Abstract
Current therapeutics for chronic infection with hepatitis B virus (HBV) rarely induce functional cure due to the immunotolerant status of patients. Small molecule agonists targeting toll‐like receptor 7 (TLR7) have been shown to elicit a functional cure in animal models of HBV but sometimes with poor tolerability due to immune‐related toxicities. In an effort to increase the therapeutic window of TLR7 agonists to treat chronic hepatitis B (CHB), we developed an oral TLR7 agonist, APR002, designed to act locally in the gastrointestinal tract and liver, thus minimizing systemic exposure and improving tolerability. Here, we describe the pharmacokinetic/pharmacodynamic (PK/PD) profile of APR002 in mice and uninfected woodchucks as well as the safety and antiviral efficacy in combination with entecavir (ETV) in woodchucks with CHB. Treatment of woodchucks chronically infected with woodchuck hepatitis virus (WHV) with weekly oral doses of APR002 was well‐tolerated. While APR002 and ETV single agents did not elicit sustained viral control, combination therapy resulted in durable immune‐mediated suppression of the chronic infection. These woodchucks also had detectable antibodies to viral antigens, enhanced interferon‐stimulated gene expression, and loss of WHV covalently closed circular DNA. Conclusion: APR002 is a novel TLR7 agonist exhibiting a distinct PK/PD profile that in combination with ETV can safely attain a functional cure in woodchucks with chronic WHV infection. Our results support further investigation of liver‐targeted TLR7 agonists in human CHB.
Abbreviations
- ALT
alanine aminotransferase
- APR002
toll‐like receptor 7 agonist from Apros Therapeutics, Inc.
- ccc DNA
covalently closed circular DNA
- CHB
chronic hepatitis B
- ETV
entecavir
- GS‐9620
toll‐like receptor 7 agonist from Gilead Sciences, Inc.
- CD
clusters of differentiation
- HBV
hepatitis B virus
- IFN‐α
interferon‐alpha
- IL‐6
interleukin‐6
- IP‐10
interferon‐gamma‐induced protein 10
- ISG
interferon‐stimulated gene
- ISG15
interferon‐induced 17 kDa protein
- LLOD
lower limit of detection
- NK
natural killer
- OAS1
2′‐5′‐oligoadenylate synthetase
- OATP
organic‐anion‐transporting polypeptide
- OD
optical density
- PCR
polymerase chain reaction
- PD
pharmacodynamic
- PK
pharmacokinetic
- SDH
sorbitol dehydrogenase
- TLR
toll‐like receptor
- TNF‐α
tumor necrosis factor alpha
- WHeAg
woodchuck hepatitis virus e antigen
- WHsAg
woodchuck hepatitis virus surface antigen
- WHV
woodchuck hepatitis virus
It is estimated that 257 million people worldwide are chronic carriers of hepatitis B virus (HBV).1 Current treatment options for chronic hepatitis B (CHB) involve direct‐acting antivirals (i.e., nucleos(t)ide analogs) for suppression of viral replication; however, this conventional antiviral therapy rarely cures the disease, and infinite treatment is required. Use of nucleos(t)ide analogs is hampered by the emergence of resistant variants during treatment, the risk of relapse following treatment discontinuation, and unwanted side effects.2 Because HBV persistence is thought to be the result of a functionally impaired immune response, patients are also treated with pegylated interferon‐alpha (IFN‐α), most often in addition to conventional antiviral therapy.3, 4 However, only a small number of patients achieve a sustained antiviral response after 1 year of monotherapy or combination therapy. Moreover, prolonged systemic IFN‐α administration is associated with treatment‐limiting adverse effects.3, 4, 5 Therefore, an effective and well‐tolerated immunotherapy that leads to a functional cure of CHB after a finite course of treatment is needed.
One area of major focus for CHB immunotherapy has been the development of synthetic agonists targeting toll‐like receptors (TLRs), mainly TLR7 but recently also TLR8 and TLR9.6, 7, 8 TLR7 is highly expressed in plasmacytoid dendritic cells and B lymphocytes and is located within the endosome where it normally functions as a sensor for single‐stranded viral RNA.7 Activation of TLR7 results in enhanced antigen processing and presentation mechanisms, the up‐regulation of costimulatory molecules critical for the cross‐priming of cytotoxic T cells, and the production of type I IFNs, such as IFN‐α, numerous T‐cell‐attractant chemokines (such as IFN‐γ‐induced protein 10 [IP‐10], also known as chemokine (C‐X‐C motif) ligand 10 [CXCL10]), and often proinflammatory cytokines (such as tumor necrosis factor alpha [TNF‐α]).9 Importantly, it has been shown that IFN‐α can epigenetically inhibit the transcription of HBV covalently closed circular DNA (cccDNA), thereby contributing to the suppression of viral replication.10 The totality of the above processes then contributes to the orchestration of a robust adaptive immune response.9 The therapeutic use of a synthetic TLR7 agonist in the context of CHB has been exemplified by GS‐9620 from Gilead Sciences; GS‐9620 demonstrated suppression of HBV and woodchuck hepatitis virus (WHV) in chimpanzees and woodchucks, respectively.11, 12 In the fully immunocompetent woodchuck animal model of vertical HBV transmission,13 sustained reduction in viral load and loss of surface antigen were associated with seroconversion in nearly one third of the animals, an unprecedented finding with any single‐agent therapy explored so far in this model.12 These data have demonstrated a compelling proof‐of‐concept that TLR7 agonism can drive sustained immune control for inducing a functional cure of CHB. However, similar to pegylated IFN‐α, dose‐limiting adverse events have emerged in all species treated with GS‐9620, including woodchucks, chimpanzees, and humans.11, 12, 14, 15, 16 Although GS‐9620 has been reported to induce IFN‐stimulated genes (ISGs) without serum IFN and to augment T‐ and natural killer‐ (NK) cell responses at low doses in preclinical studies and in phase 1/2 clinical trials,14, 16, 17 antiviral efficacy was not observed in these trials as single agent or in combination with a nucleoside analog.16, 18, 19 This could imply that GS‐9620 was capped at a suboptimal dose due to tolerability issues in patients, as reported recently16; therefore, a better tolerated TLR7 agonist with a wider therapeutic index is warranted to test this class of immunotherapy for CHB.
We reasoned that targeting a TLR7 agonist to the liver would more precisely focus innate immune priming in proximity to infected hepatocytes and in turn widen the therapeutic window. To achieve this goal, a novel orally administered TLR7 agonist, APR002, was designed and optimized using medicinal chemistry principles for hepatoselective drugs.20 APR002 exhibited similar TLR7 activity compared to the clinical benchmark GS‐9620 but displayed a distinct pharmacologic profile. Herein, we present the pharmacokinetic/pharmacodynamic (PK/PD) parameters of APR002 in mice and WHV‐negative woodchucks and the safety and efficacy evaluation in WHV‐positive woodchucks.
Materials and Methods
In Vitro Assays
We carried out assays in human embryonic kidney 293 cells (HEK293) with TLR7/8, and in peripheral blood mononuclear cells (PBMC) and splenocytes. For details refer to the Supporting Material.
Transporter Substrate Assay
For determining whether APR002 serves as a substrate of the organic‐anion‐transporting polypeptide (OATP) transporter, OATP1B1 and OATP1B3, assays in Madin‐Darby canine kidney II cells were performed by Optivia Biotechnology (Santa Clara, CA), as described in the Supporting Material.
Mouse Studies
In all studies using mice and woodchucks, animals received humane care as outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
PK Study
Male Balb/c mice (Vital River Laboratory Technology), 6‐8 weeks of age, were dosed with APR002 or GS‐9620 at 10 mg/kg by oral gavage or at 3 mg/kg by intravenous injection at 3D BioOptima (Suzhou, China). Mice were bled at specified time points or sacrificed for tissue collection. Compound concentrations in serum and liver were determined using liquid chromatography–tandem mass spectrometry.
Liver Microsomal Stability Assay
For details refer to the Supporting Material.
PD Study
For determining the dose dependency of the PD effects of APR002 and GS‐9620, female C57BL/6 mice (Charles River Laboratories), 7‐8 weeks of age, were dosed orally at 0.3, 3, 30, or 300 mg/kg at Explorabiolabs (San Diego, CA). Following oral dosing, mice were bled at 3 hours to examine serum cytokines (IP‐10, interleukin‐6 [IL‐6], and TNF‐α), and livers were removed at 5 hours for analyzing IFN‐induced 17 kDa protein (ISG15) expression.
Cytokine Assay
For assessing cytokine levels from either in vitro PBMC supernatants or in serum from in vivo mouse studies, cytokines were quantified, as described in the Supporting Material.
ISG15 Expression in Mouse Liver
RNA was isolated from mouse livers following oral dosing, as described above, converted into complementary DNA, and subjected to quantitative polymerase chain reaction (PCR) analysis for ISG15, as described in the Supporting Material. Relative ISG15 gene expression was determined after normalizing the cycle threshold (Ct) values to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) as the internal housekeeping gene.
Woodchuck Studies
Experimental Drugs
APR002 was dissolved in vehicle (0.5% carboxymethyl cellulose and 0.5% tween‐80 in water), sonicated, stirred, and mixed with woodchuck diet powder (Dyets, Bethlehem, PA). Entecavir (ETV) was purchased from BrightGene Bio‐Medical Technology (Suzhou, China) and dissolved in vehicle (phosphate‐buffered saline) and mixed with woodchuck diet powder. Both drugs were orally administered to woodchucks within 30 minutes after preparation. Control woodchucks were administered vehicle ETV or vehicle APR002 mixed with woodchuck diet powder.
PK/PD Study in WHV‐Negative Woodchucks
For details refer to the Supporting Material.
Efficacy Study in WHV‐Positive Woodchucks
Safety and sustained antiviral activity of treatment with APR002 alone or in combination with ETV was determined in woodchucks chronically infected with WHV. At the start of the study, adult woodchucks of both sexes were confirmed positive for serum WHV DNA, WHV surface antigen (WHsAg), and WHV e antigen (WHeAg) and had undetectable antibodies to WHsAg (anti‐WHs antibodies) and WHeAg (anti‐WHe antibodies). Woodchucks were assigned and stratified by sex, body weight, and by pretreatment serum viral markers (WHV DNA, WHsAg, and WHeAg loads) and serum liver enzyme activities (sorbitol dehydrogenase [SDH], aspartate aminotransferase [AST], and alanine aminotransferase [ALT]) into four groups (n = 5) (Supporting Table S1).
Woodchucks receiving ETV monotherapy (Group 1) were treated once daily orally with ETV (0.1 mg/kg/day) for 4 weeks; thereafter, ETV was administered in combination with once weekly oral vehicle APR for 12 weeks followed by ETV only, for an additional 4 weeks. Woodchucks undergoing APR002 monotherapy (Group 2) were dosed once daily orally with vehicle ETV for 4 weeks; thereafter, vehicle ETV was administered in combination with once weekly oral APR002 for 12 weeks (15.0 mg/kg/week for 6 weeks, 30.0 mg/kg/week for 6 weeks) followed by vehicle ETV only, for an additional 4 weeks. Woodchucks receiving combination therapy (Groups 3 and 4) were treated once daily orally with ETV for 4 weeks; thereafter, ETV was administered in combination with once weekly oral APR002 for 12 weeks (Group 3: 5.0 mg/kg/week for 7 weeks, 30.0 mg/kg/week for 5 weeks; Group 4: 15.0 mg/kg/week for 12 weeks) followed by ETV only, for an additional 4 weeks. Animals were monitored until the end of the study at week 36.
Drug Safety Parameters
Clinical observations and changes in body weight, body temperature, clinical chemistry, and hematology were obtained regularly for monitoring drug safety. Mortality associated with APR002 therapy was not observed. Woodchuck F6022 (Group 2) was found dead during week 8; however, this animal had already presented with changed hematology and serum chemistry parameters at week 4 before the first dose of APR002 was administered in week 5. Death was attributed to underlying renal dysfunction/kidney disease. Following treatment withdrawal, one woodchuck each in Groups 1 and 4 was euthanized at weeks 30 and 33, respectively, due to the development of hepatocellular carcinoma.
Serum WHV Parameters
Serum WHV DNA concentration was assayed by slot blot hybridization.21 Samples with WHV DNA concentrations below the lower limit of detection (LLOD) of this assay were further subjected to PCR (LLOD, ~6.0 × 102 WHV genomic equivalents [ge]/mL).21 Serum WHsAg level (LLOD, ~5 ng/mL) and anti‐WHs antibody titer (LLOD, ~100 standard units [StdU/mL]) were determined by enzyme immunoassays.22 Serum WHeAg and anti‐WHe antibody levels were assayed using cross‐reactive enzyme‐linked immunosorbent assay (DiaSorin, Minneapolis, MN). Results were provided as an optical density (OD) readout. An OD ≤0.058 indicated absence of WHeAg in serum, while an OD ≥1.870 indicated presence of anti‐WHe antibodies in serum.
Hepatic WHV Parameters
For details refer to the Supporting Material.
Host Immune Response Parameters
ISG induction associated with a single dose of APR002 in WHV‐negative woodchucks was measured over a 24‐hour period after dosing, by using PCR23 to examine changes in the transcript levels of IFN‐α and IFN‐induced guanosine triphosphate‐binding protein (Mx1) in liver and woodchuck‐specific primers and probes (Supporting Table S2). Woodchuck GAPDH expression was used to normalize target gene expression. Induction of ISGs associated with ETV/APR002 therapy of WHV‐positive woodchucks was determined 24 hours after dosing by changes in the transcript levels of 2′‐5′‐oligoadenylate synthetase (OAS1) and ISG15 in blood (weeks 8, 16, and 22) and liver (weeks 16 and 36). 18S ribosomal RNA expression was used to normalize target gene expression. Transcription levels of target genes were calculated as fold change relative to predose (weeks –2 to –1.5) or pretreatment level (T0) in blood or liver (predose, weeks –2 to –1.5; pretreatment level, week –1) using the formula 2–ΔΔ Ct.
Statistical Analyses
Parameters were compared to the values at pretreatment and between the four treatment groups using the unpaired Student t test with equal variance. P < 0.05 was considered statistically significant.
Results
APR002 Is A TLR7 Agonist With A Distinct PK/PD Profile
APR002 is derived from a low molecular weight TLR7 pharmacophore and modified with a liver‐targeting moiety.24 The liver‐targeting moiety was designed following medicinal chemistry principles inspired from hepatoselective drugs for metabolic disease.20 The chemistry and structural activity relationship of this novel series of TLR7 agonists is presented elsewhere.24 It was hypothesized that this design feature would enable more precise spatiotemporal control of drug distribution to the liver (after absorption through the gut) while minimizing systemic exposure that is not needed for effective immune priming. Extensive in vitro profiling of APR002 compared to GS‐9620 across species demonstrated that a liver‐targeting moiety can be incorporated into a TLR7 pharmacophore without the loss of TLR7 activity (Supporting Fig. S1).
For determining whether the liver‐targeting moiety of APR002 can result in high hepatic uptake and low systemic exposure, mice were orally administered a single dose of APR002 or GS‐9620 and the compound concentrations in serum and liver were assayed (Fig. 1A,B). In contrast to GS‐9620, which exhibited a liver‐to‐serum ratio of 5.6, APR002 was highly retained in liver, with a ratio of ~30. As shown in Fig. 1C, the enhanced hepatic exposure of APR002 may be explained by active uptake by OATP1B1‐ and OATP1B3‐mediated transport (12‐fold and 3‐fold, respectively). In addition, APR002 also exhibited a low volume of distribution (Supporting Table S3A) and low clearance in mouse and human liver microsomes (Supporting Table S3B). This is in contrast to GS‐9620, which was reported to display a high volume of distribution in mice and fast clearance in mouse and human liver microsomes.17, 25 Hence, the PK profile of APR002 is distinct from that of GS‐9620.
Figure 1.
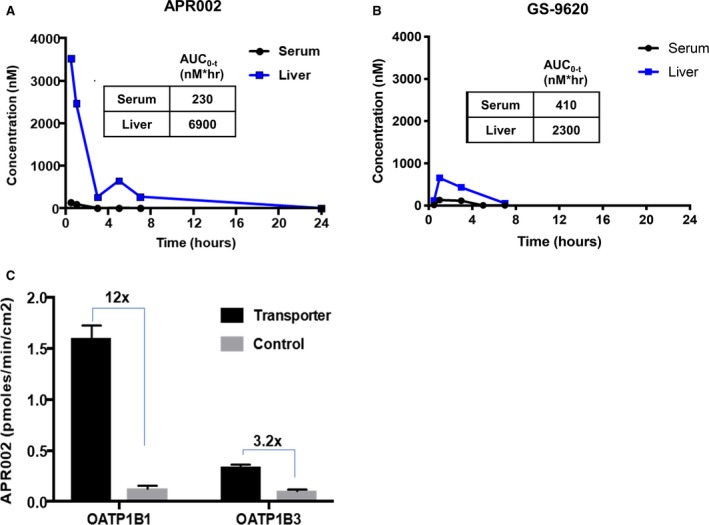
APR002 is highly retained in liver, mediated in part by OATP transporters. The concentration of (A) APR002 or (B) GS‐9620 in serum and liver of Balb/c mice over a 24‐hour period following a single oral dose of compound at 10 mg/kg. Values are the mean of two mice per time point. (C) APR002 uptake in a cell‐based assay specific for active OATP1B1 and OATP1B3 transport versus passive transport (control). Values represent average uptake ± SD. Abbreviation: AUC, area under the curve.
Following TLR7 activation, type I IFNs are induced but are typically transient and difficult to measure. Thus, to more accurately capture the IFN response, ISG15 expression in liver and the induction of the IFN‐dependent chemokine IP‐10 as well as the proinflammatory cytokines IL‐6 and TNF‐α were measured in blood following a single oral dose of APR002 and GS‐9620 over a 1,000‐fold range in mice. As shown in Fig. 2A, while GS‐9620 exhibited a dose‐dependent increase in serum IP‐10 with a steep dose response (slope, 2.8), APR002 induced a more dose‐proportional response (slope, 0.9). Likewise, the hepatic expression of ISG15 was augmented in a similar dose‐proportional manner following increasing APR002 dosage in contrast to GS‐9620 (Fig. 2B). Interestingly, while both compounds at high doses achieved near comparable levels of IFN‐driven endpoints, serum IP‐10, and hepatic ISG15 expression, GS‐9620 elicited significantly higher levels of proinflammatory cytokines, such as IL‐6 and TNF‐α, exhibiting >6‐fold and >4‐fold higher levels, respectively (Fig. 2C,D). Because IL‐6 and TNF‐α are believed to contribute to poor tolerability in humans,26 we hypothesized that APR002 may uncouple efficacy from poor tolerability. Overall, these data highlight that while in vitro activity is comparable between the two compounds, they exhibit distinct dose dependency and PD activity, which may be the result of their respective PK properties. Due to the favorable profile, APR002 was selected for further evaluation of PK and PD parameters in WHV‐negative woodchucks and for safety and efficacy in WHV‐positive woodchucks.
Figure 2.
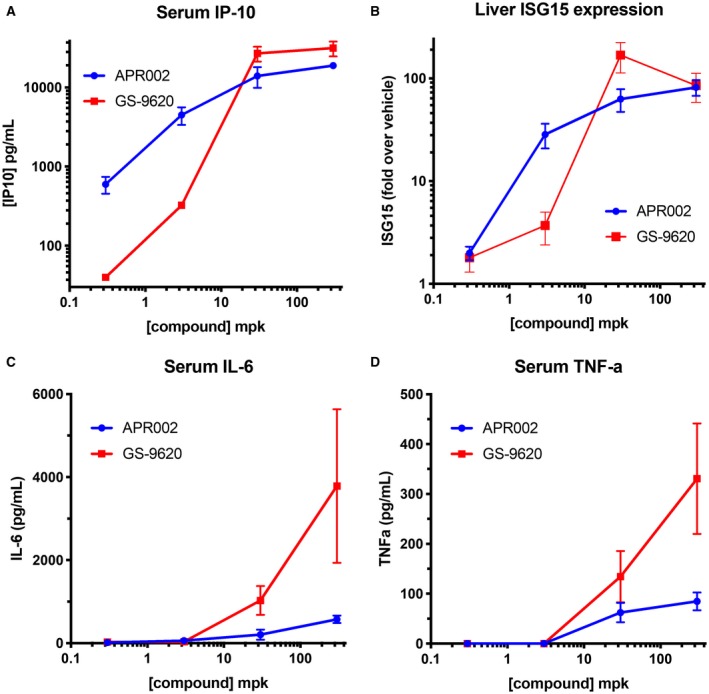
APR002 exhibits dose‐proportional IFN‐dependent PD responses with minimal proinflammatory cytokine induction. Following a single oral dose of increasing dosages over a 1,000‐fold range given to C57BL/6 mice, the levels of (A) serum IP‐10, (B) hepatic ISG15 expression, (C) serum IL‐6, and (D) serum TNF‐α are shown as a function of the dose of APR002 or GS‐9620. Cytokine data represent the average pg/mL amount in serum ± SD. Liver ISG15 expression is shown as average fold over vehicle ± SD. Abbreviation: mpk, mg per kg.
TLR7 Agonist APR002 Is Active in WHV‐Negative Woodchucks
To first ensure that APR002 is sufficiently absorbed and active in woodchucks, APR002 PK/PD activity was assessed in healthy WHV‐naive animals. Woodchucks were orally administered a single increasing dose, and serum PK levels over a 24‐hour period in addition to terminal liver PK levels at 24 hours were assessed. Woodchucks exhibited dose‐dependent increases in serum and liver concentrations of APR002 (Supporting Fig. S2A,B). Furthermore, to ensure that TLR7 is engaged in liver tissue, IFN‐α expression and ISG expression were assessed at two time points after dosing. As expected, APR002 induced both IFN‐α and ISGs, such as Mx1, in liver (Supporting Fig. S2C,D). While a dose‐dependent effect was not apparent, this may be due to the shallow dose‐response curve seen with this compound in this dose range (Fig. 2).
ETV/APR002 Combination Therapy Resulted in Sustained Suppression of WHV Replication in Periphery and Liver in A Subset of Woodchucks
To assess the therapeutic efficacy of APR002, woodchucks with chronic WHV infection were stratified into four comparable cohorts (Supporting Table S2). The efficacy of APR002 and ETV alone was assessed as well as the combination of APR002 and ETV to more accurately reflect the intended clinical use of APR002. As expected, ETV monotherapy (Group 1) induced rapid and marked declines in serum WHV DNA in all woodchucks (maximum average reduction, 7.72 log10) (Fig. 3; Supporting Table S4). After drug withdrawal, all five woodchucks experienced recrudescence of WHV replication. Declines in viremia during APR002 monotherapy (Group 2) were less pronounced (maximum average reduction, 1.02 log10), and the four surviving woodchucks showed minor signs of viral relapse immediately after treatment cessation. ETV/APR002 combination therapy at increasing doses (Groups 3 and 4) mediated comparable efficacy as ETV monotherapy, although the antiviral response was somewhat diminished in individual animals (maximum average reduction, 6.64 log10 and 7.33 log10, respectively). Importantly, after completion of ETV/APR002 combination therapy, suppression of viral load was sustained in two of five surviving woodchucks, each in Groups 3 and 4, until the end of the study, while the other animals demonstrated viral relapse. Because viral loads in both groups were comparable to Group 1 during almost the entire study, add‐on APR002 treatment apparently was unable to augment the antiviral effect mediated by ETV on serum viremia in all woodchucks.
Figure 3.
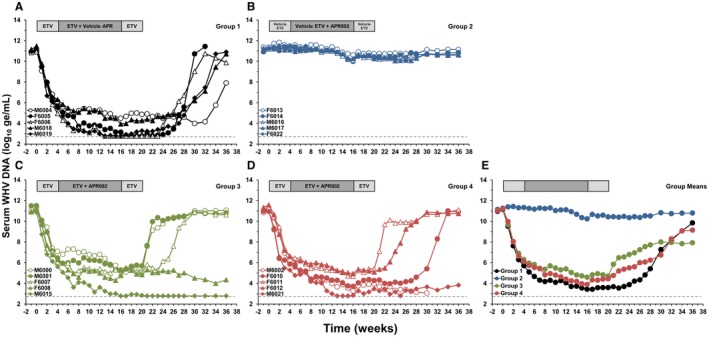
ETV/APR002 combination therapy results in sustained suppression or undetectable serum WHV DNA in a subset of woodchucks. Changes in serum WHV DNA relative to pretreatment (T0) in woodchucks during (A) ETV monotherapy (Group 1), (B) APR002 monotherapy (Group 2), (C,D) ETV/APR002 combination therapy (Groups 3 and 4), (E) geometric group means. WHV DNA levels in Groups 1, 2, 3, and 4 were significantly reduced compared to pretreatment during weeks 1‐30 (Group 1), week 11 and during weeks 13‐36 (Group 2), during weeks 1‐27 (Group 3), and during weeks 1‐27 (Group 4) (P < 0.05). WHV DNA levels in Groups 3 and 4 were comparable to Group 1 during the study except for week 7 when viremia in Group 4 was significantly higher (P < 0.05). Abbreviation: ge/mL, genome equivalents or copy numbers per milliliter.
ETV monotherapy also caused pronounced, albeit transient, declines in serum antigenemia in all woodchucks, with four and five animals that presented with undetectable WHsAg or WHeAg, respectively, during the study (Fig. 4; Supporting Fig. S3; Supporting Table S4). Following treatment cessation, all five woodchucks had relapses in antigenemia. The pattern of WHeAg decline and increase in animals of this group were comparable to WHsAg. As noted before for serum viremia, the reductions in WHsAg and WHeAg during APR002 monotherapy were much less pronounced. ETV/APR002 combination therapy in Groups 3 and 4 suppressed WHsAg and WHeAg in two and three woodchucks, respectively, but the antiviral effect on antigenemia was diminished when compared to ETV monotherapy. At the end of the study, ETV/APR002 combination therapy resulted in the loss of detectable WHsAg and WHeAg in two of five woodchucks, each in Groups 3 and 4, and these were the same animals with suppressed or undetectable WHV DNA. The WHsAg load in Group 1 was significantly lower when compared to Group 3 but similar to Group 4 throughout the study, indicating again that add‐on APR002 treatment apparently was incapable of enhancing the antiviral effect induced by ETV on serum antigenemia in all woodchucks.
Figure 4.
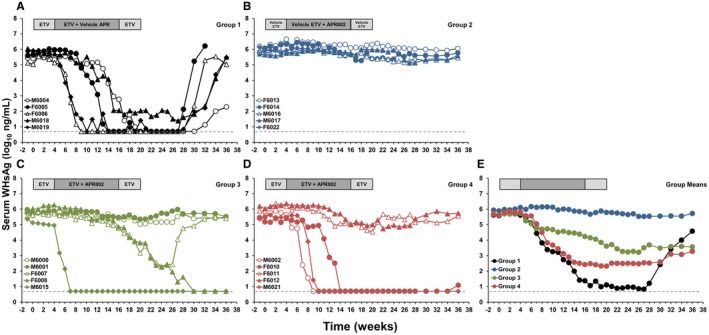
ETV/APR002 combination therapy produces loss of serum WHsAg in a subset of woodchucks. Changes in serum WHsAg relative to pretreatment (T0) in woodchucks during (A) ETV monotherapy (Group 1), (B) APR002 monotherapy (Group 2), (C,D) ETV/APR002 combination therapy (Groups 3 and 4), and (E) geometric group means. WHsAg levels in Groups 1, 3, and 4 were significantly reduced compared to pretreatment during weeks 11‐30 (Group 1), at week 20 and during weeks 22‐26 (Group 3), and during weeks 13‐30 (Group 4) (P < 0.05). WHsAg level in Group 3 was significantly higher compared to Group 1 during weeks 15‐23 and 25‐27 (P < 0.05). WHsAg level in Group 4 was comparable to Group 1 during the study.
ETV monotherapy and ETV/APR002 combination therapy also produced marked but transient declines in viral nucleic acids within liver of most woodchucks (Supporting Fig. S4). APR002 monotherapy somewhat reduced viral nucleic acids in some but not all animals. Compared to ETV monotherapy, the antiviral response was more varied in individual woodchucks of Groups 3 and 4 and overall somewhat diminished, as especially noted for WHV cccDNA and WHV RNA, but comparable for WHV DNA replicative intermediates. Importantly again, three woodchucks across Groups 3 and 4 and one additional animal in Group 3 had undetectable or markedly suppressed viral nucleic acids, respectively, at the end of the study, and these were the same animals with suppressed or undetectable WHV DNA and loss of WHsAg and WHeAg in serum.
ETV/APR002 Combination Therapy Induced Durable Host Immune Responses in Periphery and Liver in A Subset of Woodchucks
Elicitation of anti‐WHs antibodies was observed in a subset of woodchucks administered ETV/APR002 combination therapy but did not occur in any animal that underwent ETV or APR002 monotherapy (Fig. 5A; Supporting Table S4). Anti‐WHs antibodies were detected in one of five woodchucks in Group 3 and in three of five woodchucks in Group 4, with a total of three animals from both groups that presented with antibodies at the end of the study. Antibodies were mainly detected after cessation of combination therapy and after prolonged loss of serum WHsAg. The same three woodchucks of Groups 3 and 4 with durable antibody response to WHsAg also demonstrated long‐lasting elicitation of anti‐WHe antibodies (Fig. 5B; Supporting Table S4); this occurred following add‐on APR002 treatment and after prior loss of serum WHeAg. In addition, one animal developed anti‐WHe antibodies during ETV monotherapy; however, the response was transient and antibodies were absent at the end of the study, consistent with the presence of serum WHeAg during this time. Taken together, these results suggest that anti‐WHs and anti‐WHe antibodies were only elicited together when ETV and APR002 were administered in combination.
Figure 5.
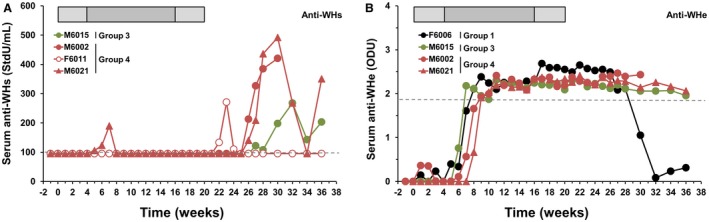
ETV/APR002 combination therapy elicits durable antibody response to WHsAg and WHeAg in a subset of woodchucks. Changes in (A) serum anti‐WHs antibody titers and (B) anti‐WHe antibody levels relative to pretreatment (T0) in individual woodchucks during ETV monotherapy (Group 1) and ETV/APR002 combination therapy (Groups 3 and 4). Abbreviations: ODU, optical density unit; StdU, standard unit.
ETV monotherapy was usually not associated with the induction of ISGs, such as OAS1 and ISG15, in periphery (Fig. 6A,B) and liver (Fig. 6C,D). One animal had a marked but transient increase in blood ISG15 transcript level, while two animals showed somewhat increased OAS1 transcript levels in liver. A few animals also had elevated expression of both ISGs in blood and/or liver later during the study, suggesting an immune response of the host to the recurrence of WHV replication following treatment withdrawal. Although APR002 monotherapy did not mediate pronounced antiviral efficacy, it induced marked and transient ISG expression in a few woodchucks in blood and liver during treatment, indicating on‐target activation of TLR7 in the host following dosing with APR002 that was independent of ETV treatment. Importantly, pronounced increases in ISG expression were not noted after treatment cessation. A similar pattern was observed during ETV/APR002 combination therapy, with most or all animals of Group 4 presenting with elevated and transient ISG expression in blood and liver. Somewhat different was the pattern for ISG expression in woodchucks of Group 3 during ETV/APR002 combination therapy. OAS1 transcript levels were elevated in blood and liver, while increased ISG15 expression was observed early on in blood but not in liver at later time points. A correlation of timing and/or magnitude of ISG expression and the sustained suppression or undetectability of WHV markers and elicitation of anti‐WHV antibodies were not apparent, although most of these woodchucks in Groups 3 and 4 had pronounced increases in transcript levels in either blood or liver or in both tissues. However, it appeared that maximum expression of ISG15, and to a lesser degree of OAS1, in blood temporally correlated with the marked decline in serum WHsAg (and WHeAg) level in animals with undetectable or suppressed WHV replication and anti‐WHs and anti‐WHe antibody elicitation at the end of the study (Fig. 7A‐D). Pronounced but transient increases in the serum activity of liver enzymes occurred in a few woodchucks during ETV monotherapy and ETV/APR002 combination treatment (Supporting Fig. S5A‐C). Marked elevations in SDH were observed in two animals and coincided with the initial period of viral load reduction by ETV treatment, indicating nucleoside‐associated effects. Other increases in liver enzymes were mainly present in animals of Groups 3 and 4 with suppressed or undetectable WHV markers in periphery and liver at the end of the study, suggesting immune effects mediated by add‐on APR002. Interestingly, Group 3 animals had maximally elevated SDH and/or ALT levels after 4 weeks of ETV treatment and before dosing with APR002 was initiated, while Group 4 animals presented with maximum liver enzyme increases after 4‐8 weeks of add‐on APR002 treatment (Fig. 7A‐D). It appeared that the peak in liver enzyme activity in Group 3 correlated with the initial decline in serum viremia, as also observed in Group 1 during ETV monotherapy. This peak was delayed in Group 4 and appeared to correlate with the marked reductions in serum antigenemia and maximum expression of blood ISGs. Nevertheless, liver enzyme increases reversed thereafter in these woodchucks and normalized during the remainder of treatment. One woodchuck in Group 2 also presented with elevated liver enzymes early on and was found dead thereafter (mortality was attributed to underlying renal dysfunction/kidney disease).
Figure 6.
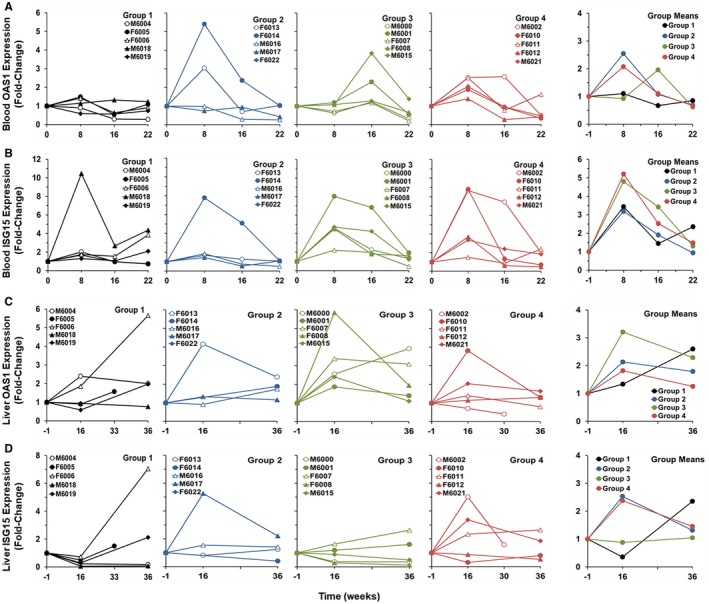
ETV/APR002 combination therapy induces ISG expression in periphery and liver in a subset of woodchucks. Fold changes in blood expression of (A) OAS1 and (B) ISG15 and in liver expression of (C) OAS1 and (D) ISG15 relative to pretreatment (T0 or week –1) in woodchucks during ETV monotherapy (Group 1), APR002 monotherapy (Group 2), ETV/APR002 combination therapy (Groups 3 and 4), and group means. For blood, OAS1 expression in Group 4 was significantly higher compared to pretreatment at week 8 (P < 0.05). OAS1 expression in Groups 3 and 4 was significantly higher compared to Group 1 at weeks 16 and 8, respectively (P < 0.05). ISG15 expression was significantly higher compared to pretreatment at weeks 8 and 16 for Group 3 and at week 8 for Group 4 (P < 0.05). For liver, OAS1 expression was significantly higher compared to pretreatment at weeks 36 in Group 2 and weeks 16 and 36 for Group 3 (P < 0.05). OAS1 expression in Group 3 was higher compared to Group 1 at week 16 (P < 0.05). ISG15 expression in Group 1 was significantly reduced compared to pretreatment at week 16 (P < 0.05). ISG15 expression in Group 4 was significantly elevated compared to Group 1 at week 16 (P < 0.05).
Figure 7.
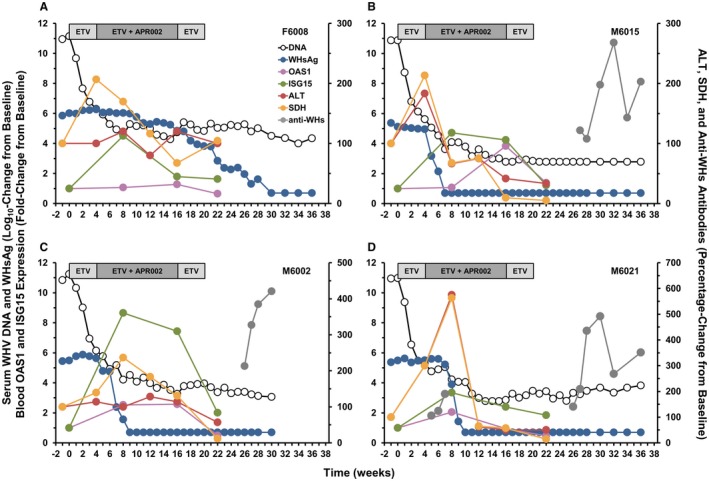
Relationship between serum WHV markers, serum activity of liver enzymes, peripheral ISG expression, and anti‐WHV antibody elicitation in woodchucks with undetectable or suppressed WHV replication induced by ETV/APR002 combination therapy. Log10 changes in serum WHV DNA and WHsAg, fold changes in blood OAS1 and ISG15 expression, and percentage changes in ALT and SDH levels and anti‐WHs antibody titers relative to pretreatment (T0) in woodchucks (A) F6008, (B) M6015, (C) M6002, and (D) M6021 during ETV/APR002 combination therapy (Groups 3 and 4).
Discussion
To improve the therapeutic window of oral TLR7 agonists for the treatment of CHB, we developed a novel liver‐targeted TLR7 agonist, APR002. APR002 showed comparable TLR7 activity to the clinical benchmark GS‐9620 but demonstrated higher liver exposure and lower volume of distribution following oral and intravenous dosing, respectively. By design, APR002 clearly differentiates from the high absorption, high hepatic extraction, and high volume of distribution characteristics of GS‐9620.17, 25 Compared to GS‐9620, the distinct PK properties of APR002 afforded a more dose‐proportional PD dose response and significantly less proinflammatory cytokine induction in mice; this is predicted to widen the therapeutic window and improve overall tolerability.
To test this, we first evaluated PK and PD parameters of APR002 in WHV‐negative woodchucks and thereafter the safety, host immune response, and antiviral efficacy of this compound in WHV‐positive woodchucks. Because most immunotherapy approaches for CHB will be examined on top of standard of care (i.e., conventional) antiviral treatment, APR002 was tested alone and as an add‐on to treatment with the nucleoside analog ETV. As shown, only combination treatment resulted in sustained suppression or even undetectability of circulating viral DNA, surface and e antigens and in elicitation of antibodies to both antigens in a subset of animals. Other woodchucks in both combination therapy groups also showed marked reductions in WHV DNA and to a lesser degree in WHsAg and WHeAg, but the effect was transient and comparable to the antiviral efficacy induced by ETV monotherapy, including the absence of detectable antibodies. The PD activity in these woodchucks, as tested by the expression of ISGs in blood and in part in liver, was consistent with the activation of TLR7 by APR002. Together with the transient but sometimes pronounced elevations in serum liver enzyme activity in woodchucks with suppressed or undetectable viral markers in serum and in liver, this could indicate more liver inflammation due to immune‐mediated control of the replicating virus in hepatocytes by noncytolytic/cytolytic mechanisms. Although not measured directly in the current study, these mechanisms could involve, at least in part, antiviral cytokines, such as IFNs and ISGs, and cytotoxic activity of NK and/or clusters of differentiation (CD)8+ T cells, as shown for the TLR7 agonist GS‐9620 in woodchucks.12 This assumption is supported by recent findings that peripheral blood mononuclear cells collected from patients who were nucleos(t)ide analog‐suppressed HBeAg negative during treatment with GS‐9620 presented with increased cytokine production by HBV‐specific CD4+ and CD8+ T cells and with increased activation and function of NK cells but with reduced ability of the latter cells to suppress T‐cell responses.16 WHV‐specific cellular responses were not tested in the current study for determining if woodchucks undergoing treatment with APR002 developed a functional adaptive T‐cell response. However, a crosslink between innate and adaptive immune responses is suggested by the seroconversion to antibodies against WHsAg and WHeAg that only occurred in animals with undetectable WHV replication at the end of the study. Antibody elicitation may indicate a reversal of impaired functions of antigen‐presenting cells and activation of B cells in these animals. In addition, B‐lymphocytes express TLR7 and receptor activation leads to polyclonal cell expansion and differentiation into immunoglobulin‐secreting plasma cells.27 Thus, ETV/APR002 combination treatment activated at least the humoral arm of the adaptive immune response in a subset of animals.
While ARP002 monotherapy was also associated with transient increases in liver enzymes and type I IFN response gene transcription, it was unable to induce antiviral effects comparable to ETV monotherapy, indicating that an initial reduction of viral replication was likely a requirement for the antiviral activity of the TLR7 agonist. As also noted in the current study during ETV monotherapy, marked but transient suppression of WHV markers in serum and liver is commonly seen during treatment of woodchucks with nucleos(t)ide analogs. A relapse of WHV replication after discontinuation of conventional antiviral treatment is usually observed, indicating that the reduction of viral markers is dependent on the continued presence of nucleos(t)ide analogs.21, 28, 29, 30 Conventional antiviral treatment in woodchucks usually does not significantly affect the levels of viral surface or e antigens in serum, and the induction of an antibody response to these antigens is therefore rare.13 This is comparable to the situation in patients with CHB undergoing standard treatment with nucleos(t)ide analogs.3 It is of note that high levels of HBV surface antigen (and presumably of HBV e antigen in patients positive for HBe) are implicated in the maintenance of immunologic tolerance against the virus at the B‐ and T‐cell level.31, 32, 33 Other immunomodulators, including IFN‐α, GS‐9620, and the TLR8 agonist GS‐9688, were recently evaluated in woodchucks as a single agent and exhibited sustained antiviral effects on WHV replication, with reduced surface antigen levels and seroconversion in a subset of animals.12, 34, 35 In contrast, immunomodulators, such as TLR9 and retinoic acid‐inducible gene I agonists, only mediate pronounced and sustained antiviral efficacy in combination with nucleos(t)ide analogs in woodchucks.23, 36 Thus, a combination of interventions targeting different steps in the replication cycle of HBV and WHV may be able to address the deficiencies of conventional antiviral therapy more efficiently and effectively than the respective monotherapies and may also be safer. In this study, three of ten woodchucks on APR002/ETV combination therapies demonstrated clearance of viral surface and e antigens after treatment withdrawal, development of antibodies to both antigens, and loss of viral cccDNA, thereby implying induction of a durable antiviral response (i.e., functional cure). A fourth woodchuck undergoing combination therapy had undetectable WHV antigens and suppressed WHV cccDNA but apparently did not elicit antiviral antibodies.
Importantly, all applied regimens with ETV and APR002 in the current study were not associated with treatment‐limiting adverse effects, and weekly oral dosing with APR002 for 12 weeks was well‐tolerated by woodchucks. Changes in the expression of proinflammatory cytokines in blood and liver of woodchucks treated with ETV and APR002, including IL‐6 and TNF‐α, were comparable to those observed in animals administered ETV (data not shown). Signs of systemic immunotoxicity linked to changes in hematology (such as thrombocytopenia) and clinical chemistry parameters (such as sustained elevations of liver enzyme and bilirubin levels) were not noted. Animal F6022 of Group 2 had already presented with increased liver enzymes after placebo treatment for 4 weeks with vehicle ETV and before treatment with the TLR7 agonist was initiated in week 5. Although the elevations in liver enzymes were pronounced in this animal, they were in the range of those occasionally observed in other animals treated with ETV and/or APR002. The contribution of APR002 monotherapy on the continued increase in liver enzymes in this particular animal is unknown, but death was attributed to underlying renal dysfunction/kidney disease. Thus, the single mortality of a total of 15 animals that completed APR002 treatment appears unrelated to therapy with the TLR7 agonist. The safety profile of APR002, therefore, appears distinct compared to the previous evaluation of GS‐9620 in woodchucks where initial treatment was halted due to thrombocytopenia and continued at lower doses after a treatment break.12 While it is difficult to make a direct comparison to the previous data with GS‐9620 in the woodchuck model, it should be noted that the fold change in blood ISGs with both compounds are close (OAS1: GS‐9620, 5‐fold; APR002, 2‐fold to 4‐fold). It was observed, however, that APR002 treatment in Groups 2, 3, and 4 was associated with slightly lower body temperatures in woodchucks when compared to animals in Group 1, which underwent ETV monotherapy (data not shown). Temporary lower body temperature could indicate a slower metabolism and thus a lower conversion of the ETV prodrug into the active compound within liver. If this assumption is correct, it may explain the less pronounced antiviral activity of ETV/APR002 combination therapy as observed in a majority of woodchucks of both groups, especially on serum viremia and antigenemia, when compared to animals administered ETV. A somewhat slower metabolism, however, would not be expected to affect the observed liver distribution of APR002 through OATP uptake. It is hypothesized that local APR002 in gut and liver can activate TLR7 in the resident dendritic cells, but the exact mechanism requires further mechanistic investigation.
Efficacy of GS‐9620 in patients with CHB dosed in combination with the antiviral drug tenofovir disoproxil fumarate or other nucleos(t)ide analogs was not achieved.16, 18, 19 The discrepancy in antiviral response obtained in patients infected with HBV versus animal models of HBV may be explained by much higher doses of GS‐9620 administered to chimpanzees and woodchucks than to humans. Alternatively, these animals may have a greater sensitivity to TLR7 immunomodulation. Thus, the proinflammatory nature of GS‐9620 may be responsible for its narrow therapeutic window in humans, and as such, the highest dose evaluated in phase 2 studies was too low to observe antiviral efficacy compared to that observed in animal models. Therefore, the dose range established in the present woodchuck study for antiviral efficacy needs to be confirmed and also demonstrated to be safe in humans before application of APR002 as a new therapeutic intervention for CHB can be initiated in patients. Nevertheless, our data suggest that the liver‐targeted TLR7 agonist APR002 in combination with a nucleoside analog has the potential to safely induce immunologic control of CHB infection in patients and presents a new opportunity in the search for an HBV cure.
Supporting information
Acknowledgment
We gratefully acknowledge Christian Gonzalez and Dr. Rajen Koshy (National Institutes of Health/National Institute of Allergy and Infectious Diseases/Division of Microbiology and Infectious Diseases) for intellectual support and advice during the study. We also gratefully acknowledge Dr. Robin D. Tucker of the Department of Comparative Medicine at Georgetown University for excellent assistance with the woodchucks. We further gratefully acknowledge Supti Sen, Vinona Muralidaran, and Dr. Deborah Berry of the Histopathology and Tissue Shared Resource at Georgetown University for excellent assistance with the immunohistochemistry of liver tissues. Microsomal stability and PK studies were performed at 3D BioOptima (Suzhou, China). OATP substrate assays were performed at Optivia Biotechnology (Santa Clara, CA). PK analysis following APR002 dosing of WHV‐negative woodchucks was performed by Alliance Pharma (Malvern, PA).
Supported by the National Institute of Allergy and Infectious Diseases, Division of Microbiology and Infectious Diseases (contract HHSN2722001000011I, task order HHSN27200004 [D13] to the Department of Microbiology and Immunology, Georgetown University Medical Center).
Potential conflict of interest: Dr. Corpuz, Dr. Plouffe, and Dr. Rodrigo are employed by Apros Therapeutics. Dr. Miller and Dr. Wu own stock in and are employed by Apros Therapeutics. The other authors have nothing to report.
See Editorial on Page 1289.
Contributor Information
Andrew T. Miller, Email: Andrew_Miller@aprostx.com.
Stephan Menne, Email: Stephan.Menne@georgetown.edu.
References
Author names in bold designate shared co‐first authorship.
- 1. World Health Organization . Global hepatitis report, 2017. www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Published April 2017. Accessed November 2018. [Google Scholar]
- 2. Liang TJ, Block TM, McMahon BJ, Ghany MG, Urban S, Guo JT, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology 2015;62:1893‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. Hepatology 2017;66:1296‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoulim F, Lebosse F, Levrero M. Current treatments for chronic hepatitis B virus infections. Curr Opin Virol 2016;18:109‐116. [DOI] [PubMed] [Google Scholar]
- 5. Kwon H, Lok AS. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol 2011;8:275‐284. [DOI] [PubMed] [Google Scholar]
- 6. Zhang E, Kosinska A, Lu M, Yan H, Roggendorf M. Current status of immunomodulatory therapy in chronic hepatitis B, fifty years after discovery of the virus: search for the "magic bullet" to kill cccDNA. Antiviral Res 2015;123:193‐203. [DOI] [PubMed] [Google Scholar]
- 7. Sepehri Z, Kiani Z, Alavian SM, Arababadi MK, Kennedy D. The link between TLR7 signaling and hepatitis B virus infection. Life Sci 2016;158:63‐69. [DOI] [PubMed] [Google Scholar]
- 8. Suslov A, Wieland S, Menne S. Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B. Curr Opin Virol 2018;30:9‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyake K, Shibata T, Ohto U, Shimizu T. Emerging roles of the processing of nucleic acids and toll‐like receptors in innate immune responses to nucleic acids. J Leukoc Biol 2017;101:135‐142. [DOI] [PubMed] [Google Scholar]
- 10. Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, et al. IFN‐alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest 2012;122:529‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, et al. GS‐9620, an oral agonist of toll‐like receptor‐7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013;144:1508‐1517, 1517.e1501‐1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, et al. Sustained efficacy and seroconversion with the toll‐like receptor 7 agonist GS‐9620 in the woodchuck model of chronic hepatitis B. J Hepatol 2015;62:1237‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menne S, Cote PJ. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J Gastroenterol 2007;13:104‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gane EJ, Lim YS, Gordon SC, Visvanathan K, Sicard E, Fedorak RN, et al. The oral toll‐like receptor‐7 agonist GS‐9620 in patients with chronic hepatitis B virus infection. J Hepatol 2015;63:320‐328. [DOI] [PubMed] [Google Scholar]
- 15. Lopatin U, Wolfgang G, Tumas D, Frey CR, Ohmstede C, Hesselgesser J, et al. Safety, pharmacokinetics and pharmacodynamics of GS‐9620, an oral toll‐like receptor 7 agonist. Antivir Ther 2013;18:409‐418. [DOI] [PubMed] [Google Scholar]
- 16. Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, et al. TLR7 agonist increases responses of hepatitis B virus‐specific T cells and natural killer cells in patients with chronic hepatitis B treated with nucleos(t)ide analogues. Gastroenterology 2018;154:1764‐1777.e1767. [DOI] [PubMed] [Google Scholar]
- 17. Fosdick A, Zheng J, Pflanz S, Frey CR, Hesselgesser J, Halcomb RL, et al. Pharmacokinetic and pharmacodynamic properties of GS‐9620, a novel toll‐like receptor 7 agonist, demonstrate interferon‐stimulated gene induction without detectable serum interferon at low oral doses. J Pharmacol Exp Ther 2014;348:96‐105. [DOI] [PubMed] [Google Scholar]
- 18. Janssen H, Brunetto MR, Kim YJ, Ferrari C, Massetto B, Nguyen AH, et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS‐9620) in virally suppressed patients with chronic hepatitis B. J Hepatol 2018;68:431‐440. [DOI] [PubMed] [Google Scholar]
- 19. Agarwal K, Ahn SH, Elkhashab M, Lau AH, Gaggar A, Bulusu A, et al. Safety and efficacy of vesatolimod (GS‐9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J Viral Hepat 2018;25:1331‐1340. [DOI] [PubMed] [Google Scholar]
- 20. Tu M, Mathiowetz AM, Pfefferkorn JA, Cameron KO, Dow RL, Litchfield J, et al. Medicinal chemistry design principles for liver targeting through OATP transporters. Curr Top Med Chem 2013;13:857‐866. [DOI] [PubMed] [Google Scholar]
- 21. Menne S, Butler SD, George AL, Tochkov IA, Zhu Y, Xiong S, et al. Antiviral effects of lamivudine, emtricitabine, adefovir dipivoxil, and tenofovir disoproxil fumarate administered orally alone and in combination to woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob Agents Chemother 2008;52:3617‐3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cote PJ, Roneker C, Cass K, Schodel F, Peterson D, Tennant B, et al. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol 1993;6:161‐169. [DOI] [PubMed] [Google Scholar]
- 23. Suresh M, Korolowicz KE, Balarezo M, Iyer RP, Padmanabhan S, Cleary D, et al. Antiviral efficacy and host immune response induction during sequential treatment with SB 9200 followed by entecavir in woodchucks. PLoS One 2017;12:e0169631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu TY‐H. (WO2018/106606) Pyrimidine compound containing acidic groups. World Intellectual Property Oganization. Patentscope. Published June 14, 2018. Accessed December 2018. [Google Scholar]
- 25. Roethle PA, McFadden RM, Yang H, Hrvatin P, Hui H, Graupe M, et al. Identification and optimization of pteridinone toll‐like receptor 7 (TLR7) agonists for the oral treatment of viral hepatitis. J Med Chem 2013;56:7324‐7333. [DOI] [PubMed] [Google Scholar]
- 26. Engel AL, Holt GE, Lu H. The pharmacokinetics of toll‐like receptor agonists and the impact on the immune system. Expert Rev Clin Pharmacol 2011;4:275‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bekeredjian‐Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol 2005;174:4043‐4050. [DOI] [PubMed] [Google Scholar]
- 28. Korba BE, Cote P, Hornbuckle W, Tennant BC, Gerin JL. Treatment of chronic woodchuck hepatitis virus infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology 2000;31:1165‐1175. [DOI] [PubMed] [Google Scholar]
- 29. Mason WS, Cullen J, Moraleda G, Saputelli J, Aldrich CE, Miller DS, et al. Lamivudine therapy of WHV‐infected woodchucks. Virology 1998;245:18‐32. [DOI] [PubMed] [Google Scholar]
- 30. Genovesi EV, Lamb L, Medina I, Taylor D, Seifer M, Innaimo S, et al. Efficacy of the carbocyclic 2′‐deoxyguanosine nucleoside BMS‐200475 in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother 1998;42:3209‐3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 2012;61:1754‐1764. [DOI] [PubMed] [Google Scholar]
- 32. Boni C, Laccabue D, Lampertico P, Giuberti T, Vigano M, Schivazappa S, et al. Restored function of HBV‐specific T cells after long‐term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012;143:963‐973.e9. [DOI] [PubMed] [Google Scholar]
- 33. Corti D, Benigni F, Shouval D. Viral envelope‐specific antibodies in chronic hepatitis B virus infection. Curr Opin Virol 2018;30:48‐57. [DOI] [PubMed] [Google Scholar]
- 34. Daffis S, Chamberlain J, Zheng J, Santos R, Rowe W, Mish M, et al. Sustained efficacy and surface antigen seroconversion in the woodchuck model of chronic hepatitis B with the selective toll‐like receptor 8 agonist GS‐9688. J Hepatol 2017;66(Suppl.):S692‐S693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fletcher SP, Chin DJ, Gruenbaum L, Bitter H, Rasmussen E, Ravindran P, et al. Intrahepatic transcriptional signature associated with response to interferon‐alpha treatment in the woodchuck model of chronic hepatitis B. PLoS Pathog 2015;11:e1005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meng Z, Zhang X, Pei R, Zhang E, Kemper T, Vollmer J, et al. Combination therapy including CpG oligodeoxynucleotides and entecavir induces early viral response and enhanced inhibition of viral replication in a woodchuck model of chronic hepadnaviral infection. Antiviral Res 2016;125:14‐24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


