Abstract
Background
Treatment-resistant depression (TRD) may represent a substantial proportion of major depressive disorder (MDD); however, the risk of mortality in TRD is still incompletely assessed.
Methods
Data were obtained from Optum Clinformatics™ Extended, a US claims database. Date of the first antidepressant (AD) dispensing was designated as the index date for study entry and 6 months prior to that was considered the baseline period. Patients with MDD aged ≥ 18 years, index date between January 1, 2008 and September 30, 2015, no AD claims during baseline, and continuous enrollment in the database during baseline were included. Patients who started a third AD regimen after two regimens of appropriate duration were included in the TRD cohort. All-cause mortality was compared between patients with TRD and non-TRD MDD using a proportional hazards model and Kaplan–Meier estimate with TRD status being treated as a time-varying covariate. The model was adjusted for study year, age, gender, depression diagnosis, substance use disorder, psychiatric comorbidities, and Charlson comorbidity index.
Results
Out of 355,942 patients with MDD, 34,176 (9.6%) met the criterion for TRD. TRD was associated with a significantly higher mortality compared with non-TRD MDD (adjusted HR: 1.29; 95% CI 1.22–1.38; p < 0.0001). Survival time was significantly shorter in the TRD cohort compared with the non-TRD MDD cohort (p < 0.0001).
Conclusions
Patients with TRD had a higher all-cause mortality compared with non-TRD MDD patients.
Keywords: Major depressive disorder, Mortality, Treatment-resistant depression
Background
Approximately 4.4% of the world’s population suffers from depression at any given time, making depression the largest cause of disability world-wide (7.5% of all years lived with disability [YLDs]) [1]. In the US, depression is the second largest contributor to YLDs, after back pain [2]. Among American adults, the life-time prevalence of major depressive disorder (MDD) is 20.6% [3]. Extensive research over decades has found an association between depression and increased mortality [4–9]. A recent meta-analysis found that depression was associated with a 50% increase in mortality [10].
Though depression is a known risk factor for suicide [11], suicide alone does not entirely explain the increased mortality in depression. Depression may elicit pathophysiological changes such as peripheral inflammation, oxidative stress, and cardio-metabolic conditions that contribute to the development of chronic somatic diseases that increase the risk of mortality [5]. Various effects of depression, such as decreased treatment adherence, sedentary lifestyle, smoking, unhealthy diet, and other mental comorbidities may also contribute to the increased mortality [5]. Moreover, in patients with pre-existing chronic diseases, comorbid depression may be an independent risk factor for mortality [12–16].
Treatment-resistant depression (TRD) may represent up to 60% of patients with depression, depending on the response or remission criteria [17]. Results of the sequenced treatment alternatives to relieve depression (STAR*D) trial showed a 50% cumulative remission rate in outpatients with MDD after two different antidepressant (AD) regimens of adequate dose and duration, and the likelihood of remission substantially decreased after two regimens [18, 19]. The failure of two adequate AD regimens is a common criterion for defining TRD [20, 21].
The literature has suggested that TRD may be associated with an increased risk of mortality. Based on a review of clinical studies, Carney et al. concluded that TRD is associated with a higher cardiovascular mortality as compared with treatment responders [23]. In a Swedish study, Reutfors et al. reported that patients with TRD had a 35% higher all-cause mortality than non-TRD MDD patients [24]. However, the association seems complex, as several risk factors for TRD, as well as several detrimental outcomes from TRD, may themselves be associated with increased mortality. Among these are social and functional impairment, comorbidities such as substance use disorders (SUD), anxiety disorders, and personality disorders, frequent and recurrent episodes of depression, and frequent hospitalizations [17, 18, 22].
Taken together, the available body of research suggests that patients with TRD may have a higher all-cause-mortality than other patients with MDD. This association should be studied in different populations to strengthen the body of evidence and explore the generalizability. Such investigations should ideally be conducted in large-sized cohorts with data that allows for long term follow-up, and which contains information on socio-demographic and clinical variables known to be associated both with TRD and with increased mortality. Therefore, the current study was conducted to estimate the all-cause mortality in TRD patients compared with non-TRD MDD patients using administrative claims data in the US.
Methods
Data source
Data for the current analysis were obtained from Optum Clinformatics™ Extended Data Mart (CEDM), a claims database that contains covered lives with combined benefit structure that includes both medical and prescription coverage. The CEDM database captures information regarding health care costs, resource utilization, quality, and effectiveness. The database includes approximately 15 million covered affiliate lives annually. The population is heavily weighted to a commercial health plan population, but also includes a Medicare Advantage population.
The CEDM also contains mortality data of patients. The National Technical Information Service (NTIS) receives Death Master File (DMF) data from the Social Security Administration (SSA) and disseminates them on behalf of SSA. Effective November 1, 2011, the source of the mortality data changed to the NTIS’s Limited Access DMF, which contains data on decedents who died fewer than 3 years ago. So, death reports were missing from the data when death occurs outside medical facilities after November 1, 2011. Therefore, these data may not be suitable for absolute mortality estimates; nevertheless, assuming the data are missing at random with respect to TRD status, they are useful for comparative assessment.
Study design and sample selection
Date of the first AD dispensing was considered as the index date for inclusion in the cohort. A period of 6 months prior to the index date was set as baseline (Fig. 1). International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM) diagnosis codes were used for data retrieval related to different diagnoses (Table 1).
Fig. 1.
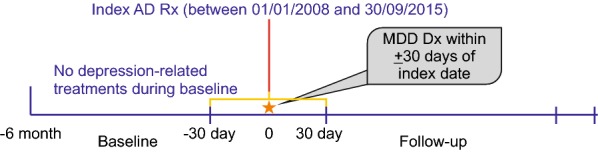
Study design. AD antidepressant, MDD major depressive disorder, Rx prescription, Dx diagnosis
Table 1.
ICD-9 and ICD-10 codes of the conditions mentioned in this study
| Condition | ICD-9 | ICD-10 |
|---|---|---|
| Major depressive disorder | 296.2×, 296.3×, 300.4, 309.0, 309.1, 309.28, 311 | F32–F34 |
| Depression comorbidity | ||
| Anxiety | 300.0×, 300.2×, 300.3 | F40, F41, F42 |
| Personality disorder | 301.× | F34, F60, F21 |
| Post-traumatic stress disorder | 309.81 | F43 |
| Self-harm | E950–E959 | X60–X84, Y87.0, T14.91 |
| Suicide ideation | V62.84 | R45.851 |
Patients aged ≥ 18 years who filled a prescription for AD medication between January 1, 2008 and September 30, 2015, who were diagnosed with ICD 296.2× (major depressive disorder single episode), 296.3× (major depressive disorder recurrent episode), 300.4 (dysthymic disorder), 309.0 (adjustment disorder with depressed mood), 309.1 (prolonged depressive reaction), 309.28 (adjustment disorder with mixed anxiety and depressed mood), 311 (depressive disorder, not elsewhere classified) within ± 30 days from the index date, and had no claims for an AD with continuous enrollment in the insurance plan throughout the baseline period were included in the analysis. Among patients with an ICD depression diagnosis code 300.4, 309.0, 309.1, or 311 there was also the restriction that only those who had at least two consecutive AD dispenses with a gap of ≤ 30 days (to ensure some level of compliance) post index date were included. Patients diagnosed with psychosis, mania and bipolar disorder, or dementia during the baseline period, and those who had received treatment with lithium, thyroid hormone, antipsychotics, mood stabilizers (carbamazepine, lamotrigine, valproate), electroconvulsive therapy or repetitive transcranial magnetic stimulation during the baseline period were excluded. The classification of ICD-codes into an overall category of MDD varies between studies. We chose to follow the ICD-9 Code-Drug Match POS Prior Authorization Guideline of UnitedHealthcare (2014) because the data source of Optum is from the insurance policies sponsored by UnitedHealthcare [25].
An MDD patient who started a third AD regimen after two AD regimens of adequate duration was classified as a patient with TRD. The first medication had to be an AD but the second and the third could be an AD either with or without an augmentation therapy (see Additional file 1). Because claims databases do not capture reasons for medication change, the failure of a medication was determined operationally by quantifying an ‘adequate duration’ for that medication with a lower and an upper limit. Results of data exploration suggest that about 20% of patients had a duration of first AD < 28 days before change (switched to or augmented with the second AD) and about 15% had a duration of second AD < 28 days, and it is unlikely that a patient and caregiver would continue a regimen with inadequate results for more than 3 months. We therefore set an upper limit of 180 days on the time from the start of the first AD regimen to the start of the third AD regimen [21] (Additional file 2). The starting date for the third AD was defined as the TRD start date. Patients were followed up from the TRD start date until death, or were censored if they dropped out of the insurance plan; were diagnosed with psychosis, mania/bipolar disorder or dementia; or until the end of data collection.
Use of the Optum database was reviewed by the New England Institutional Review Board (IRB) and was determined to be exempted from IRB approval, as this project does not involve human subject research. Confidentiality of patient records was always maintained. The study report contains aggregate data only and does not identify individual patients or physicians.
Assessment
The outcome of interest was all-cause mortality. Survival probability was estimated, and the relative risk of all-cause mortality was compared between the TRD and non-TRD MDD cohorts. Relative risk of mortality in the TRD and non-TRD MDD cohorts was further assessed on subgroups based on gender, age (categorized as 18–24, 25–34, 35–44, 45–54, 55–64, 65+ years), study year (from 2008 to 2015), depression diagnosis (categorized as diagnosis with MDD, dysthymic disorder, adjustment disorder with depressed mood, or depressive disorder), psychiatric comorbidity status (yes/no), self-harm status (yes/no), and substance use disorder (SUD) status (yes/no) at baseline.
Statistical analysis
Patient characteristics and outcome measures for the TRD and the non-TRD MDD cohorts were summarized using descriptive statistics. All-cause mortality between TRD and non-TRD MDD patients was compared using a proportional hazards model with TRD status being treated as a time-varying covariate. The model was adjusted for the following covariates evaluated during the baseline period: study year, age, gender, depression diagnosis, SUD, other psychiatric comorbidities (diagnosis of anxiety, post-traumatic stress disorder, or personality disorder), and Charlson comorbidity index (CCI). The CCI is a method of measuring the burden of comorbidities, in which each comorbidity category is assigned a weight, based on the adjusted risk of mortality [26]. A composite score of morbidity, the Quan-Charlson comorbidity index (QCI) was used [27]. To assess the relative risk of mortality on the TRD and non-TRD MDD cohorts in a subgroup, this subgroup variable was not included in the model adjustment. Relative risk of all-cause mortality was estimated as the hazard ratio (HR) with 95% confidence interval (CI). The Kaplan–Meier survival estimates of the TRD and non-TRD MDD cohorts were compared using the log-rank test.
Results
A total of 355,942 pharmacologically treated MDD patients were included in the analysis. At baseline, the mean age was 46.4 years, most of the patients were women (62.4%), 3.0% had SUD, 0.5% had a diagnosis of self-harm, and 37.1% had other psychiatric comorbid diagnoses. During the study, 34,176 (9.6%) patients met the criteria for TRD (Table 2).
Table 2.
Demographic and baseline characteristics
| All MDD patients N = 355,942 |
TRD patients N = 34,176 |
|
|---|---|---|
| Age, mean years (SD) | 46.4 (18.01) | 44.5 (17.35) |
| Charlson comorbidity score, mean (SD) | 0.063 (0.372) | 0.183 (0.630) |
| Age groups, years (%) | ||
| 18–24 | 40,681 (11.4) | 4686 (13.7) |
| 25–34 | 68,137 (19.1) | 6669 (19.5) |
| 35–44 | 69,687 (19.6) | 6970 (20.4) |
| 45–54 | 63,632 (17.9) | 6344 (18.6) |
| 55–64 | 46,306 (13.0) | 4369 (12.8) |
| ≥ 65 | 67,499 (19.0) | 5138 (15.0) |
| Gender, n (%) | ||
| Women | 222,165 (62.4) | 21,443 (62.7) |
| Depression subtype, n (%) | ||
| Major depressive disorder (ICD 296.2, 296.3×) | 130,379 (36.6) | 14,622 (42.8) |
| Dysthymic disorder (ICD 300.4) | 43,115 (12.1) | 3836 (11.2) |
| Adjustment disorders with depressive symptoms (ICD 309.0, 309.1, 309.28) | 11,758 (3.3) | 950 (2.8) |
| Other depressive disorder (ICD 311) | 170,690 (48.0) | 14,768 (43.2) |
| Substance use disorder, n (%) | ||
| Yes | 10,582 (3.0) | 1263 (3.7) |
| Self-harm, n (%) | ||
| Yes | 1610 (0.5) | 275 (0.8) |
| Other psychiatric comorbidities, n (%) | ||
| Yes | 131,963 (37.1) | 13,743 (40.2) |
SD standard deviation, MDD major depressive disorder, TRD treatment-resistant depression
Survival time was significantly shorter in the TRD cohort compared with the non-TRD MDD cohort (log-rank p < 0.0001) (Fig. 2). Patients with TRD had a significantly higher overall all-cause mortality (HR: 1.29; 95% CI 1.22, 1.38) compared with non-TRD MDD patients. The all-cause mortality in the TRD cohort was significantly higher within most of the subgroups (Table 3). The subgroups whose point estimates for HRs stood out were: study year 2015 (HR: 2.28; 95% CI 1.57, 3.31), age group 25–34 years (HR: 2.35; 95% CI 1.69, 3.26), and self-harm (yes) (HR: 2.02; 95% CI 1.00, 4.05). For other subgroups defined by gender, depression diagnosis, psychiatric comorbidity, and SUD, the point estimates for HR did not vary much across the categories (Table 3).
Fig. 2.
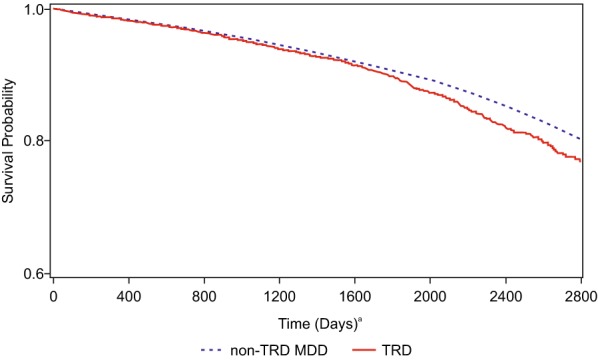
Kaplan–Meier plot. aDay 0 was the first antidepressant dispensing day for non-TRD MDD and the TRD onset date for TRD patients
Table 3.
Hazard ratios of all-cause mortality in the TRD cohort vs. the non-TRD MDD cohort
| Event number/N | HR (95% CI) | p value | |
|---|---|---|---|
| Overall | 13,124/355,942 | 1.29 (1.22, 1.38) | < 0.0001 |
| Year | |||
| 2008 | 3331/59,997 | 1.33 (1.19, 1.49) | < 0.0001 |
| 2009 | 2684/49,834 | 1.25 (1.09, 1.44) | 0.0015 |
| 2010 | 2076/44,272 | 1.33 (1.14, 1.55) | 0.0003 |
| 2011 | 1534/44,567 | 1.20 (0.99, 1.45) | 0.0644 |
| 2012 | 1239/43,556 | 1.48 (1.20, 1.82) | 0.0002 |
| 2013 | 1058/41,992 | 1.49 (1.18, 1.87) | 0.0008 |
| 2014 | 775/40,490 | 1.47 (1.09, 1.98) | 0.0105 |
| 2015 | 427/31,234 | 2.28 (1.57, 3.31) | < 0.0001 |
| Gender | |||
| Women | 6837/222,165 | 1.34 (1.23, 1.46) | < 0.0001 |
| Men | 6287/133,777 | 1.25 (1.14, 1.37) | < 0.0001 |
| Age group | |||
| 18–24 | 108/40,681 | 1.50 (0.84, 2.68) | 0.1698 |
| 25–34 | 232/68,137 | 2.35 (1.69, 3.26) | < 0.0001 |
| 35–44 | 486/69,687 | 1.38 (1.04, 1.83) | 0.0282 |
| 45–54 | 1032/63,632 | 1.31 (1.07, 1.61) | 0.0097 |
| 55–64 | 1745/46,306 | 1.35 (1.15, 1.57) | 0.0001 |
| 65+ | 9521/67,499 | 1.24 (1.15, 1.34) | < 0.0001 |
| Depression subtype | |||
| Major depressive disorder (ICD 296.2, 296.3×) | 4358/130,379 | 1.33 (1.20, 1.47) | < 0.0001 |
| Dysthymic disorder (ICD 300.4) | 1007/43,115 | 1.40 (1.13, 1.74) | 0.0024 |
| Adjustment disorders (ICD 309.0, 309.1, 309.28) | 420/11,758 | 1.31 (0.90, 1.89) | 0.1586 |
| Other depressive disorder (ICD 311) | 7339/170,690 | 1.25 (1.15, 1.37) | < 0.0001 |
| Other psychiatric comorbidities | |||
| No | 9880/223,979 | 1.29 (1.20, 1.39) | < 0.0001 |
| Yes | 3244/131,963 | 1.32 (1.18, 1.49) | < 0.0001 |
| Self-harm | |||
| No | 13,067/354,332 | 1.29 (1.22, 1.38) | < 0.0001 |
| Yes | 57/1610 | 2.02 (1.00, 4.05) | 0.0495 |
| Substance use disorder | |||
| No | 12,519/345,360 | 1.30 (1.22, 1.38) | < 0.0001 |
| Yes | 605/10,582 | 1.24 (0.95, 1.61) | 0.1136 |
Hazard ratios are presented overall and stratified on subgroups
TRD treatment-resistant depression, HR hazard ratio, CI confidence interval
Discussion
The current large cohort study showed a significantly increased all-cause mortality in patients with TRD compared with non-TRD MDD patients, after adjusting for several risk factors. MDD is associated with significantly elevated risk of early death, which may be attributed to the association between MDD and a variety of chronic physical health disorders, and with increased suicide risk [28, 29]. Results of the current study suggest that the increased mortality in patients with MDD is concentrated among those with TRD.
The proportion of patients with TRD in the current analysis (9.6%) was similar to that reported in two other claims database analyses (6.6%, 11%) [30, 31], but was substantially lower than the prevalence concluded from the STAR*D trial, in which approximately 50% of the patients did not have complete remission with two AD trials [32]. The prevalence reported in the claims database studies may not be comparable to the prevalence reported in the STAR*D study due to differences in populations and in TRD criteria. In retrospective claims database studies, the TRD criteria are based only on the count of medication regimens, but the STAR*D study was also able to consider clinical assessments [33]. In addition, patients receiving protocol-defined treatment, as in STAR*D study, may have moved faster through therapies than would be typical of real-world treatment patterns. Either or both of these, or the different patient populations, may explain the higher proportion of TRD in the STAR*D study.
Although no previous study has investigated the mortality risk in patients with TRD in the US population, a recent cohort study in Swedish patients, using a similar definition of TRD as in the present study, showed that patients with TRD had a 35% higher all-cause mortality compared with non-TRD MDD patients (HR: 1.35; 95% CI 1.21, 1.50) [24]. Findings of the current study, which showed a 29% higher all-cause mortality in patients with TRD compared with non-TRD MDD patients (HR: 1.29; 95% CI 1.22, 1.38), are in agreement with the Swedish study. Reutfors et al. reported that the increase in mortality comparing TRD vs. non-TRD MDD was most prominent in the younger subgroup (aged 18–29 years) (HR: 2.03; 95% CI 1.55, 2.64); in the current study, the relative increase in mortality was highest among TRD patients between 25 and 34 years of age (HR: 2.35; 95% CI 1.69, 3.26).
Multiple factors could contribute to increased mortality among patients with TRD. Walker et al. observed increased mortality among patients with mental health disorders overall due to unnatural, natural, and unknown causes [8], and this increased mortality risk has previously been observed in TRD [34, 35]. Although patients in both cohorts had MDD, prior data have shown patients with TRD have longer episodes compared with those with non-TRD MDD [30] and risk of suicide is elevated during a depressive episode [36]. Therefore, the increased mortality could in part reflect the higher proportion of the follow-up period the TRD cohort likely spends in an episode in this analysis. Exiting an episode and attaining remission could be particularly clinically important for TRD patients because, in contrast to non-TRD MDD patients, an increased risk of suicide has been observed in TRD patients even at mild levels of depression symptoms [37]. Additionally, Amos et al., reported an increased proportion of patients with TRD have an inpatient stay compared with Non-TRD MDD patients [38]. Multiple reports have found a large increase in suicide risk following a mental-health-related inpatient stay [34, 39–41]. It is not likely that the mental-health-related inpatient stay is causally related to the increased risk of suicide, but that other acute aspects of the patient’s depression contribute to both the risk of inpatient stay and suicide. Because mortality from natural causes is also associated with TRD, suicide can only partly explain increased mortality. It is possible that TRD modifies lifestyle factors that increase the risk of developing physical comorbidities that impact mortality, or lead to poorer management of these comorbidities once they occur. It is also likely that physical comorbidities among patients with MDD increase the risk of developing TRD, but we attempted to adjust for that possibility in this analysis. In totality, our findings add to the literature suggesting the potential importance of helping patients with depression manage their symptoms and exit an episode, and TRD patients face greater hurdles in achieving these goals.
Limitations of the current study include uncertain disease coding in claims databases, which are usually captured for billing purposes, and may not reflect clinically and systematically verified definitions of medical conditions. It is not possible to determine from claims data whether regimens were changed due to lack of efficacy or other reasons such as lack of tolerability or poor adherence or any other reasons. In addition, this study did not examine the adequacy of the antidepressant doses and included patients with depression who may not have met the criteria for MDD. Considering that the data did not include patients’ entire histories, the notion of incident cases in this study was limited to the current episode. Because many individuals reside in claims database for fewer than 3 years, the portions of the curves in Fig. 2 that describe the mortality experience of patients over substantially longer time periods, may not be generalizable. Though we attempted to adjust for confounding, some confounding may have remained. For example, confounding by medical comorbidities may not have been entirely removed by adjustment for the Charlson comorbidity index. Additionally, the data source did not have information about MDD severity, cause of death, or suicide; so, effect of these variables on outcome could not be estimated. Finally, the population of Optum CEDM is primarily representative of commercial claims patients with lower proportion of Medicare population, hence the study population is likely to have higher socio-economic status compared with the overall US population.
Strengths of the current study include use of a large data set collected from an administrative database that provided medication exposure information based on dispensing details of medications, adjusting for several potential confounders, and being based on information from real-world patients who were receiving usual care.
Conclusions
Patients with TRD may have a significantly greater risk of all-cause mortality compared with non-TRD MDD patients. The current study has added to the emerging literature as, to best of our knowledge, it is the first study in the US on increased all-cause mortality in patients with TRD as compared with non-TRD MDD patients. Results are likely to be fairly generalizable as the study was conducted using a large data set that reflects current clinical practices in the US.
Supplementary information
Additional file 1. List of antidepressant medications and minimum adequate dose. Provides a detailed list of antidepressants and their minimum adequate dose considered during the analysis.
Additional file 2. Method of determination of adequate duration of antidepressant therapy. Detailed explanation of algorithm used to estimate adequate duration of antidepressant therapy.
Acknowledgements
The authors acknowledge Prabhakar Pandey (SIRO Clinpharm Pvt. Ltd.) for providing writing assistance and Harry Ma, Ph.D. (Janssen Global Services, LLC) for additional editorial support.
Abbreviations
- AD
Antidepressant
- CCI
Charlson comorbidity index
- CEDM
Clinformatics™ Extended Data Mart
- CI
Confidence interval
- DMF
Death master file
- HR
Hazard ratio
- ICD
International Classification of Diseases
- IRB
Institutional Review Board
- MDD
Major depressive disorder
- NTIS
National Technical Information Service
- SSA
Social Security Administration
- STAR*D
Sequenced treatment alternatives to relieve depression
- SUD
Substance use disorder
- TRD
Treatment-resistant depression
- YLD
Years lived with disability
Authors’ contributions
Conception and design: GL, DF, GW, RB, LB, PB, JR, AD; collection and assembly of data: GL, DF, GW, AD; data analysis and interpretation: GL, DF, GW, JJS, RB, LB, PB, JR, AD; manuscript writing: All authors. All authors read and approved the final manuscript.
Funding
This study was sponsored by Janssen Research & Development, LLC. RB is supported by the Swedish Research Council (Grant 2016-02362).
Availability of data and materials
The data sets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Use of the Optum database was reviewed by the New England Institutional Review Board (IRB) and was determined to be exempted from IRB approval, as this project does not involve human subject research. Confidentiality of patient records was always maintained. The study report contains aggregate data only and does not identify individual patients or physicians.
Consent for publication
Not applicable.
Competing interests
GL, DF, GW, CB, JS, and AD are employees of Janssen Research & Development, LLC and hold stocks in the parent company. LB, PB, RB, and JR are in research collaboration with Janssen for which grant support has been received by Karolinska Institutet. JR has conducted research in collaboration with Abbvie, AstraZeneca, and Pfizer, for which grant/research support has been received by Karolinska Institutet.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gang Li, Email: GLi@its.jnj.com.
Daniel Fife, Email: DFife@its.jnj.com.
Grace Wang, Email: GWang22@its.jnj.com.
John J. Sheehan, Email: Jsheeha2@its.jnj.com
Robert Bodén, Email: robert.boden@neuro.uu.se.
Lena Brandt, Email: Lena.Brandt@ki.se.
Philip Brenner, Email: philip.brenner@ki.se.
Johan Reutfors, Email: Johan.Reutfors@ki.se.
Allitia DiBernardo, Email: ADiBerna@its.jnj.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12991-019-0248-0.
References
- 1.World Health Organization. Depression and other common mental disorders. http://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf;jsessionid=9AFDFABE4DFBCB868100C34A39B99CC8?sequence=1. Accessed 16 Sept 2018.
- 2.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72(3):227–236. doi: 10.1016/S0165-0327(01)00413-X. [DOI] [PubMed] [Google Scholar]
- 5.Machado MO, Veronese N, Sanches M, Stubbs B, Koyanagi A, Thompson T, et al. The association of depression and all-cause and cause-specific mortality: an umbrella review of systematic reviews and meta-analyses. BMC Med. 2018;16(1):112. doi: 10.1186/s12916-018-1101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartoli F, Lillia N, Lax A, Crocamo C, Mantero V, Carra G, et al. Depression after stroke and risk of mortality: a systematic review and meta-analysis. Stroke Res Treat. 2013;2013:862978. doi: 10.1155/2013/862978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broadhead WE, Blazer DG, George LK, Tse CK. Depression, disability days, and days lost from work in a prospective epidemiologic survey. JAMA. 1990;264(19):2524–2528. doi: 10.1001/jama.1990.03450190056028. [DOI] [PubMed] [Google Scholar]
- 8.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–341. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Q, Kling JM. Depression and the risk of myocardial infarction and coronary death: a meta-analysis of Prospective Cohort Studies. Medicine (Baltimore). 2016;95(6):e2815. doi: 10.1097/MD.0000000000002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171(4):453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- 11.Bolton JM, Gunnell D, Turecki G. Suicide risk assessment and intervention in people with mental illness. BMJ. 2015;351:h4978. doi: 10.1136/bmj.h4978. [DOI] [PubMed] [Google Scholar]
- 12.Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol. 2005;32(6):1013–1019. [PubMed] [Google Scholar]
- 13.Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44(4):470–477. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin EH, Heckbert SR, Rutter CM, Katon WJ, Ciechanowski P, Ludman EJ, et al. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med. 2009;7(5):414–421. doi: 10.1370/afm.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mykletun A, Bjerkeset O, Dewey M, Prince M, Overland S, Stewart R. Anxiety, depression, and cause-specific mortality: the HUNT study. Psychosom Med. 2007;69(4):323–331. doi: 10.1097/PSY.0b013e31803cb862. [DOI] [PubMed] [Google Scholar]
- 16.Onitilo AA, Nietert PJ, Egede LE. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. Gen Hosp Psychiatry. 2006;28(5):396–402. doi: 10.1016/j.genhosppsych.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord. 2009;116(1–2):4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Murphy JA, Sarris J, Byrne GJ. A review of the conceptualisation and risk factors associated with treatment-resistant depression. Depress Res Treat. 2017;2017:4176825. doi: 10.1155/2017/4176825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 20.Conway CR, George MS, Sackeim HA. Defining treatment-resistant depression-reply. JAMA Psychiatry. 2017;74(7):759. doi: 10.1001/jamapsychiatry.2017.0970. [DOI] [PubMed] [Google Scholar]
- 21.Fife D, Feng Y, Wang MY, Chang CJ, Liu CY, Juang HT, et al. Epidemiology of pharmaceutically treated depression and treatment resistant depression in Taiwan. Psychiatry Res. 2017;252:277–283. doi: 10.1016/j.psychres.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Rizvi SJ, Grima E, Tan M, Rotzinger S, Lin P, McIntyre RS, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014;59(7):349–357. doi: 10.1177/070674371405900702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry. 2009;166(4):410–417. doi: 10.1176/appi.ajp.2008.08081239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reutfors J, Andersson TM, Brenner P, Brandt L, DiBernardo A, Li G, et al. Mortality in treatment-resistant unipolar depression: a register-based cohort study in Sweden. J Affect Disord. 2018;238:674–679. doi: 10.1016/j.jad.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 25.UnitedHealthcare Community & State. Guideline: ICD-9 Code-Drug Match POS Prior Authorization. 2014.
- 26.Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why summary comorbidity measures such as the Charlson comorbidity index and Elixhauser score work. Med Care. 2015;53(9):e65–e72. doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 28.Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35(1):1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman DP, Perry GS, Strine TW. The vital link between chronic disease and depressive disorders. Prev Chronic Dis. 2005;2(1):A14. [PMC free article] [PubMed] [Google Scholar]
- 30.Kubitz N, Mehra M, Potluri RC, Garg N, Cossrow N. Characterization of treatment resistant depression episodes in a cohort of patients from a US commercial claims database. PLoS ONE. 2013;8(10):e76882. doi: 10.1371/journal.pone.0076882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberg P, Corey-Lisle PK, Birnbaum H, Marynchenko M, Claxton A. Economic implications of treatment-resistant depression among employees. Pharmacoeconomics. 2004;22(6):363–373. doi: 10.2165/00019053-200422060-00003. [DOI] [PubMed] [Google Scholar]
- 32.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 33.Vieta E, Colom F. Therapeutic options in treatment-resistant depression. Ann Med. 2011;43(7):512–530. doi: 10.3109/07853890.2011.583675. [DOI] [PubMed] [Google Scholar]
- 34.Olin B, Jayewardene AK, Bunker M, Moreno F. Mortality and suicide risk in treatment-resistant depression: an observational study of the long-term impact of intervention. PLoS ONE. 2012;7(10):e48002. doi: 10.1371/journal.pone.0048002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston KM, Powell LC, Anderson IM, Szabo S, Cline S. The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord. 2019;242:195–210. doi: 10.1016/j.jad.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 36.Holma KM, Melartin TK, Haukka J, Holma IA, Sokero TP, Isometsa ET. Incidence and predictors of suicide attempts in DSM-IV major depressive disorder: a five-year prospective study. Am J Psychiatry. 2010;167(7):801–808. doi: 10.1176/appi.ajp.2010.09050627. [DOI] [PubMed] [Google Scholar]
- 37.Dold M, Bartova L, Fugger G, Kautzky A, Souery D, Mendlewicz J, et al. Major depression and the degree of suicidality: results of the European Group for the Study of Resistant Depression (GSRD) Int J Neuropsychopharmacol. 2018;21(6):539–549. doi: 10.1093/ijnp/pyy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amos TB, Tandon N, Lefebvre P, Pilon D, Kamstra RL, Pivneva I, et al. Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a US commercial claims database. J Clin Psychiatry. 2018;79:2. doi: 10.4088/JCP.17m11725. [DOI] [PubMed] [Google Scholar]
- 39.Katz IR, Peltzman T, Jedele JM, McCarthy JF. Critical periods for increased mortality after discharge from inpatient mental health units: opportunities for prevention. Psychiatr Serv. 2019;70(6):450–456. doi: 10.1176/appi.ps.201800352. [DOI] [PubMed] [Google Scholar]
- 40.Haglund A, Lysell H, Larsson H, Lichtenstein P, Runeson B. Suicide immediately after discharge from psychiatric inpatient care: a cohort study of nearly 2.9 million discharges. J Clin Psychiatry. 2019;80:2. doi: 10.4088/JCP.18m12172. [DOI] [PubMed] [Google Scholar]
- 41.Forte A, Buscajoni A, Fiorillo A, Pompili M, Baldessarini RJ. Suicidal risk following hospital discharge: a review. Harv Rev Psychiatry. 2019;27(4):209–216. doi: 10.1097/HRP.0000000000000222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. List of antidepressant medications and minimum adequate dose. Provides a detailed list of antidepressants and their minimum adequate dose considered during the analysis.
Additional file 2. Method of determination of adequate duration of antidepressant therapy. Detailed explanation of algorithm used to estimate adequate duration of antidepressant therapy.
Data Availability Statement
The data sets during and/or analyzed during the current study available from the corresponding author on reasonable request.


