nicotine- and THC-seeking behaviors in both active addiction and relapse models, as well as cue-induced reinstatement of self-administration of cocaine [47].
Importantly, AM4113 is devoid of many untoward effects associated with CB1R inverse agonists. Pretreatment with AM4113 had no effect on food intake or anxiety, yet demonstrated antidepressant-like effects [48]. Unlike another antagonist/inverse agonist AM251, AM4113 did not enhance retention of contextual fear conditioning in rats [49]. The whole gut transit time in mice was not reduced by AM4113, in contrast to the shortened transit time seen with CB1R inverse agonists [50]. AM4113 attenuated some of the depressive effects of WIN55,212–2 in behavioral profiles in open-field studies, including ambulation, rearing, circling, and the increased latency to leave the middle start area of the open-field arena [42]. However, like SR141716, AM4113 dose-dependently increased the level of scratching and grooming [42], altered the natural satiation in rats such as excessive grooming [51], and precipitated cannabinoid withdrawal signs [52].
Pharmacokinetic studies indicated that AM4113 had poor bioavailability, but was able to penetrate the BBB, and was subsequently eliminated more slowly from brain than plasma [44]. Though useful as a research tool, the lack of oral bioavailability limits its development as a clinical candidate [53].
AM6527
Replacement of the 4-chlorophenyl substituent by the 4-iodophenyl group at the C5 of the pyrazole template led to AM6527 which is an orally active CB1R neutral antagonist. AM6527 has lower binding affinity to rat CB1R and maintained the same 100-fold selectivity in relative to the hCB2R like AM4113. In the food-reinforced behavior experiments in rats, both AM6257 and AM4113 had similar ED50 values when administered intraperitoneally; however, only AM6527 was effective following oral administration (ED50 = 1.49 mg/kg), whereas AM4113 showed no effects up to oral doses of 32 mg/kg [53].
Like AM4113, AM6527 pretreatment prevented naloxone-precipitated morphine withdrawal in the conditioned place aversion (CPA) paradigm. However, orally administered AM6527 was also observed to suppress locomotor activity during conditioning [43].
NESS06SM
NESS06SM is a condensed tricyclic pyrazole derivative of SR14716 [54]. It exhibited nanomolar affinity to CB1R (Ki = 10.25 nM) and good selectivity relative to CB2R (Ki CB2R > 5000 nM). It behaved as a neutral antagonist in the [35S]GTPγS assay [54]. In silico parameters suggested that NESS06SM exhibited sparing BBB permeability. Chronic treatment with NESS06SM reduced body weight and cardiovascular risk factors in diet induced obesity mice fed with fat diet. More importantly, chronic administration of NESS06SM did not alter mRNA expression of both monoaminergic transporters and neurotrophins associated with anxiety and mood disorders [54]. Furthermore, cotreatment with NESS06SM reduced food intake and weight increment, attenuated common side effects caused by chronic administration of the atypical antipsychotic, olanzapine and restored all blood parameters without altering the positive behavioral effects of olanzapine [55].
ABD459
ABD459 exhibited a binding affinity Ki of 8.6 nM for murine CB1R. It antagonized CP55,940-induced [35S]GTPγS binding without affecting the basal receptor constitutive activity, thus demonstrating properties as a CB1R neutral antagonist. Intraperitoneal administration of ABD459 (3–20 mg/kg) inhibited food consumption in nonfasted mice with no rebound after washout. It did not alter motor activity. ABD459 decreased rapid eye movement (REM) sleep with no effects on wakefulness and non-REM sleep [56].
Other Pyrazole derivatives
Using bioisoteric replacement of the C3-carboxamide of the SR141716 with vinyl fluoride, the Lazzari lab reported the pyrazole derivatives (±)-12 and (Z)-13 [57]. These two compounds exhibited lower binding affinities and selectivity for CB1R than SR141716. Pharmacological characterization in the [35S]GTPγS binding and mouse vas deferens assays supported their neutral antagonist profiles. (Z)-13 at 20 mg/kg (i.p.) produced the same reduction in food intake as 10 mg/kg of SR141716 yet no observable adverse effects were evidenced for (Z)-13 at 20 mg/kg [57].
2.2. Non-pyrazole-based CB1R neutral antagonists
Thioamide
Bostrӧm et al from AstraZeneca performed scaffold hopping to replace the pyrazole core of SR141716 with a pyrazine and modified the carboxamide to thioamide [58]. Pyrazine-2-thioamide 14 was confirmed to be a neutral antagonist in the [35S]GTPγS binding assay with the same potency as SR141716 (14, IC50 = 2.4 nM). Compound 14 was found to be able to cross the BBB; however, its solubility remains poor (< 0.1 µM). Oral administration of 14 reduced body weight in cafeteria diet induced obese mice which remained after a 1–2 week washout period, a phenomenon not seen with SR141716 [58].
Tetrahydrocannabivarin (THCV)
Δ9-Tetrahydrocannabivarin (THCV), naturally found in Cannabis, is a homologue of Δ9-THC with a propyl side chain instead of a pentyl group (Fig. 3). [35S]GTPγS binding studies indicated that THCV acted as a CB1R neutral antagonist at low doses [32]. However, at higher doses, it can behave as a CB1 agonist like Δ9-THC. THCV could act either as CB2R agonist or antagonist depending on the assays [59–62].
THCV produced hypophagic effects in both fasted and non-fasted mice [63]. While it did not significantly impact food intake or weight gain, THCV produced an early and transient increase in energy expenditure. THCV dose-dependently reduced glucose intolerance in genetically obese mice and improved glucose tolerance and enhanced insulin sensitivity in diet-induced obese mice [64].
In human trials, THCV treatment increased neural responding to rewarding and aversive stimuli [65]. Using magnetic resonance imaging, another study in humans revealed that THCV decreased resting state functional connectivity in the default mode network and increased connectivity in the cognitive control and dorsal visual stream networks, brain regions where functional connectivity are found to be altered in obesity [66].
Cannabidiol-based derivatives O-2050 and O-2654
6”-Azidohex-2”-yne-cannabidiol (O-2654) was suggested to be a CB1R neutral antagonist as it could antagonize the inhibitory effect of WIN55,212–2 in the mouse vas deferens but did not amplify the electrically evoked contractions like SR141716A [67]. No other studies have been reported on this compound.
A sulfonamide analogue of Δ8-THC with an acetylenic side chain, O-2050, was initially proposed as CB1R neutral antagonist as it produced potent antagonism of WIN55,212–2 and CP55,940 but showed no inverse agonist property in the myenteric plexus-longitudinal muscle (MPLM) preparation of guinea-pig small intestine [32]. However, subsequently, the neutral antagonist property of O-2050 was found to be assay-dependent. Even though O-2050 antagonized the cannabinoid effects in the [35S]GTPγS binding and mouse vas deferens assays without exhibiting any activity alone in either assay, it inhibited forskolin-stimulated cAMP signaling and produced similar effect in a mouse drug discrimination procedure like THC [68]. O-2050 also facilitated noradrenaline release and potentiate the CB1R inverse agonistic effects of SR141716 in the guinea pig hippocampus [69]. These latter results suggest that O-2050 is not a viable CB1R neutral antagonist.
Amauromine
Diketopiperazine alkaloid amauromine from the fungus Auxarthron reticulatum is derived from the marine sponge Ircinia variabilis. It had a moderate binding affinity to hCB1R (Ki = 178 nM). cAMP assays suggested that it behaved as a CB1R neutral antagonist with Kb = 66.6 nM [70], although further studies are needed.
JWH-018-N-(5-hydroxypentyl) β-D-glucuronide (018-gluc)
018-Gluc is a glucuronide metabolite of JWH-018, a synthetic cannabinoid found in K2/Spice preparations produced through hydroxylation by cytochrome P450s and subsequent glucuronidation by UDP-glucuronosyltransferases. 018-Gluc was reported to be a CB1R neutral antagonist based on the characterization from the [35S]GTPγS binding assay [71].
3. PERIPHERALLY RESTRICTED ANTAGONISTS
The main concerns about rimonabant that led to its withdrawal from the market were its CNS-mediated adverse effects. Growing evidence suggests that blockage of CB1R activity in peripheral tissues such as liver, muscle, adipocytes, and pancreas is adequate to suppress food intake, increase energy expenditure and reduce lipogenesis in both liver and adipose tissues [15,17,27–28]. Thus, a straightforward strategy to retain peripherally-mediated cannabinoid receptor therapeutic activity in metabolic disorders while eliminating adverse centrally-mediated effects is to develop peripherally restricted CB1R antagonists.
Common strategies to minimize CNS penetration in the development of peripherally restricted ligands include introduction of polar functional groups and addition of hydrogen bond donors. The topological polar surface area (TPSA), defined as the surface sum of all polar atoms such as oxygen and nitrogen, is a widely used parameter to predict the passive diffusion of a molecule through the BBB. Molecules with TPSA greater than 120 Å2 typically have poor membrane permeability [72]. A TPSA value of less than 70 Å2 tends to favor BBB permeability [72–73]. Thus, the most common strategy to design peripherally restricted ligands is to incorporate polar groups that increase TPSA values. A second strategy is to include permanently charged moieties that cannot pass through BBB passively and can only be transported across the BBB by special transporters. A third strategy is to introduce molecular features that enhance the propensity to become substrates of efflux proteins such as P-glycoproteins.
As the development of CB1R peripherally restricted antagonists up to 2013 has been reviewed in detail before [74], herein we will focus on the updates in this area since then. Similar to CB1R neutral antagonists, the pyrazole core of SR141716 also serves as the starting point for side chain modifications and scaffold hopping in the development of peripherally restricted CB1R antagonists. These compounds can act as either inverse agonists or neutral antagonists.
3.1. Pyrazole-based peripherally restricted CB1R antagonists.
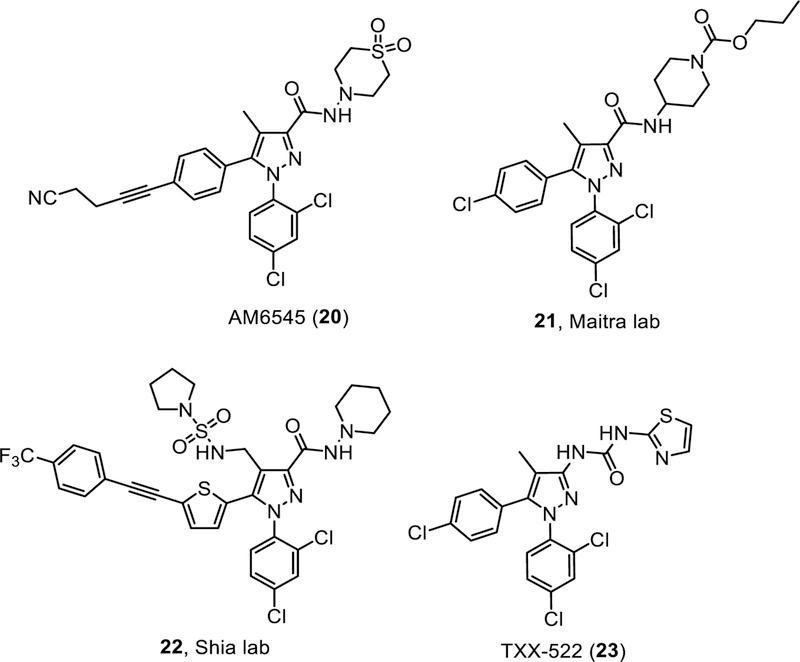
AM6545
AM6545 is a peripherally restricted CB1R neutral antagonist. AM6545 has reasonable binding affinity to rat CB1R with Ki value of 1.7 nM, and exhibited a 302-fold or 38-fold selectivity in affinity relative to mouse CB2R and human CB2R, respectively [75]. As a neutral antagonist, AM6545 had no effect on the forskolin-stimulated cAMP level up to 3 µM, unlike the inverse agonist AM251 which stimulated cAMP production [75].
As one of the first reported peripherally restricted CB1 antagonists, AM6545 has been assessed in numerous behavioral models. AM6545 strongly suppressed the intake of high carbohydrate, high fat, and high sucrose diet with no or little impact on standard lab chow diet [76–77]. AM6545 inhibited refeeding in fasted rats [78]. It inhibited food intake in CB1R gene-deficient mice, but not in CB1R/CB2R double knockout mice [75]. AM6545 did not affect food handling, instead, it reduced time spent feeding and feeding rate [76]. Unlike SR141716, AM6545 did not induce the initial transient decrease in food intake but a slight decrease was evident later during the treatment period [79]. However, AM6545 did not affect total caloric intake [79].
AM6545 demonstrated a marked reduction of body weight and fat mass in obese mice [79] [80]. Although AM6545 did not have an impact on lean mass, the metabolic profile of these obese mice was significantly improved [79]. Dyslipidemia (elevated serum triglyceride, total cholesterol, free fatty acids), glucose intolerance, and hyperinsulinemia, hormone dysregulation and hepatic steatosis were alleviated upon treatment with AM6545 [79–81]. Beneficial for diabetic nephropathy, AM6545 reduced diabetes-induced albuminuria and prevented nephrin loss both in vivo and in vitro in podocytes exposed to glycated albumin [82]. AM6545 exhibited synergistic effect with the CB2R agonist AM1241, displaying better efficacy in abolishing diabetes-induced renal monocyte infiltration and M1/M2 macrophage imbalance in vivo [82].
Pretreatment of AM6545 (i.p.) reversed the depressant effects of CP55,940 for all four cardiovascular parameters into stimulatory ones (diastolic blood pressure, systolic blood pressure, mean blood pressure, and heart rate) [83]. AM6545 (i.p.) markedly attenuated or delayed the lung inflammation and fibrosis in a murine model of radiation-induced pulmonary fibrosis and enhanced animal survival as in the genetic deletion studies [84]. AM6545 did not cause liver injury [38], and was able to reverse the slowing effect of WIN55,212–2 on colonic motility [75].
AM6545 is devoid of many centrally mediated untoward effects. In contrast to AM251, AM6545 had no/little nausea and vomiting side effects in animal studies. It did not produce gaping or conditioned taste avoidance in rats [75]. In addition, AM6545 did not potentiate LiCl-induced conditioned gaping nor reduced sucrose palatability [85]. Unlike AM4113, AM6545 did not precipitate cannabinoid withdrawal signs [52], or substitute for SR141716 in the rimonabant-induced conditioned suppression of saccharin drinking [45]. Also different from AM251, AM6545 did not affect the fixed consecutive number task, a test to measure choice to terminate a chain of responses prematurely following systemic blockade of WAY100,635, an 5HT1A antagonist in rats [86].
Though AM6545 has a more limited brain penetration than AM4113, its brain-to-plasma ratio remains high at 0.20 to 0.41 for several hours after intraperitoneal administration, thus complicating the assessment of the contributions of CB1R-mediated peripheral and central mechanisms to its behavioral effects [75].
TM-38837
TM-38837 (structure not disclosed) was discovered internally at 7TM Pharma. In 2010, 7TM Pharma announced that it successfully completed a Phase I clinical trial for TM-38837 demonstrating that TM-38837 did not display the typical psychomimetic effects of THC at therapeutically relevant doses [87]. TM-38837 at 100 mg (p.o.) had no measurable feeling of high or body sway effects and limited heart rate effects [88]. TM-38837 was found to induce larger effects on inhibition of THC-induced heart rate increases than on THC-induced visual analogue scale of feeling high, consistent with its preference for peripheral target sites [89]. In PET studies in cynomolgus monkeys, expected clinical therapeutic doses of 20 mg demonstrated low levels of in vivo brain CB1R occupancy compared with SR141716 [90]. Unfortunately, 7TM Pharma has discontinued its business and there has been no further news on the development of TM-38837.
Pyrazole derivatives (Maitra lab)
In an effort to limit brain penetration, the Maitra lab modified the C3-carboxamide function of the pyrazole scaffold by incorporating permanently charged moieties such as alkyl pyridinium salts and N-oxides and groups with high TPSA values such as sulfonamides, sulfamides, piperidines functionalized with carbamates, amides, and sulfonamides [91–93]. Compound 21 (Fig. 4) was identified to have good CB1R antagonist potency, moderate selectivity over CB2R, and good metabolic stability. In vivo pharmacokinetic testing indicated that 21 has good oral bioavailability and minimal brain penetration [93].
Fig. (4).
Structures of pyrazole-based peripherally restricted CB1R antagonists.
Pyrazole derivative (Shia lab)
After several rounds of optimization at the C5 position of the pyrazole scaffold [94–95], the Shia lab presented compound 22 (Fig. 4) with excellent binding affinity (CB1R Ki = 0.3 nM), in vitro potency (EC50 = 3 nM), and limited CNS penetration (brain-to-plasma ratio 1:64). 22 had significant efficacy in reducing weight in a diet-induced obese mouse model and displayed a clean off-target binding screen against 163 other receptors [96].
TXX-552
TXX-552 is a peripherally restricted CB1R neutral antagonist disclosed recently with a brain:plasma ratio of 0.02 in rats [97]. It dose-dependently reversed WIN55,212–2-induced inhibition of forskolin-stimulated cAMP accumulation but did not increase the forskolin-induced cAMP level alone, indicating a lack of inverse agonist activity. It demonstrated good CB1 binding affinity and selectivity over the CB2R. In vivo studies confirmed that TXX-552 had minimal brain penetration and good oral bioavailability. TXX-552 decreased body weight of diet-induced obese mice, although the effect was less pronounced than observed with SR141716 at the same dose of 5 mg/kg. In contrast to SR141716, TXX-552 had no impact on acute food consumption by mice; however, TXX-552 was found to decrease fasting blood glucose and insulin levels, ameliorate glucose intolerance, reduce both serum triglyceride and total cholesterol levels. No adverse effects on physical appearance, behavior, or other signs of toxicity including lethargy, mania, or alteration of excreta were observed during the treatment with TXX-552 [97].
3.2. Non-pyrazole-based peripherally restricted CB1R antagonists
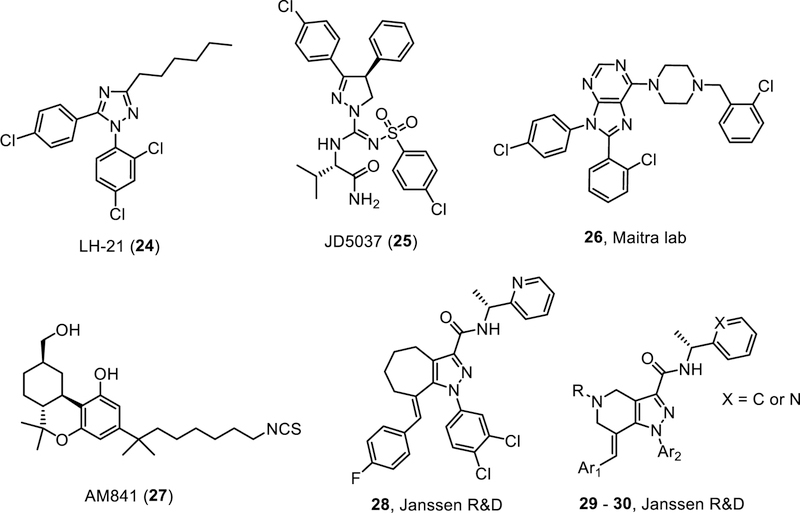
Triazole LH-21:
A medicinal chemistry effort to investigate triazoles as the core structure in place of the pyrazole of SR141716 led to the identification of LH-21 as a CB1R neutral antagonist based on the mouse vas deferens and guinea pig ileum assays [98]. LH-21 has relatively low binding affinity to both human and rat CB1R (Ki values in the 630 – 855 nM range) [98–99]. While it initially appeared to have limited brain penetration and have silent antagonist activity [98], subsequent studies by Chen et al indicated that LH-21 was brain penetrant (~1:1 brain-to-plasma ratio), and acted as an inverse agonist in the cAMP assay [99].
LH-21 had no effect on nociception, temperature, spontaneous motility, and catalepsy in cannabinoid tetrad test [98]. LH-21 did not affect feeding by acute administration in free feeding animals but was highly effective in blocking ghrelin-induced hyperphagia [100]. LH-21 was found to improve glucose handling in diet-induced obese pre-diabetic mice [101]. However, unlike SR141716, chronic administration of LH-21 (3 mg/kg) did not improve hypertriglyceridaemia, hypercholesterolaemia or liver fat deposits in Zucker rats [102]. LH-21 reduced feeding and body weight gain in both wild-type and CB1R knockout mice, suggesting that its appetite suppressant and anti-obesity effects are independent of action at the CB1R [99,102–104].
Anxiety-like behaviors and induction of complex motor activities such as grooming and scratching sequences were not observed with LH-21 [99]. Instead, it was observed to counteract obesity-induced anxiety, though this effect was proposed to be mediated by GPR55 receptor [101]. LH-21 only slightly reduced alcohol self-administration at a high dose of 10 mg/kg [104].
Pyrazoles JD5037 and JD5006
JD5037 and its analogue JD5006 are two peripherally restricted CB1 antagonists derived from the structure of ibipinabant (SLV-319) with a pyrazoline core instead of the pyrazole under a collaboration between Jenrin Discovery Inc. and National Institute of Health [105]. Both compounds showed good potency at the CB1 receptor (IC50 = 2 and 18 nM, respectively), lower BBB penetration as determined in the MDCK-mdr1 assay, and reduced brain receptor occupancy upon oral administration compared to SLV-319.
Preclinical studies in rodents and primates support the therapeutic benefits of JD5037 in suppressing progression of hepatic fibrosis. JD5037 was highly effective in inhibiting β–arrestin-2 activation, a marker in fibrotic disease progression [106]. When administered in the prediabetic stage, JD5037 prevented or reversed progression of type 2 diabetic nephropathy development in Zucker diabetic fatty rats [107]. JD5037 also attenuated the induction of tumor promoting genes in chemically-induced hepatocellular carcinoma [108].
JD5037 has demonstrated positive effects on metabolic parameters associated with Type 2 diabetes and obesity in a variety of murine models. JD5037 was as effective as the globally acting CB1R inverse agonist, SLV319, in reducing appetite, body weight, hepatic steatosis, and insulin resistance, and improving glucose tolerance in diet-induced obese mice [109–110]. These effects on appetite and weight reduction are mediated by decreasing leptin expression and secretion by adipocytes, increasing leptin clearance via the kidney [109], and restoring sensitivity to endogenous leptin which elicits hypophagia via the re-activation of melanocortin signaling in the arcuate nucleus [111] and/or decrease in ceramide levels [110]. Daily chronic treatment with JD5037 (3 mg/kg/day for 28 days) reduced body weight, reversed hyperphagia, and improved metabolic parameters in an extreme obesity Prader-Willi syndrome (PWS) mouse model [112]. JD5037 exhibited stronger affinity than SR141716 to a human CB1R isoform (CB1b) which is highly expressed in β-cells and hepatocytes, but not in brain. It potentiated adenylyl cyclase activation, thus stimulating insulin secretion in β-cells [113].
In December 2017, Jenrin Discovery Inc. received approval to start the Investigational New Drug (IND) application for JD5037 as a drug candidate to treat nonalcoholic steatohepatitis (NASH).
Purine derivatives
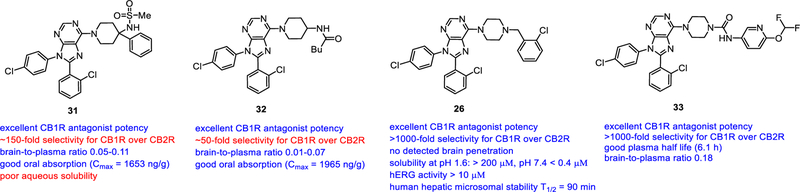
Though otenabant (5), a CB1 antagonist developed by Pfizer, has physicochemical properties usually associated with peripherally restricted compound, it is CNS permeable. X-ray structure suggested that the atypical lower polarity of otenabant was attributed to the intramolecular H-bonding between the primary amide and the ethyl amine portion of the molecule [114]. Therefore, the Maitra lab sought to replace this portion of otenabant with other polar groups incapable of forming intramolecular H-bonding. This led to compound 31 with excellent potency on CB1R, 153-fold selectivity over CB2R, and good stability in human S9 microsomal fractions (>90% remaining after 120 min of incubation). In a rat PK assay, 31 demonstrated limited CNS penetration (brain to plasma ratios ranging from 0.05 to 0.11) [115]. Further optimization led to compound 33 which maintained good potency at CB1R, 50-fold selectivity over CB2R, low CNS penetration and good oral bioavailability. Oral administration of 33 did not reverse any centrally-mediated cannabimimetic effects of Δ9-THC in the tetrad assay [116], consistent with its restriction to the periphery.
Switching the piperidine to piperazine for exploration of substitution on this piperazine ring led to compound 26 with good potency in calcium mobilization assay, excellent selectivity for CB1R relative to CB2R, good solubility in gastric pH, though limited solubility at pH 7.4. It has no detectable hERG activity in the [3H]astemizole displacement assay. It demonstrated good half-life (T1/2 = 90 min, CL=14 ml/min/kg) against human hepatic microsome preparations. After 10mg/kg oral administration, compound 26 was found predominantly in liver with some in plasma, and very little in brain. Compound 26 showed little to no blocking of temperature drop in rodent hypothermia model, confirming the low brain penetration. More importantly, p.o. administration of 26 was effective in reducing hepatic lipid accumulation in the murine alcohol-induced liver steatosis assay [117]. The urea analogue 33 was reported to have excellent CB1R antagonist potency, selectivity, oral bioavailability, and reduced brain penetration (Fig. 6) [118].
Fig. (6).
Structures of purine-based peripherally restricted CB1R inverse agonists developed by the Maitra group.
AM841
The hexahydrocannabinol AM841 is a peripherally-restricted covalent CB1R antagonist with relatively high CB1 affinity (Ki = 9 nM) [119]. Studies showed that AM841 reduced GI motility in normal and stressed mice, but demonstrated very little brain penetration and no characteristic centrally mediated CB1 receptor-mediated effects (analgesia, hypothermia or hypolocomotion) [120].
Tetrahydroindazole and Tetrahydropyrazolo[4,3-c]pyridine derivatives
Janssen Research & Development LLC reported two series of tetrahydroindazole (28) and tetrahydropyrazolo[4,3-c]pyridine (29 – 30) derivatives (Fig. 5) as peripherally selective CB1R inverse agonists (brain-to-plasma ratio < 0.1) [121–122]. These compounds exhibited nanomolar potency against CB1R [121–122]. Oral administration of 28 once a day for 3 weeks resulted in lower plasma glucose levels in diet-induced mice [122].
Fig. (5).
Structures of non-pyrazole-based peripherally restricted CB1R antagonists.
4. ALLOSTERIC MODULATORS
The discovery that the CB1R contains allosteric binding site(s) has sparked an interest in pursuing CB1R allostery as an alternate approach to achieve therapeutic benefits while avoiding the inherent side effects of orthosteric ligands [123]. Allosteric modulators are generally categorized into positive allosteric modulators (PAMs) or negative allosteric modulators (NAMs), which positively or negatively modulate the affinity and/or efficacy of the orthosteric agonists, respectively [124]. A typical NAM reduces binding affinity of an orthosteric agonist, resulting in weakening signaling efficacy. In contrast, a subset of allosteric modulators demonstrates enhanced binding affinity of the orthosteric agonists but reduced functional efficacy of the orthosteric agonists. They are termed PAM-antagonists to distinguish its distinct operational model of allosterism compared to typical NAMs. Upon binding, these PAM-antagonists turn the receptor-G protein-orthosteric ligand-allosteric modulator complexes into inactive conformations, resulting in attenuation of efficacy of the orthosteric agonist through a “seek-and-destroy” mechanism. PAM-antagonists provide unique advantages in contrast to orthosteric antagonists and typical NAMs such as better reversal of ongoing persistent pathological overactivation and favorable target coverage in vivo [125].
Both PAMs and NAMs have been described recently in several reviews, including a detailed one from us [126], and herein we provide an update on CB1R NAMs with emphasis on those newly disclosed since 2017.
Indole-2-carboxamides
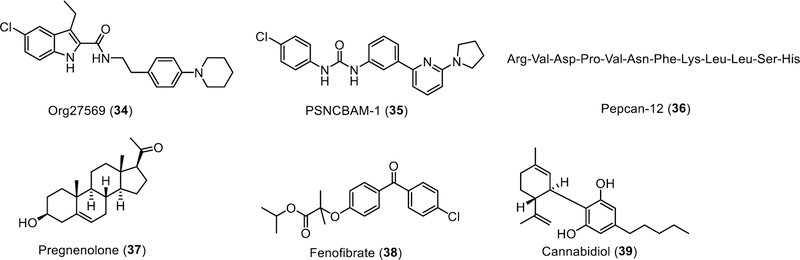
Organon reported the first CB1R allosteric modulators with an indole-2-carboxamide scaffold in 2005. Among these indole derivatives, the more widely investigated Org27569 displays a CB1R selective PAM-antagonist profile with positive binding cooperativity with the orthosteric agonist CP55,940, and reduction of efficacy of agonists in in vitro functional assays [123]. Org27569 demonstrates probe dependence with varying degrees of binding cooperativity with different orthosteric agonists. In a sharp contrast to strong positive binding cooperativity with CP55,490, Org27569 had close to neutral cooperativity with other orthosteric agonists such as HU-210, WIN55,212–2, Δ9-THC, methanandamide, anandamide, and 2-AG [127–128]. Org27569 also displays a probe-dependent CB1R allosteric modulatory profile in a variety of in vitro functional assays, while it exhibits little or no inverse agonist activity alone [126].
Though only resulting in modest improvement of potency, structure-activity relationship (SAR) studies on Org27569 revealed the indole core and the 2-carboxamide function are critical. The ethylene linker and 4-dimethylamino or 4-diethylamino on the ring B are optimal. Shorter alkyl side chains at the C3 and electron-withdrawing substituents at C5 position of the indole ring are preferred [129–133].
The CNS-penetrant Org27569 reduced food intake in both CB1-deficient and wild-type mice [134]. It significantly attenuated the hypothermic effect of CP55,940 and anandamide [135] as well as both cue- and drug-induced reinstatement of cocaine- and methamphetamine seeking behavior like SR141716, supporting the potential benefits of Org27569 in treatment of drug addiction [126,135].
Diarylureas
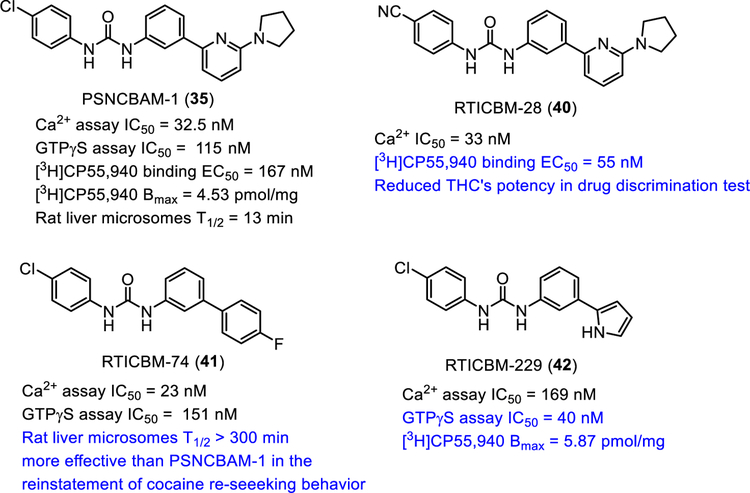
Another CB1R modulator, PSNCBAM-1, reported by Prosidion Limited in 2007 shares a similar PAM-antagonist profile in vitro as Org27569 [30,126].
The first SAR studies revealed that alkyl substitution at the 2-aminopyridine moiety and electron-deficient aromatic groups such as cyano at the 4-chlorophenyl position are important for CB1 allosteric modulation [136]. The urea moiety was found to be critical for the modulatory activity [137]. Subsequently, it was found that the pyrrolidine ring is not required for the allosteric modulation [138], and the pyridine of PSNCBAM-1 could be replaced by other aromatic groups such as phenyl or five-membered heterocyclic rings [138–141]. Saturation binding studies on some of these compounds revealed that they increased [3H]CP55,950 Bmax but did not alter Kd values, implying their modulating CB1R by stabilizing low affinity receptors into a high affinity state [141].
Replacement of the 5-pyrrolidinylpyridine in PSCNABM-1 with 4-fluorophenyl resulted in RTICBM-74 (Fig. 8) with similar potencies as PSNCBAM-1 in calcium mobilization and [35S]GTPγS binding assays but possessing significantly improved metabolic stability against rat liver microsomes (RTICBM-74: T1/2 > 6 h, CL < 4.6 µL/min/mg vs. PSNCBAM-1 T1/2= 13 min, CL = 114 µL/min/mg) [138]. Replacement of the 5-pyrrolidinylpyridine in PSCNABM-1 with 2-pyrrolyl group yielded RTICBM-229 (Fig. 8) with better potency in the [35S]GTPγS binding assay (RTICBM-229 IC50 = 40 nM; PSNCBAM-1 IC50 = 115 nM), and greater cooperativity evidenced by significant increase of the maximal binding level of [3H]CP55,490 (RTICBM-229 Bmax = 5.87 pmol/mg, PSNCBAM-1 Bmax = 4.53 pmol/mg) [141].
Fig. (8).
Structures of diarylurea-based CB1R PAM-Antagonists.
PSNCBAM-1 significantly reduced food consumption and body weight in an acute feeding rat model without any adverse effects on animal behavior and apparent signs of toxicity [30]. PSNCBAM-1 attenuated the reinstatement of extinguished cocaine-seeking behavior in rats [138]. Interestingly, the metabolically stable diarylurea RTICBM-74 is more effective than PSNCBAM-1 in this cocaine-seeking paradigm [138]. Unlike Org27569, PSNCBAM-1 caused a small but significant attenuation of Δ9-THC-induced antinociception in mice [142]. Its structural analogue RTICBM-28 (Fig. 8) was able to reduce the potency of Δ9-THC in drug discrimination assay, a phenomenon not seen with PSNCBAM-1 [142]. These differences in in vivo activities between these closely related analogues with similar in vitro potencies could be the result of variations in pharmacokinetics or binding kinetics to the CB1R target.
Pepcans
The α-hemoglobin-derived dodecapeptide (RVDPVNFKLLSH, pepcan-12) exhibited a typical allostery profile as expected for typical NAMs: decreasing both [3H]CP55,940 and [3H]WIN55,212–2 binding levels and attenuating effects of agonists in a number of in vitro functional assays [143–144]. However, it also behaved as a partial agonist [143,145] and CB2R PAM in other assays [146].
Pregnenolone
Pregnenolone, an endogenous steroid hormone, was proposed to function as a CB1R NAM based on its ability to reduce Δ9-THC-induced pERK1/2 activation in human CB1-CHO cells [147]. However, it had no effect on [3H]CP55940 and [3H]WIN55212–2 equilibrium binding, WIN55,212–2-inhibited pERK activation [127], or 2-AG signaling in autapic hippocampal neurons [144]. Animal studies showed that pregnenolone blocked the effects Δ9-THC on locomotion, antinociception, hypothermia, catalepsy, food intake, memory impairment, glutamate and dopamine release [147], but had no impact on the effect of Δ9-THC in drug discrimination paradigm [142].
Fenofibrate
Fenofibrate, a PPAR agonist, was reported as a CB1R NAM at high concentrations of 10–100 µM in radioligand binding assay, [35S]GTPγS binding, total ERK expression and β-arrestin recruitment. At concentrations of less than 10 µM, it exhibited agonist activity in these assays [148].
Cannabidiol
Cannabidiol, a nonpsychotropic constituent of marijuana was reported to act as a CB1R NAM, reducing the efficacy and potency of 2-AG and Δ9-THC on PLCβ, ERK, β-arrestin-2 recruitment, and CB1R internalization [149], as well as antagonizing the agonist activities of CP55,940 and WIN55,212–2 in the mouse vas deferens [150]. However, it demonstrated very weak binding affinity to CB1R (reported Ki values range from 4 to more than 10 µM) [150], and acted as a weak partial agonist in functional assays at concentrations more than 1 µM [149].
5. CONCLUSIONS AND FUTURE DIRECTIONS
The development of CB1R neutral antagonists, peripherally restricted antagonists and allosteric modulators has provided promising alternate approaches to the first generation globally acting orthosteric CB1 receptor antagonist/inverse agonist, rimonabant. These research efforts have significantly expanded our understanding of the CB1R receptor signaling pathways and pharmacology and demonstrated proof of concept for several alternative approaches to improve therapeutic utility and decrease adverse side effects. For example, reduction of food intake and weight gain and the production of other desired therapeutic actions can be achieved by selectively targeting peripheral CB1 receptor-mediated signaling processes or through allosteric of CB1 receptor conformation and function.
CB1 neutral antagonists provide the first “proof of concept” that “cleaner” compounds with little inverse agonism may achieve CB1R blockade as traditional antagonists/inverse agonist but have a more favorable pharmacological profile. For example, both CB1R inverse agonists and neutral antagonists are able to reduce food intake and food-reinforced behaviors, but the latter do not cause nausea and vomiting [151]. In addition, neutral antagonists such as AM4113 appear to lack anxiogenic or depression-like effects associated with the inverse agonism at CB1 receptors in classical animal models such as elevated plus maze test. However, some of the side effects of CB1R inverse agonists still linger, such as AM4113 which produced anxiety-like effects similar to that seen with rimonabant in an open field assay [42]. Another complicating factor is the challenge to separate the pharmacological effects between pure antagonism and inverse agonism in a living system [152–153]. Finally, the long term anxiogenic or depressive effects of CB1R neutral antagonists have been investigated.
Similarly, peripherally restricted CB1R antagonists have been shown to retain many global antagonist effects, such as reducing feeding and weight gain and improving metabolic profile in coronary artery disease [154–155], liver and pancreatic disease [156], inflammatory disorders [156], and arthritis [157]. Importantly, many peripherally restricted CB1R antagonists do not show the centrally-mediated psychiatric side effects commonly observed with inverse agonists SR141716 and AM251. Among the two most advanced compounds in this class, TM-38857 was evaluated in a Phase I clinical trial before 7TM went out of business, whereas JD5037 was recently approved for IND application for the treatment of NASH. A positive clinical outcome of the front-running candidate JD5037 would validate this peripherally-restricted antagonist approach in targeting CB1R receptor for therapeutic benefits.
Allosteric modulation is a unique approach to target GPCRs such as the CB1R. Allosteric modulators offer many unique advantages over orthosteric ligands. They exert their effects only in the presence of endogenous ligands, allowing fine-tuning of their signaling instead of completely blocking the receptor function with exogenous cannabinoids. The probe- and pathway-dependence of allosteric modulators result in differential responses in different signaling pathways. Therefore, allosteric modulators can selectively target one specific endogenous agonist or a certain pathway for desirable therapeutic outcome. As previously discussed [126], allosteric modulators Org27569 and PSNCBAM-1 showed reduced inverse agonist effect, and therefore may have lower psychiatric side effects compared to SR141716 and other orthosteric antagonists/inverse agonists. Allosteric modulation can be useful for both central and peripheral therapeutic indications.
Mounting evidence supports CB1R antagonism as an important mechanism to manipulate the endocannabinoid signaling for the treatment of obesity, metabolic disorders, and drug dependence/substance abuse. Although the first generation CB1R antagonists have an unsatisfactory safety profile, newer classes of CB1R-targeted inhibitors such as neutral antagonists, peripherally restricted antagonists, and allosteric modulators offer promising alternate pharmacological approaches for the treatment of CB1R-mediated disorders. The exciting results discussed here represent the next step in a long journey, and much work is needed from both medicinal chemistry and pharmacology researchers to develop clinically useful CB1R-mediated therapeutic agents.
Fig. (7).
Structures of reported CB1R NAMs.
ACKNOWLEDGEMENT
This work was supported by the National Institute on Drug Abuse, National Institutes of Health, USA (grants DA040693, DA040460, and DA045910).
LIST OF ABBREVIATIONS
- ALT
Alanine Aminotransferase
- AST
Aspartate Aminotransferase
- BBB
Blood-brain barrier
- CB1R
Cannabinoid 1 Receptor
- CB2R
Cannabinoid 2 Receptor
- CBD
Cannabidiol
- CL
Clearance
- CNS
Central Nervous System
- i.p.
intraperitoneal
- p.o.
oral administration
- LDH
Lactic Acid Dehydrogenase
- MPLM
Myenteric Plexus-Longitudinal Muscle
- NAM
Negative Allosteric Modulator
- PAM
Positive Allosteric Modulator
- SAR
Structure-Activity Relationship
- Δ9-THC
(−)-delta 9-Tetrahydrocannabinol
- TPSA
Topological Polar Surface Area
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article has no conflict of interest.
REFERENCES
- [1].Pertwee RG Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Philos. Trans. R. Soc. Lond. B Biol. Sci, 2012, 367, 3353–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Klein TW Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol, 2005, 5, 400–411. [DOI] [PubMed] [Google Scholar]
- [3].De Petrocellis L; Cascio MG; Di Marzo V The endocannabinoid system: A general view and latest additions. Br. J. Pharmacol, 2004, 141, 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Porter AC; Felder CC The endocannabinoid nervous system: Unique opportunities for therapeutic intervention. Pharmacol. Ther, 2001, 90, 45–60. [DOI] [PubMed] [Google Scholar]
- [5].Harkany T; Guzmán M; Galve-Roperh I; Berghuis P; Devi LA; Mackie K The emerging functions of endocannabinoid signaling during cns development. Trends Pharmacol. Sci, 2007, 28, 83–92. [DOI] [PubMed] [Google Scholar]
- [6].Kreitzer AC; Regehr WG Retrograde signaling by endocannabinoids. Curr. Opin. Neurobiol, 2002, 12, 324–330. [DOI] [PubMed] [Google Scholar]
- [7].Di Marzo V Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov, 2008, 7, 438–455. [DOI] [PubMed] [Google Scholar]
- [8].Muccioli GG; Lambert DM Current knowledge on the antagonists and inverse agonists of cannabinoid receptors. Curr. Med. Chem, 2005, 12, 1361–1394. [DOI] [PubMed] [Google Scholar]
- [9].Jagerovic N; Fernandez-Fernandez C; Goya P Cb1 cannabinoid antagonists: Structure-activity relationships and potential therapeutic applications. Curr. Top. Med. Chem, 2008, 8, 205–230. [DOI] [PubMed] [Google Scholar]
- [10].Azar S; Sherf-Dagan S; Nemirovski A; Webb M; Raziel A; Keidar A; Goitein D; Sakran N; Shibolet O; Tam J; Zelber-Sagi S Circulating endocannabinoids are reduced following bariatric surgery and associated with improved metabolic homeostasis in humans. Obes. Surg, 2018, [DOI] [PubMed]
- [11].Monteleone AM; Di Marzo V; Monteleone P; Dalle Grave R; Aveta T; Ghoch ME; Piscitelli F; Volpe U; Calugi S; Maj M Responses of peripheral endocannabinoids and endocannabinoid-related compounds to hedonic eating in obesity. Eur. J. Nutr, 2016, 55, 1799–1805. [DOI] [PubMed] [Google Scholar]
- [12].van Eyk HJ; van Schinkel LD; Kantae V; Dronkers CEA; Westenberg JJM; de Roos A; Lamb HJ; Jukema JW; Harms AC; Hankemeier T; van der Stelt M; Jazet IM; Rensen PCN; Smit JWA Caloric restriction lowers endocannabinoid tonus and improves cardiac function in type 2 diabetes. Nutr. Diabetes, 2018, 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Perkins JM; Davis SN Endocannabinoid system overactivity and the metabolic syndrome: Prospects for treatment. Curr. Diab. Rep, 2008, 8, 12–19. [DOI] [PubMed] [Google Scholar]
- [14].Duffy D; Rader D Endocannabinoid antagonism: Blocking the excess in the treatment of high-risk abdominal obesity. Trends. Cardiovasc. Med, 2007, 17, 35–43. [DOI] [PubMed] [Google Scholar]
- [15].Despres JP; Golay A; Sjostrom L; Rimonabant in Obesity-Lipids Study, G. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med, 2005, 353, 2121–2134. [DOI] [PubMed] [Google Scholar]
- [16].Van Gaal LF; Rissanen AM; Scheen AJ; Ziegler O; Rossner S; Group, R. I.-E. S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the rio-europe study. Lancet, 2005, 365, 1389–1397. [DOI] [PubMed] [Google Scholar]
- [17].Pi-Sunyer FX; Aronne LJ; Heshmati HM; Devin J; Rosenstock J; Group, R. I.-N. A. S. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: Rio-north america: A randomized controlled trial. JAMA, 2006, 295, 761–775. [DOI] [PubMed] [Google Scholar]
- [18].Scheen AJ; Finer N; Hollander P; Jensen MD; Van Gaal LF; Group, R. I.-D. S. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: A randomised controlled study. Lancet, 2006, 368, 1660–1672. [DOI] [PubMed] [Google Scholar]
- [19].Rosenstock J; Hollander P; Chevalier S; Iranmanesh A; Group, S. S. Serenade: The study evaluating rimonabant efficacy in drug-naive diabetic patients: Effects of monotherapy with rimonabant, the first selective cb1 receptor antagonist, on glycemic control, body weight, and lipid profile in drug-naive type 2 diabetes. Diabetes Care, 2008, 31, 2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Despres JP; Ross R; Boka G; Almeras N; Lemieux I; Investigators, A. D.-L. Effect of rimonabant on the high-triglyceride/ low-hdl-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: The adagio-lipids trial. Arterioscler. Thromb. Vasc. Biol, 2009, 29, 416–423. [DOI] [PubMed] [Google Scholar]
- [21].Steinberg MB; Foulds J Rimonabant for treating tobacco dependence. Vasc. Health Risk Manag, 2007, 3, 307–311. [PMC free article] [PubMed] [Google Scholar]
- [22].Huestis MA; Boyd SJ; Heishman SJ; Preston KL; Bonnet D; Le Fur G; Gorelick DA Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl), 2007, 194, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kirkham TC Taranabant cuts the fat: New hope for cannabinoid-based obesity therapies? Cell Metab, 2008, 7, 1–2. [DOI] [PubMed] [Google Scholar]
- [24].Bergman J; Delatte MS; Paronis CA; Vemuri K; Thakur GA; Makriyannis A Some effects of cb1 antagonists with inverse agonist and neutral biochemical properties. Physiol. Behav, 2008, 93, 666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Greasley PJ; Clapham JC Inverse agonism or neutral antagonism at g-protein coupled receptors: A medicinal chemistry challenge worth pursuing? Eur. J. Pharmacol, 2006, 553, 1–9. [DOI] [PubMed] [Google Scholar]
- [26].Beltramo M; Brusa R; Mancini I; Scandroglio P Detecting constitutive activity and protean agonism at cannabinoid-2 receptor. Methods Enzymol, 2010, 484, 31–51. [DOI] [PubMed] [Google Scholar]
- [27].Osei-Hyiaman D; Liu J; Zhou L; Godlewski G; Harvey-White J; Jeong WI; Batkai S; Marsicano G; Lutz B; Buettner C; Kunos G Hepatic cb1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Invest, 2008, 118, 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bensaid M; Gary-Bobo M; Esclangon A; Maffrand JP; Le Fur G; Oury-Donat F; Soubrie P The cannabinoid cb1 receptor antagonist sr141716 increases acrp30 mrna expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol. Pharmacol, 2003, 63, 908–914. [DOI] [PubMed] [Google Scholar]
- [29].Ahn KH; Mahmoud MM; Kendall DA Allosteric modulator org27569 induces cb1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and gi protein-independent erk1/2 kinase activation. J. Biol. Chem, 2012, 287, 12070–12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Horswill JG; Bali U; Shaaban S; Keily JF; Jeevaratnam P; Babbs AJ; Reynet C; Wong Kai In P Psncbam-1, a novel allosteric antagonist at cannabinoid cb1 receptors with hypophagic effects in rats. Br. J. Pharmacol, 2007, 152, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fong TM Constitutive activity in cannabinoid receptors. Adv. Pharmacol, 2014, 70, 121–133. [DOI] [PubMed] [Google Scholar]
- [32].Pertwee RG Inverse agonism and neutral antagonism at cannabinoid cb1 receptors. Life Sci, 2005, 76, 1307–1324. [DOI] [PubMed] [Google Scholar]
- [33].Kenakin T The physiological significance of constitutive receptor activity. Trends Pharmacol. Sci, 2005, 26, 603–605. [Google Scholar]
- [34].Hurst DP; Lynch DL; Barnett-Norris J; Hyatt SM; Seltzman HH; Zhong M; Song ZH; Nie J; Lewis D; Reggio PH N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1h-pyrazole −3-carboxamide (sr141716a) interaction with lys 3.28(192) is crucial for its inverse agonism at the cannabinoid cb1 receptor. Mol. Pharmacol, 2002, 62, 1274–1287. [DOI] [PubMed] [Google Scholar]
- [35].Pan X; Ikeda SR; Lewis DL Sr 141716a acts as an inverse agonist to increase neuronal voltage-dependent ca2+ currents by reversal of tonic cb1 cannabinoid receptor activity. Mol. Pharmacol, 1998, 54, 1064–1072. [DOI] [PubMed] [Google Scholar]
- [36].Fride E; Braun H; Matan H; Steinberg S; Reggio PH; Seltzman HH Inhibition of milk ingestion and growth after administration of a neutral cannabinoid cb1 receptor antagonist on the first postnatal day in the mouse. Pediatr. Res, 2007, 62, 533–536. [DOI] [PubMed] [Google Scholar]
- [37].Hurst D; Umejiego U; Lynch D; Seltzman H; Hyatt S; Roche M; McAllister S; Fleischer D; Kapur A; Abood M; Shi S; Jones J; Lewis D; Reggio P Biarylpyrazole inverse agonists at the cannabinoid cb1 receptor: Importance of the c-3 carboxamide oxygen/lysine3.28(192) interaction. J. Med. Chem, 2006, 49, 5969–5987. [DOI] [PubMed] [Google Scholar]
- [38].Seltzman HH; Maitra R; Bortoff K; Henson J; Reggio PH; Wesley D; Tam J Metabolic profiling of cb1 neutral antagonists. Methods Enzymol, 2017, 593, 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sink KS; McLaughlin PJ; Wood JA; Brown C; Fan P; Vemuri VK; Peng Y; Olszewska T; Thakur GA; Makriyannis A; Parker LA; Salamone JD The novel cannabinoid cb1 receptor neutral antagonist am4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology, 2008, 33, 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chambers AP; Vemuri VK; Peng Y; Wood JT; Olszewska T; Pittman QJ; Makriyannis A; Sharkey KA A neutral cb1 receptor antagonist reduces weight gain in rat. Am. J. Physiol. Regul. Integr. Comp. Physiol, 2007, 293, R2185–2193. [DOI] [PubMed] [Google Scholar]
- [41].Cluny NL; Chambers AP; Vemuri VK; Wood JT; Eller LK; Freni C; Reimer RA; Makriyannis A; Sharkey KA The neutral cannabinoid cb(1) receptor antagonist am4113 regulates body weight through changes in energy intake in the rat. Pharmacol. Biochem. Behav, 2011, 97, 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jarbe TU; LeMay BJ; Olszewska T; Vemuri VK; Wood JT; Makriyannis A Intrinsic effects of am4113, a putative neutral cb1 receptor selective antagonist, on open-field behaviors in rats. Pharmacol. Biochem. Behav, 2008, 91, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wills KL; Vemuri K; Kalmar A; Lee A; Limebeer CL; Makriyannis A; Parker LA Cb1 antagonism: Interference with affective properties of acute naloxone-precipitated morphine withdrawal in rats. Psychopharmacology (Berl), 2014, 231, 4291–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Balla A; Dong B; Shilpa BM; Vemuri K; Makriyannis A; Pandey SC; Sershen H; Suckow RF; Vinod KY Cannabinoid-1 receptor neutral antagonist reduces binge-like alcohol consumption and alcohol-induced accumbal dopaminergic signaling. Neuropharmacology, 2018, 131, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jarbe TU; LeMay BJ; Vemuri VK; Vadivel SK; Zvonok A; Makriyannis A Central mediation and differential blockade by cannabinergics of the discriminative stimulus effects of the cannabinoid cb1 receptor antagonist rimonabant in rats. Psychopharmacology (Berl), 2011, 216, 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kangas BD; Delatte MS; Vemuri VK; Thakur GA; Nikas SP; Subramanian KV; Shukla VG; Makriyannis A; Bergman J Cannabinoid discrimination and antagonism by cb(1) neutral and inverse agonist antagonists. J. Pharmacol. Exp. Ther, 2013, 344, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schindler CW; Redhi GH; Vemuri K; Makriyannis A; Le Foll B; Bergman J; Goldberg SR; Justinova Z Blockade of nicotine and cannabinoid reinforcement and relapse by a cannabinoid cb1-receptor neutral antagonist am4113 and inverse agonist rimonabant in squirrel monkeys. Neuropsychopharmacology, 2016, 41, 2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gueye AB; Pryslawsky Y; Trigo JM; Poulia N; Delis F; Antoniou K; Loureiro M; Laviolette SR; Vemuri K; Makriyannis A; Le Foll B The cb1 neutral antagonist am4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int. J. Neuropsychopharmacol, 2016, 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sink KS; Segovia KN; Collins LE; Markus EJ; Vemuri VK; Makriyannis A; Salamone JD The cb1 inverse agonist am251, but not the cb1 antagonist am4113, enhances retention of contextual fear conditioning in rats. Pharmacol. Biochem. Behav, 2010, 95, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Storr MA; Bashashati M; Hirota C; Vemuri VK; Keenan CM; Duncan M; Lutz B; Mackie K; Makriyannis A; Macnaughton WK; Sharkey KA Differential effects of cb(1) neutral antagonists and inverse agonists on gastrointestinal motility in mice. Neurogastroenterol. Motil, 2010, 22, 787–796, e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hodge J; Bow JP; Plyler KS; Vemuri VK; Wisniecki A; Salamone JD; Makriyannis A; McLaughlin PJ The cannabinoid cb1 receptor inverse agonist am 251 and antagonist am 4113 produce similar effects on the behavioral satiety sequence in rats. Behav. Brain Res, 2008, 193, 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tai S; Nikas SP; Shukla VG; Vemuri K; Makriyannis A; Jarbe TU Cannabinoid withdrawal in mice: Inverse agonist vs neutral antagonist. Psychopharmacology (Berl), 2015, 232, 2751–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sink KS; Vemuri VK; Wood J; Makriyannis A; Salamone JD Oral bioavailability of the novel cannabinoid cb1 antagonist am6527: Effects on food-reinforced behavior and comparisons with am4113. Pharmacol. Biochem. Behav, 2009, 91, 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mastinu A; Pira M; Pinna GA; Pisu C; Casu MA; Reali R; Marcello S; Murineddu G; Lazzari P Ness06sm reduces body weight with an improved profile relative to sr141716a. Pharmacol. Res, 2013, 74, 94–108. [DOI] [PubMed] [Google Scholar]
- [55].Lazzari P; Serra V; Marcello S; Pira M; Mastinu A Metabolic side effects induced by olanzapine treatment are neutralized by cb1 receptor antagonist compounds co-administration in female rats. Eur. Neuropsychopharmacol, 2017, 27, 667–678. [DOI] [PubMed] [Google Scholar]
- [56].Goonawardena AV; Plano A; Robinson L; Ross R; Greig I; Pertwee RG; Hampson RE; Platt B; Riedel G Modulation of food consumption and sleep-wake cycle in mice by the neutral cb1 antagonist abd459. Behav. Pharmacol, 2015, 26, 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Manca I; Mastinu A; Olimpieri F; Falzoi M; Sani M; Ruiu S; Loriga G; Volonterio A; Tambaro S; Bottazzi ME; Zanda M; Pinna GA; Lazzari P Novel pyrazole derivatives as neutral cb(1) antagonists with significant activity towards food intake. Eur. J. Med. Chem, 2013, 62, 256–269. [DOI] [PubMed] [Google Scholar]
- [58].Bostrom J; Olsson RI; Tholander J; Greasley PJ; Ryberg E; Nordberg H; Hjorth S; Cheng L Novel thioamide derivatives as neutral cb1 receptor antagonists. Bioorg. Med. Chem. Lett, 2010, 20, 479–482. [DOI] [PubMed] [Google Scholar]
- [59].Pertwee RG; Thomas A; Stevenson LA; Ross RA; Varvel SA; Lichtman AH; Martin BR; Razdan RK The psychoactive plant cannabinoid, delta9-tetrahydrocannabinol, is antagonized by delta8- and delta9-tetrahydrocannabivarin in mice in vivo. Br. J. Pharmacol, 2007, 150, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Batkai S; Mukhopadhyay P; Horvath B; Rajesh M; Gao RY; Mahadevan A; Amere M; Battista N; Lichtman AH; Gauson LA; Maccarrone M; Pertwee RG; Pacher P Delta8-tetrahydrocannabivarin prevents hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid cb2 receptors. Br. J. Pharmacol, 2012, 165, 2450–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Thomas A; Stevenson LA; Wease KN; Price MR; Baillie G; Ross RA; Pertwee RG Evidence that the plant cannabinoid delta9-tetrahydrocannabivarin is a cannabinoid cb1 and cb2 receptor antagonist. Br. J. Pharmacol, 2005, 146, 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bolognini D; Costa B; Maione S; Comelli F; Marini P; Di Marzo V; Parolaro D; Ross RA; Gauson LA; Cascio MG; Pertwee RG The plant cannabinoid delta9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br. J. Pharmacol, 2010, 160, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Riedel G; Fadda P; McKillop-Smith S; Pertwee RG; Platt B; Robinson L Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br. J. Pharmacol, 2009, 156, 1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wargent ET; Zaibi MS; Silvestri C; Hislop DC; Stocker CJ; Stott CG; Guy GW; Duncan M; Di Marzo V; Cawthorne MA The cannabinoid delta(9)-tetrahydrocannabivarin (thcv) ameliorates insulin sensitivity in two mouse models of obesity. Nutr. Diabetes, 2013, 3, e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tudge L; Williams C; Cowen PJ; McCabe C Neural effects of cannabinoid cb1 neutral antagonist tetrahydrocannabivarin on food reward and aversion in healthy volunteers. Int. J. Neuropsychopharmacol, 2014, 18, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rzepa E; Tudge L; McCabe C The cb1 neutral antagonist tetrahydrocannabivarin reduces default mode network and increases executive control network resting state functional connectivity in healthy volunteers. Int. J. Neuropsychopharmacol, 2015, 19, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Thomas A; Ross RA; Saha B; Mahadevan A; Razdan RK; Pertwee RG 6”-azidohex-2”-yne-cannabidiol: A potential neutral, competitive cannabinoid cb1 receptor antagonist. Eur. J. Pharmacol, 2004, 487, 213–221. [DOI] [PubMed] [Google Scholar]
- [68].Wiley JL; Breivogel CS; Mahadevan A; Pertwee RG; Cascio MG; Bolognini D; Huffman JW; Walentiny DM; Vann RE; Razdan RK; Martin BR Structural and pharmacological analysis of o-2050, a putative neutral cannabinoid cb(1) receptor antagonist. Eur. J. Pharmacol, 2011, 651, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jergas B; Schulte K; Bindila L; Lutz B; Schlicker E O-2050 facilitates noradrenaline release and increases the cb1 receptor inverse agonistic effect of rimonabant in the guinea pig hippocampus. Naunyn. Schmiedebergs. Arch. Pharmacol, 2014, 387, 621–628. [DOI] [PubMed] [Google Scholar]
- [70].Elsebai MF; Rempel V; Schnakenburg G; Kehraus S; Muller CE; Konig GM Identification of a potent and selective cannabinoid cb1 receptor antagonist from auxarthron reticulatum. ACS Med. Chem. Lett, 2011, 2, 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Seely KA; Brents LK; Radominska-Pandya A; Endres GW; Keyes GS; Moran JH; Prather PL A major glucuronidated metabolite of jwh-018 is a neutral antagonist at cb1 receptors. Chem. Res. Toxicol, 2012, 25, 825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pajouhesh H; Lenz GR Medicinal chemical properties of successful central nervous system drugs. NeuroRx, 2005, 2, 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kelder J; Grootenhuis PD; Bayada DM; Delbressine LP; Ploemen JP Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharm. Res, 1999, 16, 1514–1519. [DOI] [PubMed] [Google Scholar]
- [74].Chorvat RJ Peripherally restricted cb1 receptor blockers. Bioorg. Med. Chem. Lett, 2013, 23, 4751–4760. [DOI] [PubMed] [Google Scholar]
- [75].Cluny NL; Vemuri VK; Chambers AP; Limebeer CL; Bedard H; Wood JT; Lutz B; Zimmer A; Parker LA; Makriyannis A; Sharkey KA A novel peripherally restricted cannabinoid receptor antagonist, am6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br. J. Pharmacol, 2010, 161, 629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Randall PA; Vemuri VK; Segovia KN; Torres EF; Hosmer S; Nunes EJ; Santerre JL; Makriyannis A; Salamone JD The novel cannabinoid cb1 antagonist am6545 suppresses food intake and food-reinforced behavior. Pharmacol. Biochem. Behav, 2010, 97, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Argueta DA; DiPatrizio NV Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol. Behav, 2017, 171, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].DiPatrizio NV; Igarashi M; Narayanaswami V; Murray C; Gancayco J; Russell A; Jung KM; Piomelli D Fasting stimulates 2-ag biosynthesis in the small intestine: Role of cholinergic pathways. Am. J. Physiol. Regul. Integr. Comp. Physiol, 2015, 309, R805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Boon MR; Kooijman S; van Dam AD; Pelgrom LR; Berbee JF; Visseren CA; van Aggele RC; van den Hoek AM; Sips HC; Lombes M; Havekes LM; Tamsma JT; Guigas B; Meijer OC; Jukema JW; Rensen PC Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J, 2014, 28, 5361–5375. [DOI] [PubMed] [Google Scholar]
- [80].Ma H; Zhang G; Mou C; Fu X; Chen Y Peripheral cb1 receptor neutral antagonist, am6545, ameliorates hypometabolic obesity and improves adipokine secretion in monosodium glutamate induced obese mice. Front. Pharmacol, 2018, 9, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bowles NP; Karatsoreos IN; Li X; Vemuri VK; Wood JA; Li Z; Tamashiro KL; Schwartz GJ; Makriyannis AM; Kunos G; Hillard CJ; McEwen BS; Hill MN A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc. Natl. Acad. Sci. U. S. A, 2015, 112, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Barutta F; Grimaldi S; Gambino R; Vemuri K; Makriyannis A; Annaratone L; di Marzo V; Bruno G; Gruden G Dual therapy targeting the endocannabinoid system prevents experimental diabetic nephropathy. Nephrol. Dial. Transplant, 2017, 32, 1655–1665. [DOI] [PubMed] [Google Scholar]
- [83].Grzeda E; Schlicker E; Luczaj W; Harasim E; Baranowska-Kuczko M; Malinowska B Bi-directional cb1 receptor-mediated cardiovascular effects of cannabinoids in anaesthetized rats: Role of the paraventricular nucleus. J. Physiol. Pharmacol, 2015, 66, 343–353. [PubMed] [Google Scholar]
- [84].Bronova I; Smith B; Aydogan B; Weichselbaum RR; Vemuri K; Erdelyi K; Makriyannis A; Pacher P; Berdyshev EV Protection from radiation-induced pulmonary fibrosis by peripheral targeting of cannabinoid receptor-1. Am. J. Respir. Cell Mol. Biol, 2015, 53, 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Limebeer CL; Vemuri VK; Bedard H; Lang ST; Ossenkopp KP; Makriyannis A; Parker LA Inverse agonism of cannabinoid cb1 receptors potentiates licl-induced nausea in the conditioned gaping model in rats. Br. J. Pharmacol, 2010, 161, 336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].McLaughlin PJ; Jagielo-Miller JE; Plyler ES; Schutte KK; Vemuri VK; Makriyannis A Differential effects of cannabinoid cb1 inverse agonists and antagonists on impulsivity in male sprague dawley rats: Identification of a possibly clinically relevant vulnerability involving the serotonin 5ht1a receptor. Psychopharmacology (Berl), 2017, 234, 1029–1043. [DOI] [PubMed] [Google Scholar]
- [87].7TM_Pharma. (2009). Tm38837, a cb1 receptor antagonist/inverse agonist.
- [88].Klumpers LE; Fridberg M; de Kam ML; Little PB; Jensen NO; Kleinloog HD; Elling CE; van Gerven JM Peripheral selectivity of the novel cannabinoid receptor antagonist tm38837 in healthy subjects. Br. J. Clin. Pharmacol, 2013, 76, 846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Guan Z; Klumpers LE; Oyetayo O-O; Heuberger J; Gerven J. M. A. v. ; Stevens J. Pharmacokinetic/pharmacodynamic modelling and simulation of the effects of different cannabinoid receptor type 1 antagonists on δ9-tetrahydrocannabinol challenge tests. Br. J. Clin. Pharmacol, 2015, 81, 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Takano A; Gulyas B; Varnas K; Little PB; Noerregaard PK; Jensen NO; Elling CE; Halldin C Low brain cb1 receptor occupancy by a second generation cb1 receptor antagonist tm38837 in comparison with rimonabant in nonhuman primates: A pet study. Synapse, 2014, 68, 89–97. [DOI] [PubMed] [Google Scholar]
- [91].Fulp A; Bortoff K; Zhang Y; Seltzman H; Snyder R; Maitra R Towards rational design of cannabinoid receptor 1 (cb1) antagonists for peripheral selectivity. Bioorg. Med. Chem. Lett, 2011, 21, 5711–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Fulp A; Bortoff K; Seltzman H; Zhang Y; Mathews J; Snyder R; Fennell T; Maitra R Design and synthesis of cannabinoid receptor 1 antagonists for peripheral selectivity. J. Med. Chem, 2012, 55, 2820–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Fulp A; Zhang Y; Bortoff K; Seltzman H; Snyder R; Wiethe R; Amato G; Maitra R Pyrazole antagonists of the cb1 receptor with reduced brain penetration. Bioorg. Med. Chem, 2016, 24, 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Tai CL; Hung MS; Pawar VD; Tseng SL; Song JS; Hsieh WP; Chiu HH; Wu HC; Hsieh MT; Kuo CW; Hsieh CC; Tsao JP; Chao YS; Shia KS Design, synthesis, and biological evaluation of novel alkenylthiophenes as potent and selective cb1 cannabinoid receptor antagonists. Org. Biomol. Chem, 2008, 6, 447–450. [DOI] [PubMed] [Google Scholar]
- [95].Hung MS; Chang CP; Li TC; Yeh TK; Song JS; Lin Y; Wu CH; Kuo PC; Amancha PK; Wong YC; Hsiao WC; Chao YS; Shia KS Discovery of 1-(2,4-dichlorophenyl)-4-ethyl-5-(5-(2-(4-(trifluoromethyl)phenyl)ethynyl)thiophe n-2-yl)-n-(piperidin-1-yl)-1h-pyrazole-3-carboxamide as a potential peripheral cannabinoid-1 receptor inverse agonist. ChemMedChem, 2010, 5, 1439–1443. [DOI] [PubMed] [Google Scholar]
- [96].Chang CP; Wu CH; Song JS; Chou MC; Wong YC; Lin Y; Yeh TK; Sadani AA; Ou MH; Chen KH; Chen PH; Kuo PC; Tseng CT; Chang KH; Tseng SL; Chao YS; Hung MS; Shia KS Discovery of 1-(2,4-dichlorophenyl)-n-(piperidin-1-yl)-4-((pyrrolidine-1-sulfonamido)methyl)-5 -(5-((4-(trifluoromethyl)phenyl)ethynyl)thiophene-2-yl)-1h-pyrazole-3-carboxamide as a novel peripherally restricted cannabinoid-1 receptor antagonist with significant weight-loss efficacy in diet-induced obese mice. J. Med. Chem, 2013, 56, 9920–9933. [DOI] [PubMed] [Google Scholar]
- [97].Chen W; Shui F; Liu C; Zhou X; Li W; Zheng Z; Fu W; Wang L Novel peripherally restricted cannabinoid 1 receptor selective antagonist txx-522 with prominent weight-loss efficacy in diet induced obese mice. Front. Pharmacol, 2017, 8, 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jagerovic N; Hernandez-Folgado L; Alkorta I; Goya P; Navarro M; Serrano A; Rodriguez de Fonseca F; Dannert MT; Alsasua A; Suardiaz M; Pascual D; Martin MI Discovery of 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1h-1,2,4-triazole, a novel in vivo cannabinoid antagonist containing a 1,2,4-triazole motif. J. Med. Chem, 2004, 47, 2939–2942. [DOI] [PubMed] [Google Scholar]
- [99].Chen RZ; Frassetto A; Lao JZ; Huang RR; Xiao JC; Clements MJ; Walsh TF; Hale JJ; Wang J; Tong X; Fong TM Pharmacological evaluation of lh-21, a newly discovered molecule that binds to cannabinoid cb1 receptor. Eur. J. Pharmacol, 2008, 584, 338–342. [DOI] [PubMed] [Google Scholar]
- [100].Alen F; Crespo I; Ramirez-Lopez MT; Jagerovic N; Goya P; de Fonseca FR; de Heras RG; Orio L Ghrelin-induced orexigenic effect in rats depends on the metabolic status and is counteracted by peripheral cb1 receptor antagonism. PLoS One, 2013, 8, e60918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Romero-Zerbo SY; Ruz-Maldonado I; Espinosa-Jimenez V; Rafacho A; Gomez-Conde AI; Sanchez-Salido L; Cobo-Vuilleumier N; Gauthier BR; Tinahones FJ; Persaud SJ; Bermudez-Silva FJ The cannabinoid ligand lh-21 reduces anxiety and improves glucose handling in diet-induced obese pre-diabetic mice. Sci. Rep, 2017, 7, 3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Pavon FJ; Serrano A; Perez-Valero V; Jagerovic N; Hernandez-Folgado L; Bermudez-Silva FJ; Macias M; Goya P; de Fonseca FR Central versus peripheral antagonism of cannabinoid cb1 receptor in obesity: Effects of lh-21, a peripherally acting neutral cannabinoid receptor antagonist, in zucker rats. J. Neuroendocrinol, 2008, 20 Suppl 1, 116–123. [DOI] [PubMed] [Google Scholar]
- [103].Alonso M; Serrano A; Vida M; Crespillo A; Hernandez-Folgado L; Jagerovic N; Goya P; Reyes-Cabello C; Perez-Valero V; Decara J; Macias-Gonzalez M; Bermudez-Silva FJ; Suarez J; Rodriguez de Fonseca F; Pavon FJ Anti-obesity efficacy of lh-21, a cannabinoid cb(1) receptor antagonist with poor brain penetration, in diet-induced obese rats. Br. J. Pharmacol, 2012, 165, 2274–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pavon FJ; Bilbao A; Hernandez-Folgado L; Cippitelli A; Jagerovic N; Abellan G; Rodriguez-Franco MA; Serrano A; Macias M; Gomez R; Navarro M; Goya P; Rodriguez de Fonseca F Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1h-1,2,4-triazole--lh 21. Neuropharmacology, 2006, 51, 358–366. [DOI] [PubMed] [Google Scholar]
- [105].Chorvat RJ; Berbaum J; Seriacki K; McElroy JF Jd-5006 and jd-5037: Peripherally restricted (pr) cannabinoid-1 receptor blockers related to slv-319 (ibipinabant) as metabolic disorder therapeutics devoid of cns liabilities. Bioorg. Med. Chem. Lett, 2012, 22, 6173–6180. [DOI] [PubMed] [Google Scholar]
- [106].Jenrin_Discovery, https://www.businesswire.com/news/home/20171214005128/en/Jenrin-Discovery%E2%80%99s-IND-Application-Peripherally-Restricted-Cannabinoid-1 (Accessed Sep 2018)
- [107].Jourdan T; Szanda G; Rosenberg AZ; Tam J; Earley BJ; Godlewski G; Cinar R; Liu Z; Liu J; Ju C; Pacher P; Kunos G Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc. Natl. Acad. Sci. U. S. A, 2014, 111, E5420–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mukhopadhyay B; Schuebel K; Mukhopadhyay P; Cinar R; Godlewski G; Xiong K; Mackie K; Lizak M; Yuan Q; Goldman D; Kunos G Cannabinoid receptor 1 promotes hepatocellular carcinoma initiation and progression through multiple mechanisms. Hepatology, 2015, 61, 1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tam J; Cinar R; Liu J; Godlewski G; Wesley D; Jourdan T; Szanda G; Mukhopadhyay B; Chedester L; Liow JS; Innis RB; Cheng K; Rice KC; Deschamps JR; Chorvat RJ; McElroy JF; Kunos G Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab, 2012, 16, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Cinar R; Godlewski G; Liu J; Tam J; Jourdan T; Mukhopadhyay B; Harvey-White J; Kunos G Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology, 2014, 59, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Tam J; Szanda G; Drori A; Liu Z; Cinar R; Kashiwaya Y; Reitman ML; Kunos G Peripheral cannabinoid-1 receptor blockade restores hypothalamic leptin signaling. Mol. Metab, 2017, 6, 1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Knani I; Earley BJ; Udi S; Nemirovski A; Hadar R; Gammal A; Cinar R; Hirsch HJ; Pollak Y; Gross I; Eldar-Geva T; Reyes-Capo DP; Han JC; Haqq AM; Gross-Tsur V; Wevrick R; Tam J Targeting the endocannabinoid/cb1 receptor system for treating obesity in prader-willi syndrome. Mol. Metab, 2016, 5, 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gonzalez-Mariscal I; Krzysik-Walker SM; Doyle ME; Liu QR; Cimbro R; Santa-Cruz Calvo S; Ghosh S; Ciesla L; Moaddel R; Carlson OD; Witek RP; O’Connell JF; Egan JM Human cb1 receptor isoforms, present in hepatocytes and beta-cells, are involved in regulating metabolism. Sci. Rep, 2016, 6, 33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Griffith DA; Hadcock JR; Black SC; Iredale PA; Carpino PA; DaSilva-Jardine P; Day R; DiBrino J; Dow RL; Landis MS; O’Connor RE; Scott DO Discovery of 1-[9-(4-chlorophenyl)-8-(2-chlorophenyl)-9h-purin-6-yl]-4-ethylaminopiperidine-4- carboxylic acid amide hydrochloride (cp-945,598), a novel, potent, and selective cannabinoid type 1 receptor antagonist. J. Med. Chem, 2009, 52, 234–237. [DOI] [PubMed] [Google Scholar]
- [115].Fulp A; Bortoff K; Zhang Y; Seltzman H; Mathews J; Snyder R; Fennell T; Maitra R Diphenyl purine derivatives as peripherally selective cannabinoid receptor 1 antagonists. J. Med. Chem, 2012, 55, 10022–10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Fulp A; Bortoff K; Zhang Y; Snyder R; Fennell T; Marusich JA; Wiley JL; Seltzman H; Maitra R Peripherally selective diphenyl purine antagonist of the cb1 receptor. J. Med. Chem, 2013, 56, 8066–8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Amato GS; Manke A; Harris DL; Wiethe RW; Vasukuttan V; Snyder RW; Lefever TW; Cortes R; Zhang Y; Wang S; Runyon SP; Maitra R Blocking alcoholic steatosis in mice with a peripherally restricted purine antagonist of the type 1 cannabinoid receptor. J. Med. Chem, 2018, 61, 4370–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Amato GS; Manke A; Vasukuttan V; Wiethe RW; Snyder RW; Runyon SP; Maitra R Synthesis and pharmacological characterization of functionalized 6-piperazin-1-yl-purines as cannabinoid receptor 1 (cb1) inverse agonists. Bioorg. Med. Chem, 2018, 26, 4518–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Picone RP; Khanolkar AD; Xu W; Ayotte LA; Thakur GA; Hurst DP; Abood ME; Reggio PH; Fournier DJ; Makriyannis A (−)-7’-isothiocyanato-11-hydroxy-1’,1’-dimethylheptylhexahydrocannabinol (am841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the cb1 cannabinoid receptor. Mol. Pharmacol, 2005, 68, 1623–1635. [DOI] [PubMed] [Google Scholar]
- [120].Keenan CM; Storr MA; Thakur GA; Wood JT; Wager-Miller J; Straiker A; Eno MR; Nikas SP; Bashashati M; Hu H; Mackie K; Makriyannis A; Sharkey KA Am841, a covalent cannabinoid ligand, powerfully slows gastrointestinal motility in normal and stressed mice in a peripherally restricted manner. Br. J. Pharmacol, 2015, 172, 2406–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhu B; Matthews JM; Xia M; Black S; Chen C; Hou C; Liang Y; Tang Y; Macielag MJ Tetrahydropyrazolo[4,3-c]pyridine derivatives as potent and peripherally selective cannabinoid-1 (cb1) receptor inverse agonists. Bioorg. Med. Chem. Lett, 2016, 26, 5597–5601. [DOI] [PubMed] [Google Scholar]
- [122].Matthews JM; McNally JJ; Connolly PJ; Xia M; Zhu B; Black S; Chen C; Hou C; Liang Y; Tang Y; Macielag MJ Tetrahydroindazole derivatives as potent and peripherally selective cannabinoid-1 (cb1) receptor inverse agonists. Bioorg. Med. Chem. Lett, 2016, 26, 5346–5349. [DOI] [PubMed] [Google Scholar]
- [123].Price MR; Baillie GL; Thomas A; Stevenson LA; Easson M; Goodwin R; McLean A; McIntosh L; Goodwin G; Walker G; Westwood P; Marrs J; Thomson F; Cowley P; Christopoulos A; Pertwee RG; Ross RA Allosteric modulation of the cannabinoid cb1 receptor. Mol. Pharmacol, 2005, 68, 1484–1495. [DOI] [PubMed] [Google Scholar]
- [124].Conn PJ; Christopoulos A; Lindsley CW Allosteric modulators of gpcrs: A novel approach for the treatment of cns disorders. Nat. Rev. Drug Discov, 2009, 8, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kenakin T; Strachan RT Pam-antagonists: A better way to block pathological receptor signaling? Trends Pharmacol. Sci, 2018, 39, 748–765. [DOI] [PubMed] [Google Scholar]
- [126].Nguyen T; Li JX; Thomas BF; Wiley JL; Kenakin TP; Zhang Y Allosteric modulation: An alternate approach targeting the cannabinoid cb1 receptor. Med. Res. Rev, 2017, 37, 441–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Khajehali E; Malone DT; Glass M; Sexton PM; Christopoulos A; Leach K Biased agonism and biased allosteric modulation at the cb1 cannabinoid receptor. Mol. Pharmacol, 2015, 88, 368–379. [DOI] [PubMed] [Google Scholar]
- [128].Baillie GL; Horswill JG; Anavi-Goffer S; Reggio PH; Bolognini D; Abood ME; McAllister S; Strange PG; Stephens GJ; Pertwee RG; Ross RA Cb(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Mol. Pharmacol, 2013, 83, 322–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Nguyen T; German N; Decker AM; Li JX; Wiley JL; Thomas BF; Kenakin TP; Zhang Y Structure-activity relationships of substituted 1h-indole-2-carboxamides as cb1 receptor allosteric modulators. Bioorg. Med. Chem, 2015, 23, 2195–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Piscitelli F Alessia L; Giuseppe La Regina; Coluccia Antonio; Morera Ludovica; Allara Marco; Novellino Ettore; Vincenzo Di Marzo; Silvestri Romano. Indole-2-carboxamides as allosteric modulators of the cannabinoid cb1 receptor. J. Med. Chem, 2012, 55, 5627–5631. [DOI] [PubMed] [Google Scholar]
- [131].Ahn KH; Mahmoud MM; Samala S; Lu D; Kendall DA Profiling two indole-2-carboxamides for allosteric modulation of the cb1 receptor. J Neurochem, 2013, 124, 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Greig IR; Ross RA; Pertwee RG; Trembleau L; Abdelrahman M; Baillie GL N-(arylalkyl)-1h-indole-2-sulfonic acid amide compounds and their therapeutic use as cannabinoid allosteric modulators. Greater Britain Patent WO 2012/117216 A1, 2012.
- [133].Khurana L; Ali HI; Olszewska T; Ahn KH; Damaraju A; Kendall DA; Lu D Optimization of chemical functionalities of indole-2-carboxamides to improve allosteric parameters for the cannabinoid receptor 1 (cb1). J. Med. Chem, 2014, 57, 3040–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Gamage TF; Ignatowska-Jankowska BM; Wiley JL; Abdelrahman M; Trembleau L; Greig IR; Thakur GA; Tichkule R; Poklis J; Ross RA; Pertwee RG; Lichtman AH In-vivo pharmacological evaluation of the cb1-receptor allosteric modulator org-27569. Behav. Pharmacol, 2014, 25, 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ding Y; Qiu Y; Jing L; Thorn DA; Zhang Y; Li JX Behavioral effects of the cannabinoid cb1 receptor allosteric modulator org27569 in rats. Pharmacol. Res. Perspect, 2014, 2, e00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].German N; Decker AM; Gilmour BP; Gay EA; Wiley JL; Thomas BF; Zhang Y Diarylureas as allosteric modulators of the cannabinoid cb1 receptor: Structure-activity relationship studies on 1-(4-chlorophenyl)-3-{3-[6-(pyrrolidin-1-yl)pyridin-2-yl]phenyl}urea (psncbam-1). J. Med. Chem, 2014, 57, 7758–7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Thakur GA; Kulkarni PM Allosteric modulators of cb1 cannabinoid receptors. United States Patent US 2015/0005346 A1, 2015.
- [138].Nguyen T; German N; Decker AM; Langston TL; Gamage TF; Farquhar CE; Li JX; Wiley JL; Thomas BF; Zhang Y Novel diarylurea based allosteric modulators of the cannabinoid cb1 receptor: Evaluation of importance of 6-pyrrolidinylpyridinyl substitution. J. Med. Chem, 2017, 60, 7410–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Khurana L; Fu BQ; Duddupudi AL; Liao YH; Immadi SS; Kendall DA; Lu D Pyrimidinyl biphenylureas: Identification of new lead compounds as allosteric modulators of the cannabinoid receptor cb1. J. Med. Chem, 2017, 60, 1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Bertini S; Chicca A; Gado F; Arena C; Nieri D; Digiacomo M; Saccomanni G; Zhao P; Abood ME; Macchia M; Gertsch J; Manera C Novel analogs of psncbam-1 as allosteric modulators of cannabinoid cb1 receptor. Bioorg. Med. Chem, 2017, 25, 6427–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Nguyen T; Gamage TF; Decker AM; German N; Langston TL; Farquhar CE; Kenakin TP; Wiley JL; Thomas BF; Zhang Y Diarylureas containing 5-membered heterocycles as cb1 receptor allosteric modulators: Design, synthesis, and pharmacological evaluation. ACS Chem. Neurosci, 2018, In press. [DOI] [PMC free article] [PubMed]
- [142].Gamage TF; Farquhar CE; Lefever TW; Thomas BF; Nguyen T; Zhang Y; Wiley JL The great divide: Separation between in vitro and in vivo effects of psncbam-based cb1 receptor allosteric modulators. Neuropharmacology, 2017, 125, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bauer M; Chicca A; Tamborrini M; Eisen D; Lerner R; Lutz B; Poetz O; Pluschke G; Gertsch J Identification and quantification of a new family of peptide endocannabinoids (pepcans) showing negative allosteric modulation at cb1 receptors. J. Biol. Chem, 2012, 287, 36944–36967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Straiker A; Mitjavila J; Yin D; Gibson A; Mackie K Aiming for allosterism: Evaluation of allosteric modulators of cb1 in a neuronal model. Pharmacol. Res, 2015, 99, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gomes I; Grushko JS; Golebiewska U; Hoogendoorn S; Gupta A; Heimann AS; Ferro ES; Scarlata S; Fricker LD; Devi LA Novel endogenous peptide agonists of cannabinoid receptors. FASEB J, 2009, 23, 3020–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Petrucci V; Chicca A; Viveros-Paredes J; Gertsch J In: Peptide endocannabinoids (pepcans) are pams of cb2 receptors and involved in the innate immune response, Proceedings of the 24th annual symposiums on the cannabinoids, International Cannabinoid Research Society, 2014. [Google Scholar]
- [147].Vallee M; Vitiello S; Bellocchio L; Hebert-Chatelain E; Monlezun S; Martin-Garcia E; Kasanetz F; Baillie GL; Panin F; Cathala A; Roullot-Lacarriere V; Fabre S; Hurst DP; Lynch DL; Shore DM; Deroche-Gamonet V; Spampinato U; Revest JM; Maldonado R; Reggio PH; Ross RA; Marsicano G; Piazza PV Pregnenolone can protect the brain from cannabis intoxication. Science, 2014, 343, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Priestley RS; Nickolls SA; Alexander SP; Kendall DA A potential role for cannabinoid receptors in the therapeutic action of fenofibrate. FASEB J, 2015, 29, 1446–1455. [DOI] [PubMed] [Google Scholar]
- [149].Laprairie RB; Bagher AM; Kelly ME; Dupre DJ; Denovan-Wright EM Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J. Biol. Chem, 2014, 289, 24845–24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Laprairie RB; Bagher AM; Kelly ME; Denovan-Wright EM Cannabidiol is a negative allosteric modulator of the cannabinoid cb1 receptor. Br. J. Pharmacol, 2015, 172, 4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Salamone JD; McLaughlin PJ; Sink K; Makriyannis A; Parker LA Cannabinoid cb1 receptor inverse agonists and neutral antagonists: Effects on food intake, food-reinforced behavior and food aversions. Physiol. Behav, 2007, 91, 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kenakin T Efficacy as a vector: The relative prevalence and paucity of inverse agonism. Mol. Pharmacol, 2004, 65, 2–11. [DOI] [PubMed] [Google Scholar]
- [153].Giraldo J How inverse can a neutral antagonist be? Strategic questions after the rimonabant issue. Drug Discov. Today, 2010, 15, 411–415. [DOI] [PubMed] [Google Scholar]
- [154].Nissen SE; Nicholls SJ; Wolski K; Rodes-Cabau J; Cannon CP; Deanfield JE; Despres JP; Kastelein JJ; Steinhubl SR; Kapadia S; Yasin M; Ruzyllo W; Gaudin C; Job B; Hu B; Bhatt DL; Lincoff AM; Tuzcu EM; Investigators S Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: The stradivarius randomized controlled trial. JAMA, 2008, 299, 1547–1560. [DOI] [PubMed] [Google Scholar]
- [155].Sugamura K; Sugiyama S; Nozaki T; Matsuzawa Y; Izumiya Y; Miyata K; Nakayama M; Kaikita K; Obata T; Takeya M; Ogawa H Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation, 2009, 119, 28–36. [DOI] [PubMed] [Google Scholar]
- [156].Izzo AA; Camilleri M Emerging role of cannabinoids in gastrointestinal and liver diseases: Basic and clinical aspects. Gut, 2008, 57, 1140–1155. [DOI] [PubMed] [Google Scholar]
- [157].Richardson D; Pearson RG; Kurian N; Latif ML; Garle MJ; Barrett DA; Kendall DA; Scammell BE; Reeve AJ; Chapman V Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthrit. Res. Ther, 2008, 10, R43. [DOI] [PMC free article] [PubMed] [Google Scholar]