Ammonia in water ice found in a tectonic region on Pluto is a clue to the geological and geochemical history of the dwarf planet.
Abstract
We report the detection of ammonia (NH3) on Pluto’s surface in spectral images obtained with the New Horizons spacecraft that show absorption bands at 1.65 and 2.2 μm. The ammonia signature is spatially coincident with a region of past extensional tectonic activity (Virgil Fossae) where the presence of H2O ice is prominent. Ammonia in liquid water profoundly depresses the freezing point of the mixture. Ammoniated ices are believed to be geologically short lived when irradiated with ultraviolet photons or charged particles. Thus, the presence of NH3 on a planetary surface is indicative of a relatively recent deposition or possibly through exposure by some geological process. In the present case, the areal distribution is more suggestive of cryovolcanic emplacement, however, adding to the evidence for ongoing geological activity on Pluto and the possible presence of liquid water at depth today.
INTRODUCTION
Some small bodies in the solar system preserve a record of the events and chemistry of the solar nebula in which they formed and thereby carry chemical signatures of the molecular cloud from which the nebula condensed. Those bodies that have not been geologically and geochemically reprocessed carry the most pristine records. Ammonia is a potentially important source of nitrogen in the solar system and plays a pivotal role in planetary chemistry; until the work presented here, ammonia had not been detected spectroscopically on Pluto. The ammonia found in molecular clouds (1) and incorporated into condensing planetesimals, and comets (2), is especially important in the synthesis of organic materials found in certain meteorites (3, 4) and is therefore a critical component in the chain of abiotic events, leading to planetary biochemical environments. In H2O-rich bodies, NH3 also has the effect of lowering the melting temperature, thereby helping to maintain liquid in their interiors (5). Although NH3 is stable against evaporation for the age of the solar system on distant small bodies (6, 7), it can be readily destroyed by ultraviolet (UV) radiation or ion bombardment as a free molecule or when frozen in ice. This process is enhanced when NH3 occurs in H2O ice (8). Therefore, NH3 presence on a surface suggests a geologically relatively short lifetime and can be indicative of a young or renewed surface (9). However, its presence on the ancient surfaces of Pluto’s small satellites indicates that ammonia may be able to survive for a long time in that environment, perhaps by being in a more durable chemical form such as a hydrate (NH3•nH2O) or as an ammoniated salt (10). In general, the form of the ammonia deduced from the spectral evidence available in current astronomical observations is ambiguous because absorption features in solid NH3, hydrates, and ammoniated salts are weak combination bands with broad shapes and central wavelengths that overlap.
Pluto’s surface was observed at high spatial resolution by the near-infrared spectral imager [Linear Etalon Imaging Spectral Array (LEISA), ~2700 m per pixel] and charge-coupled device camera [Multi-spectral Visible Imaging Camera (MVIC), ~650 m per pixel] system (11) on the New Horizons spacecraft during the flyby in 2015 (12–14). In the narrow geographic region of Pluto scanned at highest resolution, Cthulhu (some of the place and feature names on Pluto used in this paper are informal) is a central feature, with a dark red-brown color indicating a substantial non-ice composition rich in materials processed both in the atmosphere and on the surface and regarded as tholins (13). In the northern part of Cthulhu, two geographic features stand out: Elliot crater and the adjacent Virgil Fossae troughs, whose main component (hereafter Virgil Fossa) has a unique red color as seen in the color-enhanced MVIC image (Fig. 1B). A few small craters nearby share this distinctive coloration. The red-colored region in and around the fossa is spatially coincident with a prominent exposure of H2O ice (as shown in Fig. 2B) that is seen in only a few other regions of Pluto’s surface (15–18). In the enlarged view of the western portion of the fossa from the Pluto base map (Fig. 2A), shown also with the corresponding H2O ice map derived from LEISA observations (Fig. 2B), muted topography (pit craters and fossa troughs) supports the contention that cryoclastic material was deposited from one or more erupting fountains. The apparent source of the fountains is within the main trough of the fossa, probably along the dip-slip fault defining the southern wall. The affected area is >5000 km2, and other sources of fountaining and flow may occur in the same and adjacent areas that carry the H2O and color signature.
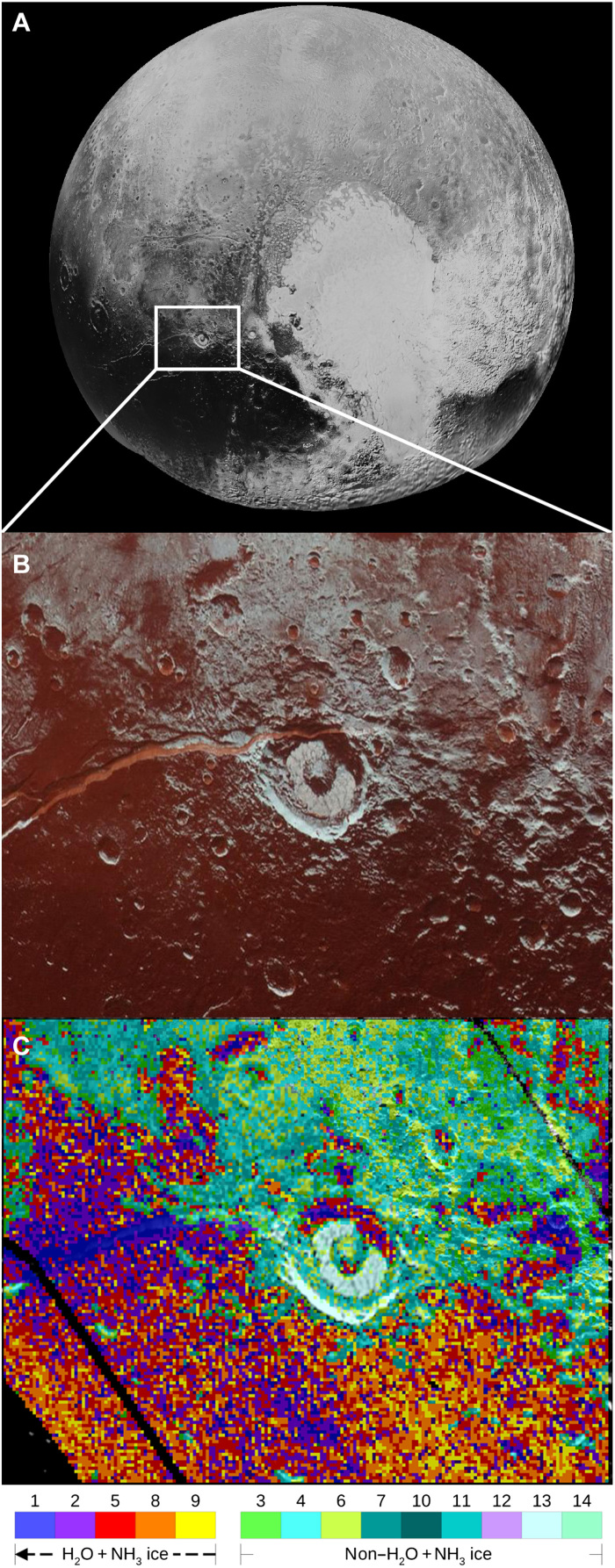
Fig. 1. Distribution of red-tinted H2O ice exhibiting the spectral signature of NH3 in Virgil Fossae and surrounding terrain.
(A) Pluto’s encounter hemisphere as seen by New Horizons during the 14 July 2015 flyby. (B) Selected ROI in the MVIC image illustrating the uniquely bright red coloring of Virgil Fossa. (C) Geographical distribution of the 14 clusters where clusters 1 (dark blue), 2 (purple), 5 (red), 8 (orange), and 9 (yellow) show the H2O + NH3–rich clusters in a gradation from maximum to minimum (indicated by the arrow direction). Image credit: NASA, Johns Hopkins University, Southwest Research Institute.
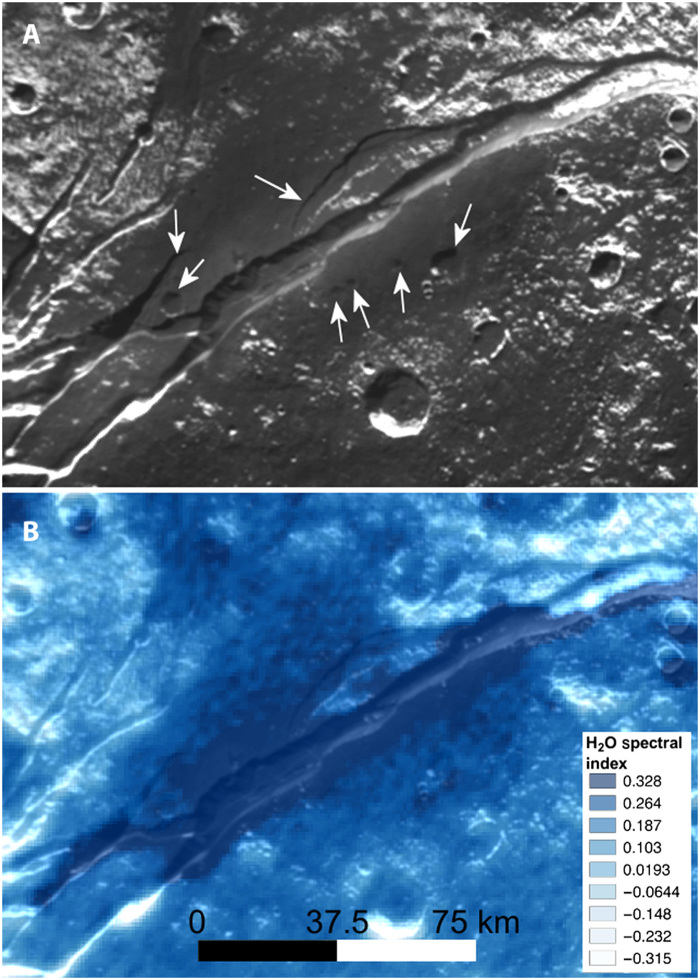
Fig. 2. Detail of the site of the putative cryovolcanic emission of NH3-bearing H2O in the main trough of Virgil Fossae.
(A) Section of Virgil Fossae from the Pluto base map. Arrows point to topographic features that are muted in form by an apparent mantling by material ejected in a cryovolcanic event within the fossa main channel. (B) Same region shown with the H2O ice distribution (17, 18) in blue overlain on the base map: Darker shade of blue indicates greater concentration based on the strength of the ice absorption bands. Image credit: NASA, Johns Hopkins University, Southwest Research Institute.
Proceeding on the premise that Virgil Fossae and surroundings represent the site of cryovolcanic activity in the fractured crust of Pluto in a region of planet-wide stress induced by the formation of Sputnik Planitia (19, 20) and subsequent events, we have investigated the composition of this region of interest (ROI) to understand its characteristics and the coincidence of the ammonia signature and red coloration with exposures of H2O ice.
RESULTS
Spectral analysis
We have studied the spectral signature of the ROI using a multivariate statistical analysis that classifies the spectra by their shape, in conjunction with radiative transfer modeling of the relevant spectra obtained from the classification process. We describe the approach in detail in the Materials and Methods.
The spectral averages obtained from the classification appear very similar to the eye, as shown in Fig. 3A, but map in a consistent way away from Virgil Fossa, as shown in Fig. 1C. To understand the differences that justify the classification, we adopted the spectrum of the class farthest away from the Virgil Fossa channel as our standard against which we compared, by means of a ratio, the other cluster averages. The ratios are shown in Fig. 3B and highlight spectral absorption bands characteristic of H2O ice in a progression of increasing strength. A closer inspection revealed an anomalously deep 1.65-μm band and a depression in the shoulder of one of the H2O bands at ~2.2 μm, typical of the spectral signature of NH3. To confirm the detection, we computed a preliminary model of the spectrum of the ratio, a mixture of H2O and tholin but no NH3, shown in Fig. 4At and described in the Materials and Methods. The residuals of the observation ratioed to the model show a clear band at 2.2 μm (Fig. 4Ab).
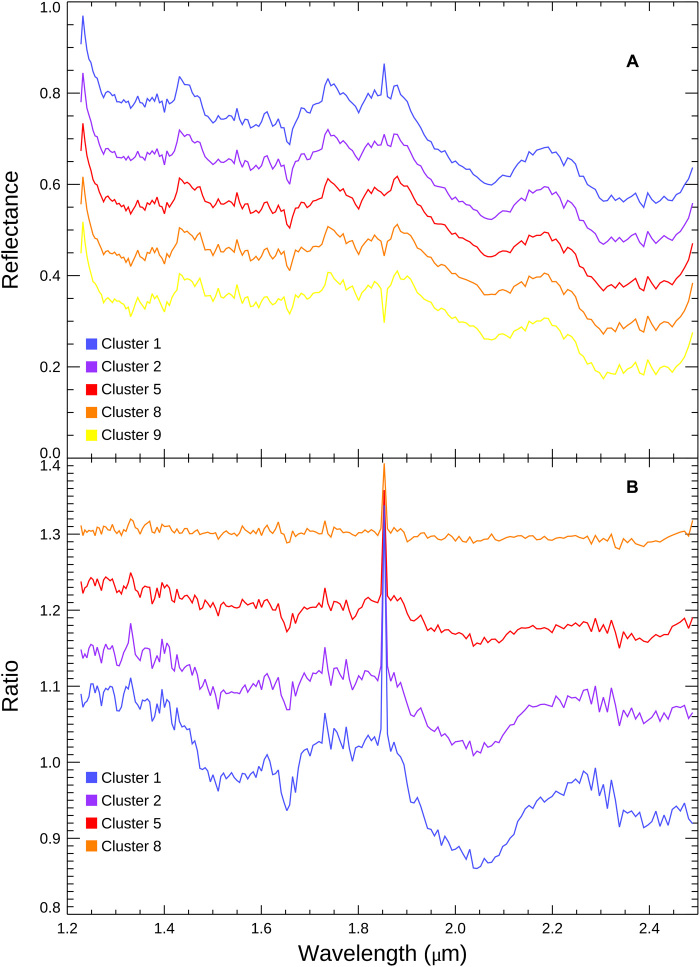
Fig. 3. Spectral signature of NH3 in Virgil Fossae and surrounding terrain.
(A) Averaged spectra for the CH4-poor clusters 1, 2, 5, 8, and 9. Note that the raw spectra are similar. (B) Spectra of cluster 9 divided by clusters 1, 2, 5, and 8. The ratioed spectra reveal H2O ice absorptions at 1.5, 1.65, and 2.0 μm and a depression at 2.2 μm characteristic of NH3 products.
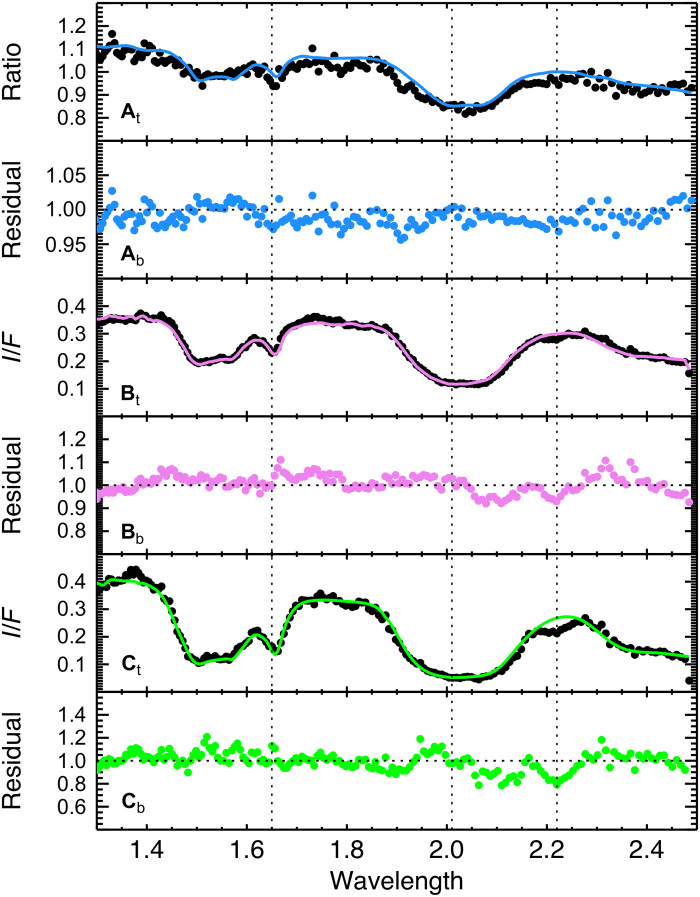
Fig. 4. Radiative transfer model fits to the derived spectra of NH3-rich region of Virgil Fossae.
Model fits (solid line, upper section of each panel) and residuals (lower section of each panel) obtained by dividing Pluto’s ratio of cluster 9 to cluster 1 (At and Ab), Charon’s disk-averaged spectrum (Bt and Bb), and Nix’s disk-averaged spectrum (Ct and Cb) by their corresponding best fits shown as a colored solid line. Models were calculated by means of either Hapke (46) or Shkuratov (47) approaches, including H2O ice and a tholin, but not NH3. Dashed vertical lines point to the wavelengths characteristic of the NH3 spectrum. I/F, reflected intensity divided by incident solar flux.
Comparison to satellites
A number of investigations of Pluto’s largest moon Charon, as well as its smaller moons Nix and Hydra, have reported the presence of NH3 or an ammoniated species revealed as a broad absorption band in the 2.2-μm spectral region (21–25, 10). New Horizons observed Charon and the smaller satellites during the July 2015 flyby with the LEISA spectral imager at a spatial resolution of 4.87 km per pixel for Charon, 3.6 km per pixel for Nix, and 14.5 km per pixel for Hydra. NH3 products were identified on Charon’s surface in specific regions often corresponding to craters (26). In Fig. 4, we compare the NH3 signature of Pluto (Fig. 4A), Charon (Fig. 4B), and Nix (Fig. 4C). The stronger absorption of Pluto’s differential spectrum with respect to that of Charon is visible at 2.2 μm. The 2.2-μm absorption on Pluto and Nix is instead similar in depth and in the shape of the right wing of the band, as seen in the residual spectra, where the absorption on Pluto extends further toward shorter wavelengths. This may be due to the presence of other unidentified material(s) in the Virgil Fossa region. Overall, the consistent location and shape of the 2.2-μm band on Pluto’s surface compared to the satellites demonstrate the strong presence of NH3 and related species in some form on regions of Pluto’s surface.
DISCUSSION
As noted, H2O ice and the unique red coloration are spatially coherent, and the distribution of the H2O-rich pixels, obtained with our multivariate analysis, shows areas where they clump in higher spatial concentration. These areas are located mainly along the Fossa, in a crater east of Elliot crater and possibly in a few patches north of the Fossa and Elliot crater. More isolated pixels are spread south and a few north of the Fossa.
Because H2O ice is stable against evaporation at Pluto’s temperatures, and in view of its sparse distribution across the surface, H2O is regarded as the bedrock mostly covered from view by more volatile ices (N2, CO, and CH4) and non-ice components (tholins) and exposed only in some areas. Layers of more volatile ices alternate in cycles of sublimation and condensation with Pluto’s seasons, contributing to the tenuous atmosphere of the planet (27) and accumulations of one or another of the ices in regions of different surface temperature [e.g., the concentration of N2 ice in Sputnik Planitia (17)].
The coincidence of both H2O and NH3 absorption bands on localized regions of the surface may indicate exposure of NH3 hydrate ice, which naturally forms from crystallization of H2O-NH3 fluids (28), or an ammoniated salt such as NH4Cl or NH4CO3. Whereas in the hydrates, the central wavelength of the broad absorption band is 2.21 to 2.22 μm, in the salts, the broad band is generally centered between ~2.14 and 2.18 μm. Cook et al. (10) found that NH4Cl gives a good fit to the shape and position of the ammonium band in both Nix and Hydra observed with New Horizons. Both of these small bodies also show strong bands of H2O ice in the crystalline phase.
The ammonia or ammonium spectral signature is destroyed as the molecule is dissociated, and knowledge of its durability in Pluto’s space environment is essential to an estimate of the age of the surface in which it is detected. The three principal sources of ammonia dissociation on the surface are UV photons, charged particles from the solar wind, and galactic cosmic rays (GCRs). UV photons come directly from the Sun and also from the interplanetary medium. The Lyman-α flux from the two sources combined is ~7 × 108 photons cm−2 s−1 (29). The Lyman-α flux reaching the surface is modulated by the gaseous CH4 content of the atmosphere. Models of the atmospheric content of CH4 on annual and millennial time scales predict that the transparency of the atmosphere to Lyman-α flux varies from ~0.01 to 10%. Longer-wavelength UV radiation also contributes to the destruction of NH3 (9). When NH3 is dissociated, the fragments can combine quickly with other components in proximity. In a natural system, numerous other molecules and molecular fragments are expected, and in the presence of carbon species, a number of new molecules and radicals will form. Bernstein et al. (30), for example, found that in a mix of H2O:CH3OH:CO:NH3 ices, about half of the N in NH3 was incorporated into complex organic residues after exposures of ~1 × 1020 photons/cm2. At times when Pluto’s atmosphere is 10% transparent to Lyman-α photons, this flux corresponds to a time scale of ~4 × 105 years, indicating a geologically short lifetime. At times of lower atmospheric transparency, the equivalent lifetime for the ammonia is ~4 × 108 years.
Charged particles (e−, H+, and He2+) from the solar wind interact with Pluto’s tenuous atmosphere, and much of the flux is deflected around the planet (31). Some high-energy particles penetrate to the surface, although the flux is variable with atmospheric density, and the interaction with the surface materials is not well established. Both UV photons and solar wind charged particles deposit their energy in the uppermost few micrometers of Pluto’s surface (32), and even for energetic particles, the secondary electrons initiate most of the chemical reactions (33). The spectral evidence reported here also represents the uppermost few micrometers of the surface and corresponds to the optical depth to which near-infrared reflectance spectroscopy is sensitive. Loeffler et al. (9) showed that the spectral signature of NH3 in H2O ice is quickly destroyed by 100-keV protons at 120 K but is much slower at lower temperatures more characteristic of Pluto’s surface (~40 to 60 K). With specific reference to the NH3 signature on Charon, Loeffler et al. (9) estimate that ~40% of the original ammonia has been destroyed over the 4.5–billion year age of the solar system.
GCRs penetrate several meters into a surface of pure H2O ice or a mix of NH3 in H2O ice, depositing energy through secondary and higher-order interactions, ionizing molecules and atoms in their pathways. At the calculated rate of energy deposition for energies >10.5 eV, slightly greater than the dissociation energy of the N─H bond in NH3, the destruction of all NH3 molecules in the upper 1-m layer of an ice composed of 30/70 NH3:H2O occurs in ~1.1 × 109 years. In an ice of this composition with no other impurities, dissociated NH3 can readily recombine with an available electron from an H2O molecule and indefinitely maintain a level of concentration. In a realistic ice, dissociated ammonia fragments will combine with other components, as noted above, and lose their spectral identity. Thus, in a real ice, 109 years can be taken as an upper limit to the longevity of the ammonia signature in the NH3-H2O ice surface in the Virgil Fossae region.
The ammonia spectral signature in ammoniated salts is expected to be more robust against destruction because ionic bonds are stronger than covalent bonds, but we are unaware of direct experimental evidence in support of this assertion. If the ammonia in Virgil Fossae is the signature of ammoniated salts, then the age of its deposition can be greater than if it arises instead from more fragile ammonia hydrates. In addition, if ammonia is replenished in the optical layer by diffusion of NH3 upward through the H2O ice [e.g., (34)], then the age of the initial deposition becomes indeterminate, but greater than if it comes from the hydrates.
While the age of the deposit of red-colored H2O ice in the Virgil Fossae region is not well constrained by the durability of the NH3 signature, it presents the appearance of a geologically recent cryovolcanic eruption or eruptions, emanating from a source of liquid water beneath Pluto’s frozen surface ices (temperature, ~40 K), either now or in the past. In a concentration of ~33% NH3 in liquid H2O, the freezing point is depressed to 176 K. Presumably, such a mixture could remain as a fluid in the interior of Pluto, where the temperature is naturally higher than the surface because of the decay or radioactive elements in the rocky material comprising the majority of Pluto’s mass (35). The liquid H2O–NH3 mixture could be part of a subsurface ocean (20) or a more localized crustal reservoir. Cracks or conduits in the icy crust could be routes of egress for the liquid H2O–NH3 that, upon reaching the vacuum and cold at the surface, both freezes and boils, forming fountains that shower the Fossa surroundings with icy particles.
The blue pixels in Fig. 1C, markers of areas of H2O enriched with NH3, are distributed south for more than 150 km and partly north of Virgil Fossa. They are concentrated as well as in sections of the Fossa itself, a channel through which liquid H2O–NH3, colored with the unique red chromophore, may have flowed from one or more vents before freezing in place. Outside the Fossa, the pixel distribution is suggestive of ejected dissemination of H2O-NH3 ice particles. The deeper H2O bands could be due to differences in grain size where the larger grains might have been deposited closer to the eruption source or sources.
The ice in and around Virgil Fossa, which consists of H2O contaminated with NH3, has a unique red coloration, as seen in Fig. 1B. Non-ice surface materials having pronounced yellow-to-red coloration are thought to be products of energetic processing of CH4 (or other carbon-bearing molecules) and N2 (13) and are chemically similar to a strongly colored refractory organic solid [Pluto ice tholin (PIT)] produced in the laboratory by UV photolysis and charged-particle radiolysis of a mixture of CH4 and N2 ices (36, 37). Energetic processing of the same molecular mixture in the gas phase also produces colored organic tholins (38), and similar chemistry enhanced by the presence of NH3 may have occurred in subsurface liquid reservoirs on Pluto, if dissolved H2CO was also present (39).
The Virgil Fossae complex is part of a tectonic pattern radiating away from Sputnik Planitia and the basin in which it lies (14). The ejection of fluid onto the surface through faults or cracks could be propelled by fluid pressure due to ocean or crustal reservoir freezing (40) and or by gas pressure due to exsolution in a cryovolcanic event, perhaps of the type described by Neveu et al. (41). Both the pattern and the relative freshness of the Virgil Fossa scarp are indicative of geologically relative youth or renewal of faults driven by basin-related stresses (19, 20). Therefore, if cryovolcanism is involved in the creation of the ammonia-rich spectral signature described here, then it is further suggestive of both ongoing tectonism and escape of ammonia-bearing aqueous fluids presumably derived from an internal ocean or from a crustal reservoir ultimately sourced from the ocean.
MATERIALS AND METHODS
Multivariate analysis
To isolate the spectral signature of Virgil Fossa in the LEISA data, we used an unsupervised statistical clustering tool, i.e., a k-means classification tool made unsupervised by means of the Calinski-Harabasz (C-H) criterion (42). The clustering algorithm was applied to the spectral region between ~1.75 and ~2.22 μm. The C-H criterion selected three as the ideal number of clusters to represent the main variations in the area: no data, CH4-rich, and CH4-poor. However, we chose 14 classes of differing spectra (or clusters) in the adopted ROI to highlight subtle differences that go beyond the abundance of CH4 ice. Of these classes, clusters 10 and 12 were too noisy to be considered, and another seven were dominated by absorption bands of CH4 ice and mostly located in the northeast half of the region (colored teal in Fig. 1C). The remaining five classes were spread over the southwest part of the ROI, showing a spectral signature that could not be readily attributed either to an ice or to a refractory material. Figure 1C shows the distribution of the 14 clusters.
We focused our analysis on clusters 1, 2, 5, 8, and 9 whose spectral averages, offset for clarity, are shown in Fig. 3A. All clusters have a SD of 0.08 ≤ σ ≤ 0.10 corresponding to the spectral variation of the spectra in the region adopted by the clustering. The cluster population varies from ~1500 to ~4000 pixels but does not correlate with the SD, confirming the fact that the SD is not a statistical measurement. One of them, cluster 1, included a strong concentration of pixels on and around Virgil Fossa (shown in dark blue in Fig. 1C). Although the spectral signature of this region is similar to those of the other four CH4-poor classes (Fig. 3A), further analysis revealed important variations. To distinguish subtle differences between the average spectrum of Virgil Fossa (cluster 1) and spectra of its surroundings, we compared the spectral signatures by taking the ratio of the spectrum of cluster 9 by that of every other cluster average. Cluster 9 is the class that is geographically most distant from the Fossa and is believed to be the most H2O-ice poor on the basis of H2O distribution maps (Fig. 2B) (18). The resulting spectra (Fig. 3B) display evident H2O ice signatures, particularly for the cluster 1 pixel average. In (Fig. 3B), the spectrum of cluster 9 is not shown again as it is adopted as the standard for comparing the spectra. We note that the trace showing the differential of cluster 8, with very shallow bands, is highlighting pixels that are spectrally very similar to those in cluster 9. Similarly, with the other class averages, the main difference highlighted in the traces is a progression in the depth of H2O ice bands, culminating with the most H2O-rich cluster average, cluster 1, geographically coincident with the main trough of Virgil Fossa.
Upon closer inspection of the differential spectra, the varying depth of the 1.65-μm H2O ice band becomes evident. The depth of this band exceeds that of the 1.5-μm band for all classes, a behavior that is anomalous for pure H2O ice. The spectra are also characterized by a depression of varying strength in the spectral region between 2.1 and 2.25 μm. Bands at these wavelengths are characteristic of NH3 and its stoichiometric hydrates (43, 44) as well as ammoniated salts. NH3 ice has also an absorption band at ~2.0 μm that blends in with the strong H2O band.
Geographical mapping
The spatial distribution of the pixels showing a contribution of NH3 in H2O according to the differential analysis described before is presented in Fig. 1C. It is overlain on a base map obtained from the corresponding LOng Range Reconnaissance Imager (LORRI) observations obtained during the flyby. Figure 1B shows the MVIC map for comparison. The strongest H2O and NH3 signature (blue pixels) are found in and near Virgil Fossa, with a few spread out in the neighboring areas, while regions with the shallowest H2O and NH3 bands (yellow pixels) are located away from Virgil Fossa. The remaining clusters are spread between these two extremes in a geographically homogeneous distribution. The different depths of the H2O bands could be due either to variations in concentration or to the near-infrared path length in the ice. While more precise modeling will be needed to determine which of the two possibilities is correct, for the purpose of this paper, we identified all pixels in the clusters adopted for this study as H2O-rich.
Modeling
To highlight the presence of NH3 ice on Pluto, we calculated a rough model of the differential spectrum of cluster 1 shown in Fig. 3B and corresponding to the blue pixels in Fig. 1C. We used the following three components: H2O ice, amorphous carbon (AC), and a refractory organic solid made by electron radiolysis of an ice mixture of N2, CH4, and CO (37), denoted as PIT, mixed intimately using the Shkuratov computational formulation (45). For the H2O ice component, we used the optical constants in (45) and a grain size of 3 μm, contributing 83.5% to the mixture. The low-reflectance components, AC in 20-μm grains and PIT in grains of 15 μm, contributed at concentrations of 1.5 and 15%, respectively. PIT was produced in the laboratory by Materese et al. (37), and its optical constants were extracted from the reflectance spectrum. We note that the adopted grain size of the H2O ice is very small and marginally acceptable in terms of the geometrical optics assumptions in the Shkuratov formulation. However, the purpose of the model is purely to highlight the presence of NH3, and therefore, the relative abundances and grain sizes of the components included in the model should not be used in a quantitative way. We compared the model to the observations by calculating the residuals, shown in Fig. 4Ab along with corresponding spectra, models, and residuals of Charon and the smaller satellite Nix. Pluto’s residual spectrum, obtained by dividing the observations by the model, shows a clear dip at both 1.65 and 2.2 μm. The 2.2-μm depression is wider than expected from contributions of pure NH3 and NH3 in H2O (hydrates) and might include signatures of other compounds presently unidentified.
The 1.5- and 2.0-μm bands characteristic of a NH3 signature are somewhat inconsistent. The NH3 2.0-μm band is blended with the H2O band, which is a much stronger feature in the H2O than in the NH3 spectrum. For this reason, it is difficult to disentangle the two contributions, even with a modeling approach. In our model (Fig. 4At), the residual spectrum shows a value close to 1 at 2.0 μm, indicative of a good fit to the depth of the band. The model is imperfect, however, leaving small discrepancies in band strength and continuum levels that might indicate the presence of another component aside from NH3, presently unidentified. For example, the 1.65-μm band is too strong in the data when compared to a pure crystalline H2O ice spectrum. Methane has a band close to this wavelength, but the lack of other stronger CH4 bands in our data excludes this possibility.
Modeling the spectra of the satellites
Radiative transfer models based on Hapke or Shkuratov approaches have been applied to Charon (26) and Pluto’s small satellites Nix and Hydra (10). Hapke as well as Shkuratov theory (46, 47) rely on knowing the optical constants of each material included in the model. However, optical constants for ammonia hydrates are poorly known for several reasons, including the coarse wavelength sampling, the lack of data in the relevant wavelength range, and also the detailed structure (stoichiometry) of the material. Optical constants for ammoniated salts have not, to our knowledge, been measured. To model these spectra, the data were given lower statistical weight over the wavelength range of 2.18 to 2.24 μm, and the models consist of a mixture of amorphous and crystalline H2O ice and PIT. The difference between the model and the observations highlighted the large absorption feature with a minimum at 2.21 μm, consistent with ammonia hydrates and ammoniated salts (e.g., NH4Cl), all of which show the ammonia band at a slightly longer wavelength than pure NH3.
Supplementary Material
Acknowledgments
Funding: D.P.C., S.P., C.M.D.O., and other New Horizons team members acknowledge support from NASA’s New Horizons Project. S.P, also gratefully acknowledges NASA grant no. NNX16AC83G for partial funding that supported this work. B.S. acknowledges the Centre National d’Etudes Spatiales (CNES) for its financial support through its “Système Solaire” program. Author contributions: C.M.D.O. and D.P.C.: data processing and evaluation; S.P., F.S., B.S., and J.C.C.: modeling of spectra; W.B.M., O.M.U., K.N.S., J.M.M., J.C.C., A.H.P., and S.A.S.: interpretation of geological setting; W.M.G., A.V., and C.B.O.: preparation of the initial LEISA datasets and interpretation of the spectra; H.A.W., L.A.Y., and K.E.: critical to New Horizons operations before and during spacecraft encounter with Pluto. All members of the New Horizons Surface Composition Science Theme Team contributed to the overall planning and execution of the mission and to the acquisition and processing of the data, and laid the foundation for understanding of Pluto’s composition and geology upon which the results present here are based. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper. New Horizons spacecraft data are deposited in the NASA Planetary Data System (PDS) without restrictions. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/5/eaav5731/DC1
List of members of the New Horizons Surface Composition Science Theme Team.
Contributor Information
Collaborators: New Horizons Surface Composition Science Theme Team
REFERENCES AND NOTES
- 1.Öberg K. I., Boogert A. C. A., Pontoppidan K. M., The Spitzer ice legacy: Ice evolution from cores to protostars. Astrophys. J. 740, 109 (2011). [Google Scholar]
- 2.Mumma M. J., Charnley S. B., The chemical composition of comets—Emerging taxonomies and natal heritage. Annu. Rev. Astron. Astrophys. 49, 471–524 (2011). [Google Scholar]
- 3.O’D. Alexander C. M., Nittler L. R., Davidson J., Ciesla F. J., Measuring the level of interstellar inheritance in the solar protoplanetary disk. Meteorit. Planet. Sci. 52, 1797–1821 (2017). [Google Scholar]
- 4.Pizzarello S., Williams L. B., Ammonia in the early solar system: An account from carbonaceous meteorites. Astrophys. J. 749, 161 (2012). [Google Scholar]
- 5.Neveu M., Desch S. J., Castillo-Rogez J. C., Aqueous geochemistry in icy world interiors: Equilibrium fluid, rock, and gas compositions, and fate of antifreezes and radionuclides. Geochim. Cosmochim. Acta 212, 324–371 (2017). [Google Scholar]
- 6.Watson K., Murray B. C., Brown H., The stability of volatiles in the solar system. Icarus 1, 317–327 (1963). [Google Scholar]
- 7.Lebofsky L. A., Stability of frosts in the solar system. Icarus 25, 205–217 (1975). [Google Scholar]
- 8.Moore M. H., Ferrante R. F., Hudson R. L., Stone J. N., Ammonia–water ice laboratory studies relevant to outer solar system surfaces. Icarus 190, 260–273 (2007). [Google Scholar]
- 9.Loeffler M. J., Raut U., Baragiola R. A., Radiation chemistry in ammonia-water ices. J. Chem. Phys. 132, 054508 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Cook J. C., Dalle Ore C. M., Protopapa S., Binzel R. P., Cartwright R., Cruikshank D. P., Earle A., Grundy W. M., Ennico K., Howett C., Jennings D. E., Lunsford A. W., Olkin C. B., Parker A. H., Philippe S., Reuter D., Schmitt B., Stansberry J. A., Alan Stern S., Verbiscer A., Weaver H. A., Young L. A., Composition of Pluto’s small satellites: Analysis of New Horizons spectral images. Icarus 315, 30–45 (2018). [Google Scholar]
- 11.Reuter D. C., Stern S. A., Scherrer J., Jennings D. E., Baer J. W., Hanley J., Hardaway L., Lunsford A., McMuldroch S., Moore J., Olkin C., Parizek R., Reitsma H., Sabatke D., Spencer J., Stone J., Throop H., van Cleve J., Weigle G. E., Young L. A., Ralph: A visible/infrared imager for the New Horizons Pluto/Kuiper Belt mission. Space Sci. Rev. 140, 129–154 (2008). [Google Scholar]
- 12.Stern S. A., Bagenal F., Ennico K., Gladstone G. R., Grundy W. M., McKinnon W. B., Moore J. M., Olkin C. B., Spencer J. R., Weaver H. A., Young L. A., Andert T., Andrews J., Banks M., Bauer B., Bauman J., Barnouin O. S., Bedini P., Beisser K., Beyer R. A., Bhaskaran S., Binzel R. P., Birath E., Bird M., Bogan D. J., Bowman A., Bray V. J., Brozovic M., Bryan C., Buckley M. R., Buie M. W., Buratti B. J., Bushman S. S., Calloway A., Carcich B., Cheng A. F., Conard S., Conrad C. A., Cook J. C., Cruikshank D. P., Custodio O. S., Dalle Ore C. M., Deboy C., Dischner Z. J. B., Dumont P., Earle A. M., Elliott H. A., Ercol J., Ernst C. M., Finley T., Flanigan S. H., Fountain G., Freeze M. J., Greathouse T., Green J. L., Guo Y., Hahn M., Hamilton D. P., Hamilton S. A., Hanley J., Harch A., Hart H. M., Hersman C. B., Hill A., Hill M. E., Hinson D. P., Holdridge M. E., Horanyi M., Howard A. D., Howett C. J. A., Jackman C., Jacobson R. A., Jennings D. E., Kammer J. A., Kang H. K., Kaufmann D. E., Kollmann P., Krimigis S. M., Kusnierkiewicz D., Lauer T. R., Lee J. E., Lindstrom K. L., Linscott I. R., Lisse C. M., Lunsford A. W., Mallder V. A., Martin N., McComas D. J., McNutt R. L. Jr., Mehoke D., Mehoke T., Melin E. D., Mutchler M., Nelson D., Nimmo F., Nunez J. I., Ocampo A., Owen W. M., Paetzold M., Page B., Parker A. H., Parker J. W., Pelletier F., Peterson J., Pinkine N., Piquette M., Porter S. B., Protopapa S., Redfern J., Reitsema H. J., Reuter D. C., Roberts J. H., Robbins S. J., Rogers G., Rose D., Runyon K., Retherford K. D., Ryschkewitsch M. G., Schenk P., Schindhelm E., Sepan B., Showalter M. R., Singer K. N., Soluri M., Stanbridge D., Steffl A. J., Strobel D. F., Stryk T., Summers M. E., Szalay J. R. Jr., Tapley M., Taylor A., Taylor H., Throop H. B., Tsang C. C. C., Tyler G. L., Umurhan O. M., Verbiscer A. J., Versteeg M. H., Vincent M., Webbert R., Weidner S., Weigle G. E., White O. L., Whittenburg K., Williams B. G., Williams K., Williams S., Woods W. W., Zangari A. M., Zirnstein E., The Pluto system: Initial results from its exploration by New Horizons. Science 350, aad1815 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Grundy W. M., Binzel R. P., Buratti B. J., Cook J. C., Cruikshank D. P., Dalle Ore C. M., Earle A. M., Ennico K., Howett C. J. A., Lunsford A. W., Olkin C. B., Parker A. H., Philippe S., Protopapa S., Quirico E., Reuter D. C., Schmitt B., Singer K. N., Verbiscer A. J., Beyer R. A., Buie M. W., Cheng A. F., Jennings D. E., Linscott I. R., Parker J. W., Schenk P. M., Spencer J. R., Stansberry J. A., Stern S. A., Throop H. B., Tsang C. C. C., Weaver H. A., Weigle G. E. II, Young L. A.; New Horizons Science Team , Surface compositions across Pluto and Charon. Science 351, aad9189 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Moore J. M., McKinnon W. B., Spencer J. R., Howard A. D., Schenk P. M., Beyer R. A., Nimmo F., Singer K. N., Umurhan O. M., White O. L., Stern S. A., Ennico K., Olkin C. B., Weaver H. A., Young L. A., Binzel R. P., Buie M. W., Buratti B. J., Cheng A. F., Cruikshank D. P., Grundy W. M., Linscott I. R., Reitsema H. J., Reuter D. C., Showalter M. R., Bray V. J., Chavez C. L., Howett C. J. A., Lauer T. R., Lisse C. M., Parker A. H., Porter S. B., Robbins S. J., Runyon K., Stryk T., Throop H. B., Tsang C. C. C., Verbiscer A. J., Zangari A. M., Chaikin A. L., Wilhelms D. E.; New Horizons Science Team , The geology of Pluto and Charon through the eyes of New Horizons. Science 351, 1284–1293 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Olkin C. B., Spencer J. R., Grundy W. M., Parker A. H., Beyer R. A., Schenk P. M., Howett C. J. A., Stern S. A., Reuter D. C., Weaver H. A., Young L. A., Ennico K., Binzel R. P., Buie M. W., Cook J. C., Cruikshank D. P., Dalle Ore C. M., Earle A. M., Jennings D. E., Singer K. N., Linscott I. E., Lunsford A. W., Protopapa S., Schmitt B., Weigle E.; New Horizons Science Team , The global color of Pluto from New Horizons. Astron. J. 154, 258 (2017). [Google Scholar]
- 16.J. C. Cook, D. P. Cruikshank, C. M. Dalle Ore, K. Ennico, W. M. Grundy, C. B. Olkin, S. Protopapa, S. A. Stern, H. A. Weaver, L. A. Young; New Horizons surface composition theme team, The identification and distribution of Pluto’s non-volatile inventory, in 47th Lunar Planetary Science Conference, The Woodlands, Texas, 21 to 26 March 2016. [Google Scholar]
- 17.Protopapa S., Grundy W. M., Reuter D. C., Hamilton D. P., Dalle Ore C. M., Cook J. C., Cruikshank D. P., Schmitt B., Philippe S., Quirico E., Binzel R. P., Earle A. M., Ennico K., Howett C. J. A., Lunsford A. W., Olkin C. B., Parker A., Singer K. N., Stern S. A., Verbiscer A. J., Weaver H. A., Young L. A., Pluto’s global surface composition through pixel-by-pixel Hapke modeling of New Horizons Ralph/LEISA data. Icarus 287, 218–228 (2017). [Google Scholar]
- 18.Schmitt B., Philippe S., Grundy W. M., Reuter D. C., Cote R., Quirico E., Protopapa S., Young L. A., Binzel R. P., Cook J. C., Cruikshank D. P., Dalle Ore C. M., Earle A. M., Ennico K., Howett C. J. A., Jennings D. E., Linscott I. R., Lunsford A. W., Olkin C. B., Parker A. H., Singer K. N., Spencer J. R., Stansberry J., Stern S. A., Tsang C. C. C., Verbiscer A. J., Weaver H. A.; New Horizons Science Team , Physical state and distribution of materials at the surface of Pluto from New Horizons LEISA imaging spectrometer. Icarus 287, 229–260 (2017). [Google Scholar]
- 19.Keane J. T., Matsuyama I., Kamata S., Steckloff J. K., Reorientation and faulting of Pluto due to volatile loading within Sputnik Planitia. Nature 540, 90–93 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Nimmo F., Hamilton D. P., McKinnon W. B., Schenk P. M., Binzel R. P., Bierson C. J., Beyer R. A., Moore J. M., Stern S. A., Weaver H. A., Olkin C. B., Young L. A., Smith K. E.; New Horizons Geology, Geophysics &Imaging Theme Team , Reorientation of Sputnik Planitia implies a subsurface ocean on Pluto. Nature 540, 94–96 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Buie M. W., Grundy W. M., The Distribution and physical state of H2O on Charon. Icarus 148, 324–339 (2000). [Google Scholar]
- 22.Brown M. E., Calvin W. M., Evidence for crystalline water and ammonia ices on Pluto’s satellite Charon. Science 287, 107–109 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Dumas C., Terrile R. J., Brown R. H., Schneider G., Smith B. A., Hubble Space Telescope NICMOS spectroscopy of Charon’s leading and trailing hemispheres. Astron. J. 121, 1163–1170 (2001). [Google Scholar]
- 24.Cook J. C., Desch S. J., Roush T. L., Trujillo C. A., Geballe T. R., Near-infrared spectroscopy of Charon: Possible evidence for cryovolcanism on Kuiper Belt objects. Astrophys. J. 663, 1406–1419 (2007). [Google Scholar]
- 25.A. J. Verbiscer, D. E. Peterson, M. F. Skrutskie, M. Cushing, M. J. Nelson, J. D. Smith,J. C. Wilson, Simultaneous spatially-resolved near-infrared spectra of Pluto and Charon, in 38th Lunar Planetary Science Conference XXXVIII, League City, Texas 12 to 16 March 2007. [Google Scholar]
- 26.Dalle Ore C. M., Protopapa S., Cook J. C., Grundy W. M., Cruikshank D. P., Verbiscer A. J., Ennico K., Olkin C. B., Stern S. A., Weaver H. A., Young L. A.; New Horizons Science Team , Ices on Charon: Distribution of H2O and NH3 from New Horizons LEISA observations. Icarus 300, 21–32 (2017). [Google Scholar]
- 27.Gladstone G. R., Stern S. A., Ennico K., Weaver H. A., Young L. A., Summers M. E., Strobel D. F., Hinson D. P., Kammer J. A., Parker A. H., Steffl A. J., Linscott I. R., Parker J. W., Cheng A. F., Slater D. C., Versteeg M. H., Greathouse T. K., Retherford K. D., Throop H., Cunningham N. J., Woods W. W., Singer K. N., Tsang C. C. C., Schindhelm E., Lisse C. M., Wong M. L., Yung Y. L., Zhu X., Curdt W., Lavvas P., Young E. F., Tyler G. L.; New Horizons Science Team , The atmosphere of Pluto as observed by New Horizons. Science 351, aad8866 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Uras N., Devlin J. P., Rate study of ice particle conversion to ammonia hemihydrate: Hydrate crust nucleation and NH3 diffusion. J. Phys. Chem. A 104, 5770–5777 (2000). [Google Scholar]
- 29.G. R. Gladstone, W. R. Pryor, S. A. Stern, K. Ennico, C. B. Olkin, J. R. Spencer, H. A. Weaver, L. A. Young, F. Bagena, A. F. Chen, N. J. Cunningham, H. A. Elliott, T. K. Greathouse,D. P. Hinson, J. A. Kammer, I. R. Linscott, J. W. Parker, K. D. Retherford, A. J. Steff,D. F. Strobel, M. E. Summers, H. Throop, M. H. Versteeg, M. W. Davis, The Lyman; α sky background as observed by New Horizons. Geophys. Res. Lett. 45, 8022–8028 2018 10.1029/2018GL078808. [DOI]
- 30.Bernstein M. P., Sandford S. A., Allamandola L. J., H, C, N, and O isotopic substitution studies of the 2165 wavenumber (4.62 micron) “XCN” Feature produced by ultraviolet photolysis of mixed molecular ices. Astrophys. J. 542, 894–897 (2000). [Google Scholar]
- 31.McComas D. J., Elliott H. A., Weidner S., Valek P., Zirnstein E. J., Bagenal F., Delamere P. A., Ebert R. W., Funsten H. O., Horanyi M., McNutt R. L., Moser C., Schwadron N. A., Strobel D. F., Young L. A., Ennico K., Olkin C. B., Stern S. A., Weaver H. A., Pluto’s interaction with the solar wind. J. Geophys. Res. Space Phys. 121, 4232–4246 (2016). [Google Scholar]
- 32.Bennett C. J., Pirim C., Orlando T. M., Space-weathering of solar system bodies:A laboratory perspective. Chem. Rev. 113, 9086–9150 (2013). [DOI] [PubMed] [Google Scholar]
- 33.R. L. Hudson, M. E. Palumbo, G. Strazzulla, M. H. Moore, J. F. Cooper,S. J. Sturner, Laboratory studies of the chemistry of transneptunian object surface materials, in The Solar System Beyond Neptune, M. A. Barucci, H. Boehnhardt, D. P. Cruikshank, A. Morbidelli, Eds. (University of Arizona Press, 2009), pp. 507–523. [Google Scholar]
- 34.Holler B. J., Young L. A., Buie M. W., Grundy W. M., Lyke J. E., Young E. F., Roe H. G., Measuring temperature and ammonia hydrate ice on Charon in 2015 from Keck/OSIRIS spectra. Icarus 284, 394–406 (2017). [Google Scholar]
- 35.McKinnon W. B., Stern S. A., Weaver H. A., Nimmo F., Bierson C. J., Cook J. C., Grundy W. M., Cruikshank D. P., Parker A. H., Moore J. M., Spencer J. R., Young L. A., Olkin C. B., Smith K. E.; New Horizons Geology, Geophysics & Imaging and Composition Theme Teams , Origin of the Pluto-Charon system: Constraints from the New Horizons flyby. Icarus 287, 2–11 (2017). [Google Scholar]
- 36.Materese C. K., Cruikshank D. P., Sandford S. A., Imanaka H., Nuevo M., White D., Ice chemistry on outer solar system bodies: Carboxylic acids, nitriles, and urea detected in refractory residues produced from the UV Photolysis of N2:CH4:Co-containing ices. Astrophys. J. 788, 111 (2014). [Google Scholar]
- 37.Materese C. K., Cruikshank D. P., Sandford S. A., Imanaka H., Nuevo M., Ice chemistry on outer solar system bodies: Electron radiolysis of N2-, CH4-, and CO-containing ices. Astrophys. J. 812, 150 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imanaka H., Khare B. N., Elsila J. E., Bakes E. L. O., McKay C. P., Cruikshank D. P., Sugita S., Matsui T., Zare R. N.. Laboratory experiments of Titan tholin formed in cold plasma at various pressures: Implications for nitrogen-containing polycyclic aromatic compounds in Titan haze. Icarus 168, 344–366 (2004). [Google Scholar]
- 39.Kebukawa Y., Cody G. D., A kinetic study of the formation of organic solids from formaldehyde: Implications for the origin of extraterrestrial organic solids in primitive Solar System objects. Icarus 248, 412–423 (2015). [Google Scholar]
- 40.Manga M., Wang C.-Y., Pressurized oceans and the eruption of liquid water on Europa and Enceladus. Geophys. Res. Lett. 34, L07202 (2007). [Google Scholar]
- 41.Neveu M., Desch S. J., Shock E. L., Glein C. R., Prerequisites for explosive cryovolcanism on dwarf planet-class Kuiper belt objects. Icarus 246, 48–64 (2015). [Google Scholar]
- 42.Caliński T., Harabasz J., A dendrite method for cluster analysis. Commun. Stat. 3, 1–27 (1974). [Google Scholar]
- 43.Sill G., Fink U., Ferraro J. R., Absorption coefficients of solid NH3 from 50 to 7000 cm−1. J. Opt. Soc. Am. 70, 724–730 (1980). [Google Scholar]
- 44.U. Fink, G. T. Sill, The infrared spectral properties of frozen volatiles, in Comets, L. L. Wilkening, Ed. (University of Arizona Press, 1982), pp. 164–202. [Google Scholar]
- 45.Mastrapa R. M. E., Bernstein M. P., Sandford S. A., Roush T. L., Cruikshank D. P., Dalle Ore C. M., Optical constants of amorphous and crystalline H2O-ice in the near infrared from 1.1 to 2.6 μm. Icarus 197, 307–320 (2008). [Google Scholar]
- 46.B. Hapke, Theory of Reflectance and Emittance (Topics in Remote Sensing) (Cambridge Univ. Press, 1993). [Google Scholar]
- 47.Shkuratov Y., Starukhina L., Hoffmann H., Gabriel A., A model of spectral albedo of particulate surfaces: Implications for optical properties of the Moon. Icarus 137, 235–246 (1999). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/5/eaav5731/DC1
List of members of the New Horizons Surface Composition Science Theme Team.