Abstract
Malaria during pregnancy due to Plasmodium falciparum or P. vivax is a major public health problem in endemic areas, with P. falciparum causing the greatest burden of disease. Increasing resistance of parasites and mosquitoes to existing tools, such as preventive antimalarial treatments and insecticide-treated bed nets respectively, is eroding the partial protection that they offer to pregnant women. Thus, development of effective vaccines against malaria during pregnancy is an urgent priority. Relevant animal models that recapitulate key features of the pathophysiology and immunology of malaria in pregnant women could be used to accelerate vaccine development. This review summarizes available rodent and nonhuman primate models of malaria in pregnancy, and discusses their suitability for studies of biologics intended to prevent or treat malaria in this vulnerable population.
Among Plasmodium species that infect humans, P. falciparum is the most deadly. Despite long-term exposure to P. falciparum infection, women are again susceptible to P. falciparum infection during pregnancy, particularly primigravidae1,2. Similarly, susceptibility to P. vivax increases during pregnancy, and while the susceptibility to P. vivax infection is greatest in primigravidae, the risk of disease is greatest in multigravidae3,4. The adverse effects of malaria in pregnancy (MiP) on the fetus as well as the mother are well-documented, particularly for P. falciparum1–3. Abortion, stillbirth, miscarriage, intrauterine growth retardation (IUGR; see Box 1 for glossary), pre-term delivery (PTD), low birth weight (LBW), severe maternal anemia, and multiple clinical symptoms, including acute severe syndromes, have been reported among malaria-infected pregnant women1–8. These sequelae lead to increased maternal and fetal mortality and morbidity rates5,7.
Box 1 |. Glossary of terms and concepts
Chondroitin sulfate A (CSA):
A low sulfated glycoaminoglycan expressed on the surface of syncytiotrophoblasts, in intervillous spaces, and within fibrinoid deposits. CSA is the major placental receptor to which IEs bind in the placenta, causing placental sequestration.
Infected Erythrocyte (IE):
During malaria infection, parasites invade erythrocytes where they develop within a parasitophorous vacuole in the erythrocyte cytoplasm. The parasite exports proteins to the infected erythrocyte surface membrane.
Intermittent preventive treatment during pregnancy (IPTp):
Regular anti-malarial treatments during pregnancy that lead to improved pregnancy outcomes in malaria-endemic areas. Doses of anti-malarial drugs are administrated to pregnant women no more than once a month after the first trimester of pregnancy. This preventive strategy is now implemented in many malaria-endemic countries, despite the emergence of drug-resistant parasites.
Intrauterine growth restriction (IUGR):
Also known as intrauterine growth retardation, it refers to poor growth of the fetus as determined by weight-for-gestational age during the pregnancy. Restriction of oxygen and nutrients required for normal development of the fetus may be one of the causes of IUGR. IUGR has been associated with pre-term delivery and is a major risk factor for infant mortality.
Low birth weight (LBW):
LBW has been defined by the World Health Organization (WHO) to classify neonates with birth weights below 2500 g. LBW is a common sequela of placental malaria and has been associated with infant mortality.
Malaria in pregnancy (MiP):
Infection with Plasmodium spp during pregnancy. P. falciparum infection during pregnancy leads to placental malaria with sequestration of parasitized erythrocytes in intervillous spaces. Other species of Plasmodium cause systemic malaria infection during pregnancy without placental sequestration. Many terms are used to designate malaria infection detected in pregnant women, named MiP in this review.
Multigravidae:
In malaria-endemic areas, women become resistant over successive pregnancies to PM-related pregnancy outcomes. Multigravid women develop protective immunity against CSA-binding P. falciparum parasites, preventing parasite sequestration in the placenta.
Primigravidae:
In malaria-endemic areas, first-time pregnant women are the most susceptible to PM and its related sequelae, due to the lack of immunity to PM parasites. Primigravid women have low levels of antibodies against CSA-binding P. falciparum parasites.
P. falciparum erythrocyte membrane protein 1 (PfEMP1):
PfEMP1 proteins constitute a major VSA family expressed on P. falciparum infected erythrocytes. These proteins are encoded by a family of var genes comprising approximately 60 copies within every parasite genome. The proteins localize to electron-dense IE surface knobs and mediate interactions between the P. falciparum IE and endothelial receptors to avoid splenic clearance and the immune system. Different members of this family of proteins have been associated with adhesion to various receptors and therefore sequestration in corresponding tissues, such as CSA in the placenta.
Placental malaria (PM):
A hallmark of malaria infection during pregnancy, PM is characterized by sequestration of P. falciparum-infected erythrocytes in the maternal intervillous spaces. Although P. knowlesi is also known as a placenta sequestering parasite in nonhuman primates, no evidence of P. knowlesi placental sequestration in humans exists. Placental sequestration leads to inflammation of the placenta.
Pre-term delivery (PTD):
According to the World Health Organization, a PTD is defined as a baby born alive before 37 weeks of pregnancy are completed. Pregnant women who develop PM are more likely to experience PTD.
Sequestration:
Sequestration is defined as accumulation of IEs in deep vascular beds including the placenta, and not just evidence of IEs “adhering” to tissue.
VAR2CSA:
A member of the PfEMP1 family highly expressed by P. falciparum isolated from the placenta. VAR2CSA-expressing parasites selectively bind placental tissue on the placental receptor chondroitin sulfate A (CSA), and are differentially recognized by antibodies from protected pregnant women. VAR2CSA is the leading candidate to be developed as a vaccine against placental malaria.
Variant surface antigens (VSAs):
Large families of protein paralogs expressed on the surface of IEs by different pathogens in order to evade the immune response as part of their survival mechanism. Parasites sequentially switch the expression of different VSAs that are targeted by the immune system. Different families of proteins are expressed as VSAs by Plasmodium and other pathogens. The expression of the Plasmodium interspersed repeat (PIR) multigene family of proteins is common to all Plasmodium species, although their function(s) remains unknown. Other VSAs are species-specific, such as SICAvar (in P. knowlesi) or PfEMP1 (in P. falciparum).
Malaria presentation differs between women with low and high malaria immunity. The former often experience acute severe syndromes, such as respiratory distress and cerebral malaria, and these women are at high risk for mortality without prompt treatment9. The latter may remain asymptomatic or paucisymptomatic during chronic infections which nevertheless lead to poor outcomes like severe maternal anemia that increase mortality risk.
The sequestration of P. falciparum-infected erythrocytes (IEs) in the intervillous spaces of the placenta is a hallmark of placental malaria (PM) pathology10,11. Although P. vivax-MiP also causes maternal anemia and LBW12–14, disease severity is generally less than that of P falciparum-MiP, and there has been no evidence of placental sequestration of P. vivax-IEs. Placental P. falciparum parasites, as well as those isolated from women during pregnancy, selectively bind to chondroitin sulfate A (CSA), a glycosaminoglycan expressed by syncytiotrophoblast, which localizes to the surface of placental villi as well as to fibrinoid in the intervillous spaces15–21. Placental sequestration of parasites can elicit an inflammatory infiltrate in the intervillous spaces, a typical feature in primigravidae that is specifically associated with poor outcomes including severe maternal anemia and LBW of newborns2.
VAR2CSA, a member of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family, is highly expressed by placental P. falciparum, and has been identified as the major parasite surface ligand for the CSA receptor22–24. The var genes that encode PfEMP 1 proteins are unique to the P. falciparum genome25,26, and regulation of var genes ensures that only one PfEMP1 variant is expressed on the surface of each IE20. Pregnant women become resistant to PM as they acquire antibodies against CSA-binding parasites over successive pregnancies, suggesting a role in protection of women against poor pregnancy outcomes22,27–30. These findings support the development of a PM vaccine based on VAR2CSA that targets placental P. falciparum parasites31. No orthologs of VAR2CSA have been identified in P. vivax, and as the pathogenesis of P. vivax-MiP remains unclear, there is no similar path to develop a vaccine for this syndrome.
In areas of stable transmission, current malaria preventive measures include intermittent preventive therapy during pregnancy (IPTp) with antimalarial drugs and the use of insecticide treated nets. However, emerging resistance to both antimalarial drugs and insecticides can render such measures ineffective. In areas of unstable transmission, women with low immunity to malaria must be actively screened and treated promptly to prevent severe sequelae32. Therefore, an effective vaccine that can protect pregnant women against MiP in high and low transmission zones is urgently needed. However, the lack of suitable models to understand biological mechanisms involved in disease and protective immunity impedes vaccine development. Furthermore, the lack of suitable models to assess vaccine efficacy limits testing of candidate immunogens to laborious clinical trials that may yield less effective vaccines.
In this review, we describe existing animal models of MiP and discuss the relevance of each model in the context of PM vaccine development (see Box 2 and Fig. 1 for summary of models).
Box 2 |. Key features of P. falciparum malaria in pregnant women by which to assess animal models
Parasitemia
Higher density of parasites in placental versus peripheral blood.
Chronicity of infection: long term parasite carriage.
Parasite recrudescence during pregnancy.
Placental sequestration
Accumulation of mature stage parasites in maternal vascular spaces of placenta.
Inflammatory infiltrate in maternal vascular spaces of placenta.
Evidence of placental damage: syncytiotrophoblastic necrosis, partial loss of microvilli, fibrinoid deposits.
Phenotype of the parasites
Adhesion of placental parasites to CSA expressed by syncytiotrophoblast.
Upregulation of the parasite ligand VAR2CSA involved in placental sequestration.
Acquisition of protective immunity over successive malaria-exposed pregnancies
clinical resistance acquired over successive pregnancies.
Antibodies to placental parasites associated with clinical resistance.
Antibodies to placental parasites with broadly neutralizing activity.
FIGURE 1 |.
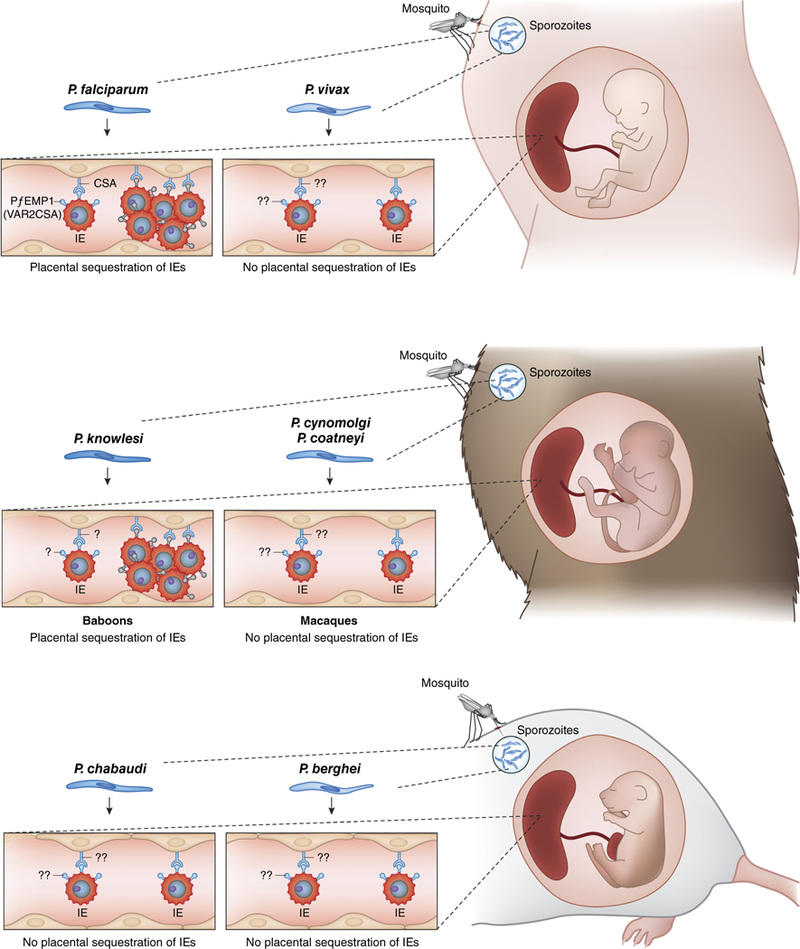
Schematic summarizing different malarial parasites and current knowledge of infected erythrocytes (IEs) and mechanisms in humans, nonhuman primates, and rodent models of malaria in pregnancy. For more details, see Table 1.
Rodent models for placental malaria
Rodents are attractive for MiP research because of their short reproductive cycles, highly reproducible course of parasitemia in inbred animals, and less restrictive ethical concerns. Study conditions can be easily manipulated to model aspects of human epidemiology, such as timing of parasite exposure (for example, before pregnancy, during pregnancy, or both), or intensity of exposure (to model high, moderate and low transmission settings).
Rodent studies have demonstrated that MiP can result in complications to both dams and their offspring similar to those seen in humans, such as abortions and poor placental development9,33. Some evidence for a distinct pregnancy parasite phenotype, parasite adhesion to placental receptors, placental sequestration of parasites, and immunity to placental parasites, have all been reported34–40. However, placental sequestration of parasites has generally not been a prominent feature of rodent MiP, unlike human MiP. Further, the absence of PfEMP1 homologs in rodent malaria parasites impedes mechanistic studies of these key virulence markers, as well as pre-clinical testing of VAR2CSA-based interventions. In this review, sequestration is defined as accumulation of IE in the placenta, and not just evidence of IE “adhering” to placental tissue.
Infection during pregnancy (non-immune model)
The use of non-immune pregnant rodents provides opportunities to understand the pathological outcomes of infection during pregnancy. This may be particularly relevant to MiP in women in areas of unstable transmission and travelers, who lack immunity and are at high risk of acute severe syndromes.
In non-pregnant non-immune C57B/6 mice, control of P chabaudi infection is mediated first by pro-inflammatory cytokines and then by antibody-mediated responses41,42. In pregnant non-immune mice, P. chabaudi infection increases levels of pro-inflammatory cytokines such as interferon-γ, tumor necrosis factor-α, and inter-leukin-1β (IL) in the plasma. High levels of anti-inflammatory cytokines (such as IL-10) have also been observed, though these were insufficient to prevent fetal loss37,39. Conversely, P berghei in non-immune pregnant mice was reported to cause increased levels of IL-17 and reduced levels of IL-10 that were associated with lower birth weights of offspring35, although other studies have not seen these relationships43–45. Increased levels of complement C5a and C5a receptor in infected mice have been associated with poor fetal growth, and mutant mice lacking the C5a receptor delivered significantly heavier offspring46. P berghei infection during pregnancy leads to dysregulated angiopoietin levels, which are also associated with fetal growth restriction47. These observations with P. chabaudi and P berghei suggest that dysregulated levels of cytokines, complement factors, and angiogenic factors (such as angiopoietin 1 and 2) may impair placental formation, resulting in poor placental function38,46,48, that contributes to poor fetal outcomes47,49.
In non-pregnant rodents, P. chabaudi can sequester in various organs34, but it remains unclear the degree to which sequestration occurs in the placenta. Poovassery et al. reported that P. chabaudi can accumulate in the placentae of infected pregnant mice, with significantly higher parasite densities in placental than peripheral blood only in mice that suffered abortion50. Meanwhile, a recent study of P chabaudi-infected pregnant mice showed no significant difference in placental and peripheral parasite densities, and no apparent accumulation of mature parasite forms in the placenta by histological examination40. The absence of var genes, especially VAR2CSA, could explain why P chabaudi parasites do not sequester in the placenta, although the absence of var genes does not preclude sequestration in other vascular beds. Of note, P vivax also lacks var genes and shares several protein orthologs such as pir genes that encode a variant antigen family with P chabaudi45. Since P vivax MiP does not involve placental parasite sequestration12,13, and in vitro studies have failed to identify a P vivax adhesion phenotype related to MiP51, systemic infection in the mother may more likely explain poor pregnancy outcomes. P. chabaudi infection in pregnant mice does not result in placental abnormalities or significant monocyte infiltration, but nevertheless offspring had significantly lower birth weights compared to offspring from naive dams40,50. Hence, P. chabaudi should be further evaluated as a model of P vivax MiP.
P. berghei has been demonstrated to bind to mouse placenta ex vivo, and this adhesion was reduced by pre-incubating P. berghei IEs with CSA and/or hyaluronic acid (HA)9,36; the involvement of HA in the binding of P. falciparum-IEs to human placenta is questionable52. In a subsequent study using intravital imaging techniques, the authors provided insight into interactions between P. berghei-IEs and trophoblast: no stationary IEs were observed in areas of high blood flow, whereas in low flow regions IEs remained stationary, indicating preferential IE adhesion in these regions53. The authors suggested that in maternal blood spaces with low microcirculation, IEs might establish a stable IE-trophoblast interaction that was associated with IE adhesion in the placenta, indicating that the parasite takes advantage of the heterogeneous placental blood flow pattern to accumulate in the mouse placenta53.
Because var genes are absent in P berghei, the antigen(s) that mediates placental binding in rodent models could be identified and might be studied as an alternative vaccine target(s). Conversely, the absence of var genes represents a limitation for studying VAR2CSA-based vaccines in this model. This limitation has been partially overcome in a recent report54, where a transgenic P. berghei parasite was engineered to express the full-length VAR2CSA extracellular region fused to a full-length P. berghei exported protein (EMAP1). BALB/c mice infected at mid-gestation with P. berghei-VAR2CSA suffered a higher incidence of stillbirth, lower fetal weight, maternal mortality and fetal loss as compared to those infected by wild-type P. berghei. Notably, the parasite loads in infected placentas were similar with both parasite lines, indicating that increased virulence did not depend on parasite burden54. VAR2CSA expression increased adhesion of P. berghei to placental tissue in in vitro assays, and induced VAR2CSA antibodies in infected mice54. The P berghei-VAR2CSA model has some limitations. Despite increased adherence to placental tissue, VAR2CSA expression by P berghei exacerbated MiP pathogenicity without enhancing placental parasite load, which is unlike the pattern seen in women54. The fact that placental binding is likely to occur in both P berghei-VAR2CSA and P berghei models complicates the use of the P berghei-VAR2CSA model to assess VAR2CSA-based vaccine candidates under development.
Of note, placentas collected from P. berghei-infected mice demonstrate significant monocyte infiltration, abnormal placental formation, and the presence of P. berghei-IEs and hemozoin9,55. However, the intense peripheral parasitemia (more than 50% infected erythrocytes) during P. berghei infections may have also contributed to or been responsible for the high parasite density and pathology in the placenta9.
The different non-immune rodent MiP models may offer an opportunity to understand pathological outcomes during pregnancy. The dysregulation of both pro-inflammatory and anti-inflammatory cytokine responses (as well as complement factors) has parallels to changes observed in infected pregnant women. In mice, these result in dysregulated vasculogenesis (differentiation of precursor cells) and angiogenesis (formation of new blood vessels) in malaria-infected placentas, and in turn, poorer birth outcomes; these relationships should be further studied in pregnant women. While the different models display some evidence of IE adhesion to placenta or of placental inflammation, the degree of sequestration does not approach that seen with P. falciparum, suggesting that the rodent MiP models may have limited relevance to P. falciparum MiP in women. Transgenic parasites expressing VAR2CSA should be further investigated to understand their usefulness to assess VAR2CSA-based vaccines. One alternative might be to establish a model using a rodent strain with no known affinity for binding to the placenta, where the placental sequestration phenotype can be induced by introducing VAR2CSA expression in transgenic parasites.
Infection before pregnancy (recrudescence model)
Parasite recrudescence is an established feature of MiP due to either P. falciparum or P vivax in women56,57. Models of recrudescence in pregnant animals could be exploited to understand its basis and any distinct effects on placental function, fetal development, and acquisition of MiP-specific immunity. In one study, mice challenged with P. berghei carried low grade parasitemia for months, and upon pregnancy, experienced recrudescent parasitemia which was often lethal within the first few days of pregnancy58. Anemia, poor pregnancy outcomes, and enrichment of mature stage parasites in the placenta have been observed36,43. Interestingly, recrudescence was less frequent in subsequent pregnancies and pregnancy outcomes were improved, suggesting the acquisition of specific immunity9,36. Further, plasma antibodies acquired from infections before pregnancy were not reactive toward P berghei parasites that were recrudescent during pregnancy, suggesting that distinct variant surface antigens (VSAs) were expressed36.
With P chabaudi, most mice experienced a non-lethal recrudescence during pregnancy associated with anemia40, similar to the clinical presentation of malaria in many women. The acquisition of antibodies against the parasites has not been investigated in the P. chabaudi MiP model. More studies are needed to better understand the basis for recrudescence, and whether these models can be useful for PM vaccine development.
Exposure before and during pregnancy (immune model)
A model that incorporates malaria exposure before pregnancy is better suited to mimic first-time pregnant mothers in endemic regions, as most of these women have experienced malaria before pregnancy. Such models can yield insights into the impact of preexisting immunity on malaria-related poor pregnancy outcomes, the differences and similarities between MiP-specific immunity versus general malaria immunity, and the potential for pre-existing immunity to be boosted during MiP.
P. chabaudi has the advantage of several antigenically distinct parasite lines that allow serial heterologous infections59. Sequential infection with P chabaudi AS before pregnancy and then P chabaudi CB during pregnancy causes death and impaired post-natal growth of pups that is associated with inflammatory cytokines. Notably, offspring delivered by re-infected dams were more resistant to P. chabaudi infection compared to those by never-infected dams40. Similarly, infants born in areas of stable malaria transmission display resistance to malaria infection and disease for the first months of life, and the transfer of maternal antibodies may contribute to such protection, though the evidence for this is not yet conclusive (reviewed in ref. 60).
Both heterologous re-infection with P. berghei NK65 during pregnancy and homologous re-infection with P. berghei K173 during pregnancy led to dysregulated inflammatory responses and significantly lower birth weight of pups44,45. The observations seen in re-infection models suggest that, despite previously acquired immunity, mice remain susceptible to re-infection of the same species during pregnancy, likely by parasite expression of VSA to evade preexisting immunity as observed in the recrudescent model36. Therefore, immunity acquired before pregnancy may be inadequate to confer protection during pregnancy36, a pattern also seen in human studies2.
Overall, rodent models that allow parasite infection before and during pregnancy may better reflect the experience of women in endemic areas, who are also exposed to malaria both before and during pregnancy. These models can be used to dissect immunopathology that ensues during pregnancy despite previously acquired immunity. They can also be interrogated to discriminate the benefits of immunity acquired against malaria generally versus MiP specifically, as well as the effect of maternal antibodies to modulate protection in offspring. Finally, such studies can lead to the identification of antigen(s) relevant to these different forms of acquired immunity, which might prompt studies of these non-VAR2CSA antigens as targets of human immunity during MiP and thereby inform future vaccine development and testing.
Limitations of rodent models for pregnancy malaria vaccine development
With their ease of use, rodent models present an attractive approach to study MiP pathogenesis and immunity. However, the absence of PfEMP1 homologs and understanding of surface proteins that mediate pathology in rodent malaria impede their use to better understand MiP pathogenesis (see Box 3 for rodent model limitations). Rodent parasites possess the pir multigene family that is related to the P. falciparum rif and stevor gene families, but little evidence regarding their function(s) is known61. On the other hand, the recent use of transgenic P. berghei parasites expressing VAR2CSA, which were shown to bind more effectively to the placenta than control parasites, may provide a viable platform for vaccine studies54. Such models can be useful to assess anti-VAR2CSA antibodies in preventing poor pregnancy outcomes in both dams and pups. However, the limited evidence of placental sequestration with both transgenic and wild-type P berghei raises concerns for their relevance to human PM.
Box 3 |. Challenges in rodent models for PM vaccine development
Parasite proteins?
Parasite proteins that may play a role in rodent models of MiP are unclear. No var gene encoded proteins or homologs exist in rodent parasites. Rodent parasites possess the pir multigene family that is related to the P. falciparum rif and stevor gene families, but little evidence regarding their function in placental adherence is known.
P. berghei binding?
Transgenic P. berghei parasites expressing VAR2CSA bind in vitro at higher numbers to placental tissue compared to wild-type P. berghei, however these parasites yield similar placental parasite load in vivo. This model needs further optimization to assess VAR2CSA-based PM vaccines, as placental binding can occur with both P. berghei-VAR2CSA and P. berghei models.
Placental anatomy and microcirculation?
Placental anatomy differs substantially between humans and rodents. These differences may contribute to different microcirculation flow and vascular niches in the placenta, and may be associated with different mechanisms of placental sequestration in rodent models.
In the P. chabaudi-MiP model, adverse pregnancy outcomes with limited evidence of placental sequestration suggest it may be an attractive model to study P. vivax-MiP40. Although CIR protein has been implicated in rosette formation (clusters in which uninfected erythrocytes aggregate around an IE)62, this feature is uncommon during P. falciparum-MiP63.
Overall, the majority of rodent MiP studies have been done using blood-stage parasite inoculation, which does not represent the natural route of infection. Future studies with rodent models should further explore sporozoite inoculation to establish infection, and whether this alters MiP presentation or acquisition of immunity. Notably, there are substantial differences in placental anatomy between humans and rodents (see Fig. 2). For instance, in the human placenta, the chorionic plate (involved in blood flow in maternal space) has a tree branch-like pattern, in contrast to the mouse placenta that is interconnected like a maze (reviewed in ref. 64). Such differences may contribute to different microcirculation rates and vascular niches in the placenta, and must be taken into consideration when assessing MiP features in these rodent models.
FIGURE 2 |.
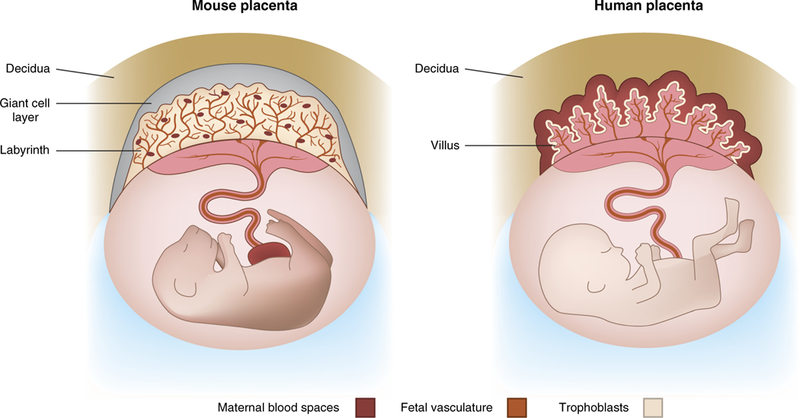
Illustration demonstrating key differences in placental anatomy between humans and mice.
Pregnancy malaria in non-human primates
Non-human primates (NHPs) and humans share strong developmental, immunological, anatomical, and biochemical similarities, as well as physiologically similar reproductive systems65,66. The hemochorial type placenta is shared by many NHPs and humans, emphasizing the potential importance of these models to understand PM pathogenesis67,68. NHP models of MiP have mainly focused on two monkey species: rhesus macaques (Macaca mulatta)69,70 and baboons (Papio anubis)71,72. While rhesus monkeys and baboons are susceptible to many Plasmodium species, only a few (P coatneyi and P. cynomolgi for rhesus macaques and P. knowlesi for baboons) have been studied as potential MiP models. For most of these studies, only non-immune pregnant monkeys were used, so that observations from these studies to date may be most relevant to MiP in non-immune women.
P. coatneyi and P. cynomolgi in pregnant rhesus monkeys
P. coatneyi has several biological features in common with P. falciparum, such as a 48-h intra-erythrocytic developmental cycle, electron-dense knob protrusions on the surface of IE, rosette formation73–76, and complete deep vascular sequestration of mature-stage IEs with predilection for specific tissues77–80. In non-splenectomized pregnant rhesus monkeys of various parities, high peak parasitemia was observed seven days post-inoculation of blood stage P. coatneyi during the first trimester of gestation, which coincided with the loss of pregnancy in 3 out of 4 infected pregnant monkeys70. Other adverse pregnancy outcomes were observed in 6 additional pregnant monkeys infected with reduced P coatneyi inoculum (along with one monkey from the first cohort) who carried their gestations to term, including LBW, intrauterine growth restriction (IUGR), congenital infection, and infant mortality70. Placental histology studies of P coatneyi-infected pregnant monkeys revealed fibrinoid deposits, infarcts, syncytiotrophoblast disruption, inflammatory cell infiltration, malaria pigment (hemozoin), and activated macrophages69. These placental damages are similar to those seen in humans12,81,82. However, placental sequestration of parasites does not appear to be a feature of P.coatneyi MiP (see section below on “Placental sequestration patterns”).
P cynomolgi infection in pregnant rhesus monkeys has also been studied as a non-sequestering parasite model for P. vivax infection in pregnancy. By infecting pregnant rhesus monkeys in the third trimester of pregnancy with either P cynomolgi cynomolgi or P. cynomolgi Bastianelli, Kamboj and Dutta83 showed intensified parasitemia and significant pathology, including delayed placental retention as well as fetal, infant, and maternal mortality. Saxena and colleagues subsequently studied early gestation P. cynomolgi infections in rhesus monkeys to relate biochemical abnormalities with placental pathology and clinical outcomes84–87. In four pregnant monkeys, inoculation of P. cynomolgi during the first trimester of pregnancy resulted in maternal, fetal, and newborn deaths. Histochemical studies revealed changes in hydrolytic enzyme activity in the placenta, which might directly impact functional and morphological integrity of the tissue by impairing the enzyme-mediated cellular transport mechanisms84,88, and thereby contribute to embryonic deaths and abortions84,86,87.
The pregnancy outcomes described in both these rhesus models are similar to those reported for human MiP, and thus support the use of P. coatneyi and P cynomolgi as valid models to study adverse outcomes seen in pregnant women.
P. knowlesi infection in pregnant monkeys
P. knowlesi naturally infects the long-tailed macaque (Macaca fascicularis), and can infect other primates including baboons and humans89,90. Although P. knowlesi clusters phylogenetically with P. vivax91, which has a non-sequestering phenotype, P knowlesi is a partially sequestering parasite known to accumulate in several organs91,92. In a study from the 1930s, Das Gupta infected a pregnant rhesus monkey with P knowlesi and observed that placental blood was massively infected, while fetal blood and other maternal organs were free of parasites93. However, this study has not been replicated, and additional histological evidence for placental sequestration in this model is still needed.
In olive baboons (Papio anubis), P. knowlesi infection can be acute (with multiple organ dysfunction and cerebral involvement) or chronic (with enlarged spleen) with similar clinical symptoms to those of P. falciparum infection in humans, including but not limited to anemia, hyperbilirubinemia, cholestasis, apathy, lethargy and coma66. P knowlesi MiP in olive baboons shares several features with human MiP, including placental sequestration; maternal anemia; accumulation of immunological cells such as macrophages, neutrophils and monocytes in the placenta; and placental tissue damage71,72,94. Studies have demonstrated the accumulation of late-stage P. knowlesi-IEs in the intervillous spaces of the baboon placenta, increased placental parasitemia compared to peripheral parasitemia, and infiltration of inflammatory cells in the placenta leading to severe placental damage72,94, demonstrating that P knowlesi parasites can cytoadhere in the placenta71,94 despite the absence of a confirmed VAR2CSA ortholog. The SICAvar multigene family responsible for antigenic variation in P knowlesi95 has been related to the var gene family in P. falciparum96,97, but their biological function in cytoadherence and sequestration is still unknown96. However, in homology blasts, erythrocyte binding proteins (EBP-alpha, EBP-beta and EBP-gamma) showed the greatest homology to VAR2CSA98.
Immunological and hematological changes related to MiP have also been investigated in the P. knowlesi-baboon model. P. knowlesi infection in pregnant baboons causes elevated concentrations of TNF-α and IL-12 in placental plasma, but decreased levels of IL-4, IL-6, IL-10 and IFN-γ cytokines72,94. These cytokine changes differ in some ways from those in the malaria-infected human placenta, where no change in the levels of IL-4 and IL-6 and increased levels of IFN-γ have been reported99. In one study, all pregnant baboons developed anemia during P knowlesi infection as compared to uninfected pregnant baboons94. These reports highlight many similarities in MiP pathology in humans and the P. knowlesi-baboon model, the most relevant being placental sequestration and adverse sequelae. These observations support the use of the olive baboon-P. knowlesi model for further MiP-related immunology studies and investigation of molecular mechanisms involved in the accumulation of non-falciparum parasites in the placenta.
Challenges in the use of NHP models for placental malaria vaccine development
The NHP models reported to date offer various opportunities to study MiP pathology and represent a major asset to help identify drug targets. However, some key limitations exist in order to mimic MiP pathology in humans and to obtain key clinical outcomes that can be monitored as endpoints for PM or MiP vaccine candidates (see Box 4 for primate model limitations).
Box 4 |. Challenges in NHP models for PM vaccine development
Placental sequestration in rhesus monkeys?
No evidence of P. coatneyi and P. cynomolgi placental sequestration exists in rhesus monkeys, suggesting that a vaccine designed to prevent placental sequestration could not be assessed in these models.
P. knowlesi ligand?
P. knowlesi sequesters in the placentas of olive baboons and possibly rhesus, however the molecular basis for sequestration is unknown. Unless the P. knowlesi ligand for placental sequestration is identified, and orthologous proteins in P. falciparum are shown to be involved in human placental sequestration, this model might not be useful in PM vaccine development.
VAR2CSA orthologs?
VAR2CSA, the current leading PM vaccine candidate in humans, is exclusively expressed by P. falciparum, and the closely related chimpanzee parasite P. reichnowi with no orthologs found in Plasmodium species used in existing NHP models.
Protective immunity?
Existing NHP models have not been studied for the acquisition of protective immunity over successive pregnancies, as observed in women living in malaria-endemic areas. These features are needed in NHP models to assess clinical PM vaccines.
Placental sequestration patterns
Placental sequestration is absent in some models (P. coatneyi and P cynomolgi MiP in rhesus monkeys), which may make them more suitable as models for P. vivax MiP. In the P coatneyi model of MiP, the parasite densities in both placental and peripheral blood smears were similar at delivery69. In addition, although IEs could be identified within the intervillous space of placental tissue sections, there was no increased accumulation of IEs within the intervillous space nor IEs adhering to the syncytiotrophoblast surface69. The failure of P. coatneyi to sequester in the placenta might be related to the absence of VAR2CSA or var gene orthologs in P. coatneyi100.
P. knowlesi sequesters in the placentas of olive baboons and possibly rhesus, but the molecular basis for sequestration is not yet known. In a previous report, P knowlesi IEs from human subjects have been shown to bind in a specific but variable manner to the inducible endothelial receptors ICAM-1 and VCAM101, suggesting that VSA (distinct from PfEMP1) may be mediating cytoadherence. These observations highlight the dearth of knowledge regarding the underlying mechanisms for placental tropism of parasites in these NHP models. This also raises the question about P. falciparum placental sequestration and the central role of VAR2CSA as the only known ligand for parasite binding to placental tissues. Of note, no study has conclusively established that VAR2CSA is required for placental sequestration. Overall, the biology of host-parasite interactions in the context of pregnancy merits further investigation in monkeys, including the relative contribution of sequestration in the placenta versus other organs to adverse pregnancy outcomes.
MiP-related parity effect and protection
The effect of parity on human susceptibility to MiP is well-known: in areas of stable transmission, primigravidae have the highest susceptibility to P. falciparum parasitemia as well as adverse pregnancy outcomes, and susceptibility decreases over successive pregnancies1,2,5–7‘102. This pattern has been explained by the acquisition of specific antibodies against the VSA of placental IE28,30. Therefore, induction of this functional immunity that protects against MiP is the ultimate goal of PM vaccine development.
Little is known about the parity effect in existing NHP models. Parity of naive pregnant rhesus monkeys does not influence whether the animal will develop a high or low parasitemia in P. coatneyi MiP69,70, but this is thought to be true in naive humans as well. Further, studies of MiP in NHP models71,83,86,94 were not designed to address this key element of immunity to MiP. Future studies should explore the impact of malaria episodes over successive pregnancies, as well as before pregnancy, on PM in the different NHP models. This can ascertain which models best mimic MiP in endemic regions, and potentially help to understand the dynamic acquisition of protective immunity against PM.
Conclusion
One hundred twenty-five million pregnant women are at risk of Plasmodium infection each year103, which causes poor outcomes including death for both mother and offspring. The underlying mechanisms of disease and death remain uncertain. Furthermore, the need for an effective vaccine has been made more urgent by the spread of drug-resistant parasites and insecticide-resistant mosquitoes. Relevant animal models could improve our understanding of MiP and accelerate development of preventive strategies. However, skepticism will remain as most of the existing models share some but not all features of MiP in humans (Table 1). A pressing need is to establish a model that is suitable to assess VAR2CSA-based vaccine candidates currently under development31. Importantly only the P. falciparum genome encodes VAR2CSA, and therefore transgenic model parasites engineered to express VAR2CSA may be useful to overcome these shortcomings in the existing models54. Alternatively, a model that can be infected by P. falciparum, which is limited at present to a few New World monkeys, might be useful for studying PM pathogenesis and evaluating vaccine activity.
Table 1 |.
Characteristics of MiP in humans, non-human primates and rodents
| Characteristics of malaria in pregnancy | Humans | Non-human primates | Rodents | ||||
|---|---|---|---|---|---|---|---|
| P. falciparum | P. vivax | P. knowlesi | P. coatneyi | P. cynomolgi | P. chabaudi | P. berghei | |
| Sequestration within the placenta | Yes15,18,19 | No12,104 | Yes71,72,94 | Unclear* | No84,85,105 | Unclear* | Unclear* |
| Inflammatory infiltrate in the placenta | Yes2 | Yes104 | Yes94 | Yes69 | Yes86 | No50 | Yes9 |
| Dysregulated cytokines levels | Yes106 | Yes107 | Yes72 | Unknown | Unknown | Yes37,39 | Yes35,44,45 |
| Placental tissue damage | Yes81,82 | Yes108 | Yes94 | Yes69 | Yes84–86 | Unknown | Yes9,43 |
| Affect placenta functions | Yes109,110 | Unknown | Unknown | Yes69 | Yes84–86 | Unknown | Unknown |
| Maternal anemia | Yes7 | Yes13,111 | Yes94 | Yes70,78 | Unknown | Yes50 | Yes36 |
| Acquisition of immunity against placental-binding parasites | Yes28 | Unknown | Unknown | Unknown | Unknown | Unknown | Yes36 |
| Outcomes of fetus | |||||||
| Congenital malaria | Uncommon12 | Uncommon14 | No94 | Common70 | Unknown | Unknown | Unknown |
| Low birthweight | Yes3,5,7 | Yes13,111 | Unknown | Yes69,70 | Unknown | Yes40 | Yes36 |
insufficient evidence or contradicting reports
ACKNOWLEDGMENTS
We are thankful to J. Patrick Gorres for extensive proofreading and editing of the manuscript.The authors are supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Brabin BJ An analysis of malaria in pregnancy in Africa. Bull. World Health Organ. 61, 1005–1016 (1983). [PMC free article] [PubMed] [Google Scholar]
- 2.Rogerson SJ, Hviid L, Duffy PE, Leke RF & Taylor DW Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 7, 105–117 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Desai M et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7, 93–104 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Nosten F, ter Kuile F, Maelankirri L, Decludt B & White NJ Malaria during pregnancy in an area of unstable endemicity. Trans. R. Soc. Trop. Med. Hyg. 85, 424–429 (1991). [DOI] [PubMed] [Google Scholar]
- 5.Menendez C et al. The impact of placental malaria on gestational age and birth weight. J. Infect. Dis. 181, 1740–1745 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Rogerson SJ, Mwapasa V & Meshnick SR Malaria in pregnancy: linking immunity and pathogenesis to prevention. Am. J. Trop. Med. Hyg. 77, 14–22 (2007). [PubMed] [Google Scholar]
- 7.Steketee RW, Nahlen BL, Parise ME & Menendez C The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64, 28–35 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Wickramasuriya G Malaria and Ankylostomiasis in the pregnant woman, London: Oxford University Press, 1936. [Google Scholar]
- 9.Neres R, Marinho CR, Goncalves LA, Catarino MB & Penha-Goncalves C Pregnancy outcome and placenta pathology in Plasmodium berghei ANKA infected mice reproduce the pathogenesis of severe malaria in pregnant women. PLoS One 2008;3:e1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews KT & Lanzer M Maternal malaria: Plasmodium falciparum sequestration in the placenta. Parasitol. Res. 88, 715–723 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Bray RS & Sinden RE The sequestration of Plasmodium falciparum infected erythrocytes in the placenta. Trans. R. Soc. Trop. Med. Hyg. 73, 716–719 (1979). [DOI] [PubMed] [Google Scholar]
- 12.McGready R et al. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am. J. Trop. Med. Hyg. 70, 398–407 (2004). [PubMed] [Google Scholar]
- 13.Nosten F et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet 354, 546–549 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Morales AJ et al. Pregnancy outcomes associated with Plasmodium vivax malaria in northeastern Venezuela. Am. J. Trop. Med. Hyg. 74, 755–757 (2006). [PubMed] [Google Scholar]
- 15.Beeson JG, Amin N, Kanjala M & Rogerson SJ Selective accumulation of mature asexual stages of Plasmodium falciparum-infected erythrocytes in the placenta. Infect. Immun. 70, 5412–5415 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doritchamou J et al. First-trimester Plasmodium falciparum infections display a typical “placental” phenotype. J. Infect. Dis. 206, 1911–1919 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Doritchamou J et al. Dynamics in the cytoadherence phenotypes of Plasmodium falciparum infected erythrocytes isolated during pregnancy. PLoS ONE 9, e98577 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried M, Domingo GJ, Gowda CD, Mutabingwa TK & Duffy PE Plasmodium falciparum: chondroitin sulfate A is the major receptor for adhesion of parasitized erythrocytes in the placenta. Exp. Parasitol. 113, 36–42 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Fried M & Duffy PE Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272, 1502–1504 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Scherf A et al. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 17, 5418–5426 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuikue Ndam NG et al. Variable adhesion abilities and overlapping antigenic properties in placental Plasmodium falciparum isolates. J. Infect. Dis. 190, 2001–2009 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Salanti A et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49, 179–191 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Tuikue Ndam NG et al. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J. Infect. Dis. 192, 331–335 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Viebig NK et al. Disruption of var2csa gene impairs placental malaria associated adhesion phenotype. PLoS ONE 2, e910 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baruch DI et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82, 77–87 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Su XZ et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82, 89–100 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Duffy PE & Fried M Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 71, 6620–6623 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried M, Nosten F, Brockman A, Brabin BJ & Duffy PE Maternal antibodies block malaria. Nature 395, 851–852 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Ndam NT et al. Protective Antibodies against Placental Malaria and Poor Outcomes during Pregnancy, Benin. Emerg. Infect. Dis. 21, 813–823 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teo A, Feng G, Brown GV, Beeson JG & Rogerson SJ Functional Antibodies and Protection against Blood-stage Malaria. Trends Parasitol. 32, 887–898 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Chene A et al. Clinical development of placental malaria vaccines and immunoassays harmonization: a workshop report. Malar. J. 15, 476 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nosten F et al. Mefloquine antimalarial prophylaxis in pregnancy: dose finding and pharmacokinetic study. Br. J. Clin. Pharmacol. 30, 79–85 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tegoshi T, Desowitz RS, Pirl KG, Maeno Y & Aikawa M Placental pathology in Plasmodium berghei-infected rats. Am. J. Trop. Med. Hyg. 47, 643–651 (1992). [DOI] [PubMed] [Google Scholar]
- 34.Brugat T et al. Sequestration and histopathology in Plasmodium chabaudi malaria are influenced by the immune response in an organ-specific manner. Cell. Microbiol. 16, 687–700 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitri LE et al. Low Fetal Weight is Directly Caused by Sequestration of Parasites and Indirectly by IL-17 and IL-10 Imbalance in the Placenta of Pregnant Mice with Malaria. Korean J. Parasitol. 53, 189–196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Megnekou R, Hviid L & Staalsoe T Variant-specific immunity to Plasmodium berghei in pregnant mice. Infect. Immun. 77, 1827–1834 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poovassery J & Moore JM Association of malaria-induced murine pregnancy failure with robust peripheral and placental cytokine responses. Infect. Immun. 77, 4998–5006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahmah Z, Sasmito SD, Siswanto B, Sardjono TW & Fitri LE Parasitemia induces high plasma levels of Interleukin-17 (IL-17) and low levels of Interleukin-10 (IL-10) and Transforming Growth Factor-ss (TGF-ss) in pregnant mice infected with malaria. Malays. J. Med. Sci. 22, 25–32 (2015). [PMC free article] [PubMed] [Google Scholar]
- 39.Sarr D, Smith GM, Poovassery JS, Nagy T & Moore JM Plasmodium chabaudi AS induces pregnancy loss in association with systemic pro-inflammatory immune responses in A/J and C57BL/6 mice. Parasite Immunol. 34, 224–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma A, Conteh S, Langhorne J & Duffy PE Heterologous infection of pregnant mice induces low birth weight and modifies offspring susceptibility to malaria. PLoS ONE 11, e0160120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su Z & Stevenson MM Central role of endogenous gamma interferon in protective immunity against blood-stage plasmodium chabaudi AS infection. Infect. Immun. 68, 4399–4406 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su Z & Stevenson MM IL-12 is required for antibody-mediated protective immunity against blood-stage Plasmodium chabaudi AS malaria infection in mice. J. Immunol. 168, 1348–1355 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Marinho CR et al. Recrudescent Plasmodium berghei from pregnant mice displays enhanced binding to the placenta and induces protection in multigravida. PLoS ONE 4, e5630 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Megnekou R, Staalsoe T & Hviid L Cytokine response to pregnancy-associated recrudescence of Plasmodium berghei infection in mice with pre-existing immunity to malaria. Malar. J. 12, 387 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mineo S, Niikura M, Inoue S, Kuroda M & Kobayashi F Development of severe pathology in immunized pregnant mice challenged with lethal malaria parasites. Infect. Immun. 81, 3865–3871 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conroy AL et al. Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell Host Microbe 13, 215–226 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Umbers AJ, Aitken EH & Rogerson SJ Malaria in pregnancy: small babies, big problem. Trends Parasitol. 27, 168–175 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Silver KL, Zhong K, Leke RG, Taylor DW & Kain KC Dysregulation of angiopoietins is associated with placental malaria and low birth weight. PLoS ONE 5, e9481 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorman EK et al. Impaired uteroplacental blood flow in pregnancies complicated by falciparum malaria. Ultrasound Obstet. Gynecol. 19, 165–170 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Poovassery J & Moore JM Murine malaria infection induces fetal loss associated with accumulation of Plasmodium chabaudi AS-infected erythrocytes in the placenta. Infect. Immun. 74, 2839–2848 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin-Menendez A et al. Rosetting in Plasmodium vivax: a cytoadhesion phenotype associated with anaemia. PLoS Negl. Trop. Dis. 7, e2155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muthusamy A et al. Chondroitin sulfate proteoglycan but not hyaluronic acid is the receptor for the adherence of Plasmodium falciparum-infected erythrocytes in human placenta, and infected red blood cell adherence up-regulates the receptor expression. Am. J. Pathol. 170, 1989–2000 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Moraes LV, Tadokoro CE, Gomez-Conde I, Olivieri DN & Penha-Goncalves C Intravital placenta imaging reveals microcirculatory dynamics impact on sequestration and phagocytosis of Plasmodium-infected erythrocytes. PLoS Pathog. 9, e1003154 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Moraes LV et al. Murine Model for Preclinical Studies of Var2CSA-Mediated Pathology Associated with Malaria in Pregnancy. Infect. Immun. 84, 1761–1774 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hviid L, Marinho CR, Staalsoe T & Penha-Goncalves C Of mice and women: rodent models of placental malaria. Trends Parasitol. 26, 412–419 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Al Hammadi A, Mitchell M, Abraham GM & Wang JP Recrudescence of plasmodium falciparum in a primigravida after nearly 3 years of Latency. Am. J. Trop. Med. Hyg. 96, 642–644 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rijken MJ et al. Chloroquine resistant vivax malaria in a pregnant woman on the western border of Thailand. Malar. J. 10, 113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Zon AA & Eling WM Depressed malarial immunity in pregnant mice. Infect. Immun. 28, 630–632 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLean SA, Pearson CD & Phillips RS Antigenic variation in plasmodium chabaudi: analysis of parent and variant populations by cloning. Parasite Immunol. 8, 415–424 (1986). [DOI] [PubMed] [Google Scholar]
- 60.Dobbs KR & Dent AE Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology 143, 129–138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janssen CS, Phillips RS, Turner CM & Barrett MP Plasmodium interspersed repeats: the major multigene superfamily of malaria parasites. Nucleic Acids Res. 32, 5712–5720 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yam XY et al. Characterization of the plasmodium interspersed repeats (PIR) proteins of plasmodium chabaudi indicates functional diversity. Sci. Rep. 6, 23449 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogerson SJ, Beeson JG, Mhango CG, Dzinjalamala FK & Molyneux ME Plasmodium falciparum rosette formation is uncommon in isolates from pregnant women. Infect. Immun. 68, 391–393 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Georgiades P, Ferguson-Smith AC & Burton GJ Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23, 3–19 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Esch E et al. Summary comparison of female reproductive system in human and the cynomolgus monkey (Macaca fascicularis). Toxicol. Pathol. 36, 171S–172S (2008). [Google Scholar]
- 66.Ozwara H et al. Experimental infection of the olive baboon (Paplio anubis) with Plasmodium knowlesi: severe disease accompanied by cerebral involvement. Am. J. Trop. Med. Hyg. 69, 188–194 (2003). [PubMed] [Google Scholar]
- 67.Saxena N, Mohan A, Upadhyay SN & Maitra SC Rhesus monkey placenta: a light and electron microscopic study. Lab. Anim. India. 1, 11–23 (1992). [Google Scholar]
- 68.Seeley R, Stephens T & Tate P Development, growth, aging, and genetics: anatomy and physiology, New York, McGraw Hill, 2000. [Google Scholar]
- 69.Davison BB et al. Placental changes associated with fetal outcome in the Plasmodium coatneyi/rhesus monkey model of malaria in pregnancy. Am. J. Trop. Med. Hyg. 63, 158–173 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Davison BB et al. Plasmodium coatneyi in the rhesus monkey (Macaca mulatta) as a model of malaria in pregnancy. Am. J. Trop. Med. Hyg. 59, 189–201 (1998). [DOI] [PubMed] [Google Scholar]
- 71.Barasa M, Gicheru M, Kagasi A & Ozwara S Characterisation of placental malaria in olive baboons (Papio anubis) infected with Plasmodium knowlesi H strain. Int J Integ Biol 9, 54–58 (2010). [Google Scholar]
- 72.Barasa M et al. Cytokine expression in malaria-infected non-human primate placentas. Open Vet J 2, 58–64 (2012). [PMC free article] [PubMed] [Google Scholar]
- 73.Aikawa M, Rabbege JR, Udeinya I & Miller LH Electron microscopy of knobs in Plasmodium falciparum-infected erythrocytes. J. Parasitol. 69, 435–437 (1983). [PubMed] [Google Scholar]
- 74.Kawai S, Aikawa M & Suzuki M A nonhuman primate model for severe human malaria: Plasmodium coatneyi-infected Japanese macaque (Macaca fuscata). Tokai J. Exp. Clin. Med. 23, 101–102 (1998). [Google Scholar]
- 75.Kilejian A, Abati A & Trager W Plasmodium falciparum and Plasmodium coatneyi: immunogenicity of “knob-like protrusions” on infected erythrocyte membranes. Exp. Parasitol. 42, 157–164 (1977). [DOI] [PubMed] [Google Scholar]
- 76.Tegoshi T et al. Ultrastructure of rosette formation by Plasmodium coatneyi-infected erythrocytes of rhesus. Parasitol. Res. 79, 611–613 (1993). [DOI] [PubMed] [Google Scholar]
- 77.Desowitz RS, Miller LH, Buchanan RD & Permpanich B The sites of deep vascular schizogony in Plasmodium coatneyi malaria. Trans. R. Soc. Trop. Med. Hyg. 63, 198–202 (1969). [DOI] [PubMed] [Google Scholar]
- 78.Desowitz RS, Miller LH, Buchanan RD, Yuthasastrkosol V & Permpanich B Comparative studies on the pathology and host physiology of malarias. I. Plasmodium coatneyi. Ann. Trop. Med. Parasitol. 61, 365–374 (1967). [DOI] [PubMed] [Google Scholar]
- 79.Sein KK et al. Sequestration pattern of parasitized erythrocytes in cerebrum, mid-brain, and cerebellum of Plasmodium coatneyi-infected rhesus monkeys (Macaca mulatta). Am. J. Trop. Med. Hyg. 49, 513–519 (1993). [DOI] [PubMed] [Google Scholar]
- 80.Smith CD, Brown AE, Nakazawa S, Fujioka H & Aikawa M Multi-organ erythrocyte sequestration and ligand expression in rhesus monkeys infected with Plasmodium coatneyi malaria. Am. J. Trop. Med. Hyg. 55, 379–383 (1996). [DOI] [PubMed] [Google Scholar]
- 81.Walter PR, Garin Y & Blot P Placental pathologic changes in malaria. A histologic and ultrastructural study. Am. J. Pathol. 109, 330–342 (1982). [PMC free article] [PubMed] [Google Scholar]
- 82.Yamada M et al. Plasmodium falciparum associated placental pathology: a light and electron microscopic and immunohistologic study. Am. J. Trop. Med. Hyg. 41, 161–168 (1989). [DOI] [PubMed] [Google Scholar]
- 83.Kamboj K & Dutta GP Severity of blood-induced Plasmodium cynomolgi B and Plasmodium cynomolgi cynomolgi infection in pregnant rhesus monkeys. Indian J. Malariol. 20, 1–5 (1983). [Google Scholar]
- 84.Saxena N & Murthy PS Hydrolytic enzyme activity in rhesus monkey placenta during early gestational malaria: histochemical studies. J. Vector Borne Dis. 42, 135–140 (2005). [PubMed] [Google Scholar]
- 85.Saxena N & Murthy PS Oxidoreductases in early gestational monkey placenta during maternal malarial infection: histochemical localisation. J. Vector Borne Dis. 44, 116–121 (2007). [PubMed] [Google Scholar]
- 86.Saxena N, Pandey VC, Saxena PN & Upadhyay S Hydrolytic enzymes of rhesus placenta during Plasmodium cynomolgi infection: ultrastructural and biochemical studies. Indian J. Exp. Biol. 31, 54–56 (1993). [PubMed] [Google Scholar]
- 87.Saxena N, Upadhyay SN, Dutta GP, Kazim M & Maitra SC Scanning electron microscopic studies on rhesus monkey placenta during early gestational malarial infection. Indian J. Exp. Biol. 26, 712–714 (1988). [PubMed] [Google Scholar]
- 88.Wislocki G & Padykula H Sex and internal secretions, v II, Baltimore: William and Wilkins; (1961). [Google Scholar]
- 89.Coatney GR The simian malarias: zoonoses, anthroponoses, or both? Am. J. Trop. Med. Hyg. 20, 795–803 (1971). [DOI] [PubMed] [Google Scholar]
- 90.Garnham P Malaria parasites and other haemosporidia, Oxford: Blackwell Scientific Publications, 1966. [Google Scholar]
- 91.Escalante AA, Barrio E & Ayala FJ Evolutionary origin of human and primate malarias: evidence from the circumsporozoite protein gene. Mol. Biol. Evol. 12, 616–626 (1995). [DOI] [PubMed] [Google Scholar]
- 92.Miller LH, Fremount HN & Luse SA Deep vascular schizogony of Plasmodium knowlesi in Macaca mulatta. Distribution in organs and ultrastructure of parasitized red cells. Am. J. Trop. Med. Hyg. 20, 816–824 (1971). [DOI] [PubMed] [Google Scholar]
- 93.Das Gupta B Malarial infection in the placenta and transmission to the foetus. Ind. Med. Gaz. 74, 397–399 (1939). [PMC free article] [PubMed] [Google Scholar]
- 94.Onditi FI et al. Parasite accumulation in placenta of non-immune baboons during Plasmodium knowlesi infection. Malar. J. 14, 118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.al-Khedery B, Barnwell JW & Galinski MR Antigenic variation in malaria: a 3’ genomic alteration associated with the expression of a P. knowlesi variant antigen. Mol. Cell 3, 131–141 (1999). [DOI] [PubMed] [Google Scholar]
- 96.Galinski MR et al. Plasmodium knowlesi: a superb in vivo nonhuman primate model of antigenic variation in malaria. Parasitology 17, 1–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Korir CC & Galinski MR Proteomic studies of Plasmodium knowlesi SICA variant antigens demonstrate their relationship with P. falciparum EMP1. Infect. Genet. Evol. 6, 75–79 (2006). [DOI] [PubMed] [Google Scholar]
- 98.Nyamagiri JO, Onditi FI, Ochola L, Waihenya R & Ozwara HS Plasmodium knowlesi Ligand-receptor Process in Baboon (Papio anubis) Placenta. Journal of Biol. Agric. Healthc. 4, 2225–093X (2014). [Google Scholar]
- 99.Fried M, Muga RO, Misore AO & Duffy PE Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J. Immunol. 160, 2523–2530 (1998). [PubMed] [Google Scholar]
- 100.Chien JT et al. High-Quality Genome Assembly and Annotation for Plasmodium coatneyi, Generated Using Single-Molecule Real-Time PacBio Technology. Genome Announc. 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fatih FA et al. Cytoadherence and virulence - the case of Plasmodium knowlesi malaria. Malar. J. 11, 33 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brabin BJ et al. The sick placenta-the role of malaria. Placenta 25, 359–378 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Dellicour S, Tatem AJ, Guerra CA, Snow RW & ter Kuile FO Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 7, e1000221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmed R et al. Placental infections with histologically confirmed Plasmodium falciparum are associated with adverse birth outcomes in India: a cross-sectional study. Malar. J. 13, 232 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saxena N & Saxena P Placental pathology in rhesus monkey during malarial infection: histological studies. Indian J. Anim. Sci. 63, 1227–1232 (1993). [Google Scholar]
- 106.Chene A et al. Placental cytokine and chemokine profiles reflect pregnancy outcomes in women exposed to Plasmodium falciparum infection. Infect. Immun. 82, 3783–3789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Agudelo OM et al. Submicroscopic infection of placenta by Plasmodium produces Th1/Th2 cytokine imbalance, inflammation and hypoxia in women from north-west Colombia. Malar. J. 13, 122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Souza RM et al. Placental histopathological changes associated with Plasmodium vivax infection during pregnancy. PLoS Negl. Trop. Dis. 7, e2071 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chandrasiri UP et al. Insight into the pathogenesis of fetal growth restriction in placental malaria: decreased placental glucose transporter isoform 1 expression. J. Infect. Dis. 209, 1663–1667 (2014). [DOI] [PubMed] [Google Scholar]
- 110.Umbers AJ et al. Placental malaria-associated inflammation disturbs the insulin-like growth factor axis of fetal growth regulation. J. Infect. Dis. 203, 561–569 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brutus L, Santalla J, Schneider D, Avila JC & Deloron P Plasmodium vivax malaria during pregnancy, Bolivia. Emerg. Infect. Dis. 19, 1605–1611 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


