Abstract
Background.
Vaccines are among the safest medical products in use today. Hundreds of millions of vaccinations are administered in the United States each year. Serious adverse reactions are uncommon. However, temporally associated deaths can occur following vaccination. Our aim was to characterize main causes of death among reports submitted to the US Vaccine Adverse Event Reporting System (VAERS), a spontaneous vaccine safety surveillance system.
Methods.
We searched VAERS for US reports of death after any vaccination from 1 July 1997 through 31 December 2013. Available medical records, autopsy reports, and death certificates were reviewed to identify cause of death.
Results.
VAERS received 2149 death reports, most (n = 1469 [68.4%]) in children. Median age was 0.5 years (range, 0–100 years); males accounted for 1226 (57%) reports. The total annual number of death reports generally decreased during the latter part of the study period. Most common causes of death among 1244 child reports with available death certificates/autopsy reports included sudden infant death syndrome (n = 544 [44%]), asphyxia (n = 74 [6.0%]), septicemia (n = 61 [4.9%]), and pneumonia (n = 57 [4.6%]). Among 526 adult reports, most common causes of death included diseases of the circulatory (n = 247 [46.9%]) and respiratory systems (n = 77 [14.6%]), certain infections and parasitic diseases (n = 62 [11.8%]), and malignant neoplasms (n = 20 [3.8%]). For child death reports, 79.4% received >1 vaccine on the same day. Inactivated influenza vaccine given alone was most commonly associated with death reports in adults (51.4%).
Conclusions.
No concerning pattern was noted among death reports submitted to VAERS during 1997–2013. The main causes of death were consistent with the most common causes of death in the US population.
Keywords: death, vaccines, epidemiology, surveillance, vaccine safety
When a death occurs shortly following vaccination, it is important to assess whether it was related to vaccination. In 2009–2010, a close temporal association between receipt of the pandemic influenza A(H1N1) vaccine ( pH1N1) and 107 deaths (among 15 million doses of vaccine distributed in Japan) resulted in concern about a possible causal relationship, despite a lack of compelling epidemiologic or clinical evidence [1, 2]. Deaths following vaccination have had a negative impact on vaccination programs [3, 4], particularly in low- and middle-income countries implementing large-scale infant vaccination programs [5], even when investigations do not find evidence of a causal relationship.
In a review of reports of death following vaccination submitted to the Vaccine Adverse Event Reporting System (VAERS) from the early 1990s, the Institute of Medicine concluded that most were coincidental, not causally associated [6]. A separate review of 1266 death reports to VAERS from 1990 to 1997 found that almost half were attributable to sudden infant death syndrome (SIDS), which decreased in frequency following recommendations in the early 1990s to change infant sleep environment (ie, sleep on back or side) [7]. As new vaccines are added to the childhood vaccination schedule and use of existing vaccines expands, such as the universal recommendation in 2010 for influenza vaccination for all persons aged ≥6 months [8, 9], it is important to continue to monitor death reports to VAERS. We reviewed reports of death after vaccination reported to VAERS from 1997 through 2013.
METHODS
Vaccine Adverse Events Reporting System
VAERS is a US national vaccine safety surveillance system, co-administered by the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration, that receives spontaneous reports of adverse events following vaccination [10]. VAERS accepts reports from vaccine manufacturers, healthcare providers, vaccine recipients, and others. The VAERS report form collects information on age, sex, vaccines administered, the AE experienced, and health history. Signs and symptoms of adverse events are coded by trained personnel using the Medical Dictionary for Regulatory Activities (MedDRA), a clinically validated, internationally standardized terminology [11]. Each VAERS report may be assigned 1 or more MedDRA preferred terms. A report is considered serious based on the Code of Federal Regulations definition if 1 or more of the following is reported: death, life-threatening illness, hospitalization or prolongation of existing hospitalization, or permanent disability [12]. For nonmanufacturer serious reports, medical records are routinely requested and made available to VAERS personnel. For death reports, efforts are made to obtain autopsy reports and death certificates that contain information on the cause of death.
We analyzed VAERS death reports received by 1 June 2014 for individuals vaccinated with any vaccine from 1 July 1997 through 31 December 2013. Non-US and duplicate death reports were excluded. Hearsay reports (secondhand reports) with no vaccination date recorded were also excluded.
Clinical Review of Death Reports
CDC physicians reviewed the VAERS reports, available autopsy findings, death certificates, and medical records to assess causes of death. Cause of death was classified into major International Classification of Diseases, Tenth Revision (ICD-10) diagnostic categories, which have been described previously [13]. We did not attempt to assess death reports for causal relationships with vaccination, although we did review specific causes of death where causal relationships between vaccination and death have been established or a plausible theoretical risk exists; these included anaphylaxis, intussusception, Guillain–Barré syndrome (GBS), yellow fever vaccine–associated viscerotropic disease, smallpox complications leading to death, and syncope after vaccination leading to head trauma and subsequent death [14].
We calculated descriptive statistics for sex, age groups, onset interval (time from vaccination to death), year of vaccination, cause of death, and vaccines administered. Calculations were performed using SAS software, version 9.2 (SAS Institute, Cary, North Carolina). Because VAERS is a routine surveillance program that does not meet the definition of research, it is not subject to institutional review board review and informed consent requirements.
RESULTS
We identified 2149 death reports in VAERS (Table 1). Most reports involved children aged 0–17 years and males. Autopsy reports and/or death certificates were available for 1770 (82.4%) reports. The median onset interval, the period from vaccination to death, was 3 days (range, 0–2442 days) for all ages, 2 days (range, 0–1478 days) for infants (<1 year of age), 5 days (range, 0–2442 days) for children 1–17 years, and 3 days (range, 0–2011 days) for adults (≥18 years). Among the 1469 reports in children aged 0–17 years, 1166 (79.4%) received >1 vaccine on the day of vaccination; among infants (n = 1165), 1004 (86.2%) received >1 vaccine. Among the 666 reports for adults aged ≥18 years, 92 (13.8%) received >1 vaccine on the day of vaccination.
Table 1.
Death Reports in the Vaccine Adverse Event Reporting System Among Persons Vaccinated 1 July 1997–31 December 2013
| Characteristic | No. (%) |
|---|---|
| Total reports | 2149 |
| Child reports (0–17 y) | 1469 (68.4) |
| Adult reports (≥18 y) | 666 (30.9) |
| Unknown age | 14 (0.7) |
| Age, mo, median (range) | 6 (0–1204) |
| Age group, ya | |
| <1 | 1165 (54.2) |
| 1–4 | 197 (9.2) |
| 5–9 | 30 (1.4) |
| 10–17 | 77 (3.6) |
| 18–45 | 139 (6.5) |
| 46–64 | 152 (7.1) |
| ≥65 | 375 (17.5) |
| Male sexb | 1226 (57.0) |
| Onset, d, median (range), all reportsc | 3 (0–2442) |
| Onset, d, median (range), infants (<1 y) | 2 (0–1478) |
| Type of reporterd (n = 2090) | |
| Vaccine provider | 982 (47.1) |
| Other | 672 (32.2) |
| Manufacturer | 288 (13.8) |
| Parent/patient | 144 (6.9) |
| With autopsy report and/or death certificate | 1770 (82.4) |
Age unknown for 14 reports.
Sex unknown for 21 reports.
Onset unknown for 170 reports.
Type of reporter unknown for 63 reports.
The number of death reports in children exceeded those in adults in all years, and in both groups the number of reports has decreased in recent years (Figure 1).
Figure 1.
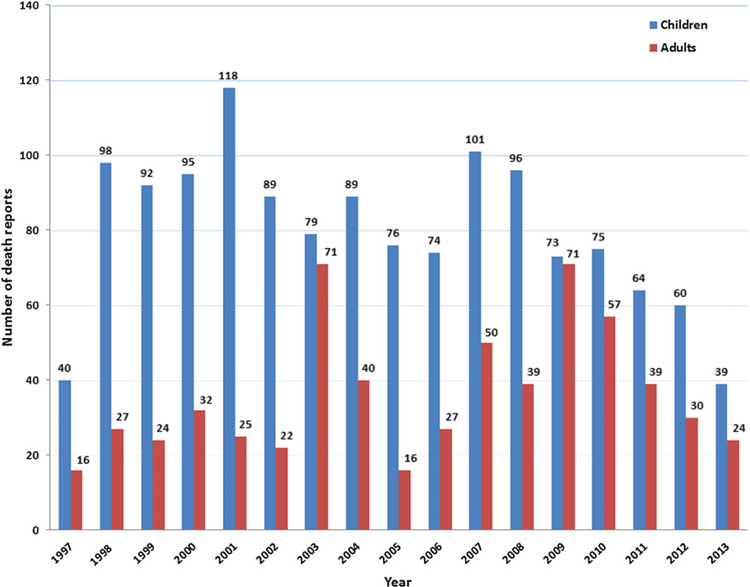
Trends in death reports in children (0–17 years old) and adults (≥18 years old) in the Vaccine Adverse Event Reporting System, 1 July 1997–31 December 2013.
Reports of Children
Causes of Death
Among reports of death in children with autopsy findings and/or death certificates available for review, the most common causes of death by ICD-10 major group (Table 2) included SIDS and diseases of the respiratory system, with pneumonia as the most common cause of death in the respiratory category. “Injury, poisoning and certain other consequences of external causes” were noted in 96 reports, with asphyxiation being the most common cause of death in this category. Septicemia or sepsis was the fourth most common cause of death. In 146 of 1244 (11.7%) reports, the autopsy report or death certificate stated the cause of death was undetermined. SIDS reports progressively decreased in frequency from a peak in 1998 (n = 50) to a nadir in 2011 (n = 21). Most SIDS cases were among infants 2–4 months of age (n = 398 [66%]) and mostly among males (n = 375 [62.2%]) (Table 3). SIDS reports were most common among children who had received DTaP-HepB-IPV (diphtheria and tetanus toxoids and acellular pertussis adsorbed, hepatitis B, and inactivated poliovirus vaccine) plus Haemophilus influenzae type b (Hib) plus 7- or 13-valent pneumococcal conjugate vaccine (PCV7 or PCV13) (11.6%) followed by HepB vaccine given alone (9%).
Table 2.
Most Common Causes of Death Among Reports in Persons Aged 0–17 Years (n = 1244) in the Vaccine Adverse Event Reporting System, 1 July 1997–31 December 2013
| ICD-10 Major Group | No.(%) |
|---|---|
| Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified | 703 (56.5) |
| Sudden infant death syndrome | 544 |
| Undetermined | 146 |
| Diseases of the respiratory system | 98 (7.9) |
| Pneumonia | 57 |
| Injury, poisoning, and certain other consequences of external causes | 96 (7.7) |
| Asphyxiation | 74 |
| Certain infections and parasitic diseases | 80 (6.4) |
| Septicemia, sepsis | 61 |
| Diseases of the nervous system | 73 (5.9) |
| Meningitis | 18 |
| Seizures | 18 |
| Diseases of the circulatory system | 69 (5.5) |
| Other forms of heart disease | 54 |
| Congenital malformations, deformations, chromosomal abnormalities | 39 (3.1) |
| Diseases of the digestive system | 25 (2.0) |
| External causes of morbidity | 16 (1.3) |
| Assault | 11 |
| Other causes of death | 45 (3.6) |
Abbreviation: ICD-10, International Classification of Diseases, Tenth Revision.
Table 3.
Characteristics of Sudden Infant Death Syndrome Reports in the Vaccine Adverse Event Reporting System, 1997–2013
| Characteristic | No. (%) |
|---|---|
| SIDS reports | 603 (28.1) |
| Male sexa | 375 (62.2) |
| Onset, d, median (range) | 3 (0–121) |
| Age, mo, median (range) Type of reporterb (n = 581) |
2 (0–37) |
| Provider | 340 (58.5) |
| Other | 171 (29.4) |
| Parent/patient | 37 (6.4) |
| Manufacturer | 33 (5.7) |
| Age group, mo (n = 603) | |
| <12 | 582 (96.5) |
| 12–48 | 20 (3.3) |
| Unknown | 1 (0.2) |
| No. of SIDS reports with autopsy reports and/or death certificate | 544 (90.2) |
| Top 4 vaccines given individually or simultaneously | |
| DTaP-HepB-IPV + Hib + PCV7 or PCV13 | 70 (11.6) |
| HepB | 54 (8.9) |
| DTaP + Hib-HepB + IPV + PCV7 or PCV13 | 52 (8.6) |
| DTaP + Hep B + Hib + IPV | 47 (7.8) |
| DTaP + Hib + IPV + PCV7 or PCV13 | 42 (6.9) |
Total death reports = 2149.
Abbreviations: DTaP, diphtheria, tetanus, and acellular pertussis vaccine; DTaP-IPV-Hib, combination diphtheria, tetanus, and acellular pertussis, inactivated poliovirus, and Haemophilus influenzae type b conjugate vaccine; HepB, hepatitis B vaccine; Hib, Haemophilus influenzae type b conjugate vaccine; Hib-HepB, combination Haemophilus influenzae type b conjugate and hepatitis B vaccine; IPV, inactivated poliovirus vaccine; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; SIDS, sudden infant death syndrome.
Sex unknown in 2 reports.
Type of reporter unknown for 22 reports.
Vaccines Administered
The most common vaccines and vaccine combinations associated with child death reports for all years combined are listed in Table 4. For child death reports, 79.4% received >1 vaccine on the same day. The most common vaccines in children were DTaP-HepB-IPV + Hib + PCV7 or PCV13 (n = 127 [8.7%]) followed by HepB vaccine given alone (n = 115 [7.8%]).
Table 4.
Five Most Common Vaccines and Vaccine Combinations (Simultaneous Administration) for Child and Adult Death Reports in the Vaccine Adverse Event Reporting System, 1 July 1997–31 December 2013
| Type of Report | No. (%) |
|---|---|
| Child reports (n = 1469) | |
| DTaP-HepB-IPV + Hib + PCV7 or PCV13 | 127 (8.7) |
| HepB | 115 (7.8) |
| DTaP + Hib-HepB + IPV + PCV7 or PCV13 | 102 (7.0) |
| DTaP-HepB-IPV + Hib + PCV7 or PCV13 + RV5 | 84 (5.7) |
| DTaP + Hep B + Hib + IPV | 77 (5.2) |
| Adult reports (n = 666) | |
| IIV3 | 342 (51.4) |
| Herpes zoster | 41 (6.2) |
| PPSV23 | 39 (5.9) |
| Influenza A(H1N1) (pandemic) inactivated | 37 (5.6) |
| IIV3 + PPSV23 | 15 (2.3) |
Abbreviations: DTaP, diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed; DTaP-HepB-IPV, diphtheria and tetanus toxoids and acellular pertussis adsorbed, hepatitis B, and inactivated poliovirus vaccine; HepB, hepatitis B vaccine; Hib, Haemophilus influenzae type b conjugate vaccine; Hib-HepB, Haemophilus influenzae type b conjugate and hepatitis B vaccine; IIV3, trivalent inactivated influenza vaccine; IPV, inactivated poliovirus vaccine; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine; RV5, rotavirus vaccine ( pentavalent).
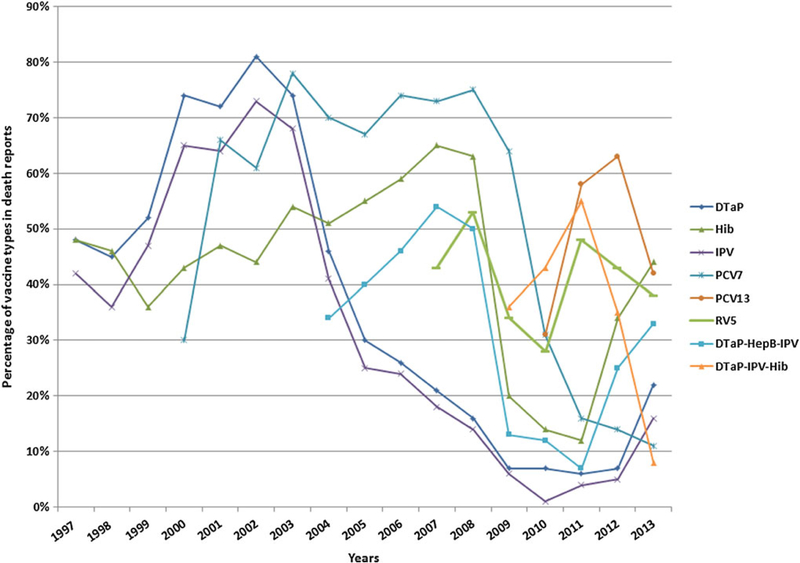
Among children aged 0–17 years, DTaP vaccine was most common among death reports from 1998 through 2002 (Figure 2). From 2003 through 2009, PCV7 became the predominant vaccine seen in death reports, and in 2011 and 2012, PCV13 was the predominant vaccine. PCV7 was licensed and recommended for use in 2000, and the first reports of death following PCV7 vaccination were reported that year (n = 20). The rotavirus pentavalent vaccine (RV5) was licensed for use in 2006, and the first reports of death following RV5 occurred that year.
Figure 2.
Trends in death reports by vaccine type in children aged 0–17 years, Vaccine Adverse Event Reporting System, 1 July 1997–31 December 2013. Only the most common vaccines associated with death reports are shown. Vaccines shown may be given alone or with other vaccines and may be single or combined antigen vaccines, so percentages of death reports in any given year may exceed 100%. Abbreviations: DTaP, diphtheria, tetanus, and acellular pertussis vaccine; DTaP-HepB-IPV, combination diphtheria, tetanus, and acellular pertussis, hepatitis B, and inactivated poliovirus vaccine; DTaP-IPV-Hib, combination diphtheria, tetanus, and acellular pertussis, inactivated poliovirus and Haemophilus influenzae type b conjugate vaccine; HepB, hepatitis B vaccine; Hib, Haemophilus influenzae type b conjugate vaccine; IPV, inactivated poliovirus vaccine; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; RV5, rotavirus vaccine ( pentavalent).
Reports of Adults
Causes of Death
Among reports of death in adults with autopsy or death certificate findings (Table 5), the most common causes of death included diseases of the circulatory system, diseases of the respiratory system, and certain infections and parasitic diseases; the most common causes of death in each of these categories included ischemic heart disease, pneumonia, and septicemia or sepsis, respectively. In 16 of 526 (3%) reports, the cause of death was undetermined.
Table 5.
Most Common Causes of Death Among Reports in Persons Aged ≥18 Years (n = 526) in the Vaccine Adverse Event Reporting System, 1 July 1997–31 December 2013
| ICD-10 Major Group | No. (%) |
|---|---|
| Diseases of the circulatory systema | 247 (46.9) |
| Ischemic heart disease | 119 |
| Other forms of heart disease | 74 |
| Cerebrovascular diseases | 15 |
| Hypertensive diseases | 16 |
| Diseases of the respiratory system | 77 (14.6) |
| Pneumonia | 34 |
| Chronic lower respiratory diseases | 20 |
| Other diseases of the respiratory system | 13 |
| Certain infections and parasitic diseases | 62 (11.8) |
| Septicemia, sepsis | 48 |
| Diseases of the nervous system | 43 (8.2) |
| Malignant neoplasms | 20 (3.8) |
| Injury, poisoning, and certain other consequences of external causes | 20 (3.8) |
| Other causes of death | 57 (10.8) |
Abbreviation: ICD-10, International Classification of Diseases, Tenth Revision.
Pulmonary heart disease and diseases of pulmonary circulation (n = 12), diseases of arteries, arterioles, and capillaries (n = 7), diseases of veins, lymphatic vessels, and lymph nodes, not elsewhere classified (n = 3).
Vaccines Administered
Among adult reports, the most commonly associated vaccines (Table 4) included IIV3 (n = 342 [51.4%]), herpes zoster (shingles) vaccine (n = 41 [6.2%]), 23-valent pneumococcal polysaccharide vaccine (n = 39 [5.9%]), and 2009 pH1N1 inactivated monovalent vaccine (n = 37 [5.6%]), all of which were given alone. Of reports of death among adults, trivalent inactivated influenza vaccine (IIV3) was the most commonly received vaccine for all years, with the exception of 2009 when the 2009 pH1N1 monovalent inactivated vaccine was the most commonly received vaccine and IIV3 was the second most common.
Prespecified Conditions as a Cause of Death
Anaphylaxis was identified as the cause of death in 6 reports; 5 after IIV3 vaccine. The onset interval for all 5 reports was <24 hours. In one report, the patient received IIV3 and ceftriaxone concomitantly. Intussusception was the stated cause of death in 6 reports; all involved administration of several vaccines simultaneously; in 5 reports patients received a rotavirus vaccine. The median onset interval was 5 days (range, 4–16 days) for these 5 reports. Two reports had an onset interval >6 days.
GBS was reported as the cause of death or a contributor to the death, or listed as a diagnosis, in 23 reports; vaccines administered included only IIV3 in 20 reports and three reports where IIV3 was given in combination with pneumococcal vaccine, 2009 pH1N1 inactivated monovalent vaccine, or HepB/varicella zoster vaccine. The median onset interval for these 23 reports was 12 days (range, 2–44 days). In 19 of 23 reports, the GBS was verified as cause of death by review of medical records. There was one report of syncope after vaccination leading to head trauma and resulting in death; details about this case have been reported previously [15]. Two reports involved deaths resulting from possible complications of smallpox vaccine. One report involved a 26-year-old male active-duty soldier who died suddenly after smallpox and IIV3 vaccination. The cause of death was eosinophilic myocarditis (hypersensitivity myocarditis) compatible with postvaccinial myocarditis. A second death report involved a 18-year-old male active-duty soldier who received anthrax, smallpox, and typhoid fever vaccines and died 2 weeks later. The cause of death was “complications from smallpox vaccination.” Autopsy findings included myocarditis with dilated cardiopathy and pulmonary edema. Yellow fever vaccine viscerotropic disease was the stated cause of death in one report involving a 22-year-old woman who received yellow fever vaccine 7 days before death.
DISCUSSION
This comprehensive review of death reports to VAERS for the period 1 July 1997 through 31 December 2013 indicates that the most common causes of death in VAERS were consistent with the leading causes of death in the US population (Table 6) [13]. The 2149 deaths described in this study were reported to VAERS during a period of time when approximately 2 billion doses of vaccine were distributed for use in the United States. This translates to roughly 1 reported death per 1 million doses of vaccine distributed. Because the majority of death reports were in children, the most common causes of death were in this age group. SIDS was the leading cause of death (28.1%) among all reports and accounted for 51.7% of death reports in infants, which is consistent with infant mortality data that place SIDS as the third leading cause of death in the United States among infants, after congenital malformations, deformations, and chromosomal abnormalities; and disorders related to short gestation and low birthweight [13, 16]. The male predominance of death reports in our study is driven by SIDS reports in which males accounted for 62%. This is consistent with studies that found males to be at higher risk of SIDS [17]. SIDS occurs rarely during the first month of life and peaks between 2–3 months of age [17]. Because SIDS peaks at a time when children are receiving many recommended vaccinations, it would not be unexpected to observe a coincidental close temporal relationship between vaccination and SIDS [18]. SIDS deaths in the United States have been declining since the early 1990s for a variety of factors that include recommended changes in sleeping position and environment, clarification of the case definition, and diagnostic coding shifts [19–22]. This downward trend in SIDS reports has also been observed in SIDS reports submitted to VAERS since the early 1990s [7] and has continued during the years of this review from 1997 through 2013. There is considerable evidence that vaccination is not causally associated with SIDS [18, 22, 23], including an Institute of Medicine (IOM) review in 2003 that rejected a causal association between the whole cell pertussis–containing vaccine (which is no longer in use in the United States) and SIDS and between exposure to multiple simultaneous vaccines and SIDS [21].
Table 6.
Age-Adjusted Mortality Rates for the 15 Leading Causes of Death in the United States, 2010a
| Cause of Death | Age-Adjusted Death Rate (per 100 000 US Standard Population) |
|---|---|
| All causes | 747.0 |
| 1. Diseases of heart (heart disease) | 179.1 |
| 2. Malignant neoplasms (cancer) | 172.8 |
| 3. Chronic lower respiratory diseases | 42.2 |
| 4. Cerebrovascular diseases (stroke) | 39.1 |
| 5. Accidents (unintentional injuries) | 38.0 |
| 6. Alzheimer’s disease | 25.1 |
| 7. Diabetes mellitus (diabetes) | 20.8 |
| 8. Nephritis, nephrotic syndrome, and nephrosis (kidney disease) | 15.3 |
| 9. Influenza and pneumonia | 15.1 |
| 10. Intentional self-harm (suicide) | 12.1 |
| 11. Septicemia | 10.6 |
| 12. Chronic liver disease and cirrhosis | 9.4 |
| 13. Essential hypertension and hypertensive renal disease (hypertension) | 8.0 |
| 14. Parkinson’s disease | 6.8 |
| 15. Pneumonitis due to solids and liquids | 5.1 |
Source: Murphy et al [16].
Other leading causes of death among VAERS reports included diseases of the circulatory system and diseases of the respiratory system. Diseases of the circulatory system, the most common causes of death among VAERS death reports in adults, are the leading causes of death in the US population. Some other leading causes of death among VAERS reports included pneumonia and septicemia/sepsis, both of which rank among the top 11 leading causes of death in the US population [13].
In different age groups, the most common vaccines temporally associated with deaths tended to be those typically recommended and given at the particular age (Table 4). Thus, for child reports, the most common vaccines were combination vaccines given simultaneously with other vaccines (ie, DTaP-HepB-IPV, Hib, PCV7 or PCV13). An exception was the first dose of HepB vaccine, generally given during the first month of life. HepB vaccine was the second most common vaccine associated with death reports. A previous study investigated neonatal death reports submitted to VAERS after HepB vaccine during 1991 through 1998 and did not find any safety pattern of concern [24], and a population-based study did not find a significant difference in the proportion of HepB-vaccinated (31%) and -unvaccinated (35%) neonates dying of unexpected causes [25].
We noted that death reports appear to follow the Weber effect [26], a tendency for new medical products or products perceived to be new to have higher reporting rates for adverse events initially, which then decline despite steadily increasing prescribing rates. For example, the peak in number of death reports during 2001 appears to coincide with an increase in PCV7 use following its licensure and recommendation for use in 2000. RV5 was licensed and recommended in 2006, and the peak in the number of death reports after RV5 occurred in 2008. DTaP-HepB-IPV was first licensed and recommended in 2002 and the first death reports in VAERS were observed in 2003 with the highest number of reports in 2007, which was followed by a decline in subsequent years.
VAERS strengths include its broad national scope and timeliness, and its use for detecting signals of potential vaccine safety problems that may be further studied in other epidemiologic studies. However, any finding in VAERS needs to be interpreted with caution given the inherent limitations of passive surveillance systems, such as over- or underreporting, biased reporting, and inconsistency in quality and completeness of reports [10]. VAERS generally cannot assess if a vaccine caused an adverse event. VAERS does not collect data on the number of individuals vaccinated; therefore, with no denominator data, it is not possible to calculate rates of adverse events. Likewise, VAERS does not collect data on the total number of vaccinated individuals who died; therefore, it is not possible to calculate death rates following vaccination.
Because a large number of vaccines are given to young children (often simultaneously) at scheduled well-child visits, especially during the first year of life, deaths occurring in close temporal association following vaccination are likely to occur by chance alone. It is important for immunization programs to be aware of background rates of adverse events, including mortality rates in the population, to develop risk communication strategies to help communities understand deaths following vaccination, which can be disruptive to vaccination programs [27]. For example, Hib vaccine has been introduced progressively into some Asian countries’ immunization programs as a component of a combination pentavalent vaccine replacing diphtheria–whole-cell pertussis (DTwP) or DTwP-HepB. During introduction of these vaccines into Sri Lanka, India, and Vietnam in 2008–2010, deaths were reported among a small number of vaccine recipients, prompting authorities to suspend the use of these vaccines [5]. More recently, 4 deaths among elderly individuals who received the IIV3 vaccine in Italy prompted the Italian Medicines Agency to temporarily suspend the use of that vaccine in that country [28]. Investigations into the causes of death in all these examples found that the vaccines were not implicated. Other examples of how deaths following vaccinations can be disruptive to immunization programs and public health have been discussed in the scientific literature [27].
Few epidemiologic studies have investigated the occurrence of deaths following vaccination or assessed mortality rates in vaccinated and unvaccinated populations. A previous review of death reports in VAERS during 1990–1997 [7] did not identify any pattern of concern. The findings in our review are consistent with previous findings, especially related to SIDS reports. A study using electronic health record databases in the Vaccine Safety Datalink (VSD) between 2005 and 2008 estimated the mortality rate among vaccinated individuals and also assessed major causes of death [29]. The age-adjusted death rate within 60 days of vaccination was 442.5 deaths per 100 000 person-years, which is lower than the US death rate during 2008 reported by the National Center for Health Statistics of 758.3 per 100 000 population [29]. The authors attributed the lower death rate in the VSD vaccinated population to a “healthy vaccinee effect,” meaning that people are more likely to receive a vaccine when they are relatively healthy and free of disease. The leading causes of death in the VSD vaccinated population were similar to those reported by the National Center for Health Statistics for the general US population.
In our VAERS review, we did not detect any concerning patterns that would suggest causal relationships between vaccination and deaths. With rare exceptions (eg, anaphylaxis), the evidence from multiple VAERS reviews in combination with findings from IOM reviews and a VSD study using electronic health record databases do not suggest a causal relationship or increased risk of death following vaccination. Continuous monitoring and assessment of death reports in VAERS is warranted to ensure public confidence in the immunization program. Risk assessment and communication strategies should be in place to rapidly respond to reports of deaths following vaccination.
Acknowledgments.
We thank Dr Frank DeStefano for his valuable comments and advice.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the US Food and Drug Administration.
Footnotes
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Nakada H, Narimatsu H, Tsubokura M, et al. Risk of fatal adverse events after H1N1 influenza vaccination. Clin Infect Dis 2010; 50:1548–9. [DOI] [PubMed] [Google Scholar]
- 2.McNeil MM, Broder KR, Vellozzi C, DeStefano F. Risk of fatal adverse events after H1N1 influenza vaccine: limitations of passive surveillance data. Clin Infect Dis 2010; 51:871–2. [DOI] [PubMed] [Google Scholar]
- 3.Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare of Japan. Safety review results of pneumococcal conjugate vaccine and Haemophilus influenzae type b (Hib) vaccine for pediatrics, 2011. Available at: http://www.pmda.go.jp/files/000153722.pdf Accessed 5 June 2015.
- 4.Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare of Japan. Pharmaceuticals and medical devices safety information No. 280, 2011. Available at: https://www.pmda.go.jp/files/000153635.pdf Accessed 5 June 2015.
- 5.Global Advisory Committee on Vaccine Safety, 12–13 June 2013. Wkly Epidemiol Rec 2013; 88:301–12. [PubMed] [Google Scholar]
- 6.Institute of Medicine. Stratton KR, Howe CJ, Johnston RB Jr, eds. Adverse events associated with childhood vaccines: evidence bearing on causality. Washington, DC: National Academy Press, 1994:P274–304. [PubMed] [Google Scholar]
- 7.Silvers LE, Ellenberg SS, Wise RP, et al. The epidemiology of fatalities reported to the Vaccine Adverse Event Reporting System 1990–1997. Pharmacoepidemiol Drug Saf 2001; 10:279–85. [DOI] [PubMed] [Google Scholar]
- 8.Bridges CB, Coyne-Beasley T; Advisory Committee on Immunization Practices (ACIP), ACIP Adult Immunization Work Group, Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older—United States, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:110–2. [PMC free article] [PubMed] [Google Scholar]
- 9.Akinsanya-Beysolow I; Advisory Committee on Immunization Practices (ACIP); ACIP Child/Adolescent Immunization Work Group; Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 0 through 18 years—United States, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:108–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J 2004; 23:287–94. [DOI] [PubMed] [Google Scholar]
- 11.Medical Dictionary for Regulatory Activities. Available at: http://www.meddramsso.com/ Accessed 28 April 2011.
- 12.Food and Drug Administration. 21 CFR Part 600.80. Postmarketing reporting of adverse experiences. Vol 62: Federal Register, 1997:52252–53. [Google Scholar]
- 13.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep 2012; 61:1–51. [PubMed] [Google Scholar]
- 14.Miller E, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; doi: 10.1016/j.vaccine.2015.05.023. [DOI] [PMC free article] [PubMed]
- 15.Woo EJ, Ball R, Braun MM. Fatal syncope-related fall after immunization. Arch Pediatr Adolesc Med 2005; 159:1083. [DOI] [PubMed] [Google Scholar]
- 16.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep 2013; 61:1–116. [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics 2005; 116:1245–55. [DOI] [PubMed] [Google Scholar]
- 18.Brotherton JM, Hull BP, Hayen A, Gidding HF, Burgess MA. Probability of coincident vaccination in the 24 or 48 hours preceding sudden infant death syndrome death in Australia. Pediatrics 2005; 115:e643–6. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Sudden unexpected infant death (SUID), 2011. Available at: http://www.cdc.gov/sids/ Accessed 19 August 2014.
- 20.Jorch G, Tapiainen T, Bonhoeffer J, et al. Unexplained sudden death, including sudden infant death syndrome (SIDS), in the first and second years of life: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2007; 25:5707–16. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine. Immunization safety review: vaccinations and sudden unexpected death in infancy. Washington, DC: The National Academies Press, 2003. [PubMed] [Google Scholar]
- 22.Vennemann MM, Höffgen M, Bajanowski T, Hense HW, Mitchell EA. Do immunisations reduce the risk for SIDS? A meta-analysis. Vaccine 2007; 25:4875–9. [DOI] [PubMed] [Google Scholar]
- 23.Vennemann MM, Butterfass-Bahloul T, Jorch G, et al. GeSID Group. Sudden infant death syndrome: no increased risk after immunisation. Vaccine 2007; 25:336–40. [DOI] [PubMed] [Google Scholar]
- 24.Niu MT, Salive ME, Ellenberg SS. Neonatal deaths after hepatitis B vaccine: the vaccine Adverse Event Reporting System, 1991–1998. Arch Pediatr Adolesc Med 1999; 153:1279–82. [DOI] [PubMed] [Google Scholar]
- 25.Eriksen EM, Perlman JA, Miller A, et al. Lack of association between hepatitis B birth immunization and neonatal death: a population-based study from the vaccine safety datalink project. Pediatr Infect Dis J 2004; 23:656–62. [DOI] [PubMed] [Google Scholar]
- 26.Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 2004; 24: 743–9. [DOI] [PubMed] [Google Scholar]
- 27.Black S, Eskola J, Siegrist CA, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet 2009; 374:2115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Medicines Agency. No evidence that Fluad vaccine caused deaths in Italy. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2014/12/news_detail_002228.jsp&mid=WC0b01ac058004d5c1 Accessed 28 May 2015.
- 29.McCarthy N, Weintraub E, Vellozzi C, et al. Mortality rates and cause-of-death patterns in a vaccinated population. Am J Prev Med 2013; 45: 91–7. [DOI] [PubMed] [Google Scholar]