Abstract
Purpose of review
Muscle-invasive bladder cancer (MIBC) comprises approximately one-third of bladder cancers and is associated with significant morbidity and mortality. Accurate staging of bladder cancer is essential because of significantly different treatment options and the consequences of inaccurate staging. The current recommended method for staging is transurethral resection of the bladder tumor followed by contrast- enhanced computed tomography (CT). In this review, we discuss cross-sectional imaging approaches used to assess local, nodal, and distant metastases in MIBC.
Recent findings
Determining the most accurate imaging method for staging MIBC is a contentious issue. CT with contrast is a practical approach; however, there is potential for understaging of small lymph nodes or foci of metastasis. Multiparametric MRI is emerging as the imaging modality of choice in tumor staging, with a reported accuracy of more than 90%. Locoregional lymph node metastasis can also be accurately evaluated using functional MRI and specific contrast agents with paramagnetic characteristics. PET/CT with conventional radiotracers is a common imaging modality for staging distant metastases.
Summary
Conventional imaging methods for evaluating MIBC are of limited use. However, recent advances in molecular imaging, targeted contrast agents, and functional MRI have shown promising results for the staging of bladder cancer.
Keywords: bladder cancer, diffusion-weighted-MRI, muscle-invasive, PET/computed tomography, ultra-small superparamagnetic iron oxide
INTRODUCTION
Bladder cancer is the most common type of urothelial malignancy, with over 74 000 new cases diagnosed each year and more than 15 000 deaths expected in 2015 [1]. About one-third of cases will present with invasion into the bladder muscle. These are a biologically heterogeneous group of cancers with different molecular and histopathologic subtypes and variable responses to chemotherapy and, thus, variable clinical outcomes [2]. Radical cystectomy with pelvic lymph node dis-section is the standard treatment for muscle-invasive bladder cancer (MIBC). However, radical surgery is often associated with significant morbidity and complications. Recent data suggest comparable survival rates by employing bladder-preserving methods combined with chemotherapy and radiation therapy in a selected group of patients with MIBC [3,4]. This is why accurate staging of bladder cancer is of paramount importance for choosing the best treatment. The current recommendation of the National Comprehensive Cancer Network for initial staging of MIBC is abdominal and pelvic computed tomography (CT) or MRI with noncontrast chest CT [5■■]. Here, we review the various imaging techniques being used to stage known bladder cancer, and discuss the advantages and disadvantages of each (Tables 1–3) [6–19,20■21–25].
Table 1.
Selected publications on imaging for tumor staging in muscle-invasive and metastatic bladder cancer
| Tumor staging | ||||||||
|---|---|---|---|---|---|---|---|---|
| Modality | Author/date [refs] | n | Accuracy % | Overstaging % |
Understaging % |
Sensitivity % | Specificity % | Comments |
| CT | Tritschler (2012) [6] | 276 | 49 | 23.4 | 24.7 | NR | NR | |
| CT | Blick (2012) [7] | 778 | NR | NR | NR | 95 | 83 | |
| CT | Wang (2010) [8] | 44 | 96.4 | NR | NR | 97.7 | 95.9 | |
| FDG PET/CT | Lodde (2010) [9] | 44 | NR | NR | NR | PET/CT 85 CT 77 | PET/CT 25 CT 50 | |
| MRI | Ghafoori (2013) [10] | 86 | NR | 54.5 | 45.5 | 93 | 94 | Figures are for differentiating organ-con-fined from nonorgan-confined tumors |
| MRI | Liedberg (2013) [11] | 53 | NR | 48.9 | 12.7 | NR | NR | |
| MRI (DWI cf T2WI) | Wu (2013) [12] | 362 | 90–95 DWI | NR | NR | 83–88 DWI | 91–96 DWI | Figures are for differentiating Tis and T2 from T3 and T4 tumors |
| 83–92 T2WI 93–99 DWI and T2WI |
72–85 T2WI 87–92 DWI and T2WI |
85–94 T2WI 94–100 DWI and T2WI |
||||||
| MRI | Rajesh (2011) [13] | 100 | 63 | NR | NR | 90.5 | 60 | Figures are for differentiating organ confined (≤T2) from non organ confined (≥T3) tumors |
Table 3.
Selected publications on imaging for staging distant metastases in muscle-invasive and metastatic bladder cancer
| Distant metastases | ||||||||
|---|---|---|---|---|---|---|---|---|
| Modality | Author/date [ref] | n | Accuracy % |
Overstaging % |
Understaging % |
Sensitivity % | Specificity % | Comments |
| FDG PET/CT | Goodfellow (2014) [16] | 233 | PET 86 CT 83 | NR | NR | PET/CT 54 CT 41 | PET/CT 97 CT 98 | |
| FDG PET/CT | Apolo (2010) [25] | 57 | NR | NR | NR | 40.7 | 91.5 | FDG PET/CT detected more malignant disease than CT/ MRI in 40% of patients |
COMPUTED TOMOGRAPHY
CT, which is widely available and inexpensive, is considered the gold standard for staging nodal and distant metastases. Guidelines of the European Association of Urology recommend CT for initial staging and follow-up of patients with MIBC [26]. However, CT has several limitations for tumor staging of bladder cancer, mainly lack of visualization of the bladder wall muscle layers and lack of specificity for early detection of extravesical invasion of the tumor. Findings suggestive of extravesical invasion of the tumor are nonspecific and include perivesical fat stranding and adjacent soft tissue nodularity (Fig. 1) [27]. The other major disadvantage of CT is a lack of functional data and heavy reliance on morphology for staging of nodal and distant meta-stases, which often leads to understaging of cancers with small foci of metastases. A retrospective study of 276 patients undergoing radical cystectomy demonstrated a low rate of overstaging (8.3%) and a high rate of understaging (29.4%) [6]. Even more troublesome was CT’s inability to accurately predict the depth of bladder tumor invasion (accuracy rate: 49%), leading to overstaging in 23.4% and understaging in 24.7% of cases, as confirmed by cystectomy [6]. Despite these limitations, CT is a reliable and cost-effective method for the initial staging and follow-up of patients with MIBC who undergo radical cystectomy [26].
FIGURE 1.
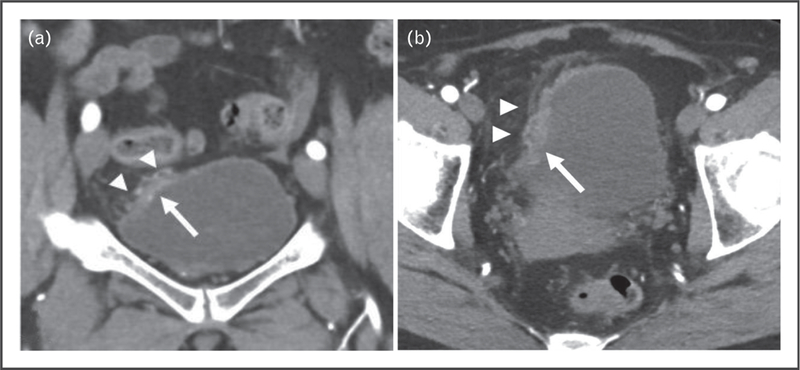
A 63-year-old woman presented with hematuria. (a) A focal area of wall thickening at the dome of the bladder was shown on coronal CT postcontrast image of the pelvis (arrow). (a,b) Areas of fat stranding and nodularity adjacent to focal wall thickening (arrowheads) were highly suspicious for invasion of bladder wall. TURBT disease showed invasion only of the lamina propria. CT, computed tomography; TURBT, transurethral resection of the bladder tumor.
PET/COMPUTED TOMOGRAPHY
By itself, CT provides limited functional information. However, when combined with PET, CT is a powerful tool for detecting local metabolically active foci of cancer that might otherwise be too small to visualize (Fig. 2) [28]. The most common radiotracer in clinical practice is fluorine-18 2 fluoro-2-deoxy-D-glucose (18F-FDG). However, use of this radiotracer to image local bladder cancer is limited because of urinary excretion that leads to a high concentration of radio-tracer within the bladder [28]. This interferes with visualization of the bladder tumor and adjacent locoregional lymph nodes, which often have lower metabolic activity compared with bladder content. Several methods have been proposed for overcoming this limitation, the most practical being the use of a diuretic, voiding before the examination, and delayed imaging. In a study of 25 patients with bladder cancer, forced diuresis with intravenous furosemide was a more sensitive method than contrast-enhanced CT for detecting the primary tumor (96 vs. 92%). In addition, PET/CT was more sensitive than CT for the detection of locoregional lymph node metastasis (78 vs. 44%) [29]. Others have reported similarly promising results. In a study of 11 patients with bladder cancer in intact bladders [30], PET/CT showed 100% sensitivity and specificity for detection of bladder wall cancers, and 41% of patients were upstaged following delayed-phase imaging. Others have proposed using radiotracers such as 11C-meth-ionine and 11C-choline with minimal urinary excretion. When combined with PET/CT, these radiotracers have the advantage of minimal urinary excretion and better definition of bladder wall and lumen.
FIGURE 2.
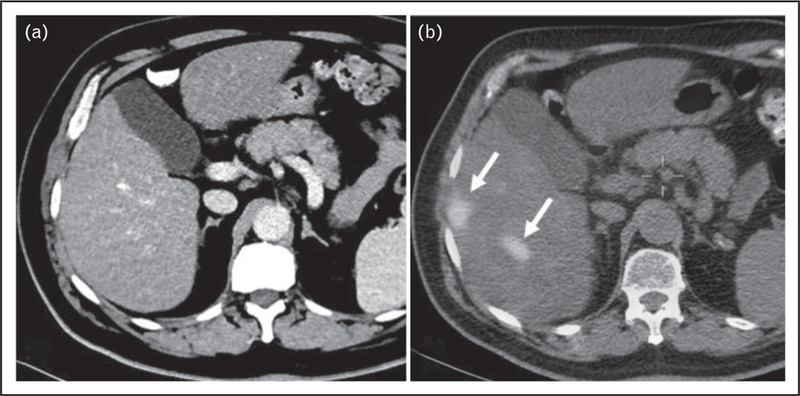
A 65-year-old man with MIBC underwent screening CT examination of chest, abdomen, and pelvis (CAP) with contrast. (a) CT showed no evidence of metastasis to the liver. (b) PET/CT showed foci of increased radiotracer uptake within segments V and VI of the liver, with SUVmax of 9.2 compatible with metastases (arrows). Restaging follow-up CT CAP 8 weeks after this study confirmed metastasis to the liver. CT, computed tomography; MIBC, muscle-invasive bladder cancer.
Choline is an integral part of the cell membrane. It incorporates into the tumor cell wall by final conversion into phosphatidylcholine and becoming a component of the cell membrane. Choline can be tagged with 11C and used as a radiotracer to detect cellular turnover [31]. The disadvantage of using 11C-choline is its short half-life of 20 min, which limits its use to imaging centers with an onsite cyclotron. However, 11C-choline can improve visualization of the primary tumor [32] and aid in preoperative staging [33,18]. In a recent study comparing 11C-choline imaging with histology in 59 patients [20■], 11C-choline detected lymph node metastasis in 62.7% of patients. Interestingly, the study also reported a reasonably high detection rate for primary cancer or local relapse in 54.2% of patients. Overall, 11C-choline yields sensitivity (59%), specificity (90%), positive predictive value (71%), negative predictive value (84%), and accuracy (81%) for nodal staging. In a study of 20 patients with bladder cancer who underwent local and metastatic staging with 11C-choline and 18F-FDG PET/CT, 11C-choline PET/CT showed a high positive predictive value (84.7% in 51 detected lesions) [34]. However, the positive predictive value of 18F-FDG PET/CT was higher (90.7%). In addition, the maximum standardized uptake value of 18F-FDG was significantly higher than that for 11C-choline for bladder and extravesical metastatic lesions. In sum, 11C-choline did not provide additional benefit for staging of MIBC and was inferior to 18F-FDG for the detection of extravesical disease.
Another radiotracer proposed for tumor staging in patients with MIBC is 11C-methionine. Methionine is an amino acid that can be used to detect metabolically active lesions with high protein metabolism and amino acid transport. 11C-methionine can reliably detect bladder cancer in patients with large lesions. However, the major limiting factor for use of this radiotracer is its lack of specificity and increased background uptake, which limits the evaluation of small lymph node metastases [35]. In a study of 11C-methionine PET in patients with biopsy-proven urinary bladder carcinoma, 18 of 23 primary tumors were detected, and higher 11C-methionine uptake was associated with higher tumor grade [36].
The true value of PET/CT, however, is in staging of distant bladder cancer metastasis. A study investigating the value of 18F-FDG PET/CT imaging in the management of 47 patients with advanced bladder cancer reported an overall sensitivity and specificity of 87 and 88%, respectively, in the detection of malignant metastatic lesions [25]. The study demonstrated superior detection power for 18F-FdG PET/CT compared with conventional MRI or CT alone, with more lesions detected in 40% of patients. More importantly, patients’ planned management was altered in 68% of cases based on the 18F-FDG PET/CT results. In a meta-analysis of six studies, including the previous study, the pooled sensitivity and specificity of 18F-FDG PET/CT for staging and restaging of bladder cancer was 82 and 89%, respectively [28].
MRI
MRI is an excellent method for detailed evaluation of pelvic anatomy because of its superior soft-tissue contrast resolution (Fig. 3). In addition, diffusion-weighted MRI (DW-MRI) and dynamic contrast-enhanced MRI (DCE-MRI) can provide functional information.
FIGURE 3.
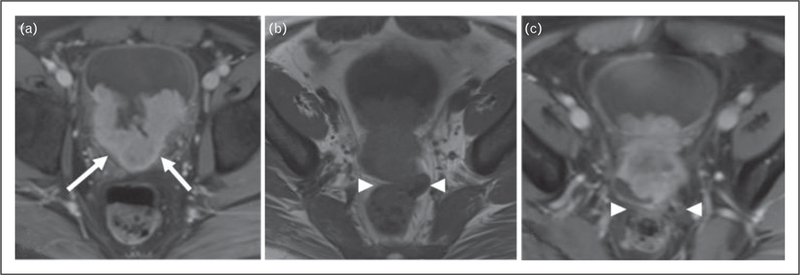
A 37-year-old man presented with a large enhancing mass within the left body of the bladder. (a) Contrastenhanced pelvic MRI showed fat suppression extending into the trigon (arrows). (b) There is obliteration of the fat plane between bladder and rectum on nonfat-saturated T1-weighted images (arrowheads) and (c) Postcontrast T1-weighted images compatible with extravesical extension of the bladder mass and invasion of the rectum.
Morphologic MRI
Key elements of the MRI imaging protocol at the National Cancer Institute include Tl-weighted imaging without fat suppression, isotropic T2-weighted imaging with and without fat saturation, DW-MRI, and DCE-MRI. One of the main challenges for imaging of the bladder is properly distending the bladder at the time of imaging. Compression of surrounding fat in an overdistended bladder can lead to underestimation of extravesical extension of the tumor, whereas an underdistended bladder can obscure small lesions due to bladder wall thickening. Therefore, it is recommended that the patients empty the bladder approximately 2h before the examination [37]. Tl-weighted imaging is a sound method for visualizing perivesical fat as the frontline of extravesical tumor extension, with low signal intensity of the tumor as opposed to the background fat [38■■]. The disadvantage of Tl-weighted imaging without contrast is lack of differentiation between the tumor and the bladder wall because of similar signal characteristics. Therefore, evaluating the depth of bladder wall invasion is severely limited with Tl-weighted imaging. In contrast, T2-weighted imaging can use the inherently high T2 signal intensity of urine to delineate the intraluminal portion of the mass. In addition, interruption of low Tl signal detrusor muscle is indicative of transmurality and extravesical invasion of the tumor [11]. The main disadvantage of anatomical MRI of the bladder is lack of detailed tissue characterization and, thus, scarce data on tumor aggressiveness. There is also a risk of overestimating the extent of the tumor following transurethral resection of the bladder tumor (TURBT) or radiation and chemotherapy [39].
Dynamic contrast-enhanced MRI
DCE-MRI relies on contrast materials with paramagnetic capabilities to evaluate tumor vascularity and permeability. DCE-MRI uses Tl-weighted sequences with fast acquisition capabilities, which yield high-resolution sequential images of the imaging field. DCE curves can be drawn by measuring signal intensity within the desired volume of mass as an indirect method of assessing angiogenesis [40]. The normal bladder wall does not enhance significantly on early postcontrast images; tumors with more vascularity demonstrate higher signal intensity following injection of contrast. In a study of l22 patients with MIBC, DCE-MRI demonstrated a high accuracy rate (74%) in differentiating node-negative organ-confined bladder cancer from non-organ-confined bladder cancer. Yet, despite high accuracy (80.3%) in detecting positive nodes, DCE-MRI shows low sensitivity (40.7%) in determining positive nodal disease [23]. Contrast within the bladder lumen on delayed-phase images helps to delineate the mass’s intraluminal contour.
One of the more promising recent developments in the use of imaging for staging metastatic lymph nodes is the use of ultra-small superparamagnetic iron oxide (USPIO) contrast agents. A USPIO contrast agent, such as ferumoxtran-10, is injected intravenously and ultimately taken up by macrophages. Deposition of macrophages within normal lymph nodes leads to a significant signal drop on Tl-weighted and T2-weighted images. Lack of macrophages and replacement of lymph node cells by metastatic cancer cells mean that metastatic lymph nodes do not take up the USPIO, leading to higher T1 and T2 signals [41]. USPIO has been used to evaluate nodal staging of various pelvic malignancies [42–44]. One study involved 75 patients with prostate or bladder cancer who were initially staged as N0 by conventional cross-sectional imaging [21]. Investigators compared the combined USPIO DW-MRI findings with histopathologic results from extended pelvic lymph node dissection. Three readers reported rates of sensitivity (65–75%) and specificity (94–97%) for the combined imaging method for detection of metastatic lymph nodes.
Diffusion-weighted MRI
DW-MRI makes use of the diffusivity of water molecules in the tumor microenvironment; more restricted diffusion of water molecules means more signal detection and brighter signal [45]. Bladder cancer’s high cellularity, high nucleus:cytoplasm ratio, decreased extracellular space, and tissue disorganization results in increased signal on DW-MRI images [46■■]. The degree of restricted diffusion can be quantified using a calculated apparent diffusion coefficient (ADC) map and drawing the region of interest over the lesion [45]. The lower the ADC value, the more restricted diffusivity of water molecules in the tumor microenvironment.
By the same principle, DW-MRI can detect primary bladder cancer or foci of metastases that have more restricted diffusion compared with normal background tissue. In a study of 104 bladder cancer patients, DW-MRI showed 90% sensitivity in detecting bladder cancer, which was comparable with T2-weighted MRI [47]. Similarly, DW-MRI can be used to detect malignant lymph nodes. Another study showed that because metastatic lymph nodes have lower ADC values than normal lymph nodes, DW-MRI had a sensitivity of 76% and specificity of 89% [24]. Several methods have been proposed to increase the sensitivity and specificity of DW-MRI for the detection of metastatic lymph nodes. One proposed method is using fusion of stronger b-values (750 s/mm2) with conventional T2-weighted images to increase the visibility of lymph nodes [48].
Lower ADC value can be helpful in differentiating high-grade from low-grade bladder cancer. A study evaluating 37 bladder cancer patients showed significantly lower ADC values in high-grade tumors compared with low-grade tumors [49]. The area under the curve for differentiating low-grade and high-grade tumors was higher for ADC relative to the size of the bladder tumor. In another study in bladder cancer, ADC values and tumor markers were compared with histology and correlated with clinicopathologic prognostic criteria such as tumor size, grade, and muscle invasiveness. ADC values were also correlated with immunohistochemically derived biomarkers such as p53, p21, and ki67. Low ADC values and p53 were associated with tumor stage, pathologic tumor grade, and lymphovascular invasion [50■]. Significantly, lower ADC values in MIBCs and high-grade T1 bladder cancers compared with less aggressive phenotypes with sensitivity of 88%, specificity of 85%, and accuracy of 87% have been reported [47].
DW-MRI is also helpful in differentiating recurrence from post-treatment changes. In a study of 11 patients with suspected tumor recurrence, DW-MRI was shown to be more sensitive and specific than DCE-MRI (92.6 and 100% vs. 59.3 and 82.3%, respectively) [51].
FUTURE DIRECTIONS
MRI can provide valuable morphologic and functional information for staging of MIBC. At the same time, PET agents have been proven invaluable in tumor, nodal, and metastatic staging of bladder cancer. Combining these two methods in a hybrid PET/MRI machine is a powerful modality for local and systemic staging of bladder cancer, with potential applications for evaluating treatment response. The main advantage of hybrid PET/MRI scanners is the ability to perfectly coregister PET and MRI data, which is particularly challenging in bladder imaging because of changes in the size of the bladder during imaging [52]. Another area of research and potential improvement is new radiopharmaceuticals, including agents targeted against specific cancer antigens. This technique has shown promising results in labeling cancer cells for gross visualization by cystoscopy (endoscopic molecular imaging of bladder cancer using a CD47 antibody). Many other methods for macroscopic and microscopic labeling of cancer cells are in development [53].
CONCLUSION
Although there is currently no ideal imaging method for diagnosing and staging MIBC, PET/CT is the preferred modality for initial staging of nodal and distant metastases in patients with MIBC. In the absence of data from large prospective studies, tumor staging depends on invasive pathologic assessment via TURBT. There are, however, several promising areas of research, including high-resolution multiparametric MRI and PET/MRI, and targeted molecular imaging.
Table 2.
Selected publications on imaging for staging of lymph node metastases in muscle-invasive and metastatic bladder cancer
| Lymph nodes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Modality | Author/date [refs] | n | Accuracy % |
Overstaging % |
Understaging % |
Sensitivity % | Specificity % | Comments |
| CT | Tritschler (2012) [6] | 276 | 54 | 8.3 | 29.4 | NR | NR | |
| FDG PET/CT | Jeong (2015) [14] | 61 | NR | PET/CT 3.3 CT 6.6 | PET/CT 26.2 CT 21.3 | PET/CT 47.1 CT 29.4 | PET/CT 93.2 CT 97.7 | |
| [11C] Choline PET-/CT | Brunocilla (2014) [15] | 26 | NR | NR | NR | PET/CT 42 CT 14 | PET/CT 84 CT 89 | Patient-based analysis |
| [18F] FDG PET-CT cf CT & PET | Goodfellow (2014) [16] | 233 | PET 82 CT 83 PET + CT 87 | NR | NR | PET 46 CT 46 PET/CT 69 | PET 97 CT 98 PET/CT 95 | |
| FDG PET/CT | Hitier-Berthault (2013) [17] | 52 | PET/CT 65.4 CT 55.7 | NR | NR | PET/CT 36.4 CT 9.1 | PET/CT 86.7 CT 90 | |
| [11C] Choline PET-/CT | Maurer (2011) [18] | 44 | PET/CT 91 CT 90 | NR | NR | PET/CT 28 CT 39 | PET/CT 95 CT 92 | Identification of involved lymph nodes |
| FDG PET/CT | Lodde (2010) [9] | 44 | NR | NR | NR | PET/CT 57 CT 33 | PET/CT 100 CT 100 | |
| FDG PET/CT | Swinnen (2010) [19] | 51 | PET/CT 84 CT 80 | NR | NR | PET/CT 46 CT 46 | PET/CT 97 CT 92 | |
| [11C] Choline PET-CT | Ceci (2015) [20■] | 59 | 81 | NR | NR | 59 | 90 | |
| MRI (DW and USPIO) | Birkhauser (2013) [21] | 75 | NR | NR | NR | 65–75 | 93–96 | |
| MRI (DW & USPIO) | Thoeny (2009) [22] | 21 | 90 | NR | NR | 80 | 73 | |
| MRI (DGE) | Daneshmand (2012) [23] | 122 | 80.3 | NR | NR | 40.7 | 91.5 | |
| MRI (DW) | Papalia (2011) [24] | 36 | NR | NR | NR | 76.4 | 89.4 | |
KEY POINTS.
Although CT with or without contrast is not an accurate method for initial assessment of local bladder tumor invasion, CT after radical cystectomy is the primary modality for nodal and distant metastases surveillance, although small nodes and metastatic foci may be understaged.
PET/CT with conventional radiotracers is commonly used for staging distant metastases. PET/MRI is a newer modality under investigation for local and systemic staging of bladder cancer, with potential applications for evaluating treatment response.
Multiparametric MRI is emerging as the imaging modality of choice in tumor staging, with a reported accuracy of more than 90%.
Locoregional lymph node metastases can also be accurately evaluated using functional MRI and specific contrast agents with paramagnetic characteristics.
Acknowledgements
The authors thank Bonnie L. Casey for editorial assistance in the preparation of this article.
Financial support and sponsorship
None.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014; 25:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray PJ, Fedewa SA, Shipley WU, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol 2013; 63:823–829. [DOI] [PubMed] [Google Scholar]
- 4.Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014; 32:3801–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer. http://www.cus.cz/wp-content/uploads/2012/10/NCCN-C67-2014.pdf [Accessed May 2015]■■ Current recommendations for MIBC staging from the National Comprehensive Cancer Network
- 6.Tritschler S, Mosler C, Straub J, et al. Staging of muscle-invasive bladder cancer: can computerized tomography help us to decide on local treatment? World J Urol 2012; 30:827–831. [DOI] [PubMed] [Google Scholar]
- 7.Blick CG, Nazir SA, Mallett S, et al. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cyst-scopy and voided urine cytology: results for 778 patients from a hospital haematuria clinic. BJU Int 2012; 110:84–94. [DOI] [PubMed] [Google Scholar]
- 8.Wang LJ, Wong YC, Ng KF, et al. Tumor characteristics of urothelial carcinoma on multidetector computerized tomography urography. J Urol 2010; 183:2154–2160. [DOI] [PubMed] [Google Scholar]
- 9.Lodde M, Lacombe L, Friede J, et al. Evaluation of fluorodeoxyglucose positron-emission tomography with computed tomography for staging of urothelial carcinoma. BJU Int 2010; 106:658–663. [DOI] [PubMed] [Google Scholar]
- 10.Ghafoori M, Shakiba M, Ghiasi A, et al. Valueof MRI in local staging ofbladder cancer. Urol J 2013; 10:866–872. [PubMed] [Google Scholar]
- 11.Liedberg F, Bendahl PO, Davidsson T, et al. Preoperative staging of locally advanced bladder cancer before radical cystectomy using 3 tesla magnetic resonance imaging with a standardized protocol. Scand J Urol 2013; 47:108–112. [DOI] [PubMed] [Google Scholar]
- 12.Wu LM, Chen XX, Xu JR, et al. Clinical value of T2-weighted imaging combined with diffusion-weighted imaging in preoperativeT staging of urinary bladder cancer: a large-scale, multiobserver prospective study on 3.0-T MRI. Acad Radiol 2013; 20:939–946. [DOI] [PubMed] [Google Scholar]
- 13.Rajesh A, Sokhi HK, Fung R, et al. Bladder cancer: evaluation of staging accuracy using dynamic MRI. Clin Radiol 2011; 66:1140–1145. [DOI] [PubMed] [Google Scholar]
- 14.Jeong IG, Hong S, You D, et al. FDG PET-CT for lymph node staging of bladder cancer: a prospective study of patients with extended pelvic lym-phadenectomy. Ann Surg Oncol 2015. [DOI] [PubMed] [Google Scholar]
- 15.Brunocilla E, Ceci F, Schiavina R, et al. Diagnostic accuracy of (11)C-choline PET/CT in preoperative lymph node staging of bladder cancer: a systematic comparison with contrast-enhanced CT and histologic findings. Clin Nucl Med 2014; 39:e308–e312. [DOI] [PubMed] [Google Scholar]
- 16.Goodfellow H, Viney Z, Hughes P, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int 2014; 114:389–395. [DOI] [PubMed] [Google Scholar]
- 17.Hitier-Berthault M, Ansquer C, Branchereau J, et al. 18 F-fluorodeoxyglucose positron emission tomography-computed tomography for preoperative lymph node staging in patients undergoing radical cystectomy for bladder cancer: a prospective study. Int J Urol 2013; 20:788–796. [DOI] [PubMed] [Google Scholar]
- 18.Maurer T, Souvatzoglou M, Kubler H, et al. Diagnostic efficacy of [11C]choline positron emission tomography/computed tomography compared with conventional computed tomography in lymph node staging of patients with bladder cancer prior to radical cystectomy. Eur Urol 2012; 61:1031–1038. [DOI] [PubMed] [Google Scholar]
- 19.Swinnen G, Maes A, Pottel H, et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol 2010; 57: 641–647. [DOI] [PubMed] [Google Scholar]
- 20.Ceci F, Bianchi L, Graziani T, et al. 11C-choline PET/CT and bladder cancer:lymph node metastasis assessment with pathological specimens as reference standard. Clin Nucl Med 2015; 40:e124–e128.■ Authors compared histology with 11C-choline for the detection of recurrence and locoregional lymph node metastasis and demonstrated high specificity, negative predictive value, and accuracy for 11C-choline.
- 21.Birkhauser FD, Studer UE, Froehlich JM, et al. Combined ultrasmall super-paramagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol 2013; 64:953–960. [DOI] [PubMed] [Google Scholar]
- 22.Thoeny HC, Triantafyllou M, Birkhaeuser FD, et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol 2009; 55:761–769. [DOI] [PubMed] [Google Scholar]
- 23.Daneshmand S, Ahmadi H, Huynh LN, Dobos N. Preoperative staging of invasive bladder cancer with dynamic gadolinium-enhanced magnetic resonance imaging: results from a prospective study. Urology 2012; 80:1313–1318. [DOI] [PubMed] [Google Scholar]
- 24.Papalia R, Simone G, Grasso R, et al. Diffusion-weighted magnetic resonance imaging in patients selected for radical cystectomy: detection rate of pelvic lymph node metastases. BJU Int 2012; 109:1031–1036. [DOI] [PubMed] [Google Scholar]
- 25.Apolo AB, Riches J, Schoder H, et al. Clinical value of fluorine-18 2-fluoro-2- deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol 2010; 28:3973–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol 2011; 59:1009–1018. [DOI] [PubMed] [Google Scholar]
- 27.Lawrentschuk N, Lee ST, Scott AM. Current role of PET, CT, MR for invasive bladder cancer. Curr Urol Rep 2013; 14:84–89. [DOI] [PubMed] [Google Scholar]
- 28.Lu YY, Chen JH, Liang JA, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: a systemic review and meta-analysis. Eur J Radiol 2012; 81:2411–2416. [DOI] [PubMed] [Google Scholar]
- 29.Nayak B, Dogra PN, Naswa N, Kumar R. Diuretic 18F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. EurJ Nucl Med Mol Imaging 2013; 40:386–393. [DOI] [PubMed] [Google Scholar]
- 30.Anjos DA, Etchebehere EC, Ramos CD, et al. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med 2007; 48:764–770. [DOI] [PubMed] [Google Scholar]
- 31.Bouchelouche K, Turkbey B, Choyke PL. PET/CT and MRI in bladder cancer. J Cancer Sci Ther 2012; S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jong IJ, Pruim J, Elsinga PH, et al. Visualisation of bladder cancer using (11)C-choline PET: first clinical experience. Eur J Nucl Med Mol Imaging 2002; 29:1283–1288. [DOI] [PubMed] [Google Scholar]
- 33.Gofrit ON, Mishani E, Orevi M, et al. Contribution of 11C-choline positron emission tomography/computerized tomography to preoperative staging of advanced transitional cell carcinoma. J Urol 2006; 176:940–944; discussion 944. [DOI] [PubMed] [Google Scholar]
- 34.Golan S, Baniel J, Lask D, et al. Transurethral resection of bladder tumour complicated by perforation requiring open surgical repair: clinical characteristics and oncological outcomes. BJU Int 2011; 107:1065–1068. [DOI] [PubMed] [Google Scholar]
- 35.Letocha H, Ahlstrom H, Malmstrom PU, et al. Positron emission tomography with L-methyl-11C-methionine in the monitoring of therapy response in muscle-invasive transitional cell carcinoma of the urinary bladder. Br J Urol 1994; 74:767–774. [DOI] [PubMed] [Google Scholar]
- 36.Ahlstrom H, Malmstrom PU, Letocha H, et al. Positron emission tomography in the diagnosis and staging of urinary bladder cancer. Acta Radiol 1996; 37:180–185. [DOI] [PubMed] [Google Scholar]
- 37.Verma S, Rajesh A, Prasad SR, et al. Urinary bladder cancer: role of MR imaging. Radiographics 2012; 32:371 –387. [DOI] [PubMed] [Google Scholar]
- 38.de Haas RJ, Steyvers MJ, Futterer JJ. Multiparametric MRI of the bladder: ready for clinical routine? AJR Am J Roentgenol 2014; 202:1187–1195.■■ Review of literature and pictorial essay on the role of MRI in the evaluation of bladder cancer.
- 39.Raza SA, Jhaveri KS. MR imaging of urinary bladder carcinoma and beyond. Radiol Clin North Am 2012; 50:1085–1110. [DOI] [PubMed] [Google Scholar]
- 40.Sourbron S Technical aspects of MR perfusion. EurJ Radiol 2010; 76:304–313. [DOI] [PubMed] [Google Scholar]
- 41.Sankineni S, Brown AM, Fascelli M, et al. Lymph node staging in prostate cancer. Curr Urol Rep 2015; 16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atri M, Zhang Z, Marques H, et al. Utility of preoperative ferumoxtran-10 MRI to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: results of ACRIN 6671/GOG 0233. European J Radiol Open 2015; 2:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fortuin AS, Smeenk RJ, Meijer HJ, et al. Lymphotropic nanoparticle-enhanced MRI in prostate cancer: value and therapeutic potential. Curr Urol Rep 2014; 15:389. [DOI] [PubMed] [Google Scholar]
- 44.Koh DM, George C, Temple L, et al. Diagnostic accuracy of nodal enhancement pattern of rectal cancer at MRI enhanced with ultrasmall superpara- magnetic iron oxide: findings in pathologically matched mesorectal lymph nodes. AJR Am J Roentgenol 2010; 194:W505–513. [DOI] [PubMed] [Google Scholar]
- 45.Malayeri AA, El Khouli RH, Zaheer A, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics 2011;31:1773–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida S, Koga F, Kobayashi S, et al. Diffusion-weighted magnetic resonance imaging in management of bladder cancer, particularly with multimodal bladder-sparing strategy. World J Radiol 2014; 6:344–354.■■Evaluation of the role of DW-MRI in diagnosis, staging, and posttreatment response.
- 47.Kobayashi S, Koga F, Yoshida S, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol 2011; 21:2178–2186. [DOI] [PubMed] [Google Scholar]
- 48.Mir N, Sohaib SA, Collins D, Koh DM. Fusion of high b-value diffusion- weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis. J Med Imaging Radiat Oncol 2010; 54:358–364. [DOI] [PubMed] [Google Scholar]
- 49.Rosenkrantz AB, Haghighi M, Horn J, et al. Utility of quantitative MRI metrics for assessment of stage and grade of urothelial carcinoma of the bladder: preliminary results. AJR Am J Roentgenol 2013; 201:1254–1259. [DOI] [PubMed] [Google Scholar]
- 50.Sevcenco S, Haitel A, Ponhold L, et al. Quantitative apparent diffusion coefficient measurements obtained by 3-Tesla MRI are correlated with biomarkers of bladder cancer proliferative activity. PLoS One 2014; 9:e106866.■ Study showing that lowADCvalues in DW-MRI were associated with tumorstage, pathologic tumor grade, and lymphovascular invasion.
- 51.Wang HJ, Pui MH, GuoY, et al. Diffusion-weighted MRI in bladder carcinoma: the differentiation between tumor recurrence and benign changes after resection. Abdom Imaging 2014; 39:135–141. [DOI] [PubMed] [Google Scholar]
- 52.Rosenkrantz AB, Balar AV, Huang WC, et al. Comparison of coregistration accuracy of pelvic structures between sequential and simultaneous imaging during hybrid PET/MRI in patients with bladdercancer. Clin Nucl Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zlatev DV, Altobelli E, Liao JC. Advances in imaging technologies in the evaluation of high-grade bladder cancer. Urol Clin North Am 2015; 42:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]


