Abstract
Background/Aims
Semen hyperviscosity (SHV) is one of the significant factors involved in poor semen quality and male infertility. It also leads major problems during assisted reproduction techniques and in vitro fertilization process. Although influence of SHV on sperm quality, fertilization rate and male infertility have been widely considered, molecular and cellular mechanisms for these abnormalities are not well understood. In this review, we aimed to discuss the proposed cellular and molecular mechanisms of SHV on male reproductive system, the importance of oxidative stress (OS) and the mechanisms by which SHV induces OS and impairment of other antioxidants.
Methods
A PubMed/Medline and EM-BASE search was performed using keywords: “hyperviscosity semen”, “oxidative stress”, and “male infertility”.
Conclusion
OS induced by reactive oxygen species can be considered as a major mechanism in patients with hyperviscosity semen that is associated with DNA fragmentation, lipid peroxidation and sperm membrane disintegrity, apoptosis, depletion of antioxidants, and subsequently poor sperm quality and male infertility. Therefore, antioxidant therapy may improve main pathological effects of hyperviscosity semen, especially oxidative damages and inflammation, on sperm quality and function. Further, randomized controlled studies are necessary to confirm these results and make a comparison between effects of various antioxidants such as N-acethyl-cysteine and Curcumin on fertility problem in patients with hyperviscous semen.
Key Words: Antioxidants, Lipid peroxidation, Male infertility, Oxidative stress, Semen hyperviscosity
Introduction
Infertility is now considered as one of the significant health problems among couples worldwide. There are many factors that affect sperm parameters, function and subsequently male fertility [1]. Genetic abnormalities, molecular mutations, hormonal defects, impaired sper-matogenesis, nutritional deficiency of some trace elements and vitamins, obstructive problems and structural damages such as varicocele, environmental agents, and life style are grouped as popular factors that affect human sperm function and fertilization processes [2,3].
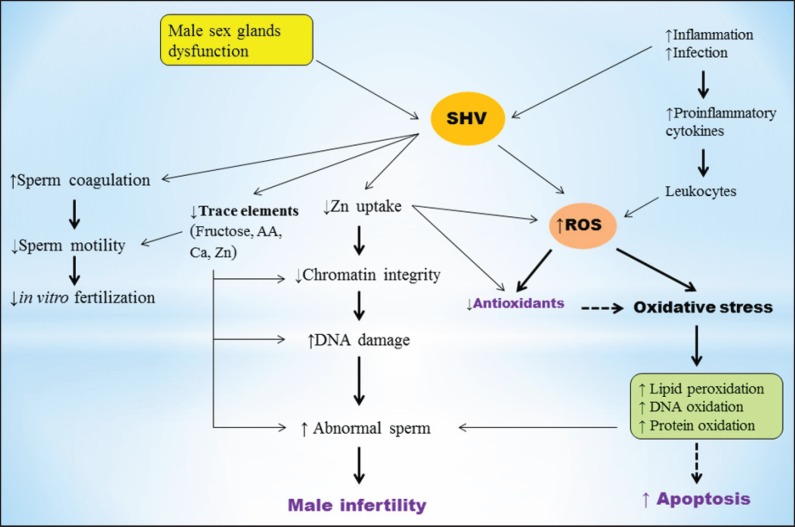
Semen hyperviscosity (SHV), which is characterized by a thick and coagulated appearance, is a condition that affects the physical and chemical characteristics of human seminal fluid [4]. Semen with normal viscose plays critical roles for sperm function and fertilization process. It facilitates the entry of spermatozoa into cervical mucus [5], maintains sperm swimming speed after mucus pen-etration, regulates the distribution of surface charges on the sperm membrane during the maturation process [6], prevents of the lipid peroxidation reaction [7], and maintains the chromatin integrity of spermatozoa [8]. SHV is probably caused by dysfunction, infection and inflammation of the male accessory glands or the immune system [9]. Recent studies have reported that SHV occurs in 12-29% of ejaculates and can be considered as a main reason for male infertility [9,10,11]. It has been shown to be contributed in poor sperm motility and semen quality, as well as a poor outcome with in vitro fertilization [12]. Although the pathogenic aspects of SHV are now clarified, the exact mechanism in which hyperviscous semen is associated with abnormal sperm and male infertility are still not clear. Altered sperm motility, technical difficulties in in vitro fertilization, inflammation, oxidative stress (OS) and changes in trace elements are the major proposed mechanisms of SHV on male infertility (Fig. 1). In the following sections we will discuss these proposed mechanisms in which SHV induces impaired spermato-genesis and male infertility.
Fig. 1.
Proposed mechanisms for the effects of hyperviscous semen on poor sperm quality and male infertility. SHV decreases sperm quality and fertilization rate through several mechanisms including: impairment of sperm motility, increased number of inflammatory cytokines and leukocytes, enhanced ROS production and OS, reduced uptake of Zn, impairment of seminal plasma antioxidants, changes in some trace elements and apoptosis. OS induced by overproduction of ROS and antioxidants impairment is the major mechanism of SHV which is associated with sperm DNA oxidation, membrane lipid peroxidation, and subsequently poor sperm quality.
Sperm Motility
Impairment of normal sperm movement is considered as one of the significant mechanisms by which SHV leads to male infertility [13]. Numerous studies have demonstrated that SHV is associated with reduced sperm motility because of a trapping effect of hypervis-cous semen [11]. It is contributed to the pathogenesis of different forms of asthenozoospermia and male infertility [14]. Recent studies have found significant negative correlations between SHV and sperm motility, grade of motility, total motile sperm and sperm vitality [11,14,15]. Normal motility of sperm is a critical factor for the entry of sperm into the cervical mucus, sperm-egg interaction and fertilization process. Therefore, hyperviscous semen traps sperm cells in the fibrous or mucoid mass and prevents their normal progression through the female genital tract.
In vitro Fertilization
As seminal plasma has an important role in events leading to fertilization, SHV can also lead to in vivo and in vitro complications which have negative consequences in assisted reproductive technology setting [11,12]. Hy-perviscous semen causes certain difficulties for proper separation of spermatozoa and its number. SHV is reported to be associated with poor outcome of controlled ovarian hyperstimulation and intrauterine insemination [16]. It reduces fertilization rates in patients undergoing in vitro fertilization programs [11]. SHV can also result in certain technical difficulties in the handling of semen samples, when using Percoll gradients to prepare sperm for in vitro fertilization [11].
Deficiency in Zinc Uptake
SHV, which is caused by the seminal vesicle hypo-function, can lead to enhanced proportion of prostatic fluid that contains zinc (Zn) [12]. Seminal plasma zinc is originated primarily from the prostate gland and may reflect prostatic secretary function [17]. Increased level of zinc in absence of a seminal vesicle zinc ligand can inhibit sperm chromatin decondensation which may result in chromatin instability [11]. Recent studies have reported increased defects in chromatin integrity and packaging in patients with hyperviscous semen [18]. Poor chromatin packaging or integrity is associated with sperm DNA damage and increased risk of infertility and pregnancy outcome [19,20]. Furthermore, zinc has anti-oxidative properties and plays as a cofactor for different antioxidants such as Cu/Zn-superoxide dismutase [21,22,23]. Given the antioxidative properties of zinc, reduced intake of zinc in patients with HSV can result in OS and subsequently sperm DNA damage, membrane lipid per-oxidation, apoptosis and poor semen quality [24,25].
Changes in Trace Elements
SHV not only stimulates overproduction of reactive oxygen species (ROS) and oxidative damages through induction of inflammation, but also it leads to alterations in the contents of seminal plasma fructose, ascorbic acid, calcium, and zinc, which in turn exert negative influences on sperm function or fertilizing capacity of spermatozoa [14]. Mahran et al. [14] reported a significant negative correlation between SHV and seminal plasma levels of fructose, ascorbic acid, zinc, and calcium. They also observed that enhanced level of leukocytes is correlated negatively with ascorbic acid, fructose, zinc and calcium concentrations. In another study, Gonzales et al. [26] reported reduced levels of fructose in SHV patients. These trace elements are necessary for the spermatogen-esis, energy production, normal function of spermatozoa, sperm motility, protection of sperm against ROS and fertilization process [21,27,28,29,30]. Therefore, reduced levels of these elements in patients with hyperviscous semen exert negative impacts on spermatogenesis, semen quality and male fertility. Furthermore, fructose and ascorbic acid are biomarkers of seminal vesicle function, while calcium and zinc are biomarkers of prostatic function. Thus, physical analysis of ejaculate including viscosity can be clinically useful for the evaluation of the secretory activity of the these male accessory glands [14].
OS
OS induced by free radicals, especially ROS, is now considered as one of the main idiopathic factors that affect human spermatozoa [3]. It is now considered as one of the main reason for male infertility, that is, 30 to 40% of infertile men have enhanced contents of ROS in their seminal plasma [31,32]. Recent studies have indicated that OS is the main mechanism of HSV effects on poor semen quality [4,33]. OS is a condition in which free radicals contents overwhelm the levels of antioxidants. ROS such as hydroxyl radicals (OH·), superoxide anion radical (O2· -) and hydrogen peroxide (H2O2) are very reactive agents that interact with cellular macromolecules to compensate their deficit electron [21,30,34]. Although free radicals are essential for sperm capacitation and acrosome reaction at physiological concentration, they can oxide DNA, proteins and lipids at high concentrations [35].
Human sperm cells are particularly susceptible to ROS because they have high levels of polyunsaturated fatty acids at their membrane. Additionally, they lose most of their cytoplasmic antioxidants during spermio-genesis [36,37]. Therefore, enhanced contents of ROS and subsequently reduced levels of seminal plasma an-tioxidants can increase sperm DNA damage, membrane lipid peroxidation, and eventually sperm cells damage and increased risk of male infertility [38]. 8-OHdG, malondialdehyde and protein carbonyl are biomarkers of DNA, protein and lipid oxidation. Numerous studies reported increased contents of these biomarkers in seminal plasma of infertile patients.
Leukocytes, especially neutrophils and macrophages, and dysfunctional spermatozoa are the major endogenous sources of ROS production in human semen [39,40]. Overproduction of ROS in human semen affects sperm cells mitochondrial function and subsequently sperm mo-tility [41]. During infection or inflammation, leukocytes can discharge up to 100 times more ROS than normal and contribute to OS [42,43]. A great number of studies have reported leukocytospermia in infertile patients [31,40,44,45]. Increased number of seminal plasma leukocytes and proinflammatory cytokines such as IL-6, IL-8, and tumor necrosis factor is associated with overproduction of ROS, oxidative damages to sperm DNA, membrane lipids and subsequently apoptosis and sperm abnormalities [46].
Leukocytes are particularly responsible in the development of SHV, as they are increased during the infection and produce high levels of ROS [47]. Patients with hyperviscous semen have high percentage of leukocytes compared with non-hyperviscous men. Recent studies have indicated a significant positive correlation between leukocytospermia and hyperviscosity semen [14]. In a study, Mahran et al. [14] observed leukocytospermia in 37.5% of the infertile men with hyperviscosity semen. Furthermore, increased number of leukocytes in SHV patients was correlated negatively with sperm motility and vitality. They suggested that SHV seems to be a result of infection or inflammation in 75% cases. Elis et al. [48] proposed that anti-inflammatory therapy can successfully treat mild SHV.
Recent evidences have demonstrated that OS induced by ROS is the major mechanism of HSV on poor quality of sperm and male infertility among patients with hyperviscous semen [4,33]. OS has been also reported to be associated with hyperviscous blood [49,50]. In a study by Harisa et al. [49] they observed a rise in malon-dialdehyde and protein carbonyl levels in patients with higher blood viscosity. They also showed that increasing concentrations of malondialdehyde and protein carbonyl is correlated with increasing degrees of viscosity. In another study by Kasperczyk et al. [50] they reported whole blood viscosity is associated with OS, erythrocyte aggregation and decreased level of malondialdehyde.
Although these data suggest that OS is a contributor to SHV, the exact mechanism in which SHV increase OS is not well understood. One of these mechanisms is probably related to increased level of abnormal sperms in patients with SHV. Patients with hyperviscous semen have higher percentage of abnormal spermatozoa in their semen compared to healthy individuals [4]. As dysfunctional spermatozoa are one of the major sources of ROS production, increased number of immature and abnormal sperm cells in SHV patients may explain the higher existence of OS in their semen. Furthermore, the number of leukocytes, as the other significant source of ROS generation, in semen of HSV patients is greater than that in men with normal semen. Therefore, increased number of leukocytes as a result of inflammation or infection in HSV patients can be considered as the other main reasons for overproduction of ROS and OS in these patients. Enhanced ROS in SHV patients can decline the effective concentration of seminal plasma antioxidants and as the result increases the harmful effects of ROS to spermatozoa. Thus, impairment of semen antioxidants can be considers as one of the other main mechanism SHV action on poor semen quality [4]. In a study by Siciliano et al. [51] they reported a severe impairment of both the high and low molecular weight antioxidants in SHV patients. Layali et al. [4] indicated that hyperviscous semen impairs seminal plasma total antioxidants capacity, which is eventually associated with sperm membrane lipid per-oxidation. Similarly, Aydemir et al. [33] found higher malondialdehyde levels in seminal plasma of patients with hyperviscous semen compared to non-viscous individuals. These results suggest that increased number of abnormal sperms and inflammatory cytokines as well as severe impairment of antioxidants, which are associated with increased sperm membrane lipid peroxidation, can be the main reason for low quality of sperm among SHV patients.
Conclusion
SHV can be considered as one of the main reason for poor quality of sperm in men with hyperviscous semen. Impairment of sperm motility, deficiency in zinc uptake, changes in trace elements, poor quality of semen, increased number of leukocytes and ROS production, and OS are the major mechanisms in which SHV induces sperm abnormalities and male infertility. Impairment of seminal plasma antioxidant and OS is the major mechanism of SHV, which can be associated with sperm DNA damage, membrane lipid peroxidation, sperm chromatin instability and low fertilization rate. Therefore, treatment with antioxidants may be helpful in patients showing hyperviscous semen to protect sperm cells by oxidative damages. However, further studies into possible treatments for and causes of SHV are essential in order to improve fertility and the success of assisted reproductive technology procedures.
Acknowledgments
We are deeply indebted to past and present collaborators.
References
- 1.Tahmasbpour E, Balasubramanian D, Agar-wal A. A multi-faceted approach to understanding male infertility: gene mutations, molecular defects and assisted reproductive techniques (ART) J Assist Reprod Genet. 2014;31:1115–1137. doi: 10.1007/s10815-014-0280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Wang SM. Clinical relevance of oxidation-reduction potential in the evaluation of male infertility. Urology. 2017;104:84–89. doi: 10.1016/j.urology.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Re-prod Biomed Online. 2017;34:48–57. doi: 10.1016/j.rbmo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Layali I, Tahmasbpour E, Joulaei M, Jorsaraei SG, Farzanegi P. Total antioxidant capacity and lipid peroxidation in semen of patient with hyperviscosity. Cell J. 2015;16:554–559. doi: 10.22074/cellj.2015.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overstreet JW, Coats C, Katz DF, Hanson FW. The importance of seminal plasma for sperm penetration of human cervical mucus. Fertil Steril. 1980;34:569–572. doi: 10.1016/s0015-0282(16)45197-6. [DOI] [PubMed] [Google Scholar]
- 6.Clavert A, Montagnon D, Cranz C, Rum-pler Y. Soluble seminal fluid proteins from various animal species. Arch Androl. 1985;14:177–179. doi: 10.3109/01485018508988295. [DOI] [PubMed] [Google Scholar]
- 7.Jones R, Mann T, Sherins R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril. 1979;31:531–537. doi: 10.1016/s0015-0282(16)43999-3. [DOI] [PubMed] [Google Scholar]
- 8.Huret JL. Nuclear chromatin decondensation of human sperm: a review. Arch Androl. 1986;16:97–109. doi: 10.3109/01485018608986928. [DOI] [PubMed] [Google Scholar]
- 9.Andrade-Rocha F. Physical analysis of ejaculate to evaluate the secretory activity of the seminal vesicles and prostate. Clin Chem Lab Med. 2005;43:1203–1210. doi: 10.1515/CCLM.2005.208. [DOI] [PubMed] [Google Scholar]
- 10.Stephanus du Plessis S, Gokul S, Agarwal A. Semen hyperviscosity: causes, consequences, and cures. Front Biosci (Elite Ed) 2013;5:224–231. doi: 10.2741/e610. [DOI] [PubMed] [Google Scholar]
- 11.Esfandiari N, Burjaq H, Gotlieb L, Casper RF. Seminal hyperviscosity is associated with poor outcome of in vitro fertilization and embryo transfer: a prospective study. Fertil Steril. 2008;90:1739–1743. doi: 10.1016/j.fertnstert.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Du Plessis SS, Gokul S, Agarwal A. Semen hyperviscosity: causes, consequences, and cures. Front Biosci (Elite Ed) 2013;5:224–231. doi: 10.2741/e610. [DOI] [PubMed] [Google Scholar]
- 13.Mendeluk GR, Munuce MJ, Carizza C, Sardi M, Bregni C. Sperm motility and ATP content in seminal hyperviscosity. Arch Androl. 1997;39:223–227. doi: 10.3109/01485019708987920. [DOI] [PubMed] [Google Scholar]
- 14.Mahran Z, El-Eraki Saleh M. Human semen hyperviscosity: prevalence and effects on physical and biochemical semen parameters in subfertile Egyptian men. Egypt J Dermatol Venerol. 2014;34:135–139. [Google Scholar]
- 15.Elzanaty S, Malm J, Giwercman A. Vis-co-elasticity of seminal fluid in relation to the epididymal and accessory sex gland function and its impact on sperm motility. Int J Androl. 2004;27:94–100. doi: 10.1046/j.1365-2605.2003.00455.x. [DOI] [PubMed] [Google Scholar]
- 16.Esfandiari N, Gotlieb L, Casper RF. Seminal hyperviscosity is associated with poor outcome of controlled ovarian stimulation and intrauterine insemination: a prospective study. Int J Fertil Womens Med. 2006;51:21–27. [PubMed] [Google Scholar]
- 17.Khan MS, Zaman S, Sajjad M, Shoaib M, Gilani G. Assessment of the level of trace element zinc in seminal plasma of males and evaluation of its role in male infertility. Int J Appl Basic Med Res. 2011;1:93–96. doi: 10.4103/2229-516X.91152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopalkrishnan K, Padwal V, Balaiah D. Does seminal fluid viscosity influence sperm chro-matin integrity? Arch Androl. 2000;45:99–103. doi: 10.1080/014850100418783. [DOI] [PubMed] [Google Scholar]
- 19.Gorczyca W, Traganos F, Jesionowska H, Darzynkiewicz Z. Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: analogy to apoptosis of somatic cells. Exp Cell Res. 1993;207:202–205. doi: 10.1006/excr.1993.1182. [DOI] [PubMed] [Google Scholar]
- 20.Manicardi GC, Bianchi PG, Pantano S, Az-zoni P, Bizzaro D, Bianchi U, Sakkas D. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod. 1995;52:864–867. doi: 10.1095/biolreprod52.4.864. [DOI] [PubMed] [Google Scholar]
- 21.Colagar AH, Marzony ET, Chaichi MJ. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res. 2009;29:82–88. doi: 10.1016/j.nutres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Li Y, Li Z, Cao Y, Wang F, Li C. Effect of dietary zinc on morphological characteristics and apoptosis related gene expression in the small intestine of Bama miniature pigs. Acta Histochem. 2017;119:235–243. doi: 10.1016/j.acthis.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Perera NC, Godahewa GI, Lee J. Cop-per-zinc-superoxide dismutase (CuZnSOD), an antioxidant gene from seahorse (Hippocampus abdominalis); molecular cloning, sequence characterization, antioxidant activity and potential peroxidation function of its recombinant protein. Fish Shellfish Immunol. 2016;57:386–399. doi: 10.1016/j.fsi.2016.08.052. [DOI] [PubMed] [Google Scholar]
- 24.Zheng JL, Zeng L, Xu MY, Shen B, Wu CW. Different effects of low- and high-dose waterborne zinc on Zn accumulation, ROS levels, oxidative damage and antioxidant responses in the liver of large yellow croaker Pseudosciaena crocea. Fish Physiol Biochem. 2017;43:153–163. doi: 10.1007/s10695-016-0275-6. [DOI] [PubMed] [Google Scholar]
- 25.Seth R, Corniola RS, Gower-Winter SD, Morgan TJ, Jr, Bishop B, Levenson CW. Zinc deficiency induces apoptosis via mitochon-drial p53- and caspase-dependent pathways in human neuronal precursor cells. J Trace Elem Med Biol. 2015;30:59–65. doi: 10.1016/j.jtemb.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Gonzales GF, Villena A. Influence of low corrected seminal fructose levels on sperm chromatin stability in semen from men attending an infertility service. Fertil Steril. 1997;67:763–768. doi: 10.1016/s0015-0282(97)81380-5. [DOI] [PubMed] [Google Scholar]
- 27.Said L, Galeraud-Denis I, Carreau S, Saâd A. Relationship between semen quality and seminal plasma components: alpha-glucosidase, fructose and citrate in infertile men compared with a normospermic population of Tunisian men. Andrologia. 2009;41:150–156. doi: 10.1111/j.1439-0272.2008.00906.x. [DOI] [PubMed] [Google Scholar]
- 28.Skandhan KP, Mazumdar B, Sumangala B, Jaya V. Seminal plasma calcium in normal and infertile patients. Urologia. 2017;84:35–37. doi: 10.5301/uro.5000167. [DOI] [PubMed] [Google Scholar]
- 29.Blomberg Jensen M, Gerner Lawaetz J, An-dersson AM, Petersen JH, Nordkap L, Bang AK, Ekbom P, Joensen UN, Prætorius L, Lundstrøm P, Boujida VH, Lanske B, Juul A, Jørgensen N. Vitamin D deficiency and low ionized calcium are linked with semen quality and sex steroid levels in infertile men. Hum Reprod. 2016;31:1875–1885. doi: 10.1093/humrep/dew152. [DOI] [PubMed] [Google Scholar]
- 30.Colagar AH, Marzony ET. Ascorbic Acid in human seminal plasma: determination and its relationship to sperm quality. J Clin Biochem Nutr. 2009;45:144–149. doi: 10.3164/jcbn.08-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanzafame FM, La Vignera S, Vicari E, Ca-logero AE. Oxidative stress and medical anti-oxidant treatment in male infertility. Reprod Biomed Online. 2009;19:638–659. doi: 10.1016/j.rbmo.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Aydemir B, Onaran I, Kiziler AR, Alici B, Akyolcu MC. The influence of oxidative damage on viscosity of seminal fluid in infertile men. J Androl. 2008;29:41–46. doi: 10.2164/jandrol.107.003046. [DOI] [PubMed] [Google Scholar]
- 34.Hosseinzadeh Colagar A, Pouramir M, Tah-masbpour Marzony E, Ali Jorsaraei., SG Relationship between seminal malondialde-hyde levels and sperm quality in fertile and infertile men. Braz Arch Biol Technol. 2009;52:1387–1392. [Google Scholar]
- 35.Mahfouz RZ, du Plessis SS, Aziz N, Sharma R, Sabanegh E, Agarwal A. Sperm viability, apoptosis, and intracellular reactive oxygen species levels in human spermatozoa before and after induction of oxidative stress. Fertil Steril. 2010;93:814–821. doi: 10.1016/j.fertnstert.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal A, Nandipati KC, Sharma RK, Zippe CD, Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl. 2006;27:335–347. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005;95:503–507. doi: 10.1111/j.1464-410X.2005.05328.x. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963–974. [PubMed] [Google Scholar]
- 39.Agarwal A, Mulgund A, Sharma R, Sabanegh E. Mechanisms of oligozoospermia: an oxi-dative stress perspective. Syst Biol Reprod Med. 2014;60:206–216. doi: 10.3109/19396368.2014.918675. [DOI] [PubMed] [Google Scholar]
- 40.Oborna I, Fingerova H, Novotny J, Brezinova J, Svobodova M, Aziz N. Reactive oxygen species in human semen in relation to leukocyte contamination. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:53–57. doi: 10.5507/bp.2009.009. [DOI] [PubMed] [Google Scholar]
- 41.Durairajanayagam D, Agarwal A, Ong C, Prashast P. Lycopene and male infertility. Asian J Androl. 2014;16:420–425. doi: 10.4103/1008-682X.126384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophys-iology of human reproduction. Fertil Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 43.Lavranos G, Balla M, Tzortzopoulou A, Syriou V, Angelopoulou R. Investigating ROS sources in male infertility: a common end for numerous pathways. Reprod Toxicol. 2012;34:298–307. doi: 10.1016/j.reprotox.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, Tinneberg HR, Schill WB, Kruger TF. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–642. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 45.Rengan AK, Agarwal A, van der Linde M, du Plessis., SS An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Re-prod Biol Endocrinol. 2012;10:92. doi: 10.1186/1477-7827-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castiglione R, Salemi M, Vicari LO, Vicari E. Relationship of semen hyperviscosity with IL-6, TNF-alpha, IL-10 and ROS production in seminal plasma of infertile patients with prostatitis and prostato-vesiculitis. Androlo-gia. 2014;46:1148–1155. doi: 10.1111/and.12207. [DOI] [PubMed] [Google Scholar]
- 47.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 48.Elia J, Delfino M, Imbrogno N, Capogreco F, Lucarelli M, Rossi T, Mazzilli F. Human semen hyperviscosity: prevalence, pathogen-esis and therapeutic aspects. Asian J Androl. 2009;11:609–615. doi: 10.1038/aja.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harisa GI. Blood viscosity as a sensitive indicator for paclitaxel induced oxidative stress in human whole blood. Saudi Pharm J. 2015;23:48–54. doi: 10.1016/j.jsps.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasperczyk A, S?owi?ska-?o?y?ska L, Do-brakowski M, Zalejska-Fiolka J, Kasperczyk S. The effect of lead-induced oxidative stress on blood viscosity and rheological properties of erythrocytes in lead exposed humans. Clin Hemorheol Microcirc. 2014;56:187–195. doi: 10.3233/CH-131678. [DOI] [PubMed] [Google Scholar]
- 51.Siciliano L, Tarantino P, Longobardi F, Rago V, De Stefano C, Carpino A. Impaired seminal antioxidant capacity in human semen with hyperviscosity or oligoasthenozoospermia. J Androl. 2001;22:798–803. [PubMed] [Google Scholar]