Abstract
Weight loss is the primary intervention for nonalcoholic fatty liver disease (NAFLD). A decrease in resting metabolic rate (RMR) out of proportion to the degree of weight loss may promote weight regain. We aimed to determine the impact of hepatic steatosis on weight loss‐associated changes in RMR and metabolic adaptation, defined as the difference between predicted and measured RMR after weight loss. We retrospectively analyzed prospectively collected data from 114 subjects without diabetes (52 with NAFLD), with body mass index (BMI) >35, and who enrolled in a 6‐month weight loss intervention. Hepatic steatosis was determined by unenhanced computed tomography scans by liver:spleen attenuation ratio <1.1. RMR was measured by indirect calorimetry. At baseline, patients with hepatic steatosis had higher BMI, fat mass (FM), fat‐free mass (FFM), and RMR (RMR, 1,933 kcal/day; 95% confidence interval [CI], 841‐2,025 kcal/day; versus 1,696; 95% CI, 1,641‐1,751; P < 0.0001). After 6 months, the NAFLD group experienced larger absolute declines in weight, FM, and FFM, but percentage changes in weight, FFM, and FM were similar between groups. A greater decline in RMR was observed in patients with NAFLD (−179 kcal/day; 95% CI, −233 to −126 kcal/day; versus −100; 95% CI, −51 to −150; P = 0.0154) for the time × group interaction, and patients with NAFLD experienced greater metabolic adaptation to weight loss (−97 kcal/day; 95% CI, −143 to −50 kcal/day; versus −31.7; 95% CI, −74 to 11; P = 0.0218) for the prediction × group interaction. The change (Δ) in RMR was significantly associated with ΔFM, ΔFFM, and baseline RMR, while metabolic adaptation was significantly associated with female sex and ΔFM only. Conclusion: Hepatic steatosis is associated with a greater reduction in FM, which predicts RMR decline and a higher metabolic adaptation after weight loss, potentially increasing the risk of long‐term weight regain.
Abbreviations
- AEE
energy expenditure in physical activity
- BMI
body mass index
- CI
confidence interval
- CT
computed tomography
- DO
diet alone
- FFM
fat‐free mass
- FM
fat mass
- L/S ratio
liver:spleen ratio
- NAFLD
nonalcoholic fatty liver disease
- PA
physical activity
- RMR
resting metabolic rate
- TDEE
total daily energy expenditure
Multiple studies have proven the efficacy of weight loss for treating nonalcoholic fatty liver disease (NAFLD). There appears to be a dose‐response relationship between weight loss and histologic improvements as 10% or greater weight loss is associated with marked reductions in inflammation and fibrosis.1 Despite the clinical benefit, sufficient weight loss in ambulatory patients with NAFLD remains challenging. In an analysis of a large tertiary hepatology practice, we reported that only 19.8% of patients achieve 5% weight loss.2 Furthermore, successful maintenance of weight loss outside of clinical research trials is elusive; follow‐up studies of clinical weight loss trials demonstrated that 77% of patients regain weight within 3 years of initial weight loss.3 Therefore, there is an unmet need to understand barriers to successful weight loss in patients with NAFLD.
Metabolic adaptation may represent one explanation for poor weight loss rates in NAFLD. Metabolic adaptation refers to physiologic declines in energy expenditure and resting metabolic rate (RMR) in response to weight loss that exceed changes predicted by body composition.4, 5 Reduced RMR after initial weight loss is an independent predictor of further weight loss,5, 6 and low RMR is a strong predictor of future weight regain6, 7, 8 across all overweight classifications. It is unclear, however, if metabolic adaptation also negatively impacts weight loss attempts in patients with NAFLD.
The aim of our study was to examine the effect of hepatic steatosis on RMR responses to weight loss interventions. Using body composition and energy expenditure data acquired from patients with severe obesity who were undergoing structured weight loss treatment,9, 10, 11 we examined the relationship between weight loss interventions, RMR, and NAFLD. We hypothesized that NAFLD is associated with a greater metabolic adaptation to weight loss.
Participants and Methods
Study Design and Participants
This was a retrospective analysis of a 6‐month clinical trial comparing dietary weight loss intervention to multimodal treatment with diet and physical activity (RENEW; ClinicalTrials.gov trail registration identifier, NCT00712127).9 The Institutional Review Board at the University of Pittsburgh approved the study, and all participants provided written informed consent before enrollment. From February 2007 to March 2009, men and women between 30 and 55 years of age were enrolled in a prospective, single‐blind, randomized control trial. Inclusion criteria included World Health Organization (WHO) class II or III obesity (defined as body mass index [BMI] ≥35 kg/m2) and the ability to 1) walk without assistance, 2) obtain medical clearance for interventions, and 3) commit to scheduling assessment and intervention visits. Exclusion criteria included recent cancer within 5 years of enrollment, coronary artery disease, diabetes mellitus, uncontrolled hypertension, and pregnancy within 6 months of participation. Subjects were excluded if they had undergone prior bariatric surgery, had lost >5% of current weight in the prior 6 months, or had previously enrolled in a weight loss reduction program within the prior year. Individuals with liver enzyme elevations over 30% above the upper limit of normal were also excluded.
Details of the dietary and physical activity interventions have been described in detail.9 Dietary therapy included scheduled meetings with a prescribed diet shown to achieve sustained 8%‐10% weight loss12 and portions of the diet were provided in liquid supplements. Adherence was assessed through self‐recording. Physical activity intervention consisted of brisk walking for up to 60 minutes/day 5 days/week, and activity was monitored with pedometers and self‐reporting in a diary. All clinical, radiographic, laboratory, and metabolic data described below were obtained at baseline and after 6 months in a cohort of 114 subjects.
Determination of NAFLD
Hepatic steatosis was determined using hepatic and splenic attenuation measurements from nonenhanced abdominal computed tomography (CT) scans.9, 11 Liver:spleen attenuation ratio (L/S ratio) strongly correlates with hepatic steatosis, and an L/S ratio <1.1 is over 80% accurate for identification of individuals at 30% steatosis.13 Therefore, NAFLD in the current study was defined as an L/S ratio <1.1.
Demographic, Clinical, and Anthropometric Assessment
Race was self‐reported, and all subjects completed the Cut down/Annoyed/Guilty/Eye‐opener (CAGE) questionnaire to screen for alcohol‐use disorder and were asked to quantify the average frequency of drinking and number of drinks per episode. There was no difference in alcohol intake between the group with or without CT‐determined NAFLD in the 12‐month period before study enrollment.9, 14 Body weight and height were measured to calculate BMI. Fat‐free mass (FFM) and fat mass (FM) were quantified using either dual X‐ray absorptiometry (DXA) or air‐displacement plethysmography in subjects exceeding weight capacity limits for DXA.9
Energy Expenditure and Resting Metabolic Rate Measurement
Total daily energy expenditure (TDEE) was measured using doubly labeled water, as described.15 The measured CO2 production rate was then multiplied by the energy equivalent of CO2 at an assumed respiratory quotient (RQ) of 0.86 to determine TDEE. RMR was determined using indirect calorimetry.15 The thermic effect of food was assumed to be 10% of TDEE, and energy expended in physical activity (AEE) was calculated as follows:
Physical activity monitors (Sensewear Pro3; BodyMedia, Pittsburgh, PA) were worn during assessments of TDEE at baseline and at 6 months, and these were used to measure steps per day.15
Serum Leptin Measurement
Fasting whole‐blood samples collected with the RENEW trial9 and serum leptin levels were measured by enzyme‐linked immunosorbent assay.10
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 6.0 (GraphPad Software, Inc., La Jolla, CA) and Stata version 13.0 (StataCorp, Boston, MA). Continuous variables were reported as means and 95% confidence intervals (CIs), and categorical variables were reported as absolute frequencies and percentages. Continuous variables were compared between groups using Welch's t test, and categorical variables were compared using Fisher's exact test. Two‐way repeated measures analysis of variance (ANOVA) was used to compare posttreatment changes between groups. Post‐hoc paired t tests were performed to determine significance of within‐group (time) differences, and unpaired t tests were used to determine between‐group differences. Holm‐Sidak methods were used to correct for multiple comparisons. P < 0.05 was considered statistically significant.
Results
Baseline Clinical and Demographic Features
Among 114 participants, 52 (45.6%) met imaging criteria for NAFLD. Twelve subjects (10.5%) were men, and 11 of these men had NAFLD. There were no differences in age and ethnic distribution between groups (Table 1). The mean BMI of the study cohort was 43.6 (95% CI, 42.6‐44.6), and subjects with NAFLD had higher BMI, FM, and FFM than those without NAFLD; percentage FM and percentage FFM were similar between groups. Mean TDEE was higher in the NAFLD cohort, but there were no differences in RQ, AEE, or physical activity (as measured by steps per day) between groups. Instead, higher baseline RMR in subjects with NAFLD was responsible for increased TDEE, and RMR remained significantly elevated in NAFLD even after adjustment for age, sex, FM, and FFM. Despite higher FM in subjects with NAFLD, leptin levels were similar in subjects with and without NAFLD.
Table 1.
Baseline Demographic, Clinical, and Metabolic Features of the Study Cohort
| Variable | All (N = 114) | NAFLD (n = 52) | No NAFLD (n = 62) | P Value |
|---|---|---|---|---|
| Age, years | 47.1 (46.0‐48.3) | 46.5 (44.7‐48.3) | 47.6 (46.1‐49.2) | 0.3403 |
| Male sex, n (%) | 12 (10.5%) | 11 (21.2%) | 1 (1.6%) | 0.001 |
| Non‐white race, n (%) | 38 (33.3%) | 14 (26.95%) | 24 (38.7%) | 0.232 |
| Weight, kg | 118.8 (115.5‐122.1) | 127.1 (122.3‐131.9) | 111.9 (108.1‐115.6) | <0.0001 |
| BMI, kg/m2 | 43.6 (42.6‐44.6) | 45.6 (44.1‐47.1) | 41.9 (40.7‐43.2) | 0.0003 |
| Intervention, n (%) | 0.710 | |||
| Diet alone | 55 (48.2%) | 24 (46.1%) | 31 (50.0%) | |
| Diet and exercise | 59 (51.8%) | 28 (53.8%) | 31 (50.0%) | |
| Liver/spleen ratio | 1.075 (1.031‐1.118) | 0.884 (0.826‐0.942) | 1.231 (1.207‐1.256) | <0.0001 |
| FM, kg | 60.0 (57.7‐62.2) | 63.9 (60.6‐67.3) | 56.7 (53.8‐59.5) | 0.0011 |
| Percentage FM | 50.3% (49.4%‐51.1%) | 50.2% (48.7%‐51.6%) | 50.3% (49.2%‐51.4%) | 0.8451 |
| FFM, kg | 57.5 (55.9‐59.1) | 61.9 (59.2‐64.6) | 53.8 (52.3‐55.3) | <0.0001 |
| Percentage FFM | 48.6% (47.7%‐49.5%) | 48.8% (47.4%‐50.2%) | 48.6% (47.3%‐49.5%) | 0.6645 |
| Unadjusted RMR, kcal/day | 1,804 (1,749‐1,859) | 1,933 (1,841‐2,025) | 1,696 (1,641‐1,751) | <0.0001 |
| Adjusted RMR,* kcal/day | 1,803 (1,776‐1,832) | 1,944 (1,911‐1,976) | 1,868 (1,655‐1,718) | <0.0001 |
| Respiratory quotient | 0.806 (0.799‐0.814) | 0.808 (0.798‐0.818) | 0.805 (0.794‐0.816) | 0.6842 |
| TDEE kcal/day | 3,182 (3,093‐3,272) | 3,321 (3,173‐3,469) | 3,066 (2,963‐3,169) | 0.0045 |
| AEE, kcal/day | 1,060 (1,004‐1,116) | 1,056 (975‐1,138) | 1,063 (985‐1,142) | 0.5517 |
| Leptin, ng/mL | 53.30 (49.10‐57.49) | 56.28 (49.33‐63.23) | 50.75 (45.61‐55.89) | 0.1945 |
| Physical activity, steps/day | 7,406 (6,815‐7,998) | 7,073 (6,165‐7,981) | 7,696 (6,905‐8,487) | 0.1500 |
RMR was adjusted for age, sex, FM, and FFM.
Changes in Body Composition After Weight Loss Interventions Differ Between Patients With and Without NAFLD
Study participants underwent a 6‐month supervised weight loss intervention consisting of either dietary modification alone (DO) or with physical activity (DO + PA; Table 1).11 There was no difference in prescribed treatment regimens between groups as similar proportions of subjects with and without NAFLD were assigned to DO (24 subjects [46.2%] versus 31 [50.0%], respectively) and DO + PA arms (28 [53.8%] versus 31 [50.0%], respectively; P = 0.170). As we previously reported, subjects with NAFLD lost more weight, but the proportion of patients achieving 5% and 10% weight loss goals did not differ between subjects with and without NAFLD. Although patients with NAFLD experienced greater absolute declines in FM and FFM, percentage reductions in BMI, FM, and FFM were similar between groups (Supporting Table S1). Interestingly, subjects with NAFLD lost a greater proportion of their weight as FM (80.2%; 95% CI, 71.3%‐89.1%; versus 62.8%; 95% CI, 49.0%‐76.5%; P = 0.0215), while subjects with and without NALFD lost a similar proportion of their weight as FFM (19.8%; 95% CI, 11.7%‐29.2%; versus 32.2%; 95% CI, 19.1%‐45.4%, respectively; P = 0.1530) (Fig. 1).
Figure 1.
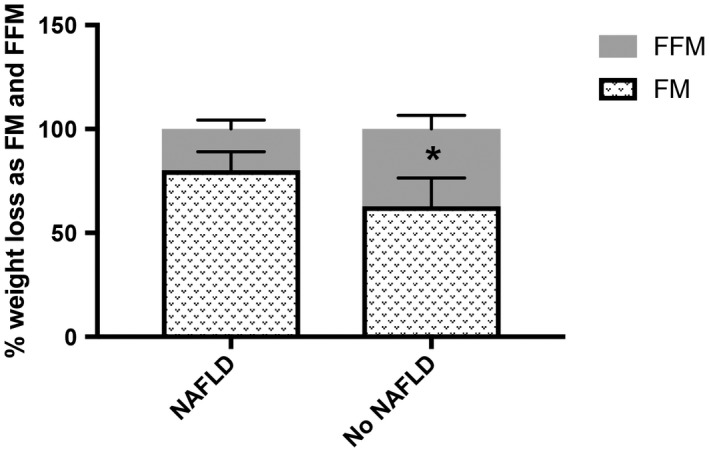
Percentage of total weight loss from FM and FFM. The percentage of total weight loss from FM significantly differed between subjects with and without NAFLD. The percentage weight loss from FFM did not differ between groups. *P < 0.05. Data represent mean ± 95% CI.
Energy Expenditure Responses to Weight Loss Interventions Differ Between Patients With and Without NAFLD
We next assessed changes in energy homeostasis after weight loss intervention (Table 2). Subjects with NAFLD experienced greater reductions in RMR than subjects without NAFLD, but the change in TDEE was not significantly different between groups. This may be related to differences in AEE responses to weight loss therapy as subjects without NAFLD had greater reductions in AEE than subjects with NAFLD. Participants with NAFLD had increased physical activity after 6 months, while subjects without NAFLD demonstrated no changes in daily step counts. Together, these findings suggest that observed AEE reductions in NAFLD may be related to enhanced metabolic efficiency of activity.
Table 2.
Changes in Energy Expenditure and Activity After Lifestyle Intervention in Subjects With and Without NAFLD
| Variable | NAFLD | P (Time) | No NAFLD | P (Time) | P (NAFLD) | P (Time × NAFLD) |
|---|---|---|---|---|---|---|
| RMR change, kcal/day | −179 (−233 to −126) | <0.0001 | −100 (−51 to −149) | <0.0001 | <0.0001 | 0.0154 |
| Percentage RMR change | −8.7 (−11.0 to −6.4) | −5.3 (−7.5 to −3.0) | 0.0170 | |||
| TDEE change, kcal/day | −170 (−264 to −76) | 0.0001 | −205 (−291 to −118) | <0.0001 | 0.0011 | 0.5378 |
| AEE change, kcal/day | 26 (−111 to 59) | 0.7366 | −83 (−161 to −4) | 0.0371 | 0.0453 | 0.0353 |
| PA change, steps/day | 1,126 (373.7‐1,879) | 0.0020 | 566 (−123 to 1,254) | 0.1259 | 0.2479 | 0.2148 |
RMR changed by −179 kcal/day (95% CI, −232 to −125) and −100 kcal/day (95% CI, −149 to −51) in subjects with and without NAFLD, respectively (P = 0.0154 for time × NAFLD interaction; Table 2). To account for alterations in body mass and body composition with weight loss, we used baseline data to generate a least‐squares linear regression equation to calculate predicted RMR after weight loss intervention as follows:
Using this equation, postintervention RMR was predicted to be 1,851 kcal/day (95% CI, 1,770‐1,931) in subjects with NAFLD and 1,627 kcal/day (95% CI, 1,586‐1,689) in subjects without NAFLD. However, RMR decreased by −98 kcal/day (95% CI, −143 to −50) more than expected in patients with NAFLD, while there was no statistically significant difference in predicted and measured RMR after weight loss intervention in participants without NAFLD (P = 0.0218; Table 3). Together, these findings demonstrate that only subjects with NAFLD experienced metabolic adaptation to weight loss.
Table 3.
Metabolic Adaptation to Weight Loss Interventions in Subjects With and Without NAFLD
| Variable | NAFLD | No NAFLD | P (NAFLD) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | P (Time) | Baseline | 6 Months | P (Time) | ||
| Measured RMR, kcal/day | 1,933 (1,841‐2,025) | 1,754 (1,677‐1,830) | <0.0001 | 1,696 (1,641‐1,751) | 1,596 (1,552‐1,639) | <0.0001 | <0.0001 |
| Predicted RMR, kcal/day | 1,851 (1,770‐1,931) | 1,627 (1,586‐1,669) | <0.0001 | ||||
| P (Zero) | P (Zero) | ||||||
| Metabolic adaptation, kcal/day | −97 (−143 to −50) | <0.0001 | −32 (−74 to 11) | 0.1774 | 0.0218 | ||
Using linear regression, we examined changes in clinical variables to identify factors associated with changes in RMR and metabolic adaption after weight loss. In univariate models of RMR change, female sex, presence of NAFLD, and changes in leptin, FM, and FFM exhibited positive linear relationships with change in RMR, while Caucasian race and baseline RMR were inversely associated with change in RMR. In the multivariate (adjusted) model, only FM change, FFM change, and baseline RMR were associated with RMR change (Table 4).
Table 4.
Linear Regression Analysis of Factors Associated With RMR Change After Weight Loss
| Feature | Beta (UV) | 95% CI (UV) | P (UV) | Beta (MV) | 95% CI (MV) | P (MV) |
|---|---|---|---|---|---|---|
| Age | −2.061 | −7.295 to 3.172 | 0.437 | |||
| Sex | 106.385 | 2.175‐210.594 | 0.045 | −36.455 | −126.672 to 53.761 | 0.425 |
| Diet + exercise | −52.610 | −117.021 to 11.801 | 0.108 | |||
| White race | −99.376 | −165.893 to −32.859 | <0.004 | −29.313 | −79.424 to 20.799 | 0.249 |
| FM change | 17.525 | 12.884‐22.167 | <0.001 | 9.128 | 4.428‐13.828 | <0.001 |
| FFM change | 24.829 | 13.411‐36.247 | <0.001 | 12.722 | 2.427‐23.018 | 0.016 |
| Leptin change | 3.338 | 1.658‐5.016 | <0.001 | 0.476 | −0.988 to 1.939 | 0.521 |
| No NAFLD | 79.083 | 15.410‐142.756 | 0.015 | −20.649 | −72.118 to 30.819 | 0.428 |
| Baseline RMR | −0.356 | −0.443 to −0.269 | <0.001 | −0.298 | −0.396 to −0.201 | <0.001 |
Abbreviations: MV, multivariate; UV, univariate.
In univariate models of metabolic adaptation, female sex, FM change, and presence of NAFLD were positively linearly related with metabolic adaptation, while Caucasian race was inversely associated with metabolic adaptation. In adjusted models, however, only FM change and female sex were independently associated with metabolic adaptation (Table 5). Metabolic adaptation increased linearly with FM loss, but no such relationship was observed with FFM loss (Fig. 2). Although both FM and FFM change are important determinants of RMR responses to weight loss interventions, these findings suggest that only FM change influences metabolic adaptation to weight loss. Furthermore, the univariate association between presence of NAFLD and metabolic association may be explained by greater declines in FM loss after weight loss intervention. Finally, treatment intervention (diet versus diet plus exercise) had no effect on either RMR change or metabolic adaptation (Tables 4 and 5).
Table 5.
Linear Regression Analysis of Factors Associated With Metabolic Adaptation to Weight Loss
| Feature | Beta (UV) | 95% CI (UV) | P (UV) | Beta (MV) | 95% CI (MV) | P (MV) |
|---|---|---|---|---|---|---|
| Age | 1.188 | −3.323 to 5.701 | 0.603 | |||
| Female sex | −142.222 | −229.589 to −54.857 | 0.002 | −110.919 | −197.075 to −24.763 | 0.012 |
| Diet + exercise | −1.592 | −57.686 to 54.500 | 0.955 | |||
| White race | 78.726 | 21.122‐136.330 | 0.008 | 48.840 | −6.183 to 103.863 | 0.081 |
| FM change | −9.443 | −14.006 to −4.881 | 0.000 | −7.467 | −12.034 to −2.901 | 0.002 |
| FFM change | 0.816 | −9.796 to 11.428 | 0.879 | |||
| Leptin change | −0.625 | −2.167 to 0.916 | 0.423 | |||
| No NAFLD | −65.068 | −120.010 to −10.127 | 0.021 | −22.917 | −76.654 to 30.821 | 0.400 |
| Baseline RMR | 0.064 | −0.030 to 0.158 | 0.178 |
Abbreviations: MV, multivariate; UV, univariate.
Figure 2.
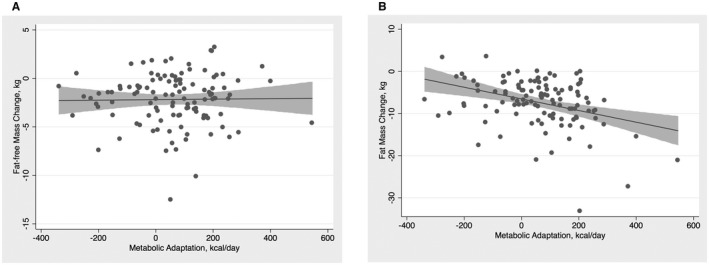
Scatter plots with fitted linear regression curves between metabolic adaptation. (A) FFM change and (B) FM change. Gray bands represent 95% CIs.
We performed Spearman correlations between change in L/S ratio and change in RMR (ρ = 0.166 and P = 0.0828) and between L/S ratio and metabolic adaptation (ρ = −0.034 and P = 0.7247). Thus, we did not find a significant correlation between either of these outcomes and L/S ratio change.
Discussion
In this study, we performed a post‐hoc analysis to compare RMR changes in subjects with and without NAFLD who had severe obesity without diabetes mellitus and who were enrolled in an interventional weight loss trial. We report several important findings with respect to the association between hepatic steatosis and systemic energy balance. First, RMR reductions after weight loss were greater in subjects with NAFLD, while TDEE changes were similar between groups. Second, we demonstrated that metabolic adaptation to weight loss is greater in patients with NAFLD. Finally, we found that only FM change was associated with metabolic adaptation in this cohort even though both changes in FFM and FM affect RMR responses to weight loss.
Weight loss remains the most effective treatment of NAFLD, and with increased weight loss, greater clinical benefit is conferred. While as little as 5% weight reductions are associated with improvement in aminotransferases,16, 17 weight loss of up to 7% is associated with reduced intra‐hepatic triglyceride content,18, 19 and 10% or greater weight loss is associated with marked reductions in inflammation and fibrosis.1 Weight loss attempts in NAFLD are largely unsuccessful.2 Furthermore, weight maintenance is challenging as approximately 80% of patients will regain within 6‐12 months after initial weight loss.20, 21
Alterations in physical activity, hunger, and metabolic efficiency have been described after weight loss,22 with a central feature being metabolic adaptation to weight loss characterized by an RMR that exceeds changes predicted by body composition alone.23 Responses of RMR to weight loss have important implications for future weight maintenance as multiple studies have demonstrated that both low RMR5, 6 and increased metabolic adaptation15, 23 are associated with decreased future weight loss and increased weight regain. Metabolic adaptation appears with even small amounts of weight loss,24 and the metabolic impact of weight loss can persist for years after an initial weight loss intervention.23, 25 Although physiologic regulation of metabolic adaptation remains incompletely understood, previous work suggests that hepatic glycogen depletion and reduced insulin secretion contribute to early declines in RMR with weight loss.4, 26 In contrast, long‐term control of metabolic adaptation may be under the influence of leptin, and administration of leptin after initial weight loss prevented weight regain during maintenance of weight loss.26, 27, 28
Given the critical importance of weight loss in the management of NAFLD, we sought to determine physiologic responses to weight loss in subjects with NAFLD. We found that individuals with hepatic steatosis exhibited a metabolic adaptation to weight loss that was not observed in subjects without NAFLD. One potential explanation for this finding may be higher baseline RMR in subjects with NAFLD as prior studies have shown positive associations between baseline RMR and declines in both RMR and metabolic adaptation after starvation‐induced weight loss.4 In the current study, baseline RMR was associated with a greater reduction in RMR with weight loss. However, there was no statistically significant relationship between baseline RMR and metabolic adaption, thus implying other factors, including NAFLD itself, may affect systemic energy regulation in response to weight loss interventions.
In the current study, changes in RMR were associated with changes in FFM and FM while only changes in FM influenced metabolic adaptation. This suggests that metabolic adaptation defends an FM set point rather than total body mass or FFM. This finding is consistent with earlier reports demonstrating that FM change but not FFM change was positively correlated with starvation‐induced metabolic adaptation.29 More recent work has demonstrated that reduction of fat energy stores below a critical threshold triggers metabolic adaptation.30, 31 In the current study, subjects with NAFLD had a greater baseline FM and a greater fraction of weight loss was in the form of FM compared to subjects without NAFLD. Furthermore, we previously demonstrated that visceral adipose tissue mass was greater in individuals with NAFLD compared to those without NAFLD and that individuals lost a greater proportion of weight in the form of visceral adipose tissue.11 Together, these observations indicate that, although FFM is the primary determinant of RMR, metabolic adaption defends against depletion of energy stores during calorie deprivation.32 In NAFLD, it is possible that enhanced visceral adipose tissue mass adversely raises adipose tissue “set points” to increase metabolic adaptation to weight loss and hence predisposes to weight regain. Future studies are planned to test this hypothesis.
A few limitations are noted. First, this was a single‐center study where weight loss and not RMR was the primary endpoint. Second, the majority of participants were women without diabetes with WHO class II and III obesity. Therefore, further studies are required to validate these findings in other populations with NAFLD, particularly in individuals with nonalcoholic steatohepatitis or high degrees of hepatic steatosis because the exclusion criterion of high alanine aminotransferase may have biased against the selection of these subpopulations. Third, steatosis was defined qualitatively using a CT‐derived L/S ratio and no biopsies were available to assess the severity of underlying fibrosis. In addition, a CT‐determined L/S ratio has limited sensitivity for the identification of mild steatosis (<30%); therefore, patients with lesser degrees of steatosis may have been misclassified. On the other hand, this study was performed on a well‐phenotyped cohort with prospectively collected data in a prespecified protocol.
In conclusion, individuals with hepatic steatosis compared to those without have a greater RMR decline and higher metabolic adaptation after weight loss, potentially increasing the risk of long‐term weight regain.
Supporting information
Supported by the Pennsylvania State Department of Health.
Potential conflict of interest: Dr. Behari received grants from General Electric. The other authors have nothing to report.
Contributor Information
Vikrant P. Rachakonda, Email: rachakondavp@upmc.edu.
Jaideep Behari, Email: behajx@upmc.edu.
References
- 1. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, Torres‐Gonzalez A, Gra‐Oramas B, Gonzalez‐Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367‐378.e365. [DOI] [PubMed] [Google Scholar]
- 2. Dudekula A, Rachakonda V, Shaik B, Behari J. Weight loss in nonalcoholic fatty liver disease patients in an ambulatory care setting is largely unsuccessful but correlates with frequency of clinic visits. PLoS One 2014;9:e111808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Penn L, White M, Lindstrom J, den Boer AT, Blaak E, Eriksson JG, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PLoS One 2013;8:e57143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, et al. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr 2015;102:807‐819. [DOI] [PubMed] [Google Scholar]
- 5. Tremblay A, Chaput JP. Adaptive reduction in thermogenesis and resistance to lose fat in obese men. Br J Nutr 2009;102:488‐492. [DOI] [PubMed] [Google Scholar]
- 6. Dulloo AG, Schutz Y. Adaptive thermogenesis in resistance to obesity therapies: issues in quantifying thrifty energy expenditure phenotypes in humans. Curr Obes Rep 2015;4:230‐240. [DOI] [PubMed] [Google Scholar]
- 7. Buscemi S, Verga S, Caimi G, Cerasola G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. Int J Obes (Lond) 2005;29:287‐291. [DOI] [PubMed] [Google Scholar]
- 8. Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A. Clinical significance of adaptive thermogenesis. Int J Obes (Lond) 2007;31:204‐212. [DOI] [PubMed] [Google Scholar]
- 9. Goodpaster BH, Delany JP, Otto AD, Kuller L, Vockley J, South‐Paul JE, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010;304:1795‐1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeLany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH. Effect of physical activity on weight loss, energy expenditure, and energy intake during diet induced weight loss. Obesity (Silver Spring) 2014;22:363‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rachakonda V, Wills R, DeLany JP, Kershaw EE, Behari J. Differential impact of weight loss on nonalcoholic fatty liver resolution in a North American cohort with obesity. Obesity (Silver Spring) 2017;25:1360‐1368. [DOI] [PubMed] [Google Scholar]
- 12. Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24‐month weight loss maintenance in overweight women. Arch Intern Med 2008;168:1550‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwasaki M, Takada Y, Hayashi M, Minamiguchi S, Haga H, Maetani Y, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation 2004;78:1501‐1505. [DOI] [PubMed] [Google Scholar]
- 14. Rachakonda VP, Reeves VL, Aljammal J, Wills RC, Trybula JS, DeLany JP, et al. Serum autotaxin is independently associated with hepatic steatosis in women with severe obesity. Obesity (Silver Spring) 2015;23:965‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeLany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH. High energy expenditure masks low physical activity in obesity. Int J Obes (Lond) 2013;37:1006‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, et al. Fatty Liver Subgroup of the Look AHEAD Research Group. Effect of a 12‐month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010;33:2156‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51:121‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrison SA, Fecht W, Brunt EM, Neuschwander‐Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology 2009;49:80‐86. [DOI] [PubMed] [Google Scholar]
- 19. Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non‐alcoholic fatty liver disease (NAFLD): a systematic review and meta‐analysis of randomised trials. Diabetologia 2012;55:885‐904. [DOI] [PubMed] [Google Scholar]
- 20. Anderson JW, Konz EC, Frederich RC, Wood CL. Long‐term weight‐loss maintenance: a meta‐analysis of US studies. Am J Clin Nutr 2001;74:579‐584. [DOI] [PubMed] [Google Scholar]
- 21. Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, et al. Long‐term weight loss maintenance in the United States. Int J Obes (Lond) 2010;34:1644‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Westerterp KR. Control of energy expenditure in humans. Eur J Clin Nutr 2017;71:340‐344. [DOI] [PubMed] [Google Scholar]
- 23. Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring) 2016;24:1612‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nymo S, Coutinho SR, Torgersen LH, Bomo OJ, Haugvaldstad I, Truby H, et al. Timeline of changes in adaptive physiological responses, at the level of energy expenditure, with progressive weight loss. Br J Nutr 2018;120:141‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butte NF, Brandt ML, Wong WW, Liu Y, Mehta NR, Wilson TA, et al. Energetic adaptations persist after bariatric surgery in severely obese adolescents. Obesity (Silver Spring) 2015;23:591‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller MJ, Enderle J, Bosy‐Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep 2016;5:413‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low‐dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 2005;115:3579‐3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kissileff HR, Thornton JC, Torres MI, Pavlovich K, Mayer LS, Kalari V, et al. Leptin reverses declines in satiation in weight‐reduced obese humans. Am J Clin Nutr 2012;95:309‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dulloo AG, Jacquet J. Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. Am J Clin Nutr 1998;68:599‐606. [DOI] [PubMed] [Google Scholar]
- 30. Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond) 2010;34(Suppl. 1):S47‐S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenbaum M, Leibel RL. Models of energy homeostasis in response to maintenance of reduced body weight. Obesity (Silver Spring) 2016;24:1620‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tremblay A, Royer MM, Chaput JP, Doucet E. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int J Obes (Lond) 2013;37:759‐764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


