ABSTRACT
Mutations in the Abelson-helper integration site 1 (AHI1) gene are associated with neurological/neuropsychiatric disorders, and cause the neurodevelopmental ciliopathy Joubert syndrome (JBTS). Here, we show that deletion of the transition zone (TZ) protein Ahi1 in mouse embryonic fibroblasts (MEFs) has a small effect on cilia formation. However, Ahi1 loss in these cells results in: (1) reduced localization of the JBTS-associated protein Arl13b to the ciliary membrane, (2) decreased sonic hedgehog signaling, (3) and an abnormally elongated ciliary axoneme accompanied by an increase in ciliary IFT88 concentrations. While no changes in Arl13b levels are detected in crude cell membrane extracts, loss of Ahi1 significantly reduced the level of non-membrane-associated Arl13b and its stability via the proteasome pathway. Exogenous expression of Ahi1–GFP in Ahi1−/− MEFs restored ciliary length, increased ciliary recruitment of Arl13b and augmented Arl13b stability. Finally, Ahi1−/− MEFs displayed defects in cell motility and Pdgfr-α-dependent migration. Overall, our findings support molecular mechanisms underlying JBTS etiology that involve: (1) disruptions at the TZ resulting in defects of membrane- and non-membrane-associated proteins to localize to primary cilia, and (2) defective cell migration.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Ahi1, Arl13b, Cilia, Migration, Shh, Stability
Summary: The Joubert syndrome-causing protein, Ahi1, acts as a gatekeeper in regulating primary cilia protein composition and length. Ahi1 also regulates Arl13b stability, Shh signaling and cell migration.
INTRODUCTION
Mutations in the Abelson-helper integration site 1 (AHI1) gene are one of the more common genetic causes of the neurodevelopmental disorder Joubert syndrome (JBTS). JBTS is characterized by midbrain/hindbrain malformations and a broad spectrum of clinical features, involving other organ systems (Brancati et al., 2010; Dixon-Salazar et al., 2004; Ferland et al., 2004; Parisi et al., 2007). The majority of identified AHI1 pathogenic variants in JBTS result in truncated/non-functional protein products (Valente et al., 2006). AHI1 variants have also been associated with neuropsychiatric disorders, including schizophrenia and autism (Alvarez Retuerto et al., 2008; Ingason et al., 2010). The AHI1 gene encodes for the Ahi1 protein, which contains WD40 repeats and an SH3 domain (Jiang et al., 2002). Subcellularly, Ahi1 preferentially localizes to the distal end of the mother centriole, including the transition zone (TZ), and participates in the formation of primary cilia in epithelial cells (Hsiao et al., 2009). Recently, JBTS has been proposed to result from disruption of the ciliary TZ architecture, leading to defective ciliary signaling (Shi et al., 2017).
The primary cilium, a slender microtubule-based extension (axoneme) of the cell membrane, is critical for embryonic development and tissue homeostasis (Goetz and Anderson, 2010). In non-dividing cells that form cilia, migration and docking of the basal body (a modified mother centriole) to the apical membrane, intraflagellar transport (IFT) and microtubule dynamics are required for assembly and elongation of the axoneme (Rosenbaum and Witman, 2002; Sorokin, 1962; Stephens, 1997). IFT is an evolutionary conserved transportation system powered by IFT particles and molecular motors moving structural and functional components into and out of the cilium (Kozminski et al., 1993; Rosenbaum and Witman, 2002). Between the basal body and cilium lies the TZ, a subdomain that selectively controls the entrance and exit of ciliary components (Reiter et al., 2012). The TZ is thought to restrict lateral diffusion of ciliary membrane components to the remaining plasma membrane (Chih et al., 2011; Hu et al., 2010; Williams et al., 2011), thereby maintaining a distinct protein composition between these two cellular compartments.
ADP-ribosylation factor-like protein-13b (Arl13b) is a ciliary membrane-associated GTPase, mutations in which cause defects in ciliary architecture, ciliogenesis and sonic hedgehog (Shh) signaling (Caspary et al., 2007; Larkins et al., 2011; Mariani et al., 2016). The canonical Shh pathway acts through the secreted glycoprotein Shh, and controls embryonic development. When Shh signaling is not active, the membrane receptor Patched1 (Ptch1) localizes to cilia, inhibits the activation of the G protein-coupled receptor Smoothened (Smo) and regulates the activity of Gli transcription factors. Once Shh binds Ptch1, it is inactivated via cellular internalization. Smo is then constitutively trafficked to the primary cilium, leading to upregulation of Gli1 and Ptch1 mRNAs (Bai et al., 2002; Corbit et al., 2005; Denef et al., 2000; Rohatgi et al., 2007).
In addition to ciliary Arl13b regulating transcriptional Shh signaling, Arl13b has also been implicated in interneuron migration during brain development and in MEF migration (Higginbotham et al., 2012; Mariani et al., 2016). Missense mutations in ARL13B that result in altered Arl13b function have been identified in individuals with JBTS (Cantagrel et al., 2008; Rafiullah et al., 2017). Individuals with JBTS can also present with neuronal migration disorders, including periventricular, interpeduncular, cortical, and other hindbrain heterotopias (Doherty, 2009; Harting et al., 2011; Poretti et al., 2011; Tuz et al., 2014). Finally, mutations in AHI1 in JBTS have been linked to polymicrogyria, a late neurodevelopmental stage migration disorder (Dixon-Salazar et al., 2004; Gleeson et al., 2004).
Despite the known participation of Ahi1 in primary cilia biogenesis, its participation at the ciliary TZ and in mediating cell migration remains elusive. The present study sought to further investigate the involvement of Ahi1 in cilia function using Ahi1−/− MEFs. Consistent with a role for Ahi1 in TZ function, Ahi1 depletion in MEFs disrupts ciliary trafficking of Arl13b and reduces Shh signaling. Ahi1−/− cells also display abnormally elongated cilia lengths, associated with an increase in ciliary recruitment of IFT88. In addition, our findings reveal novel molecular pathways of Arl13b regulation mediated by Ahi1 and the involvement of Ahi1 as a centrosome protein important for migration processes that provide insight into Ahi1 dysfunction in human disease pathogenesis.
RESULTS
Ahi1 localizes at the TZ and its deletion has a small effect on ciliogenesis in MEFs
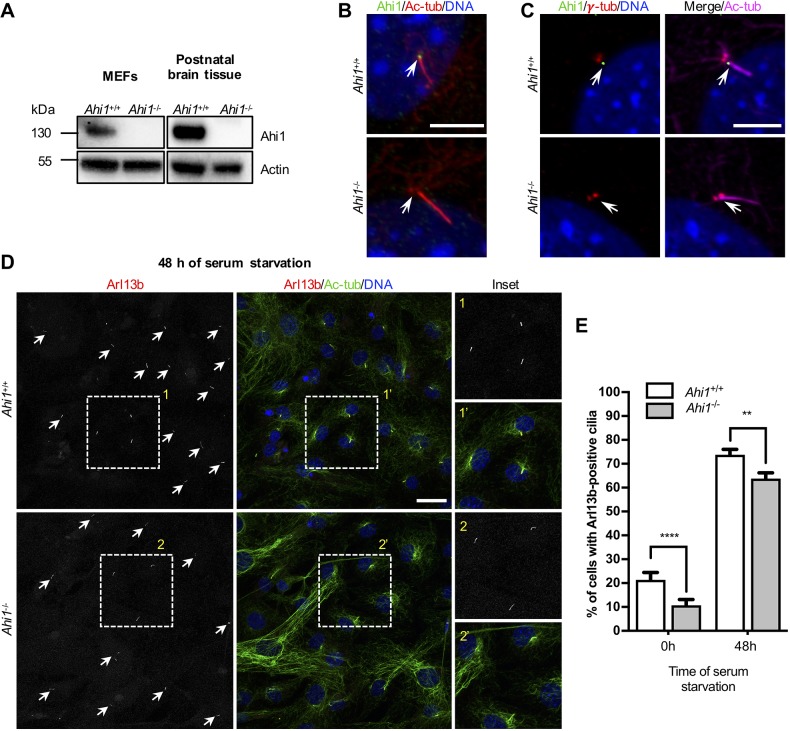
We previously reported that Ahi1 regulates primary cilia formation in epithelial cell lines (Hsiao et al., 2009). Subsequent studies, using human-derived fibroblasts from individuals with AHI1 missense mutations, have shown diverse ciliary phenotypes associated with different pathological conditions (Nguyen et al., 2017; Tuz et al., 2013). Here, we further explore the involvement of Ahi1 in cilia function, analyzing Ahi1-null MEFs. First, we sought to characterize expression and subcellular localization of Ahi1 in MEFs. Immunoblotting of Ahi1 in MEFs and postnatal brain tissue lysates from wild-type and Ahi1−/− mice demonstrate the specificity of our anti-Ahi1 antibody (Fig. 1A). Immunofluorescence analysis of cells in G0/G1 phase with primary cilia showed Ahi1 localization at the base of the ciliary axoneme, colocalized with acetylated α-tubulin (Ac-tub) (Fig. 1B). More detailed observations of Ahi1 localization utilizing the basal body marker, γ-tubulin, in addition to Ahi1 and acetylated α-tubulin, revealed that Ahi1 was detected between the basal body and ciliary axoneme (Fig. 1C), a domain recognized as the ciliary TZ. The specificity of Ahi1 localization was further confirmed using immunocytochemistry in Ahi1−/− cells (Fig. 1B,C). In cells at G2/M transition and S phase, Ahi1 was also detected near and adjacent to centrioles (visualized with γ-tubulin; Fig. S1A). In wild-type MEFs treated with nocodazole, Ahi1 is detected in proximity to one of the centrioles (mother centriole) (Hsiao et al., 2009), independent of microtubule polymerization (Fig. S1B). These observations demonstrate that Ahi1 is primarily paired with the mother centriole during the cell cycle, including its localization in proximity to the basal body in ciliated cells (Hsiao et al., 2009; Lee et al., 2014).
Fig. 1.
Ahi1 localizes at the ciliary TZ and facilitates cilia formation. (A) Immunoblotting of MEFs and brain tissue lysates from Ahi1+/+ and Ahi1−/− mice probed with Ahi1 and actin antibodies. No Ahi1 bands were detected in Ahi1−/− mice samples using an antibody directed against the C-terminal domain of the Ahi1 protein. (B) Immunofluorescence for Ahi1 (green) and acetylated α-tubulin (Ac-tub; red) with DNA/nuclei (blue) in Ahi1+/+ and Ahi1−/− MEFs. Arrows point to base of cilia where Ahi1 is detected only in Ahi1+/+ MEFs. Scale bar: 5 µm. (C) Ahi1+/+ and Ahi1−/− MEFs serum-starved for 48 h and immunostained for Ahi1 (green), the basal body marker, γ-tubulin (γ-tub; red) and Ac-tub (magenta). Arrows point to the ciliary transition zone where Ahi1 is detected in Ahi1+/+ MEFs. Scale bar: 5 µm. (D) Ahi1+/+ and Ahi1−/− MEFs immunolabeled for the membrane-associated cilia protein Arl13b (red) and Ac-tub (green), after 48 h of serum starvation. Arrowheads indicate Arl13b-positive cilia staining in grayscale images. Scale bar: 30 µm. (E) Percentage of MEFs with Arl13b-positive cilia in cultures grown in 10% FBS (0 h) and serum-starved for 48 h. n=4 per genotype, experiments were done in quadruplicate and at least two fields taken with a 40× magnification objective were considered per experiment (n>400 cells/group). Error bars represent s.e.m. ****P<0.0001; **P<0.01 (χ-squared test).
We then examined ciliogenesis in MEFs assessed by immunofluorescence analysis using the ciliary markers, Arl13b and acetylated α-tubulin (Fig. 1D). A modest but significant reduction in Arl13b-positive cilia was observed in Ahi1−/− MEFs grown in 10% FBS (0 h; 10% versus 21% in wild-type cells) or serum-starved (48 h; 63% versus 73% in controls) (Fig. 1E). Similarly, a significant reduction in acetylated α-tubulin-positive cilia was detected in serum-starved (48 h) Ahi1−/− MEFs (∼53% versus 63% in controls) (Fig. S1C) with all acetylated α-tubulin-positive cilia (in both genotypes) colabeling with Arl13b. In contrast to the substantial reduction in primary cilia biogenesis previously observed in Ahi1-knockdown mouse inner medullary collecting duct cells (IMCD3) and Ahi1−/− MEFs from mice on a C57BL6/J background (Hsiao et al., 2009), the modest effect on cilia formation observed here in Ahi1−/− MEFs suggests a cell type (organ)-specific effect as well as genetic modifier effects. Differences in cilia phenotypes associated with the cell type (organ) have also been reported in mice mutants for other TZ proteins (Garcia-Gonzalo et al., 2011; Weatherbee et al., 2009).
Ahi1 facilitates ciliary recruitment of Arl13b and Htr6, and its deletion upregulates ciliary length
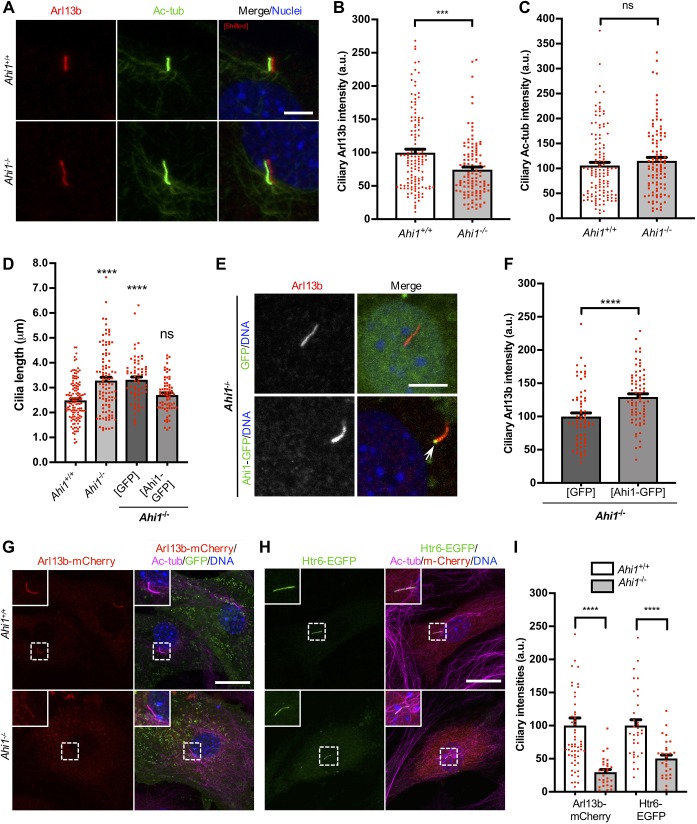
Disruption of TZ components cause defects in ciliary protein composition and differences in cilia length (Cevik et al., 2013; Craige et al., 2010; Garcia-Gonzalo et al., 2011; Gerhardt et al., 2015). Thus, we evaluated the role of Ahi1 with regard to ciliary localization of the membrane-associated protein Arl13b and on cilia length in Ahi1−/− cells. Wild-type and Ahi1−/− MEFs were serum-starved and immunolabeled for Arl13b and acetylated α-tubulin (Fig. 2A). A 25% decrease in ciliary Arl13b intensity was observed in Ahi1-null cells relative to wild type (Fig. 2A,B). Despite the reduction of ciliary Arl13b signal in Ahi1−/− MEFs, no changes in intensity were detected for ciliary acetylated α-tubulin (Fig. 2A,C). Significant reductions in ciliary signal in Ahi1−/− MEFs were also noted for Arl13b:Ac-tub ratios and measuring Arl13b across a line segmenting cilia (Fig. S2A,B).
Fig. 2.
Ahi1 facilitates recruitment of Arl13b and Htr6 to primary cilia and regulates cilia length. (A) Cilia immunolabeling in serum-starved Ahi1+/+ and Ahi1−/− MEFs (48 h). Cells were labeled for Arl13b (red) and acetylated α-tubulin (Ac-tub; green), and DNA/nuclei (blue). Scale bar: 5 µm. (B–D) Graphs of all cilia measured for (B) Arl13b and (C) Ac-tub intensities and (D) cilia length from Ahi1+/+ and Ahi1−/− cultures. Ciliary intensities for the two cilia markers were normalized to Ahi1+/+ values. n=4/genotype, experiments were carried out in quadruplicate and n>100 cilia/group. (E,F) Ahi1−/− MEFs electroporated with GFP or Ahi1–GFP (green) and serum-starved for 48 h (24 h post-transfection). Cells were analyzed by immunofluorescence for Arl13b (red). Scale bar: 5 µm. Note that in cells expressing Ahi1–GFP, the fusion protein is localized at the base of the primary cilium (arrow), cells display normal cilia and ciliary Arl13b labeling is more prominent than in cells expressing GFP only. These parameters were quantified and represented as graphs; cilia length in D and Arl13b ciliary intensity in F. Graphs represent data obtained from Ahi1−/− cell lines (n=3; n>75 cilia). (G,H) Ahi1+/+ and Ahi1−/− MEFs were co-transfected with either (G) GFP and Arl13b–mCherry or (H) Htr6–EGFP and mCherry (green and red, respectively). Cells were serum-starved for 48 h (24 h post-transfection) and labeled for acetylated α-tubulin (Ac-tub; magenta) and DNA/nuclei (blue). Scale bars: 20 µm. (I) Graph of Arl13b–mCherry and Htr6–EGFP ciliary intensities normalized to Ahi1+/+ values. Bars represent means from n=3 cell lines/genotype (n≥28 cilia/group). All error bars represent s.e.m. ****P<0.0001; ***P<0.001; ns, not significant (Mann–Whitney test for B and C, Dunnett's multiple comparison test for D, unpaired two-tailed t-tests for F, Mann–Whitney test and unpaired two-tailed t-tests, respectively, for Arl13b–mCherry and Htr6–EGFP intensities for I).
Interestingly, cilia length analyses showed significantly longer cilia in Ahi1−/− MEFs (mean=3.28 µm) compared to wild-type controls (mean=2.48 µm) (Fig. 2A,D). Exogenous expression of full-length Ahi1–GFP in Ahi1−/− MEFs restored wild-type ciliary length (mean=2.70 µm) (Fig. 2D,E) and significantly reversed ciliary Arl13b reductions (∼30% more Arl13b intensity in Ahi1−/− cells compared to GFP-transfected Ahi1−/− cells; Fig. 2E,F). These results confirm the role of Ahi1 in Arl13b ciliary recruitment and in regulating ciliary length. Notably, the cilia enlargement observed here in Ahi1−/− MEFs (FVB/NJ) is in contrast to the severe defects in ciliogenesis previously reported in MEFs lacking Ahi1 from mice on a C57BL/6J background (Hsiao et al., 2009) suggesting genetic modifier effects associated with strain background.
If less trafficking of Arl13b to cilia in Ahi1−/− cells is due to diminished cellular levels of this protein, Arl13b exogenous overexpression should reestablish its ciliary translocation. To examine this, MEFs were transfected with Arl13b–mCherry and cells were immunolabeled with acetylated α-tubulin after primary cilia induction (Fig. 2G,I). In transfected cells, ciliary Arl13b–mCherry intensity was still decreased (∼70%) in Ahi1-null cultures compared to Ahi1+/+ controls (Fig. 2G,I). Interestingly, ectopic expression of a Myc-tagged Arl13b mutant (Arl13b C8S/C9S-myr), which is not palmitoylated but is able to traffic to cilia owing to the myristoylation (myr) sequence (Roy et al., 2017), is not detected at primary cilia in Ahi1−/− MEFs (Fig. S2C). These results indicate that deficits in ciliary trafficking of Arl13b in Ahi1-null cells are not related to Arl13b palmitoylation. We also examined ciliary Arl13b distribution in Ahi1−/− and wild-type embryonic brain tissue, showing a significant reduction in Arl13b-positive cilia in Ahi1−/− mice compared to wild-type brains (Fig. S2D). Together, this supports a role for Ahi1 at the TZ in translocating Arl13b into the cilium, independent of Arl13b expression levels.
We next assessed ciliary translocation of another ciliary membrane-associated protein (in neurons), the serotonin 6 receptor (Htr6), in MEFs (Brailov et al., 2000; Mitchell and Neumaier, 2005). Heterologous expression of Htr6–EGFP in MEFs showed translocation of this receptor to the primary cilium of transfected wild-type cells (Fig. 2H,I). Consistent with our results obtained for Arl13b overexpression, a significant reduction in the fluorescence signal for Htr6–EGFP was detected in cilia of Ahi1−/− MEFs compared to wild-type cells (Fig. 2H,I).
Besides the ciliary deficiency of Arl13b in Ahi1−/− MEFs, we examined whether ciliary recruitment of platelet-derived growth factor receptor α (Pdgfr-α) in Ahi1-null MEFs. In fibroblasts, Pdgfr-α is selectively targeted to primary cilia assisting in coordinated cell migration (Schneider et al., 2010, 2005). Our results showed no significant changes in the ciliary intensity of Pdgfr-α immunolabeling between Ahi1−/− and wild-type cells in serum-starved cultures (Fig. S3A,B). Moreover, no differences were found in cellular Pdgfr-α expression levels (Fig. S3C–E) or in the activation of the Pdgfr-α signaling pathway (Fig. S3F–H). Overall, these findings suggest that Ahi1 in MEFs selectively disrupts ciliary trafficking of Arl13b and Htr6 and regulates ciliary length (a process that requires proper IFT machinery).
Ahi1 depletion leads to an abnormal distribution of IFT88 and unaltered IFT140 localization in cilia
Renal primary cilia in Ift88-null mice are shorter than normal (Pazour et al., 2000) and have increased anterograde velocities (base-to-tip) of IFT88–EYFP upon the addition of compounds that upregulate cilia length (Besschetnova et al., 2010). IFT88 is a member of the core IFT-B protein complex, which serves as a ‘tubulin module’ that binds and transport tubulin within cilia (Bhogaraju et al., 2013). To examine whether changes in anterograde IFT components were associated with abnormally elongated cilia in Ahi1-depleted cells, we specifically analyzed expression levels and ciliary distribution of IFT88 in MEFs (Fig. 3; Fig. S4). Immunoblot analysis showed no differences in IFT88 protein levels in whole-cell extracts between wild-type and Ahi1−/− MEFs either in the presence of FBS (0 h) or in serum-starved culture conditions (48 h) (Fig. S4A,B). Following primary cilia induction, MEFs were immunolabeled for IFT88, acetylated α-tubulin and γ-tubulin (Fig. 3A–D). IFT88 accumulation was evident at the proximal region of the axoneme (or TZ) (arrows) as well as at cilia tips (asterisks) (Fig. 3A). IFT88 accumulation at cilia tips was markedly increased in Ahi1−/− cells, cells whose cilia are significantly longer than wild-type cells (Fig. 3A). These observations are consistent with an increase in anterograde and unaltered retrograde (tip-to-base movement) velocities of the IFT system as previously reported (Besschetnova et al., 2010). Quantification of the intensity of IFT88 at primary cilia showed a significant increase in Ahi1−/− MEFs (Fig. 3B), and analysis of the staining patterns revealed an altered IFT88 ciliary distribution in Ahi1−/− cells when compared with wild-type cells (Fig. 3C,D). Notably, we found a higher distribution of IFT88 in a punctate pattern in Ahi1−/− MEFs (Fig. 3D). This indicates a positive correlation between elongated cilia (Fig. 2D) and the ciliary concentration of IFT88 in Ahi1−/− MEFs that is independent of the protein levels of IFT88 in the cell (Fig. S4A,B). In order to investigate how Ahi1 may be regulating IFT-A subunits, which control retrograde IFT, we examined endogenous subcellular localization of IFT140 (a core IFT-A component) by immunofluorescence in Ahi1−/− MEFs (Fig. 3E). IFT140 was predominantly detected at the ciliary base (open arrows) but is also present at the cilia tip (arrows) (Fig. 3E). No differences in fluorescence intensity of IFT140 were detected either at the base or cilia tip in Ahi1-depleted cells compared to controls (Fig. 3F,G). Collectively, analysis of IFT proteins suggest that the absence of Ahi1 preferentially impaired ciliary localization of IFT-B components, while IFT-A core members were not affected.
Fig. 3.
Ahi1 depletion disrupts ciliary distribution of IFT88 while IFT140 is not significantly affected. (A) Serum-starved Ahi1+/+ and Ahi1−/− MEFs (48 h) were fixed and immunolabeled for IFT88 (green), γ-tubulin (γ-tub; red), Ac-tub (magenta), and DNA/nuclei (blue). Arrowheads indicate the proximal region of the axoneme, and asterisks denote cilia tips. Scale bar: 10 µm. (B) Graph of IFT88 intensities at primary cilia with ciliary intensities being normalized to Ahi1+/+ values. n=4 per genotype, experiments were carried out in duplicate (n≥92 cilia/group). (C) IFT88 (green) immunolabeling patterns at primary cilia in Ahi1+/+ and Ahi1−/− MEFs. The base of cilia was identified by γ-tubulin staining (red) with DNA/nuclei (blue). Scale bar: 5 µm. (D) IFT88 distribution at primary cilia represented as stacked bar graphs and expressed as percentages in Ahi1+/+ and Ahi1−/− MEFs. n, number of cilia per group. Graph represents data obtained from n≥3 cell lines per genotype. (E) Serum-starved Ahi1+/+ and Ahi1−/− MEFs (48 h) were immunostained for IFT140 (green) and Ac-tub (red) with DNA/nuclei (blue). Open arrows indicate cilia bases and arrows denote cilia tips. Scale bar: 5 µm. IFT140 intensities at the base (F) and cilia tip (G) in n=3 cell lines per genotype (n>55 cilia). Error bars represent s.e.m. ****P<0.0001; ***P<0.001; ns, not significant (Mann–Whitney test for B, F and G and Pearson's χ-squared test for D).
Deletion of Ahi1 decreases Shh pathway signaling
Canonical Shh signaling, which plays an important role in development and homeostasis, is coupled to the primary cilium and its activation requires Arl13b and IFT machinery (Huangfu et al., 2003; Liem et al., 2012). Given that Ahi1 deletion impacts ciliary trafficking of both IFT88 and Arl13b, we hypothesized that Ahi1−/− cells may have a disruption in the localization of Shh pathway proteins and therefore in Shh transcriptional signaling. After primary cilia induction, we activated the Shh pathway with the small molecule Smoothened agonist (SAG) in Ahi1+/+ and Ahi1−/− MEFs, and analyzed the trafficking of Smo to the cilium by immunofluorescence (Fig. 4A). Smo enrichment to the cilium was only evident upon SAG treatment (right panel, Fig. 4A) and, surprisingly, no differences were detected in the ciliary intensity of this Shh pathway effector between wild-type and Ahi1−/− MEFs (Fig. 4B). Previous studies have reported that SAG-treated MEFs resulted in a depletion of the ciliary TZ protein, Rpgrip1l, suggesting that the regulation of the Shh pathway may also occur via Gli3 transcriptional activation (Gerhardt et al., 2015). To evaluate cellular Shh transcriptional activity, Ahi1+/+ and Ahi1−/− MEFs were treated with SAG (Chen et al., 2002) for 24 h and Gli1 and Ptch1 mRNA levels were measured using quantitative real-time PCR analysis. Upregulation of both mRNAs were found in SAG-treated MEFs with the magnitude of the relative expression for Gli1 being higher than Ptch1 (Fig. 4C,D). These differences in relative expression can be explained by the fact that Gli1 is not expressed unless Shh is active (Bai et al., 2002). Conversely, Ptch1 is expressed at a baseline level to serve as a Shh signaling receptor with its upregulation after Shh activation functioning as a negative-feedback loop for the pathway (Goodrich et al., 1996). Statistical analysis showed a significant reduction in the levels of Gli1 mRNA in SAG-treated Ahi1−/− MEFs when compared to wild-type cells (Fig. 4C). These results indicate that Ahi1 regulates Shh transcriptional signaling independently of Smo ciliary recruitment. Recently, analysis of cells expressing Arl13b with mutations in its GTPase domain have shown a reduced Shh response, with unaltered Smo enrichment in cilia after Shh activation (Mariani et al., 2016). Furthermore, it was shown that Arl13b has the ability to regulate Shh signaling downstream of Smo activation (Caspary et al., 2007). We propose that reduced ciliary Arl13b in the absence of Ahi1 contributes to the aberrant activation of the Shh pathway. Because there is also a small reduction in cilia biogenesis in the absence of Ahi1 in MEFs, it is difficult to determine whether the reduced Shh response is caused also by these defects or by a combination of both observed phenotypes in Ahi1−/− cells.
Fig. 4.
Lack of Ahi1 leads to reduced Shh signaling activation but unaltered ciliary trafficking of Smo. (A) Serum-starved Ahi1+/+ and Ahi1−/− MEFs (24 h) were treated with DMSO (control) or SAG (a Shh activator) for 24 h. Cells were co-immunolabeled for Smo (red) and acetylated α-tubulin (Ac-tub; green) with DNA/nuclei (blue). Smo localization is only seen in cells treated with SAG (arrows). Scale bar: 5 µm. (B) Graph showing Smo intensities at primary cilia after Shh activation by SAG. n>100 cilia per group (n=4/genotype). Relative expression of Gli1 (C) and Ptch1 (D) mRNAs in Ahi1+/+ and Ahi1−/− MEFs after DMSO (control) or SAG treatment for 24 h. Fold change was normalized to Ahi1+/+ cells (set at 1) treated with DMSO after normalization (Rpl13a mRNA). Bars represent means from n≥3 cell lines/genotype and experiments were carried out in duplicate. Error bars represent s.e.m. ****P<0.0001; **P<0.01; *P<0.05; ns, not significant (Mann–Whitney test for B and unpaired two-tailed t-tests for C and D).
The loss of Ahi1 decreases Arl13b levels in soluble cell fractions and at the base of the primary cilium
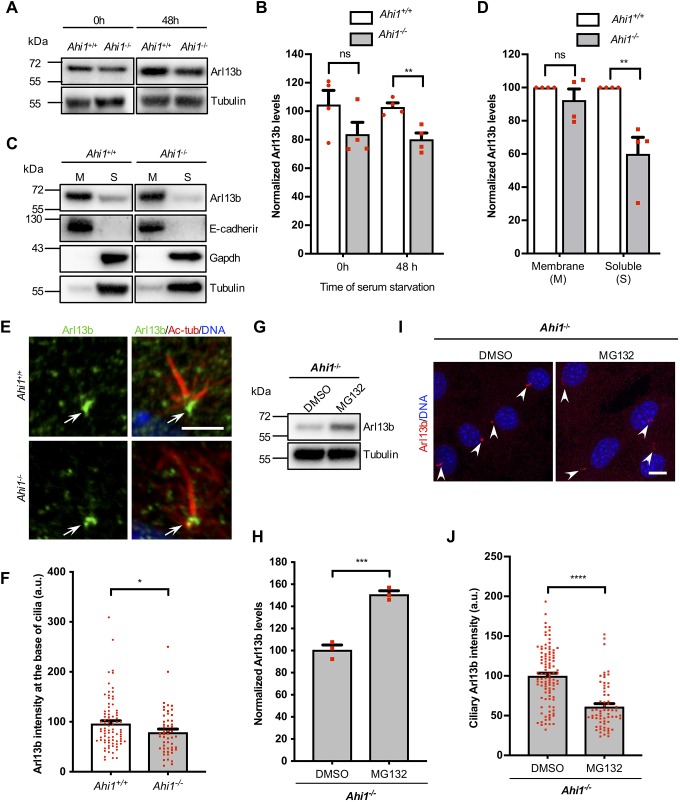
Given the decrease of ciliary Arl13b observed in Ahi1-null cells, we next examined Arl13b levels by immunoblotting total cell extracts of MEFs grown in FBS (0 h) or serum-starved for 48 h (Fig. 5A). In serum-starved conditions, a significant decrease in Arl13b levels (∼20%) was found in Ahi1−/− cultures compared to Ahi1+/+ controls (Fig. 5B). Conversely, no differences were detected in acetylated α-tubulin levels in total cell extracts between the wild-type and Ahi1−/− samples (Fig. S4C,D). Next, Arl13b levels were assessed in membrane and soluble fractions from wild-type and Ahi1−/− MEFs grown in serum-starved conditions (48 h). Subcellular fractionation followed by immunoblotting revealed that Arl13b was present in both membrane and soluble cell fractions, using E-cadherin and Gapdh as subcellular compartmentalization markers, respectively (Fig. 5C). The Arl13b signal is more prominent in the fractions enriched for the membrane-associated protein E-cadherin, indicating preferential association of Arl13b with cell membranes (which include ciliary membranes). Supporting this observation, Pdgfr-α, which is compartmentalized almost exclusively to fibroblast cilia (Schneider et al., 2005), also co-fractionated with E-cadherin, indicating that membrane fractions are enriched in ciliary membrane components (Fig. S3A,C,E). Decreased levels of Arl13b were identified in soluble fractions of Ahi1−/− MEFs compared to wild-type cells. Despite this reduction in the cytosol, no changes in Arl13b expression were detected in cell membrane extracts (Fig. 5D). Visualization of ciliary Arl13b by immunofluorescence in cultured cell lines required preservation of membrane components necessitating the avoidance of high-concentrations of detergent post-fixation (Hua and Ferland, 2017; Larkins et al., 2011). Interestingly, when MEFs were treated with an extraction buffer containing 0.5% Triton X-100 prior to fixation, Arl13b was detected at the base of cilia (Fig. 5E). Quantification of Arl13b intensity at the ciliary base revealed an ∼20% reduction in Arl13b intensity at the base of the cilium in Ahi1−/− cells compared wild-type cells (Fig. 5F). These results indicate that Ahi1 appears not to have a global effect on translocation of Arl13b to cell membranes, but modulates Arl13b levels in the cytosol, ciliary base and ciliary membrane (Fig. 2).
Fig. 5.
Loss of Ahi1 decreases Arl13b levels in soluble cell fractions and at the ciliary base. (A) Immunoblotting of MEF lysates from Ahi1+/+ and Ahi1−/− cultures grown in 10% FBS (0 h) and serum-starved for 48 h probed for Arl13b and tubulin. (B) Quantification of chemiluminescent signals normalized to Ahi1+/+ values; tubulin was used as a loading control. n=4/genotype and experiments were performed in duplicate. (C) Arl13b analysis in membrane (M) and soluble (S) fractions by immunoblotting in Ahi1+/+ and Ahi1−/− cell cultures. E-cadherin was used as a control for membrane-bound proteins and Gapdh as a control for soluble proteins. (D) Quantification of Arl13b signals in n=4 cell lines/genotype, experiments were performed in duplicate. Tubulin was used as a loading control for M and S fractions, and bars represent normalization to Ahi1+/+ values. (E) Ahi1+/+ and Ahi1−/− MEFs immunolabeled for Arl13b (green) and acetylated α-tubulin (Ac-tub; red) with DNA/nuclei (blue). Cells were pre-extracted with buffer containing 0.5% Triton X-100 and fixed with methanol. Scale bar: 3 µm. Arrows highlight Arl13b immunolabeling at the ciliary base. (F) Arl13b intensities at the ciliary base normalized to Ahi1+/+ values. (G,I) Immunoblotting and immunofluorescence of Arl13b in Ahi1−/− MEFs treated with vehicle (DMSO) or MG132 for 6 h. Prior to MG132 treatment, Ahi1−/− MEFs were serum-starved (48 h) to induce formation of primary cilia (I). Arl13b, red, arrowheads, and DNA/nuclei, blue. Scale bar: 10 µm. (H,J) Quantitative analysis of Arl13b chemiluminescence in cells (H) and Arl13b fluorescent signals at primary cilia (J). Experiments were carried out in n=3 cell lines in duplicate. Bars represent values normalized to controls (DMSO/vehicle) and error bars represent s.e.m. ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05; ns, not significant [Mann–Whitney test in B (0 h), D (M), F and J, and unpaired two-tailed t-tests B (48 h), D (S), and H].
As Arl13b is degraded via the proteasomal pathway (Roy et al., 2017), we evaluated whether pharmacological inhibition of the proteasome could increase total cell levels of Arl13b and ciliary Arl13b in Ahi1−/− cells. Serum-starved Ahi1−/− cultures were treated with MG132 (proteasome inhibitor) for 6 h followed by immunoblotting and immunofluorescence microscopic analyses (Fig. 5G,I). Ahi1−/− cells treated with MG132 showed a significant increase in total Arl13b levels, but an ∼40% reduction of ciliary Arl13b intensity compared to controls (DMSO, vehicle) (Fig. 5H,I,J). Subcellular fractionation analysis showed that MG132-treated Ahi1−/− cells, specifically, increased soluble Arl13b levels without noticeable changes of the protein in membrane fractions [including ciliary membranes (Fig. S5D,E)].
Despite MG132 increasing Arl13b expression in Ahi1−/− cells (Fig. 5H), this increase is not sufficient to restore Arl13b ciliary recruitment in Ahi1−/− cells (Fig. 5I,J). In addition, proteasome inhibition accelerated cilia disassembly and increased cilia length (Fig. S5A–C). How proteasome inhibition regulates cilia formation and axoneme length has been previously reported and indicates a functional relationship between negative regulators of cilia biogenesis and their degradation through the ubiquitin proteasome system (Kasahara et al., 2014). Depletion of these negative regulators, which includes the centrosome protein NDE1, also increases ciliary length by modulating the IFT system (Kim et al., 2011; Maskey et al., 2015). Despite Arl13b directly interacting with IFT-B components, this association apparently is not required for Arl13b ciliary localization (Nozaki et al., 2017). In regard to why decreased Arl13b degradation in the presence of MG132 significantly impacts its ciliary localization in Ahi1−/− MEFs, our interpretations are limited due to the unknown mechanism that regulates Arl13b trafficking to primary cilia. However, proteasome inhibition demonstrated a negative correlation between ciliary length and Arl13b ciliary concentration in cells lacking Ahi1. Overall, these findings suggest that the absence of TZ proteins, which includes Ahi1, decrease proteasomal activity at the ciliary base affecting cilia signaling components including Arl13b.
Ahi1 regulates Arl13b stability
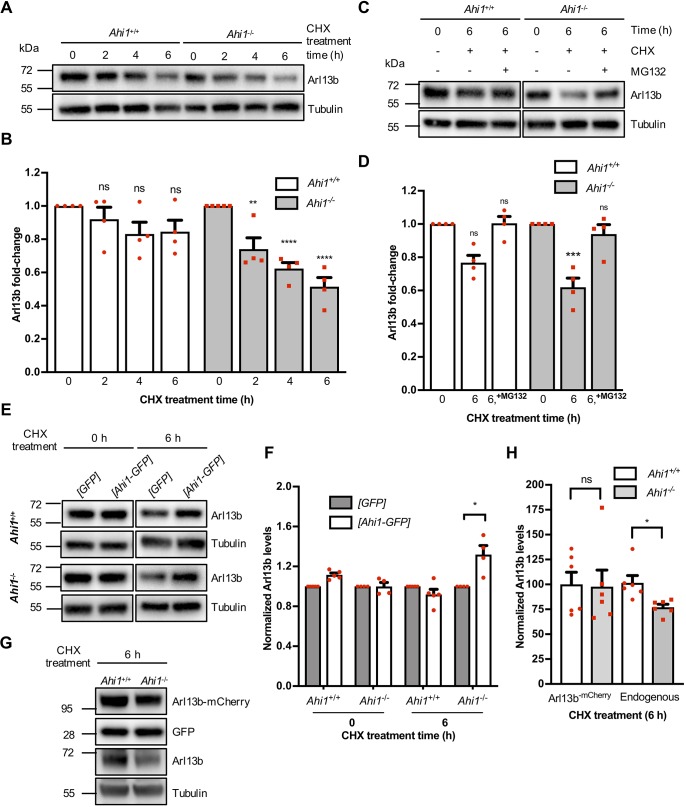
To further elucidate regulation of Arl13b mediated by Ahi1, we examined degradation rates of Arl13b as function of time in Ahi1−/− MEFs treated with the protein synthesis inhibitor cycloheximide (Fig. 6A). Time course analysis of Arl13b levels indicated a significantly faster degradation of Arl13b in Ahi1−/− cells relative to wild-type controls (Fig. 6A,B). After 6 h of cycloheximide treatment, there was a non-significant effect on Arl13b levels in wild-type cells, but a 50% decrease of Arl13b in Ahi1−/− cultures (Fig. 6B). This significant reduction of Arl13b levels in Ahi1−/− cells treated with cycloheximide was blocked in the presence of MG132 (Fig. 6C,D). This result is consistent with the proteasome degradation pathway described for Arl13b (Roy et al., 2017). Moreover, a 30% increase in Arl13b levels was observed in cycloheximide-treated (6 h) Ahi1−/− cells expressing Ahi1–GFP as compared to controls (GFP-transfected cells) (Fig. 6E,F). To address potential concerns regarding the specificity of Ahi1–GFP transient transfection and the increased stability of Arl13b in Ahi1−/− cells in the presence of cycloheximide, we also performed the same experimental strategy in wild-type MEFs. Our results confirm that the augmented levels of Arl13b are not an indirect effect of Ahi1–GFP overexpression (Fig. 6E,F). Additional results in MEFs transiently transfected with Ahi1–GFP and treated with either MG132 or MG132 and cycloheximide indicated that Arl13b levels observed after cycloheximide treatment (6 h, Fig. 6F) are the result of Arl13b stability and not de novo synthesis of the protein (not shown). To explore whether stability defects in Arl13b in Ahi1−/− MEFs could be rescued by Arl13b–mCherry, serum-deprived Ahi1+/+ and Ahi1−/− MEFs were transiently co-transfected with Arl13b–mCherry and GFP constructs and treated with cycloheximide, with cell lysates analyzed by immunoblotting (Fig. 6G). Quantitative analysis showed that ectopic expression of Arl13b–mCherry was not able to recue Arl13b stability defects in Ahi1−/− MEFs and that Arl13b–mCherry stability was not affected in the absence of Ahi1 (Fig. 6H). The latter suggests that overexpressed Arl13b–mCherry has different degradation rates than endogenous Arl13b or that overexpression per se masks any Arl13b–mCherry stability defects or a combination of both. However, this interpretation has a caveat since biochemical characterization of the fluorescent-tagged Arl13b protein remains elusive. Together, these results establish a relationship between the presence of Ahi1 and Arl13b stability.
Fig. 6.
Ahi1 increases the stability and protects Arl13b from proteasomal degradation. (A) Immunoblotting of Ahi1+/+ and Ahi1−/− MEF lysates treated with vehicle (DMSO) or cycloheximide (CHX) for 0, 2, 4 or 6 h, and probed for Arl13b. (B) Arl13b fold-change over the CHX time course (n=4/genotype). (C) Immunoblots of Arl13b from Ahi1+/+ and Ahi1−/− MEF lysates treated with vehicle (DMSO), CHX, or CHX and MG132 for 6 h. (D) Quantification of Arl13b fold-change (with or without CHX and/or MG132). n=3/genotype; experiments were carried out in duplicate. Bars represent results normalized to control values (DMSO/vehicle). (E) Immunoblots of Ahi1−/− and wild-type MEF lysates transfected with either GFP or Ahi1–GFP and probed for Arl13b and tubulin. At 24 h post-transfection cells were lysed (0 h) or treated with CHX for 6 h. For A, C and E, tubulin was used as a loading control. (F) Quantification of Arl13b chemiluminescent signals. n≥4/genotype; experiments were carried out in duplicate. Bars represent data normalized to GFP values. (G) Immunoblotting of Ahi1+/+ and Ahi1−/− MEF lysates co-transfected with Arl13b–mCherry and GFP. At 24 h post-transfection, cells were serum-deprived for 48 h and treated with CHX for 6 h and probed with the indicated antibodies. (H) Quantification of Arl13b and Arl13b–mCherry levels normalized to wild-type values. Tubulin was used as a loading control; similar results were obtained when GFP was used as a loading control (data not shown). Error bars represent s.e.m. ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05; ns, not significant [Dunnett's multiple comparison test for B and D, unpaired two-tailed t-tests for F and H (endogenous) and Mann–Whitney test for H (Arl13b-mCherry)].
Ahi1 promotes cell migration and its loss disrupts Pdgfr-α mediated chemotaxis
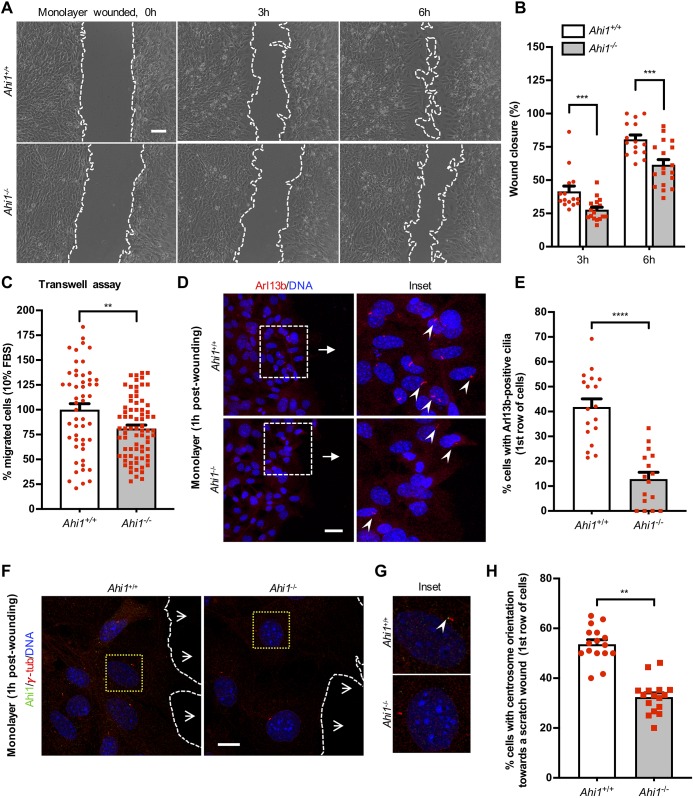
Previous work has described a role for Arl13b and Ahi1 in neuronal migration (Guo et al., 2015; Higginbotham et al., 2012). Here, we assessed directional migration in Ahi1-null cultures using two different approaches: wound-healing assays recorded by live-cell microscopy and transwell assays (Fig. 7). A significant reduction in cell wound closure was observed in Ahi1−/− cultures compared to wild type over a 6 h imaging period (Fig. 7A,B). At 6 h, the wound in Ahi1+/+ cultures was nearly closed (80.7%) in comparison to a 61.54% wound closure in Ahi1−/− cells. Similar results were obtained in sub-confluent MEF monolayers (Fig. S6A,B). No differences in cell proliferation were observed between Ahi1+/+ and Ahi1−/− MEFs as assessed by analyzing Ki-67 immunoreactivity (data not shown). For transwell migration assays, FBS (10%) was used as a chemoattractant to stimulate migration of MEFs. Migration towards FBS was significantly reduced (∼20%) in Ahi1−/− cultures relative to Ahi1+/+ cells (Fig. 7C). Complementary analysis performed in previously established Ahi1-knockdown IMCD3 cells (Hsiao et al., 2009) showed a ∼40% reduction in migrating cells towards FBS (10%) compared to control-scramble cells (Fig. S6C–E).
Fig. 7.
Ahi1 facilitates cell motility and centrosome re-orientation in wound healing assays. (A) Images of Ahi1+/+ and Ahi1−/− MEF cultures at 0, 3 and 6 h after monolayers were scratched. Cell cultures were grown to confluence, scratched and migration recorded with bright-field microscopy in the presence of FBS (10%). Scale bar: 100 µm. (B) Quantitative analysis of wound closure after 3 and 6 h was performed as described in the Materials and Methods. n=3/genotype, experiments in triplicate with at least three different fields along the wound being analyzed per experiment. (C) Migration analysis expressed as percentage of Ahi1+/+ and Ahi1−/− MEFs towards FBS (10%) using transwell inserts. Counting was performed in ≥10, 20× magnification fields/experiment. n=3/genotype, experiments were done in triplicate. (D) Ahi1+/+ and Ahi1−/− monolayers, 1 h post-wounding, immunolabeled for Arl13b (red) with DNA/nuclei (blue). Scale bar: 30 µm. Arrows indicate the direction of migration towards the wound and insets show magnified images of the boxed region. Arrowheads show Arl13b cilia labeling. (E) Percentage of cells with Arl13b-positive cilia facing towards the scratch in Ahi1+/+ and Ahi1−/− cultures. n=3/genotype; experiments were performed at least in triplicate. (F) Ahi1+/+ and Ahi1−/− MEF confluent monolayers were scratched. Cells were allowed to recover for 1 h post-wounding, fixed and immunostained for Ahi1 (green), γ-tubulin (red) and DNA/nuclei (blue). Scale bar, 10 µm. Open arrows depict the direction of the migration towards the wound with the discontinuous white lines marking the wound. (G) Insets show a magnification of the dotted yellow square in F. Ahi1 is only detected in wild-type cells in proximity to the centrioles (arrowhead). (H) Percentage of cells with centrosomes that are directed toward the wound edge after 1 h in Ahi1+/+ and Ahi1−/− MEF monolayers (n>150 cells/genotype). Error bars represent s.e.m. ****P<0.0001; ***P<0.001; **P<0.01 (unpaired two-tailed t-tests in B and C and χ-squared tests for E and H).
Arl13b localization during MEF migration was analyzed in monolayers of confluent Ahi1+/+ and Ahi1−/− cells, 1 h after they were scratched, and immunolabeled for Arl13b. Arl13b localization was evident at primary cilia (Fig. 7D). Analysis of the number of cells with Arl13b-positive cilia facing the wound showed a significant decrease in Ahi1−/− monolayers compared to Ahi1+/+ controls (Fig. 7D,E). To address concerns over the potential participation of non-ciliary Arl13b in migration (Mariani et al., 2016), we analyzed chemotaxis in response to SAG stimulation in non-ciliated MEFs (Fig. S7). No differences in chemotactic response were detected between Ahi1−/− and wild-type cells suggesting that the reduction in migration is not due to the demonstrated increased degradation of Arl13b in MEFs lacking Ahi1.
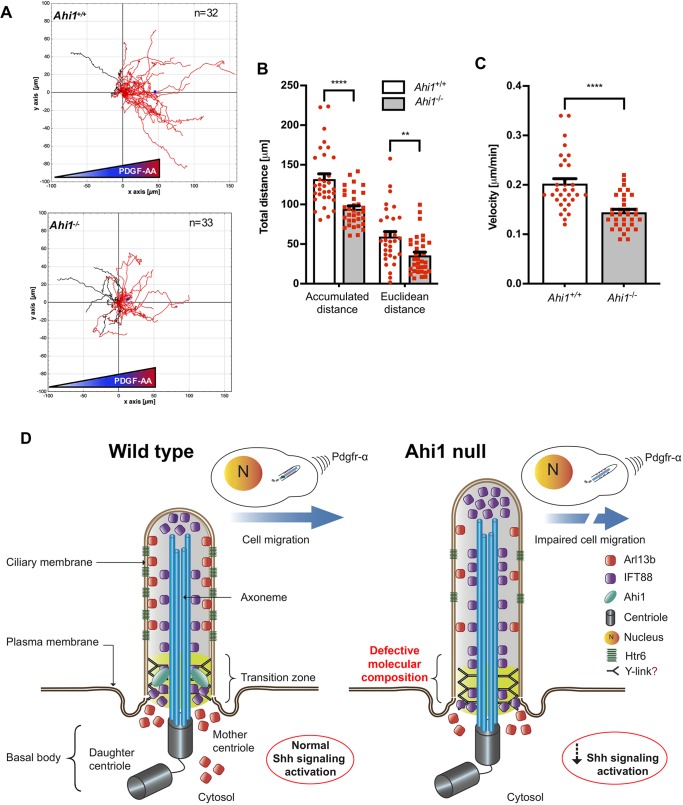
In directionally migrating cells, including fibroblasts, the centrosome becomes oriented between the nucleus and the leading edge facing the movement (Tang and Marshall, 2012). Importantly, Ahi1 localizes at the centrosome at interphase in MEFs (Fig. S1A). To investigate centrosome position in migrating Ahi1+/+ and Ahi1−/− MEFs, confluent monolayers were fixed 1 h after the introduction of a scratch, and immunolabeled for γ-tubulin and Ahi1 (Fig. 7F,G). In wild-type cultures, 50% of the cells reorientate the centrosome towards the scratch-wound in comparison to 30% in Ahi1−/− MEFs (Fig. 7H). This reduction is associated with decreased cell migration in Ahi1-null cells (Fig. 7) and possibly implicates Ahi1 in determining the direction of cell movement. We further tested the ability of Ahi1−/− MEFs to undergo directional migration along a gradient of PDGF-AA in a chemotaxis assay. PGDGF-AA is a ligand for the ciliary receptor Pdgfr-α, which controls directed fibroblast migration (Schneider et al., 2010). We analyzed whole-cell motility behaviors from time lapse movies by tracking the movement of individual Ahi1+/+ and Ahi1−/− cells over 12 h (Fig. 8A). Results showed that the percentage of Ahi1−/− MEFs that are able to respond towards the PDGF-AA source were similar to wild-type values (∼72% versus 87%, respectively; red tracks, Fig. 8A), supporting no alterations in Pdgfr-α signaling activation between cells of these two genotypes (Fig. S3). However, Ahi1−/− cells migrated significantly less distance (94.4 µm) than wild-type cells (132.0 µm) (Fig. 8B), and moved with a significantly lower velocity to the PDGF-AA source compared to control cells (0.15 µm/min versus 0.2 µm/min for Ahi1−/− and Ahi1+/+ cells, respectively) (Fig. 8C). Overall, our migration analyses using different assays indicates that Ahi1 modulates directional cell migration in fibroblasts downstream of Pdgfr-α, and possibly by cellular mechanisms that involve centrosome-dependent pathways or other cytoskeletal components.
Fig. 8.
Chemotactic response of Ahi1-null cells to a PDGF-AA gradient and a schematic model depicting how Ahi1 mediates ciliary protein composition and cell migration in MEFs. (A) Track plots (12 h) of serum-deprived Ahi1+/+ and Ahi1−/− MEFs in the presence of a PDGF-AA gradient. The starting point for all cell trajectories is set to (x=0, y=0); n represents the number of aggregated trajectories of individual cells. The blue mark in the graphs depict the ‘center of mass’ for all cell end points for each population. Migrated distances (B) and velocities (C) of wild-type and Ahi1−/− MEFs along the PDGF-AA gradient. Error bars represent s.e.m. ****P<0.0001; **P<0.01 (unpaired two-tailed t-tests in B and C). (D) Illustration of an Ahi1 wild-type cell with localization of Arl13b, Htr6 and IFT88 proteins at the primary cilium. In Ahi1-null cells, the levels of these membrane-associated and soluble proteins are reduced in the cilium with concomitant axoneme lengthening. Lacking Ahi1 at the transition zone not only reduces Arl13b ciliary recruitment but also diminishes stability and levels of this protein in the cytosol. Importantly, Shh signaling activation and cell migration processes mediated by Pdgfr-α are also impaired in Ahi1 null cells. Of note, in this model, proteins that retain the ability to access the ciliary compartment in absence of Ahi1 are not represented, which includes IFT140, Smo and Pdgfr-α. Further structural characterization is required to elucidate the Y-link organization in Ahi1-null cells.
DISCUSSION
Human mutations in AHI1 and ARL13B genes, which often result in non-functional protein products, are linked to JBTS. However, the molecular relationship between these two JBTS-associated genes and their function in the cell and at primary cilia was unclear. Here, using Ahi1−/− MEFs, we found that the TZ protein, Ahi1, (1) promotes ciliary recruitment of membrane-associated and soluble proteins, including Arl13b and IFT88, (2) controls axoneme length, (3) regulates Arl13b stability by preventing its proteasomal degradation, (4) modulates Shh signaling activation, and (5) regulates cell migration. As such, our findings contribute to a better understanding of primary cilia function and should aid in understanding the intricate molecular processes of JBTS and other ciliopathies (Fig. 8D).
Genomic studies and characterization of protein–protein interactions in mammalian cells have revealed the existence of two main TZ modules referred as the NPHP and the MKS complex (Garcia-Gonzalo and Reiter, 2017). Ahi1 is considered part of the of the Meckel syndrome (MKS) TZ module given that it interacts with B9d1 (Dowdle et al., 2011). Recent studies, employing fluorescence recovery after photobleaching and biomolecular fluorescence complementation assays, have revealed that there is a large immobile fraction of Ahi1 at the base of cilia and Ahi1 interacts with transiting transmembrane and soluble ciliary proteins as well as axoneme-associated proteins (Takao et al., 2017). In addition, super-resolution imaging has revealed Ahi1 localization to a ring-shape domain whose diameter corresponds to that of the ciliary membrane (Lee et al., 2014). These findings suggest that Ahi1 is a stable component localized in proximity to the peripheral membrane of the ciliary gating zone, which controls the entry of both soluble and transmembrane proteins to primary cilia.
We have previously reported that Ahi1 localizes to the TZ of ciliated photoreceptors and Ahi1 deletion causes abnormal protein trafficking to photoreceptor outer segments leading to retinal degeneration (Westfall et al., 2010). Subsequent observations have shown a decrease in the number of Arl13b-positive cilia in fibroblasts obtained from individuals with AHI1 mutations and JBTS (Tuz et al., 2013), suggesting a role for Ahi1 in trafficking of ciliary membrane components. Consistent with a role in TZ function, here we demonstrated that Ahi1 selectively regulates trafficking of membrane-associated components and soluble components (Arl13b, Htr6 and IFT88) to cilia. Moreover, a reduction in Arl13b-positive cilia in embryonic brain tissue was also observed in Ahi1-null mice. However, we found no differences in the ciliary recruitment of IFT140, Pdgfr-α and Smo in Ahi1-depleted MEFs, despite changes in transcriptional regulation of Gli1. As such, Ahi1 joins the list of TZ proteins implicated in Shh signaling and speculatively in serotonin neurotransmission. Interestingly, conditional deletion of Ahi1 in neuronal cells results in decreased levels of serotonin in brain tissue as well as concomitant depressive-like behaviors in mice (Ren et al., 2014). Given the involvement of Htr6 in cognition and memory (Mitchell and Neumaier, 2005; Wesołowska, 2010) as well as the association of AHI1 variants in neuropsychiatric and other neurodevelopmental disorders, such as autism and schizophrenia (Alvarez Retuerto et al., 2008; Dixon-Salazar et al., 2004; Ferland et al., 2004; Ingason et al., 2010), future studies are required to elucidate how pathological alleles in AHI1 alter serotonin signaling pathways and behavior.
Despite major progress, the precise molecular mechanism of how cells modulate cilia length is not well understood. Mutations or absence of ciliary TZ components associated with JBTS result in cilia length differences (Garcia-Gonzalo et al., 2011; Gerhardt et al., 2015; Srivastava et al., 2017), implicating cilia length dysregulation in human disease. Here, we found that depletion of Ahi1 abnormally elongates cilia in MEFs with this phenotype being rescued by transient transfection of wild-type Ahi1–GFP. Ciliary length regulation requires an equilibrium between assembly and disassembly at the ciliary tip, which relies on IFT machinery and microtubule dynamics (Kozminski et al., 1993; Marshall and Rosenbaum, 2001; Stephens, 1997). In mice, depletion of the central component of the IFT complex B, IFT88, which transports proteins from the base to the tip of the cilium, results in shorter or absent cilia and polycystic kidney disease (Davenport et al., 2007; Pazour et al., 2000). Conversely, depletion of retrograde IFT transport, which modulates cargo trafficking from the tip to the base of the cilium, produces an abnormal swollen morphology of this organelle and altered Shh signaling (Fu et al., 2016; Liem et al., 2012; Qin et al., 2011). Neither of these cilia morphologies were detected in Ahi1-null cells based on our immunofluorescence analysis using different ciliary markers. However, a positive correlation was observed between elongated cilia, ciliary concentration of IFT88, and defective Shh signaling activation in Ahi1−/− MEFs. Complementary to our findings, an increase in anterograde and unaltered retrograde velocities of IFT88–EYFP were reported upon the addition of compounds that increase cilia lengths in IMCD3 cells (Besschetnova et al., 2010). Conversely, our immunofluorescence analysis in Ahi1-knockout cells for IFT140, a core component of the IFT-A complex that controls retrograde IFT, showed no differences in IFT140 accumulation at the ciliary base or tip compared to wild-type cells. Collectively, analysis of IFT complexes suggests that the absence of Ahi1 preferentially impairs ciliary trafficking of IFT-B rather than IFT-A proteins (Fig. 8D).
Recently, it has been shown that Arl13b interacts with IFT-B subunits IFT46 and IFT56 through its C-terminal domain with presumably no interactions occurring with IFT-A proteins (Cevik et al., 2013; Nozaki et al., 2017). However, Arl13b interaction with IFT-B components appears to have no impact on its ciliary localization (Higginbotham et al., 2012; Nozaki et al., 2017). Here, we found that Ahi1–GFP exogenously expressed in Ahi1-null MEFs localizes at the ciliary base, restores cilia length to wild-type MEF cilia length, and, importantly, rescues ciliary Arl13b levels. These observations indicate a negative association between cilia length and the amount of ciliary Arl13b in Ahi1-null cells. Similar phenotypes have also been reported in MEFs in the absence of other TZ proteins including, Rpgrip1l, Tcntn2 and B9d1, which suggest this group of proteins regulates Arl13b ciliary membrane composition and cilia length (Dowdle et al., 2011; Garcia-Gonzalo et al., 2011; Gerhardt et al., 2015). Therefore, we hypothesize that the absence of Ahi1 at the ciliary TZ triggers cell signals to accelerate axoneme assembly at the expense of ciliary membrane components; phenotypes observed for other TZ proteins (Garcia-Gonzalo et al., 2011). We propose that the reduced ciliary levels of Arl13b in the absence of Ahi1 contribute to the abnormal activation of the Shh pathway downstream of Smo (Fig. 8D). In agreement with previous reports, reductions in ciliary Arl13b and alterations in Shh signaling have been also noted in cilia from Rpgrip1l−/− MEFs, independent of the activation of Smo (Gerhardt et al., 2015). Because there is a small reduction in ciliated Ahi1−/− MEFs, it is difficult to dissect whether the reduced Shh response is also caused by the reductions in cilia frequency. In addition to IFT participation, cilia length regulation requires other intracellular cues, such as gene transcription and protein degradation of cilia-associated components, indicating active involvement of other cell organelles (Gibbons et al., 1994; Kasahara et al., 2014; Tang et al., 2013). As such, decreased proteasomal activity at the ciliary base was associated with abnormal cilia elongation in Rpgrip1l-null MEFs (Gerhardt et al., 2015). However, Rpgrip1l levels at the TZ were similar between Ahi1+/+ and Ahi1−/− MEFs suggesting differences in ciliary molecular pathways, which converge in cilia length regulation (Fig. S8).
Interestingly, Ahi1 deficiency decreased the amount of Arl13b at the ciliary base and cytosol without noticeable alteration in cell membrane levels. We also observed a reduction of Arl13b stability due to the proteasomal pathway in Ahi1-null cells using pharmacological approaches. Arl13b stability in Ahi1−/− MEFs was increased by transient transfection of wild-type Ahi1–GFP demonstrating that Ahi1 is sufficient for Arl13b stabilization. The ciliary phenotype observed in rescue experiments, involving overexpression of Arl13b and Htr6, and pharmacological inhibition of Arl13b degradation, eliminates defective trafficking of ciliary Arl13b in Ahi1-null cells as a consequence of reduced levels of Arl13b in the cytosol.
Palmitoylation is generally found to stabilize proteins by preventing ubiquitylation and degradation. The Arl13b mutant (C8S/C9S) protein, which disrupt palmitoylation sites within the protein and its localization to cilia membranes, are dramatically degraded via the proteasome. Additionally, ciliary resorption after heat shock treatment reduces Arl13b protein levels by ∼50% in the cell, whereas depalmitoylation blockers mitigate degradation of the protein (Roy et al., 2017). The mechanism by which this post-translational modification protects Arl13b from degradation remains unclear, but one possibility is that the soluble fraction of Arl13b is more liable to be degraded, while membrane-associated proteins are protected. We also ruled out that defective Arl13b translocation in Ahi1-null cells relies on Arl13b palmitoylation processes (Fig. S2C), instead our findings suggest that Ahi1 disrupts TZ architecture, affecting Arl13b ciliary recruitment. It is possible that Ahi1 at the TZ protects Arl13b from premature degradation, thus facilitating its translocation to the ciliary membrane, and this may explain the reduced levels of the protein in Ahi1-depleted cells at the cytosol. Further investigation is required to examine the stability of Arl13b mutants that are not able to localize to cilia in the absence of Ahi1 to better understand the molecular relationship between Ahi1 and Arl13b proteasome degradation.
Previous studies have suggested a role of Arl13b and Ahi1 in cell migration (Casalou et al., 2014; Guo et al., 2015; Higginbotham et al., 2012), but the precise mechanism remains elusive. Other studies have also shown that cilia coordinate cell migration in fibroblasts through Pdgfr-α ciliary signaling (Schneider et al., 2010). The cilia-related defects, including the deficits in Arl13b function/stability, observed in the absence of Ahi1 led us to explore directional migration in Ahi1-null MEFs by different approaches. Our results showed reduced motility with a smaller number of ciliated cells along the wound edge of scratched monolayers. Deficits in cell motility were also detected in Ahi1−/− MEFs by using transwell assays. Interestingly, we also found that a smaller proportion of Ahi1−/− MEFs reorientate the centrosome towards the wound. To better delineate ciliary pathways involved in MEF migration in cells lacking Ahi1, we analyzed the chemotaxic response to a PDGF-AA gradient in serum-deprived MEF cultures. Like wild-type cells, Ahi1−/− MEFs were able to migrate towards the PDGF-AA gradient. Statistical analysis indicates no significant differences in directionality, which is supported by an unaffected Pdgfr-α ciliary recruitment and Pdgfr-α signaling activation in cells lacking Ahi1 (Fig. S3). However, analysis of the parameters describing cell movement (migrated distance and velocity) confirmed a reduced motility in Ahi1-null cells compared to wild-type in the presence of PDGF-AA. Overall, these results suggest that Ahi1 regulates cell migration downstream Pdgfr-α signaling and possibly through other molecular mechanisms that involve centrosome or ciliary Arl13b-dependent pathways or a combination of both (Fig. 8D).
MATERIALS AND METHODS
Animals
Generation of Ahi1-knockout (Ahi1−/−) mice has been described previously (Hsiao et al., 2009). Mice were bred onto an FVB/NJ genetic background (Bourgeois and Ferland, 2019) and genotyped by PCR using genomic tail DNA as a template. Mice were maintained on a normal 12-h-light–12-h-dark cycle with lights off at 19:00 h. All experimental procedures involving mice were performed under approval from the Institutional Animal Care and Use Committee (IACUC) of the Albany Medical College, in accordance with The National Institutes of Health's Guide for the Use and Care of Laboratory Animals.
Cell culture, transfection, and drug treatments
Wild-type (Ahi1+/+) and Ahi1-knockout (Ahi1−/−) mouse embryonic fibroblasts (MEFs) were prepared from embryonic day (E)14.5 embryos derived from intercrossing Ahi1+/− mice. Following visceral organ removal, tissue was minced with a sterile razor blade in cold Hank's balanced salt solution. Tissue was incubated with 0.25% trypsin/EDTA (Gibco) for 30 min at 37°C and passed multiple times through an 18-gauge needle. The cell suspensions were transferred to gelatin coated (0.1% v/v in water) 10 cm tissue culture dishes containing DMEM (4500 mg/l glucose, Sigma-Aldrich), 10% fetal bovine serum (FBS; SH30070, HyClone), 100 units/ml penicillin and 100 mg/ml streptomycin (Gibco). Cells were split upon reaching confluency. For experiments, MEFs were used until passage five with cultures maintained at 37°C in a humidified atmosphere containing 5% CO2.
MEF transfection was performed using an Amaxa Nucleofector II (Lonza) in accordance with the manufacturer's instructions using Ingenio electroporation solution (Mirus). Briefly, 106 cells were electroporated with 1 µg of DNA of the indicated construct and then seeded on gelatinized-coverslips.
For rescue experiments, mouse full-length Ahi1 GFP-tagged plasmids were electroporated (as described above) in MEFs and then seeded onto gelatin-coated plates or coverslips. After electroporation, cells were maintained in culture with media containing 10% FBS for 24 h, followed by serum starvation for 48 h to induce robust primary cilia formation. For all the starvation experiments, media was supplemented with 0.1% FBS. The proteasome inhibitor, MG-132, the protein translation inhibitor, cycloheximide (CHX), and nocodazole were from Sigma (474790, C1988, M1404, respectively). PDGF-AA (R&D Systems) stock solutions were prepared at 100 µg/ml in 4 mM HCl as suggested by the manufacturer.
Antibodies and plasmids
For immunocytochemistry, primary antibodies were as follows: 1:1000 dilution for Ahi1 (Doering et al., 2008), Smo and Pdgfrα (Santa Cruz Biotechnology; sc-166685 and sc-338, respectively), 1:2000 dilution for γ-tubulin, acetylated α-tubulin (Sigma-Aldrich; T6557 and T6793, respectively), Arl13b (UC Davis/NIH NeuroMab Facility; N295B/66), Arl13b, IFT88 and IFT140 (Proteintech; 17711-1-1-AP, 13967-1-AP and 17460-I-AP, respectively). Secondary fluorescent antibodies used were: Alexa Fluor 488 and Alexa Fluor 546 (Life Technologies; A11001, A21202, A11034 and A11030, A11035 and A11035), and Dylight 649 (Jackson ImmunoResearch; 715-495-151), all at 1:2000 dilutions. For western blot analysis, the following primary antibodies were used at 1:2000 dilutions, Arl13b (Proteintech; 17711-1-AP), E-cadherin (BD Biosciences; 610181), α-tubulin (Abcam; ab4074), GFP and m-Cherry (Clonetech; 632381 and 632543), 1:5000 for actin (Millipore; MAB1501), and 1:7000 for Gapdh (Abcam; ab8245). Horseradish peroxidase (HRP)-conjugated secondary antibodies were used at a 1:10,000 dilution (Invitrogen; 32230 and 32260).
Expression plasmids pEGFPN3-Htr6 and pAGGS-Arl13b-mCherry vectors were donated by Dr Kirk Mykytyn (Ohio State University) and Dr Kathryn Anderson (Sloan Kettering Institute), respectively. pmCherry-N1 and pEGFP-N1 were obtained from Clontech. pcDNA-Ahi1-GFP was previously generated in the laboratory (Hsiao et al., 2009). Arl13b C8S/C9S was synthesized with an N-terminal myristoylation (myr) sequence and a C-terminal myc-tag (Roy et al., 2017), and then cloned in-frame into pCDNA3.1. This myr-Arl13b C8S/C9S-myc construct was generated by Gene Universal.
Western blotting (immunoblotting) analyses
MEFs in six-well cell culture plates were rinsed with cold PBS, scraped off the plate, and lysed in 150 µl of RIPA buffer supplemented with protease and phosphatase inhibitors. Cell lysates were then incubated for 20 min on a nutating mixer and centrifuged at 16,000 g for 20 min at 4°C. The supernatant was transferred to a clean tube and protein concentrations were determined with the Advanced Protein Assay Reagent (Cytoskeleton; ADV01). MEF lysates were mixed with loading buffer, resolved by 10% SDS-PAGE or 10% TGX stain-free polyacrylamide gels (Bio-Rad; 161-0183) and transferred to PVDF membranes (Millipore; IPFL00010). Membranes were incubated in blocking buffer [5% (w/v) nonfat dry milk in Tris-buffered saline with 0.1% Tween (TBST)] for 1 h at room temperature followed by overnight incubation of primary antibodies (diluted in blocking buffer) at 4°C. Membranes were incubated for 1 h at room temperature with HRP-conjugated secondary antibodies in TBST. Chemiluminescent detection was performed using the SuperSignal kit (Thermo Fisher Scientific; 34095) and quantified using a ChemiDoc MP imaging system with Image Lab software (Bio-Rad).
Immunocytochemistry and fluorescence imaging
MEFs (3×104 cells/cm2) were seeded onto gelatin-coated glass coverslips for varying durations and fixed with 4% paraformaldehyde (PFA) for 18 min at room temperature or in ice-cold methanol for 5 min at −20°C. Cells were permeabilized in 0.04% Triton X-100, blocked with 1% bovine serum albumin (BSA), and incubated with primary antibodies overnight at 4°C or 1 h at room temperature. Fluorescent-dye-conjugated secondary antibodies were incubated for 1 h at room temperature. All primary and secondary antibodies were added in blocking solution and cells were then incubated on an orbital shaker. After primary and secondary antibody incubations, cells were extensively washed with phosphate-buffered saline (PBS). DNA was labeled with Hoechst 33342 dye (1 µg/ml) and coverslips were mounted using Fluoromount-G (Southern Biotech). To visualize Ahi1 and Arl13b at the base of the primary cilium, cells were pre-extracted for 1 min in an extraction buffer (100 mM PIPES, 1 mM EGTA, 4% PEG800 and 0.5% Triton-X-100) followed by methanol fixation. Images were acquired using a Zeiss confocal microscope LSM800 with Airyscan and a 40×/1.4 numerical aperture (NA) objective or a 63×/1.4 NA oil objective. Images were processed with Zen black 2.1 or Zen blue lite 2.3 (Zeiss).
Stimulation of MEFs with SAG and quantitative RT-PCR analysis
Wild-type and Ahi1−/− MEFs were plated on coverslips, serum-starved for 24 h and treated with 100 nM SAG (Santa Cruz Biotechnology) for an additional 24 h. Cells were fixed, immunolabeled and analyzed for Smo localization. For quantitative RT-PCR analysis, MEFs were plated on six-well plates (3.5×105 cells/well) (maintained in culture for 24 h) and treated with 3 nM SAG (Chen et al., 2002) or vehicle (DMSO) for 24 h after plating. Cells were scraped from the well and total RNA was isolated using RNeasy Plus Mini Kit 50 (Qiagen) according to the manufacturer's instructions. Isolated RNA was then converted into cDNA using the Verso cDNA synthesis kit (Thermo Fisher Scientific). Expression levels from cell lines (n=3, for both genotypes) were analyzed using SYBR Green master mix (Bio-Rad) and gene-specific qPCR primers using a CFX96 Real Time PCR machine (Bio-Rad) with reactions performed in triplicate (experiments were carried out in duplicate). The expression levels of Gli1 and Ptch1 were normalized to Rpl13a with the latter serving as the internal control. Primers for Gli1, Ptch1 and Rpl13a were obtained from Qiagen (QT01551984, QT00149135 and QT00267197, respectively). Melt curve data analyses were also performed after each experiment to ensure the amplification of only one product and the specificity of the primer sequences.
Crude cell membrane isolation
MEFs (3×104 cells/cm2) were grown on gelatinized six-well cell culture plates for 24 h and serum-deprived for 48 h. Cells were washed with cold PBS, scrapped in 150 µl of detergent-free homogenization buffer (250 mM sucrose, 1 mM EDTA, 1.5 mM MgCl2, 20 mM HEPES pH 7.4) containing a protease inhibitor cocktail (Roche; 04693159001). Cell suspensions were collected in 1.5 ml tubes, placed on ice for 10 min, followed by three repeated freeze–thaw cycles with liquid nitrogen and ice. Nuclei and unbroken cells were removed by centrifugation at 800 g for 10 min. The supernatant was collected and centrifuged again at 28,000 g for 30 min in an Allegra X-30R centrifuge (Beckman Coulter). The resulting pellet, containing membrane (M) proteins, was washed with fractionation buffer and the supernatant with soluble (S) proteins was concentrated using acetone precipitation. M and S fractions were resuspended in 30 µl of RIPA buffer (150 mM NaCl, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, and 50 mM Tris-HCl pH 8.0) supplemented with protease inhibitor cocktail and phosphatase inhibitors (Roche; 04906837001) and used for western blot analyses.
Cell migration assays and analyses
MEFs were plated in 12-well cell culture plates and, upon reaching confluence (∼48 h), the cell monolayer was scratched using a 200 µl pipette tip across the bottom of the dish. Cells were washed extensively and allowed to migrate in medium with 10% FBS in a 37°C incubation chamber with 5% CO2. This live-cell imaging was performed, immediately after the monolayers were scratched, using a Leica DM IRB microscope at 10× magnification using bright field microscopy. The area of cell migration from the initial wound edge was measured in three random areas per well using ImageJ (Schindelin et al., 2012). Wound closure (as a percentage) was determined as follows: [(wound area at t0h−wound area at t3h or 6h)/wound area at t0h]×100.
Transwell assays were performed using modified Boyden chambers. In brief, cells were serum-starved overnight, trypsinized and resuspended in serum-free medium. The lower chamber was filled with 500 µl of DMEM supplemented with either 10% FBS or FBS-free. Cells (4×104 in 200 µl) were added to the culture inserts (8 µm, Costar) and allowed to migrate through the membrane for 6 h at 37°C in a 5% CO2 atmosphere. Cells in the upper surface of the membrane were gently removed with cotton pads. Migratory cells, attached to the lower side of the membrane, were fixed with ice-cold methanol for 5 min at −20°C and stained with Hoechst 33342 dye (1 µg/ml). The number of cells migrating to the bottom of the well were counted using nuclei staining (with Hoechst) and fluorescent microscopy. Images were obtained with a Zeiss AxioImager-Z1 microscope equipped with an AxioCam MRm camera and processed with AxioVision Rel. 4.5 software (Zeiss).
Chemotaxis assay
For chemotaxis experiments, µ-slide chemotaxis chambers (Ibidi) were used according to the manufacturer's instructions with slight modifications. In brief, MEFs (6 µl of a 3×106 cells/ml suspension) were loaded into the central channel of the µ-slide chemotaxis chamber that had previously been coated with gelatin (0.1% v/v in water). MEFs were allowed to attach in the culture hood for 40 min, transferred to a 37°C incubator with 5% CO2 and maintained in culture for 24 h. Then, cells were serum deprived for 48 h. Of note, chambers were placed in a sterile 10 cm Petri dish with a wet tissue around it to prevent evaporation. Using the manufacturer's protocol, a PDGF-AA gradient was created in the chambers [C100 (maximum concentration)=200 ng/ml]. Cell migration was recorded over 12 h with an image acquired every 10 min on an inverted microscope (10× objective) having a 37°C incubator and 5% CO2 atmosphere. For trajectory analysis, cells were tracked using the manual tracking plug-in for ImageJ, and tracks were analyzed using the chemotaxis and migration tool plug-in for ImageJ (Ibidi) software (Schindelin et al., 2012). The software was also used to quantify chemotactic or chemokinetic responses including accumulated distance in µm (total cell path traveled), Euclidean distance in µm (shortest distance between cell start and end points) and cell velocity in µm/min.
Quantification and statistics
For all experiments, results were obtained using at least four separate litters with values obtained for Ahi1−/− MEFs being compared to wild-type littermates. All experiments included 2–4 technical replicates. All images in the same set of experiments were acquired with identical confocal microscope settings with quantification of cilia intensity performed using ImageJ (Schindelin et al., 2012). To compare the intensity of Arl13b, Smo, Pdgfrα and IFT88 localization in the primary cilium of MEFs, integrated density values were generated for individual axonemes by selecting the entire cilium (as labeled by acetylated α-tubulin) and colocalization of both proteins was corroborated in the overlay images. Integrated densities for IFT88 labeling were corrected using γ-tubulin to ensure the inclusion of both base and cilia tip immunostaining. For IFT140, integrated density values were obtained by drawing a line with the freehand selection tool around the IFT140 signal at the ciliary base. To obtain IFT140 tip intensity values, a rectangle with similar dimensions was positioned at ciliary tips (cilia were identified by acetylated α-tubulin labeling). For all ciliary protein measurements, background correction was performed by subtracting the integrated density of an adjacent area of identical dimensions to the region of interest (ROI). The integrated density of the ROI (cilia base or cilia tip) was calculated in maximum intensity projections. Note that integrated density (IntDen) in the ROI represents the product of area and mean gray value; IntDen=[area in µm2(sum of the gray values of all pixels÷number of pixels)]. For cilia length, a segmented line was drawn from the base to the tip of the cilium, using the acetylated α-tubulin or Arl13b channel, and length was measured. To determine the percentage of ciliogenesis, the total number of cilia and total number of nuclei were manually counted.
For all data, we first assessed whether data were normally distributed. For experiments with a small sample size (n=3 or 4), normality was tested using the Shapiro-Wilk test. Depending on the distribution, data were analyzed by Student's t-test or using the Mann–Whitney test for datasets without a normal distribution. The tests used are indicated in the corresponding figure legend. Prism 7 (v7.0c) was used for all statistical analyses. Statistical significance was set to P<0.05. Significance is marked with asterisks in all figures (*P<0.05; **P<0.01; ***P<0.001; ****P<0.0001; ns, not significant). Red symbols in bar graphs represent individual data points.
Supplementary Material
Acknowledgements
We wish to thank Dr Joseph Mazurkiewicz for helpful discussions and Ms Julia Nalwalk for critically reviewing our manuscript. We wish to thank Dr Kirk Mykytyn (Ohio State University) and Dr Kathryn Anderson (Sloan Kettering Institute) for the gift of the pEGFPN3-5Ht6 and pAGGS-Arl13b-mCherry vectors, respectively.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.M.-E., R.J.F.; Methodology: J.M.-E., R.J.F.; Validation: J.M.-E.; Formal analysis: J.M.-E., R.J.F.; Investigation: J.M.-E., R.J.F.; Resources: J.M.-E., R.J.F.; Writing - original draft: J.M.-E.; Writing - review & editing: J.M.-E., R.J.F.; Visualization: J.M.-E., R.J.F.; Supervision: R.J.F.; Project administration: R.J.F.; Funding acquisition: R.J.F.
Funding
This work was supported in part by a grant from National Institutes of Health (NIH/NINDS; R01NS092062 to R.J.F.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.230680.supplemental
References
- Alvarez Retuerto A. I., Cantor R. M., Gleeson J. G., Ustaszewska A., Schackwitz W. S., Pennacchio L. A. and Geschwind D. H. (2008). Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum. Mol. Genet. 17, 3887-3896. 10.1093/hmg/ddn291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C. B., Auerbach W., Lee J. S., Stephen D. and Joyner A. L. (2002). Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129, 4753-4761. [DOI] [PubMed] [Google Scholar]
- Besschetnova T. Y., Kolpakova-Hart E., Guan Y., Zhou J., Olsen B. R. and Shah J. V. (2010). Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr. Biol. 20, 182-187. 10.1016/j.cub.2009.11.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogaraju S., Cajanek L., Fort C., Blisnick T., Weber K., Taschner M., Mizuno N., Lamla S., Bastin P., Nigg E. A. et al. (2013). Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 341, 1009-1012. 10.1126/science.1240985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J. R. and Ferland R. J. (2019). Loss of the neurodevelopmental Joubert syndrome causing protein, Ahi1, causes motor and muscle development delays independent of central nervous system involvement. Dev. Biol. 448, 36-47. 10.1016/j.ydbio.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailov I., Bancila M., Brisorgueil M.-J., Miquel M.-C., Hamon M. and Vergé D. (2000). Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 872, 271-275. 10.1016/S0006-8993(00)02519-1 [DOI] [PubMed] [Google Scholar]
- Brancati F., Dallapiccola B. and Valente E. M. (2010). Joubert Syndrome and related disorders. Orphanet J. Rare Dis. 5, 20 10.1186/1750-1172-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantagrel V., Silhavy J. L., Bielas S. L., Swistun D., Marsh S. E., Bertrand J. Y., Audollent S., Attié-Bitach T., Holden K. R., Dobyns W. B. et al. (2008). Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am. J. Hum. Genet. 83, 170-179. 10.1016/j.ajhg.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalou C., Seixas C., Portelinha A., Pintado P., Barros M., Ramalho J. S., Lopes S. S. and Barral D. C. (2014). Arl13b and the non-muscle myosin heavy chain IIA are required for circular dorsal ruffle formation and cell migration. J. Cell Sci. 127, 2709-2722. 10.1242/jcs.143446 [DOI] [PubMed] [Google Scholar]
- Caspary T., Larkins C. E. and Anderson K. V. (2007). The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell 12, 767-778. 10.1016/j.devcel.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Cevik S., Sanders A. A. W. M., Van Wijk E., Boldt K., Clarke L., van Reeuwijk J., Hori Y., Horn N., Hetterschijt L., Wdowicz A. et al. (2013). Active transport and diffusion barriers restrict Joubert Syndrome-associated ARL13B/ARL-13 to an Inv-like ciliary membrane subdomain. PLoS Genet. 9, e1003977 10.1371/journal.pgen.1003977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. K., Taipale J., Young K. E., Maiti T. and Beachy P. A. (2002). Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. USA 99, 14071-14076. 10.1073/pnas.182542899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B., Liu P., Chinn Y., Chalouni C., Komuves L. G., Hass P. E., Sandoval W. and Peterson A. S. (2011). A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat. Cell Biol. 14, 61-72. 10.1038/ncb2410 [DOI] [PubMed] [Google Scholar]
- Corbit K. C., Aanstad P., Singla V., Norman A. R., Stainier D. Y. R. and Reiter J. F. (2005). Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018-1021. 10.1038/nature04117 [DOI] [PubMed] [Google Scholar]
- Craige B., Tsao C.-C., Diener D. R., Hou Y., Lechtreck K.-F., Rosenbaum J. L. and Witman G. B. (2010). CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190, 927-940. 10.1083/jcb.201006105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport J. R., Watts A. J., Roper V. C., Croyle M. J., van Groen T., Wyss J. M., Nagy T. R., Kesterson R. A. and Yoder B. K. (2007). Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 17, 1586-1594. 10.1016/j.cub.2007.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef N., Neubüser D., Perez L. and Cohen S. M. (2000). Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell 102, 521-531. 10.1016/S0092-8674(00)00056-8 [DOI] [PubMed] [Google Scholar]
- Dixon-Salazar T., Silhavy J. L., Marsh S. E., Louie C. M., Scott L. C., Gururaj A., Al-Gazali L., Al-Tawari A. A., Kayserili H., Sztriha L. et al. (2004). Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am. J. Hum. Genet. 75, 979-987. 10.1086/425985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering J. E., Kane K., Hsiao Y.-C., Yao C., Shi B., Slowik A. D., Dhagat B., Scott D. D., Ault J. G., Page-McCaw P. S. et al. (2008). Species differences in the expression of Ahi1, a protein implicated in the neurodevelopmental disorder Joubert syndrome, with preferential accumulation to stigmoid bodies. J. Comp. Neurol. 511, 238-256. 10.1002/cne.21824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D. (2009). Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin. Pediatr. Neurol. 16, 143-154. 10.1016/j.spen.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W. E., Robinson J. F., Kneist A., Sirerol-Piquer M. S., Frints S. G. M., Corbit K. C., Zaghloul N. A., van Lijnschoten G., Mulders L., Verver D. E. et al. (2011). Disruption of a ciliary B9 protein complex causes Meckel syndrome. Am. J. Hum. Genet. 89, 94-110. 10.1016/j.ajhg.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland R. J., Eyaid W., Collura R. V., Tully L. D., Hill R. S., Al-Nouri D., Al-Rumayyan A., Topcu M., Gascon G., Bodell A. et al. (2004). Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat. Genet. 36, 1008-1013. 10.1038/ng1419 [DOI] [PubMed] [Google Scholar]
- Fu W., Wang L., Kim S., Li J. and Dynlacht B. D. (2016). Role for the IFT-A complex in selective transport to the primary cilium. Cell Rep. 17, 1505-1517. 10.1016/j.celrep.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo F. R. and Reiter J. F. (2017). Open sesame: how transition fibers and the transition zone control ciliary composition. Cold Spring Harb. Perspect. Biol. 9, a028134 10.1101/cshperspect.a028134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo F. R., Corbit K. C., Sirerol-Piquer M. S., Ramaswami G., Otto E. A., Noriega T. R., Seol A. D., Robinson J. F., Bennett C. L., Josifova D. J. et al. (2011). A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 43, 776-784. 10.1038/ng.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt C., Lier J. M., Burmühl S., Struchtrup A., Deutschmann K., Vetter M., Leu T., Reeg S., Grune T. and Rüther U. (2015). The transition zone protein Rpgrip1l regulates proteasomal activity at the primary cilium. J. Cell Biol. 210, 115-133. 10.1083/jcb.201408060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons B. H., Asai D. J., Tang W. J., Hays T. S. and Gibbons I. R. (1994). Phylogeny and expression of axonemal and cytoplasmic dynein genes in sea urchins. Mol. Biol. Cell 5, 57-70. 10.1091/mbc.5.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson J. G., Keeler L. C., Parisi M. A., Marsh S. E., Chance P. F., Glass I. A., Graham J. M. Jr., Maria B. L., Barkovich A. J. and Dobyns W. B. (2004). Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am. J. Med. Genet. A 125A, 125-134; discussion 117 10.1002/ajmg.a.20437 [DOI] [PubMed] [Google Scholar]
- Goetz S. C. and Anderson K. V. (2010). The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331-344. 10.1038/nrg2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich L. V., Johnson R. L., Milenkovic L., McMahon J. A. and Scott M. P. (1996). Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 10, 301-312. 10.1101/gad.10.3.301 [DOI] [PubMed] [Google Scholar]
- Guo J., Higginbotham H., Li J., Nichols J., Hirt J., Ghukasyan V. and Anton E. S. (2015). Developmental disruptions underlying brain abnormalities in ciliopathies. Nat. Commun. 6, 7857 10.1038/ncomms8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting I., Kotzaeridou U., Poretti A., Seitz A., Pietz J., Bendszus M. and Boltshauser E. (2011). Interpeduncular heterotopia in Joubert syndrome: a previously undescribed MR finding. AJNR Am. J. Neuroradiol. 32, 1286-1289. 10.3174/ajnr.A2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H., Eom T.-Y., Mariani L. E., Bachleda A., Hirt J., Gukassyan V., Cusack C. L., Lai C., Caspary T. and Anton E. S. (2012). Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev. Cell 23, 925-938. 10.1016/j.devcel.2012.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y.-C., Tong Z. J., Westfall J. E., Ault J. G., Page-McCaw P. S. and Ferland R. J. (2009). Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking. Hum. Mol. Genet. 18, 3926-3941. 10.1093/hmg/ddp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Milenkovic L., Jin H., Scott M. P., Nachury M. V., Spiliotis E. T. and Nelson W. J. (2010). A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436-439. 10.1126/science.1191054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K. and Ferland R. J. (2017). Fixation methods can differentially affect ciliary protein immunolabeling. Cilia 6, 5 10.1186/s13630-017-0045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D., Liu A., Rakeman A. S., Murcia N. S., Niswander L. and Anderson K. V. (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83-87. 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- Ingason A., Giegling I., Cichon S., Hansen T., Rasmussen H. B., Nielsen J., Jürgens G., Muglia P., Hartmann A. M., Strengman E. et al. (2010). A large replication study and meta-analysis in European samples provides further support for association of AHI1 markers with schizophrenia. Hum. Mol. Genet. 19, 1379-1386. 10.1093/hmg/ddq009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Hanna Z., Kaouass M., Girard L. and Jolicoeur P. (2002). Ahi-1, a novel gene encoding a modular protein with WD40-repeat and SH3 domains, is targeted by the Ahi-1 and Mis-2 provirus integrations. J. Virol. 76, 9046-9059. 10.1128/JVI.76.18.9046-9059.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K., Kawakami Y., Kiyono T., Yonemura S., Kawamura Y., Era S., Matsuzaki F., Goshima N. and Inagaki M. (2014). Ubiquitin-proteasome system controls ciliogenesis at the initial step of axoneme extension. Nat. Commun. 5, 5081 10.1038/ncomms6081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Zaghloul N. A., Bubenshchikova E., Oh E. C., Rankin S., Katsanis N., Obara T. and Tsiokas L. (2011). Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat. Cell Biol. 13, 351-360. 10.1038/ncb2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski K. G., Johnson K. A., Forscher P. and Rosenbaum J. L. (1993). A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 90, 5519-5523. 10.1073/pnas.90.12.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins C. E., Aviles G. D. G., East M. P., Kahn R. A. and Caspary T. (2011). Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol. Biol. Cell 22, 4694-4703. 10.1091/mbc.e10-12-0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. L., Santé J., Comerci C. J., Cyge B., Menezes L. F., Li F.-Q., Germino G. G., Moerner W. E., Takemaru K.-I. and Stearns T. (2014). Cby1 promotes Ahi1 recruitment to a ring-shaped domain at the centriole-cilium interface and facilitates proper cilium formation and function. Mol. Biol. Cell 25, 2919-2933. 10.1091/mbc.e14-02-0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem K. F. Jr., Ashe A., He M., Satir P., Moran J., Beier D., Wicking C. and Anderson K. V. (2012). The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J. Cell Biol. 197, 789-800. 10.1083/jcb.201110049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani L. E., Bijlsma M. F., Ivanova A. I., Suciu S. K., Kahn R. A. and Caspary T. (2016). Arl13b regulates Shh signaling from both inside and outside the cilium. Mol. Biol. Cell 27, 3687-3790. 10.1091/mbc.e16-03-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. F. and Rosenbaum J. L. (2001). Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 155, 405-414. 10.1083/jcb.200106141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskey D., Marlin M. C., Kim S., Kim S., Ong E.-C., Li G. and Tsiokas L. (2015). Cell cycle-dependent ubiquitylation and destruction of NDE1 by CDK5-FBW7 regulates ciliary length. EMBO J. 34, 2424-2440. 10.15252/embj.201490831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell E. S. and Neumaier J. F. (2005). 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol. Ther. 108, 320-333. 10.1016/j.pharmthera.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Nguyen T.-M. T., Hull S., Roepman R., van den Born L. I., Oud M. M., de Vrieze E., Hetterschijt L., Letteboer S. J. F., van Beersum S. E. C., Blokland E. A. et al. (2017). Missense mutations in the WD40 domain of AHI1 cause non-syndromic retinitis pigmentosa. J. Med. Genet. 54, 624-632. 10.1136/jmedgenet-2016-104200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki S., Katoh Y., Terada M., Michisaka S., Funabashi T., Takahashi S., Kontani K. and Nakayama K. (2017). Regulation of ciliary retrograde protein trafficking by the Joubert syndrome proteins ARL13B and INPP5E. J. Cell Sci. 130, 563-576. 10.1242/jcs.197004 [DOI] [PubMed] [Google Scholar]
- Parisi M. A., Doherty D., Chance P. F. and Glass I. A. (2007). Joubert syndrome (and related disorders) (OMIM 213300). Eur. J. Hum. Genet. 15, 511-521. 10.1038/sj.ejhg.5201648 [DOI] [PubMed] [Google Scholar]
- Pazour G. J., Dickert B. L., Vucica Y., Seeley E. S., Rosenbaum J. L., Witman G. B. and Cole D. G. (2000). Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709-718. 10.1083/jcb.151.3.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti A., Huisman T. A., Scheer I. and Boltshauser E. (2011). Joubert syndrome and related disorders: spectrum of neuroimaging findings in 75 patients. AJNR Am. J. Neuroradiol. 32, 1459-1463. 10.3174/ajnr.A2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Lin Y., Norman R. X., Ko H. W. and Eggenschwiler J. T. (2011). Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc. Natl. Acad. Sci. USA 108, 1456-1461. 10.1073/pnas.1011410108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiullah R., Long A. B., Ivanova A. A., Ali H., Berkel S., Mustafa G., Paramasivam N., Schlesner M., Wiemann S., Wade R. C. et al. (2017). A novel homozygous ARL13B variant in patients with Joubert syndrome impairs its guanine nucleotide-exchange factor activity. Eur. J. Hum. Genet. 25, 1324-1334. 10.1038/s41431-017-0031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter J. F., Blacque O. E. and Leroux M. R. (2012). The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 13, 608-618. 10.1038/embor.2012.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Qian X., Zhai L., Sun M., Miao Z., Li J. and Xu X. (2014). Loss of Ahi1 impairs neurotransmitter release and causes depressive behaviors in mice. PLoS ONE 9, e93640 10.1371/journal.pone.0093640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R., Milenkovic L. and Scott M. P. (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372-376. 10.1126/science.1139740 [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. L. and Witman G. B. (2002). Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3, 813-825. 10.1038/nrm952 [DOI] [PubMed] [Google Scholar]
- Roy K., Jerman S., Jozsef L., McNamara T., Onyekaba G., Sun Z. and Marin E. P. (2017). Palmitoylation of the ciliary GTPase ARL13b is necessary for its stability and its role in cilia formation. J. Biol. Chem. 292, 17703-17717. 10.1074/jbc.M117.792937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L., Clement C. A., Teilmann S. C., Pazour G. J., Hoffmann E. K., Satir P. and Christensen S. T. (2005). PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 15, 1861-1866. 10.1016/j.cub.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Schneider L., Cammer M., Lehman J., Nielsen S. K., Guerra C. F., Veland I. R., Stock C., Hoffmann E. K., Yoder B. K., Schwab A. et al. (2010). Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell. Physiol. Biochem. 25, 279-292. 10.1159/000276562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Garcia G. III, Van De Weghe J. C., McGorty R., Pazour G. J., Doherty D., Huang B. and Reiter J. F. (2017). Super-resolution microscopy reveals that disruption of ciliary transition-zone architecture causes Joubert syndrome. Nat. Cell Biol. 19, 1178-1188. 10.1038/ncb3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. (1962). Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15, 363-377. 10.1083/jcb.15.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Ramsbottom S. A., Molinari E., Alkanderi S., Filby A., White K., Henry C., Saunier S., Miles C. G. and Sayer J. A. (2017). A human patient-derived cellular model of Joubert syndrome reveals ciliary defects which can be rescued with targeted therapies. Hum. Mol. Genet. 26, 4657-4667. 10.1093/hmg/ddx347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. E. (1997). Synthesis and turnover of embryonic sea urchin ciliary proteins during selective inhibition of tubulin synthesis and assembly. Mol. Biol. Cell 8, 2187-2198. 10.1091/mbc.8.11.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao D., Wang L., Boss A. and Verhey K. J. (2017). Protein interaction analysis provides a map of the spatial and temporal organization of the ciliary gating zone. Curr. Biol. 27, 2296-2306.e3. 10.1016/j.cub.2017.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N. and Marshall W. F. (2012). Centrosome positioning in vertebrate development. J. Cell Sci. 125, 4951-4961. 10.1242/jcs.038083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Lin M. G., Stowe T. R., Chen S., Zhu M., Stearns T., Franco B. and Zhong Q. (2013). Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature 502, 254-257. 10.1038/nature12606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuz K., Hsiao Y.-C., Juárez O., Shi B., Harmon E. Y., Phelps I. G., Lennartz M. R., Glass I. A., Doherty D. and Ferland R. J. (2013). The Joubert syndrome-associated missense mutation (V443D) in the Abelson-helper integration site 1 (AHI1) protein alters its localization and protein-protein interactions. J. Biol. Chem. 288, 13676-13694. 10.1074/jbc.M112.420786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuz K., Bachmann-Gagescu R., O'Day D. R., Hua K., Isabella C. R., Phelps I. G., Stolarski A. E., O'Roak B. J., Dempsey J. C., Lourenco C. et al. (2014). Mutations in CSPP1 cause primary cilia abnormalities and Joubert syndrome with or without Jeune asphyxiating thoracic dystrophy. Am. J. Hum. Genet. 94, 62-72. 10.1016/j.ajhg.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente E. M., Brancati F., Silhavy J. L., Castori M., Marsh S. E., Barrano G., Bertini E., Boltshauser E., Zaki M. S., Abdel-Aleem A. et al. (2006). AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders. Ann. Neurol. 59, 527-534. 10.1002/ana.20749 [DOI] [PubMed] [Google Scholar]
- Weatherbee S. D., Niswander L. A. and Anderson K. V. (2009). A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum. Mol. Genet. 18, 4565-4575. 10.1093/hmg/ddp422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesołowska A. (2010). Potential role of the 5-HT6 receptor in depression and anxiety: an overview of preclinical data. Pharmacol. Rep. 62, 564-577. 10.1016/S1734-1140(10)70315-7 [DOI] [PubMed] [Google Scholar]
- Westfall J. E., Hoyt C., Liu Q., Hsiao Y.-C., Pierce E. A., Page-McCaw P. S. and Ferland R. J. (2010). Retinal degeneration and failure of photoreceptor outer segment formation in mice with targeted deletion of the Joubert syndrome gene, Ahi1. J. Neurosci. 30, 8759-8768. 10.1523/JNEUROSCI.5229-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. L., Li C., Kida K., Inglis P. N., Mohan S., Semenec L., Bialas N. J., Stupay R. M., Chen N., Blacque O. E. et al. (2011). MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 192, 1023-1041. 10.1083/jcb.201012116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.