Abstract
The chikungunya virus (CHIKV) infection epidemic has emerged as a significant public health concern in the last 10–15 years, especially in Asian and south American countries. However, with ever-expanding tourism and migration, cases have now been reported in north America and Europe. CHIKV infection predominantly causes musculoskeletal symptoms with a chronic polyarthritis which may resemble autoimmune inflammatory arthritis. CHIKV infection should always be suspected in a returning traveller presenting with fever, skin rash and arthralgia. Though first reported in the last century, a series of epidemics since 2004 have substantially improved our knowledge. There has also been a significant increase in our understanding of the immunopathogenesis of chikungunya infection. This knowledge is being used in the development of new treatment strategies and preventive measures. In this narrative review, we discuss some of the recent advances in the epidemiology, immunopathogenesis, and management of CHIKV arthritis.
KEYWORDS: Chikungunya infection, chronic inflammatory rheumatism, chronic arthritis, methotrexate, DMARDs, viral arthritis
Introduction
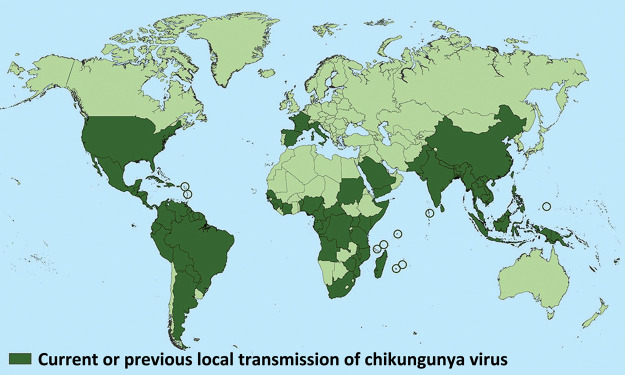
Chikungunya virus (CHIKV), an alphavirus of the Togaviridae family is now an established arboviral infection in the tropical areas of the world. With ever-increasing tourism and migration of populations between continents, CHIKV infection has now been identified in over 60 countries in Asia, Africa, Europe and the Americas.1 The global spread of CHIKV infection is shown in Fig 1. In a returning traveller presenting with fever, skin rash and arthralgia, CHIKV infection is emerging as an important differential diagnosis along with other arboviral infections such as dengue and Zika.2,3 This vector born disease is transmitted to humans by the bite of infected female Aedes aegypti (predominantly in tropical countries) or Aedes albopictus (predominantly in temperate countries) mosquitoes. Other species of mosquitoes, like Culex and Anopheles stephensi, have been shown experimentally to support transmission of CHIKV.4,5 Chikungunya virus was first isolated in 1953 in the Newala district of Tanzania.6 Since then, major outbreaks have been documented across the world and the global CHIKV epidemic from 2004–2011 affected around 6.5 million individuals.4 Increasing tourism, infection in naive populations and viral mutations, have been postulated as reasons behind its recent extensive spread.5 The recent epidemiological studies have shown that CHIKV infection is a multisystem disorder with predominant involvement of musculoskeletal, neurological and gastrointestinal system.8–11 In this narrative review, we present an up to date appraisal of the general aspects of CHIKV infection with a focus on the different treatment strategies in acute and chronic phase of chikungunya arthritis.
Fig 1.
Countries with the presence of chikungunya infection. Does not include countries or territories where only imported cases have been documented. Reproduced with permission from Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-Borne Diseases (DVBD). Geographic distribution: where has chikungunya virus been found? Atlanta: CDC, 2018. www.cdc.gov/chikungunya/geo/index.html [Accessed on 15 January 2019].
Clinical manifestations
Chikungunya infection may be divided into an acute phase (<3 months) and chronic phase (>3 months). The acute phase may be further subdivided into viraemic (5–10 days) and sub-acute post-viraemic (6–21 days) phases.12 The viraemic phase is characterised by sudden onset of high-grade fever (often >39°C), severe polyarthralgia/polyarthritis, myalgias, conjunctivitis and exanthema. The exanthema can present as diffuse or focal skin rash. Many patients also develop pruritus, vesicles, purpura and skin hyperesthesia. In the sub-acute phase, fever settles but articular symptoms and fatigue persist. Polyarthritis is usually symmetrical and involves small and large joints. Tenosynovitis and bursitis can also occur which are usually very painful.13,14 Patients who have concomitant co-morbidities such as osteoarthritis, heart failure, respiratory disease, renal disease and diabetes are likely to have more severe manifestations of acute infection.8 Acute CHIKV infection may also exacerbate underlying rheumatic diseases with studies showing CHIKV infection causing relapses of autoimmune arthritis in patients who were in remission prior to infection.9
The reported prevalence of CHIKV arthritis patients progressing to a chronic stage (>3 months) ranges from 4.1–78.6%.10,19 The reasons for this significant variability are not clear from available epidemiological studies. The prevalence of chronic joint pain in a cohort study from Latin America is reported to be approximately 25% after a median follow-up of 20 months.11 The prevalence of chronic arthritis after acute CHIKV infection has been reported at approximately 14%.10 Factors such as an age of more than 45 years, high viral load (>109/mL) during the acute phase and severe immunologic response in post-viraemic phase are predictors of development of chronic symptoms.14,19
The chronic phase of the disease may follow a pattern similar to rheumatoid arthritis, peripheral spondyloarthritis, undifferentiated arthritis and fibromyalgia. The ankles, knees, hips, wrists, elbows and metacarpo-phalangeal joints are mainly involved. A recent epidemiologic study from Colombia has found that about 25% of patients remained symptomatic with joint pain after 20 months follow-up.10 Similar findings were reported in a meta-analysis of studies of post-CHIKV infection; chronic inflammatory rheumatism (CIR) was present in 25% of cases and chronic arthritis in 14% of cases.11 In a study from the island of Martinique in the Caribbean, 128 patients (all had positive serologic findings and medical histories consistent with diagnosis of CHIKV arthritis); about 21% of patients progressed to seronegative rheumatoid arthritis in 1 year. In the same study, 37% of patients had flare of underlying degenerative arthritis, 35% had relapse of previous clinically inactive spondyloarthritis and 7% patients had fibromyalgia.9
About 20% of patients with chronic CHIKV infection may have chronic pain with neuropathic features including paraesthesia, tingling, numbness and itching.16 A study of pregnant women conducted on the island of Réunion during 2005–2006 epidemic showed that the mother to child (vertical) transmission rate was 50% when CHIKV infection occurred in the intra-partum (2 days either side of delivery) period. Delivery by caesarean section did not prevent the transmission of virus, the babies were born healthy, however they developed fever, weakness and thrombocytopenia between 3–7 days.17
Immunological response
In the acute phase of CHIKV infection, intense viremia is associated with activation of host type I interferon and interleukin (IL)-6. Other pro-inflammatory cytokines, chemokines, and soluble factors (IL-4, IL-7, macrophage migration inhibition factor (MIF), CCL2, CCL4, CXCL10 etc) are also activated. This strong immune response leads to the clearing of the virus by macrophages, cluster of differentiation (CD)8+ T and natural killer (NK) cells within 7–10 days of acute infection and virus levels become undetectable.7,14 For this reason, after first week of infection, diagnosis using CHIKV polymerase chain reaction (PCR) is discouraged. Immunological studies have shown decreased viral clearance due to immune dysregulation in the elderly and in patients with co-morbidities such as type 2 diabetes, chronic kidney disease and chronic heart diseases, explaining the occurrence of more intense infection in these patients.18
Chronic infection is associated with high levels of monocyte chemoattractant protein (MCP-1), macrophage inflammatory protein (MIP-1), IL-6 and IL-12 with CD4+ T cells playing a major role in ongoing inflammatory arthritis.7,14 Persistence of CHIKV ribonucleic acid in perivascular synovial macrophages has been linked to high immunoglobulin M (IgM) response and chronic arthritis symptoms.14,18 However, the exact immunological basis for ongoing persistent inflammation causing musculoskeletal symptoms is still not clear.
Diagnosis
The diagnosis of CHIKV infection should be strongly suspected in endemic areas or travellers from affected regions where patients report new onset debilitating polyarthralgia with high-grade fever. Other causes of febrile illness such as dengue and Zika virus infection, malaria and typhoid fever should also be considered depending on the disease prevalence in the visited country.2,3 Blood investigations in the initial stages can show transient anaemia, leukopenia, lymphopenia, mild thrombocytopenia, raised liver enzymes and serum creatinine levels.15,34 Chikungunya virus can be detected by the reverse transcription (RT) – PCR method from 0 to 7 days of infection, after which PCR detection becomes unreliable.12 IgM becomes detectable in 5–10 days after the onset of infection; positivity is at a maximum after 3 weeks and it remains positive up to 2–3 months. Immunoglobulin G (IgG) becomes positive after first week of infection remaining positive for years.13,14 As a general rule, serological markers (IgM and IgG) should not be checked in first week of infection. In chronic arthritis (>3 months) post CHIKV infection, checking serology for autoimmune arthritis such as rheumatoid factor (RF), anti-citrullinated peptide antibody (anti-CCP) and human leukocyte antigen (HLA) B27 is advised to ascertain the possibilities of coexisting rheumatoid arthritis and spondyloarthritis (Table 1).24,31
Table 1.
Diagnosis of chikungunya infection and arthritis
| Phase | Modality |
|---|---|
| Acute phase (<3 months) | Viral PCR (0–5 days) |
| IgM CHIKV (day 7 till 2–3 months) | |
| IgG CHIKV (day 7 onward) | |
| Chronic phase (>3 months) | IgG CHIKV |
| RF, anti CCP, HLA B27 (if indicated) |
Anti-CCP = anti-citrullinated peptide antibody; CHIKV = chikungunya virus; HLA = human leukocyte antigen; IgG = immunoglobulin G; IgM = immunoglobulin M; PCR = polymerase chain reaction; RF = rheumatoid factor.
Management of chikungunya arthritis
Management of CHIKV arthritis has evolved during epidemics in Asia, Indian Ocean islands, French overseas territories and the Americas. It is to be noted that high quality randomized control trials are lacking, and current guidelines are based on observational and prospective epidemiological studies from endemic regions.19 There are several recent publications addressing the management of CHIKV arthritis.19–24,32 The management is usually divided in to two groups; acute (<3 months) and chronic (>3 months). French and Brazilian guidelines have further subdivided acute phase in to acute phase (1–3 weeks) and sub-acute (4–12 weeks).24,32 This division into stages is to guide treatment according to immunological response and clinical manifestation of CHIKV infection.19 At present no specific antiviral therapy is available for CHIKV infection. Prophylactic antibiotics administration is discouraged in acute cases.
The therapeutic options for acute and chronic CHIKV musculoskeletal manifestations including arthritis follow.
Management of musculoskeletal manifestations in acute phase (<3 months)
Most CHIKV infections are self-limiting and patients improve with supportive care with hydration and analgesia. Paracetamol remains the preferred initial choice for fever and musculoskeletal pain symptoms. World Health Organization (WHO) guidelines published in 2009 for CHIKV infection recommends home treatment for non-complicated acute cases.20 This constitutes adequate rest, hydration, paracetamol (up to 4 g maximum in divided dosages) and avoidance of aspirin. All major published guidelines advise avoiding non-steroidal anti-inflammatory drugs (NSAIDs) until dengue is ruled out, as dengue may be complicated by haemorrhage.24,32 Ibuprofen, diclofenac, naproxen or aceclofenac are the NSAIDs which can be taken along with paracetamol for intractable symptoms once dengue infection has been ruled out. Analgesia may be required for a few weeks until pain resolves. French and Brazilian guidelines advice against use of corticosteroids in acute phase (<3 weeks). In the sub-acute phase, for intractable synovitis and tenosynovitis, low dose oral prednisolone can be used, at a dose of 10 mg daily for 5 days and further tapering over the next 10 days. The maximum dose of steroids recommended is 0.5 mg/kg and steroids are not advised for more than 4 weeks.24 Adding low dose oral steroids to NSAIDs has been shown to aid resolution of symptoms.25 Weak opioids like codeine and tramadol can be added to the previous regimen. For peripheral neuropathy symptoms pregabalin or amitriptyline should be used.24,32
Studies on the use of chloroquine phosphate (CQ) and hydroxychloroquine (HCQ) in the acute phase have shown inconsistent effects, though some benefit cannot be ruled out.26,27 Brazilian guidelines advice use of HCQ for persistent symptoms (>3–4 weeks) in the dosages of 5 mg/kg/day.32 WHO guidelines also support using HCQ 200 mg once daily or CQ 300 mg daily for 4 weeks for resistant musculoskeletal symptoms.20 Table 2 gives a summary of current treatment options for acute CHIKV arthritis.
Table 2.
Management of acute chikungunya arthritis (<3 months)
| Treatment | Comments |
|---|---|
| Supportive | Rest, hydration |
| Paracetamol | up to 4 g/day maximum in divided dosages |
| Weak opioids | Oral codeine 30 mg two to three times daily, oral tramadol 50 mg two to three times daily |
| NSAIDs (after ruling out other arboviral infections like dengue infection) | Ibuprofen 400 mg three times daily, diclofenac 50 mg twice daily, naproxen 250 mg twice daily, aceclofenac 200 mg daily |
| Oral prednisolone and intra-articular steroids | 5–10 mg/day; in sub-acute cases post 3 weeks, with confirmed synovitis and tenosynovitis, to taper in 1–2 weeks, maximum for 4 weeks |
| Tricyclic antidepressants and anticonvulsants | Oral amitriptyline 25–50 mg/day, oral pregabalin 50–150 mg two to four times a day and oral gabapentin 300 mg two to three times daily |
NSAIDs = non-steroidal anti-inflammatory drugs.
Management of musculoskeletal manifestations in chronic phase (>3 months)
Disease-modifying anti-rheumatic drugs (DMARDs) such as methotrexate (MTX), sulfasalazine (SSZ) and HCQ have shown benefits in the chronic phase of CHIKV arthritis with ongoing synovitis or tenosynovitis.22,23,25–27 In a study from Réunion island, in patients with post CHIKV-CIR with median follow-up of 21 months, MTX with mean dose of 15 mg weekly led to clinical improvement in 75% (54/72) and 8% (6/72) achieved partial recovery while 9% (7/72) patients had radiographic worsening of joints and the remaining patients had to stop MTX because of side effects.23 Patients with RF seropositivity had better response to MTX. An observational 16-week follow-up study from India showed the efficacy of combination therapy with MTX and HCQ in 49% of the patients with chronic CHIKV arthritis.27 In an another small study, addition of MTX to patients with poor response to combination therapy with HCQ and SSZ at the end of 3 months of treatment lead to improvement in 93% of cases in next 2 years of follow-up with only 7% patients being still symptomatic.27 However, in this study, 69% (11/16) of studied population was seropositive (RF or anti-CCP) which might explain the high response rate of MTX. A recent, open-label 24 week study randomised patients who had persistent arthritis (>1 year) and were taking HCQ in to two groups; one group received fixed-dose combination (methotrexate 15 mg/week, sulfasalazine 1 g daily and HCQ 400 mg daily) and another group continued to take HCQ (with dose optimised to 400 mg daily).29 Both groups also took oral prednisolone for 6 weeks. At 24 weeks, the combination therapy group showed significant improvement in both disease activity and disability. At the study end, pain visual analogue score (VAS) was also significantly less in the combination therapy group. The French guidelines advice use of DMARDs (methotrexate, leflunomide, sulfasalazine) according to the clinical stage of CIR. Brazilian guidelines recommend use of MTX along with HCQ and low dose oral steroids for 6–8 weeks. Guidelines also advise on usage of biologic agents such as tumour necrosis factor-α inhibitors (TNFi) in resistant cases, after fulfilling the local criteria for their usage.24,32 Table 3 gives a summary of current treatment options for chronic CHIKV arthritis.
Table 3.
Management of chronic chikungunya arthritis (>3 months)
| Treatment | Comments |
|---|---|
| NSAIDs, paracetamol and weak opioids | Ibuprofen 400 mg three times daily, diclofenac 50 mg twice daily, naproxen 250 mg twice daily, aceclofenac 200 mg daily |
| Oral prednisolone | 5–10 mg/day; in confirmed synovitis and tenosynovitis, to taper in 1–2 weeks, maximum for 4 weeks (French guidelines) and 6–8 weeks (Brazilian guidelines) |
| Hydroxychloroquine | 5 mg/kg/day, maximum 400 mg daily |
| Methotrexate | 10–25 mg/week with folic acid 1 mg/day |
| Sulfasalazine | 1–2 g/day |
| Biologic therapy | TNFi (in resistant cases as per local guidelines) |
| Tricyclic antidepressants and anticonvulsants | Oral amitriptyline 25–50 mg/day, oral pregabalin 50–150 mg two to four times a day and oral gabapentin 300 mg two to three times daily |
NSAIDs = non-steroidal anti-inflammatory drugs; TNFi = tumor necrosis factor inhibitor.
Newer therapies and vaccines
Various in vitro studies have shown the effects of antivirals on CHIKV replication. Favipiravir, ribavirin with a combination of interferon α, umifenovir (antiviral) and suramin (antiprotozoal) are drugs which have shown some beneficial effects.9 A combination therapy of abatacept (selective T-cell co-stimulation blocker) and 4N12 monoclonal neutralising antibody, administered in mouse models has shown a significant decrease in periarticular swelling with decreasing proinflammatory cytokines. Animal studies have also shown that monoclonal antibodies given in acute CHIKV infection can reduced viraemia, and have therapeutic as well as prophylactic effects.14,30
Recombinant measles and chikungunya virus (MV-CHIK) vaccine and virus-like particles (VLP) vaccine are two of the many vaccines which are currently been investigated for CHIKV infections, which have shown promising results in inducing immunologic response and are at different stages of development.30 Recently published results of a phase 2 trial of MV-CHIK vaccine showed good immunogenicity, safety and tolerability profile and hopefully will emerge as a viable vaccine for clinical use.33
Conclusions
In summary, CHIKV infection may result in significant acute and chronic musculoskeletal co-morbidities which cause personal, social and economic distress with loss of productive working hours. It has worse manifestations in the more vulnerable subgroups of the population comprising children, elderly and patients with chronic hypertension, renal diseases, diabetes, heart diseases and previous rheumatological disorders. Post chikungunya arthritis may persist for months to years, contributing to the disease burden in the society. Available research has shown considerable benefits with DMARDs in chronic CHIKV arthritis patients. In the absence of high quality randomised controlled trials, at present there are no universal agreed management recommendations or validated management protocols for treatment of acute and chronic post-CHIKV arthritis. Although CHIKV infection has become a major public health issue in tropical countries; ever-increasing tourism and intercontinental immigration have made CHIKV infection a very real threat to temperate countries also. There is an urgent need to formulate universal guidelines for diagnosis and management of CHIKV infection, from concerned stakeholders including clinicians, patient representatives and national societies.
Key practice suggestions.
Chikungunya virus (CHIKV) infection and other arboviral infections should be considered in returning travellers from endemic regions who present with fever, skin rash and arthralgia.
For the diagnosis of acute CHIKV infection viral reverse transcription-polymerase chain reaction should be used in the first 5–7 days, after which test becomes unreliable. After the first week CHIKV immunoglobulin M should be used to confirm the diagnosis.
Management of CHIKV infection in acute phase is supportive. Haemorrhagic virus/dengue should be excluded before prescribing NSAIDs.
In patients having persistent arthritis for more than 3 months, other rheumatological conditions must be considered, such as rheumatoid arthritis and spondyloarthritis.
The severity of acute illness, high viral titres during acute infection and age >45 years are predictors of progression to chronic CHIKV arthritis.
References
- 1.World Health Organization . Chikungunya. Geneva: WHO. www.who.int/news-room/fact-sheets/detail/chikungunya [Accessed 10 April 2019]. [Google Scholar]
- 2.Eckerle I, Briciu VT, Ergönül O, et al. Emerging souvenirs—clinical presentation of the returning traveller with imported arbovirus infections in Europe. Clinical Microbiol Infect 2018;24:240–5. [DOI] [PubMed] [Google Scholar]
- 3.Vasquez V, Haddad E, Perignon A, et al. Dengue, chikungunya, and Zika virus infections imported to Paris between 2009 and 2016: Characteristics and correlation with outbreaks in the French overseas territories of Guadeloupe and Martinique. Int J Infect Dis 2018;72:34–9. [DOI] [PubMed] [Google Scholar]
- 4.Yadav P, Gokhale MD, Barde PV, et al. Experimental transmission of Chikungunya Virus by Anopheles Stephensi mosquitoes. Acta virologica 2003;47:45–7. [PubMed] [Google Scholar]
- 5.Pialoux G, Gaüzère BA, Jauréguiberry S, et al. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 2007;7:319–27. [DOI] [PubMed] [Google Scholar]
- 6.Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond) 1956;54:177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma SK, Jain S. Chikungunya: A rheumatologist’s perspective. Int J Rheum Dis 2018;21:584–601. [DOI] [PubMed] [Google Scholar]
- 8.Staikowsky F, Talarmin F, Grivard P, et al. Prospective study of chikungunya virus acute infection in the island of la Réunion during the 2005–2006 outbreak. PLoS ONE 2009;4:e7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blettery M, Brunier L, Polomat K, et al. Brief report: management of chronic post-chikungunya rheumatic disease: the Martinican experience. Arthritis Rheumatol 2016;68:2817–24. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzón S, et al. Prevalence of post-chikungunya infection chronic inflammatory arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2016;68:1849–58. [DOI] [PubMed] [Google Scholar]
- 11.Chang AY, Encinales L, Porras A, et al. Frequency of chronic joint pain following chikungunya virus infection: a Colombian cohort study. Arthritis Rheumatol 2018;70:578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews AJ, Ravindran V. Infections and arthritis. Best Pract Res Clin Rheumatol 2014;28:935–59. [DOI] [PubMed] [Google Scholar]
- 13.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015;372:1231–9. [DOI] [PubMed] [Google Scholar]
- 14.Zaid A, Gérardin P, Taylor A, et al. Review: chikungunya arthritis: implications of acute and chronic inflammation mechanisms on disease management. Arthritis Rheumatol 2018;70:484–95. [DOI] [PubMed] [Google Scholar]
- 15.Dorléans F, Hoen B, Najioullah F, et al. Outbreak of chikungunya in the French Caribbean islands of Martinique and Guadeloupe: findings from a hospital-based surveillance system (2013–2015). Am J Trop Med Hyg 2018,98:1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Andrade DC, Jean S, Clavelou P, Dallel R, Bouhassira D. Chronic pain associated with the Chikungunya fever: long lasting burden of an acute illness. BMC Infect Dis 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gérardin P, Barau G, Michault A, et al. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Réunion. PLoS Med 2008;5:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol 2010;184:5914–27. [DOI] [PubMed] [Google Scholar]
- 19.Martí-Carvajal A, Ramon-Pardo P, Javelle E, et al. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: A systematic review. PLoS One 2017;12:e0179028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Regional Office for South-East Asia . Guidelines on clinical management of chikungunya fever. India: WHO, 2008. http://apps.who.int/iris/handle/10665/205178 [Accessed 15 October 2018]. [Google Scholar]
- 21.Erin Staples J, Hills SL, Powers AM, Angelo KM. Chikungunya. In: Centers for Disease Control and Prevention . CDC Yellow Book 2018. Oxford: Oxford University Press, 2018. www.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/chikungunya [Accessed 15 October 2018]. [Google Scholar]
- 22.Amaral JK, Sutaria R, Schoen RT. Treatment of chronic chikungunya arthritis with methotrexate: A systematic review. Arthritis Care Res (Hoboken) 2018;70:1501–8. [DOI] [PubMed] [Google Scholar]
- 23.Javelle E, Ribera A, Degasne I, et al. Specific management of post-chikungunya rheumatic disorders: a retrospective study of 159 cases in Reunion Island from 2006-2012. PLoS Negl Trop Dis 2015;9:e0003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon E, Javelle A, Cabie E, et al. French guidelines for the management of chikungunya (acute and persistent presentations). Med Mal Infect 2015;45:243–63. [DOI] [PubMed] [Google Scholar]
- 25.Padmakumar B, Jayan JB, Menon RM, et al. Comparative evaluation of four therapeutic regimes in chikungunya arthritis: a prospective randomized parallel-group study. Ind J Rheumatol 2009;4:94–101. [Google Scholar]
- 26.Chopra A, Saluja M, Venugopalan A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol 2014;66:319–26. [DOI] [PubMed] [Google Scholar]
- 27.Pandya S. Methotrexate and hydroxychloroquine combination therapy in chronic chikungunya arthritis: a 16-week study. Ind J Rheumatol 2008;3:93–7. [Google Scholar]
- 28.Ganu MA, Ganu AS. Post-chikungunya chronic arthritis: Our experience with DMARDs over two year follow up. J Assoc Physicians India 2011;59:83–6. [PubMed] [Google Scholar]
- 29.Ravindran V, Alias G. Efficacy of combination DMARD therapy vs hydroxychloroquine monotherapy in chronic persistent chikungunya arthritis: a 24week randomized controlled open label study. Clinical Rheumatol 2017;36:1335–40. [DOI] [PubMed] [Google Scholar]
- 30.Tharmarajah K, Mahalingam S, Zaid A. Chikungunya: vaccines and therapeutics. F1000Res 2017;6:2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marques CDL, Duarte ALBP, Ranzolin A, et al. Recommendations of the Brazilian Society of Rheumatology for diagnosis and treatment of chikungunya fever. Part 1 – Diagnosis and special situations. Rev Bras Reumatol Engl Ed 2017;57:421–37. [DOI] [PubMed] [Google Scholar]
- 32.Marques CDL, Duarte ALBP, Ranzolin A, et al. Recommendations of the Brazilian Society of Rheumatology for the diagnosis and treatment of chikungunya fever. Part 2 – Treatment. Rev Bras Reumatol Engl Ed 2017;57:438–51. [DOI] [PubMed] [Google Scholar]
- 33.Reisinger EC, Tschismarov R, Beubler E, et al. Immunogenicity, safety, and tolerability of the measles-vectored chikungunya virus vaccine MV-CHIK: a double-blind, randomised, placebo-controlled and active-controlled phase 2 trial. Lancet 2018;392:2718–27. [DOI] [PubMed] [Google Scholar]
- 34.Simon F, Javelle E, Oliver M, et al. Chikungunya virus infection. Curr Infect Dis Rep 2011;13:218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]