Abstract
Immunizing pregnant women is a promising strategy to reduce infectious disease-related morbidity and mortality in pregnant women and their infants. Important pre-requisites for the successful introduction of new vaccines for immunization in pregnancy include political commitment and adequate financial resources: trained, committed and sufficient numbers of healthcare workers to deliver the vaccines; close integration of immunization programs with antenatal care and Maternal and Child Health services; adequate access to antenatal care by pregnant women in the country (especially in low and middle-income countries (LMIC)); and a high proportion of births occurring in health facilities (to ensure maternal and neonatal follow-up can be done). The framework needed to advance a vaccine program from product licensure to successful country-level implementation includes establishing and organizing evidence for anticipated vaccine program impact, developing supportive policies, and translating policies into local action. International and national coordination efforts, proactive planning from conception to implementation of the programs (including country-level policy making, planning, and implementation, regulatory guidance, pharmacovigilance) and country-specific and cultural factors must be taken into account during the vaccines introduction.
Keywords: Vaccines, Immunisation, Pregnancy, Maternal Immunization, Introduction, Program, Regulatory, Safety, Vaccination coverage, Health policies, Antenatal care, Global policies, Country-level policy making, Pharmacovigilance, Healthcare providers, Antenatal care, Vaccine hesitancy
1. Introduction
Immunization in pregnancy is a promising strategy to reduce infectious disease-related morbidity and mortality in pregnant women and their infants [1,2]. Pregnant women and their infants are at high risk of adverse pregnancy outcomes from infections e.g. influenza in the last trimester can lead to severe maternal disease and rubella and zika infections in the mother can lead to congenital anomalies, such as congenital microcephaly. Approximately 1.8 million children die within the first month of life [3] with many deaths due to infections with the potential to be prevented through existing vaccines or vaccines under development for delivery to pregnant women. Immunization in pregnancy protects the mother, the fetus and the newborn through the transplacental delivery of high concentrations of protective IgG (Immunoglobulin G) antibodies, particularly before active immunization of the infant can be initiated. There is also an indirect protective effect of the immunization by preventing infection in the mother, blocking subsequent transmission of infection to the infants (cocooning) [4].
Vaccines against tetanus, pertussis and seasonal influenza have been recommended for routine immunization in pregnant women in high-income countries (HIC) and in some low and middle-income countries (LMIC) for many years and have been determined to be safe and effective at preventing infections [1,2,5]. In spite of this, their uptake has been variable and for some of them e.g. influenza vaccine, well below desired levels. Despite a recommendation by WHO, influenza immunization for pregnant women has not been incorporated into immunization programs in many LMIC. Concerns about immunization of pregnant women include safety of the vaccine for the mother and fetus, vaccine effectiveness in disease prevention, limited data on the overall disease burden in pregnant women and their infants, and the lack of concern for disease by the health care provider and the mother. New vaccines for administration to pregnant women are currently under development such as respiratory syncytial virus (RSV), group B streptococcus (GBS), cytomegalovirus (CMV) [2,6,7] and monovalent pertussis vaccines [8].
In 2015, the World Health Organization (WHO) Strategic Advisory Group on Immunization (SAGE) reviewed progress toward implementation of maternal influenza immunization in pregnancy globally [9]. SAGE noted that there are still limited data from pregnant women, healthcare providers, and immunization program managers to inform implementation, communications, and advocacy, particularly in low resource settings.
2. Implementation of immunization in pregnancy
While awaiting the licensure of new maternal vaccines, generalizable data can and should be collected from existing maternal immunization programs regarding important pre-requisites for their success. These include: political commitment and adequate financial resources; trained, committed and sufficient numbers of healthcare workers (HCW) to deliver the vaccines; close integration of antenatal care and existing maternal and child health and immunization programs, especially in LMIC; adequate access to antenatal care by pregnant women in the country; and a high number of pregnant women delivering in health facilities (so that maternal, neonate and infant follow-up can be adequately done) [10–13].
2.1. Framework for country-level implementation for a new vaccine for pregnant women
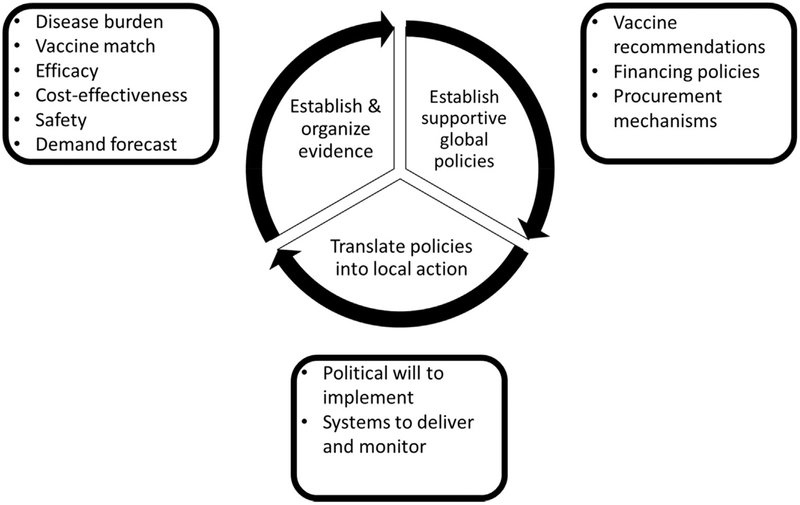
Based on the relatively recent adoption of Haemophilus influenzae type b (Hib), rotavirus and pneumococcal conjugate vaccines for infants in LMIC and on an existing set of WHO guidelines for new vaccine introduction [10], a group of vaccine authorities developed a theoretical framework for the supportive data, policies, and activities needed to advance a vaccine program from product licensure to successful country-level implementation (Fig. 1) [11]. This schematic organizes the major steps in the process of establishing and organizing evidence, developing supportive global policies, and translating policies into local action. International and national coordination efforts, proactive planning from conception to implementation and country-specific, cultural and local factors must be considered during the implementation of the immunization in pregnancy programs [11–13]. We will use this framework developed for implementation of infant immunizations to discuss the specific approach to immunization in pregnancy.
Fig. 1.
Schematic describing the evidence, policies, and actions required to achieve the successful implementation of a new vaccine strategy. Note: Adapted from a previously published work [12].
2.2. Supportive global vaccine policy
The WHO has recommended routine immunization of pregnant women with tetanus, pertussis, and influenza vaccines in certain contexts [14–16]. Other vaccines are recommended in pregnant women with high-risk exposure during outbreaks, including meningococcal and yellow fever vaccines [17,18]. WHO has also produced guidance for new vaccine introduction into routine immunization schedules [10], a global guidance document for maternal influenza immunization implementation, as well as a broader maternal immunization field guide for the Americas [19,13]. WHO antenatal care guidelines also recommend strengthening immunization platforms during pregnancy [20]. WHO’s Global Advisory Committee on Vaccine Safety has extensively reviewed and published on the safety of immunization during pregnancy. They found no evidence of adverse pregnancy outcomes from maternal immunization with inactivated virus or bacterial vaccines, or toxoid [21].
For LMIC eligible for financing by GAVI, financial support is an important prerequisite for new vaccine introduction. GAVI’s financing decisions are determined by their Vaccine Investment Strategy (VIS) recommendations, which are reviewed every 5 years. These recommendations are based on the vaccine’s impact on health, equity and social protection, value for money, economic impact and global health security. WHO works closely with GAVI to determine the new vaccines that will be included in the next VIS cycle and helps to develop the evaluation criteria based on potential cost, vaccine impact and implementation feasibility.
2.3. Country level policy making
The Expanded Programme on Immunization (EPI) managers in countries make a preliminary, decision regarding the introduction of new vaccines or the expanded use of existing vaccines based on the political, financial, technical, programmatic, and feasibility considerations. The individual country National Immunization Technical Advisory Groups (NITAG) must support any decision [22]. Once there is a technical consensus, the proposal can be presented to the political decision-makers [14].
According to the Global Vaccine Action Plan, endorsed by the WHO Member States, every country has committed to having independent NITAGs. They assist their governments in immunization policy formation by providing evidence-based advice [22]. Small countries in close proximity, with similar epidemiologic profiles, and with other commonalities may participate in sub-regional immunization technical advisory groups e.g. Caribbean Immunization Technical Advisory Group (CITAG) (a sub-regional NITAG) for the 22 small countries/territories in the Caribbean [23].
Each NITAG interprets available data to determine the policy recommendations that make the most sense within the national context. Some of the evidence that may be reviewed by the NITAGs for the introduction of vaccines for pregnant women is listed in Table 1 [14,24]. Existence of these data would help to assess whether the introduction of the vaccines for pregnant women into the national immunization program would achieve the desired national public health objectives.
Table 1.
Some evidence needed by the National Immunization Technical Advisory Group (NITAG) for the introduction of Immunization in Pregnancy vaccines.
|
Guidance for the introduction of immunization in pregnancy programs is also available from national public health agencies and medical societies like the American College of Obstetricians and Gynecologists [25].
2.4. Planning and implementation
After the decision to introduce a vaccine, a plan of action for the introduction of the new vaccines for pregnant women should be developed. Some suggested points for consideration are included in Table 2 [13,14].
Table 2.
Factors to consider for the introduction of new vaccines for pregnant women and the programmatic challenges for LMIC.
| Factors to consider for the introduction of new vaccines for pregnant women | Programmatic challenges for LMC |
|---|---|
|
|
| |
| |
Training for stakeholders
|
3. Vaccine offered to pregnant women
The vaccine could be offered to the pregnant women as part of antenatal care (ANC) visits (along with other health interventions routinely offered in the country), outpatient care (as part of general practice, gynecology, or high-risk clinics), vaccination on demand and at other health services that pregnant women might seek for their own care or for the care of their other children. Strengthening existing maternal and neonatal initiatives such as prevention of mother to child transmission of HIV or syphilis and incorporating immunization of pregnant women into these initiatives would be useful. Outreach strategies could include house-to-house vaccination, vaccination campaigns, vaccinating pregnant women at work and educational facilities, and mobile vaccination clinics.
The timing of immunization is important, especially in LMIC where pregnant women often access ANC initially in their second or third trimesters. This also avoids concerns of effects on fetal development or association mistakenly with fetal loss (which is common in the first trimester). Placental transport is most effective from approximately 34 weeks of gestation and higher levels of maternal antibodies are available for transportation to the fetus, resulting in optimal protection of the newborn infant. The first dose of the routine immunization is started early in the neonatal period in LMIC, hence the transplacental antibodies are required to persist for a relatively short duration to protect the infant. For example, for Tetanus Toxoid (TT) vaccines in India, if the immunization status of the pregnant woman is unknown, the recommendation is immunization as soon as the pregnancy is detected followed by a second dose after 4 weeks. If the woman received two doses of TT in her previous pregnancy within 3 years, then one dose of TT is recommended 4 weeks prior to the expected date of delivery.
Vaccination coverage in pregnant women could be used as an indicator for the integration of immunization in pregnancy with ANC services. Latin American countries have successfully implemented these health policies [14,26].
4. Advantages of immunisation in pregnancy programs integration with antenatal care services
Immunization in pregnancy could provide additional benefits beyond the prevention of infectious diseases in the mother and infant. Health systems for antenatal care delivery, in general, could benefit from efforts to strengthen vaccine delivery, with increased attention to evidence-based policy-making, regulation, staffing, training, documentation, cold chain, supply chain, monitoring and evaluation of coverage, adverse event assessment, and quality of care metrics [27]. It would potentially improve coverage, reduce costs, and increase the efficiency of both the antenatal care and immunization programs [14]. Pregnant women and their families can also benefit if vaccine introduction encourages increased antenatal care attendance, counselling, and recommended screening and interventions during pregnancy [28,29]. Systemic factors that are important to improve ANC utilization in LMIC include the quality and satisfaction associated with the service. Provision of immunization might help to improve both these factors and encourage ANC uptake. Further, accompanying family members could also receive catch-up immunization or care during these interactions if the services for them were available at the health centers [30]. This would decrease the time that families must invest in traveling to health facilities and the economic costs to the families and society. However, for these integrated efforts to be successful, health system strengthening is critical. This is currently often not the case in some HIC and LMIC, especially in smaller health care clinics in these countries, as there currently are different days for ANC and childhood immunizations.
5. Challenges for immunisation in pregnancy implementation
While safe and effective vaccines and global policies recommending their use are necessary for the success of a new vaccine program, they are not sufficient. Nowhere is this observation more relevant than with the maternal influenza immunization strategy. Influenza vaccines have an excellent safety profile in pregnant women [31], have been shown to prevent maternal influenza [32], and have been documented to confer protection against influenza in infants [33]. However, although pregnant women are recommended to be prioritized for influenza vaccine receipt by the WHO, only 42 percent of WHO Member States have adopted recommendations for immunization of pregnant women with influenza vaccines, mostly in the American Region [34]. The coverage of influenza immunization of pregnant women in the United States is less than 50 percent despite recommendations for many years from the Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices (ACIP) and the American College of Obstetricians and Gynecologists (ACOG) [25]. Maternal influenza immunization has not been adopted by most LMIC. Reasons for not having a national maternal influenza vaccination policy are multifactorial and differ among countries. Countries with policies tend to be HIC, use more new vaccines, have more robust NITAGs to formulate vaccine policy recommendations, and have stronger health systems to administer vaccines [34].
In the case of maternal influenza immunization, key data gaps and challenges remain. Expert reviews of the strategy have identified lack of robust estimates for severe influenza disease in mothers or their infants as a major obstacle to the strategy [33,35]. Effective mechanisms for the purchase and procurement of influenza vaccines are also critical for successful immunization programs [36].
To implement an immunization in pregnancy program for a new vaccine, programmatic challenges exist in using the antenatal care platform to immunize pregnant women. The capacity of antenatal care to deliver vaccines is LMIC is poorly characterized, however in these settings antenatal care is likely to be in need of strengthening. WHO has recommended that pregnant women should have at least 8 antenatal visits [37] and has highlighted the need to improve the quality and monitoring of antenatal care. Only about 42 percent of women from LMIC have at least four antenatal care visits, and 35 percent still only have one antenatal care visit [38,39]. Robust antenatal care involves the delivery of many evidence-based interventions across several visits [40]. A review of the quality of antenatal care found the coverage of important evidence-based interventions to be suboptimal in many LMIC [41].
Some of the factors which add further to the complexity of the introduction of new vaccines for pregnant women are mentioned in Table 2 [42,43,2,14]. Integrated approaches would need to be supported by the availability of adequate numbers and trained HCW, financial resources, logistics of vaccine acquisition, storage, administration, and tracking, and consistent tools and documents across different programs and services [44]. It is unknown how integrated service delivery can affect the smooth operations of health care delivery. On the one hand, it may decrease the time and the economic costs to the families and society, but on the other hand, it may possibly increase HCW’s daily burden of work and may require additional training, resources and support. Some of the barriers that affect HCW provision of vaccines during pregnancy and measures to address the issues are listed in Table 3 [1,14,45–47].
Table 3.
Some barriers that impact Healthcare Workers (HCW) provision of vaccines during pregnancy and measures to address them.
Barriers
|
Measures to address the barriers
|
6. Vaccine hesitancy
Vaccine hesitancy is the refusal of vaccines or a delay in acceptance despite the availability of immunization services. It is often cited as a problem that can be particularly acute during pregnancy, as pregnant women are encouraged to avoid medicines with known or uncertain risks to the fetus. Some of the factors influencing vaccine hesitancy and the measures to address it in pregnant women, their partners, and extended families are mentioned in Table 4 [1,14,43,46–48].
Table 4.
Some factors influencing Vaccine Hesitancy in pregnant women and measures to address barriers.
Factors influencing Vaccine Hesitancy
|
Measures to address barriers
|
Lower uptake of vaccination in pregnancy has been associated with younger age, not being married, lacking healthcare insurance or an obstetric care provider, having a history of pre-term delivery, belonging to a lower socioeconomic status, having a lower educational level, and being a racial or ethnic minority [1,14,43,46–48].
To address this issue, communication, advocacy, and social mobilization are useful to increase awareness among key stake-holders about the importance of immunization of pregnant women, adhering to the vaccination schedule, the safety and effectiveness of the vaccines, avoiding miscommunication and rumors, improving immunization coverage and rapid detection, reporting and addressing of possible adverse events (AE) following immunizations (AEFIs). Research from HIC and LMIC has shown that understanding the benefits of immunization by pregnant women (and especially in LMIC, their partners, and extended families) comes through education by HCW, and high-quality obstetric care with acceptable and affordable immunization services offered through well-staffed clinics, pharmacies, churches, and other settings. Recent studies support the development of interventions to promote vaccine uptake which are evidence-based and highlight vaccine safety during pregnancy to reduce these concerns [2,49].
7. System considerations
7.1. International regulatory guidance
National Regulatory Authorities in LMIC often refer to international guidelines from the Food and Drug Administration (FDA) and European Medicines Agency (EMA). Available guidance from the FDA and EMA and International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) highlight a lack of harmonization and safety monitoring expectations for immunization in pregnancy [2,50]. These vaccines present significant regulatory challenges because their safety and efficacy must be determined both in the mother and infant. In the ICH and FDA guidelines, general guidance is available and specific requirements are now emerging, with the inclusion of available data in the vaccine label on immunization of pregnant women [2,51].
In the United States, no vaccine currently has an approved label for an indication for use during pregnancy. This is due to a lack of evidence from controlled clinical trials in pregnant women at the time of initial licensure of the vaccine, on the safety of the vaccine in the vaccinated woman and infant and the effectiveness of the vaccine to protect the infant. This is needed to include an indication for use in pregnant women in the vaccine label. Pregnant women were excluded from most pre-licensure trials for licensed vaccines in HIC. Public health authorities have recommended the same vaccine for immunization in pregnancy based on reproductive toxicity studies in the preclinical phase, post-licensure surveillance, observational studies, data from small numbers of pregnant women inadvertently vaccinated in vaccination campaigns and clinical trials, and the vaccine’s perceived benefit and minimal risk for the mother and infant [2,51,52]. Currently, vaccines recommended by the ACIP (e.g. Tetanus, Diphtheria and Pertussis (Tdap), and inactivated influenza) can be used in pregnant women, as they are approved for use in adults and not contraindicated for use during pregnancy [2,51–53].
Differences between the public health recommendations regarding the vaccine use in pregnant women and the absence of the indication on the vaccine label can lead to confusion in HCW and pregnant women and result in lower compliance with national vaccination recommendations [54]. The language in the vaccine inserts may be misinterpreted to suggest safety concerns [2,51,53]. To address the potential for misinterpretation of -pregnancy-related language in vaccine inserts, the FDA issued the Pregnancy and Lactation Labeling Rule (PLLR) to improve understanding of vaccine risks [2,50,52,55]. The PLLR eliminates the earlier letter categories (A, B, C, D, X) meant to signify risk, which were difficult to practically implement [2,54].
The revised PLLR regulation requires narrative descriptions of clinically relevant information on the risks of using the vaccine in pregnant and lactating women to be included in the label. The data can come from clinical trials, epidemiologic studies, pregnancy registries, and case series reporting rare events. As new information becomes available, the vaccine label needs to be updated. This may help towards informing HCW’s prescriptions for immunization, counselling pregnant women and communicating information on the benefits and risks of the vaccine use in pregnancy and lactation and in males and females with reproductive potential [2,52,55,56].
In 2015, the Vaccines and Related Biological Products Advisory Committee of the FDA determined that the regulatory approval process for vaccines for use in pregnant women to prevent disease in the infant will be guided by regulations in Title 21 of the Code of Federal Regulations (21 CFR) and standards mentioned in applicable guidance documents i.e. the ICH and FDA Guidance documents. The vaccine development program and licensure will be product-specific to support the particular indication. Key considerations could include the use of immunological endpoints as markers of infant protection, evaluation of immune interference of transplacentally derived antibody with childhood vaccines and the duration of immunity in both the vaccinated woman and infant. Observational studies could be used for the study of licensed vaccines already recommended for use during pregnancy [2,54].
Using standardized case definitions for maternal and neonatal outcomes will enable the pooling of data from clinical and observational studies for the vaccine labels [2]. Having clarity regarding vaccine labeling related to pregnancy will help reduce variability in the labels and ensure that health care providers and pregnant women have a higher level of confidence in vaccines to be administered in pregnancy. This will hopefully increase the uptake of immunization in pregnancy [2,52,53]. Well-defined international guidance could provide a roadmap for regulation of vaccines used during pregnancy in LMIC.
For new RSV and GBS vaccines under development, advance agreement on the design and parameters of clinical trials that could support licensure for immunization in pregnancy by regulatory authorities in countries will be useful. Important work done by WHO to define the technological roadmap and preferred product characteristics for the RSV and GBS vaccines has been accomplished [57,58]. Another area of importance will be to agree on plans regarding post-licensure (either full or conditional licensure) follow-up to gather further safety and efficacy information from the field if investigational candidate vaccines are deployed in LMIC that currently do not have systems for such surveillance in place.
7.2. Pharmacovigilance of immunisation in pregnancy
Monitoring the safety of vaccines in the woman and infant should be an important part of any immunization in pregnancy program. HIC have developed vaccine safety monitoring systems that have been used to monitor the safety of vaccines for pregnant women [59–63]. Unlike HIC, LMIC face challenges in their efforts to monitor the safety of immunization in pregnancy. Some limitations encountered by LMIC include lack of background population data on important pregnancy or infant outcomes necessary for immunization in pregnancy safety surveillance [2,64]. Prematurity, small for gestational age, and pregnancy complications are not uncommon in pregnancies, even in the absence of vaccination. The baseline rates of adverse pregnancy outcomes in a population should be enumerated to understand expected baselines of AE to inform vaccine safety assessments and clinically important effect sizes, if there were safety signals that must be captured by safety monitoring systems [2,65].
Adverse events following immunization (AEFI) surveillance should be established to identify AE if they were to occur. This is to ensure that the risk–benefit balance remains positive in pregnant women and their newborns [66]. Vaccine pharmacovigilance includes three stages: signal detection, development of a causality hypothesis, and testing of the hypothesis [67].
7.2.1. Spontaneous reporting systems
Signal detection in the post-marketing setting mainly relies on spontaneous reports of AEFI and literature case reports (passive pharmacovigilance) [67].
Many LMIC have spontaneous reporting systems with different degrees of pharmacovigilance [68]. There are national spontaneous reporting databases that collaborate with the WHO Programme for International Drug Monitoring. The Global Individual Case Safety Reports Database System (VigiBase) is a WHO global database which contains over 16 million individual case safety reports with member countries of the WHO Programme for International Drug Monitoring submitting reports since 1968. VigiBase is linked to medical and drug classifications such as WHO ART (Adverse Reactions Terminology), MedDRA (Medical Dictionary for Regulatory Activities), WHO ICD (International Classification of Diseases), and WHODrug to enable structured data entry, retrieval and analysis [69]. Analyses of spontaneous reports may provide a signal for higher than expected AE rates or potentially previously unrecognized AE. However, there are many limitations to the evaluation of passive reports including extremely low reporting of AEFIs by healthcare providers [2,66], selective reporting of AE, lack of denominator data, incomplete data on AE, lack of evidence supporting the diagnosis submitted in many reports and the lack of control groups [70].
AEFI causal relationships can usually not be determined based on individual reports [71]. Determining if there is a causal relationship between vaccination in early pregnancy and birth defects would require a statistically significant difference in the group of pregnant women receiving the vaccines compared to a control group. This is often limited by an inadequate sample size/power to detect differences. A false positive finding, where an association between the vaccine and outcome is incorrectly identified can potentially affect public trust in vaccination and/or the rates of vaccine coverage [2,72,73].
7.2.2. Active surveillance
Signals coming from spontaneous reports warrant additional epidemiological and clinical investigations and further confirmation in a controlled study may be required [73]. Pharmacoepidemiological studies using large electronic health record databases make it possible to study the association of rare AE and provide timely answers to meet the short deadlines of the pharmacovigilance decision-making process. This not only simplifies the logistics and reduces the costs of pharmacoepidemiological research, but can also increase it validity [74]. However, these electronic databases contain observational data which has not been collected for research purposes. Vaccine safety studies that use these databases are limited by the quality of the information. As a result, biased results due to potential misclassification of the exposure (vaccination) and/or outcome (AE) are possible [75]. The combined use of multiple healthcare databases for the conduct of post-marketing active surveillance studies for vaccine safety is being increasingly done especially in HIC to increase the statistical sample size and heterogeneity of exposure. There are issues associated with this approach, including the different underlying healthcare systems, type of information collected, lack of harmonized case definitions, medical event coding systems, language and programs selected for data management and analyses. These large databases also need to respect country-specific data anonymization and privacy regulations. Specific software (e.g. Jerboa) deal with privacy issues by using a common data model and sharing only aggregated and anonymized data. Providing remote research environments for storage and safe access to the data from different databases is necessary [76].
Several HIC have developed active surveillance systems for AEFI for analyses of the association between a vaccine and one or more pre-specified adverse health outcomes e.g. CDC’s Vaccine Safety Datalink (VSD), the US FDA’s Post-Licensure Rapid Immunization Safety Monitoring (PRISM) and IMPACT, Canada’s Immunization Monitoring program. Rapid Cycle Analysis (RCA) (frequent (e.g., weekly) analysis) of the VSD allows more timely analysis of pre-specified AE of special interest so the general public can be informed rapidly of possible risks of recently licensed vaccines or new immunization schedules. TreeScan (https://www.treescan.org/), a free analytical software for large datasets, can be used to identify previously unspecified outcomes by simultaneously evaluating thousands of groups of AE and potential AE, to determine if there is a higher probability of any adverse event occurring among people exposed to a particular vaccine [77].
Some LMIC have surveillance and survey systems in place that have been established to collect population health data including maternal health data e.g. health and demographic surveillance systems (HDSS). However, such databases might contain aggregated data making analysis impossible at the individual level. Households are visited regularly and this could serve as a platform to collect data on the safety of immunization in pregnancy. Pregnancy registries may be set up and used to collect immunization in pregnancy safety data, but their use in LMIC has been limited. Health information systems (HIS) can collect data on subjects who attend health facilities; however, they can lack standardized definitions and be biased. One potential approach for safety monitoring of maternal vaccines in LMIC is the use of a network of hospitals or health centers within a country or in several countries using case-only analytic methods to circumvent the difficulty of obtaining denominator data to calculate rates of AE. An example of this is monitoring conducted by the Pediatric Investigators Collaborative Network on Infections (PICNIC) in Canada for RSV-associated deaths in pediatric patients [78]. A similar approach could be used to study the safety of vaccines for pregnant women as was done to assess the safety of the pandemic H1N1 influenza vaccine in pregnant women in Taiwan [79]. Landscape analysis could be used to identify sentinel surveillance sites in countries to study the safety of vaccines administered to pregnant women.
The Latin American Center for Perinatology (CLAP) was established in 1970 with the aim of strengthening health care services especially primary care for mothers and neonates. In recent years, CLAP has compiled data from 29 countries in Latin America using an electronic, standardized perinatology clinical record. This tool has helped with the analyses of health outcomes of interest for Maternal and Neonatal Immunisation. CLAP is also establishing a surveillance network of sentinel hospital sites across the Region. These sentinel centers will actively look for and investigate pregnancy and infant outcomes following immunization in pregnancy [80].
A limitation, not unique to LMIC but also present in some HIC, is the lack of harmonized methods for vaccine safety studies, lack of standard definitions for important pregnancy and infant outcomes such as fetal loss, stillbirth, birth defects, and maternal morbidity and causality assessment for maternal or fetal AEFI in pregnancy [2,64,81]. This is being addressed through the Global Alignment of Immunization safety Assessment in pregnancy (GAIA) project, which was set up in 2014 in response to the WHO call for a globally concerted approach to actively monitor the safety of vaccines and immunization in pregnancy programs, with a specific focus on LMIC needs and requirements [2,64]. The project has developed a core set of 25 globally standardized case definitions of selected key obstetric and neonatal outcomes to determine the level of diagnostic certainty in the assessment of an event to ensure comparability and harmonization of data collected in different resource settings [81,82]. In addition, guidelines and tools for assessment of the safety of vaccines used in clinical trials in pregnant women have been developed. The guidelines have been supported by WHO Global Advisory Committee on Vaccine Safety [2,64,82,83]. The GAIA outputs have been developed based on a standard global consensus process with investigators, academia, vaccine manufacturers and public health institutes. They are being increasingly utilized in the field of immunization in pregnancy and maternal and child health [2,64].
The surveillance of vaccine safety in pregnant women in LMIC is especially challenging. The small differences in risk that would be expected need big sample sizes to perform studies with adequate statistical power. To date, most published studies on vaccine safety in pregnancy have been from HIC [68]. This may be explained in part by the lack of appropriate databases in LMIC. The strengthening, adaptation, and use of the expanding information sources are the next step to generate complementary information for the continuous benefit-risk assessment process.
7.3. Ethical considerations for immunization in pregnancy programs
The principle of fair distribution of research benefits requires that research addresses diverse health needs across different classes/groups of people and not disproportionately focus on the health needs of a limited class of people. In the past, groups considered vulnerable have had additional protections to participate in research, often including investigations designed to exclude them (e.g. women of reproductive age, pregnant women, children etc.) [84–86]. Due to the exclusions, information about the prevention, diagnosis, and treatment of diseases that affect such groups is limited. This has resulted in injustice to pregnant women from a justice, equity, medical need, ethical and public health perspective [2,84–87]. Pregnant women were denied autonomy and a right to decide on research participation for themselves.83Not vaccinating pregnant women deprives them of the protection they deserve against infectious diseases [84–87]. The International Ethical Guidelines for Health-related Research Involving Humans by the Council for International Organizations of Medical Sciences (CIOMS), in collaboration with WHO, note that pregnant women are not vulnerable simply because they are pregnant [84]. There is a need to redress these injustices by encouraging the participation of previously excluded groups, including pregnant women, in medical research and offering immunization to pregnant women if the vaccines have been licensed or recommended for use in pregnancy in those countries [2,84–87].
Based on the principle of autonomy, in no case must the permission of another person replace the requirement of individual informed consent by the pregnant woman to receive the vaccine. In case the pregnant women so desires, the information about the vaccines can be shared with her spouse and extended family [85,86]. Providing easily understandable, culturally sensitive, comprehensive information in the locally spoken language on the benefits and risks of immunization in pregnancy is important, so that pregnant women can make their own decision to get immunized [2,84–87].
Active dialogue between pregnant women and HCW in the community is necessary to understand pregnant women’s beliefs about the necessity and safety of immunization in pregnancy. Taking the perspectives of women seriously contributes to the ethical justification and trustworthiness of the program. Women should also be adequately represented in decision-making bodies that influence national- and international-level policy decisions about immunization in pregnancy programs (including the NITAG, community advisory groups, policymakers, scientific bodies’ etc.) [2,84–87].
8. Advancing of immunisation of pregnant women
Robust disease burden and cost-effectiveness estimates for RSV and GBS are being collected and will serve to inform expectations of vaccine program impact [6,7]. These vaccines are also being developed with WHO programmatic suitability criteria in mind, so clinical development will consider issues of product storage volume, cold chain, injection devices, and waste disposal that are of particular concern in LMIC [88]. GBS and RSV vaccines have several advantages over influenza vaccines, as they will not require annual reformulation; they will likely have longer shelf lives, and will not require frequent stock rotations [6,7]. However, the kinetics of the antibody responses to these vaccines will need to be characterized [6,7]. Maternal antibodies generally wane over a period of less than 6 months. If there are higher titers of maternal antibody present in the neonate after birth, they will persist for a longer time in the infant. Immunizing pregnant women during the late second or third trimester (after 20 weeks and, preferably at 27–36 weeks of gestation) is recommended [89,90].
There is reason to be optimistic about the implementation of immunization in pregnancy for a number of maternal and neonatal pathogens. Using GBS as an example, it will be far easier to administer a vaccine than to culture clinical specimens from every pregnant woman for GBS carriage and then treat each colonized women with antibiotics to prevent a single case of early-onset GBS disease [91]. Routinely collecting clinical specimens for culture from pregnant women for GBS is not done in LMIC. Even with the development of highly specific and sensitive rapid bedside GBS diagnostic tests, their use would likely not be possible in LMIC due to logistic and financial considerations. In addition, the culturing and treatment approach has no effect on the prevention of late-onset GBS meningitis in the infant, as shown by the existing data from the United States [92].
9. Conclusion
An investment in an immunization in pregnancy strategy would provide public health benefit by preventing infectious diseases in pregnant women and their infants and strengthening country antenatal care and health care delivery systems. To implement an immunization in pregnancy program for a new vaccine, programmatic challenges exist in using the antenatal care platform to deliver vaccines, especially in LMIC. These are mentioned in Table 2. Based on the lessons learned from current immunization in pregnancy programs, if the introduction of new vaccines for pregnant women is systemically done this may considerably decrease the morbidity and mortality associated with vaccine-preventable infectious diseases in pregnant women and their infants.
Acknowledgements
We thank the CDC reviewers for their careful and detailed review and valuable feedback.
Footnotes
Conflict of interest
There is no COI to report and no funding was received to prepare the manuscript.
Publisher's Disclaimer: Disclaimer
The findings, opinions, conclusions, and assertions contained in this consensus document are those of the individual members. They do not necessarily represent the official positions of any participant’s organization (e.g., government, university, or corporations) and should not be construed to represent any Agency determination or policy.
References
- [1].Marshall H, McMillan M, Andrews RM, Macartney K, Edwards K. Vaccines in pregnancy: The dual benefit for pregnant women and infants. Hum Vaccin Immunother 2016;12(4):848–56. 10.1080/21645515.2015.1127485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kochhar S, Bonhoeffer J, Jones CE, Muñoz FM, Honrado A, Bauwens J, et al. Imunization in pregnancy clinical research in low- and middle-income countries - Study design, regulatory and safety considerations. Vaccine 2017;35(48 Pt A):6575–81. 10.1016/j.vaccine.2017.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392(10159):1736–88. 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Swamy GK, Heine RP. Vaccinations for pregnant women. Obstet Gynecol 2015;125(1):212–26. 10.1097/AOG.0000000000000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Skoff TH, Blain AE, Watt J, Scherzinger K, McMahon M, Zansky SM, et al. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants <2 months of age: a case-control evaluation. Clin Infect Dis 2017;65(12):1977–83. 10.1093/cid/cix724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Heath PT, Culley FJ, Jones CE, Kampmann B, Le Doare K, Nunes MC, et al. Group B streptococcus and respiratory syncytial virus immunisation during pregnancy: a landscape analysis. Lancet Infect Dis 2017;17(7):e223–34. 10.1016/S1473-3099(17)30232-3. [DOI] [PubMed] [Google Scholar]
- [7].Marchant A, Sadarangani M, Garand M, Dauby N, Verhasselt V, Pereira L, et al. Maternal immunisation: collaborating with mother nature. Lancet Infect Dis 2017;17(7):e197–208. 10.1016/S1473-3099(17)30229-3. [DOI] [PubMed] [Google Scholar]
- [8].New acellular pertussis vaccine may solve waning immunogenicity problem. Accessed on 20 April 2018 at https://www.mdedge.com/pediatricnews/article/144892/vaccines/new-acellular-pertussis-vaccine-may-solve-waning.
- [9].Maternal Immunization Research and Implementation Portfolio. Accessed on 20 April 2018 at http://www.who.int/immunization/research/development/Portfolio_maternal_immunization_activities.pdf.
- [10].World Health Organization. Vaccine introduction guidelines. Adding a vaccine to the national immunizaiton programme Decision and implementation. In: Organization WH, editor. Geneva, Switzerland: World Health Organization; 2005. Accessed on 20 April 2018 at http://www.who.int/immunization/hpv/plan/vaccine_introduction_guidelines_who_2005.pdf. [Google Scholar]
- [11].Levine OS, Hajjeh R, Wecker J, Cherian T, O’Brien KL, Knoll MD, et al. A policy framework for accelerating adoption of new vaccines. Hum Vacc 2010;6:1021–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kochhar S, Rath B, Seeber LD, Rundblad G, Khamesipour A, Ali M. Vienna Vaccine Safety Initiative. Introducing new vaccines in developing countries. Expert Rev Vacc 2013;12(12):1465–78. 10.1586/14760584.2013.855612. [DOI] [PubMed] [Google Scholar]
- [13].Pan American Health Organization. Maternal and Neonatal Immunization Field Guide for Latin America and the Caribbean In: Pan American Health Organization, editor. Washington, DC: Pan American Health Organization; 2017. Accessed on 4 April 2018 at http://iris.paho.org/xmlui/handle/123456789/34150. [Google Scholar]
- [14].Tetanus vaccines: WHO position paper - February 2017. Wkly Epidemiol Rec. 2017;92(6):53–76. [PubMed] [Google Scholar]
- [15].Pertussis vaccines: WHO position paper - September 2015. Wkly Epidemiol Rec. 2015; 90(35):433–58. [PubMed] [Google Scholar]
- [16].World Health Organization. Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec 2012;87(47):461–76. [PubMed] [Google Scholar]
- [17].World Health O. WHO position paper, Meningococcal A conjugate vaccine: Updated guidance, February 2015. Vaccine 2018;36(24):3421–2. pii: S0264-410X(17)30971-4. 10.1016/j.vaccine.2017.07.063. [DOI] [PubMed] [Google Scholar]
- [18].Vaccines and vaccination against yellow fever. WHO position paper – June 2013. Wkly Epidemiol Rec 2013;88:269–83. [PubMed] [Google Scholar]
- [19].World Health Organization. How to implement influenza vaccination of pregnant women: An introduction manual for national immunization programme managers and policy makers. In: Organization WH, editor. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- [20].World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. In: Organization WH, editor. Geneva, Switzerland: World Health Organization; 2016. Accessed on 20 April 2018 at http://apps.who.int/iris/bitstream/handle/10665/250796/9789241549912-eng.pdf;jsessionid=AFEAE63F78D2FF5897F9B66D4B05EB7A?sequence=1 [PubMed] [Google Scholar]
- [21].Safety of Immunization during Pregnancy- A review of the evidence. Global Advisory Committee on Vaccine Safety. World Health Organization 2014. Accessed on 22 January, 2019 at https://www.who.int/vaccine_safety/publications/safety_pregnancy_nov2014.pdf?ua=1. [Google Scholar]
- [22].World Health Organization. Global vaccine action plan 2011–2020. 2013. Accessed on 28 June on file:///C:/Users/S.K/Downloads/9789241504980_eng.pdf.
- [23].World Health Organization. Global Vaccine Action Plan Secretariat Annual Report 2017. In: World Health Organization, editor. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- [24].Duclos P. National Immunization Technical Advisory Groups (NITAGs): guidance for their establishment and strengthening. Vaccine 2010;28(Suppl1):A18–25. [DOI] [PubMed] [Google Scholar]
- [25].American College of Obstetricians and Gynecologists. Maternal Influenza Review Program- A Tool Kit for State and Local Health Departments. The Ameican College of Obstretricians and Gynecologists; 2016. Accessed on 7 June 2018 at http://immunizationforwomen.org/uploads/MIRP_%20toolkit_Final.pdf. [Google Scholar]
- [26].Vizzotti C, Neyro S, Katz N, Juárez MV, Perez Carrega ME, Aquino A, et al. Maternal immunization in Argentina:A storyline from the prospective of a middle income country. Vaccine 2015;33(47):6413–9. 10.1016/j.vaccine.2015.07.109. [DOI] [PubMed] [Google Scholar]
- [27].Ortiz JR, Neuzil KM. Influenza immunization of pregnant women in resource-constrained countries: an update for funding and implementation decisions. Curr Opin Infect Dis 2017;30:455–62. 10.1097/QCO.0000000000000392. [DOI] [PubMed] [Google Scholar]
- [28].Hodgins S, D’Agostino A. The quality-coverage gap in antenatal care: toward better measurement of effective coverage. Global Health, Sci Pract 2014;2:173–81. 10.9745/GHSP-D-13-00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hodgins S, Tielsch J, Rankin K, Robinson A, Kearns A, Caglia J. A new look at care in pregnancy: simple, effective interventions for neglected populations. PLoS One 2016;11:. 10.1371/journal.pone.0160562e0160562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].World Health Organization. Methodology for the assessment of missed opportunities for vaccination. In: World Health Organization, editor. Geneva, Switzerland: World Health Organization; 2017. Accessed on 28 June 2018 at http://apps.who.int/iris/bitstream/handle/10665/259201/9789241512954-eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- [31].Keller-Stanislawski B, Englund JA, Kang G, Mangtani P, Neuzil K, Nohynek H, et al. Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 2014;32:7057–64. 10.1016/j.vaccine.2014.09.052. [DOI] [PubMed] [Google Scholar]
- [32].Sakala IG, Honda-Okubo Y, Fung J, Petrovsky N. Influenza immunization during pregnancy: Benefits for mother and infant. Hum Vaccin Immunother 2016;12(12):3065–71. 10.1080/21645515.2016.1215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fell DB, Azziz-Baumgartner E, Baker MG, Batra M, Beaute J, Beutels P, et al. Influenza epidemiology and immunization during pregnancy: Final report of a World Health Organization working group. Vaccine. 2017;35:5738–50. 10.1016/j.vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ortiz JR, Perut M, Dumolard L, Wijesinghe PR, Jorgensen P, Ropero AM, et al. A global review of national influenza immunization policies: Analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization. Vaccine 2016;34:5400–5. 10.1016/j.vaccine.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sobanjo-Ter Meulen A, Abramson J, Mason E, Rees H, Schwalbe N, Bergquist S, et al. Path to impact: A report from the Bill and Melinda Gates Foundation convening on maternal immunization in resource-limited settings; Berlin - January 29–30, 2015. Vaccine 2015;33:6388–95. 10.1016/j.vaccine.2015.08.047. [DOI] [PubMed] [Google Scholar]
- [36].Lambach P, Hombach J, Ortiz JR. A global perspective of maternal influenza immunization. Vaccine 2015;33:6376–9. 10.1016/j.vaccine.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].WHO recommendations on antenatal care for a positive pregnancy experience Accessed on 28 June 2018 at http://apps.who.int/iris/bitstream/10665/250796/1/9789241549912-eng.pdf?ua=1. [PubMed]
- [38].UNICEF. Only half of women worldwide receive the recommended amount of care during pregnancy. Accessed on 10 April 2017 at: https://data.unicef.org/topic/maternal-health/antenatal-care/#.
- [39].World Health Organization Global Health Observatory (GHO) data: Antenatal Care . World Health Organization Web site. Accessed on 10 April 2018 at http://www.who.int/gho/maternal_health/reproductive_health/antenatal_care_text/en/. [Google Scholar]
- [40].Hodgins S, D’Agostino A. The quality-coverage gap in antenatal care: toward better measurement of effective coverage. Global Health, Sci Pract 2014;2:173–81. 10.9745/GHSP-D-13-00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Accessed on 10 April 2018 at http://apps.who.int/iris/bitstream/10665/250796/1/9789241549912-eng.pdf. [PubMed] [Google Scholar]
- [42].Lambach P, Alvarez AM, Hirve S, Ortiz JR, Hombach J, Verweij M, et al. Considerations of strategies to provide influenza vaccine year round. Vaccine 2015;33:6493–8. 10.1016/j.vaccine.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moniz MH, Beigi RH. Maternal immunization. Clinical experiences, challenges, and opportunities in vaccine acceptance. Hum Vaccin Immunother 2014;10(9):2562–70. 10.416/21645515.2014.970901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maternal immunization against tetanus. Accessed on 28 June on http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/immunization_tetanus.pdf.
- [45].The Partnership for Maternal, Newborn and Child Health, Integrating immunization and other services for women and children. Accessed on 28 June on http://www.who.int/pmnch/knowledge/publications/summaries/ks25/en/.
- [46].MacDougall DM, Halperin SA. Improving rates of maternal immunization: Challenges and opportunities. Hum Vaccin Immunother 2016;12(4):857–65. 10.1080/21645515.2015.1101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wilson RJ, Paterson P, Jarrett C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine. 2015. Nov 25;33(47):6420–9. 10.1016/j.vaccine. [DOI] [PubMed] [Google Scholar]
- [48].Arriola CS, Vasconez N, Thompson M, Mirza S, Moen AC, Bresee J, et al. Factors associated with a successful expansion of influenza vaccination among pregnant womenin Nicaragua. Vaccine 2016;34(8):1086–90. 10.1016/j.vaccine.2015.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chamberlain AT, Seib K, Ault KA, Orenstein WA, Frew PM, Malik F, et al. Factors Associated with Intention to Receive Influenza and Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccines during Pregnancy: A Focus on Vaccine Hesitancy and Perceptions of Disease Severity and Vaccine. Safety. PLoS Curr 2015;7 10.1371/currents.outbreaks.d37b61bceebae5a7a06d40a301cfa819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Labelling Information of Inactivated Influenza Vaccines for Use in Pregnant Women. Geneva: World Health Organization; 2016. Available at: http://www.who.int/entity/biologicals/expert_committee/Label_after_ECBS_HK_28_Oct_2016.clean.pdf. [Google Scholar]
- [51].Roberts JN, Gruber MF. Regulatory considerations in the clinical development of vaccines indicated for use during pregnancy. Vaccine. 2015;33(8):966–72. 10.1016/j.vaccine.2014.12.068. [DOI] [PubMed] [Google Scholar]
- [52].Gruber MF, The US. FDA pregnancy lactation and labeling rule - Implications for maternal immunization. Vaccine 2015;33(47):6499–500. 10.1016/j.vaccine.2015.05.107. [DOI] [PubMed] [Google Scholar]
- [53].Neels P, Southern J, Abramson J, Duclos P, Hombach J, Marti M, et al. Off-label use of vaccines. Vaccine 2017;35(18):2329–37. 10.1016/j.vaccine.2017.02.056. [DOI] [PubMed] [Google Scholar]
- [54].Top KA, Arkell C, Scott H, McNeil SA, Mannerfeldt J, Ortiz JR, et al. Effect of package insert language on health-care providers’ perceptions of influenza vaccination safety during pregnancy. Lancet Glob Health 2016;4(10):e690–1. 10.1016/S2214-109X(16)30182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pregnancy and Lactation Labeling (Drugs) Final Rule, FDA, accessed on 2 June, 2018 at http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm.
- [56].Gruber Marion. Regulatory Issues for Maternal Immunization. NVAC, 2014. Accessed on 20 May, 2018 at https://www.hhs.gov/sites/default/files/nvpo/nvac/meetings/pastmeetings/2014/gruber_maternal_immunization_septnvac2014.pdf. [Google Scholar]
- [57].Vekemans J, Moorthy V, Giersing B, Friede M, Hombach J, Arora N, et al. Respiratory syncytial virus vaccine research and development: World Health Organization technological roadmap and preferred product characteristics pii: S0264-410X(17)31364-6. Vaccine 2018. 10.1016/j.vaccine.2017.09.092. [DOI] [PubMed] [Google Scholar]
- [58].Vekemans J, Moorthy V, Friede M, Alderson MR, Sobanjo-Ter Meulen A, Baker CJ, et al. Maternal immunization against Group B streptococcus: World Health Organization research and development technological roadmap and preferred product characteristics. Vaccine. 2018. 10.1016/j.vaccine.2017.09.087. pii: S0264-410X(17)31359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine 2013. Jun 12;31(27):2898–903. 10.1016/j.vaccine.2013.03.069. [DOI] [PubMed] [Google Scholar]
- [60].Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System e1–9. Am J Obstet Gynecol 2011;205(5):473 10.1016/j.ajog.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Baxter R, Bartlett J, Fireman B, Lewis E, Klein NP. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics 2017;139(5). 10.1542/peds.2016-4091. [DOI] [PubMed] [Google Scholar]
- [62].Moro PL, McNeil MM, Sukumaran L, Broder KR. The Centers for Disease Control and Prevention’s public health response to monitoring Tdap safety in pregnant women in the United States. Hum Vaccin Immunother 2015;11(12):2872–9. 10.1080/21645515.2015.1072664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women – Advisory Committee on Immunization Practices (ACIP). 2012 MMWR 2013;62(07):131–5. [PMC free article] [PubMed] [Google Scholar]
- [64].Kochhar S, Bauwens J, Bonhoeffer J. Safety assessment of immunization in pregnancy. Vaccine 2017;35(48 Pt A):6469–71. 10.1016/j.vaccine.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Orenstein LA, Orenstein EW, Teguete I, Kodio M, Tapia M, Sow SO, et al. Background rates of adverse pregnancy outcomes for assessing the safety of maternal vaccinetrials in sub-Saharan Africa. PLoS ONE 2012;7(10):. 10.1371/journal.pone.0046638e46638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bill and Melinda Gates Foundation & Global Alliance to Prevent Prematurity and Stillbirth. Maternal Immunization Safety Monitoring in Low- and Middle-Income Countries: A Roadmap for Program Development. Building an approach that is practical, affordable, and sustainable (2016). Accessed on 20 June 2018 at http://apps.who.int/medicinedocs/en/m/abstract/Js23275en/.
- [67].Chandler RE. Safety concerns with HPV vaccines continue to linger: are current vaccine pharmacovigilance practices sufficient? Drug Saf. 2017;40(12):1167–70. 10.1007/s40264-017-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cassidy C, MacDonald NE, Steenbeek A, Ortiz JR, Zuber PL, Top KA. A global survey of adverse event following immunization surveillance systems for pregnant women and their infants. Hum Vaccin Immunother 2016;12(8):2010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].The Uppsala Monitoring Centre. Accessed on 21 June 2018 at https://www.who-umc.org/vigibase/vigibase/.
- [70].Halsey NA, Proveaux T. Value of an in-depth analysis of unpublished data on the safety of influenza vaccines in pregnant women. Vaccine 2017;35(45):6154–9. 10.1016/j.vaccine.2017.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Centers for Disease Control and Prevention (CDC).Update on overall prevalence of major birth defects–Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008;57(1):1–5. [PubMed] [Google Scholar]
- [72].Sato APS, Ferreira VLR, Tauil MC, Rodrigues LC, Barros MB, Martineli E, et al. Use of electronic immunization registry in the surveillance of adverse events following immunization. Rev Saude Publica 2018;52:4 10.11606/S1518-8787.2018052000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015;33(36):4398–405. 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].De Abajo FJ. Improving pharmacovigilance beyond spontaneous reporting. Int J Pharm Med 2005;19(4):209–18. [Google Scholar]
- [75].Xu S, Newcomer S, Nelson J, Qian L, McClure D, Pan Y, et al. Signal detection of adverse events with imperfect confirmation rates in vaccine safety studies using self-controlled case series design. Biom J. 2014;56(3):513–25. 10.1002/bimj.201300012. [DOI] [PubMed] [Google Scholar]
- [76].Trifirò G, Coloma PM, Rijnbeek PR, Romio S, Mosseveld B, Weibel D, et al. Combining multiple healthcare databases for postmarketing drug and vaccine safety surveillance: why and how? J Intern Med 2014;275(6):551–61. 10.1111/joim.12159. [DOI] [PubMed] [Google Scholar]
- [77].Kochhar S, Excler JL, Bok K, Gurwith M, McNeil M, Seligman S, et al. Defining the interval for monitoring potential adverse events following immunisation (AEFIs) after receipt of live viral vectored vaccines. Accepted for publication. Vaccine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tam J, Papenburg J, Fanella S, Asner S, Barton M, Bergeron C, et al. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) Study of Respiratory Syncytial Virus-Associated Deaths in Pediatric Patients in Canada: 2003 to 2013. Clin Infect Dis 2018. 10.1093/cid/ciy413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Huang WT, Tang FW, Yang SE, Chih YC, Chuang JH. Safety of inactivated monovalent pandemic (H1N1) 2009 vaccination during pregnancy: a population-based study in Taiwan. Vaccine 2014;32(48):6463–8. 10.1016/j.vaccine.2014.09.054. [DOI] [PubMed] [Google Scholar]
- [80].Ropero Alvarez AM, Jauregui B, El Omeiri N. Progress towards a comprehensive approach to maternal and neonatal immunization in the Americas. Rev Panam Salud Publica 2017;41 10.26633/RPSP.2017.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Fulton TR, Narayanan D, Bonhoeffer J, Ortiz JR, Lambach P, Omer SB. A systematic review of adverse events following immunization during pregnancy and the newborn period. Vaccine 2015;33(47):6453–65. 10.1016/j.vaccine.2015.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Harmonising Immunisation Safety Assessment in Pregnancy, Vaccine. Accessed on 20 July 2018 at https://www.sciencedirect.com/journal/vaccine/vol/34/issue/49.
- [83].Harmonising Immunisation Safety Assessment in Pregnancy - Part II. Vaccine Accessed on 20 July 2018 at https://www.sciencedirect.com/journal/vaccine/vol/35/issue/48/part/PA.
- [84].Gomes MF, de la Fuente-Núñez V, Saxena A, Kuesel AC. Protected to death: systematic exclusion of pregnant women from Ebola virus disease trials. Reprod Health. 2017;14(Suppl 3):172 10.1186/s12978-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].International Ethical Guidelines for Health-related Research Involving Humans, CIOMS, Geneva: 2016. Accessed on 20 July, 2018 at https://cioms.ch/wp-content/uploads/2017/01/WEB-CIOMS-EthicalGuidelines.pdf. [DOI] [PubMed] [Google Scholar]
- [86].Chamberlain AT, Lavery JV, White A, Omer SB. Ethics of maternal vaccination. Science 2017;358(6362):452–3. 10.1126/science.aao4219. [DOI] [PubMed] [Google Scholar]
- [87].Verweij M, Lambach P, Ortiz JR, Reis A. Maternal immunisation: ethical issues. Lancet Infect Dis 2016;16(12):e310–4. 10.1016/S1473-3099(16)30349-8. Epub 2016 Sep 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].World Health Organization. Assessing the programmatic suitability of vaccines candidates for WHO prequalification. In: World Health Organization, editor. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- [89].Abu Raya B, Srugo I, Kessel A, Peterman M, Bader D, Gonen R, et al. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels - a prospective study. Vaccine 2014;32(44):5787–93. 10.1016/j.vaccine.2014.08.038. [DOI] [PubMed] [Google Scholar]
- [90].Abu Raya B, Edwards KM, Scheifele DW, Halperin SA. Pertussis and influenza immunisation during pregnancy: a landscape review. Lancet Infect Dis 2017;17(7):e209–22. 10.1016/S1473-3099(17)30190-1. [DOI] [PubMed] [Google Scholar]
- [91].Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine 2013;31 (Suppl 4):D20–6. 10.1016/j.vaccine.2012.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al. Bacterial meningitis in the United States, 1998–2007. New Engl J Med 2011;364:2016–25. 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]