Abstract
Purpose of the study:
The purpose of this study was to evaluate the potential value of gallium-68 (Ga-68)-DOTATATE positron emission tomography/computed tomography (PET/CT) in predicting the risk of progression in nonbenign meningioma after definite irradiation. We retrospectively reviewed our patients with meningiomas who had the highest risk of progression: WHO histological Grade II and III tumors and with macroscopic disease as identified in Ga-68-DOTATATE PET/CT.
Materials and Methods:
Thirteen patients were included in this study. For each tumor, the following quantifiers were measured: maximum and mean standardized uptake volume (SUV), standard deviation, metabolic tumor volume (MTV), total lesion activity, and coefficient of variation. Each of the quantifiers except for maximum SUV was obtained with three different SUV thresholds: muscle based (ms), liver based (liv), and gradient based (gb). The quantifiers were analyzed in univariate Cox model for their prognostic value for progression-free survival (PFS) and overall survival (OS).
Results:
Mean follow-up of the patients was 28.2 months. The 2-year PFS and OS was 28.1% and 76.9%, respectively. The MTVgb was a significant predictor for PFS (risk of progression of disease above vs. below the 34 cm3 threshold: 100% vs. 28.3%, P = 0.0003). Clinically, the male sex also influenced PFS (Hazard ratio =13.06; 95% confidence interval: 1.56–109.25; P = 0.018). The mean SUVms(P = 0.041) and SUVgb(P = 0.048) had a prognostic value for predicting the risk of death.
Conclusion:
Ga-68-DOTATATE PET/CT has potential to predict disease progression in nonbenign meningioma patients. Further prospective studies for validating and standardizing these findings are indicated.
Keywords: Atypical meningioma, DOTATATE, gallium-68, malignant meningioma, positron emission tomography, radiotherapy
Introduction
Meningioma is a tumor originating from normal meninx; it is the most common neoplasm of the central nervous system. It can be found anywhere within the craniospinal axis, but the great majority is located intracranially.[1] Tumor grade according to WHO classification is the most important clinical factor, determining both the management and patient outcome. WHO grade I meningiomas are very often an incidental finding and should only be treated when they become symptomatic or radiologically progressive.[2] The treatment options include surgery, conventional fractionated radiotherapy, or radiosurgery - all as standalone modalities, with indications for adjuvant radiotherapy after surgery practically limited to recurrent tumors. The treatment outcome remains excellent with local control rates well exceeding 90% in 5 years for all of the mentioned treatment options.[3] The prognosis of Grade II and III meningiomas is dramatically worse. The extent of resection according to Simpson grade is one of the crucial clinical prognostic factors. Even in most optimally operated cases, a 30% risk of disease progression can be expected. For patients who underwent micro- or macro-scopically incomplete surgery (Simpson III-IV) as well as inoperable ones (Simpson V), this risk increases up to as much as 70% in 5-year follow-up,[4,5] and therefore, radiotherapy should be applied to all such high-risk patients. Since great majority of failures after radiotherapy are local,[6] a proper delineation of the tumor volume and its subclinical margins appears to be of importance. Supplementary magnetic resonance imaging (MRI) is always required for definite radiotherapy planning as the meninx is not adequately visualized on computed tomography (CT). A useful additional imaging modality is positron emission tomography/CT (PET/CT) utilizing 68-Gallium 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid octreotate (Ga-68-DOTATATE).[7] It binds specifically to somatostatin receptors type 2 (SSTR2) that are commonly overexpressed in meningioma but not in normal meningeal tissue.[8] Ga-68-DOTATATE PET/CT can therefore support the delineation by differentiation between tumor mass and postoperative lesions or identifying increased uptake corresponding to probable tumor spread in meninx that was not operated and looks unsuspicious in MRI. In addition, certain quantifiable parameters of tumor radiotracer uptake in PET/CT may correspond to extent or aggressiveness of the disease and help predict the recurrence risk. This has been vastly studied for 18-Fluoride-fluorodeoxyglucose (F-18-FDG) PET/CT,[9] yet for Ga-68-DOTATATE PET/CT in nonbenign meningioma, the data are very limited. The purpose of this study is therefore to investigate the value of Ga-68-DOTATATE PET/CT in predicting the patient outcome in patients with Grade II-III meningioma featuring the highest risk of local failure because of having a residual or inoperable disease.
Materials and Methods
Patients
Fourteen meningiomas of 13 patients treated at our institution between March 2013 and January 2015 were included in this retrospective study. Included were patients who (1) had a histologic diagnosis of WHO Grade II or III meningioma, (2) had a Ga-68-DOTATATE PET/CT scan performed for radiotherapy planning, (3) had a macroscopic tumor identifiable in MRI and PET/CT at the time of radiotherapy planning, and (4) completed the radiotherapy with definite intention (patients who underwent palliative treatment of low total biological dose were excluded). One patient had a confirmed multineoplastic genetic syndrome (neurofibromatosis Type 2).
Radiotherapy and follow-up
The radiation treatment modality and doses were established individually by the local brain tumor board basing on the patient diagnosis and previous treatment received, in all cases attempting to reach or exceed 60 Gy. The patients were immobilized using thermoplastic masks in which the imaging and treatment were performed. In addition to PET/CT, all patients had a contrast-enhanced MRI for treatment planning. The conventional irradiation was delivered with intensity-modulated radiotherapy (IMRT) technique by 6 MV linear accelerator (either Clinac 2300EX or TrueBeam, Varian Medical Systems Inc., Palo Alto, United States). To increase the radiation conformality and therefore maintain the tumor prescribed dose while avoiding unnecessary toxicity, CyberKnife (Accuray, Sunnyvale CA, United States) was used for certain patients depending on the tumor size and location, either standalone or as a boost after IMRT. The posttreatment follow-up involved clinical and neurological examination and periodic MRI scans. Their frequency was prescribed individually basing on recurrence risk and the disease extent; typically ranging from 3 to 12 months. The progression of disease (PD) was reported according to RECIST 1.1 criteria and defined as at least 20% increase of the sum of longest diameters of the tumors. In case of a clinically suspected lesion size increase not meeting the progression criteria, a follow-up Ga-68-DOTA PET/CT was performed. Any increase of both the size of the tumor in MRI and of mean standardized uptake volume (SUV) in PET/CT was interpreted as PD. To meet its aims, the present study refers the outpoint PD to each particular irradiated tumor and not to the patient.
Positron emission tomography/computed tomography protocol
The PET/CT scans were acquired randomly on one of the two hybrid scanners available at the Institution: Philips Gemini XL (Philips Healthcare, Eindhoven, the Netherlands) or Siemens® Biograph™ mCT (Siemens AG, Erlangen, Germany). The scanners are periodically calibrated against the same reference activity probe to maintain equal readouts. All examinations were performed according to the following protocol: after a 6-hour fast, the patients were intravenously administered 110–185 MBq total activity of Ga-68-DOTATATE (typically maximum 2.5 MBq per kg body weight). After 30 min of interval, the CT data were acquired on exhaust breath phase without intravenous contrasting medium, and PET data were acquired continuously during regular shallow breath in 30–40 min time. The scans ranged from the top of the head to the middle of thighs with a slice thickness of 3 mm for both CT and PET modality. Correction of attenuation was applied to the PET layer before fused analysis to eliminate breathing artifacts.
Quantitative tumor analysis in positron emission tomography/computed tomography
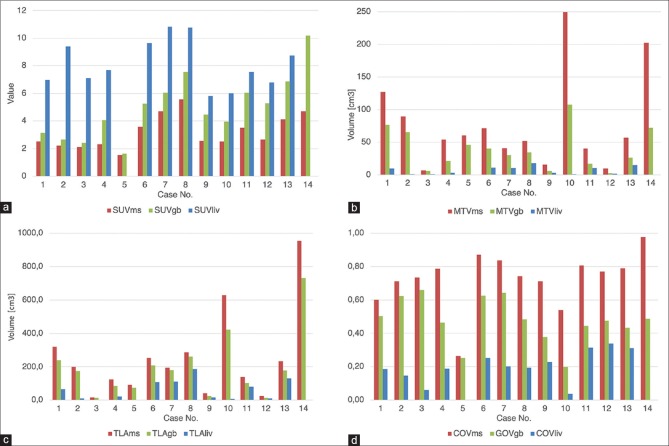
Quantitative parameters of each separate tumor in PET/CT study were evaluated using Philips Intellispace Portal version 8.0 Software. The following primary quantifiers were reported: maximum SUV (SUVmax), the mean SUV (SUVmean), and metabolic tumor volume (MTV). Two composite parameters were also calculated for each tumor: total lesion activity (TLA = SUVmean*MTV) and coefficient of variation (COV = SD/SUVmean; where SD is the standard deviation of SUVmean) that corresponds to radiotracer uptake heterogeneity in the tumor. The SUVmean, MTV, and the composite parameters were also recorded for three differently defined tumor volumes. The first two that have been previously described in the literature[10] used the patient-derived SUVmean thresholds of representative gluteal muscle (referred to asms later in the text) and liver mean SUVs (liv). Three circular three-dimensional regions of interest (ROIs) of 10 mm in diameter were placed on different parts of gluteal muscle and liver, respectively. The mean SUV of the three ROIs was the threshold value to be recorded into the database. The third segmentation method involved a gradient-based (gb) approach. As opposed to the two previous algorithms, it does not feature a fixed SUV threshold to include or not a particular voxel into the tumor volume. Using an in-built function of the software, the voxel SUV values are analyzed on a grid, and the cutoff border is placed between the two neighboring voxels with the highest SUV difference, using the phenomenon that for most radiotracers, the lesion-to-background SUV falloff is not linear but sharply demarcated. The resulting SUVmean and MTV values were intermediate between the liver and muscle-based thresholds. The mutual correlations of the three thresholds for each quantifier are shown in Figure 1a–d.
Figure 1.
(a-d) Comparison of quantifier values for muscle-, liver-, and gradient-based thresholds: (a) standardized uptake volume mean, (b) metabolic tumor volume, (c) total lesion activity, (d) coefficient of variation
Statistical analysis
All statistical calculations were performed using Stata 15 software (StataCorp LLC, College Station, TX, USA). The prognostic usability of Ga-68-DOTATATE PET/CT was assessed by correlating the quantitative metabolic parameters with the patient outcome. The primary study endpoint was progression-free survival (PFS), defined as the time between the PET/CT study and the date of the follow-up study when PD was reported. The secondary endpoint was the overall survival (OS), defined as the time between the PET/CT study and death from any cause. The prognostic value of PET/CT quantifiers for PFS and OS was analyzed in Cox proportional hazard model along with clinical variables. Thresholds for significant quantifiers were established by receiver operating characteristic (ROC) analysis. The stratified patient groups were then analyzed as binominal variables. Results within 95% confidence level (P < 0.05) were considered significant.
Results
The characteristics of the patient cohort and the data regarding treatment are presented in Table 1. The mean patient follow-up was 28.2 months (range: 20.9–41.9 months). During the follow-up, 3 of 13 patients died (all deaths were due to local tumor progression) and PD was observed in 9 of 14 tumors (in all cases local within the irradiated field) with a median time to PD of 10.3 months (range: 3.6–16.9). Five patients are alive and without PD. The estimated 2-year PFS and OS is 28.1% and 76.9%, respectively. The survival curves are demonstrated in Figure 2. The treatment of recurrences was highly individualized and involved surgery, reirradiation and somatostatin analogues or combinations of these modalities.
Table 1.
Clinical characteristics of the patients (n=13) and meningiomas (n=14)
| Characteristics | Value (%) |
|---|---|
| Median patient age (years), range | 58.7, 18-77 |
| Patient sex | |
| Male | 8 (61.5) |
| Female | 5 (38.5) |
| Total number of meningiomas | |
| 1 | 5 (38.5) |
| 2-3 | 3 (23) |
| >3 | 5 (38.5) |
| Tumor location | |
| Anterior cranial fossa | 5 (35.7) |
| Medial cranial fossa | 6 (42.9) |
| Falx | 2 (14.3) |
| Orbit | 1 (7.1) |
| Histology according to WHO | |
| Grade II | 9 (64.3) |
| Grade III | 5 (35.7) |
| Surgery | |
| Yes | 12 (85.7) |
| No | 2 (14.3) |
| Radiation treatment modality | |
| IMRT | 4 (28.5) |
| CK | 8 (57.1) |
| IMRT + CK boost | 2 (14.3) |
| Median biological radiation dose (gray), range | 60.8, 54-87.8 |
CK: CyberKnife, IMRT: Intensity-modulated radiation therapy
Figure 2.
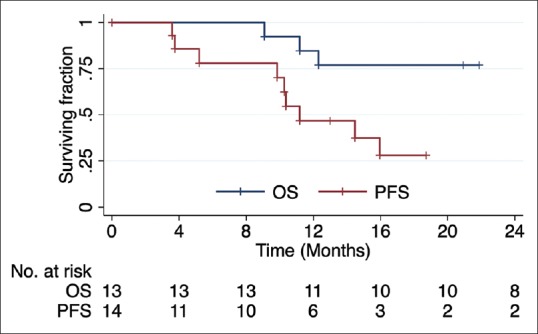
Kaplan–Meier survival curves for progression-free survival (red) and overall survival (blue)
All patients were eligible for evaluation of PET/CT quantifiers – in all cases, the tumors demonstrated tracer uptake noticeably higher than the background level (median SUVmax= 12.9, range: 4.2–29.2). Two tumors of 14 were not eligible for assessment of the liver threshold-based parameters as the liver uptake was in all ROIs measured higher than the one of tumor; other quantifiers could be evaluated.
In risk factor analysis, the MTVgb was the only metabolic quantifier identified as prognostic for PFS (HR = 1.02; 95% confidence interval [CI]: 1.003–1.048; P = 0.021). Male sex was also found to be a significant risk factor (HR = 13.06; 95%CI: 1.56-109.25; P = 0.018). For OS, SUVms(HR = 6.2, 95% CI: 1.1–35.4: P =0.041) and SUVgb(HR = 1.53, 95% CI: 1–2.35; P = 0.048) were identified as significant prognosticators. None of the clinical variables were identified as significant predictors for this endpoint.
For all three statistically significant metabolic quantifiers, ROC analysis identified relevant cutoff thresholds. The results of this analysis are displayed in Table 2; the PFS of patients stratified according to the MTVgb values is shown on Figure 3.
Table 2.
Comparison of metabolic quantifiers statistically significant in univariate models
| Endpoint | Quantifier | Threshold | AUC | Risk above threshold (%) | Risk below threshold (%) | Log-rank P |
|---|---|---|---|---|---|---|
| PFS | MTVgb | 34 cm3 | 0.844 | 100 | 28.6 | 0.0003 |
| OS | SUVms | 3.5 | 0.996 | 75 | 0 | 0.002 |
| SUVgb | 7.5 | 0.9 | 100 | 10 | 0.002 |
AUC: Area under curve (1.0 would correspond to an ideal predictor of 100% specificity and 100% sensitivity), PFS: Progression-free survival, OS: Overall survival, MTV: Metabolic tumor volume, SUV: Standardized uptake volume
Figure 3.
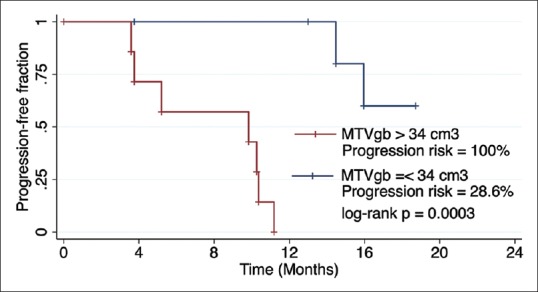
Kaplan–Meier survival curves for progression-free survival stratified according to the metabolic tumor volumegb threshold value
Discussion
The application of PET/CT in radiation oncology is an increasingly promising topic. For Ga-68-DOTA conjugates in meningioma, its primary established value is the improvement of tumor visualization. At least three groups have observed a significant planning tumor volume alteration when referring to the Ga-68-DOTA PET/CT in addition to MRI in 65%–77% of patients.[11,12,13] The Ga-68-DOTATATE PET/CT as a potential source of prognostic information in meningioma is also likely to draw clinicians' attention. One of the two studies of Ga-68-DOTATATE PET/CT in high-grade meningiomas was published aimed to analyze the recurrence pattern of these tumors. The authors did not identify any prognostic factors for outcome; the only analyzed metabolic quantifier was the SUVmax which was not significant (P = 0.09 and 0.88 for PFS and OS, respectively).[6] Another group evaluated 64 inoperable meningiomas of 23 patients and found the SUVmax of intracranial (WHO Grade I-II) and transosseous (all WHO grade I) tumors to be significantly correlating with their growth rate (r = 0.757, P < 0.001 and r = 0.819, P = 0.024, respectively). The only subgroup for which this correlation was not observed were the anaplastic meningiomas.[14]
The array of the tracer uptake parameters that was preestablished for analysis in this study has not been previously described for Ga-68-DOTA in meningioma. These quantifiers have been used with F-18-FDG PET/CT, with the prognostic significance of different particular values reported across various tumor locations.[15,16,17] In the present study, the MTV was identified as a prognostic factor for PFS, suggesting that it is the greater tumor volume that corresponds to poor disease outcome. Observations in line with this hypothesis have already been published for meningiomas; it must be noted that these studies did not use functional imaging (CT and MRI were performed). The first group found the treated tumor volume to be prognostic for death risk (HR: 1.04, P = 0.0095).[18] Another study reported a significantly increased risk of death and recurrence in meningiomas above 14.6 cm3 in size.[19] Finally, already during the study design, it was expected that if any dependence of PFS on the tumor volume is identified, then the gradient-based volumetric assessment would be the most significant prognosticator or closest to being one. A number of studies comparing the gradient-based and target-to-background metabolic volume calculation methods and validating them against the physical volume of the specimen after surgical removal are available for other tumors including laryngeal,[20] lung,[21] and esophageal cancer.[22] All of these groups identified the gradient-based assessment as the most accurate method.
The main drawback of the present study is its limited cohort size. This can be attributed to the epidemiology and management of meningiomas: currently, there are no guidelines recommending pre-surgical DOTA PET/CT for all patients, and due to the fact that about 90% of meningiomas are histologically WHO Grade I[1] and have excellent outcomes after radiotherapy,[3] we would not expect that it is widely introduced. On the contrary, our study includes highly negatively selected patients featuring the biggest risk of progression and death. Patients with subtotally resected nonbenign meningiomas have been reported to have significantly worse outcomes than ones in whom an oncologically radical resection (Simpson Grade I and II) was performed for both PFS (31%–36% vs. 63%–74%[4,5]) and OS (particularly notable for WHO Grade III tumors: as low as 10%–17% vs. 45%–75%[23,24]). Furthermore, while the MTV parameter seems to be less dependent on the examination protocol and potentially well reproducible, the same cannot be said about the mean SUV. A change in the resolution or acquisition time would significantly alter the mean and maximum SUV values; we are therefore aware of the limited practical usability of the mean SUVs that our study found prognostic for OS. The most notable application of well-standardized Ga-68-DOTA PET/CT quantifiers would be a trial escalating the radiation dose in meningiomas most prone to local treatment failure. Before this is possible, bigger patient cohorts must be prospectively studied. Such analysis should also be multicentric to increase the chance of finding enough individuals and evaluate the reproducibility of PET/CT quantifiers across different protocols, scanners, or software.
Conclusion
Ga-68-DOTATATE PET/CT is a potentially valuable supplementary imaging modality in the planning of radiotherapy of nonbenign meningioma. In addition to its primary application, which is the support of tumor visualization in the treatment planning, it may be also used to predict the disease progression in tumors at highest risk. The gradient-based metabolic volume measurement appears to be the method of choice. Validation on bigger patient cohorts and across institutions is indicated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wen PY, Quant E, Drappatz J, Beroukhim R, Norden AD. Medical therapies for meningiomas. J Neurooncol. 2010;99:365–78. doi: 10.1007/s11060-010-0349-8. [DOI] [PubMed] [Google Scholar]
- 2.Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–91. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- 3.Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA. Stereotactic radiosurgery provides equivalent tumor control to simpson grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55:1000–5. doi: 10.1016/s0360-3016(02)04356-0. [DOI] [PubMed] [Google Scholar]
- 4.Hammouche S, Clark S, Wong AH, Eldridge P, Farah JO. Long-term survival analysis of atypical meningiomas: Survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir (Wien) 2014;156:1475–81. doi: 10.1007/s00701-014-2156-z. [DOI] [PubMed] [Google Scholar]
- 5.Nanda A, Bir SC, Konar S, Maiti T, Kalakoti P, Jacobsohn JA, et al. Outcome of resection of WHO grade II meningioma and correlation of pathological and radiological predictive factors for recurrence. J Clin Neurosci. 2016;31:112–21. doi: 10.1016/j.jocn.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Zollner B, Ganswindt U, Maihöfer C, Corradini S, Alber LN, Schichor CH, et al. Recurrence pattern analysis after [Ga-68]-DOTATATE-PET/CT -planned radiotherapy of high-grade meningiomas. Rad Oncol. 2018;13:110. doi: 10.1186/s13014-018-1056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorwarth D, Henke G, Müller AC, Reimold M, Beyer T, Boss A, et al. Simultaneous 68Ga-DOTATOC-PET/MRI for IMRT treatment planning for meningioma:First experience. Int J Radiat Oncol Biol Phys. 2011;81:277–83. doi: 10.1016/j.ijrobp.2010.10.078. [DOI] [PubMed] [Google Scholar]
- 8.Silva CB, Ongaratti BR, Trott G, Haag T, Ferreira NP, Leães CG, et al. Expression of somatostatin receptors (SSTR1-SSTR5) in meningiomas and its clinicopathological significance. Int J Clin Exp Pathol. 2015;8:13185–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Subramaniam RM. PET/CT and Patient Outcomes. In: Vassallo J, editor. PET Clinics. Part. I. Vol. 10. Philadelphia: Elsevier; 2015. pp. 125–279. [Google Scholar]
- 10.Prasad V, Baum RP. Biodistribution of the ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: Characterization of uptake in normal organs and tumor lesions. Q J Nucl Med Mol Imaging. 2010;54:61–7. [PubMed] [Google Scholar]
- 11.Gehler B, Paulsen F, Oksüz MO, Hauser TK, Eschmann SM, Bares R, et al. [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. 2009;4:56. doi: 10.1186/1748-717X-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milker-Zabel S, Zabel-du Bois A, Henze M, Huber P, Schulz-Ertner D, Hoess A, et al. Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int J Radiat Oncol Biol Phys. 2006;65:222–7. doi: 10.1016/j.ijrobp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Graf R, Nyuyki F, Steffen IG, Michel R, Fahdt D, Wust P, et al. Contribution of 68Ga-DOTATOC PET/CT to target volume delineation of skull base meningiomas treated with stereotactic radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:68–73. doi: 10.1016/j.ijrobp.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Sommerauer M, Burkhardt JK, Frontzek K, Rushing E, Buck A, Krayenbuehl N, et al. 68Gallium-DOTATATE PET in meningioma: A reliable predictor of tumor growth rate? Neuro Oncol. 2016;18:1021–7. doi: 10.1093/neuonc/now001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi H, Kitajima K, Fukushima K, Kawanaka Y, Mouri M, Yamamoto S, et al. SUVmax on FDG-PET is a predictor of prognosis in patients with maxillary sinus cancer. Jpn J Radiol. 2016;34:349–55. doi: 10.1007/s11604-016-0531-9. [DOI] [PubMed] [Google Scholar]
- 16.Alluri KC, Tahari AK, Wahl RL, Koch W, Chung CH, Subramaniam RM. Prognostic value of FDG PET metabolic tumor volume in human papillomavirus-positive stage III and IV oropharyngeal squamous cell carcinoma. AJR Am J Roentgenol. 2014;203:897–903. doi: 10.2214/AJR.14.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bundschuh RA, Dinges J, Neumann L, Seyfried M, Zsótér N, Papp L, et al. Textural parameters of tumor heterogeneity in 18F-FDG PET/CT for therapy response assessment and prognosis in patients with locally advanced rectal cancer. J Nucl Med. 2014;55:891–7. doi: 10.2967/jnumed.113.127340. [DOI] [PubMed] [Google Scholar]
- 18.Ferraro DJ, Funk RK, Blackett JW, Ju MR, DeWees TA, Chicoine MR, et al. Aretrospective analysis of survival and prognostic factors after stereotactic radiosurgery for aggressive meningiomas. Radiat Oncol. 2014;9:38. doi: 10.1186/1748-717X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Stereotactic radiosurgery of world health organization grade II and III intracranial meningiomas: Treatment results on the basis of a 22-year experience. Cancer. 2012;118:1048–54. doi: 10.1002/cncr.26362. [DOI] [PubMed] [Google Scholar]
- 20.Geets X, Lee JA, Bol A, Lonneux M, Grégoire V. A gradient-based method for segmenting FDG-PET images: Methodology and validation. Eur J Nucl Med Mol Imaging. 2007;34:1427–38. doi: 10.1007/s00259-006-0363-4. [DOI] [PubMed] [Google Scholar]
- 21.Wanet M, Lee JA, Weynand B, De Bast M, Poncelet A, Lacroix V, et al. Gradient-based delineation of the primary GTV on FDG-PET in non-small cell lung cancer: A comparison with threshold-based approaches, CT and surgical specimens. Radiother Oncol. 2011;98:117–25. doi: 10.1016/j.radonc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Zhang G, Han D, Ma C, Lu J, Sun T, Liu T, et al. Gradient-based delineation of the primary GTV on FLT PET in squamous cell cancer of the thoracic esophagus and impact on radiotherapy planning. Radiat Oncol. 2015;10:11. doi: 10.1186/s13014-014-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moliterno J, Cope WP, Vartanian ED, Reiner AS, Kellen R, Ogilvie SQ, et al. Survival in patients treated for anaplastic meningioma. J Neurosurg. 2015;123:23–30. doi: 10.3171/2014.10.JNS14502. [DOI] [PubMed] [Google Scholar]
- 24.Anvari K, Hosseini S, Rahighi S, Toussi MS, Roshani N, Torabi-Nami M, et al. Intracranial meningiomas: Prognostic factors and treatment outcome in patients undergoing postoperative radiation therapy. Adv Biomed Res. 2016;5:83. doi: 10.4103/2277-9175.182214. [DOI] [PMC free article] [PubMed] [Google Scholar]