Abstract
Many studies in the literature have been carried out to evaluate the various cellular and molecular processes involved in osteogenesis.
Angiogenesis and bone formation work closely together in this group of disorders. Hypoxia-inducible factor (HIF) which is stimulated in tissue hypoxia triggers a cascade of molecular processes that helps manage this physiological deficiency.
However, there still remains a paucity of knowledge with regard to how sickle cell bone pathology, in particular avascular necrosis, could be altered when it comes to osseointegration at the molecular level.
Hypoxia-inducible factor has been identified as key in mediating how cells adapt to molecular oxygen levels.
The aim of this review is to further elucidate the physiology of hypoxia-inducible factor with its various pathways and to establish what role this factor could play in altering the disease pathophysiology of avascular necrosis caused by sickle cell disease and in improving osseointegration.
This review article also seeks to propose certain research methodology frameworks in exploring how osseointegration could be improved in sickle cell disease patients with total hip replacements and how it could eventually reduce their already increased risk of undergoing revision surgery.
Cite this article: EFORT Open Rev 2019;4:567-575. DOI: 10.1302/2058-5241.4.180030
Keywords: hypoxia-inducible factor, osseointegration, sickle cell disease: avascular necrosis, total hip replacement
Introduction
Avascular necrosis (AVN) of the femoral head remains a common complication in sickle cell disease (SCD) patients, and is frequently bilateral (Fig. 1).1 Total hip replacement (THR) surgery is often necessary to treat the degeneration which results from AVN.2 However, it has been noted that the failure rate of this procedure in SCD patients is relatively high and the failure of osseointegration has been indicated as a major underlying reason.3,4
Fig. 1.
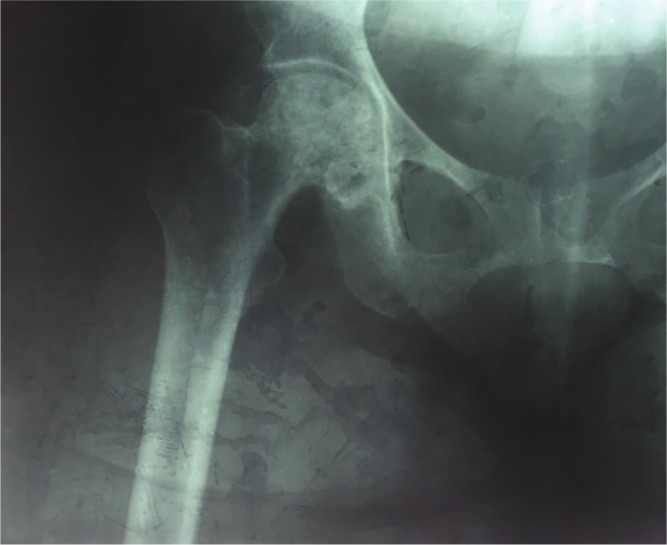
Anterioposterior radiography of a 27-year-old female sickle cell patient with right femoral head avascular necrosis.
This review seeks to outline the pathophysiology of sickle cell bone disease, its treatment and the recognized complication of implant loosening associated with the disease. It also seeks to shed light on the physiological phenomenon of osseointegration and how the study of this phenomenon has influenced orthopaedic implant design. It identifies some of the various in vitro studies which have been performed on this phenomenon, and also highlights factors contributing to the success of hip arthroplasty surgery in sickle cell disease patients.
Many studies have been carried out to evaluate the various cellular and molecular processes involved in osteogenesis. Angiogenesis and bone formation work closely together in this group of disorders.5 Hypoxia-inducible factor (HIF) which is stimulated in tissue hypoxia triggers a cascade of molecular processes that helps manage this physiological deficiency.6 However, there still remains a paucity of knowledge with regard to how sickle cell bone pathology, in particular avascular necrosis, could be altered when it comes to osseointegration at the molecular level. Hypoxia-inducible factor has been identified as key in mediating how cells adapt to molecular oxygen levels.
This review further elucidates the physiology of hypoxia-inducible factor with its various pathways and establishes what role this factor could play in altering the pathophysiology of avascular necrosis caused by sickle cell disease. It seeks to establish certain research methodology frameworks in exploring how osseointegration could be improved in sickle cell disease patients with prosthetic implants, especially total hip replacements.
One major purpose of this literature review is to highlight questions with regard to the paucity of experimental models in the current literature that will help form the basis for research to improve osseointegration in SCD patients. With considerable prior work having been undertaken to understand the mechanism of microcellular bony hypoxia, the current review sets out to bring to the fore the need to investigate further potential in vitro experimental models which will mimic the in vivo pathologic setting in sickle cell avascular necrosis. This will lead to building blocks that could help form the foundation for research directed at improving osseointegration in sickle cell disease patients.
Sickle cell bone disease
Sickle cell disease is an inherited blood condition common among, but not confined to, peoples of equatorial African ancestry. The gene for sickle haemoglobin (HbS) results in the substitution of valine for the glutamic acid normally present at the sixth position from the amino terminus of the chain of haemoglobin. It is acquired by inheriting abnormal genes from both parents, the combination giving rise to different forms of sickle cell disease. Most common at birth is homozygous sickle cell (SS) disease, also called sickle cell anaemia, in which the HbS gene is inherited from both parents.7
Sickle cell disease (SCD) encompasses various combinations of abnormal haemoglobin genes that include at least one copy of the gene for haemoglobin S paired with another structural β-chain haemoglobin variant or β-thalassemia gene.
Epidemiology
People living in Africa have the highest burden of sickle cell disease, predominantly due to four types of abnormal haemoglobin combinations: haemoglobin SS (sickle cell anaemia), haemoglobin SC, haemoglobin Sβ+ thalassemia, and haemoglobin Sβ0 thalassemia. The sickle cell trait is widespread throughout Africa with low frequencies (<1–2%) in the north and south of the continent and high but variable frequencies throughout much of equatorial Africa.8,9 An estimated 300,000–400,000 babies are born with sickle cell anaemia (SCA) worldwide every year. Approximately 75% of these births occur in sub-Saharan Africa.10–12 Population estimates in the United States suggest that approximately 100,000 people have the disease. It is also estimated that three countries in the world account for the majority of babies born with sickle cell disease. These countries are Nigeria, Democratic Republic of Congo and India. Unfortunately the mortality of children under five years with the condition can be as high as 90%.13 Demographic projections estimate that by 2050, the number of new-born babies with SCA worldwide will increase by a third and in the above mentioned countries there would an increase of 30%.14,15
Importantly for Europe there are no current data to the best of our knowledge for prevalence of sickle cell disease in the region. However, in 2010, it was reported that over 3000 children are born with SCD every year in Europe.16 This figure is unreliable bearing in mind the significant migration into Europe in recent years, and a substantial number of children are being born in areas where SCD is considered rare (for example northern and western Europe).17 The total number of patients with SCD among migrants in Germany was estimated at 2106 in 2007 and 3216 in 2015 which is an increase of 60%.18 It is also worth noting that in a multicentre sickle cell study carried out in both the United Kingdom and France, retrospective data showed a 6% mortality rate in SCD patients.19
Epidemiological profile for avascular necrosis in sickle cell disease
The femoral head is the most commonly affected site for AVN, followed by the humeral head. Studies in the literature have reported that the incidence of osteonecrosis of the femoral head (ONFH) in SCD could vary from 3–50%.20 A co-operative study of SCD reported that the estimated age for the diagnosis for AVN was 28 years and the age-specific prevalence rate was highest in patients who were over 45 years of age (34.9%).17 In contrast, the prevalence among patients under 25 years was approximately 6%. Furthermore, it is important to note that AVN is a complication associated with age, with incidence being higher among older patients. Some studies have shown that the prevalence of osteonecrosis of the femoral head is approximately 3% in patients under 15 years and could be as high as 50% in patients over 35 years of age.21 Ortiguera et al in their series noted that a 50% revision rate in patients who had THR was due to osteonecrosis; however, Johannsson et al in their study postulated that osteonecrosis in itself is not a predictor of high failure rate in THR but highlighted that the high revision rate in patients was associated with SCD.22,23
Pathophysiology
The most important pathophysiological event in sickle cell anaemia that explains most of its clinical manifestations is vascular occlusion, which may involve both the macro- and microvasculature.24 Haemoglobin polymerization, leading to erythrocyte rigidity and vascular occulsion, is central to the pathophysiology of this disease.25
Kaul et al noted in their review of the pathophysiology of vascular obstruction in sickle cell syndromes that red cell destruction leads to enhanced cell vascular adhesion which invariably led to more vascular occulsion.26
Bone involvement is the most common clinical manifestation of sickle cell disease, both in the acute setting such as painful vaso-occlusive crisis, and as a source of chronic, progressive disability such as AVN.26,27 Avascular necrosis of the femoral head is one of the significant complications affecting the musculoskeletal system in patients with sickle cell haemoglobinopathy. The reported incidence of femoral head necrosis varies from less than 10% to more than 30%.28,29 In many patients both hips and other bones are affected. The pathophysiology of osteonecrosis in sickle cell disease seems to differ from osteonecrosis because of other aetiologies. When magnetic resonance imaging (MRI) is used to quantify lesions in AVN of the femoral head, the lesions seen in sickle cell disease are larger than those seen in osteonecrosis due to other aetiologies.30 In osteonecrosis from other causes, the localization and size of the lesions is directly related to the mechanical stresses on the femoral head. The larger size and wider distribution of the lesion in sickle cell disease is due to a variety of independent factors which result in vascular occlusion.27
Non-surgical and joint-preserving treatment modalities for sickle cell disease
More conservative, evidence-based treatment modalities for SCD are well established. These include erythrocyte transfusion which forms the mainstay of treatment in SCD patients. This could be in the form of acute transfusion, which helps to improve oxygen carrying capacity and improve blood flow, or recurrent transfusions, which have been shown to prevent long-term complications by replacing rigid sickled erythrocytes with normal deformable cells and by suppressing formation of sickled erythrocytes. Other treatment methods include the administration of hydroxycarbamide, which pharmacologically increases haemoglobin F. This helps to inhibit the polymerization of haemoglobin S and hence to improve morbidity and mortality. Stem cell transplantation is considered the main curative option in SCD despite only 10–20% of patients having affected matched sibling donors. In addition, with this treatment there are still significant evidence-based concerns about transplant-related mortality and long-term toxicities, particularly relating to infertility.14,31 Joint-preserving procedures such as vascularized and non-vascularized bone grafting are indicated in the treatment of pre-collapse disease, with several studies showing successful outcomes at mid-term and long-term follow-up. However, results from core decompression procedures have been mixed.32–34
Total hip replacement for sickle cell disease
As noted earlier, AVN of the femoral head is a common consequence of vaso-occlusive attacks. The small blood vessels of the femoral head with its specific blood supply and lack of collateral circulation are particularly liable to occlusion by sickled cells. Local thrombosis gives a further reduction of the oxygen tension, resulting in increased sickling. This vicious cycle of continued hypoxia and sickling eventually produces infarction, necrosis, femoral head collapse and joint degeneration and destruction (Fig. 1). Hip symptoms are commonly seen in the second and third decades in these SCD patients. Hip replacement arthroplasty is becoming a more frequent operation in the management of those patients who have passed the stage of more conservative surgery. Decision for surgery is based on severity of pain and functional disability. Advances in medical treatment have led to improved life expectancy in sickle cell patients, which in turn has led to an increasing number of patients requiring THR.4,35
The case for cemented or cementless total hip replacement
Early published series of THR among SCD patients reported high complication and failure rates ranging from 18% to 100%. Improvement in intra- and post-operative care and possibly of the implant design has resulted in better outcomes in reports within the last decade.36 Clarke et al noted in their series of cemented THR cases, high morbidity in sickle cell disease patients due to implant loosening.37 Other complications (intra-operative and immediate post-operative) in these patients include deep infection, acetabular protrusion and femoral shaft injury.3,35 Acurio and Friedman noted a 40% revision rate at 7.5 years due to either radiologic and/or symptomatic implant loosening in cemented implants.4 It is well documented in the literature that the use of cement is likely to cause thermal necrosis of already infarcted bone, contributing to higher incidence of infection and loosening. However, Hernigou et al38,39 reported long-term results for a large series of patients with SCD who underwent cemented THR and had promising results. They postulated that the low rate of implant loosening seen in their results was associated with the stems’ rectangular geometry. It can therefore be suggested that cemented THR could be used with caution in these patients because loosening is a major concern for both femoral and acetabular components.40
Various reasons have been postulated for the high rate of implant loosening with uncemented prostheses. Due to the chronic anaemia seen in SCD patients the medullary cavity widens and there is thinning of the cortices. This usually occurs in the metaphyseal region of long bones such as the proximal femur, producing weakness, increased chance of fracture and a less than optimal environment for a femoral prosthesis. Bone marrow hyperplasia and repeated infarctions create a biological environment for the prosthesis which produces quite a high failure rate for acetabular and femoral components. It has also been suggested that lack of bone in-growth (osseointegration) in these patients could contribute to the high loosening rate, but this is yet to be verified by autopsy study.4,36 A study using histologic imaging along with concurrent MRI concluded that acetabular necrosis may be an accompaniment to aseptic necrosis of the femoral head. Further work is required to assess its significance in premature loosening of the acetabular element of total hip arthroplasty.41 Furthermore, it is worth noting that large cohort studies have shown the mean age of patients having THR for AVN in SCD to be 36 years, and so the generally high level of activity in these patients does contribute to increased implant loosening rate. Seeing that these patients are generally young, biologic fixation with modern uncemented implants could be a more attractive option. More recently published large cohort studies with long-term follow-up in SCD with uncemented THR implants have shown very encouraging results so far.40,42,43
Osseointegration
Osseointegration refers to a direct structural and functional connection between ordered, living bone and the surface of a load-carrying implant. It could also be referred to as a direct bone-to-metal interface without interposition of non-bony tissue. Researchers who first described this phenomenon, showed that titanium implants could become permanently incorporated within bone; that is, the living bone could become so fused with the titanium oxide layer of the implant that the two could not be separated without fracture.44 An implant is considered as osseointegrated when there is no progressive relative movement between the implant and the bone with which it has direct contact. Essentially, the process of osseointegration reflects an anchorage mechanism whereby non-vital components can be reliably incorporated into living bone and which persists under all normal conditions of loading. The biology of osseointegration involves processes similar to those occurring during bone healing. Various cell types, growth factors and cytokines are involved and interact throughout the stages of osseointegration, including inflammation, vascularization, bone formation and, ultimately, bone remodelling.45
Various factors may enhance or inhibit osseointegration. Factors which promote osseointegration include implant-related factors such as implant design and chemical composition, topography of the implant surface, material, shape, length, diameter, implant-surface treatment and coatings, the status of the host bone bed and its intrinsic healing potential, the mechanical stability and loading conditions applied on the implant, the use of adjuvant treatments such as bone grafting, osteogenic biological coatings, biophysical stimulation, and pharmacological agents such as simvastatin and bisphosphonates. It is important to achieve the best possible implant osseointegration into the adjacent bone and to ensure therefore long-term implant stability. For this purpose, various pharmacological, biological and biophysical modalities have been developed, such as bone-grafting materials, pharmacological agents, growth factors and bone-morphogenetic proteins. Biophysical stimulation of osseointegration includes two non-invasive and safe methods that have been initially developed to enhance fracture healing: pulsed electromagnetic fields (PEMFs) and low intensity impulse ultrasounds (LIPUS), of which most studies confirm the beneficial effects. Factors which inhibit osseointegration include: excessive implant mobility and micro-motion, inappropriate porosity of the porous coating of the implant, radiation therapy and pharmacological agents such as cyclosporin A.28,45
There have been studies looking at osseointegration in relation to biomaterials, and implant surgery. Despite advances in total hip arthroplasty, failure of acetabular cups in total hip arthroplasty remains a concern and more so when those affected are sickle cell disease patients. Kalia et al noted in their study that osseointegration improved with acetabular cups sprayed with bone marrow stromal cells.46 Other studies in the literature have concluded that other biomaterials such as zirconium had similar biocompatibility and osseointegration rates to titanium implants.47
Surface topography modifications and physicochemical treatments of surfaces to achieve an enhanced bone implant interface formation using biochemical methods offer an alternative path. These functionalization approaches require a deeper understanding of the biology and biochemistry of the host tissue at the interface in terms of the mechanisms by which cells adhere to surfaces, the role of biomolecules, functional peptide sequences and extracellular matrix proteins in influencing or regulating differentiation and remodelling of bone and tissue.48
Hypoxia-inducible factor (HIF) and its role in osseointegration
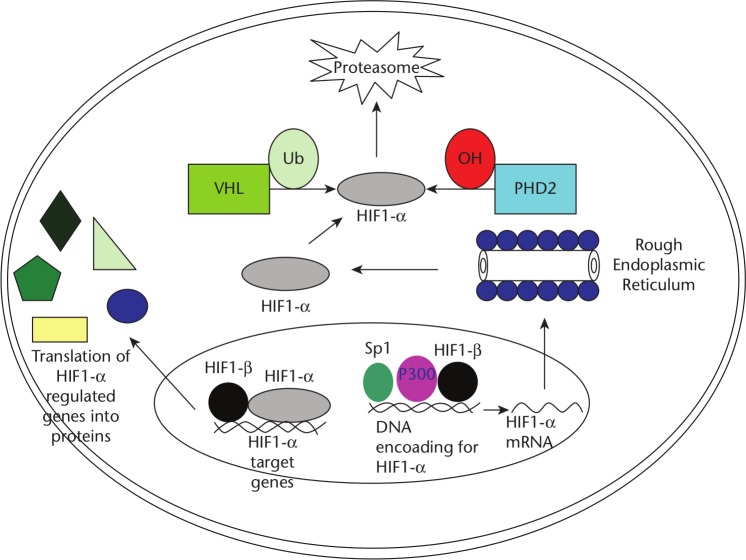
Oxygen, an indispensable metabolic substrate in various enzymatic reactions in vivo, including mitochondrial respiration, is a key regulatory signal in tissue development and homeostasis. For example, during embryonic development, cellular differentiation as well as organ growth and final shape are thought to be modulated by oxygen gradients, which, at least in part, rely on the HIF signalling pathway to mediate their effect.49 At a molecular level, a HIF complex contains an α and a β subunit, both of which can be selected from several alternatives. They are members of a large family of transcription factors which contain a basic helix–loop–helix region and a PAS domain (named for Per, Arnt/HIF-1β and Sim). HIF β subunits are constitutive and are also involved in xenobiotic responses. The α subunit is regulatory and is unique to the hypoxic response.50 The HIF family comprises three subunits: HIF-1α, HIF-2α and HIF-3α. HIF-2α and HIF-3α have limited homology with HIF-1α, but all three subunits share the conserved pVHL-binding domain (Von Hippel–Lindau) and are consequently regulated by hypoxia in the same way as HIF-1α (Fig. 2).51
Fig. 2.
The hypoxia-inducible factor (HIF)-1 pathway. The HIF-1α gene is transcribed in the nucleus with the help of specificity protein (Sp) 1, P300, and HIF-1β. Once translated in the cytoplasm, the HIF-1α protein can either become hydroxylated and ubiquinated, in which case it will be degraded by proteasomes (under normal oxygen conditions). In the setting of hypoxia, it can re-enter the nucleus and form a transcription complex with the HIF-1β subunit. If successfully stabilized with the latter subunit, the final complex ultimately will function to regulate target genes such as vascular endothelial growth factor and cathepsin D. Possible therapeutic intervention points are: the hydroxylation that leads to degradation of HIF-1α, the binding of HIF-1α to its coactivators, and the modulation of HIF-1α activity. Additionally, gene therapy approaches have been used to induce the overexpression of HIF or the disruption of the HIF pathway with antisense oligonucleotides. Abbreviations: PHD: proline-hydroxylase domain containing molecules; Ub: ubiquitin; VHL: von Hippel-Lindau protein. Image and footnote reproduced and published with the permission of Yale Journal of Biology and Medicine.71
There is established evidence that HIF-1α promotes angiogenesis and osteogenesis by elevating vascular endothelial growth factor (VEGF) levels in osteoblasts. Wang and Wan et al showed in their study that mice overexpressing HIFα in osteoblasts through selective deletion of the von Hippel–Lindau (VHL) gene expressed high levels of VEGF and developed extremely dense, heavily vascularized long bones, and they showed a model for the HIFα pathway in bone formation. In this model, osteoblasts residing on the nascent bone surface sense reduced oxygen or nutrient levels and up-regulate HIFα subunits. Elevated HIF-1α transactivates target genes such as VEGF, which then stimulate new blood vessel formation and invasion into bone. This process is exponential, with ever-increasing numbers of new blood vessels introducing more osteoblast progenitors, which then mature and function to form more individual bone-formation units.5 Wan and Shao et al also described in their own in vitro study that the HIF-1α pathway has been identified as a key component in this molecular process. They demonstrated that overexpression of HIF-1 in mature osteoblasts through disruption of the VHL protein profoundly increases angiogenesis and osteogenesis.52 However, current literature does not point to evidence of a model used in chronic hypoxia to study the role of HIF in osteogenesis.
The decisive role of the implant surface properties in molecular interactions, cellular function and bone regeneration has been demonstrated extensively in in vitro research. However, a thorough understanding at the genetic scale of the onset phase of bone regeneration at the implant interface is required prior to the development of strategies to further optimize implant osseointegration.53
It was concluded in a study that angiogenic events are crucial for the subsequent implant osseointegration. This requirement is strengthened by their finding that the gene expression of angiogenic markers is differently regulated in normal versus compromised bone at two days after implantation; this event coincides with a negatively affected osteogenic cell response and results in a suspended implant osseointegration in compromised bone.54
This calls into question the role of HIF with regard to osseointegration. Vandamme et al54 also noted in their study that HIF-1α expression increased in the initial phase of osseointegration and hence VEGF up-regulation. There are studies which have evaluated the role of implant surfaces and their role in osseointegration – Park et al showed clearly in their study that the Sr-containing oxide layer produced by hydrothermal treatment was effective in improving the osseointegration of titanium(Ti)–6aluminium–4V alloy implants by enhancing differentiation of osteoblastic cells.55 One study has also provided evidence that osteoblast attachment, as well as alkaline phosphatase (ALP) activity and calcium deposition were enhanced by the immobilized VEGF on the polysaccharide-grafted titanium. Thus, titanium substrates modified with polysaccharides conjugated with VEGF can promote osteoblast functions and concurrently reduce bacterial adhesion.56
It has also been determined that osseointegration between tissue engineered bone and dental implants was enhanced by HIF-1α.57 However, there is no evidence in the literature for the role of HIF’s molecular physiology being used in promoting the prospect of osseointegration in pathologic bony conditions involving chronic hypoxia. There could therefore be a need to study this in detail scientifically via properly directed and designed research.
The role of HIF in chronic hypoxia and how it affects osseointegration
Human mesenchymal cells (hMSCs) are multipotent cells, as they are capable of differentiating along the osteogenic, adipogenic and chondrogenic lineages as previously demonstrated by numerous studies. Potier and Ferreira et al described in their own study that temporary exposure of mesenchymal stem cells to hypoxia leads to limited stimulation of angiogenic factor secretion and also to persistent down-regulation of several osteoblastic markers, which suggests that exposure of mesenchymal cells (MSCs) transplanted in vivo to hypoxia may affect their bone-forming potential. They also established in their study that there was a two-fold up-regulation of VEGF expression by hMSCs which occurs under hypoxic conditions at both mRNA and protein levels. These findings are in line with previous reports that hypoxia increases VEGF expression.58 However, a limitation of this study was the methodology in creating a hypoxic environment for cell growth without the use of modern oxygen incubators in which accurate readings for the partial pressure of O2 at cellular level would be difficult to achieve. Diverse responses to hypoxia have been reported for cultured osteoblasts, including increased synthesis of VEGF, insulin-like growth factor II (IGF-II), and transforming growth factor β1 (TGF-β1).59,60 The long-term effects of hypoxia on the function of osteoblasts, the bone-forming cells, have received little direct attention.61 It was also highlighted in this study that exposure of osteoblasts to hypoxia (2% O2) for the first six days of culture followed by 20% O2 for the final 12 days resulted in a three-fold decrease in bone nodule formation, and the inverse led to a two-fold decrease in bone nodule formation. They saw that the amount of bone nodule formation in cultures subjected to early hypoxia added to that measured in cultures subjected to late hypoxia approached that of cultures held at 20% O2 continuously, indicating that osteoblasts are able to recover from hypoxic insult. This type of insult mimics that seen in sickle cell disease patients who may eventually develop necrosis.61
It is worth noting that HIF activation causes angiogenesis which is a prerequisite for osteogenesis and then osseointegration.5 New bone forms only in close connection to blood vessels. The mature bone cell does not survive more than 200 µm away from a blood vessel. First, the blood vessel develops and then the bone follows; a process called angiogenetic osteogenesis.62 Bone formation by osteoblasts is critically dependent on oxygen and provides further evidence for the vital role of the vasculature in maintaining bone health.61 With the understanding that osteoblasts can be genetically modified in order to cause the overexpression of HIF-1α52 leading to increased osteogenesis, it could be suggested that this concept be used in an experimental in vitro research model to study the degree of osseointegration with these modified osteoblasts when exposed to chronic and repeated hypoxic insults as seen in vivo with SCD patients with prosthetic implants. With hypoxia being both a cause and sequela of SCD,63 there are important clinical implications from the above proposal for improving osseointegration in these patients.
Future prospects
Hip implant design is crucial in addressing the problem of lack of osseointegration in sickle cell disease patients. Several studies in the literature have highlighted the use and efficacy of osteogenic coated femoral stems in total hip arthroplasty. Many orthopaedic surgeons consider the use of hydroxyapatite (HA) for the potential advantages of increasing the strength of the implant-to-host bone bond and decreasing the amount of time required to achieve stable fixation.64,65 Furthermore Sanz-Reig et al showed in their study that 98% of titanium plasma sprayed implant stems used in THA had signs of stability (endosteal bone formation and proximal adaptive bone remodelling), and 61% had endosteal spot welds indicative of bony fixation.66 In vitro and in vivo studies have shown the efficacy of osteogenic substrates such as human bone marrow stem cells seeded on poly(dl-lactic acid) scaffolds as a potential biological bone graft extender for future use in bone grafting.67 It is also worth noting that other studies including randomized controlled trials have highlighted the lack of significant difference in clinical advantage in the use of HA-coated implants.68 The reason for the disparity in the literature could be the need to further explore effective osseointegration in the design of hip implants.
With the knowledge of this common complication of implant loosening in these patients, could more improved measures be used to ensure effective osseointegration? A lot of work has been carried out in improving the titanium implant surface in order to enhance bony implant integration. Della Valle et al suggested in their work that silicon-based anodic spark deposition treatment of the titanium surface would enhance osseointegration in orthopaedic applications.69 Sandrini et al also noted in their study the novel biomimetic treatment of the titanium surface which led to faster and more durable implant-to-bone bonding through higher mineralization capacity.70 Having noted the above studies as references, could there be an experimental model which could investigate in vitro and in vivo osseointegration and thus improve the titanium surface osteogenic potential by treating with HIF-conjugated composite seeded on the implant? Implant design research aimed at improved osseointegration could benefit from these proposals and the result would inevitably be a reduction in the revision burden and high implant failure rate currently experienced by SCD patients.
Conclusion
Some light has been shed from established well documented literature on SCD and its characteristic attributes. Its common bone sequela, AVN, has also been examined, further highlighting its treatment and the fact that it is a significant factor in the outcome of hip replacement surgery in sickle cell disease patients. This review has helped to highlight the problem of implant loosening due to deficient osseointegration seen in sickle cell disease patients who have undergone uncemented THR. As discussed in the main text, the failure rate of THR in SCD patients is still a pressing matter which continues to increase the revision load in these patients. Morbidity and mortality in SCD patients following THR is significantly higher than those with THR from other indications.
The review has also shed light on the role of hypoxia-inducible factor in improving osteogenesis in physiological hypoxic conditions. Studies in the literature have elucidated its role in molecular biology and in basic science experiments but this review highlights its role in a different light in order to help improve osseointegration. This could be seen as laying down the building blocks for improved implant design tailored to reduce the surgical revision burden in SCD patients and improve their quality of life.
Footnotes
ICMJE Conflict of interest statement: ME reports board membership of Cellesce Ltd and a patent for Cellesce Bioprocess outside the submitted work.
HSG reports consultancy to JRI; expert testimony for Leigh Day Solicitors; grants/grants pending with Smith & Nephew; payment for lectures including service on speakers’ bureaus from Stryker; patents (planned, pending or issued) for personalized HTO; editorial board membership and travel expenses reimbursed for editorial board meetings for Bone & Joint Journal, all outside the submitted work.
AG declares no conflict of interest relevant to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Ilyas I, Moreau P. Simultaneous bilateral total hip arthroplasty in sickle cell disease. J Arthroplasty 2002;17:441–445. [DOI] [PubMed] [Google Scholar]
- 2. Hanker GJ, Amstutz HC. Osteonecrosis of the hip in the sickle-cell diseases: treatment and complications. J Bone Joint Surg Am 1988;70:499–506. [PubMed] [Google Scholar]
- 3. Bishop AR, Roberson JR, Eckman JR, Fleming LL. Total hip arthroplasty in patients who have sickle-cell hemoglobinopathy. J Bone Joint Surg Am 1988;70:853–855. [PubMed] [Google Scholar]
- 4. Acurio MT, Friedman RJ. Hip arthroplasty in patients with sickle-cell haemoglobinopathy. J Bone Joint Surg Br 1992;74:367–371. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y Wan C Deng L, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 2007;117:1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007;129:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serjeant GR. Sickle-cell disease. Lancet 1997;350:725–730. [DOI] [PubMed] [Google Scholar]
- 8. Pagnier J Mears JG Dunda-Belkhodja O, et al. Evidence for the multicentric origin of the sickle cell hemoglobin gene in Africa. Proc Natl Acad Sci USA 1984;81:1771–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagel RL Fabry ME Pagnier J, et al. Hematologically and genetically distinct forms of sickle cell anemia in Africa: the Senegal type and the Benin type. N Engl J Med 1985;312:880–884. [DOI] [PubMed] [Google Scholar]
- 10. Rees DC. To begin at the beginning: sickle cell disease in Africa. Lancet Haematol 2014;1:e50–e51. [DOI] [PubMed] [Google Scholar]
- 11. Kato GJ Piel FB Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers 2018;4:18010. [DOI] [PubMed] [Google Scholar]
- 12. Macharia AW Mochamah G Uyoga S, et al. The clinical epidemiology of sickle cell anemia In Africa. Am J Hematol 2018;93:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med 2011;41:S398–S405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet 2017;390:311–323. [DOI] [PubMed] [Google Scholar]
- 15. Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med 2013;10:e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piel FB Patil AP Howes RE, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun 2010;1:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thein MS, Igbineweka NE, Thein SL. Sickle cell disease in the older adult. Pathology 2017;49:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kunz JB, Cario H, Grosse R, Jarisch A, Lobitz S, Kulozik AE. The epidemiology of sickle cell disease in Germany following recent large-scale immigration. Pediatr Blood Cancer 2017;64:e26550. [DOI] [PubMed] [Google Scholar]
- 19. Perronne V Roberts-Harewood M Bachir D, et al. Patterns of mortality in sickle cell disease in adults in France and England. Hematol J 2002;3:56–60. [DOI] [PubMed] [Google Scholar]
- 20. Issa K, Naziri Q, Maheshwari AV, Rasquinha VJ, Delanois RE, Mont MA. Excellent results and minimal complications of total hip arthroplasty in sickle cell hemoglobinopathy at mid-term follow-up using cementless prosthetic components. J Arthroplasty 2013;28:1693–1698. [DOI] [PubMed] [Google Scholar]
- 21. Almeida-Matos M, Carrasco J, Lisle L, Castelar M. Avascular necrosis of the femoral head in sickle cell disease in pediatric patients suffering from hip dysfunction. Rev Salud Publica (Bogota) 2016;18:986–995. [DOI] [PubMed] [Google Scholar]
- 22. Ortiguera CJ, Pulliam IT, Cabanela ME. Total hip arthroplasty for osteonecrosis: matched-pair analysis of 188 hips with long-term follow-up. J Arthroplasty 1999;14:21–28. [DOI] [PubMed] [Google Scholar]
- 23. Johannson HR, Zywiel MG, Marker DR, Jones LC, McGrath MS, Mont MA. Osteonecrosis is not a predictor of poor outcomes in primary total hip arthroplasty: a systematic literature review. Int Orthop 2011;35:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ballas SK. Sickle cell anaemia: progress in pathogenesis and treatment. Drugs 2002;62:1143–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376:2018–2031. [DOI] [PubMed] [Google Scholar]
- 26. Kaul DK, Fabry ME, Nagel RL. The pathophysiology of vascular obstruction in the sickle syndromes. Blood Rev 1996;10:29–44. [DOI] [PubMed] [Google Scholar]
- 27. Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol 2005;129:482–490. [DOI] [PubMed] [Google Scholar]
- 28. Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC. Biology of implant osseointegration. J Musculoskelet Neuronal Interact 2009;9:61–71. [PubMed] [Google Scholar]
- 29. Ellis MJ, Chaudhuri JB. Poly(lactic-co-glycolic acid) hollow fibre membranes for use as a tissue engineering scaffold. Biotechnol Bioeng 2007;96:177–187. [DOI] [PubMed] [Google Scholar]
- 30. Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol 2007;63:16–28. [DOI] [PubMed] [Google Scholar]
- 31. Assies J, Eggelte TA, Kager PA. [Pathophysiology and treatment of sickle-cell disease]. Ned Tijdschr Geneeskd 2006;150:462–463. [PubMed] [Google Scholar]
- 32. Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg Am 2015;97:1604–1627. [DOI] [PubMed] [Google Scholar]
- 33. Sadat-Ali M, Azam MQ, Elshabouri EM, Tantawy AM, Acharya S. Stem cell therapy for avascular necrosis of femoral head in sickle cell disease: report of 11 cases and review of literature. Int J Stem Cells 2017;10:179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chughtai M, Piuzzi NS, Khlopas A, Jones LC, Goodman SB, Mont MA. An evidence-based guide to the treatment of osteonecrosis of the femoral head. Bone Joint J 2017;99-B:1267–1279. [DOI] [PubMed] [Google Scholar]
- 35. Al-Mousawi F, Malki A, Al-Aradi A, Al-Bagali M, Al-Sadadi A, Booz MM. Total hip replacement in sickle cell disease. Int Orthop 2002;26:157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al Omran A. Multiple drilling compared with standard core decompression for avascular necrosis of the femoral head in sickle cell disease patients. Arch Orthop Trauma Surg 2013;133:609–613. [DOI] [PubMed] [Google Scholar]
- 37. Clarke HJ, Jinnah RH, Brooker AF, Michaelson JD. Total replacement of the hip for avascular necrosis in sickle cell disease. J Bone Joint Surg Br 1989;71:465–470. [DOI] [PubMed] [Google Scholar]
- 38. Hernigou P, Zilber S, Filippini P, Mathieu G, Poignard A, Galacteros F. Total THA in adult osteonecrosis related to sickle cell disease. Clin Orthop Relat Res 2008;466:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hernigou P, Bachir D, Galacteros F. Avascular necrosis of the femoral head in sickle-cell disease: treatment of collapse by the injection of acrylic cement. J Bone Joint Surg Br 1993;75:875–880. [DOI] [PubMed] [Google Scholar]
- 40. Ilyas I, Alrumaih HA, Rabbani S. Noncemented total hip arthroplasty in sickle-cell disease: long-term results. J Arthroplasty 2018;33:477–481. [DOI] [PubMed] [Google Scholar]
- 41. Fink B, Assheuer J, Enderle A, Schneider T, Rüther W. Avascular osteonecrosis of the acetabulum. Skeletal Radiol 1997;26:509–516. [DOI] [PubMed] [Google Scholar]
- 42. Jack CM, Howard J, Aziz ES, Kesse-Adu R, Bankes MJ. Cementless total hip replacements in sickle cell disease. Hip Int 2016;26:186–192. [DOI] [PubMed] [Google Scholar]
- 43. Katchy AU, Anyaehie UE, Nwadinigwe CU, Eyichukwu GO. Total hip replacement in sickle cell disorder: a preliminary report of challenges and early outcome of 21 consecutive patients. Niger J Clin Pract 2018;21:492–495. [DOI] [PubMed] [Google Scholar]
- 44. Shubayev VI, Brånemark R, Steinauer J, Myers RR. Titanium implants induce expression of matrix metalloproteinases in bone during osseointegration. J Rehabil Res Dev 2004;41:757–766. [DOI] [PubMed] [Google Scholar]
- 45. Dimitriou R, Babis GC. Biomaterial osseointegration enhancement with biophysical stimulation. J Musculoskelet Neuronal Interact 2007;7:253–265. [PubMed] [Google Scholar]
- 46. Kalia P Coathup MJ Oussedik S, et al. Augmentation of bone growth onto the acetabular cup surface using bone marrow stromal cells in total hip replacement surgery. Tissue Eng Part A 2009;15:3689–3696. [DOI] [PubMed] [Google Scholar]
- 47. Möller B Terheyden H Açil Y, et al. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: an in vivo and in vitro study. Int J Oral Maxillofac Surg 2012;41:638–645. [DOI] [PubMed] [Google Scholar]
- 48. Bauer S, Schmuki P, von der Mark K, Park J. Engineering biocompatible implant surfaces Part 1: materials and surfaces. Prog Mater Sci 2013;58:261–326. [Google Scholar]
- 49. Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol 2012;8:358–366. [DOI] [PubMed] [Google Scholar]
- 50. Maxwell PH. Hypoxia-inducible factor as a physiological regulator. Exp Physiol 2005;90:791–797. [DOI] [PubMed] [Google Scholar]
- 51. Wiesener MS Turley H Allen WE, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood 1998;92:2260–2268. [PubMed] [Google Scholar]
- 52. Wan C Shao J Gilbert SR, et al. Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci 2010;1192:322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vandamme K Holy X Bensidhoum M, et al. In vivo molecular evidence of delayed titanium implant osseointegration in compromised bone. Biomaterials 2011;32:3547–3554. [DOI] [PubMed] [Google Scholar]
- 54. Vandamme K Holy X Bensidhoum M, et al. Establishment of an in vivo model for molecular assessment of titanium implant osseointegration in compromised bone. Tissue Eng Part C Methods 2011;17:311–318. [DOI] [PubMed] [Google Scholar]
- 55. Park JW, Kim HK, Kim YJ, Jang JH, Song H, Hanawa T. Osteoblast response and osseointegration of a Ti-6Al-4V alloy implant incorporating strontium. Acta Biomater 2010;6:2843–2851. [DOI] [PubMed] [Google Scholar]
- 56. Hu X, Neoh KG, Shi Z, Kang ET, Poh C, Wang W. An in vitro assessment of titanium functionalized with polysaccharides conjugated with vascular endothelial growth factor for enhanced osseointegration and inhibition of bacterial adhesion. Biomaterials 2010;31:8854–8863. [DOI] [PubMed] [Google Scholar]
- 57. Zou D He J Zhang K, et al. The bone-forming effects of HIF-1α-transduced BMSCs promote osseointegration with dental implant in canine mandible. PLoS One 2012;7:e32355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Potier E Ferreira E Andriamanalijaona R, et al. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 2007;40:1078–1087. [DOI] [PubMed] [Google Scholar]
- 59. Akeno N, Czyzyk-Krzeska MF, Gross TS, Clemens TL. Hypoxia induces vascular endothelial growth factor gene transcription in human osteoblast-like cells through the hypoxia-inducible factor-2alpha. Endocrinology 2001;142:959–962. [DOI] [PubMed] [Google Scholar]
- 60. Warren SM Steinbrech DS Mehrara BJ, et al. Hypoxia regulates osteoblast gene expression. J Surg Res 2001;99:147–155. [DOI] [PubMed] [Google Scholar]
- 61. Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res 2006;312:1693–1702. [DOI] [PubMed] [Google Scholar]
- 62. Terheyden H, Lang NP, Bierbaum S, Stadlinger B. Osseointegration: communication of cells. Clin Oral Implants Res 2012;23:1127–1135. [DOI] [PubMed] [Google Scholar]
- 63. Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood 2003;101:846–848. [DOI] [PubMed] [Google Scholar]
- 64. Cook SD, Thomas KA, Dalton JE, Volkman TK, Whitecloud TS, III, Kay JF. Hydroxylapatite coating of porous implants improves bone ingrowth and interface attachment strength. J Biomed Mater Res 1992;26:989–1001. [DOI] [PubMed] [Google Scholar]
- 65. Søballe K Hansen ES Brockstedt-Rasmussen H, et al. Gap healing enhanced by hydroxyapatite coating in dogs. Clin Orthop Relat Res 1991;272:300–307. [PubMed] [Google Scholar]
- 66. Sanz-Reig J, Lizaur-Utrilla A, Llamas-Merino I, Lopez-Prats F. Cementless total hip arthroplasty using titanium, plasma-sprayed implants: a study with 10 to 15 years of follow-up. J Orthop Surg (Hong Kong) 2011;19:169–173. [DOI] [PubMed] [Google Scholar]
- 67. Bolland BJ Kanczler JM Ginty PJ, et al. The application of human bone marrow stromal cells and poly(dl-lactic acid) as a biological bone graft extender in impaction bone grafting. Biomaterials 2008;29:3221–3227. [DOI] [PubMed] [Google Scholar]
- 68. Camazzola D, Hammond T, Gandhi R, Davey JR. A randomized trial of hydroxyapatite-coated femoral stems in total hip arthroplasty: a 13-year follow-up. J Arthroplasty 2009;24:33–37. [DOI] [PubMed] [Google Scholar]
- 69. Della Valle C, Rondelli G, Cigada A, Bianchi AE, Chiesa R. A novel silicon-based electrochemical treatment to improve osteointegration of titanium implants. J Appl Biomater Funct Mater 2013;11:e106–e116. [DOI] [PubMed] [Google Scholar]
- 70. Sandrini E, Chiesa R, Rondelli G, Santin M, Cigada A. A novel biomimetic treatment for an improved osteointegration of titanium. J Appl Biomater Biomech 2003;1:33–42. [PubMed] [Google Scholar]
- 71. Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 2007;80:51–60. [PMC free article] [PubMed] [Google Scholar]