Abstract
Immune responses to poorly immunogenic antigens, such as polysaccharides, can be enhanced by conjugation to carriers. Our previous studies indicate that conjugation to Vi polysaccharide of Salmonella Typhi may also enhance immunogenicity of some protein carriers. We therefore explored the possibility of generating a bivalent vaccine against Plasmodium falciparum malaria and typhoid fever, which are co-endemic in many parts of the world, by conjugating Vi polysaccharide, an approved antigen in typhoid vaccine, to Pfs25, a malaria transmission blocking vaccine antigen in clinical trials. Vi-Pfs25 conjugates induced strong immune responses against both Vi and Pfs25 in mice, whereas the unconjugated antigens are poorly immunogenic. Functional assays of immune sera revealed potent transmission blocking activity mediated by anti-Pfs25 antibody and serum bactericidal activity due to anti-Vi antibody. Pfs25 conjugation to Vi modified the IgG isotype distribution of antisera, inducing a Th2 polarized immune response against Vi antigen. This conjugate may be further developed as a bivalent vaccine to concurrently target malaria and typhoid fever.
Keywords: Malaria, Pfs25, Transmission blocking vaccine, Vi capsular polysaccharide, Typhoid fever, Conjugate vaccine
1. Introduction
Malaria and typhoid fever are co-endemic in large parts of the world, particularly in tropical areas [1–3]. Despite significant control efforts, approximately 21 million typhoid cases and 222 000 typhoid-related deaths were reported in 2014 [3], while 214 million malaria cases and 438 000 malaria-related deaths were reported in 2015 [4]. Typhoid fever results from S. Typhi infection transmitted through contaminated water and food [5]. Malaria is a parasitic infection transmitted by female Anopheles mosquito [6]. Though the source and route of these two infections are different, their prevalence has significant regional overlap in Africa and other tropical countries, and disproportionately affect children under 5 years of age [7,8]. In co-endemic regions, malaria infection may enhance susceptibility to typhoid fever, and co-infection may lead to misdiagnosis due to similar symptoms [9–11].
Currently, two typhoid vaccines, Vi capsular polysaccharide vaccine (Typhim Vi®) and oral live attenuated vaccine (S. Typhi Ty21a), are licensed and marketed. However, both vaccines provide poor protection for infants and children under 2 years of age, and are not recommended in this age group.
Bacterial polysaccharide conjugation to carrier proteins has become a common approach to overcome the poor immunogenicity of polysaccharides, starting with Haemophilus influenzae type b (Hib) vaccine, the first conjugate vaccine licensed in 1987 [12–14]. Conjugation technology has enabled the development of more immunogenic typhoid conjugate vaccines. Two Vi-TT (Tetanus Toxoid) conjugate vaccines, Typbar TCV® (Bharat Biotech) and Peda Typh™ (Bio-Med), have been licensed and marketed in India [15,16], while Vi-rEPA (recombinant ExoProtein A), [17–19], Vi-DT (diphtheria toxoid) [20,21], and Vi-CRM197 (nontoxic mutant of diphtheria toxin) are being evaluated for use in infants and children [22].
In studies exploring the immunogenicity of Vi conjugated to carrier proteins such as PspA (pneumococcal surface protein A), HBsAg (Hepatitis B virus surface antigen) and DT (Diphtheria Toxoid), all proteins were found to enhance the antibody response to Vi. Interestingly, conjugation also enhanced immune responses to PspA and HBsAg but not DT [23,24]. These findings suggested the potential of Vi conjugation to enhance responses to some protein antigens.
Based on this observation, we explored development of a bivalent conjugate vaccine against both typhoid fever and P. falciparum malaria. Malaria vaccine development has been hindered by the complexity of the parasite and its life cycle, as well as poor immunogenicity of many malaria antigens. The most advanced malaria vaccine candidate is a pre-erythrocytic vaccine called RTS,S, which is a virus-like particle formulated in AS01 adjuvant. RTS,S has demonstrated partial efficacy against clinical malaria in infants and young children in Phase 3 trials that wanes with time [25].
Other major vaccine efforts against malaria include pre-erythrocytic whole organism vaccines, blood stage vaccines, and transmission blocking vaccines (TBV) [26–29]. TBV have received increased attention owing to renewed interest in malaria elimination and eradication. TBV antigens are expressed in the mosquito stages of the parasite life cycle and induce antibodies that, when taken up by mosquitoes during blood meals, can prevent mosquito infection and subsequent transmission [30]. TBV might be developed as stand-alone products, or can be combined with components that prevent human infection as vaccines to interrupt malaria transmission (VIMT) [31].
Among the antigens identified as targets for TBV, Pfs25 has been the most extensively studied vaccine candidate and has received most attention for clinical development [32,33]. Pfs25 is poorly immunogenic and strategies to enhance immunogenicity have included conjugation to carrier proteins [34,35] or Outer Membrane Vesicles [36], and incorporation in virus like particles [37] or nanoparticles [38]. We have shown that conjugation of Pfs25 to different carriers increases antibody titers in animals [34–36,39,40] and humans [32].
Here we describe the functional immunogenicity of a bivalent vaccine candidate generated by conjugation of Pfs25 to Vi polysaccharide. We observed significant enhancement of antibody responses against both antigens, suggesting that this concept can be pursued as a bivalent vaccine to block malaria transmission and prevent typhoid fever.
2. Materials and methods
2.1. Vi capsular polysaccharide
Vi polysaccharide used in this study was purified from S. Typhi isolate number C6524 strain, originally obtained from a patient by the National Institute of Cholera and Enteric Diseases (NICED) in India [41]. Larger scale manufacturing including fermentation of S. Typhi, Vi purification, and Vi characterization was performed by SK Chemical, Gyunggido, South Korea. Vi contains 2.3 mmol O-acetyl per gram, and 79% of Vi was eluted from Sepharose CL-4B gel column with a distribution constant (KD) of 0.25. Protein and nucleic acid contamination in purified Vi were less than 0.05% and 0.5% respectively, and endotoxin level was 1.5 EU per microgram. Vi quality met WHO requirements for Vi polysaccharide vaccine.
2.2. Malaria transmission blocking vaccine (TBV) antigen
Recombinant Pfs25 (MW 18,735 Daltons) used for the synthesis of Vi-Pfs25 and EPA-Pfs25 conjugates was produced in Pichia pastoris according to the method previously reported by Tsai et al. [42].
2.3. Preparation of Vi-Pfs25 conjugates
Vi-Pfs25 conjugates were synthesized by two synthetic methods (Fig. S1). Details of conjugate syntheses are given in the supplementary data.
2.4. Characterization of conjugates
Pfs25 content was confirmed by sodium dodecyl sulfate–poly-acrylamide gel electrophoresis (SDS PAGE)/Western blot analysis of conjugate (2.5 µg Pfs25 equivalent), using 4–20% Tris-Glycine gel (ThermoFisher) and 30 mA constant current, and transfer to nitrocellulose membrane using iBlot device (Invitrogen). Blots were incubated with primary antibody (anti-Pfs25 mAb, 4B7) followed by secondary antibody labeled with alkaline phosphatase (goat anti-mouse IgG, KPL), and developed with BCIP/NBT phosphatase substrate (KPL).
2.5. Immunogenicity and IgG subclass composition
All animal studies were conducted per the guidelines and approval of Animal Care and Use committee at the National Institutes of Health. CD1 female mice received intramuscular injections of various conjugates in PBS or adsorbed to Alhydrogel® adjuvant (2% Alhydrogel® Brenntag, Biosector) on day 0 and 28 with 2.5 µg dose equivalent of Pfs25 in 50 µl injection volume. Alhydrogel® formulations contained 450 µg/ml of aluminum (22.5 µg/dose) and were prepared in PBS, pH 7.4. The conjugate/Alhydrogel® formulations were incubated at room temperature with gentle rotation for 1 h and stored at 2–8° C for up to 7 days until use. Control mice received 2.5 µg Vi alone in PBS or formulated in Alhydrogel®.
Mice were bled on day 42, then variously on day 83 or 91 for different groups. Mice immunized with PBS formulations were bled on day 83, and those with Alhydrogel® formulations were bled on day 91. Antibody titers against Pfs25 and Vi were measured by ELISA as described in Supplementary Data section, using un-conjugated Pfs25 and Vi respectively as coating antigens [43,44]. Vi-titer of sera diluted 1:500 is expressed in terms of optical density (OD) at 405 nm. IgG subclasses were determined in pooled sera from the second bleed using Mouse Monoclonal Antibody Isotyping Kit (ISO2, Sigma Aldrich). Each IgG subclass (IgG1, IgG2a, IgG2b and IgG3) titer was calculated as the reciprocal of serum dilution giving an OD of 0.5 for Vi and Pfs25, and expressed as a% contribution to the sum of subclasses.
2.6. Transmission blocking activity and bactericidal activity assays
2.6.1. Standard membrane feeding assay (SMFA)
Transmission blocking activity of immune sera was evaluated by SMFA, which measures the reduction of oocyst formation in mosquitos fed on P. falciparum gametocytes mixed with test sera versus control sera [33]. Briefly, Anopheles stephensi mosquitoes fed on cultured P. falciparum gametocytes mixed with sera through a Parafilm membrane stretched across a heated membrane feeding apparatus. Mouse test and control sera were tested at 1:5 dilution. In 8 days, mosquitoes were dissected, and the number of oocysts counted. A reduction in the number of midgut oocysts in mosquitoes fed on immune sera versus control sera indicates transmission blocking activity.
2.6.2. Serum bactericidal assay (SBA)
Bactericidal activity against S. Typhi was evaluated in the SBA, as described previously [45]. Serial dilutions of heat-inactivated (56 °C) sera (50 µl) were mixed with S. Typhi bacteria in triplicate, incubated for 1hr at 37 °C with 10% baby rabbit complement, then plated on square LB agar plate and overlaid with top agar. Bacterial colonies were counted after overnight incubation to determine SBA titer, defined as highest dilution giving 50% inhibition of colony formation.
2.7. Statistical analysis
ELISA data were analyzed with Prism software (GraphPad Software, Inc., La Jolla, CA) and statistical differences between groups were measured using a Kruskal-Wallis One-way ANOVA followed by a Dunn multiple comparator test for comparing three or more groups.
3. Results
3.1. Modification of Vi polysaccharide and Pfs25 with ADH (adipic acid dihydrazide) for conjugation
We examined the effect of various concentrations of EDC ((1-e thyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride) and ADH on the level of Vi modification and molecular weight of ADH-modified Vi (Method 1). EDC concentrations varied from 5 to 40 mM while maintaining ADH concentration either at 20 mM or 200 mM (referred to as low-ADH or high-ADH condition, respectively). Level of modification determined by TNBS (2,4,6-trinitrobenzene sulfonic acid) assay was 4.0–4.9% (wt.% of ADH) for Vi modified under low-ADH conditions and 17.8–25.9% under high-ADH condition, while low-ADH yielded larger molecular weight Vi than high-ADH (Table 1). This suggests that the presence of excess ADH may hinder intermolecular cross-linking to some extent. Pfs25 was modified with 20 mM ADH and 20 mM EDC, and yielded approximately 5 molecules of ADH per Pfs25.
Table 1.
Vi modification levels and molecular weights at different concentrations of EDC and ADH.
| Low-ADH Condition | |||
|---|---|---|---|
| Reaction conditions |
Modified Vi - characteristics |
||
| ADH | EDC | ADH*% (w/w) | Avg**. Mw (kDa) |
| 20 mM | 5 mM | 4.0 | 1283 |
| 10 mM | 4.5 | 1547 | |
| 20 mM | 4.9 | 1759 | |
| 30 mM | 4.8 | 1720 | |
| 40 mM | 4.4 | 2028 | |
|
High-ADH condition | |||
| Reaction conditions |
Modified Vi - characteristics |
||
| ADH | EDC | ADH% (w/w) | Avg. Mw (kDa) |
| 200 mM | 5 mM | 18.9 | 673 |
| 10 mM | 17.8 | 856 | |
| 20 mM | 25.9 | 1076 | |
| 30 mM | 25.8 | 1140 | |
| 40 mM | 25.6 | 1226 | |
Level of modification was determined by TNBS assay.
The average molecular weight was calculated by size exclusion chromatography with multi angle light scattering (SEC-MALS) detection with calibration using 12 kDa Dextran and the Vi polysaccharide (120 kDa).
3.2. Chemical conjugation of Pfs25 to Vi
Five batches of Vi-Pfs25 conjugates were synthesized initially, including four by Method 1 using low- or high-ADH conditions and one by Method 2. Conjugate syntheses by Method 1 were carried out using Vi modified with 4.0, 4.5, 18.9 and 25.9 wt% ADH, at a Pfs25/Vi input ratio of 1.3 or 1.2 by weight (Table 2), and by Method 2 at a ratio of 2. After the reaction, all conjugates were dialyzed extensively using 100 kDa molecular weight cut-off tubing against PBS containing 0.5 M NaCl to remove unconjugated Pfs25. Conjugates synthesized by Method 1 had similar molecular weight in the range of 3.8–5.2 mDa, while conjugates synthesized by Method 2 were substantially smaller with average molecular weight around 0.37 mDa (Table 2). Pfs25/Vi ratio was 0.82–1.27 and 0.53 for method 1 and 2 respectively.
Table 2.
Characteristics of Vi-Pfs25 conjugates.
| Conjugate-Lot# | Modification | Modification ADH% (wt%) | Conjugation Input ratio (Pfs25: Vi) | Final conjugate composition: Pfs25/Vi (w/w) | Average Conjugate Size (Mw) |
|---|---|---|---|---|---|
| ViADH-Pfs25–21 | Low-ADH (Method 1) | 4.0 | 1.3: 1 | 1.13 | 3.8 mDa |
| ViADH-Pfs25–22 (ViLowADH-Pfs25)* | Low-ADH (Method 1) | 4.5 | 1.2: 1 | 0.82 | 4.1 mDa |
| ViADH-Pfs25–23 | High-ADH (Method 1) | 18.9 | 1.2: 1 | 1.27 | 5.2 mDa |
| ViADH-Pfs25–24 (ViHighADH-Pfs25)* | High-ADH (Method 1) | 25.9 | 1.2: 1 | 0.92 | 5.0 mDa |
| Vi-Pfs25ADH-25 (Vi-Pfs25ADH)* | Modified Pfs25 (Method 2) | 3.8 | 2.0:1 | 0.53 | 0.37 mDa |
These conjugates were evaluated in mouse immunogenicity studies and are referred to by these labels in the text and figures describing the results from the immunogenicity studies.
3.3. SDS-PAGE & Western blot
Conjugates were analyzed by SDS-PAGE and Western blot to confirm conjugation of Pfs25 to Vi (Fig. S2). Samples (~2.5 µg Pfs25 equivalent) were separated on 4–20% Tris-Glycine gel and stained with Coomassie blue, showing high molecular weight material appearing within the well or spread across the upper half of the lane. This high molecular weight material corresponded to conjugates with Pfs25 antibody reactivity on Western blots. Unconjugated Pfs25 (both monomer and multimer) was also detected by Western blot, suggesting unconjugated protein may be retained by association with the conjugate during dialysis.
3.4. Pfs25. Antibody responses in immunized mice
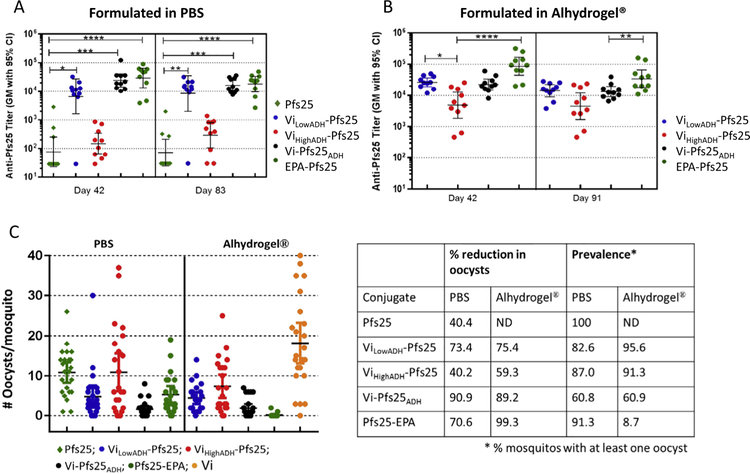
Three of the Vi-Pfs25 conjugates (ViLowADH-Pfs25, ViHighADH-Pfs25 and Vi-Pfs25ADH) formulated either in PBS or Alhydrogel® were compared to the benchmark EPA-Pfs25 conjugate as well as to unconjugated Pfs25 and Vi polysaccharide for immunogenicity in mice. In PBS, unconjugated Pfs25 induced very low titer responses, while ViLowADH-Pfs25 and Vi-Pfs25ADH (but not ViHighADH-Pfs25) increased Pfs25 titers ~2 orders of magnitude, comparable to EPA-Pfs25 (Fig. 1A). High titers observed on Day 42 persisted through Day 83. Alhydrogel® formulation increased titers induced by both ViLowADH-Pfs25 and ViHighADH-Pfs25 lots as well as EPA-Pfs25 but not Vi-Pfs25ADH (Fig. 1A and B).
Fig. 1.
Anti-Pfs25 antibody titers and transmission blocking activity of immune sera. (A) Anti-Pfs25 ELISA antibody titers on Days 42 and 83 for immune sera from mice vaccinated with unconjugated Pfs25, Vi conjugates of Pfs25, and EPA-Pfs25 formulated in PBS. (B) Anti-Pfs25 antibody titers on Days 42 and 91 for immune sera from mice vaccinated with Vi conjugates of Pfs25 and EPA-Pfs25 formulated in Alhydrogel®. ELISA data were analyzed with Prism software (GraphPad Software, Inc., La Jolla, CA) and statistical differences between groups were measured using a Kruskal-Wallis analysis followed by a Dunn multiple comparator test for comparing three or more groups. * p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001, **** p ≤ 0.0001. C) Standard Membrane Feeding Assay results showing the number of oocysts developed in each mosquito fed on P. falciparum gametocytes mixed with immune sera obtained from mice immunized with PBS or Alhydrogel formulations of various Vi-Pfs25 conjugates, EPA-Pfs25 and Pfs25. Immune sera from Day 42 was used for the assay and mice immunized with Vi was used as control. Table shows percentage reduction in mean oocyst count compared to Vi control sera.
In SMFA measurements of functional activity, antisera raised against unconjugated Pfs25 in PBS reduced oocyst numbers ~40%, while Vi-Pfs25ADH antisera reduced >90% and ViLowADH-Pfs25 antisera reduced ~70%; ViHighADH-Pfs25 antisera showed only 40% reduction (Fig. 1C). Alhydrogel® formulation improved the activity of antisera raised against ViHighADH-Pfs25 but not ViLowADH-Pfs25 or Vi-Pfs25ADH. Number of infected mosquitoes (prevalence) was also lowest for Vi-Pfs25ADH compared to other Vi conjugates. Activity of antisera against EPA-Pfs25 also improved with Alhydrogel® and was highest among all the groups.
3.5. Anti-Vi immune response
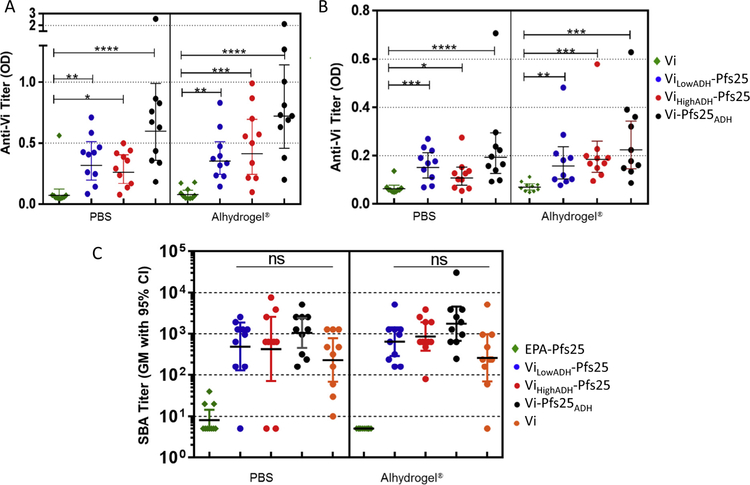
In ELISA, antisera raised against Vi alone in PBS induced low titers, while all conjugates induced significantly higher titers, with Vi-Pfs25ADH inducing highest anti-Vi titers (Fig. 2A). Alhydrogel® did not increase anti-Vi titers for any of the immunogens. Anti-Vi titer decreased for all the conjugates by day 83 or 91 (Fig. 2B).
Fig. 2.
Anti-Vi antibody titer of conjugates and serum bactericidal activity (SBA) of conjugates. Anti-VI ELISA antibodies obtained for immune sera from mice vaccinated with Vi conjugates of Pfs25, unconjugated Vi and EPA-Pfs25 formulated in PBS or Alhydrogel®. ELISA for sera obtained on (A) day 42 sera, and (B) on days 83 (for PBS formulations) and 91 (for Alhydrogel® formulations) was performed using Vi as coating antigen and sera diluted 500-fold. (C) Serum bactericidal activity (SBA) assay titer observed for sera from mice immunized with Vi-Pfs25 conjugates, unconjugated Vi and EPA-Pfs25 formulated in PBS or Alhydrogel®. Mice were vaccinated on days 0 and 28 and sera obtained on Day 83 (PBS formulations) or Day 91 (Alhydrogel® formulations) were analyzed for bactericidal activity. Data were analyzed with Prism software (GraphPad Software, Inc., La Jolla, CA) and statistical differences between groups were measured using a Kruskal-Wallis analysis followed by a Dunn multiple comparator test for comparing three or more groups. * p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001, **** p ≤ 0.0001.
Anti-Vi functional activity of immune sera was evaluated by SBA assay. Sera from mice immunized with Pfs25 or EPA-Pfs25 showed no SBA titer, whereas Vi alone and all three Vi conjugates showed significant SBA levels (Fig. 2C). All conjugates induced higher SBA than Vi alone, consistent with their higher titers. Formulation in Alhydrogel® did not significantly increase the SBA titer for any of the samples.
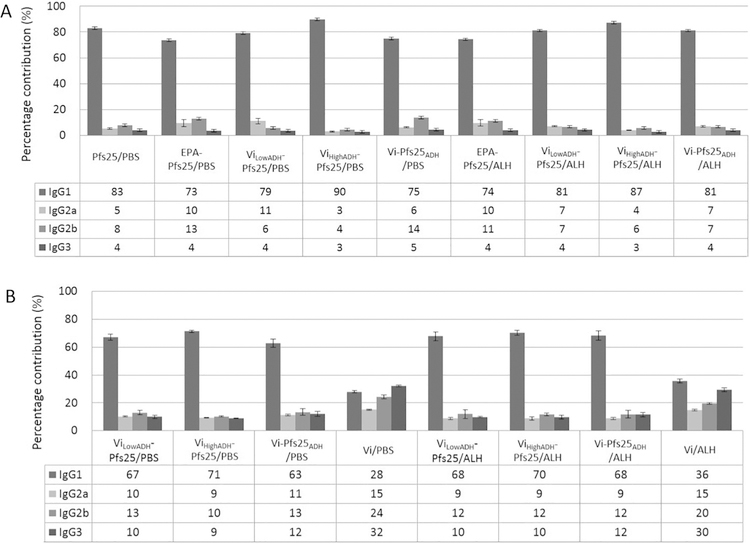
We evaluated the IgG isotype levels in the terminal bleed sera (days 83 or 91). IgG response to Pfs25 and EPA-Pfs25 conjugate is dominated by IgG1 both in PBS and Alhydrogel® formulations (Fig. 3A). All Vi conjugates, either in PBS or in Alhydrogel®, show dominant IgG1 response to Pfs25, contributing >70% of total IgG. Anti-Vi IgG isotypes (Fig. 3B) show more diversity in their response. Vi in PBS induced more IgG3 (32%) than IgG1 (28%), IgG2b (24%) or IgG2a (15%), while Vi in Alhydrogel® induced more IgG1 (36%) than IgG3 (30%) response. Chemical conjugation to Pfs25 further increased Vi specific IgG1 levels to >60%, both in PBS and Alhydrogel®.
Fig. 3.
Percentage contribution of IgG subclasses to total IgG against (A) Pfs25 and (B) Vi. Percentage contribution from IgG1, IgG2a, IgG2b and IgG3 to total IgG response against (A) Pfs25 and (B) Vi was determined from isotype specific ELISA titers obtained using pooled sera from each group of mice. Day 83 sera were analyzed for groups immunized with PBS formulations and day 91 sera were analyzed for groups received Alhydrogel® formulations of various conjugates and antigens. Percentage contribution is expressed as the percentage of subclass specific ELISA titer to the sum of all subclass titers.
We examined if chemical conjugation is necessary or simple mixing of the two antigens would be sufficient for the enhanced immunogenicity against Pfs25. Fig.S3 (supplemental data) shows comparison of anti-Pfs25 antibody titer in mice immunized with a 1:1 mixture of Pfs25 and Vi compared to a chemical conjugate of Pfs25 and Vi, formulated in PBS and Alhydrogel®. Though the antibody titer observed for Pfs25/Vi mixture was higher than for Pfs25 alone (Fig. 1A), chemical conjugates of Pfs25 and Vi induced significantly higher titers.
4. Discussion
Chemical conjugation to carriers has been a successful strategy to enhance the immunogenicity of poorly immunogenic antigens, particularly bacterial polysaccharides [46]. These carriers generally are highly immunogenic proteins or particles that convert the T-independent polysaccharide immune response to a durable T-cell dependent immune response. However, the response to the carrier protein has not been of great interest [47]. While developing a polysaccharide conjugate vaccine for typhoid fever, we observed that in certain instances, chemical conjugation of Vi polysaccharide enhanced antibody responses to the carrier protein as well [23,24]. Based on this observation, we explored the potential for developing a bivalent malaria-typhoid vaccine through chemical conjugation of Vi polysaccharide antigen to a malaria antigen.
For this initial effort, we conjugated transmission blocking vaccine antigen, Pfs25, to Vi polysaccharide. Pfs25 is a poorly immunogenic antigen and its immunogenicity can be enhanced by conjugation to carrier proteins [34,35,39]. A conjugate of Pfs25 and EPA has been undergoing clinical trials as a candidate transmission blocking vaccine [32].
Here, Vi-Pfs25 conjugates were synthesized by two general methods using ADH chemistry. Chemical conjugation of Pfs25 to Vi enhances the immunogenicity of both antigens significantly in mice, compared to the corresponding unconjugated antigens (Fig. 1A and 2A). Though two synthetic methods employed here resulted in conjugates with vastly different molecular weight and composition, both methods yielded conjugates with high immunogenicity. ADH modified Vi (Method 1) yielded conjugates with average molecular weight greater than 10 times that of ADH modified Pfs25 (Method 2) conjugate. Interestingly, one conjugate but not the other synthesized by method 1 generated high immunogenicity against Pfs25, despite similar size and composition. Formulation in Alhydrogel® had a negligible effect on Pfs25 responses with the conjugate generated with ADH-modified Pfs25, but increased Pfs25 responses of both conjugates prepared with ADH modified Vi, albeit to different degrees. The reasons for these differences are unclear. Comparing the anti-Vi immune response, all three conjugates induced significantly higher anti-Vi titers compared to unconjugated Vi (Fig. 2A). Formulation in Alhydrogel® did not further enhance their anti-Vi immunogenicity.
Functional activity induced by the conjugates was evaluated in SMFA and SBA, respectively, for anti-Pfs25 and anti-Vi activity. All conjugates induced functional activity against both antigens, though to varying levels. Among the three conjugates in PBS, Vi-Pfs25ADH induced highest functional activity in SMFA; formulation in Alhydrogel® increased activity induced by ViHighADH-Pfs25 conjugates but not ViLowADH-Pfs25 or Vi-Pfs25ADH. All three conjugates showed higher SBA activity compared to Vi alone, with conjugate Vi-Pfs25ADH showing the highest functional activity. This indicates that Vi-Pfs25 conjugates have the potential to be more effective typhoid vaccines than the unconjugated Vi, currently used in Typhim Vi®.
Antibody isotyping revealed an IgG1 dominated immune response against the Pfs25 antigen, as expected for soluble protein antigens [48,49]. Conjugation of Pfs25 to Vi increased the IgG1 response against Pfs25 with a minor contribution from IgG2 in both PBS and Alhydrogel®, indicating a Th2 polarized immune response. Vi polysaccharide alone gives a diverse response with similar contributions from IgG1, IgG2 and IgG3. On conjugation with Pfs25, anti-Vi antibody responses become more IgG1 dominated (>60%) while maintaining a broad isotype distribution as observed for other Vi conjugates such as Vi-CRM197 [50].
Chemical conjugation of a poorly immunogenic antigen to a highly immunogenic carrier can enhance antibody responses and boosting, and current conjugate vaccines as well as those under development have followed this strategy. It is interesting that in case of Vi-Pfs25 conjugate, two poorly immunogenic antigens when conjugated together result in stronger immune response to both antigens. It is clear that chemical conjugation is necessary for this effect, as mixing of the two antigens does not achieve a similar degree of enhancement. Future research should explore similar effects with other malaria antigens.
This study demonstrates that Vi polysaccharide can serve as a carrier for candidate protein or peptide vaccine antigens to increase their immunogenicity. Vi polysaccharide can be produced by extraction of surface polysaccharide from Salmonella Typhi and is the antigen in Typhim Vi® an FDA approved vaccine against Typhoid fever. This study also demonstrates that Vi-Pfs25 conjugate is a viable bifunctional vaccine candidate to prevent typhoid fever and block malaria transmission. This conjugate also may address one of the regulatory and ethical issues with TBV, which do not directly prevent infection of the vaccinee but rather reduce malaria incidence throughout the community by blocking malaria transmission [30]. A typhoid-malaria bivalent vaccine may prevent typhoid, thereby conferring a direct benefit to recipients while preventing malaria transmission in the community.
Supplementary Material
Acknowledgements
Authors would like to thank David Narum for kindly providing Pfs25, SK Chemical, Gyunggido, S. Korea for providing Vi polysaccharide, Chris Rowe for helpful suggestion on conjugate synthesis, Joo Sung Woo for assistance with Vi ELISA and J. Patrick Gorres for assistance in writing and review of this manuscript. All authors attest they meet the ICMJE criteria for authorship.
Funding
This research was funded by the Intramural Research Program of the National Institutes of Health. SA was supported by the KRIBB Research Initiative Program (Korean Biomedical Science Fellowship Program), Korea Research Institute of Bioscience and Biotechnology, Republic of Korea.
Footnotes
Conflict of Interest
The authors would like to disclose that a patent entitled “Bivalent immunogenic conjugate for malaria and typhoid” was filed on April 24, 2017. (PCT/US2017/029182).
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2018.04.035.
References
- [1].Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IR, Johnston GL, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J 2011;10:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Uneke CJ. Concurrent malaria and typhoid fever in the tropics: the diagnostic challenges and public health implications. J Vector Borne Dis 2008;45:133–42. [PubMed] [Google Scholar]
- [3].Mogasale V, Maskery B, Ochiai RL, Lee JS, Mogasale VV, Ramani E, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. The Lancet Global Health 2014;2:570–80. [DOI] [PubMed] [Google Scholar]
- [4].World malaria report 2015; World Health Organization: Geneva, Switzerland. [Google Scholar]
- [5].Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Glob Health 2012;2:010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature 2002;415:673–9. [DOI] [PubMed] [Google Scholar]
- [7].Mweu E, English M. Typhoid fever in children in Africa. Trop Med Int Health 2008;13:532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rowe AK, Rowe SY, Snow RW, Korenromp EL, Schellenberg JR, Stein C, et al. The burden of malaria mortality among African children in the year 2000. Int J Epidemiol 2006;35:691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Crump JA. Typhoid Fever and the Challenge of Nonmalaria Febrile Illness in Sub-Saharan Africa. Clin Infect Dis 2012;54:1107–9. [DOI] [PubMed] [Google Scholar]
- [10].Pradhan P. Coinfection of typhoid and malaria. J Med Lab Diagnosis 2011;2:22–6. [Google Scholar]
- [11].Church J, Maitland K. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med 2014;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stei KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis 1992;165:S49–52. [DOI] [PubMed] [Google Scholar]
- [13].Schneerson R, Barrera O, Sutton A, Robbins JB. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med 1980;152:361–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Robbins JB, Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis 1990;161:821–32. [DOI] [PubMed] [Google Scholar]
- [15].Mohan VK, Varanasi V, Singh A, Pasetti MF, Levine MM, Venkatesan R, et al. Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: a multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin Infect Dis 2015;61:393–402. [DOI] [PubMed] [Google Scholar]
- [16].Chinnasami B, Sadasivam K, Vivekanandhan A, Arunachalam P, Pasupathy S. A Study on Longevity of Immune Response after Vaccination with Salmonella Typhi Vi Conjugate Vaccine (Pedatyph) in Children. J Clin Diagn Res 2015;9: SC01–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Szu SC. Development of Vi conjugate - a new generation of typhoid vaccine. Expert Rev Vaccines 2013;12:1273–86. [DOI] [PubMed] [Google Scholar]
- [18].Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med 2001;344:1263–9. [DOI] [PubMed] [Google Scholar]
- [19].Kossaczka Z, Bystricky S, Bryla DA, Shiloach J, Robbins JB, Szu SC. Synthesis and immunological properties of Vi and di-O-acetyl pectin protein conjugates with adipic acid dihydrazide as the linker. Infect Immun 1997;65:2088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].An SJ, Yoon YK, Kothari S, Kim DR, Kim JA, Kothari N, et al. Immune suppression induced by Vi capsular polysaccharide is overcome by Vi-DT conjugate vaccine. Vaccine 2012;30:1023–8. [DOI] [PubMed] [Google Scholar]
- [21].An SJ, Yoon YK, Kothari S, Kothari N, Kim JA, Lee E, et al. Physico-chemical properties of Salmonella typhi Vi polysaccharide-diphtheria toxoid conjugate vaccines affect immunogenicity. Vaccine 2011;29:7618–23. [DOI] [PubMed] [Google Scholar]
- [22].Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect Dis 2014;14:119–29. [DOI] [PubMed] [Google Scholar]
- [23].An SJ, Woo JS, Chae MH, Kothari S, Carbis R. Preparation and testing of a Haemophilus influenzae Type b/Hepatitis B surface antigen conjugate vaccine. Vaccine 2015;33:1614–9. [DOI] [PubMed] [Google Scholar]
- [24].Kothari N, Kothari S, Choi YJ, Dey A, Briles DE, Rhee DK, et al. A bivalent conjugate vaccine containing PspA families 1 and 2 has the potential to protect against a wide range of Streptococcus pneumoniae strains and Salmonella Typhi. Vaccine 2015;33:783–8. [DOI] [PubMed] [Google Scholar]
- [25].RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015;386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Richie TL, Billingsley PF, Sim BK, James ER, Chakravarty S, Epstein JE, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 2015;33:7452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miura K. Progress and prospects for blood-stage malaria vaccines. Expert Rev Vaccines 2016;15:765–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gonçalves D, Hunziker P. Transmission-blocking strategies: the roadmap from laboratory bench to the community. Malar J 2016;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med 1991;174:1203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sauerwein RW. Malaria transmission-blocking vaccines: the bonus of effective malaria control. Microbes Infect 2007;9:792–5. [DOI] [PubMed] [Google Scholar]
- [31].A research agenda for malaria eradication: Vaccines. The malERA consultative group on vaccines Plos Med 2011;8:e1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Talaat KR, Ellis RD, Hurd J, Hentrich A, Gabriel E, Hynes NA, et al. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel(R), a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naive Adults. PLoS One 2016;11:0163144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 2008;3:e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shimp RL Jr, Rowe C, Reiter K, Chen B, Nguyen V, Aebig J, et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 2013;31:2954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Scaria PV, Chen B, Rowe CG, Jones DS, Barnafo E, Fischer ER, et al. Protein-protein conjugate nanoparticles for malaria antigen delivery and enhanced immunogenicity. PLoS One 2017;12:e0190312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu Y, Przysiecki C, Flanagan E, Bello-Irizarry SN, Ionescu R, Muratova O, et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc Natl Acad Sci U S A 2006;103:18243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jones RM, Chichester JA, Mett V, Jaje J, Tottey S, Manceva S, et al. A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS One 2013;8:e79538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kumar R, Ray PC, Datta D, Bansal GP, Angov E, Kumar N. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine 2015;33(39):5064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jones DS, Rowe CG, Chen B, Reiter K, Rausch KM, Narum DL, et al. A Method for Producing Protein Nanoparticles with Applications in Vaccines. PLoS One 2016;11:e0138761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Radtke AJ, Anderson CF, Riteau N, Rausch K, Scaria P, Kelnhofer ER, et al. Adjuvant and carrier protein-dependent T-cell priming promotes a robust antibody response against the Plasmodium falciparum Pfs25 vaccine candidate. Sci Repm 2017;7:40312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jang H, Yoon YK, Kim JA, Kim HS, An SJ, Seo JH, et al. Carbis R Optimization of Vi capsular polysaccharide production during growth of Salmonella enterica serotype Typhi Ty2 in a bioreactor. J Biotechnol 2008;135:71–7. [DOI] [PubMed] [Google Scholar]
- [42].Tsai CW, Duggan PF, Shimp RL Jr, Miller LH, Narum DL. Overproduction of Pichia pastoris or Plasmodium falciparum protein disulfide isomerase affects expression, folding and O-linked glycosylation of a malaria vaccine candidate expressed in P. pastoris. J Biotechnol 2006;121:458–70. [DOI] [PubMed] [Google Scholar]
- [43].Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine 2008;26:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Szu SC, Hunt S, Xie G, Robbins JB, Schneerson R, Gupta R, et al. A human IgG anti-Vi reference for Salmonella typhi with weight-based antibody units assigned. Vaccine 2013;31:1970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jang MS, Sahastrabuddhe S, Yun CH, Han SH, Yang JS. Serum bactericidal assay for the evaluation of typhoid vaccine using a semi-automated colony-counting method. Microb Pathog 2016;97:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 2011;17:1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bröker M, Berti F, Schneider J, Vojtek I. Polysaccharide conjugate vaccine protein carriers as a ‘‘neglected valency” - Potential and limitations. Vaccine 2017;35:3286–94. [DOI] [PubMed] [Google Scholar]
- [48].Ferrante A, Beard LJ, Feldman RG. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr Infect Dis J 1990;9:S16–24. [PubMed] [Google Scholar]
- [49].Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014;5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fiorino F, Ciabattini A, Rondini S, Pozzi G, Martin LB, Medaglini D. Immunization with the conjugate vaccine Vi-CRM197 against Salmonella typhi induces Vi-specific mucosal and systemic immune responses in mice. Vaccine 2012;30:6111–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.