Abstract
Background:
The global distribution of testicular disorders differs conforming with differences in demographic denominators. The diagnostic dictum for these disorders customarily adheres to findings at clinical assessment, relevant imaging, and laboratory evaluation. Histopathological confirmation remains the ultimate for the diagnosis of testicular malignancies and many testicular dysfunctions. The epidemiological review of the histological outcomes among Kano populace, however, is deficient.
Objective:
The aim of the study was to analyse histological pattern of testicular lesions in Kano, Nigeria.
Methodology:
The study is a 14-year retrospective review of testicular specimens subjected to histology in Kano from January 2003 to December 2016. The variables obtained were the age of patients, laterality, and histological diagnoses. These were collated and analyzed; the findings were presented as mean, patients’ age range, and laterality ratio with frequency tables.
Results:
Three hundred and forty-three testicular tissues were assessed. The nonneoplastic lesions were 79.2% with patients’ age range of 3–90 years. Atrophies and maturation arrests formed 29.4% and 18.0%, respectively. Specimens from the right were more with a ratio of 1.6:1. Neoplastic lesions were 3.5% and patients’ age range from 3 to 65 years. Seminomas were the predominant neoplastic lesion and constituted 66.7%. The right testes were more commonly affected and have a ratio of 1.4:1.
Conclusion:
This appraisal affirms that testicular lesions could be found across a wide age range and majorities are nonneoplastic. The findings in this study concur with the published African and Asian conclusions.
KEYWORDS: Neoplastic lesion, nonneoplastic lesion, testicular lesions
INTRODUCTION
The differences in the epidemiological distribution of testicular disorders are tied to demographic traits. These disorders can be neoplastic or nonneoplastic.[1] The neoplastic lesions constitute 1%–2% of all malignant tumors and have a peaked prevalence at 15 and 35 years; incidence then declined as age increases.[2] The reported trends of testicular tumors in industrialized regions of the world revealed rising rates.[3] A tumor can be germ cell, nongerm cell, or mixed testicular tumor; the risk factors include family history, Klinefelter syndrome, and cryptorchidism.[4]
Cryptorchidism which lingers in 1% of the infants in the 1st year of life could be from a short spermatic cord, narrow inguinal canal, trisomy 13, maldevelopment of the scrotum, or deficient androgen secretions.[5] This results in testicular atrophy that could as well develop due to irradiation, hypopituitarism, orchitis, prolonged antiandrogen administration, atherosclerotic narrowing of the blood supply in old age, generalized malnutrition, and exhaustion atrophy.[6]
Acute scrotal lesions include testicular torsion with specific and nonspecific acute inflammations. Tuberculosis orchitis mostly begins in the epididymis often insidiously and could clinically mimic a testicular tumor.[7] Torsion is a urologic emergency resulting from the sudden cessation of vascular supply to the testis.[8]
With this vast list of lesions and the entwined nature of these disorders, fundamentals for factual diagnoses remained the patient's clinical assessment, findings from imaging evaluation, blood biochemistry, urine antibiogram, and sometimes tissue histopathological diagnosis. The evaluations are central for screening, prognostication, predicting the natural course of the lesions, and occasionally proffers therapeutic target as well as serves as a clinical surrogate endpoint in the management of the lesions. The histopathological diagnoses from orchidectomies and biopsies specimens are the ultimate for the confirmation of certain testicular dysfunctions, malignancies as well as in some specific medicolegal vindications. Despite the significances of the histopathological outcome of the testicular lesions in patient care as well as the enunciated epidemiological relevance, appraisals on these among the Nigerian populace in the Northwestern region are lacking in the literature. The aim of this study was to construe age of occurrence, the spectrum of lesions, laterality, and frequency distribution of histologically diagnosed testicular lesions in Kano, Northwestern Nigeria.
METHODOLOGY
The appraisal was a 14-year retrospective review of all histologically diagnosed testicular lesions from January 2, 2003 to December 31, 2016 in Kano. The histology records in Aminu Kano Teaching Hospital, Kano were explored, and the registered specimens of the testicular tissue are listed out.
The histology slides of testicular specimens were retrieved and histological conclusions were collated by the authors. Fresh sections were made from archival paraffin tissue blocks if the slides could not be retrieved. Specimens for the evaluation of male infertility were received at the laboratory fixed in Bouin's solution from the theater, while the rest were submitted fixed in 10% neutral-buffered formal saline. These were processed and embedded as paraffin tissue blocks. Microtome slice was at 4 μ and sections were stained with hematoxylin and eosin. Categorization of neoplastic lesions was according to the 2016 world health organization classification for testicular tumors.[9] The variables compiled were the age, laterality, and histopathological diagnosis. The data collated were consequently analyzed using SPSS version 2010 (IBM Corp. Released 2011., IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). The results were presented as mean, ratio, and frequency tables with photomicrographs of some of the lesions.
RESULTS
Three hundred and forty-three testicular specimens were subjected to histology during the 14-year study period. One hundred and fifty-six (45.5%) of these were orchidectomy specimens from androgen deprivation therapy in patients with advanced cancer of the prostate; testicular tumors, lately presented cryptorchidism, marked testicular atrophies and testicular torsion. Across-the-board nonneoplastic specimens added up to 331 cases (96.5%), with age range of 3–90 years and the highest incident in the fourth and fifth decades. Atrophies and maturation arrests were the most common benign lesions and formed 29.4% and 18.0%, respectively, of these. Table 1 displayed histological diagnosis and frequency of age distributions of nonneoplastic testicular lesions in Kano. Sixty percent (60.29.8%) of the benign lesions were on the right side with a ratio of 1.61:1. Neoplastic lesions accounted for only 12 cases (3.5%) of these specimens with the age range of 3–65 years. Seminomas were the most common histologic type among the neoplastic lesions with eight cases (66.7%). Yolk sac tumor, embryonal carcinoma, Leydig cell tumor, mixed germ cell, and stromal tumor each had a single specimen. Table 2 shows the histological type and frequency of age distribution of testicular tumors in Kano. The tumors were more common on the right than the left side with a ratio of 1.4: Table 3 shows the laterality of nonneoplastic and neoplastic testicular lesions during the study period in Kano. There was no lesion identified in 59 specimens (17.8%) of the examined specimens. Figures 1–6 shows photomicrographs of testicular atrophy, spermatogenic maturation arrest, chronic granulomatous inflammation, Sertoli cell-only syndrome, seminoma, and yolk sac tumor, respectively.
Table 1.
Histologic diagnosis and age distribution of nonneoplastic testicular lesions in Kano
| Histologic diagnosis | 0-9 years | 10-19 years | 20-29 years | 30-39 years | 40-49 years | 50-59 years | 60-69 years | 70-79 years | 80-89 years | 90-99 years | Number of cases (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Congenital anomalies | |||||||||||
| Cryptorchidism | 1 | 4 | 7 | 1 | 1 | - | - | - | - | - | 14 (5.1) |
| Regressive changes | |||||||||||
| Atrophy | - | - | 8 | 24 | 19 | 8 | 10 | 8 | 2 | 1 | 80 (29.4) |
| Other male infertility factors | |||||||||||
| Maturation arrest | - | - | 2 | 30 | 14 | 2 | 1 | - | - | - | 49 (18.0) |
| Hypospermatogenesis | - | - | 3 | 16 | 7 | 2 | 9 | 4 | 2 | - | 43 (15.8) |
| Sertoli cell-only syndrome | - | - | 3 | 12 | 1 | - | - | - | - | - | 16 (5.9) |
| Vascular lesions | |||||||||||
| Torsion | - | 5 | 11 | 2 | 2 | 2 | 2 | 1 | - | - | 25 (9.2) |
| Inflammations | |||||||||||
| Nonspecific orchitis | 2 | 3 | 1 | - | 1 | 1 | 9 | 2 | 1 | - | 20 (7.4) |
| Specific tuberculosis | - | 4 | 2 | 4 | 4 | 2 | 2 | - | - | - | 18 (6.6) |
| Schistosomiasis | - | 4 | 1 | 1 | - | - | - | 1 | - | - | 7 (2.6) |
| Total | 3 | 20 | 38 | 90 | 49 | 17 | 33 | 16 | 5 | 1 | 272 (100) |
Table 2.
Histologic types and age distribution of testicular tumors in Kano
| Histologic types | 0-9 years | 10-19 years | 20-29 years | 30-39 years | 40-49 years | 50-59 years | 60-69 years | Number of cases (%) |
|---|---|---|---|---|---|---|---|---|
| Germ cell tumors | ||||||||
| Classic seminoma | - | - | 1 | 2 | 3 | - | - | 6 (50) |
| Spermatocytic seminoma | - | - | - | 1 | - | - | 1 | 2 (16.7) |
| Yolk sac tumor | - | - | 1 | - | - | - | - | 1 (8.3) |
| Embryonal carcinoma | 1 | - | - | - | - | - | - | 1 (8.3) |
| Stromal tumors | ||||||||
| Leydig cell tumor | - | - | - | 1 | - | - | - | 1 (8.3) |
| MGCST | - | - | 1 | - | - | - | - | 1 (8.3) |
| Total | 1 | - | 3 | 4 | 3 | - | 1 | 12 (100) |
MGCST: Mixed germ cell and stromal tumors
Table 3.
Laterality of testicular lesions in Kano
| Laterality | Side | Number of cases (%) | |
|---|---|---|---|
| Nonneoplastic lesions | Testicular tumors | ||
| Unilateral | Right | 164 (60.3) | 7 (58.3) |
| Left | 102 (37.5) | 5 (41.7) | |
| Bilateral | 6 (2.2) | - | |
| Total | 272 (100) | 12 (100) | |
Figure 1.
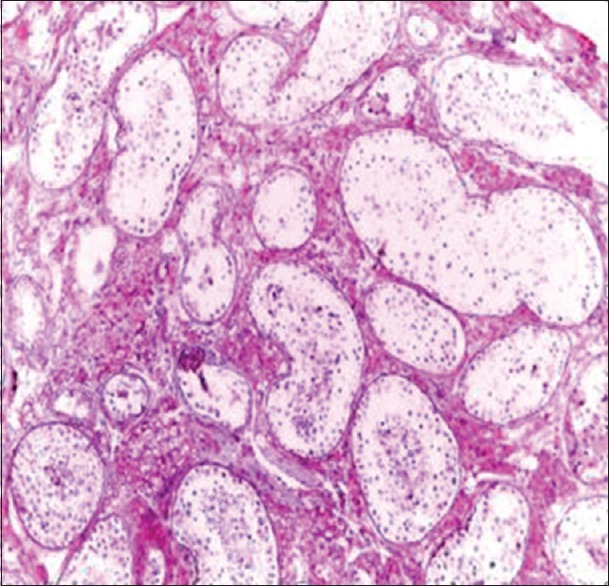
Testicular atrophy (H and E, ×20)
Figure 6.
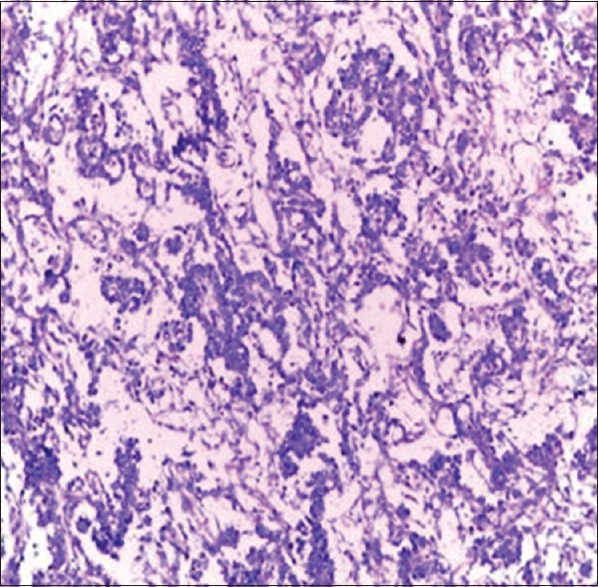
Yolk sac tumor of the testis (H and E, ×20)
Figure 2.
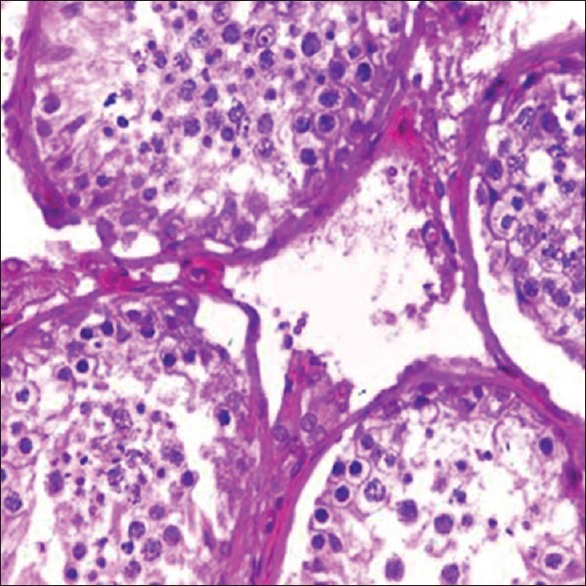
Spermatogenic Maturation arrest (H and E, ×100)
Figure 3.
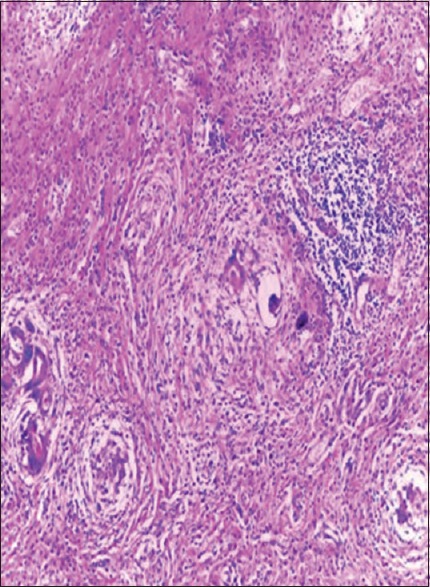
Chronic granulomatous inflammation (H and E, ×20)
Figure 4.
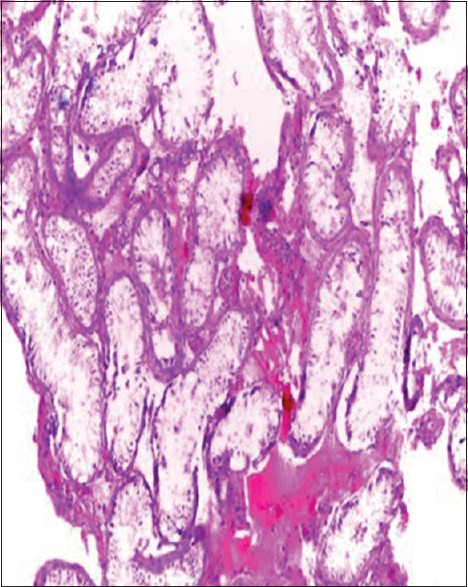
Sertoli cell-only syndrome (H and E, ×20)
Figure 5.
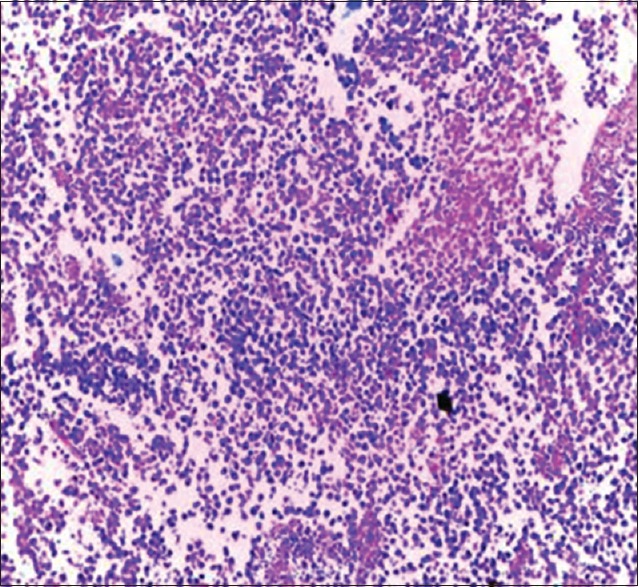
Seminoma of the testis (H and E, ×20)
DISCUSSION
Aminu Kano Teaching Hospital is the sole center that provides histopathological services for the populace in Kano State and some of its neighboring communities. The 343 testicular specimens in the study period was a small number for the state with the highest population in Nigeria.[10] The main factor for the small number could be large pools of testicular lesions do not meet the indications for biopsy and histology. Patient's clinical assessment, findings from imaging evaluation, blood biochemistry, and urine antibiogram often provide the necessary framework for the patient's management.
Most of the specimens studied in our review were nonneoplastic. This concurs with Sharma et al.’ s 97% published nonneoplastic testicular specimens.[11] In separate studies, Reddy et al. reported 86%, while Patel et al. and Baidya et al. each registered 85% as nonneoplastic.[12,13,14] The above conclusions were different from Robertson's registered 31.5 versus 68.5%.[15] Earlier studies firmly established diversity of neoplastic lesions across the regions of the world and rareness of testicular tumors in our populace.[3] Testicular atrophy was the most common nonneoplastic disorder and constituted 29.4% of benign lesions in our appraisal. This is comparable to 22.4% observed in Ibadan, Southwestern Nigeria.[16] In another review in Nnewi at Southeastern Nigeria, maturation arrest tops the list of non-neoplastic testicular lesions.[17] However, in India, Sharma et al. reported cryptorchidism as the most common notwithstanding; Patel et al. documented torsion as the more common nonneoplastic lesion.[11,13] A 2-year comparison in Nepal also recorded torsion as the most common.[14] The variations may be due to variances in genetic blueprints and exposure to environmental elements that included chemical integrals, radiations, and infective agents.
The peak prevalence of the fourth decade in this study concurs with appraisals from other Nigerian communities; however, it is higher than the second and third decades reported among Asians and the Caucasians.[11,16,17,18] The dominance of the right side nonneoplastic lesions agreed with conclusions by Sharma et al. and Patel et al. though differ from Reddy et al.[12] Moderate proportion of the specimen, i.e. 17.2% (59 cases) revealed no significant pathology; these were predominantly orchidectomy specimens in patients with advanced prostate cancers and some of the biopsies from obstructive azoospermia.
Among specific orchitis, 18 were tuberculous with the majority occurring in the second to fifth decade. This is similar to reports by Abba et al., Khandeparkar and Pinto, and Patel et al.[13,19,20] Furthermore, seven were schistosomal orchitis with a peak age in the second decade which is not unexpected in a schistosomiasis endemic region.[21,22]
Testicular tumors accounted for 3.5% (12 cases) of the specimens; generally, Africans have a lower incidence than western communities.[23] Of these tumors, seminomas were the most common histological subtypes and formed 66.7%. This agreed with most of the reports from Nigerian and Kenyan populations even though differed from a study in Ilorin, Northcentral Nigeria where yolk sac tumour was the most common histological subtype.[24,25,26,27] The peak age of seminoma was fourth to fifth decades and that of the spermatocytic tumor was seventh decade which is in concordance with the literature.[23] Right-sided involvement was more than the left with a ratio of 1.4:1. The higher proportion of right-sided undescended testes has been proposed to explain this preponderance.[5]
This review has inherent limitations of retrospective hospital-based appraisals. The study incorporated only specimens histologically analyzed at Aminu Kano Teaching Hospital, Kano. Although Aminu Kano Teaching Hospital is the only hospital in Kano State with capacity for histological assessment, component charges and extent of the study region may limit testicular tissue specimens yield. There is no evidence to affirm that entire testicular tissue specimens in Kano State are submitted for analysis in the center during the study period.
CONCLUSION
Nonneoplastic lesions formed the majority of histologically analyzed testicular lesions in all age groups in Kano. The findings were consistent with most studies in Africa and Asia but differed from those of the western world where the proportion of neoplastic lesions is comparatively higher.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rosai J. Rosai and Ackerman's Surgical Pathology. 10th ed. Vol. 1. New York: Elsevier; 2011. Male reproductive system; pp. 1335–6. [Google Scholar]
- 2.Liu S, Wen SW, Mao Y, Mery L, Rouleau J. Birth cohort effects underlying the increasing testicular cancer incidence in Canada. Can J Public Health. 1999;90:176–80. doi: 10.1007/BF03404502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhaji SA, Abdulkadir A, Sanusi HM. A 15-year pathologic review of testicular and paratesticular tumours in Kano, Northern Nigeria. Niger J Basic Clin Sci. 2016;13:114–8. [Google Scholar]
- 4.Garner MJ, Turner MC, Ghadirian P, Krewski D. Epidemiology of testicular cancer: An overview. Int J Cancer. 2005;116:331–9. doi: 10.1002/ijc.21032. [DOI] [PubMed] [Google Scholar]
- 5.Mathers MJ, Sperling H, Rübben H, Roth S. The undescended testis: Diagnosis, treatment and long-term consequences. Dtsch Arztebl Int. 2009;106:527–32. doi: 10.3238/arztebl.2009.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan PT, Schlegel PN. Diagnostic and therapeutic testis biopsy. Curr Urol Rep. 2000;1:266–72. doi: 10.1007/s11934-000-0006-4. [DOI] [PubMed] [Google Scholar]
- 7.Sanjay M, Sushma HM. Histomorphological spectrum of tumour and tumour like lesions of testis and paratesticular structures – A cross sectional study. Indian J Pathol Oncol. 2016;3:528–34. [Google Scholar]
- 8.Ringdahl E, Teague L. Testicular torsion. Am Fam Physician. 2006;74:1739–43. [PubMed] [Google Scholar]
- 9.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: Renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Nigeria Population Census. 2006. [Last accessed on 2017 Aug 30]. Available from: http://www.nigeriamasterweb.com/Nigeria06CensusFigs.html .
- 11.Sharma M, Mahajan V, Suri J, Kaul KK. Histopathological spectrum of testicular lesions – A retrospective study. Indian J Pathol Oncol. 2017;4:437–41. [Google Scholar]
- 12.Reddy H, Chawda H, Dombale VD. Histomorphological analysis of testicular lesions. Indian J Pathol Oncol. 2016;3:558–63. [Google Scholar]
- 13.Patel MB, Goswamy HM, Parikh UR, Mehta N. Histopathological study of testicular lesions. Gujarat Med J. 2015;70:41–6. [Google Scholar]
- 14.Baidya R, Sigdel B, Baidya NL. Histopathological pattern of testicular lesion. J Pathol Nepal. 2017;7:1087–90. [Google Scholar]
- 15.Robertson GS. Radical orchidectomy and benign testicular conditions. Br J Surg. 1995;82:342–5. doi: 10.1002/bjs.1800820320. [DOI] [PubMed] [Google Scholar]
- 16.Thomas JO. Histological pattern of testicular biopsies in infertile males in Ibadan, Nigeria. East Afr Med J. 1990;67:578–84. [PubMed] [Google Scholar]
- 17.Oranusi CK, Onyiaorah IV, Ukah CO. Pattern of testicular biopies as seen in a tertiary institution in Nnewi, Southeast Nigeria. Niger J Surg. 2014;20:55–8. doi: 10.4103/1117-6806.137283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adami HO, Bergström R, Möhner M, Zatoński W, Storm H, Ekbom A, et al. Testicular cancer in nine Northern European countries. Int J Cancer. 1994;59:33–8. doi: 10.1002/ijc.2910590108. [DOI] [PubMed] [Google Scholar]
- 19.Abba K, Tahir MB, Dogo HM, Nggada HA. Testicular and paratesticular non- neoplastic lesions in university of Maiduguri teaching hospital: A 10-year retrospective review. Borno Med J. 2016;13:39–44. [Google Scholar]
- 20.Khandeparkar SG, Pinto RG. Histopathological spectrum of tumor and tumor-like lesions of the paratestis in a Tertiary care hospital. Oman Med J. 2015;30:461–8. doi: 10.5001/omj.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yakubu AA, Mohammed AZ, Sheshe AA, Edino ST, Alhassan SU. Testicular schistosomiasis. An unusual cause of scrotal pain. Afr J Urol. 2005;11:258–60. [Google Scholar]
- 22.Al-Qahtani SM, Droupy SJ. Testicular schistosomiasis. Saudi Med J. 2010;31:325–7. [PubMed] [Google Scholar]
- 23.Zheng T, Holford TR, Ma Z, Ward BA, Flannery J, Boyle P, et al. Continuing increase in incidence of germ-cell testis cancer in young adults: Experience from Connecticut, USA, 1935-1992. Int J Cancer. 1996;65:723–9. doi: 10.1002/(SICI)1097-0215(19960315)65:6<723::AID-IJC2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Seleye-Fubara D, Etebu EN. Testicular tumors in Port Harcourt (a tenyear review) Niger J Clin Pract. 2004;7:56–9. [Google Scholar]
- 25.Opot EN, Magoha GA. Testicular cancer at Kenyatta national hospital, Nairobi. East Afr Med J. 2000;77:80–5. doi: 10.4314/eamj.v77i2.46402. [DOI] [PubMed] [Google Scholar]
- 26.McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE, et al. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97:63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- 27.Izegbu MC, Ojo MO, Shittu LA. Patterns of testicular malignancies in Ilorin, Nigeria. J Cancer Res Ther. 2005;1:229–31. doi: 10.4103/0973-1482.19598. [DOI] [PubMed] [Google Scholar]


