Abstract
Pulmonary arterial hypertension (PAH) is known as one of diseases with the worst prognosis. Recently, targeted PAH drugs have been developed and approved for use; therefore, the treatment strategy and goals have changed, and the prognosis has improved over two decades. We reviewed the case of a female who showed the natural disease course of heritable PAH in treatment with the targeted PAH drugs under the Korean Health Insurance policy. At the age of 15, she visited the outpatient clinic for dyspnea on exertion that occurred 3 years ago. At that time, severe pulmonary hypertension was revealed by an echocardiography and there was no evidence of significant shunt lesion or embolism. After 4 years of loss to follow-up, her performance was WHO functional class III and she still suffered from dyspnea. The initial monotherapy using an endothelin receptor antagonist was started in 2008. After 2 years, BMPR 2 mutation was detected. Her clinical symptoms gradually worsened because of poor compliance. To escalate therapy, combination therapy was given, and finally, triple maximal therapy was maintained. The next step is to consider intravenous prostanoids. Various combinations of targeted therapy have been tried, and several trials have been confirmed that improve the prognosis. Initial upfront combination therapy and a more enthusiastic approach make good a better prognosis. In this area, active support of the government insurance policy is indispensable in Korea.
Keywords: Pulmonary arterial hypertension; Combination therapy; Bone morphogenetic protein receptors, type II
Letter to the Editor
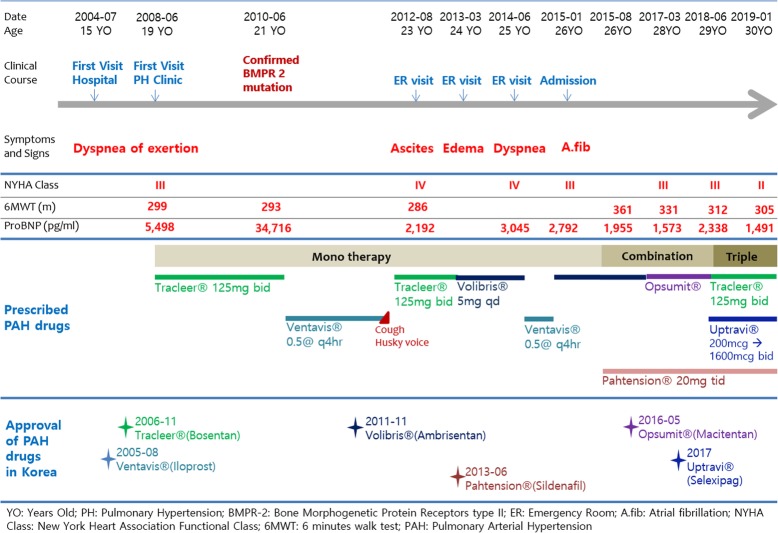
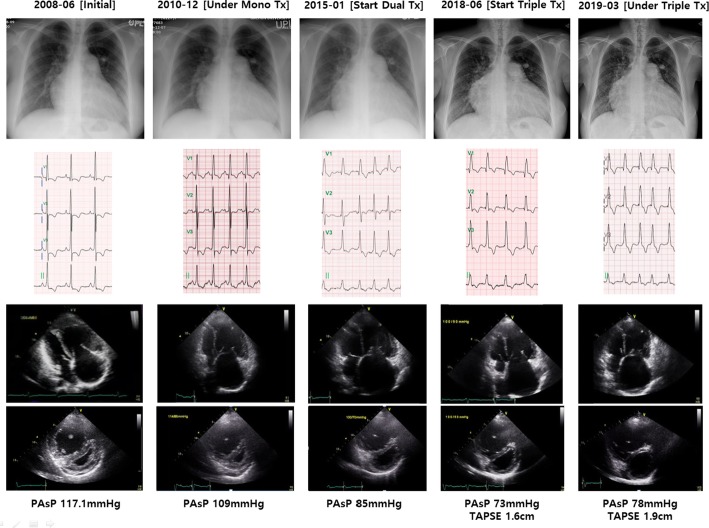
Pulmonary arterial hypertension (PAH) is known to be one of diseases with the worst prognosis. Forty years ago, the American national study reported that the estimated median survival of 194 patients who were diagnosed the primary pulmonary hypertension was 2.8 years [1]. However, the advent of targeted PAH drugs have opened a new era [2, 3]. According to evidence based studies [4–9], the drugs have been developed and approved for use, therefore, the treatment strategy and goals have changed to sequential combination therapy and upfront therapy [10–13]. The prognosis has remarkably improved over two decades [14]. The confidential treatment guidelines are proposed according to the individual risk stratification and the precise diagnosis and classification as deep-phenotyping [15]. However, the guidelines may not be similarly implemented in each country because of each government’s insurance policy. We report here on a case of a female who showed the natural disease course of heritable PAH in treatment with the targeted PAH drugs under the Korean Health Insurance policy. At the age of 15, she visited the outpatient clinic for dyspnea on exertion that initially occurred 3 years before. At that time, severe pulmonary hypertension was revealed by echocardiography and there was no evidence of significant shunt lesion or embolism. After 4 years of loss to follow-up, her performance was WHO Functional Class III as a more aggravated functional state, and she still suffered from severe PAH related symptoms. The right heart catheterization and work-up for risk stratification were performed. The mean pulmonary arterial pressure detected 83 mmHg and the vaso-reactivity tests under inhaled oxygen and iloprost were all negative, respectively. The calculated pulmonary vascular resistance was 2261.6 dyne∙sec∙cm− 5. The initial monotherapy, using an endothelin receptor antagonist, was started in 2008. After 2 years, the bone morphogenetic protein receptors (BMPR) type II mutation was confirmed. She carried exon 6 c.631C > T nonsense mutation. Her clinical symptoms gradually worsened because of poor compliance. There were some minor complications as dry cough and changing her voice, but we maintained medications and encouraged the patient. Even though much effort was given, her clinical symptoms deteriorated. To escalate therapy, combination therapy was given and finally triple maximally therapy was maintained (Fig. 1). The serial chest radiogram, electrocardiogram, and trans-thoracic echocardiography showed improvement after sequential combination therapy was administered (Fig. 2). The next step is to consider intravenous prostanoids. Unfortunately, in Korea, we have no further options. To improve prognosis, diagnosis of early disease detection and aggressive early treatment is needed. Especially, intravenous prostanoids are recommended to high risk patients, and are shown to improve outcomes [16]. For example, Japan has a remarkably good prognosis for PAH [17, 18]. What made this outcome possible is the liberal, applicable, targeted drug usage for variable situations to manage PAH patients. Upfront combination therapy and more enthusiastic approaches improve prognosis [19]. In this area, active support of the government insurance policy is indispensable and the most potent factor for improving prognosis. In conclusion, we can propose a good prognosis by appropriate targeted drugs treating the patients with pulmonary hypertension under the supportive government policy.
Fig. 1.
Chronicle of the patient’s clinical features and treatments of PAH specific drugs
Fig. 2.
Serial changes of chest radiogram, electrocardiography and trans-thoracic echocardiography
Acknowledgements
We thank Mi Ju You, RN. and Gyeong-Lim Hyun, BS. for the support of this manuscript.
Disclosures
None.
Abbreviations
- BMPR 2
Bone morphogenetic protein receptor type 2 gene
- PAH
Pulmonary arterial hypertension
- WHO
World Health Organization functional class
Authors’ contributions
KJA drafted the manuscript. AYJ revised the manuscript. WJC conceived of the case, and participated in coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by grants (2018-ER6304–00 and 2018-ER6304–01 by Research of Korea Centers for Disease Control and Prevention).
Availability of data and materials
Not applicable
Ethics approval and consent to participate
This case report was complied with the Declaration of Helsinki (6th revision).
Consent for publication
Written informed consent was obtained from the patient for publication of their individual details and accompanying images in this manuscript. The consent form is held by the authors’ institution and is available for review by the Editor-in-Chief.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2.Park YM, Chung WJ, Choi DY, Baek HJ, Jung SH, Choi IS, Shin EK. Functional class and targeted therapy are related to the survival in patients with pulmonary arterial hypertension. Yonsei Med J. 2014;55(6):1526–1532. doi: 10.3349/ymj.2014.55.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung WJ, Park YB, Jeon CH, Jung JW, Ko KP, Choi SJ, Seo HS, Lee JS, Jung HO, Investigators K. Baseline characteristics of the Korean registry of pulmonary arterial hypertension. J Korean Med Sci. 2015;30(10):1429–1438. doi: 10.3346/jkms.2015.30.10.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(6):800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 6.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347(5):322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 7.Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, Wasserman K, Oudiz R, Shapiro S, Robbins IM, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41(12):2119–2125. doi: 10.1016/S0735-1097(03)00463-7. [DOI] [PubMed] [Google Scholar]
- 8.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358(9288):1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 9.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 10.Humbert M, Barst RJ, Robbins IM, Channick RN, Galie N, Boonstra A, Rubin LJ, Horn EM, Manes A, Simonneau G. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24(3):353–359. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 11.Sitbon O, Sattler C, Bertoletti L, Savale L, Cottin V, Jais X, De Groote P, Chaouat A, Chabannes C, Bergot E, et al. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J. 2016;47(6):1727–1736. doi: 10.1183/13993003.02043-2015. [DOI] [PubMed] [Google Scholar]
- 12.van de Veerdonk Mariëlle C., Huis in t Veld Anna E., Marcus J. Tim, Westerhof Nico, Heymans Martijn W., Bogaard Harm-Jan, Vonk-Noordegraaf Anton. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. European Respiratory Journal. 2017;49(6):1700007. doi: 10.1183/13993003.00007-2017. [DOI] [PubMed] [Google Scholar]
- 13.Fox BD, Shtraichman O, Langleben D, Shimony A, Kramer MR. Combination therapy for pulmonary arterial hypertension: a systematic review and meta-analysis. Can J Cardiol. 2016;32(12):1520–1530. doi: 10.1016/j.cjca.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Tamura Y, Kumamaru H, Satoh T, Miyata H, Ogawa A, Tanabe N, Hatano M, Yao A, Abe K, Tsujino I, et al. Effectiveness and outcome of pulmonary arterial hypertension-specific therapy in Japanese patients with pulmonary arterial hypertension. Circ J. 2017;82(1):275–282. doi: 10.1253/circj.CJ-17-0139. [DOI] [PubMed] [Google Scholar]
- 15.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 16.Tokunaga N, Ogawa A, Ito H, Matsubara H. Rapid and high-dose titration of epoprostenol improves pulmonary hemodynamics and clinical outcomes in patients with idiopathic and heritable pulmonary arterial hypertension. J Cardiol. 2016;68(6):542–547. doi: 10.1016/j.jjcc.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Matsubara H, Ogawa A. Treatment of idiopathic/hereditary pulmonary arterial hypertension. J Cardiol. 2014;64(4):243–249. doi: 10.1016/j.jjcc.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa A, Ejiri K, Matsubara H. Long-term patient survival with idiopathic/heritable pulmonary arterial hypertension treated at a single center in Japan. Life Sci. 2014;118(2):414–419. doi: 10.1016/j.lfs.2014.01.077. [DOI] [PubMed] [Google Scholar]
- 19.Grignola JC. Up-front combination therapy in pulmonary arterial hypertension: from clinical trials to ‘real world’ observational studies. Int J Cardiol. 2014;173(3):349–350. doi: 10.1016/j.ijcard.2014.03.095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable