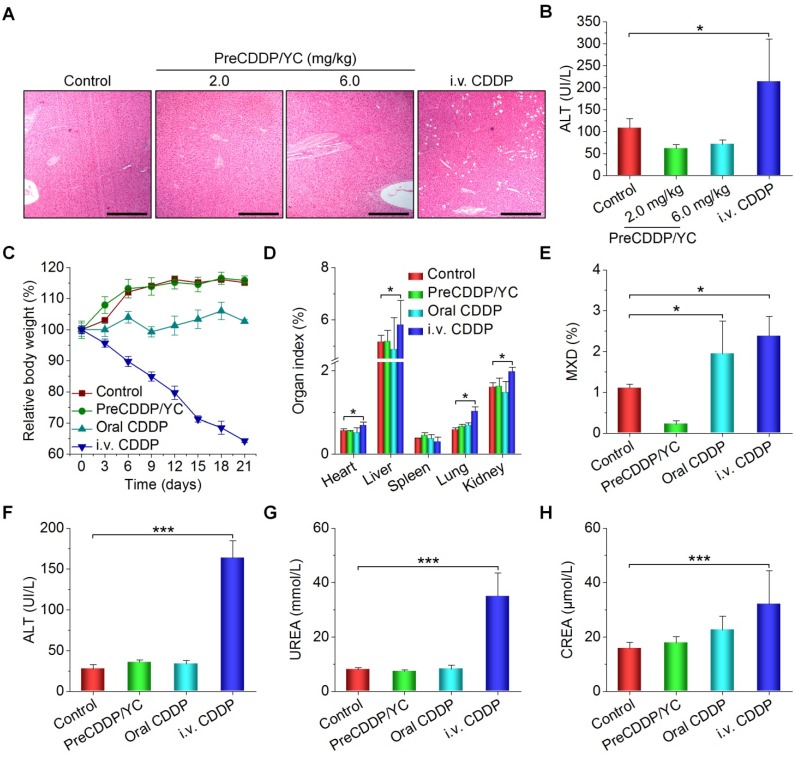
Figure 8.
In vivo safety study of different CDDP formulations in mice. (A-B) H&E-stained sections of liver isolated from A549 xenograft-bearing nude mice (A) and the serum ALT level (B) after treatment with either orally administered PreCDDP/YC or intravenously injected CDDP at 6.0 mg/kg. Scale bars, 200 μm. (C) Changes in the relative body weight of BALB/c mice during treatment with different formulations. (D-H) The organ index (D), the mixed cell count percentage (MXD) (E), as well as the levels of ALT (F), UREA (G), and CREA (H) after different treatments. Mice in the PreCDDP/YC and Oral CDDP groups were treated every three days by oral administration at a CDDP dose of 6.0 mg/kg, while the i.v. CDDP group was intravenously injected with the same dose of CDDP every three days. In all cases, mice in the control group received saline by oral gavage. Data are mean ± SD (B, n = 8; C-H, n = 6); *p < 0.05, ***p < 0.001.