Abbreviations
- FIB‐4
fibrosis‐4
- LSM
liver stiffness measurement
- NASH
nonalcoholic fatty liver disease
- VCTE
vibration‐controlled transient elastography
Nonalcoholic fatty liver disease (NAFLD) affects approximately one third of the North American population, among whom up to 5% may develop cirrhosis.1, 2 Within the spectrum of NAFLD’s histologic features, the presence of advanced fibrosis best predicts long‐term outcomes.3 Increasing awareness of NAFLD has led to an inevitable question: should we screen for NAFLD, and if so, how should we do this?
To Screen or Not to Screen?
The newly updated guidelines from the American Association for the Study of Liver Diseases recommended against routine screening for NAFLD among high‐risk groups, including diabetes and obesity.4 This recommendation is not based on evidence to support screening but rather the lack thereof. Yet, the concept of screening remains attractive. Screening is useful when the disease is serious and has a detectable and reasonably prevalent preclinical phase. Nonalcoholic steatohepatitis cirrhosis is devastating and is always preceded by fibrosis. Fibrosis, in turn, occurs in many but not all patients with NAFLD. It is unclear, however, whether there is a strategy that safely (with minimal false negatives) and cost effectively discerns a high‐risk group.
Choosing a Screening Strategy
Cost is a barrier to screening for advanced fibrosis. Inexpensive tools to detect fibrosis that developed in the past decade have made screening feasible. These tools are often validated in selected cohorts and share similar performance characteristics. Among them, the fibrosis‐4 (FIB‐4) index and liver stiffness measurements (LSMs) provided by the point‐of‐care vibration‐controlled transient elastography (VCTE) device are best studied.5, 6 Although VCTE provides better risk discrimination overall, the free‐to‐use FIB‐4 algorithm has an intrinsic advantage for cost containment. Some VCTE results can be unreliable (particularly at a high body mass index) or inaccurate (in the context of substantial hepatic inflammation or hepatic vascular congestion), while FIB‐4 may be more prone to misclassification for both the young and very old. Each test yields a continuous value that can be dichotomized to maximize negative and positive predictive values. For this reason, some investigators advocate for a staged approach that combines stringent FIB‐4 cutoffs to eliminate low‐risk persons followed by VCTE (or other tests) to discern those at high risk.7 This staged strategy is expected to reduce the cost of fibrosis screening significantly (Fig. 1). Real‐world data are limited to confirm the safety and utility of this approach.
Figure 1.
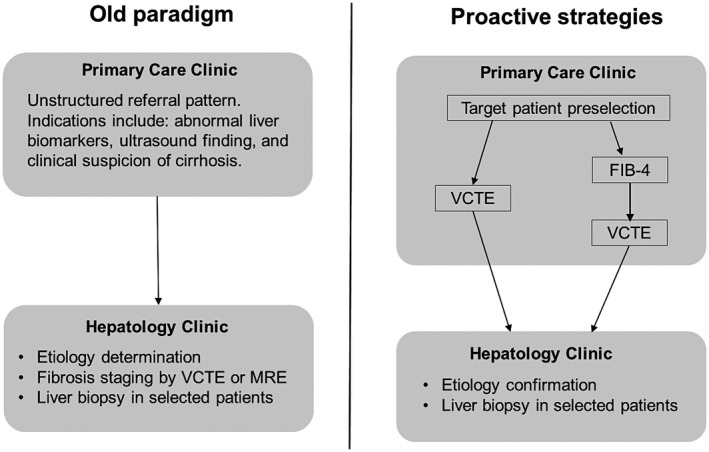
Evolving paradigms of NAFLD management. Abbreviation: MRE, magnetic resonance elastography.
Study Findings
Addressing this gap, Davyduke and colleagues8 leveraged an NAFLD referral program within the catchment of the University of Alberta to compare a two‐stage approach with the existing single‐step VCTE‐based strategy. Specifically, they evaluated 560 patients between 16‐65 years of age with elevated alanine aminotransferase or steatosis on imaging who underwent VCTE on referral. Using a threshold LSM of ≥8.0 kPa for advanced fibrosis, the authors described the impact of a “FIB‐4 first” strategy to reduce the need for VCTE and hepatology referral. Decision modeling identified a FIB‐4 value of 1.3 as an inflection point after which the posttest probability of a high LSM exceeded 12.5%. Implementing this strategy would have obviated the need for 489 (87%) VCTE examinations and prevented 50 (69%) hepatology referrals. However, this strategy would also have missed ≥41 (68%) at‐risk patients with a high LSM. Indeed, 53 of 489 subjects with FIB‐4 <1.3 had a high or invalid LSM, 29 of whom were evaluated further and 3 had F3‐F4 fibrosis on biopsy. These findings were robust across age and body mass indices.
Is This Prime Time for Fibrosis Screening?
A heightened awareness of NAFLD has led to innovative strategies to identify at‐risk patients across the globe. These programs are made possible by the use of noninvasive modalities for fibrosis risk assessment and involve a concerted effort by primary care and hepatology communities. Davyduke et al. shed light on both the cost savings as well as potential pitfalls of a FIB‐4‐first staged strategy in comparison to the one‐step VCTE approach that was in place at the time of the study. Additional data are needed to clarify the risks and benefits of this approach.
This Is Progress but Caution Is Advised
At least four concepts must be considered to contextualize these data. First, our reach may exceed our grasp. It is unknown how many patients were misclassified by the noninvasive strategy. In this post‐biopsy era, outcomes alone can be used to calibrate test cutoffs and balance the risk and benefit of screening. This demands controlled studies paired with longitudinal follow‐up. Second, patients in this study were referred. Thus, the reported prevalence of at‐risk individuals may not mirror those in the community, and this will impact test performance. Third, the conclusions of this study presuppose the availability of resources (e.g., VCTE) that may be lacking in many settings. Fourth, the generalizability of this program assumed uniform risk tolerance and practices concerning confirmatory measurements for fibrosis risk. Indeed, this strategy will miss patients with advanced fibrosis, and the cost savings require that patients and clinicians accept the results of VCTE with limited need for biopsy (e.g. to confirm advanced fibrosis). This is precisely why prospective studies that account for downstream clinical processes are needed. Regardless of the strategy design, it seems certain that proactive screening is superior to the passive waiting of fibrosis progression. Those primary care providers and hepatologists exploring innovative strategies are on the right track.
Potential conflict of interest: Dr. Jiang consults for Boehringer Ingelheim. Dr. Tapper consults for Novartis and advises Bausch; he received grants from Valeant and Gilead.
See Article on Page 1322.
References
- 1. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 2. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387‐1395. [DOI] [PubMed] [Google Scholar]
- 3. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149: 389–397.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 5. Siddiqui MS, Yamada G, Vuppalanchi R, Van Natta M, Loomba R, Guy C, et al.; NASH Clinical Research Network . Clinical Research Network. Diagnostic accuracy of non‐invasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol 2019. 10.1016/j.cgh.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, et al.; NASH Clinical Research Network . Clinical Research Network. Vibration‐controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:156‐163.e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tapper EB, Lok ASF. Use of liver imaging and biopsy in clinical practice. N Engl J Med 2017;377:756‐768. [DOI] [PubMed] [Google Scholar]
- 8. Davyduke T, Tandon P, Al-Karaghouli M, Abraldes JG, Ma MM. Impact of implementing a FIB‐4 first strategy on a pathway for patients with non‐alcoholic fatty liver disease referred from primary care. Hepatol Commun 2019;3:1322‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]


