Abstract
Objectives
The aim of this study was to assess the epidemiological, clinical, endoscopic, and pathohistological characteristics of pediatric eosinophilic esophagitis (EoE) in Serbia.
Method
All children aged 0–18 years diagnosed with EoE in the period between 2010 and 2017 at the University Children's Hospital in Belgrade, Serbia, were retrospectively enrolled.
Results
EoE was diagnosed in 35 children (12.45 ± 3.77 years) with a male predominance (74%). The median incidence rate was estimated to be 0.85 per 100,000 children per year with the highest rate estimated at 3.17 per 100,000 children in 2017. Dysphagia (71.4%) and food impaction (40%) were dominant symptoms. Inflammatory endoscopic changes were found in 74.3% and fibrostenotic changes in 62.9% of the children. The esophageal biopsy rate was low (6.8%), especially in children with reflux and nonspecific symptoms. Subepithelial fibrosis was found in only 20% of the patients. Since 2016, the number of biopsy samples has increased, but the sampling rate of lamina propria is still low (<50%). The correlation between the number of biopsies and lamina propria acquisition was strong (r<sub>s</sub> = 0.773, p < 0.05). In 2 immunocompetent adolescents, EoE was diagnosed after successful treatment of infectious esophagitis.
Conclusions
An increase in the incidence of EoE in Serbian children is evident. The biopsy rate in children with nonspecific and reflux symptoms should be increased, as well as the number of biopsy samples for the detection of subepithelial fibrosis. In immunocompetent children with infectious esophagitis, EoE should be suspected and endoscopy may be recommended after successful treatment of infection.
Keywords: Eosinophilic esophagitis, Children, Subepithelial fibrosis
Significance of the Study
The esophageal biopsy rate should be increased in children with nonspecific and reflux symptoms. In children with suspected or confirmed eosinophilic esophagitis, the number of biopsy samples should be increased for better detection of subepithelial fibrosis. Eosinophilic esophagitis should be suspected and repeat endoscopy may be recommended after successful treatment of infectious esophagitis in immunocompetent children.
Introduction
Eosinophilic esophagitis (EoE) is a chronic inflammatory disorder of the esophagus, first described in 1977, but was defined as a distinct entity only in 1993. Currently, EoE is the second most common cause of chronic esophagitis after gastroesophageal reflux disease (GERD), as well as the main cause of dysphagia and food impaction in children. EoE is defined as a chronic, local immune-mediated esophageal disease, clinically characterized by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation [1]. Due to the patchy distribution of inflammatory changes [2], at least 6 biopsies should be taken from different parts of the esophagus [1].
The first guidelines on EoE were published in 2007 [3] assuming that EoE and GERD mutually exclude each other. Moreover, for the purpose of diagnosing EoE it was necessary for the proton pump inhibitor (PPI) trial and/or pH monitoring to be negative. However, these recommendations were updated in 2011, when the term “proton pump inhibitor-responsive esophageal eosinophilia” (PPI-REE) was introduced. PPI-REE has been diagnosed in patients with clinical, endoscopic, and histological characteristics of EoE and PPI-induced clinical and histological remission, who are not necessarily diagnosed with GERD [4]. The difference in response to PPI was the key feature in distinguishing PPI-REE from EoE [5, 6, 7]. However, studies published after 2011 demonstrated that PPI-REE and EoE do not differ even on the genetic level and that both disorders are distinct from GERD [4, 8]. The latest guidelines on EoE (2017) emphasized the importance of PPI, particularly as a treatment option for EoE, while the term PPI-REE has been withdrawn [1].
Therefore, the aim of this study was to assess epidemiological, clinical, endoscopic, and pathohistological characteristics of pediatric EoE according to the current recommendations for the first time in the Serbian population.
Materials and Methods
Study Design
All children aged 0–18 years with esophageal symptoms undergoing an upper endoscopy between January 2010 and December 2017 at the Department of Gastroenterology, Hepatology and Endoscopy of the University Children's Hospital in Belgrade were retrospectively enrolled in the study.
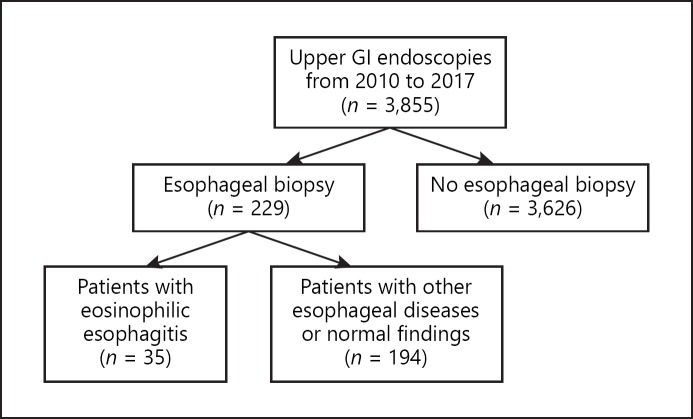
We included all children who met the histological criteria for EoE (≥15 eosinophils per HPF in esophageal mucosa, taken as the peak concentration in the specimens examined) [1]. Children with eosinophilic gastroenteritis (i.e., eosinophilic infiltration of different parts of the gastrointestinal tract), infectious esophagitis, achalasia, hypereosinophilic syndrome, hypersensitivity reactions to drugs, vasculitis, and connective tissue disorders were excluded from the study. The study design is presented in Figure 1. Esophageal biopsy rates (% of patients in whom esophageal biopsies were performed), number of esophageal biopsy samples per exam, as well as the percentage of biopsy samples with lamina propria for each year are presented in Table 1.
Fig. 1.
Study design. GI, gastrointestinal.
Table 1.
Esophageal biopsy rate, number of biopsy samples, and lamina propria acquisition in patients who underwent upper gastrointestinal endoscopy
| Year | Esophageal biopsy rate, % (n/totaln) | Esophageal biopsy samples, mean ± SD | Biopsy samples with lamina propria, % (n/totaln) |
|---|---|---|---|
| 2010 | 2.4 (8/330) | 1.2±0.4 | 10 (1/10) |
| 2011 | 2.7 (10/375) | 1.2±0.4 | 0(0/12) |
| 2012 | 9 (38/421) | 1.4±0.4 | 9.6 (5/52) |
| 2013 | 8.4 (39/461) | 1.2±0,3 | 8.3 (4/48) |
| 2014 | 2.5 (14/564) | 1.1±0.3 | 0(0/16) |
| 2015 | 4.9 (29/586) | 1.2±0.3 | 0(0/34) |
| 2016 | 8.9 (52/580) | 2.6±0.7 | 7.3 (10/137) |
| 2017 | 13.2 (71/538) | 3.2±0.8 | 10 (25/230) |
| Overall | 6.8 (261/3,855) | 2.1±0.6 | 8.3 (45/539) |
For each child, clinical presentation, endoscopic and histological findings, skin and blood allergy tests, as well as personal history of allergy and infectious esophagitis were analyzed. Additionally, the majority of children underwent multichannel intraluminal impedance (MII)-pH monitoring. We compared differences in clinical, endoscopic, and pathohistological presentation between children under the age of 10 years and adolescents.
Finally, all children who met the inclusion criteria and whose parent/caregiver gave written consent were included in the study. The study was approved by the Ethics Committee of University Children's Hospital Belgrade.
Esophagogastroduodenoscopy
The following endoscopic findings, associated with EoE, were retrospectively analyzed from endoscopy reports comprising mucosal edema, longitudinal furrowing, rings, white plaques, strictures, and narrow caliber esophagus.
Histopathological Analysis
For the pathohistological assessment, hematoxylin-eosin staining has been used. The reevaluation of old samples was performed in order to assess the following features: peak eosinophil count, eosinophil microabscesses, basal-zone hyperplasia, dilated intercellular spaces, eosinophil surface layering, papillary elongation, and lamina propria fibrosis. Additionally, Masson's trichrome staining was used to further determine the presence of subepithelial fibrosis in some patients.
MII-pH Monitoring
MII-pH monitoring was performed by a pH-MII ambulatory system (Sandhill Scientific, Denver, CO, USA). The catheter was introduced nasally after at least 3 h of fasting. The minimum required recording time was 20 h. The pH-MII recordings were automatically analyzed using the software package (AutoScan/BioView Analysis, Sandhill Scientific, Highlands Ranch, CO, USA) and reviewed manually.
Statistical Analysis
Normality of data distribution was tested using the coefficient of variation and the Kolmogorov-Smirnov test. Descriptive measures were presented according to the variable characteristics: means and standard deviations for parametric variables, medians and interquartile ranges for nonparametric numeric variables, and percentages for categorical variables. Parametric data were compared using Student's t test. Nonparametric numerical data were compared using the Mann-Whitney U test. Categorical variables were compared using the χ2 test and Fisher's exact probability. Correlation was performed by Spearman correlation. Statistical significance was established at p <0.05. SPSS 17.0 (Chicago, IL, USA) was used.
Results
EoE was diagnosed in 35 children (mean 12.45 ± 3.77 years, range 3–18) during the period from January 2010 to December 2017. Out of 35 children, 26 (74.3%) were boys (mean 13.07 ± 3.67 years), while 9 (25.7%) were girls (mean 10.67 ± 3.67 years); however, this difference in age between boys and girls was not statistically significant (t = 1.691, p > 0.05).
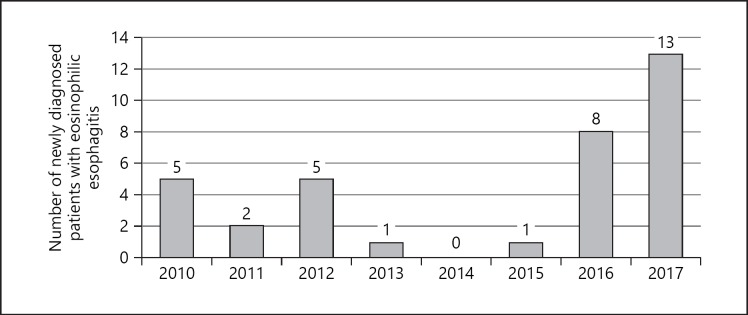
An increase was evident in the number of newly diagnosed patients with EoE in 2016 and 2017 relative to previous years as shown in Figure 2. The number of newly diagnosed EoE patients per year correlated strongly with the esophageal biopsy rate (rs = 0.743, p < 0.05) and the number of biopsies per exam (rs = 0.952, p < 0.001). In Serbia, about 30% of children diagnosed with pediatric gastrointestinal diseases are referred to University Children's Hospital. Therefore, the estimated median incidence rate of EoE in Serbian children from 2010–2017 was 0.85 per 100,000 children per year. The highest incidence rates were 3.17 per 100,000 children in 2017 and 1.95 per 100,000 children in 2016. The estimated prevalence of pediatric EoE in Serbia was 6.83 per 100,000 children. Clinical presentation and endoscopy findings in different age groups are presented in Table 2. In 6 (17.1%) patients, food impaction was the first symptom. The youngest patient was a 3-year-old girl with reflux symptoms and food refusal. Reflux symptoms were more prevalent in girls (55.6%) than in boys (11.5%) (Fisher's test, p = 0.015).
Fig. 2.
Number of newly diagnosed patients with eosinophilic esophagitis from 2010 to 2017.
Table 2.
Clinical presentation and endoscopy findings in different age groups
| Overall (n = 35) | Children<10 years (n = 11) | Children ≥10 years (n = 24) | p value | |
|---|---|---|---|---|
| Gender, boys/girls | 26/9 | 8/3 | 18/6 | 1.000 |
| Symptoms,n (%) | ||||
| Dysphagia | 25 (71.4) | 7 (63.6) | 18 (75) | 0.689 |
| Reflux symptoms | 8 (22) | 2 (18.2) | 6 (25) | 1.000 |
| Retrosternal pain | 3 (8.6) | 1 (9.1) | 2 (8.3) | 1.000 |
| Food impaction | 14 (40) | 4 (36.4) | 10 (41.7) | 1.000 |
| Upper endoscopy findings,n (%) | ||||
| Longitudinal furrowing | 25 (71.4) | 9 (81.8) | 16 (66.7) | 0.447 |
| White plaques | 11 (31.4) | 5 (45.5) | 6 (25) | 0.263 |
| Ring | 21 (60) | 5 (45.5) | 16 (66.7) | 0.283 |
| Esophageal stricture | 3 (8.6) | 0 | 3 (12.5) | 0.536 |
| Erosive esophagitis | 4 (11.4) | 1 (9.1) | 3 (12.5) | 1.000 |
| Inflammatory changes | 26 (74.3) | 9 (81.8) | 17 (70.8) | 0.685 |
| Fibrostenotic changes | 22 (48.6) | 5 (45.5) | 17 (70.8) | 0.258 |
The median time from the occurrence of the first symptoms to diagnosis was 3 ± 23.5 months (range 0–120), 3 ± 23.9 months (range 0–24) in the younger group and 3.5 ± 21.5 months (range 0–120) in the older group. This difference was not statistically significant (p > 0.05). The median time from the occurrence of the first symptoms to diagnosis was longer from 2010 to 2015 (4.5 ± 15.5 months) than from 2016 to 2017 (3.0 ± 23.9 months), with no significant difference (p > 0.05). There were no significant differences in age (Table 2) and gender concerning endoscopic findings (p > 0.05). Inflammatory changes (longitudinal furrowing, white plaques) were found in 74.3% of children, fibrostenotic changes (rings, strictures) in 62.9% of children, and both in 48.6% of children. There were no significant differences in sex, age groups, and type of symptoms (p > 0.05).
The number of endoscopic signs in each patient was analyzed. In each patient, 4 endoscopic signs were analyzed: longitudinal furrowing, rings, white plaques, and esophageal strictures. Mucosal edema, although often present, was not described in most endoscopic reports and, therefore, was not analyzed. Out of 35 patients, 4 (11.4%) children had normal endoscopic findings. A single endoscopic sign was found in 9 (25.7%) children, 2 signs in 16 (45.7%) children, and 3 signs in 3 (17.1%) children. Seven (20%) children had pathological acid reflux on pH-MII monitoring (reflux index >7%), while 4 children had erosive esophagitis.
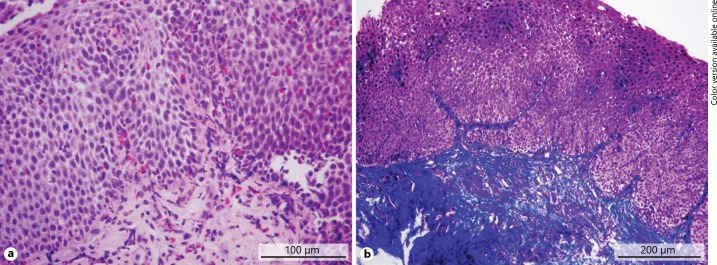
Histopathological analysis of esophageal mucosa revealed a median peak number of 77.5 ± 44 eosinophils per HPF in EoE children (range 13–146). Eosinophilic microabscesses were found in 23 (65.7%) children. There were no statistically significant differences in sex, age groups, and type of symptoms concerning these parameters (p > 0.05). The findings of the patient with marked subepithelial fibrosis in addition to eosinophilic infiltration are shown in Figure 3. Subepithelial fibrosis was found in 7 (20%) children. Unfortunately, the presence of fibrosis was rarely evaluated until 2016, when it was found to be present in 30% of patients. Subepithelial tissue was included in 16.5% of biopsy samples. The rate of acquisition of lamina propria was 31.4% (47.6% in 2016–2017 and 7.1% in 2010–2015), and the detection rate of subepithelial fibrosis was 70%. The number of esophageal biopsies per exam was low in 2010–2015. The mean number of biopsy samples was 5.0 ± 0.9 in 2016 and 6.0 ± 1.0 in 2017. The correlation between the number of biopsies and lamina propria acquisition was strong (rs = 0.773, p < 0.05). Food and/or respiratory allergies were demonstrated in 14 (40%) children with positive skin prick test and/or specific IgE antibodies. Food allergy was found in 9 (26%) children and respiratory allergy in 5 (14%) children. The diagnosis of allergic asthma was established in 3 (8.6%) children. Ten out of 14 (71.4%) children were allergic to multiple allergens. IgE-mediated milk allergy was found in 3 (8.6%) children, egg allergy in 4 (11.4%) children, and wheat allergy in 2 (5.7%) children.
Fig. 3.
Pathohistological findings of a patient with fibrostenotic phenotype of eosinophilic esophagitis. a Eosinophilic mucosal infiltration (HE staining). b Subepithelial fibrosis (blue on Masson's trichrome staining).
EoE was diagnosed in 2 brothers aged 16 and 17.5 years. In 1 sibling, esophageal candidiasis was found during the initial presentation (dysphagia, odynophagia, and epigastric pain). After successful treatment with fluconazole, a diagnosis of EoE was made. In 1 adolescent, herpes simplex virus (HSV) esophagitis was found during primary diagnostic workup. After treatment with acyclovir, additional diagnostic procedures revealed EoE. Both adolescents were immunocompetent.
Eight (22.9%) children were treated only with six food elimination diet (SFED), whereas 8 (22.9%) children were treated with PPIs and 4 (11.4%) children only with topical corticosteroids. Fifteen (42.8%) children were treated with more than 1 therapeutic modality.
Discussion
The incidence and prevalence of EoE in Serbian children is lower than previously reported in a meta-analysis from 2016 that included 13 population-based studies from North America, Europe, and Australia [9]. In the meta-analysis, the incidence of EoE in children was estimated to be 5.1 (95% CI 1.5–10.9) per 100,000 children per year, whereas the prevalence was 19.1 (95% CI 7.9–32.5) per 100,000 children. An evident increase in the incidence of pediatric EoE in Serbia in 2016 and 2017 is due to greater attention by physicians, a higher rate of esophageal biopsies, and better pathohistological analysis, as previously reported [10].
In our study, the male predominance was noted to be similar to that reported in the literature [9]. Several studies have shown that presentation of EoE is age dependent. Liacouras et al. [11], as well as Gonsalves [12], reported that the most common symptoms are feeding problems, failure to thrive, and reflux symptoms in infants and young children, vomiting, abdominal pain, and regurgitation in school children, and dysphagia and food impaction in adolescents. In adults, the most common symptoms are dysphagia (70–80%) and food impaction (33–54%) [1, 13, 14]. However, our study did not reveal a significant difference between different age groups. The reasons for these differences are a small sample size, a small number of infants and young children diagnosed with EoE, as well as selection bias. The main indications for serial biopsies in children were dysphagia and food impaction due to the high degree of suspicion for EoE. On the other hand, serial biopsies have not been routinely done in children with reflux symptoms. Some authors believe that histological analysis is not mandatory in patients with reflux symptoms, while others recommend biopsies in order to exclude other pathologies, such as EoE [15].
The median time from the onset of symptoms to the diagnosis in our study was short, probably due to the fact that mild nonspecific symptoms were not recognized (or reported). On the other hand, the time range was wide (0–120 months). A prospective study in adults demonstrated that in the absence of anti-inflammatory therapy or elimination diet, dysphagia and esophageal eosinophilia persisted with the development of subepithelial fibrosis [16]. The results of several retrospective studies have shown that the greatest risk for esophageal remodeling and stricture formation is diagnostic delay [5, 17]. Another study revealed that for every 10 years the risk of fibrostenosis was doubled (OR 2.1, 95% CI 1.7–2.7), while dysphagia increased the risk 7 times (OR 7, 95% CI 2.6–18.6) [18]. Warners et al. [19] reported that the main risk factors for stricture formation were diagnostic delay (OR 1.09, 95% CI 1.05–1.13) and male gender (OR 2.69, 95% CI 1.61–4.50). Early detection of EoE and early initiation of therapy (during the inflammatory stage) are of great importance. Therefore, the biopsy rate in children with nonspecific and reflux symptoms should be increased.
Studies have shown that fibrostenotic changes (rings, strictures, and narrow caliber esophagus) are more frequent in adults, while inflammatory changes (edema, white plaques, and longitudinal furrowing) are more frequent in children [12]. In our study, the prevalence of fibrostenotic changes (62.9%) was higher than expected. In the study of Warners et al. [19], fibrostenotic changes were found in 39% of children and in 76% of adults with EoE. Another meta-analysis showed modest sensitivity as well as modest negative and positive predictive values of endoscopic signs in patients with EoE [20]. However, this study showed greater frequencies of fibrostenotic changes in adults than in children (57 vs. 11% for rings, 25 vs. 11% for strictures). White plaques and mucosal edema were more common in children than in adults (36 vs. 19% for white plaques, 58 vs. 18% for mucosal edema). In our study, the frequency of rings was higher than previously reported (60 vs. 11%), but this did not apply to strictures (6.8 vs. 11%). Also, the frequency of erosive esophagitis was slightly lower in our patients (11 vs. 17%), as well as the frequency of normal endoscopic findings (11 vs. 17%).
In one study, subepithelial fibrosis was found in 12/21 (57%) children with EoE [21]. Dysphagia and food impaction were present only in patients with fibrosis, while 42% of patients with fibrosis had dysphagia and 80% had food impaction. However, the problem is that subepithelial fibrosis in patients with EoE can be detected only if a sufficient amount oflamina propria is obtained. Wang et al. [22] reported that the sample rate of adequate amounts of lamina propria was low in patients with EoE (43%) and controls (31%) (p =0.14). In this study, 85% of EoE patients had fibrosis, including children with only inflammatory endoscopic signs. In order to reliably detect subepithelial fibrosis, it was necessary that at least 7 biopsy samples from central and distal parts of the esophagus be taken (detection rate over 95%) [22]. As far as our study is concerned, until 2016, the number of esophageal biopsies was often inadequate and lamina propria was rarely described due to an insufficient amount of lamina propria. Since 2016, the number of biopsy samples has increased, but the sampling rate of lamina propria is still low, which needs to be analyzed further.
Atopy is present in 50–60% of patients with newly diagnosed EoE [23, 24], which is slightly above the frequency found in our study (40%). IgE-mediated food allergy was found in 15–43% of patients with EoE [23, 24], which is in concordance with our results. Milk, egg, and wheat are the most common triggers of EoE, evidenced by sequential re-introduction after elimination diet and repeated biopsies [25, 26]. In our study, multiple food allergy was found in 70% of patients, whereas IgE-mediated allergy to milk, egg, and wheat was less frequent than expected. Nevertheless, our results support the hypothesis that IgE-mediated food allergy can be considered as a predictive factor for the development of EoE in children [27].
HSV esophagitis is a severe acute viral esophagitis, which rarely occurs in immunocompetent patients. In our study, HSV esophagitis was diagnosed during the primary diagnostic workup in an immunocompetent adolescent, and subsequent evaluation revealed EoE. Zimmerman et al. [28] reported HSV esophagitis in 5 patients with EoE not previously treated with topical steroids. It is unlikely that these 2 rare diseases occur simultaneously by coincidence, suggesting that EoE may predispose to HSV infection. In a retrospective study from 2018, data on 16 children (11 immunocompetent) with HSV esophagitis diagnosed from 1982 to 2016 were analyzed [29]. EoE was diagnosed in 5 (45%) immunocompetent patients after successful treatment of HSV infection, and 4/5 children had a personal history of atopy. There are no data in the literature on the relationship of esophageal candidiasis with EoE in immunocompetent patients not treated with topical steroids. Thus, it could be hypothesized that EoE causes a reduction in local defense leading to subsequent infection, which must be further investigated.
Until 2017, topical steroids or SFED were used as first-line treatments. After new recommendations were published in 2017, PPIs became the first-line therapy. In 2 children with no response to PPIs, SFED has been started with an ongoing evaluation. In almost half of our patients, several modalities of treatment were applied due to the lack of response or a negative impact on the quality of life. However, further prospective investigations of effectiveness are necessary.
Conclusion
It is necessary to create a register of patients with EoE in Serbia. The esophageal biopsy rate in children with nonspecific and reflux symptoms needs to be increased because the early detection and treatment of EoE are of great importance. In immunocompetent children with infectious esophagitis, EoE should be suspected and repeat endoscopy may be recommended after successful treatment of infection. In order to reliably detect subepithelial fibrosis, an adequate amount of lamina propriais required, which could be achieved by increasing the number of biopsy samples.
Statement of Ethics
Written consent was obtained from the children's parents/caregivers for inclusion in the study. The study was approved by the Ethics Committee of University Children's Hospital Belgrade.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017 Apr;5((3)):335–58. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saffari H, Peterson KA, Fang JC, Teman C, Gleich GJ, Pease LF., 3rd Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: implications for endoscopic biopsy. J Allergy Clin Immunol. 2012 Sep;130((3)):798–800. doi: 10.1016/j.jaci.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007 Oct;133((4)):1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011 Feb;9((2)):110–7. doi: 10.1016/j.cgh.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011 Jul;128((1)):3–20.e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, American College of Gastroenterology ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013 May;108((5)):679–92. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulou A, Koletzko S, Heuschkel R, Dias JA, Allen KJ, Murch SH, et al. ESPGHAN Eosinophilic Esophagitis Working Group and the Gastroenterology Committee Management guidelines of eosinophilic esophagitis in childhood. J Pediatr Gastroenterol Nutr. 2014 Jan;58((1)):107–18. doi: 10.1097/MPG.0b013e3182a80be1. [DOI] [PubMed] [Google Scholar]
- 8.Dellon ES, Speck O, Woodward K, Gebhart JH, Madanick RD, Levinson S, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol. 2013 Dec;108((12)):1854–60. doi: 10.1038/ajg.2013.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias Á, Pérez-Martínez I, Tenías JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2016 Jan;43((1)):3–15. doi: 10.1111/apt.13441. [DOI] [PubMed] [Google Scholar]
- 10.Syed AA, Andrews CN, Shaffer E, Urbanski SJ, Beck P, Storr M. The rising incidence of eosinophilic oesophagitis is associated with increasing biopsy rates: a population-based study. Aliment Pharmacol Ther. 2012 Nov;36((10)):950–8. doi: 10.1111/apt.12053. [DOI] [PubMed] [Google Scholar]
- 11.Liacouras CA, Spergel J, Gober LM. Eosinophilic esophagitis: clinical presentation in children. Gastroenterol Clin North Am. 2014 Jun;43((2)):219–29. doi: 10.1016/j.gtc.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Gonsalves N. Distinct features in the clinical presentations of eosinophilic esophagitis in children and adults: is this the same disease? Dig Dis. 2014;32((1-2)):89–92. doi: 10.1159/000357078. [DOI] [PubMed] [Google Scholar]
- 13.Remedios M, Campbell C, Jones DM, Kerlin P. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc. 2006 Jan;63((1)):3–12. doi: 10.1016/j.gie.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 14.Croese J, Fairley SK, Masson JW, Chong AK, Whitaker DA, Kanowski PA, et al. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc. 2003 Oct;58((4)):516–22. doi: 10.1067/s0016-5107(03)01870-4. [DOI] [PubMed] [Google Scholar]
- 15.Genevay M, Rubbia-Brandt L, Rougemont AL. Do eosinophil numbers differentiate eosinophilic esophagitis from gastroesophageal reflux disease? Arch Pathol Lab Med. 2010 Jun;134((6)):815–25. doi: 10.5858/134.6.815. [DOI] [PubMed] [Google Scholar]
- 16.Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003 Dec;125((6)):1660–9. doi: 10.1053/j.gastro.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Lipka S, Kumar A, Richter JE. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol. 2016 Feb;50((2)):134–40. doi: 10.1097/MCG.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 18.Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014 Apr;79((4)):577–85.e4. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warners MJ, Oude Nijhuis RA, de Wijkerslooth LR, Smout AJ, Bredenoord AJ. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am J Gastroenterol. 2018 Jun;113((6)):836–44. doi: 10.1038/s41395-018-0052-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012 Sep;10((9)):988–96.e5. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007 Sep;45((3)):319–28. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Park JY, Huang R, Souza RF, Spechler SJ, Cheng E. Obtaining adequate lamina propria for subepithelial fibrosis evaluation in pediatric eosinophilic esophagitis. Gastrointest Endosc. 2018 May;87((5)):1207–1214.e3. doi: 10.1016/j.gie.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009 Oct;7((10)):1055–61. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almansa C, Krishna M, Buchner AM, Ghabril MS, Talley N, DeVault KR, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. 2009 Apr;104((4)):828–33. doi: 10.1038/ajg.2008.169. [DOI] [PubMed] [Google Scholar]
- 25.Lucendo AJ, Arias Á, González-Cervera J, Yagüe-Compadre JL, Guagnozzi D, Angueira T, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol. 2013 Mar;131((3)):797–804. doi: 10.1016/j.jaci.2012.12.664. [DOI] [PubMed] [Google Scholar]
- 26.Molina-Infante J, Arias A, Barrio J, Rodríguez-Sánchez J, Sanchez-Cazalilla M, Lucendo AJ. Four-food group elimination diet for adult eosinophilic esophagitis: A prospective multicenter study. J Allergy Clin Immunol. 2014 Nov;134((5)):1093–9.e1. doi: 10.1016/j.jaci.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7:1305–1313. doi: 10.1016/j.cgh.2009.08.030. quiz 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann D, Criblez DH, Dellon ES, Bussmann C, Pfeifer D, Froh M, et al. Acute herpes simplex viral esophagitis occurring in 5 immunocompetent individuals with eosinophilic esophagitis. ACG Case Rep J. 2016 Apr;3((3)):165–8. doi: 10.14309/crj.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritz J, Lerner D, Suchi M. Herpes simplex virus esophagitis in immunocompetent children: A harbinger of eosinophilic esophagitis? J Pediatr Gastroenterol Nutr. 2018 Apr;66((4)):609–13. doi: 10.1097/MPG.0000000000001748. [DOI] [PubMed] [Google Scholar]