Abstract
Background:
Neoadjuvant chemotherapy (NACT) for the treatment of muscle-invasive bladder cancer (MIBC) remains underutilized in the United States despite evidence supporting its use.
Objectives:
To examine the perioperative chemotherapy management of patients with MIBC by medical oncologists (MedOncs) to move toward standardization of practice
Participants and methods:
A 26-question survey was emailed to 92 MedOncs belonging to the Bladder Cancer Advocacy Network or the American Society of Clinical Oncology for completion from May to October 2011
Results:
A total of 83 MedOncs completed the survey: 52% were based in academic centers. Most referrals were from urologists (79%). NACT for treatment of MIBC and high-grade upper-tract urothelial carcinoma is offered by 80% and 46% of respondents, respectively. Adjuvant chemotherapy for treatment of MIBC and upper-tract urothelial carcinoma is offered by 46% and 42% of respondents, respectively. NACT was not offered by 49%, 29%, and 35% of respondents if Eastern Cooperative Oncology Group performance status was 3 or greater, if patients had T2 lesions without lymphovascular invasion, and if the glomerular filtration rate was <50 ml/min, respectively. Chemotherapy regimens included gemcitabine/cisplatin (90%), methotrexate/vinblastine/adriamycin/cisplatin (30%), dose-dense methotrexate, vinblastine, adriamycin, and cisplatin (20%), and gemcitabine/carboplatin (37%).
Conclusions:
Most MedOncs (79%) in this survey offer perioperative chemotherapy to all patients with MIBC. This increased use of NACT is higher than previously reported, suggesting an increase in the adoption of recommendations that follow best evidence.
Keywords: Adjuvant chemotherapy, Medical oncologist, Muscle-invasive bladder cancer, Neoadjuvant chemotherapy, Survey
1. Introduction
In the United States (US), more than 70,000 patients in 2013 presented with bladder cancer and more than 15,000 died of metastatic disease [1,2]. Approximately 20% to 25% of patients have muscle-invasive bladder cancer (MIBC), which has a high rate of disease progression, because 50% harbor micrometastatic disease that is not detectable by conventional imaging. Therefore, although patients undergo radical cystectomy, half of the patients relapse and die of metastatic disease. MIBC is potentially curable, but often fatal without effective treatment strategies. Optimal management of MIBC mandates a multidisciplinary approach with coordination of care between radiologists, pathologists, urologists, medical oncologists (MedOncs), and in some cases radiation oncologists for staging, multimodality treatment, and follow-ups.
Although radical cystectomy alone may lead to a durable cure in MIBC, the high rate of tumor recurrence suggests that early institution of systemic therapy is necessary to improve overall survival (OS) [3,4]. In MIBC, neoadjuvant chemotherapy (NACT) administered with definitive local therapy has been extensively evaluated in the hopes of improving OS. The long-term results of the international, multicenter, phase III European Organisation for Research and Treatment of Cancer (EORTC)/Medical Research Council trial that randomized 976 patients to receive 3 cycles of neoadjuvant cisplatin, methotrexate, and vinblastine or no NACT showed an absolute survival benefit of 5% and a relative reduction in the risk of death of 16% at 10 years [5]. The randomized Southwest Oncology Group (SWOG) 8710 trial also showed the median survival to be 77 months in patients with MIBC receiving neoadjuvant methotrexate/vinblastine/adriamycin/cisplatin (MVAC) followed by radical cystectomy, compared with 46 months for patients having radical cystectomy alone; a benefit in median survival from NACT of 2.5 years [6]. A meta-analysis of 3,005 patients with MIBC who received cisplatin-based NACT, including patients in the EORTC/Medical Research Council and SWOG studies, confirmed an absolute survival benefit of 5% and a 14% risk reduction in mortality at 5 years [7].
Given the level 1 evidence of a survival benefit conferred by cisplatin-based NACT, it would be expected that the use of NACT for the treatment of MIBC would be widely implemented by urologists and MedOncs. The National Comprehensive Cancer Network (NCCN) guidelines strongly support the use of cisplatin-based combination NACT for the treatment of MIBC with category 1 evidence. However, multiple retrospective studies (before 2003) using the Surveillance, Epidemiology and End Results–Medicare database or the National Cancer Database report a low use of perioperative chemotherapy (11%–12%), with NACT used in <2% of patients with MIBC [8,9]. A report documented a higher use in patients having lesions of higher T categories [10]. Unfortunately, even when NACT was given, cisplatin was usually not included. Although, practice patterns may take years to change after level 1 data have been reported, it appears that evidence-based practice change is not occurring in MIBC. A report of patients with MIBC managed at 15 institutions between 2003 and 2008 found that only 34% received perioperative chemotherapy, of which 14% was NACT and only 11% was cisplatin based [11]. A review of 17,330 cases from the Italian National Cancer Database (2003–2007) found that only 9% had received NACT for the treatment of MIBC before undergoing radical cystectomy [12]. Although there was a modest increase in NACT use from 6% in 2003 to 13% in 2007, these reports highlight the consistent underutilization of NACT for the treatment of MIBC.
The primary goal of this study was to understand the practice patterns of both academic and community MedOncs treating MIBC in the United States, including the frequency of use and type of NACT and adjuvant chemotherapy (ACT) administered, the diagnostic studies performed, and posttreatment follow-up.
2. Participants and methods
2.1. Survey
This study was approved by the US Office of Management and Budget (0925–0046). To ensure a mix of experiences and perspectives, participants were from both larger academic medical centers and smaller community-based practices. An electronic 26-question, secure, non-identifiable link to a web-based survey was emailed to 92 MedOncs belonging to the Bladder Cancer Advocacy Network or the American Society of Clinical Oncology and was also posted on the “US Oncology Portal” from May to October 2011. The US Oncology Portal is part of the I Know Med electronic medical record shared by more than 2,000 community oncologists in the US Oncology network.
Patient treatment patterns were analyzed based on type of clinical practice, referral information, type of primary tumor (bladder cancer vs. upper-tract urothelial carcinoma [UTUC], stage, age, renal function, and performance status [PS]). These data were cross-tabulated with treatment and management strategies, including frequency of use, type and dose of NACT and ACT, and imaging and diagnostic studies (computed tomography [CT] of the abdomen and pelvis, CT of the chest, ultrasound, technetium-99m bone scan, chest radiograph, magnetic resonance imaging, positron emission tomography (PET) scan, and urine cytology) at baseline and on follow-up.
The operating settings for the web survey were set up to accept only a single response (i.e., 1 survey) from 1 computer. The only identifiable information collected was the Internet Protocol address. Participants were not provided with consent forms. However, the following language was added to the invitation email and to the link in “The Oncology Portal” as an alternate for the consent form: “By clicking on the link below, you are consenting to participate in this survey.”
2.2. Analysis
A wide variety of parameters were measured. All results are reported using percentages and descriptive analysis. Percentages are based on the fraction that responded to a given question.
3. Results
3.1. Demographics
In total, 83 MedOncs completed >75% of the survey; 48% practiced in a community setting and 52% in an academic setting. Demographics of the responders and their corresponding patients with bladder cancer are in Table 1. Fig. 1 displays the percentage of responders who offer NACT or ACT or both for the treatment of MIBC and patients with high-grade UTUC.
Table 1.
Patient and medical oncology practice characteristics
| Patient and practice characteristics | All medical oncologist n = 83 |
Type of practice |
|
|---|---|---|---|
| Academic medical oncologist n = 44 (53%) |
Community medical oncologist n = 39 (47%) |
||
| Proportion of practice dedicated to genitourinary (GU) cancer | |||
| >50% | 39 (46%) | 35 (80%)* | 3 (8%)* |
| 25%−50% | 4 (5%) | 1 (2%) | 3 (8%) |
| 10%−25% | 22 (26%) | 5 (11%) | 17 (43%) |
| <10% | 19 (23%) | 3 (7%) | 17 (43%) |
| No response | 0 | 0 | 0 |
| Number of MIBC referred to your practice last year | |||
| > 20 | 25 (31%) | 22 (50%)* | 3 (8%)* |
| 13–20 | 14 (18%) | 9 (20%) | 5 (14%) |
| 6–12 | 19 (24%) | 6 (14%) | 13 (36%) |
| 1 −5 | 22 (28%) | 7 (16%) | 15 (42%) |
| No response | 4 | 0 | 4 |
| What do you estimate to be the relative contribution of each of the following in your bladder cancer referrals? | |||
| Referral source: median (range) for each category | |||
| Urologist | 80% (20%−100%) | 80% (25%−100%) | 80% (20%−100%) |
| Other medical oncologist | 10% (0%−75%) | 10% (0%−75%) | 15% (0%−75%) |
| Family physician or internist | 5% (0%−50%) | 5% (0%−20%) | 10% (0%−50%) |
| Radiation oncologist | 10% (0%−50%) | 10% (0%−50%) | 10% (0%−50%) |
| Other | 0% (0%−20%) | 0% (0%−15%) | 0% (0%−20%) |
| No response | 4 | 0 | 4 |
| Age distribution of MIBC patients: median (range) for each category | |||
| <50 y | 5% (0%−50%) | 5% (0%−50%) | 10% (0%−25%) |
| 50–64 y | 30% (0%−100%) | 30% (0%−100%) | 30% (0%−80%) |
| 65–84 y | 50% (0%−100%) | 55% (0%−85%) | 55% (0%−100%) |
| > 85 y | 5% (0%−60%) | 5% (0%−20%) | 10% (0%−60%) |
| No response | 4 | 0 | 4 |
| T-stage distribution of MIBC referred to your practice: median (range) for each category | |||
| T2: invades muscle | 40% (5%−100%) | 30% (5%−80%) | 50% (15%−100%) |
| T3: invasion of perivesical tissue | 30% (10%−85%) | 30% (10%−85%) | 30% (10%−70%) |
| T4a: invasion of adjacent organs | 10% (3%−50%) | 10% (3%−50%) | 10% (5%−30%) |
| T4b: invasion of pelvic/abdominal wall | 7.5% (2%−30%) | 5% (0%−20%) | 10% (0%−30%) |
| T (any) N (any): lymph node involvement | 10% (5%−90%) | 10% (5%−60%) | 20% (5%−90%) |
| T (any) M1: metastatic disease | 10% (0%−60%) | 5% (1%−50%) | 17.5% (0%−60%) |
| No response | 16 | 0 | 16 |
P < 0.0001 by exact 2-tailed Cochran-Armitage test for trend, comparing distribution between academic and community medical oncologists.
Fig. 1.
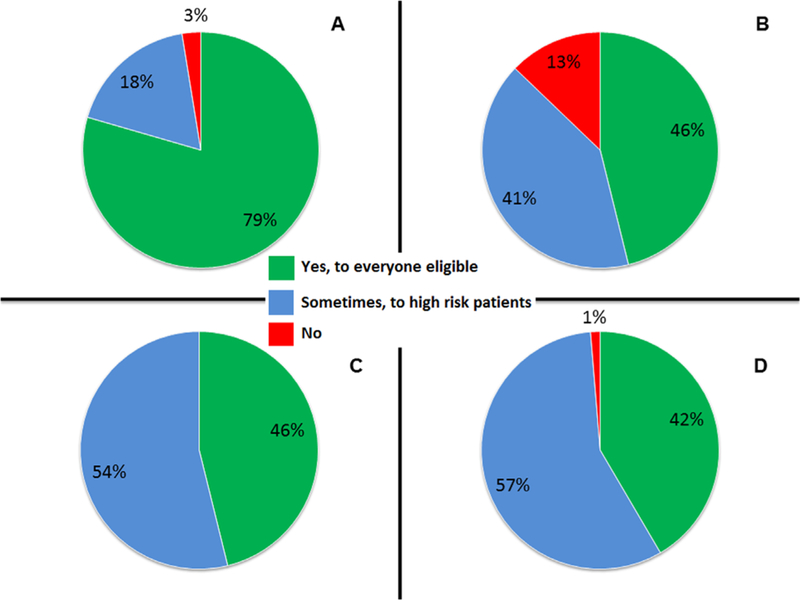
Percentage of responses to questions about perioperative chemotherapies in bladder cancer and patients with upper-tract urothelial cancer. (A) Do you offer neoadjuvant chemotherapy? (B) Do you offer neoadjuvant chemotherapy to patients with upper-tract (renal pelvis or ureter) urothelial cancer? (C) Do you offer adjuvant chemotherapy? (D) Do you offer adjuvant chemotherapy to patients with upper-tract (renal pelvis or ureter) urothelial cancer?
3.2. Use of perioperative chemotherapy
Fig. 2 shows the distribution of the results in Fig. 1 among academic vs. community MedOncs. Fig. 3 shows the average distribution of NACT vs. ACT given for the treatment of MIBC between both the groups. The collated results comparing the use of NACT in patients with MIBC with the use of NACT in patients with UTUC and ACT in patients with MIBC or UTUC showed that the MedOncs who offer NACT to eligible patients with MIBC do not offer it to everyone with UTUC (27% offer it only sometimes and 10% do not offer it all) and are split in the use of ACT for primary MIBC and UTUC between offering it to everyone and offering only sometimes to high-risk patients (data are not shown).
Fig. 2.
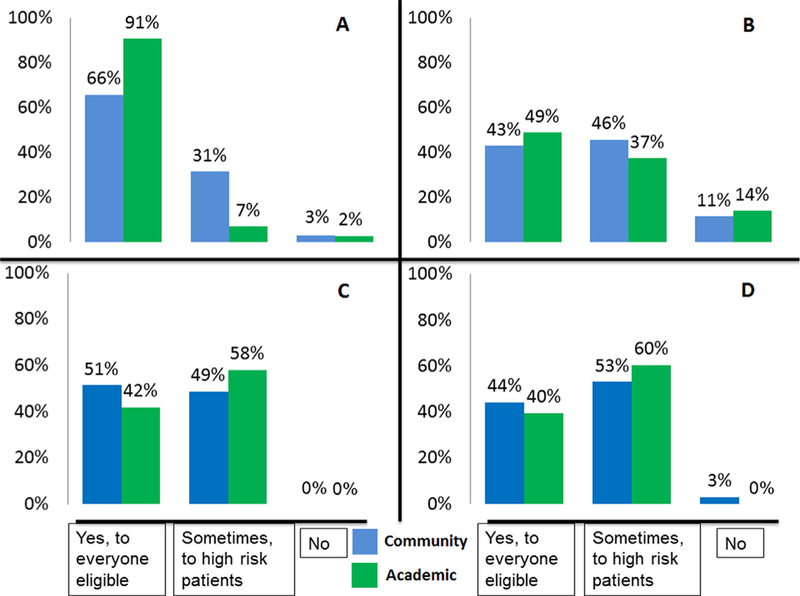
Percentage of responses to questions about perioperative chemotherapies in bladder cancer and patients with upper-tract urothelial cancer, divided by academic vs. community participants. (A) Do you offer neoadjuvant chemotherapy? (B) Do you offer neoadjuvant chemotherapy to patients with upper-tract (renal pelvis or ureter) urothelial cancer? (C) Do you offer adjuvant chemotherapy? (D) Do you offer adjuvant chemotherapy to patients with upper-tract (renal pelvis or ureter) urothelial cancer?
Fig. 3.
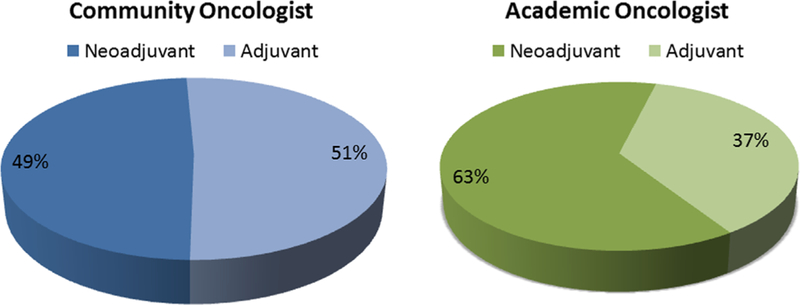
The estimated relative distribution of perioperative chemotherapies.
Fig. 4 shows the percentage of MedOncs who do not offer NACT based on certain PS, age, or renal function. Physicians were also asked about clinical T category and lymph node (LN) status at presentation in their decision making. Overall, 29% did not offer NACT to patients with T2 lesions without lymphovascular invasion and 22% did not offer NACT to patients with T4b lesions (data not shown). In terms of LN status, more than 50% of physicians do not recommend NACT if there is evidence of regional involvement. Instead, physicians suggested that patients with nodal involvement should be treated as having metastatic disease with 6 cycles of chemotherapy and considered for postchemotherapy radical cystectomy, only if there was a response to chemotherapy (data not shown). The average time interval between the final dose of NACT and radical cystectomy is 4 to 6 weeks in 92% of responders (data not shown). Fig. 5 shows the adjustments made by MedOncs in patients with renal insufficiency and how patients with pathologic residual disease are managed. Table 2 lists the most common chemotherapy regimens used. The doses commonly used were cisplatin 70 mg/m2, gemcitabine 1,000 mg/m2, carboplatin area under the curve of 5 mg/ml/min, and paclitaxel 175 mg/m2.
Fig. 4.
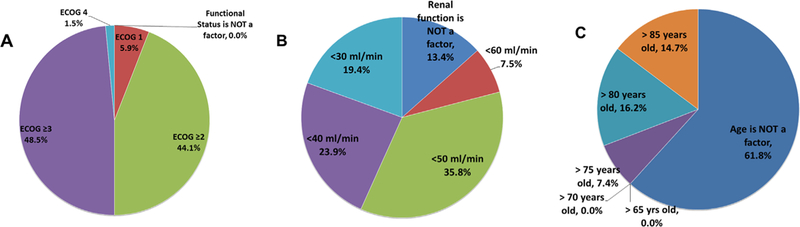
When do you NOT give neoadjuvant chemotherapy? (n = 68). (A) At what functional status would you NOT recommend neoadjuvant chemotherapy? (B) At what GFR value would you NOT recommend neoadjuvant chemotherapy? (C) At what age would you NOT recommend neoadjuvant chemotherapy?
Fig. 5.
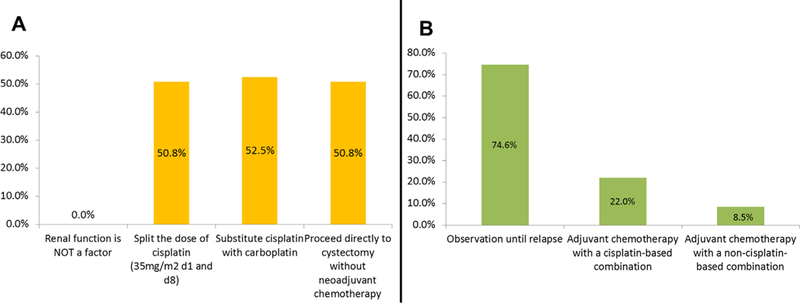
(A) Adjustments of chemotherapy for renal insufficiency. (B) Management of pathologic residual disease after neoadjuvant chemotherapy.
Table 2.
Chemotherapy regimens: “If you use any of the following neoadjuvant chemotherapy regimens, please specify the number of cycles used. Indicate all that apply”
| Chemotherapy regimen | Respondents (n = 63) |
The most commonly used number of cycles (n, %) |
|---|---|---|
| Gemcitabine/cisplatin D1, 8 of 21-d cycle | 56 (89%) | 4 cycles (40, 63%) |
| Gemcitabine/carboplatin | 23 (37%) | 4 cycles (14, 22%) |
| Gemcitabine/cisplatin D1, 8, 15 of 28-d cycle | 20 (32%) | 3 cycles (12, 19%) |
| MVAC | 18 (29%) | 3 cycles (12, 19%) |
| Dose-dense MVAC | 12 (19%) | 4 cycles (10, 16%) |
| Gemcitabine/single agent | 6 (10%) | 6 cycles (3, 5%) |
| Other (carboplatin/paclitaxel) | 2 (4%) | 3 cycles (2, 3%) |
3.3. Staging modalities
Table 3 lists the staging and follow-up modalities required by MedOncs in the management of patients with MIBC undergoing NACT; 78% of MedOncs restage only after NACT completion and not during NACT.
Table 3.
Preference of tests for initial staging and follow-up staging
| Initial staging no. of respondents (n = 68, (%) |
Follow-up staging no. of respondents (n = 63, (%) |
|
|---|---|---|
| CT abdomen and pelvis | 61 (90%) | 51 (81%) |
| CT chest | 49 (72%) | 25 (40%) |
| Cystoscopy | Not asked | 19 (30%) |
| Bone scan | 26 (38%) | 4 (5%) |
| PET/CT scan | 14 (21%) | 8 (12.7%) |
| Chest x-ray | 11 (16%) | 4 (6%) |
| MRI abdomen and pelvis | 10 (14%) | 2 (3%) |
| Urine cytology | 1 (1%) | 2 (3%) |
| Ultrasound of abdomen and pelvis | 0 (0%) | 0 (0%) |
MRI = magnetic resonance imaging; PET = positron emission tomography.
4. Discussion
This is the first survey to examine practice patterns in the management of MIBC among MedOncs in the United States. In contrast to previous reports from cancer databases [10–12], the results of this survey suggest greater adoption of level 1 evidence with 98% of MedOncs indicating they would offer NACT. However, patient selection for NACT occurs frequently with 18% of physicians offering NACT only to patients with “high-risk” MIBC based on specific tumor characteristics. Fewer physicians offer NACT for the treatment of UTUC, but ACT is very frequently offered by MedOncs for the treatment of both MIBC and UTUC. This practice pattern likely represents referral patterns in which medical oncology consults occur after patients have under-gone radical cystectomy.
Patient factors play a greater role in decision making than do tumor-specific factors. Moreover, concerns about cisplatin toxicity may be one of the factors that accounts for the gap between previously published reports, suggesting underutilization of NACT and the results of this survey. As indicated (Fig. 4), 49% of respondents do not offer NACT if the ECOG PS was >3, 35% if the glomerular filtration rate (GFR) is <50ml/min, and 16% if the patient is older than 80 years. Age was not considered a determining factor by 61%. Previously published data have demonstrated that pre-existing renal impairment significantly affects the willingness to administer perioperative cisplatin-based chemotherapy to patients with MIBC [13], and those concerns are reflected in these data. Therefore, although more MedOncs are offering cisplatin-based NACT, an older patient population with frequent renal insufficiency at risk for renal toxicity highly influences treatment recommendations.
In patients with MIBC who have renal insufficiency, MedOncs were equally divided among (1) splitting the dose of cisplatin over 2 days, (2) substituting cisplatin with carboplatin, or (3) recommending radical cystectomy without NACT. Splitting cisplatin over 2 days, by giving cisplatin 35 mg/m2 on days 1 and 8 has been studied in a phase I/II trial, and it showed an overall response rates (RR) of 65%, 4 complete responses (CR) (12.5%), and a median OS of 16 months [14]. A large ongoing Cancer and Leukemia Group B 90601 phase III trial evaluating the efficacy of bevacizumab with gemcitabine and cisplatin (GC) allows patients with mild-moderate renal insufficiency (GFR = 40–50 ml/min) to receive split-dose cisplatin providing an opportunity for a subset analysis of tolerance and efficacy (NCT00942331). In bladder cancer, carboplatin is clearly inferior to cisplatin in terms of both CR and OS [15–18]; in the SWOG phase II (S0219) study, 3 cycles of neoadjuvant paclitaxel, gemcitabine, and carboplatin showed a 60% rate of persistent cancer at radical cystectomy, despite a documented clinical CR on cystoscopy [19].
This survey highlights the need for improvement in the choice of NACT, pretreatment staging, and follow-up modalities. GC is the NACT of choice in 90% of surveyed MedOncs with 4 cycles given on a 21-day schedule or 3 to 4 cycles on a 28-day schedule being the favored regimens. The second most preferred combination, by 37% of respondents, was 3 or 4 cycles of gemcitabine and carboplatin, despite a lack of evidence. Other, cisplatin-based preferences included 3 cycles of MVAC (30%) and 4 cycles of dose-dense (dd) MVAC (20%). It is important to note that the most commonly used regimens for NACT do lack level I evidence. The data supporting the use of GC are extrapolated from a randomized phase III trial that compared MVAC vs. GC in 405 patients with metastatic-urothelial carcinoma. There was no difference in median OS, time to progression, or RR between the arms, but GC showed a more favorable safety profile with less neutropenic fever, neutropenic sepsis, and grade 3 to 4 mucositis. Likewise, the use of neoadjuvant dd-MVAC is also extrapolated from the EORTC 30924 trial performed in metastatic urothelial carcinoma [20,21]. This randomized phase III trial compared dd-MVAC plus granulocyte colony-stimulating factor with classic MVAC and showed that dd-MVAC plus granulocyte colony-stimulating factor had less neutropenic fever and higher overall RR, including a 25% CR, but no advantage in the median OS. Given the shorter duration of this regimen and high CR rate in metastatic disease, this is a reasonable neoadjuvant regimen. In fact, SWOG will be initiating a prospective randomized phase 2 trial comparing neoadjuvant dd-MVAC vs. standard-dose GC in patients with MIBC, while also evaluating a tissue biomarker reported to be predictive of chemotherapy sensitivity. Based on the current limited data and the lack of proven survival benefit from carboplatin-based combinations, patients with MIBC who are ineligible for cisplatin and cannot tolerate split-dose cisplatin are recommended to proceed directly to radical cystectomy without NACT [22].
In managing patients with residual disease after NACT, 25% of MedOncs report offering additional ACT, radiotherapy, or a clinical trial. Residual disease status following NACT is a strong negative prognostic factor [6,23–25]. Although the 5-year survival rate was more than 85% in patients with pT0 status in the phase III INT0080 trial, it was less than 40% in patients with residual muscle-invasive disease (>pT2) at the time of radical cystectomy in both arms [6]. In a retrospective analysis of 37 patients with positive pelvic LN after NACT, those receiving a variety of “adjuvant” regimens had improved recurrence-free and disease-specific survival [26]. Despite the poor prognosis in patients with residual disease following NACT and radical cystectomy, there is no high-level evidence supporting additional ACT. A clinical trial is highly desirable and needed in this setting.
This survey also highlights the lack of standardized practice for staging and follow-up. The NCCN guideline recommends imaging of the chest, abdomen, and pelvis as initial staging and for follow-up of patients with MIBC. Although almost all MedOncs image the abdomen and pelvis, a lower percentage are routinely imaging the chest with a CT. MIBC is a systemic disease with high metastatic potential, and it is logical that a CT chest should be the test of choice to rule out pulmonary metastasis before initiating chemotherapy or proceeding with definitive therapy. The disparity in practice likely reflects the lack of a prospective study confirming the utility of CT chest in staging, despite NCCN recommendations. Lastly, fluorodeoxyglucose–PET/ CT is a good adjunct to standard imaging with a sensitivity of 87% and a specificity of 88% reported in the detection of metastases in patients with bladder cancer. However, it should not be used as the only staging modality without a high-resolution CT or magnetic resonance imaging [27].
The intention of this study was to perform an exploratory analysis of the patterns of practice of MedOncs treating patients with MIBC using a survey methodology. Surveys are excellent methods of collecting a broad range of data from a large number of respondents. However, limitations include recall biases and inaccuracies in answers. Respondents often skip questions; consequently different denominators for each question may cause confusion in reported results. Lastly, this survey targeted MedOncs interested in genitourinary oncology and for whom there may be an inherent treatment bias.
5. Conclusion
Although review of older bladder cancer databases suggests underutilization of perioperative chemotherapy, most academic and community MedOncs do offer perioperative chemotherapy to eligible patients with MIBC, with a trend in favor of cisplatin-based NACT over ACT, recommendations that follow best evidence. The most common reasons for not recommending NACT were PS > 3 and renal insufficiency (GFR < 50 ml/min). Age was not considered a determining factor by most respondents. These data suggest that MedOncs utilization of perioperative chemotherapy for MIBC may be increasing owing to the adoption of evidence-based recommendations. However, multimodality treatment of MIBC also requires active participation of urologists, and a parallel survey of urologists’ referral patterns for NACT in patients with MIBC is clearly warranted.
Acknowledgment
This project was initiated at the Bladder Cancer Advocacy Network Think Tank Meeting. We would like to acknowledge the input of the members of the Standardization of care Working Group for their support.
1Funding source: The US Office of Management and Budget(0925–0046).
References
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- [2].Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005;66:4–34. [DOI] [PubMed] [Google Scholar]
- [3].Skinner DG, Lieskovsky G. Contemporary cystectomy with pelvic node dissection compared to preoperative radiation therapy plus cystectomy in management of invasive bladder cancer. J Urol 1984;131:1069–72. [DOI] [PubMed] [Google Scholar]
- [4].Herr HW, Dotan Z, Donat SM, Bajorin DF. Defining optimal therapy for muscle invasive bladder cancer. J Urol 2007;177:437–43. [DOI] [PubMed] [Google Scholar]
- [5].International Collaboration of T, Medical Research Council Advanced Bladder Cancer Working P, European Organisation for R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011; 29:2171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. [DOI] [PubMed] [Google Scholar]
- [7].Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005;48:202–5:[discussion 205–206]. [DOI] [PubMed] [Google Scholar]
- [8].David KA, Milowsky MI, Ritchey J, et al. Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: a report from the National Cancer Data Base. J Urol 2007;178:451–4. [DOI] [PubMed] [Google Scholar]
- [9].Schrag D, Mitra N, Xu F, et al. Cystectomy for muscle-invasive bladder cancer: patterns and outcomes of care in the Medicare population. Urology 2005;65:1118–25. [DOI] [PubMed] [Google Scholar]
- [10].Porter MP, Kerrigan MC, Donato BMK, Ramsey SD. Patterns of use of systemic chemotherapy for Medicare beneficiaries with urothelial bladder cancer. Urol Oncol 2011;29:252–8. [DOI] [PubMed] [Google Scholar]
- [11].Feifer A, Taylor JM, Shouery M, Steinberg GD, Stadler WM, Schoenberg M, et al. Multi-institutional quality-of-care initiative for nonmetastatic, muscle-invasive, transitional cell carcinoma of the bladder: phase I. J Clin Oncol 2011;29 [suppl 7, abstr 240]. [Google Scholar]
- [12].Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol 2011;185:72–8. [DOI] [PubMed] [Google Scholar]
- [13].Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 2006;107:506–13. [DOI] [PubMed] [Google Scholar]
- [14].Hussain SA, Stocken DD, Riley P, et al. A phase I/II study of gemcitabine and fractionated cisplatin in an outpatient setting using a 21-day schedule in patients with advanced and metastatic bladder cancer. Br J Cancer 2004;91:844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bellmunt J, Ribas A, Eres N, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer 1997;80:1966–72. [DOI] [PubMed] [Google Scholar]
- [16].Petrioli R, Frediani B, Manganelli A, et al. Comparison between a cisplatin-containing regimen and a carboplatin-containing regimen for recurrent or metastatic bladder cancer patients. A randomized phase II study. Cancer 1996;77:344–51. [DOI] [PubMed] [Google Scholar]
- [17].Dogliotti L, Carteni G, Siena S, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur Urol 2007;52:134–1. [DOI] [PubMed] [Google Scholar]
- [18].Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol 2012;23:406–10. [DOI] [PubMed] [Google Scholar]
- [19].deVere White RW, Lara PN Jr., Goldman B, et al. A sequential treatment approach to myoinvasive urothelial cancer: a phase II Southwest Oncology Group trial (S0219). J Urol 2009;181:2476–80: [discussion 2480–2471]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 2001;19: 2638–46. [DOI] [PubMed] [Google Scholar]
- [21].Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4. [DOI] [PubMed] [Google Scholar]
- [22].Apolo AB, Grossman HB, Bajorin D, et al. Practical use of perioperative chemotherapy for muscle-invasive bladder cancer: summary of session at the Society of Urologic Oncology annual meeting. Urol Oncol 2012;30:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Splinter TA, Scher HI, Denis L, et al. The prognostic value of the pathological response to combination chemotherapy before cystectomy in patients with invasive bladder cancer. European Organization for Research on Treatment of Cancer—Genitourinary Group. J Urol 1992;147:606–8. [DOI] [PubMed] [Google Scholar]
- [24].Schultz PK, Herr HW, Zhang ZF, et al. Neoadjuvant chemotherapy for invasive bladder cancer: prognostic factors for survival of patients treated with M-VAC with 5-year follow-up. J Clin Oncol 1994;12:1394–401. [DOI] [PubMed] [Google Scholar]
- [25].Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol 2001;19:4005–13. [DOI] [PubMed] [Google Scholar]
- [26].Kassouf W, Agarwal PK, Grossman HB, et al. Outcome of patients with bladder cancer with pN+ disease after preoperative chemotherapy and radical cystectomy. Urology 2009;73:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Apolo AB, Riches J, Schoder H, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol 2010;28:3973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


