Abstract
Intracellular Ca2+ signals are well organized in all cell types, and trigger a variety of vital physiological processes. The temporal and spatial characteristics of cytosolic Ca2+ increases are mainly governed by the fluxes of this ion across the membrane of the endoplasmic/sarcoplasmic reticulum and the plasma membrane. However, various Ca2+ transporters also allow for Ca2+ exchanges between the cytoplasm and mitochondria. Increases in mitochondrial Ca2+ stimulate the production of ATP, which allows the cells to cope with the increased energy demand created by the stimulus. Less widely appreciated is the fact that Ca2+ handling by mitochondria also shapes cytosolic Ca2+ signals. Indeed, the frequency, amplitude, and duration of cytosolic Ca2+ increases can be altered by modifying the rates of Ca2+ transport into, or from, mitochondria. In this review, we focus on the interplay between mitochondria and Ca2+ signaling, highlighting not only the consequences of cytosolic Ca2+ changes on mitochondrial Ca2+, but also how cytosolic Ca2+ dynamics is controlled by modifications of the Ca2+-handling properties and the metabolism of mitochondria.
Mitochondria are organelles found in nearly all cell types of eukaryotic organisms, with the notable exception of erythrocytes (Repsold and Joubert 2018). They are characterized by two membranes, the inner mitochondrial membrane (IMM) that encloses the mitochondrial matrix and the outer mitochondrial membrane (OMM). These two membranes are a few nanometers apart and delimit the so-called intermembrane space (IMS). The OMM is smooth and highly permeable to most solutes, whereas the IMM is impermeable. Besides, the IMM is folded into cristae, allowing to increase the surface of this membrane (El-Hattab et al. 2018). Mitochondria form unconnected, functionally distinct entities (Collins et al. 2002) and their number varies from tens (in lymphocytes [Potter and Ward 1942]) to thousands (in hepatocytes) per cell (Weibel et al. 1969). They are usually considered as 1-µm wide spherocylinders, varying in length from 1 to 10 µm. These organelles of widely different sizes also display heterogenous cellular distribution, with a denser aggregation of mitochondria around the nucleus than in the cell periphery (Collins et al. 2002). Heterogeneity is further amplified by the dynamic behavior of these organelles. Indeed, mitochondria continuously move along the cytoskeleton. They are able to grow, divide, or fuse with each other (Wang et al. 2012). Moreover, when submitted to stressful conditions, they can connect and form a network that allows a better adaptation to maintain cellular homeostasis (Zemirli et al. 2018).
The roles of mitochondria are numerous and notably include cell cycle regulation, apoptosis, bioenergetics, and also Ca2+ homeostasis (Murphy and Hartley 2018). Indeed, although Ca2+ signaling in nonexcitable cells mainly involves fluxes between the cytosol, the plasma membrane (PM) and the endoplasmic reticulum ([ER], the main Ca2+ storage of the cell), mitochondria, as well as other organelles such as lysosomes, are able to alter cytosolic Ca2+ signals (Dupont et al. 2011; Patel and Muallem 2011). At rest, the Ca2+ concentration inside mitochondria is of the same order as that in the cytosol (∼0.1 µm). The close apposition between mitochondria and ER membranes favors Ca2+ exchanges between these organelles (Rizzuto et al. 1998; Csordás et al. 1999, 2010). Cytosolic Ca2+ increases, mostly as a result of the release of ER Ca2+ through the inositol 1,4,5-trisphosphate receptor (IP3R), can indeed be transmitted into mitochondria via the different transporters located in the mitochondrial membranes. As mitochondria can take up large amounts of cytosolic Ca2+, this sequestration modifies the quantitative and dynamic characteristics of Ca2+ signaling in the cytosol (Nicholls and Chalmers 2004).
Ca2+ is an essential activator of mitochondrial metabolism and stimulates the synthesis of ATP molecules (Fig. 1A). Notably, Ca2+ activates three dehydrogenases involved in the Krebs cycle (Rizzuto et al. 2012). This cycle oxidizes acetyl-coenzyme A, typically issued from pyruvate generated by glycolysis. This oxidation is coupled with the reduction of NAD+ into NADH. NADH molecules in turn transfer their electrons to the electron transport chain (ETC). The four first complexes of the ETC (I–IV) use the energy provided by electrons to export protons from the matrix into the IMS, hence generating an important transmembrane potential difference between the matrix and the IMM (ΔΨ). The resting mitochondrial ΔΨ is around 150–160 mV, negative inside. Complex V, which is the F1F0-ATP synthase, consumes the energy provided by the electrochemical gradient to synthesize ATP (Murphy and Hartley 2018). The ΔΨ is crucial not only for energetic production, but also for Ca2+ fluxes in and out of mitochondria. Indeed, the Ca2+ transporters through the IMM are electrogenic and dependent on the electrochemical gradient of Ca2+ between the cytosol and the mitochondrial matrix. Thus, changes in ΔΨ affect the balance between influx and efflux of mitochondrial Ca2+. Interestingly, this is not the case for transporters in the ER where fluxes of protons and other monovalent cations compensate the potential variations due to Ca2+ transport (Meissner 1981). Perturbations in mitochondrial Ca2+ dynamics can be associated with pathological conditions: disturbances in Ca2+ fluxes, in molecular mitochondrial components, or in the distance between mitochondria and ER can lead to impaired bioenergetics of the cell or cellular death (Contreras et al. 2010; Naon and Scorrano 2014; Mishra 2016; Wang et al. 2016). Ca2+ thus constitutes a signal of life and death that is finely tuned by molecular and geometric parameters (Berridge et al. 1998).
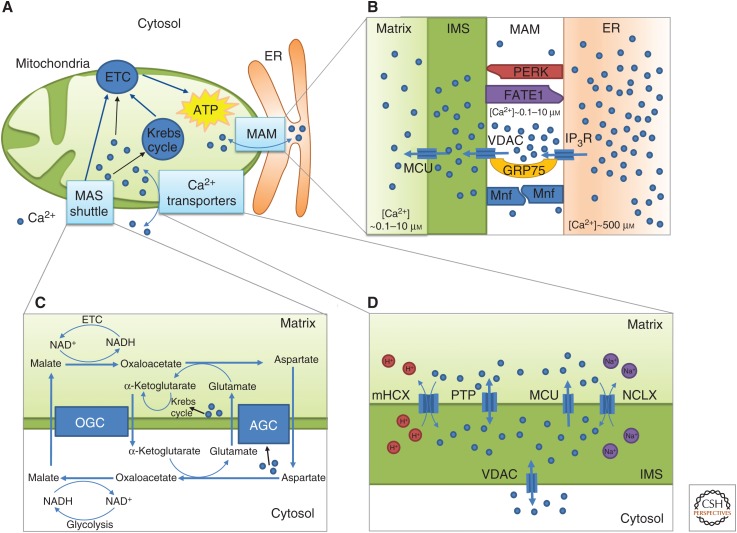
Figure 1.
Schematic representation of some key aspects of mitochondrial Ca2+ dynamics and of the interplay between Ca2+ and mitochondrial metabolism (A). Ca2+ exchanges between the cytosol and mitochondria allow the activation of mitochondrial metabolism, by boosting the Krebs cycle and the electron transport chain (ETC). (B) Membranes of the ER and the mitochondria are linked via tethering proteins (PERK, FATE, GRP75, Mfn, and others not indicated in this simplified scheme) to form mitochondria-associated membranes (MAMs). These membranes delimit areas where Ca2+ can reach high concentrations. (C) The electrons of the NADH produced in the cytosol by glycolysis are transferred into mitochondria by the malate-aspartate shuttle (MAS). The aspartate-glutamate carrier (AGC) of the MAS is activated by cytosolic Ca2+. At high mitochondrial Ca2+ concentration, the activation of the Krebs cycle consumes α-ketoglutarate and limits the impact of the MAS. OGC stands for oxoglutarate carrier (see text for details). (D) Ca2+ has to cross two membranes to enter inside a mitochondrion: the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM). The first one is passed via a voltage-dependent anion-selective channel (VDAC), a nonselective channel. More specific Ca2+ transporters are involved in the crossing of the IMM (see text for details).
In this review, we focus on the interplay between mitochondria and Ca2+ signaling, highlighting not only the consequences of cytosolic Ca2+ changes on mitochondrial Ca2+, but also how cytosolic Ca2+ dynamics can be altered by modifications of the Ca2+-handling properties and the metabolism of mitochondria. We first list the main mitochondrial Ca2+ transporters and report evidence of the impact of their activity on mitochondrial and cytosolic Ca2+ dynamics. We next focus on the mitochondrial permeability transition pore (mPTP) and on its less well-established participation in mitochondrial Ca2+ dynamics. The bidirectional relation between Ca2+ signaling and metabolism, the main physiological target of mitochondrial Ca2+ increases, is then discussed. In the last section, we report observations highlighting the importance of the spatial arrangement of the Ca2+ transporters located in the ER and in the mitochondrial membranes.
MITOCHONDRIAL Ca2+ TRANSPORTERS
On cell stimulation, cytoplasmic Ca2+ increases are transmitted to mitochondria. Ca2+ ions need to cross two membranes to pass from the cytosol into the mitochondrial matrix (Fig. 1D). Ca2+ first passes through the OMM via a highly expressed voltage-dependent anion-selective channel (VDAC). This channel behaves as a diffusion pore that is permeable to ions and small hydrophilic metabolites, thus accounting for the high permeability of the OMM. VDAC exists in three isoforms (1,2,3) and displays multiple conductance states (Shoshan-Barmatz et al. 2018). The so-called “closed” states (i.e., the states with the lower permeability to metabolites) have the highest permeability to Ca2+ (Tan and Colombini 2007). In HeLa cells stimulated by histamine, overexpression of the three VDAC isoforms induces an increase in the mitochondrial Ca2+ levels, whereas silencing their expression tends to decrease mitochondrial Ca2+ uptake (de Stefani et al. 2012). Similarly, in mouse embryonic fibroblasts, the knocking out of VDAC1 limits agonist-induced mitochondrial Ca2+ uptake (Monaco et al. 2015). In heart cells, VDAC2-dependent mitochondrial Ca2+ uptake plays a critical modulatory role in the regulation of cardiac rhythmicity, most probably by controlling the spatial and temporal extent of the Ca2+ increases in the cytosol (Shimizu et al. 2015). However, it is most often assumed that in physiological conditions the Ca2+ transport via VDAC across the OMM is kinetically not limiting.
The transfer of Ca2+ from the IMS into the mitochondrial matrix is much more complex. Various transporters are indeed expressed in the IMM (Fig. 1D). Ca2+ ions enter into mitochondria through a channel known as the mitochondrial Ca2+ uniporter (MCU). The MCU is located in the IMM, is highly selective to Ca2+, and is inhibited by ruthenium red. The opening of the uniporter has a sigmoidal dependence on cytosolic Ca2+, and is characterized by a rather low affinity for Ca2+ (Kd ∼2–10 µm) (Mallilankaraman et al. 2012; Csordás et al. 2013; Paillard et al. 2017). However, the effective dependence of the MCU on Ca2+ is intricate, as when Ca2+ enters into the mitochondrial matrix, it provokes a decrease in ΔΨ that diminishes the driving force for Ca2+ entry. Although the existence of a Ca2+ uniporter was postulated for a few decades thanks to functional studies of mitochondrial Ca2+ uptake, its molecular identity was identified much later as the 40-kDa MCU (Baughman et al. 2011; de Stefani et al. 2011). MCU oligomers form a highly selective Ca2+ pore that is part of a multiprotein complex composed of a large number of regulators, notably the mitochondria Ca2+ uptake proteins 1,2,3 (MICU 1,2,3) and the essential MCU regulator (EMRE). These regulators allow a fine tuning of the opening of the MCU pore (Mammucari et al. 2018; Nemani et al. 2018; Penna et al. 2018) and modulate the sigmoidal dependence on Ca2+ of the MCU (Csordás et al. 2013; Patron et al. 2014). Specific stoichiometries between the MCU and its regulators may be responsible for the observed tissue-specific differences of activities of the uniporter, and explains, for example, the variation in Ca2+ dynamics observed in mitochondria of heart and liver (Paillard et al. 2017; Wacquier et al. 2017). Indeed, as MICU1 is less expressed in cardiomyocytes, the Ca2+ uptake rate is higher at low cytosolic Ca2+ concentrations, but smaller at high cytosolic Ca2+ levels, as compared with hepatocytes that express much more MICU1. This tissue-specific MICU1:MCU expression ratio is highly relevant as heart cells characterized by a liver-like MICU1:MCU expression ratio display contractile dysfunction (Paillard et al. 2017). Interestingly, in modeling approaches, it is possible to provide a unifying description of mitochondrial Ca2+ dynamics in different cell types by taking into account the level of expression of the MCU (which is lower in heart cells [Fieni et al. 2012]) and the MICU1:MCU expression ratio (Wacquier et al. 2017). As a matter of fact, the regulation of the MCU is highly sophisticated and is covered in detail in recent reviews (Giorgi et al. 2018; Nemani et al. 2018; Wang et al. 2018). Although the MCU is the predominant Ca2+-import mechanism, other transport systems have been reported to enable mitochondrial Ca2+ uptake. Such systems include mitochondrial ryanodine receptors in cardiomyocytes and neurons (Jakob et al. 2014), mitochondrial connexin 43 in cardiac cells (Gadicherla et al. 2017), and the rapid mode of Ca2+ uptake (RaM) in cardiomyocytes and hepatocytes (Buntinas et al. 2001). However, the exact roles of these channels remain elusive.
Ca2+ is extruded from mitochondria via exchangers. The IMM has two main types of Ca2+ exchangers: a H+/Ca2+ exchanger (mHCX, expressed mainly in nonexcitable cells) and a Na+/Ca2+ exchanger (NCX, expressed mainly in excitable cells). The mNCX activity is attributed to the protein NCLX (Palty et al. 2010). This transporter is generally thought to import three Na+ in exchange for one Ca2+, although the exact stoichiometry of this transporter is still controversial (Kim et al. 2013). NCLX is electrogenic and its activity depends on ΔΨ. It is noncooperatively activated by mitochondrial Ca2+, whereas it is cooperatively regulated by Na+, with a Hill coefficient of 2 (Wingrove and Gunter 1986). Interestingly, it has been shown that the NCLX displays a reverse transport mode. This happens in depolarized mitochondria, under the control of the protein mitofusin 2. This reverse mode could be relevant in pathological conditions when ΔΨ is transiently or chronically collapsed, as Ca2+ uptake via the MCU is dependent on ΔΨ (Samanta et al. 2018).
The nature of the mHCX is still an ongoing and controversial issue. Some reports suggest that the leucine zipper EF hand-containing transmembrane (LETM1) protein is responsible for this activity. Indeed, in vitro, LETM1 favors an electrogenic exchange of H+ and Ca2+ (Tsai et al. 2014). Additionally, HeLa cells that do not express LETM1 show higher mitochondrial Ca2+ levels, highlighting the importance of LETM1 in Ca2+ efflux (Shao et al. 2016). However, other studies indicate that mitochondrial Ca2+ efflux is mediated by the NCLX and not by LETM1 in HeLa cells. Indeed, in cells stimulated by histamine to generate cytosolic and mitochondrial Ca2+ signals, an overexpression of LETM1 does not change the rate of Ca2+ efflux from mitochondria (de Marchi et al. 2014b). Other groups also suggest that LETM1 is a K+/H+ exchanger. In this case, LETM1 would alter NCLX activity, maybe by changing the osmotic balance (Austin et al. 2017).
Through the action of uniporter and the exchangers, cytosolic Ca2+ signals are transmitted to mitochondria. Ca2+ oscillations can thus be observed in these organelles (Ishii et al. 2006). In some cell types, mitochondria are able to integrate the cytosolic Ca2+ oscillations. For example, in cat ventricular myocytes, the cytosolic oscillations induce a progressive elevation of the mitochondrial Ca2+ level (Sedova et al. 2006). In hepatocytes, the mitochondrial Ca2+ signals themselves are integrated into different levels of activation of metabolism (Hajnóczky et al. 1995). The physiological consequences of these increases in mitochondrial Ca2+ have been much described (Jouaville et al. 1999; Denton 2009; Griffiths and Rutter 2009; Williams et al. 2015). Less emphasis has been put on the fact that mitochondria alter Ca2+ signaling in the cytosol, both by sequestering and releasing Ca2+. Experiments and mathematical models highlight well-defined differences in the phases of the Ca2+ oscillations in different compartments (ER, cytosol, and mitochondria). Interestingly, it appears that between two successive cytosolic spikes of the oscillations, mitochondria are slowly releasing Ca2+ while the ER is still replenishing. It suggests that mitochondria are able to gradually release the Ca2+ accumulated during a single spike, allowing the reactivation of the IP3R, and the triggering of a new spike (Ishii et al. 2006; Wacquier et al. 2016). Thus, Ca2+ release from mitochondria is in part responsible for the pacemaker Ca2+ increase in the cytosol that progressively activates IP3R and stimulates Ca2+ release from the ER, before the onset of each spike of the oscillations. This is supported by data obtained in different cell types when using inhibitors of the MCU or of the NCLX. For example, in HeLa cells stimulated by histamine, siRNA-mediated reduction of MCU expression tends to slow down the cytosolic oscillations (Fig. 2A,B). This is explained by a lower accumulation of Ca2+ in mitochondria, and thus a weaker subsequent reactivation of IP3R (Wacquier et al. 2016). In the same cell type, the addition of CGP37157, an NCLX inhibitor, favors fast oscillations (Hernández-SanMiguel et al. 2006). A more drastic impact of altering MCU expression is observed in mast cells, in which the proinflammatory leukotriene C4 signal induces damped Ca2+ oscillations in the cytosol. If the MCU is knocked down in these cells, the damping is hastened considerably (Samanta et al. 2014). Mitochondria also have a significant impact on the electric activity of heart cells. This activity is attributable to Ca2+ release from the sarcoplasmic reticulum (SR) and induces spontaneous heartbeats. Such a disturbance increases the risk of arrhythmia. In cardiomyocytes, this spontaneous electrical activity is sensitive to perturbations of the mitochondrial Ca2+ fluxes. Inhibition of the MCU by Ru360 indeed decreases the frequency of these beats. In a similar way, inhibiting the NCLX by CGP37157 completely inhibits spontaneous Ca2+ release, and the associated beats (Xie et al. 2018).
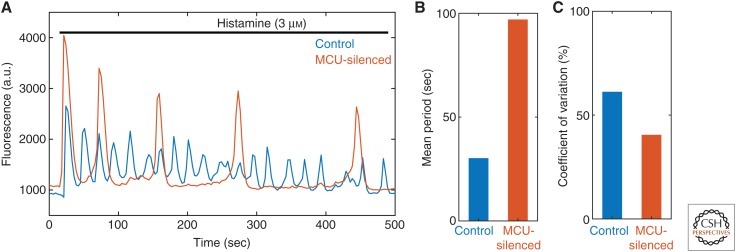
Figure 2.
Impact of the mitochondrial Ca2+ uniporter (MCU)-silencing on cytosolic Ca2+ oscillations. (A) Cytosolic Ca2+-probe fluorescence in HeLa cells stimulated with 3 µm histamine. The Ca2+ oscillations induced by histamine are drastically different in control cells (blue) compared with those in MCU-silenced cells (red), thus showing how Ca2+ handling by mitochondria can affect cytosolic Ca2+ signals. (B) Mean period of the Ca2+ oscillations shown in panel A. In MCU-silenced cells, the period of the cytosolic Ca2+ oscillations is much increased, as compared with control cells. (C) Coefficient of variation (CV) (standard deviation/mean) of the time interval between two successive peaks of these oscillations. The MCU-silenced cells display a lower CV, which is associated with more regular spikes. (The data are taken, with permission, from Wacquier et al. 2016.)
In HeLa cells, individual mitochondria of one single cell display heterogeneities in their Ca2+ profiles following a stimulation by histamine (Suzuki et al. 2014). It remains to be established whether such differences are the result of variations in the levels of expression of Ca2+ transporters, in mitochondrial Ca2+ buffering strengths, or in ER-mitochondria coupling. However, modeling approaches predict that changes in the mitochondrial properties (notably Ca2+ buffering or MCU level) greatly alter the amplitude of mitochondrial Ca2+ responses. In addition to these heterogeneities, mitochondria are likely to be the source of molecular noise because of their small volume. This is visible experimentally and in stochastic models (Lu et al. 2016; Gonze et al. 2018). Fluctuations in mitochondrial Ca2+ concentrations can be transmitted to rates of Ca2+ transport, and thus to cytosolic Ca2+ signals. In agreement with this hypothesis, considering mitochondria in stochastic models for Ca2+ oscillations increases their coefficient of variation ([CV] defined as the ratio between the standard deviation and the mean of the interspikes intervals) of ∼20% (Gonze et al. 2018). MCU-silenced HeLa cells display Ca2+ oscillations characterized by a lower CV than those of a control case (Fig. 2C; Wacquier et al. 2016). This effect is attributable both to an increase of the period, and to stochastic disturbances in Ca2+ exchanges. In other words, the coupling between cytosolic and mitochondrial Ca2+ signaling has a cost in terms of the regularity of the cytosolic Ca2+ spikes.
MITOCHONDRIAL PERMEABILITY TRANSITION PORE
Under some circumstances such as mitochondrial Ca2+ overload or metabolic stress, a sudden increase in the permeability of the IMM can be observed. This phenomenon, called permeability transition (PT), is imputed to the opening of a nonselective channel, the mitochondrial permeability transition pore (mPTP). A sustained opening of this pore leads to the leakage of ions, metabolic molecules, and proapoptotic agents, resulting in mitochondrial swelling, dissipation of the electrochemical gradient, impaired ATP production, and, finally, cell death (Giorgio et al. 2018). The properties, and the regulation, of the mPTP have been highlighted in the late 1970s by the famous works of Hunter et al. (1976) and Hunter and Haworth (1979a,b,c). The opening of the mPTP is favored by Ca2+, extramitochondrial pH, fatty acids, cyclophilin D protein (CyPD), reactive oxygen species (ROS), or inorganic phosphates. It is inhibited by other divalent ions (Mg2+, Sr2+, Mn2+), nucleotides, matrix acidification, ΔΨ, or cyclosporin A (CsA), a drug widely used to study the mPTP (Szabo and Zoratti 2014). Interestingly, mitochondrial Ca2+ is necessary for mPTP opening: in a suspension of mitochondria, the opening of the PTP (assessed by the swelling of mitochondria) can be triggered by drugs such as phenylarsine oxide (PhAsO) or p-hydroxyphenylglyoxal (OH-PGO). In the presence of the membrane permeant BAPTA-AM, responsible for mitochondrial Ca2+ chelation, the PhAsO- or OH-PGO-induced opening of the pore is blocked. This pinpoints the crucial role of mitochondrial Ca2+, even in residual amounts. However, matrix Ca2+ is by itself not always sufficient to elicit PTP opening, as no swelling is noticed in the same experiments without PhAsO or OH-PGO addition (Giorgio et al. 2018).
However, despite its early discovery and characterization, the mPTP is still subject to intensive research as its molecular nature was unsolved until recently. It was long considered that the mPTP forms at a contact site between the IMMs and the OMMs, as a complex between the VDAC and the adenine nucleotide transporter (ANT). However, this idea was rejected on the basis of experiments involving genetic deletion of these components: mitochondria lacking ANT or VDAC are still able to undergo a PT inhibited by CsA (Kokoszka et al. 2004; Baines et al. 2007). The involvement of the F1F0-ATP synthase in PT was then proposed, because CyPD, an inducer of the mPTP, was found to bind this complex and to promote its partial deactivation, an effect that can be removed by CsA (Giorgio et al. 2009). This has been validated by experiments using purified dimers of ATP synthase reconstituted in lipid bilayers. In such a system, it is possible to trigger the opening of a channel, the electrophysiological properties of which are similar to those of the mPTP (Giorgio et al. 2013).
The molecular identification of the PTP allowed a better understanding of the interplay between the inducers of PT and the pore itself. For example, the reversible protonation of a histidine residue, located in the oligomycin sensitivity-conferring protein (OSCP) unit of the ATP synthase, has been shown to inhibit the opening of the pore (Antoniel et al. 2018). The Ca2+-binding site of ATP synthase, which allows the opening of the pore, has recently been identified. Binding changes the conformation of the complex, leading to the opening of the pore (Giorgio et al. 2017).
Despite its recent molecular identification, the exact location of the pore in the ATP synthase still constitutes an ongoing issue. Two hypotheses emerge on this topic: the pore forms itself either between two monomers of ATP synthase or in the C subunit ring of the enzyme (the main transmembrane subunit forming the F0 rotor). In both cases, it seems that a dimerization step is necessary: ATP synthases purified in a lipid bilayer do not display any conductivity in their monomeric form, but are permeable when assembled in dimers (Giorgio et al. 2013, 2017). It has been further suggested that the opening of the pore is associated with the dissociation of the dimers, as the opening of the mPTP leads to an increase of the monomer/dimer ratio. As C subunits appear to play a role in this dissociation, the opening of the ATP synthase dimers would be a result of a reorganization of the C ring (Bonora et al. 2013, 2017). Nevertheless, it has also been reported that the opening of the PTP can occur even in the absence of C subunits (He et al. 2017).
As mentioned above, the opening of the mPTP is favored by ROS (Bernardi et al. 2006), which can be generated as a consequence of ATP production by mitochondria and are important for cellular signaling, although they also contribute to oxidative stress and cellular damage (Feissner et al. 2009). Mitochondrial Ca2+ overload increases ROS production through complex II disintegration. These Ca2+-induced ROS subsequently activate mPTP opening and cellular death (Hwang et al. 2014). On the other hand, depending on the targeted protein, the type and concentration of ROS, and the duration of exposure, ROS can stimulate or inhibit Ca2+ signaling (Csordás and Hajnóczky 2009; Tadic et al. 2014). For example, at the ER-mitochondria interface, H2O2 generated by Ca2+ overload modulates agonist-induced Ca2+ release and oscillations (Booth et al. 2016). This effect may be because of changes in the redox environment and thus in the redox state of IP3R1 (Joseph et al. 2018). There is thus an intricate cross talk between Ca2+ signals, ROS production, and mPTP opening, which is frequently dysregulated in severe human pathologies such as cardiovascular diseases, cancer, and neurodegeneration (Feissner et al. 2009; Marchi et al. 2017).
The physiological role of the mPTP depends on its conductance state. Indeed, the mPTP can open in two states: low and high conductance modes (Fig. 3A). The latter one is well known and occurs in stressful or pathological conditions. In this case, the pore becomes permeable to large molecules (<1.5 kDa). This opening is physiologically irreversible, leads to the dissipation of ΔΨ, and triggers cell apoptosis (Brenner and Moulin 2012). Pathological consequences linked to the mPTP are usually associated with increased Ca2+ uptake and overload, which tend to favor the opening of the pore. For example, the mPTP has a role in the ischemia/reperfusion injury (I/RI): when the blood flow in a vessel is occluded and then restored, an infarct, the size of which is reduced by inhibiting the mPTP, appears (Bulluck et al. 2016). This effect is linked to mitochondrial Ca2+ influx as the inhibition of the MCU protects neurocytes from I/RI (Yu et al. 2016). As the C subunit of the ATP synthase seems to be a core component of the mPTP, it is likely to play an important role in I/RI (Morciano et al. 2015). Drugs targeting the C subunits have recently been developed. By administrating these compounds in the reperfusion phase, the apoptotic rate of the cells is indeed attenuated (Morciano et al. 2018). Hypersensitivity of the mPTP is also encountered in neurodegenerative diseases such as Alzheimer's disease, Huntington's disease, or Parkinson's disease (Pérez and Quintanilla 2017). In contrast, a decrease in the propensity of the pore to switch to an open state can lead to cancers (Rasol et al. 2010).
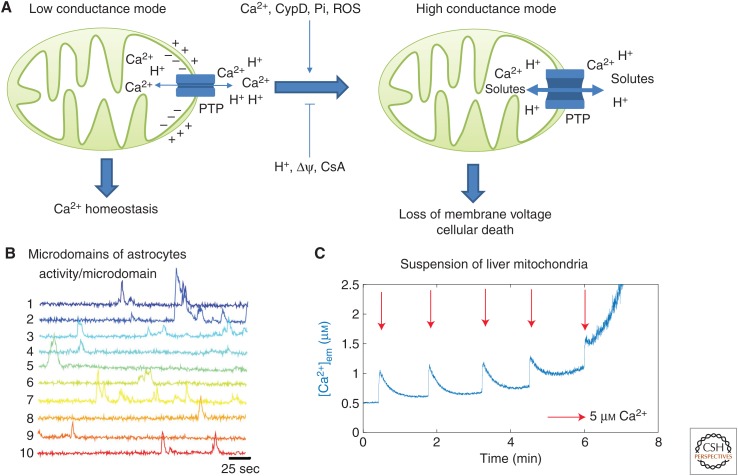
Figure 3.
The mitochondrial permeability transition pore (mPTP) can open in two conductance modes. (A) Schematic representations of the mPTP opening in the low (left) and in the high (right) conductance modes. (B) Experimental observation of transient openings of the mPTP in the low conductance mode. Each trace shows the local Ca2+ concentration in a well-defined microdomain of an astrocyte. These transient elevations are attributed to the mPTP as adding CsA decreases the probability of occurrence of these events. The data are from Agarwal et al. (2017) with permission. (C) Ca2+ measured in the extramitochondrial medium of a suspension of mitochondria extracted from hepatocytes. After several Ca2+ additions in the medium (arrows), the mPTP opens in its high conductance mode. This is visible thanks to the increase in Ca2+ in the medium, as a result of the leakage of mitochondrial Ca2+ through the mPTP in its high conductance state. See Wacquier et al. (2017) for experimental methods.
However, it seems than the PTP is not only associated with pathological conditions, in particular in its low conductance mode. This mode occurs in a transient and flickering way. In this state, the mPTP is only permeable to small ions (Ca2+, H+, K+). Although the exact mechanism of pore opening and closing in the low conductance mode remains to be fully elucidated, this conductance state seems to be involved in various physiological responses. For example, in neural progenitor cells, repetitive mPTP openings are associated with neuronal differentiation, whereas CsA (which blocks mPTP flickering) induces the proliferation of the cell (Hou et al. 2012). In mouse embryonic fibroblasts, the reprogramming into a pluripotent state seems to be favored by transient openings of the mPTP. Indeed, these openings activate the demethylation of promoter regions of pluripotency genes (Ying et al. 2018). In contrast to the high conductance mode that irreversibly leads to the dissipation of the ΔΨ, the transient opening of the mPTP in the low conductance mode could participate to Ca2+ homeostasis by allowing the release of Ca2+ from mitochondria. The low conductance mode would thereby avoid mitochondrial Ca2+ overload without impairing mitochondrial bioenergetics associated with the release of metabolic substrates. In myocyte mitochondria, these opening events are indeed associated to synchronized and CsA-dependent drops of matrix Ca2+ and mitochondrial membrane potential. In this system, these events are rare and remain localized in single mitochondria (Lu et al. 2016). These transient openings have also been indirectly observed in resting astrocytes, via the detailed observation of the local and stochastic increases of cytosolic Ca2+, which are inhibited by CsA (Fig. 3B; Agarwal et al. 2017).
As the mPTP in its low conductance mode is permeable to Ca2+, it is likely to alter cytosolic Ca2+ signaling. This is supported by experiments performed in HeLa cells: when the mPTP is inhibited by CsA, the Ca2+ oscillations in the cytosol are slightly slowed down (Wacquier et al. 2016). However, when mPTP activity is modulated by changing the expression level of the C subunit of the F1F0-ATP synthase in HeLa cells, the mitochondrial Ca2+ fluxes are not significantly altered (De Marchi et al. 2014a). A plausible explanation for these apparently contradictory results is that the frequency of the oscillations is very sensitive to small Ca2+ changes, which may not be visible when measuring global fluxes at supramaximal histamine concentrations.
The dynamics of mPTP opening in its high conductance mode has been much studied in suspensions of mitochondria submitted to additions of exogenous Ca2+ (Fig. 3C). In these conditions, most of the Ca2+ added in the extra-mitochondrial medium is rapidly taken up by mitochondria, via the MCU. Successive Ca2+ additions finally lead to the rapid release of the sequestered Ca2+, probably when the level of mitochondrial Ca2+ reaches some threshold concentration leading to the opening of the mPTP in its high conductance mode. Interestingly, before this massive Ca2+ release, the basal level of Ca2+ slowly increases in the extramitochondrial medium. This increase may reflect the progressively increasing opening of the PTP in its low conductance mode and/or a decrease in ΔΨ. Interestingly, Ichas et al. (1997) even observed traveling waves of Ca2+ in preparations of mitochondria suspended in a gel. These waves were ascribed to mitochondrial CICR relying on the low conductance mode of the PTP (Oster et al. 2011). How the regulations of the same pore in the two modes are related remains largely unknown. In a mathematical model, Oster et al. (2011) postulated two independent mechanisms of regulation for the two modes: an activation by matrix pH and by mitochondrial Ca2+ for the low and the high conductance states, respectively. Alternatively, to describe mitochondrial Ca2+ swelling in cardiac cells, it was hypothesized that the pore switches from the low to the high conductance mode if the extramitochondrial Ca2+ level increases (Chapa-Dubocq et al. 2018), which remains to be established experimentally.
MITOCHONDRIAL METABOLISM
Mitochondrial Ca2+ regulates bioenergetics in various ways. First, by increasing the NADH pool in mitochondria, which is necessary to establish the proton gradient. Ca2+ indeed activates several key enzymes of the Krebs cycle. By binding to the isocitrate and α-ketoglutarate dehydrogenases, Ca2+ increases their affinities for their substrates without altering their maximal velocities. This increases the rates of reaction of these enzymes (Williams et al. 2015). Ca2+ also indirectly activates the pyruvate dehydrogenase. This enzyme is regulated by reversible phosphorylation, catalyzed by the pyruvate dehydrogenase phosphatase (PDP). When Ca2+ binds to the PDP the activity of this enzyme is increased, leading to the activation of pyruvate dehydrogenase (Denton 2009).
Ca2+ is also an activator of the malate-aspartate shuttle (MAS), which effectively allows translocation across the IMM of electrons from the NADH molecules produced in the cytosol during glycolysis (Fig. 1C). As the mitochondrial membrane is impermeable to NADH, this coenzyme is first metabolized into malate that is conveyed into mitochondria by a α-ketoglutarate-malate transporter (OGC). There, malate is converted into aspartate, which occurs together with the reduction of NAD+ into NADH. Ca2+ regulates the activity of the aspartate-glutamate carrier (AGC), which is necessary for the replenishment of cytosolic malate. Ca2+ activates the two main AGCs with a high affinity (100 to 350 nm, depending on the tissue): citrin (in heart or liver) and aralar (in brain, skeletal muscles, or heart). Thus, MAS activation is theoretically another pathway through which Ca2+ increases in the cytosol could stimulate mitochondrial metabolism. However, this pathway is only effective near basal Ca2+ levels. Indeed, at higher mitochondrial Ca2+, the activation of the Krebs cycle triggers a decrease in α-ketoglutarate, which is a crucial metabolite of the MAS as it must be exported to allow the influx of malate (Contreras et al. 2007; Satrústegui et al. 2007).
Components of the ETC are also activated by Ca2+: the activity of complexes I, III, and IV, which extrude protons out of the matrix, is increased twofold by Ca2+ (Territo et al. 2000; Glancy et al. 2013; Williams et al. 2015). However, it is not known if this regulation is performed by Ca2+ in the matrix or in the IMS (Territo et al. 2000; Glancy et al. 2013). Furthermore, Ca2+ is able to bind and activate the F1F0-ATP synthase (Territo et al. 2000). As Ca2+ is a main activator of metabolism, it is not surprising to see that altering mitochondrial Ca2+ transporters impairs bioenergetics. LETM1 silencing induces lower mitochondrial Ca2+ levels and an impaired oxygen consumption (Doonan et al. 2014). In MICU1 mutant fibroblasts, the level of mitochondrial Ca2+ and dephosphorylated PDH are increased, although the level of ATP and the membrane potential remain unaffected (Bhosale et al. 2017).
Besides the direct activation of mitochondrial metabolism by Ca2+, the close interplay between Ca2+ signaling and metabolism is further complicated by the modulation of the rates of the Ca2+ transporters by ΔΨ. Strikingly, a large peak in cytosolic Ca2+ triggers a transient decrease of metabolic intermediates: a drop in mitochondrial/cytosolic ATP concentrations and in ΔΨ is indeed observed in HeLa cells stimulated by histamine (Jouaville et al. 1999; Griffiths and Rutter 2009). This is probably because of a transient depolarization following the Ca2+ influx into mitochondria. This effect is rapidly counterbalanced by the activation of the Krebs cycle by Ca2+ (Wacquier et al. 2016). A similar transient decrease in ATP is observed in mouse eggs when the increase in cytosolic Ca2+ is induced by the addition of the SERCA inhibitor thapsigargin (Campbell and Swann 2006). Conversely, in Xenopus oocytes, the injection of metabolic substrates induces drastic changes in cytosolic Ca2+ responses induced by IP3. These substrates allow the synchronization of the Ca2+ waves propagating in different regions of the egg. Synchronized waves display a higher amplitude and a larger velocity of propagation (Jouaville et al. 1995).
SPATIAL ASPECTS
The spatial intracellular distribution of mitochondria has a significant impact on Ca2+ signaling. For example, in mouse pancreatic acinar cells, the Ca2+ responses induced by an agonist at the apical pole of the cell remain localized in this area. This confinement is ascribed to a barrier of mitochondria, acting as a firewall by buffering Ca2+ (Tinel et al. 1999). In atrial cardiomyocytes, Ca2+ signals are generated at the periphery of the cell, but are not fully transmitted toward the cell center. If mitochondria are depolarized and thus unable to load Ca2+, the peripheral Ca2+ signal propagates in the cell and becomes global (Mackenzie et al. 2004).
The spatial arrangement of Ca2+ channels also needs to be considered: to transport Ca2+ efficiently, the MCU needs to be confronted with high Ca2+ concentrations, because of its affinity for this ion is low (KD ∼2–10 µm) (Mallilankaraman et al. 2012; Csordás et al. 2013; Paillard et al. 2017). However, the global (i.e., spatially averaged) concentration in the cytosol varies between ∼0.1 µm at rest and ∼1 µm at the peak of a Ca2+ oscillation. The low affinity of the MCU for Ca2+ prevents overloading mitochondria with Ca2+, but physiological Ca2+ sequestration requires specific mechanisms to reach sufficiently high Ca2+ concentrations to promote significant MCU activity. Hotspots of high Ca2+ concentration that promote Ca2+ uptake via the MCU can be achieved locally in regions known as mitochondria-associated membranes (MAMs). These are confined areas delimited by the membranes of the ER and the mitochondria, which are associated thanks to tethering proteins (Fig. 1B; Csordás et al. 2010). These zones contain Ca2+ channels, notably IP3R, VDAC1 (Szabadkai et al. 2006) or MCU (de la Fuente et al. 2016). Following a Ca2+ release by IP3R, the Ca2+ signal first stays localized in the MAMs as this ion diffuses quite slowly in the cytoplasm. Thus, the Ca2+ concentrations are transiently higher in MAMs than in the rest of the cytoplasm, by a factor ∼5–10 (Csordás et al. 2010; Giacomello et al. 2010; Wacquier et al. 2017). These spatially restricted areas with high Ca2+ concentrations allow a better Ca2+ uptake by the MCU.
Accumulating evidence highlights the importance of these MAMs for physiological responses inside mitochondria but also on Ca2+ signaling in the cytosol. Indeed, mathematical modeling indicates that the drastically different kinetics of mitochondrial Ca2+ increases in intact cells and in suspensions of mitochondria can be ascribed to the absence of MAMs in the latter experimental conditions (Wacquier et al. 2017). At the MAMs, VDAC1 forms a complex with IP3R via the glucose-regulated protein 75 (GRP75) (Szabadkai et al. 2006). The formation of such a complex is favored by apoptotic stimuli, which pinpoints the importance of direct routes between the ER and mitochondria on the establishment of specific pathophysiological responses. It has been shown that overexpression of the three isoforms of VDAC increases physiological mitochondrial Ca2+ uptake, but that only the overexpression of VDAC1 enhances proapoptotic Ca2+ transfer (de Stefani et al. 2012; Monaco et al. 2015). Interestingly, the Ca2+ concentration and thus the rate of Ca2+ transport is also tuned by the size of the MAMs, in particular by the distance between the membranes of the ER and those of mitochondria (Qi et al. 2015). This space is tightly controlled by tethering proteins, such as the fetal and adult testis expressed 1 (FATE1), the protein kinase RNA-like ER kinase (PERK), or mitofusin 2 (Mfn2) (Naon and Scorrano 2014; Kerkhofs et al. 2017). For example, increasing FATE1 expression will enhance the distance between ER and mitochondria, and thereby decrease Ca2+ uptake, thus making cells more resistant against apoptotic stimuli. Such a mechanism is exploited in testicular cancer (Doghman-Bouguerra et al. 2016). On the other hand, knocking down FATE1 sensitizes cells to death stimuli (Doghman-Bouguerra et al. 2016). Mfn2 has long been reported as a tethering protein, strengthening ER-mitochondria interactions. Mfn2 ablation leads to a lower Ca2+ uptake and to impaired energetic response (de Brito and Scorrano 2008; Chen et al. 2012). However, other studies pinpoint an increase in the number of close contacts between mitochondria and ER in Mfn2 knocked-out cells, compared with wild-type cells. This is associated with an improved Ca2+ transfer at the ER-mitochondria interface. The low Ca2+ uptake observed in the first studies would occur because of a lower MCU expression and not because of any structural reason (Filadi et al. 2015, 2018). MAMs are also enriched in PERK proteins. The ablation of PERK induces weaker ER-mitochondria interactions and disturbs Ca2+ signaling (Verfaillie et al. 2012). Considering this link between MAMs and Ca2+ uptake, oncogenes and tumor suppressors may promote/prevent the survival of cancer cells by modulating MAMs size and hence the Ca2+ exchanges that act as life and death signals (Bittremieux et al. 2016; Danese et al. 2017). Tethering proteins thus constitute a target of choice for chemotherapeutics (Kerkhofs et al. 2018). In a general way, alterations of the spatial characteristics of the MAMs, and thus of Ca2+ dynamics in these microdomains, potentially lead to various pathological disorders, such as obesity, neurodegenerative diseases, or cancers (Marchi et al. 2017; Pinton 2018).
Intramitochondrial spatial aspects also play a key role in Ca2+ sequestration. In heart cells, the MCU, which is much less expressed than the NCLX, is mainly found in areas close to the junctional SR. Strikingly, the NCLX is excluded of these areas. There is thus a spatial separation of Ca2+ uptake and extrusion spots. This segregation could allow an effective mitochondrial response. Indeed, in a “mixed” situation in which the NCLX is also expressed near the junctional SR, the NCLX would extrude mitochondrial Ca2+ too fast. This limits the cost in terms of depolarization of the IMM, as the NCLX is electrogenic and induces depolarization of the membrane (de la Fuente et al. 2016, 2018).
CONCLUDING REMARKS
Ca2+ signaling and its physiological consequences have been intensively studied for decades. Ca2+ exchanges between the cytoplasm and the ER/SR play a primary role in this pathway. Although mitochondria are not constitutively loaded with Ca2+ in the absence of cell stimulation, various Ca2+ transporters allow for Ca2+ exchanges between the cytoplasm and these organelles (Csordás et al. 2013; Samanta et al. 2018; Shoshan-Barmatz et al. 2018). Thus, Ca2+ handling by mitochondria plays an important role in Ca2+ signaling of stimulated cells. Importantly, changes in the mitochondrial Ca2+ fluxes modify the frequency of cytosolic Ca2+ oscillations by reshaping the pacemaker-like Ca2+ increase that progressively sensitizes the IP3R (Hernández-SanMiguel et al. 2006; Wacquier et al. 2016). Ca2+ dynamics are also important for bioenergetics as mitochondrial Ca2+ uptake stimulates the production of ATP, which allows cells to cope with the increased energy demand created by stimuli. Reciprocally, mitochondrial metabolism can to some extent control Ca2+ signaling. In this review, we have mainly focused on the less studied impact of mitochondrial Ca2+ dynamics and metabolism on cytosolic Ca2+ signaling.
The regulation and molecular nature of the mitochondrial Ca2+ transporters have been well characterized, but some important questions are still open. For example, the nature of the mHCX and its participation in mitochondrial Ca2+ efflux in different cell types remain to be established (Austin et al. 2017). Similarly, the involvement of the mPTP in its low conductance mode for cellular Ca2+ homeostasis has been suggested (Lu et al. 2016; Wacquier et al. 2016; Agarwal et al. 2017), but the dynamical and quantitative aspects of this flux have still to be characterized. Such progress could also help deciphering the mechanisms that regulate the transition of this pore from the low- to the high-conductance mode.
Mitochondrial Ca2+ impacts on metabolism by activating the MAS shuttle, the Krebs cycle, and the ETC (Denton 2009; Williams et al. 2015). Computational modeling predicts that in electrically nonexcitable cells such as HeLa cells or hepatocytes, the activation of the MAS shuttle by Ca2+ is only significant at low cytosolic Ca2+. At higher Ca2+ concentrations the activation of the glutamate-aspartate transporter is counterbalanced by the decrease in α-ketoglutarate brought about by the activation of the Krebs cycle. The generality of this prediction remains to be tested experimentally. Other parameters affect the relation between Ca2+ increases and mitochondrial metabolism. First, changes in the ratio between cytosolic and mitochondrial Ca2+ concentrations modify ΔΨ, which controls all mitochondrial Ca2+ fluxes and reaction rates in the Krebs cycle and in the ETC. The intracellular spatial arrangement of mitochondria is also important. Various studies have indeed highlighted that mitochondria and ER are tightly coupled by microdomains (Csordás et al. 2010). The size of these areas is a crucial parameter because it can modulate Ca2+ uptake and induce either impaired bioenergetics or cellular death (Chen et al. 2012; Doghman-Bouguerra et al. 2016). The number, the size, and the dynamics of individual mitochondria are other characteristics of these organelles that require further investigation to fully understand the interplay between mitochondria and Ca2+ signaling in physiological and pathological conditions.
ACKNOWLEDGMENTS
G.D. is Research Director at the Belgian FNRS. G.D., L.C., and B.W. benefited from a WBI-France exchange program (Wallonie-Bruxelles International, Fonds de la Recherche Scientifique, Ministère Français des Affaires étrangères et européennes, Ministère de l'Enseignement supérieur et de la Recherche dans le cadre des Partenariats Hubert Curien).
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D, Bergles DE. 2017. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93: 587–605.e7. 10.1016/j.neuron.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniel M, Jones K, Antonucci S, Spolaore B, Fogolari F, Petronilli V, Giorgio V, Carraro M, Di Lisa F, Forte M, et al. 2018. The unique histidine in OSCP subunit of F-ATP synthase mediates inhibition of the permeability transition pore by acidic pH. EMBO Rep 19: 257–268. 10.15252/embr.201744705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S, Tavakoli M, Pfeiffer C, Seifert J, Mattarei A, de Stefani D, Zoratti M, Nowikovsky K. 2017. LETM1-mediated K+ and Na+ homeostasis regulates mitochondrial Ca2+ efflux. Front Physiol 8: 839 10.3389/fphys.2017.00839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. 2007. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9: 550–555. 10.1038/ncb1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. 2011. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–347. 10.1038/nature10234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. 2006. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 273: 2077–2099. 10.1111/j.1742-4658.2006.05213.x [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Lipp P. 1998. Calcium—A life and death signal. Nature 395: 645–648. 10.1038/27094 [DOI] [PubMed] [Google Scholar]
- Bhosale G, Sharpe JA, Koh A, Kouli A, Szabadkai G, Duchen MR. 2017. Pathological consequences of MICU1 mutations on mitochondrial calcium signalling and bioenergetics. Biochim Biophys Acta 1864: 1009–1017. 10.1016/j.bbamcr.2017.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittremieux M, Parys JB, Pinton P, Bultynck G. 2016. ER functions of oncogenes and tumor suppressors: Modulators of intracellular Ca2+ signaling. Biochim Biophys Acta 1863: 1364–1378. 10.1016/j.bbamcr.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Bonora M, Bononi A, de Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, et al. 2013. Role of the c subunit of the F0 ATP synthase in mitochondrial permeability transition. Cell Cycle 12: 674–683. 10.4161/cc.23599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora M, Morganti C, Morciano G, Pedriali G, Lebiedzinska-Arciszewska M, Aquila G, Giorgi C, Rizzo P, Campo G, Ferrari R, et al. 2017. Mitochondrial permeability transition involves dissociation of F1F0 ATP synthase dimers and C-ring conformation. EMBO Rep 18: 1077–1089. 10.15252/embr.201643602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DM, Enyedi B, Geiszt M, Várnai P, Hajnóczky G. 2016. Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Mol Cell 63: 240–248. 10.1016/j.molcel.2016.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C, Moulin M. 2012. Physiological roles of the permeability transition pore. Circ Res 111: 1237–1247. 10.1161/CIRCRESAHA.112.265942 [DOI] [PubMed] [Google Scholar]
- Bulluck H, Yellon DM, Hausenloy DJ. 2016. Reducing myocardial infarct size: Challenges and future opportunities. Heart 102: 341–348. 10.1136/heartjnl-2015-307855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buntinas L, Gunter KK, Sparagna GC, Gunter TE. 2001. The rapid mode of calcium uptake into heart mitochondria (RaM): Comparison to RaM in liver mitochondria. Biochim Biophys Acta 1504: 248–261. 10.1016/S0005-2728(00)00254-1 [DOI] [PubMed] [Google Scholar]
- Campbell K, Swann K. 2006. Ca2+ oscillations stimulate an ATP increase during fertilization of mouse eggs. Dev Biol 298: 225–233. 10.1016/j.ydbio.2006.06.032 [DOI] [PubMed] [Google Scholar]
- Chapa-Dubocq X, Makarov V, Javadov S. 2018. Simple kinetic model of mitochondrial swelling in cardiac cells. J Cell Physiol 233: 5310–5321. 10.1002/jcp.26335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Csordás G, Jowdy C, Schneider TG, Csordás N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, et al. 2012. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ Res 111: 863–875. 10.1161/CIRCRESAHA.112.266585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JC, Berridge MJ, Lipp P, Bootman MD. 2002. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J 21: 1616–1627. 10.1093/emboj/21.7.1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras L, Gomez-Puertas P, Iijima M, Kobayashi K, Saheki T, Satrústegui J. 2007. Ca2+ activation kinetics of the two aspartate-glutamate mitochondrial carriers, aralar and citrin: Role in the heart malate-aspartate NADH shuttle. J Biol Chem 282: 7098–7106. 10.1074/jbc.M610491200 [DOI] [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T. 2010. Mitochondria: The calcium connection. Biochim Biophys Acta 1797: 607–618. 10.1016/j.bbabio.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Csordás G, Hajnóczky G. 2009. SR/ER-mitochondrial local communication: Calcium and ROS. Biochim Biophys Acta 1787: 1352–1362. 10.1016/j.bbabio.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Thomas AP, Hajnóczky G. 1999. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J 18: 96–108. 10.1093/emboj/18.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, Balla T, Hajnóczky G. 2010. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39: 121–132. 10.1016/j.molcel.2010.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Golenár T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, et al. 2013. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab 17: 976–987. 10.1016/j.cmet.2013.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Patergnani S, Bonora M, Wieckowski MR, Previati M, Giorgi C, Pinton P. 2017. Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim Biophys Acta Bioenerg 1858: 615–627. 10.1016/j.bbabio.2017.01.003 [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. 2008. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–611. 10.1038/nature07534 [DOI] [PubMed] [Google Scholar]
- de la Fuente S, Fernandez-Sanz C, Vail C, Agra EJ, Holmstrom K, Sun J, Mishra J, Williams D, Finkel T, Murphy E, et al. 2016. Strategic positioning and biased activity of the mitochondrial calcium uniporter in cardiac muscle. J Biol Chem 291: 23343–23362. 10.1074/jbc.M116.755496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente S, Lambert JP, Nichtova Z, Fernandez Sanz C, Elrod JW, Sheu SS, Csordás G. 2018. Spatial separation of mitochondrial calcium uptake and extrusion for energy-efficient mitochondrial calcium signaling in the heart. Cell Rep 24: 3099–3107.e4. 10.1016/j.celrep.2018.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi E, Bonora M, Giorgi C, Pinton P. 2014a. The mitochondrial permeability transition pore is a dispensable element for mitochondrial efflux. Cell Calcium 56: 1–13. 10.1016/j.ceca.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi U, Santo-Domingo J, Castelbou C, Sekler I, Wiederkehr A, Demaurex N. 2014b. NCLX protein, but not LETM1, mediates mitochondrial Ca2+ extrusion, thereby limiting Ca2+-induced NAD(P)H production and modulating matrix redox state. J Biol Chem 289: 20377–20385. 10.1074/jbc.M113.540898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM. 2009. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787: 1309–1316. 10.1016/j.bbabio.2009.01.005 [DOI] [PubMed] [Google Scholar]
- de Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. 2011. A 40 kDa protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340. 10.1038/nature10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stefani D, Bononi A, Romagnoli A, Messina A, de Pinto V, Pinton P, Rizzuto R. 2012. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ 19: 267–273. 10.1038/cdd.2011.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doghman-Bouguerra M, Granatiero V, Sbiera S, Sbiera I, Lacas-Gervais S, Brau F, Fassnacht M, Rizzuto R, Lalli E. 2016. FATE1 antagonizes calcium- and drug-induced apoptosis by uncoupling ER and mitochondria. EMBO Rep 17: 1264–1280. 10.15252/embr.201541504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan PJ, Chandramoorthy HC, Hoffman NE, Zhang X, Cárdenas C, Shanmughapriya S, Rajan S, Vallem S, Chen X, Foskett JK, et al. 2014. LETM1-dependent mitochondrial Ca2+ flux modulates cellular bioenergetics and proliferation. FASEB J 28: 4936–4949. 10.1096/fj.14-256453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G, Combettes L, Bird GS, Putney JW. 2011. Calcium oscillations. Cold Spring Harb Perspect Biol 3: a004226 10.1101/cshperspect.a004226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hattab AW, Suleiman J, Almannai M, Scaglia F. 2018. Mitochondrial dynamics: Biological roles, molecular machinery, and related diseases. Mol Genet Metab 10.1016/j.ymgme.2018.10.003 [DOI] [PubMed] [Google Scholar]
- Feissner RF, Skalska J, Gaum WE, Sheu SS. 2009. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci 14: 1197–1218. 10.2741/3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieni F, Bae Lee S, Jan YN, Kirichok Y. 2012. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun 3: 1317 10.1038/ncomms2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P. 2015. Mitofusin 2 ablation increases endoplasmic reticulum–mitochondria coupling. Proc Natl Acad Sci 112: E2174–E2181. 10.1073/pnas.1504880112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filadi R, Greotti E, Pizzo P. 2018. Highlighting the endoplasmic reticulum-mitochondria connection: Focus on mitofusin 2. Pharmacol Res 128: 42–51. 10.1016/j.phrs.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Gadicherla AK, Wang N, Bulic M, Agullo-Pascual E, Lissoni A, de Smet M, Delmar M, Bultynck G, Krysko DV, Camara A, et al. 2017. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res Cardiol 27: 112–127. 10.1007/s00395-017-0618-1 [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, Pozzan T. 2010. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not activation of store-operated Ca2+ channels. Mol Cell 38: 280–290. 10.1016/j.molcel.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Giorgi C, Marchi S, Pinton P. 2018. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol 19: 713–730. 10.1038/s41580-018-0052-8 [DOI] [PubMed] [Google Scholar]
- Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, Forte MA, Bernardi P, Lippe G. 2009. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem 284: 33982–33988. 10.1074/jbc.M109.020115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, et al. 2013. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci 110: 5887–5892. 10.1073/pnas.1217823110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V, Argenton F, Forte M, Tosatto S, Lippe G, et al. 2017. Ca2+ binding to F-ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep 18: 1065–1076. 10.15252/embr.201643354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, Guo L, Bassot C, Petronilli V, Bernardi P. 2018. Calcium and regulation of the mitochondrial permeability transition. Cell Calcium 70: 56–63. 10.1016/j.ceca.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Glancy B, Willis WT, Chess DJ, Balaban RS. 2013. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 52: 2793–2809. 10.1021/bi3015983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonze D, Gérard C, Wacquier B, Woller A, Tosenberger A, Goldbeter A, Dupont G. 2018. Modeling-based investigation of the effect of noise in cellular systems. Front Mol Biosci 5: 34 10.3389/fmolb.2018.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EJ, Rutter GA. 2009. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim Biophys Acta 1787: 1324–1333. 10.1016/j.bbabio.2009.01.019 [DOI] [PubMed] [Google Scholar]
- Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. 1995. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424. 10.1016/0092-8674(95)90430-1 [DOI] [PubMed] [Google Scholar]
- He J, Ford HC, Carroll J, Ding S, Fearnley IM, Walker JE. 2017. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc Natl Acad Sci 114: 3409–3414. 10.1073/pnas.1702357114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-SanMiguel E, Vay L, Santo-Domingo J, Lobatón CD, Moreno A, Montero M, Alvarez J. 2006. The mitochondrial Na+/Ca2+ exchanger plays a key role in the control of cytosolic Ca2+ oscillations. Cell Calcium 40: 53–61. 10.1016/j.ceca.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Hou Y, Ouyang X, Wan R, Cheng H, Mattson MP, Cheng A. 2012. Mitochondrial superoxide production negatively regulates neural progenitor proliferation and cerebral cortical development. Stem Cells 30: 2535–2547. 10.1002/stem.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA. 1979a. The Ca2+-induced membrane transition in mitochondria. I: The protective mechanisms. Arch Biochem Biophys 195: 453–459. 10.1016/0003-9861(79)90371-0 [DOI] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA. 1979b. The Ca2+-induced membrane transition in mitochondria. III: Transitional Ca2+ release. Arch Biochem Biophys 195: 468–477. 10.1016/0003-9861(79)90373-4 [DOI] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA. 1979c. The Ca2+-induced membrane transition in mitochondria. II: Nature of the Ca2+ trigger site. Arch Biochem Biophys 195: 460–467. 10.1016/0003-9861(79)90372-2 [DOI] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA, Southard JH. 1976. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem 251: 5069–5077. [PubMed] [Google Scholar]
- Hwang M-S, Schwall CT, Pazarentzos E, Datler C, Alder NN, Grimm S. 2014. Mitochondrial Ca2+ influx targets cardiolipin to disintegrate respiratory chain complex II for cell death induction. Cell Death Differ 21: 1733–1745. 10.1038/cdd.2014.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichas F, Jouaville L, Mazat JP. 1997. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89: 1145–1153. 10.1016/S0092-8674(00)80301-3 [DOI] [PubMed] [Google Scholar]
- Ishii K, Hirose K, Iino M. 2006. Ca2+ Shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep 7: 390–396. 10.1038/sj.embor.7400620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob R, Beutner G, Sharma VK, Duan Y, Gross RA, Hurst S, Jhun BS, O-Uchi J, Sheu SS. 2014. Molecular and functional identification of a mitochondrial ryanodine receptor in neurons. Neurosci Lett 575: 7–12. 10.1016/j.neulet.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SK, Young MP, Alzayady K, Yule DI, Ali M, Booth DM, Hajnóczky G. 2018. Redox regulation of type-I inositol trisphosphate receptors in intact mammalian cells. J Biol Chem 293: 17464–17476. 10.1074/jbc.RA118.005624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. 1995. Synchronization of calcium waves by mitochondrial substrates in Xenopus Laevis oocytes. Nature 377: 438–441. 10.1038/377438a0 [DOI] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. 1999. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc Natl Acad Sci 96: 13807–13812. 10.1073/pnas.96.24.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhofs M, Giorgi C, Marchi S, Seitaj B, Parys JB, Pinton P, Bultynck G, Bittremieux M. 2017. Alterations in Ca2+ signalling via ER-mitochondria contact site remodelling in cancer. Adv Exp Med Biol 997: 225–254. 10.1007/978-981-10-4567-7_17 [DOI] [PubMed] [Google Scholar]
- Kerkhofs M, Bittremieux M, Morciano G, Giorgi C, Pinton P, Parys JB, Bultynck G. 2018. Emergin molecular mechanisms in chemotherapy: Ca2+ signaling at the mitochondria-associated endoplasmic reticulum membranes. Cell Death Dis 9: 334 10.1038/s41419-017-0179-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Takeuchi A, Koga O, Hikida M, Matsuoka S. 2013. Mitochondria Na+–Ca2+ exchange in cardiomyocytes and lymphocytes. Adv Exp Med Biol 961: 193–201. 10.1007/978-1-4614-4756-6_16 [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. 2004. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427: 461–465. 10.1038/nature02229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kwong JQ, Molkentin JD, Bers DM. 2016. Individual cardiac mitochondria undergo rare transient permeability transition pore openings. Circ Res 118: 834–841. 10.1161/CIRCRESAHA.115.308093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. 2004. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci 117: 6327–6337. 10.1242/jcs.01559 [DOI] [PubMed] [Google Scholar]
- Mallilankaraman K, Doonan P, Cárdenas C, Chandramoorthy HC, Müller M, Miller R, Hoffman NE, Gandhirajan RK, Molgó J, Birnbaum MJ, et al. 2012. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 151: 630–644. 10.1016/j.cell.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Raffaello A, Reane DV, Gherardi G, de Mario A, Rizzuto R. 2018. Mitochondrial calcium uptake in organ physiology: From molecular mechanism to animal models. Pflüg Arch Eur J Phys 470: 1165–1179. 10.1007/s00424-018-2123-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Bittremieux M, Missiroli S, Morganti C, Patergnani S, Sbano L, Rimessi A, Kerkhofs M, Parys JB, Bultynck G, et al. 2017. Endoplasmic reticulum-mitochondria communication through Ca2+ signaling: The importance of mitochondria-associated membranes (MAMs). Adv Exp Med 997: 49–67. 10.1007/978-981-10-4567-7_4 [DOI] [PubMed] [Google Scholar]
- Meissner G. 1981. Calcium transport and monovalent cation and proton fluxes in sarcoplasmic reticulum vesicles. J Biol Chem 256: 636–643. [PubMed] [Google Scholar]
- Mishra P. 2016. Interfaces between mitochondrial dynamics and disease. Cell Calcium 60: 190–198. 10.1016/j.ceca.2016.05.004 [DOI] [PubMed] [Google Scholar]
- Monaco G, Decrock E, Arbel N, van Vliet AR, La Rovere RM, de Smedt H, Parys JB, Agostinis P, Leybaert L, Shoshan-Barmatz V, et al. 2015. The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J Biol Chem 290: 9150–9161. 10.1074/jbc.M114.622514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morciano G, Giorgi C, Bonora M, Punzetti S, Pavasini R, Wieckowski MR, Campo G, Pinton P. 2015. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J Mol Cell Cardiol 78: 142–153. 10.1016/j.yjmcc.2014.08.015 [DOI] [PubMed] [Google Scholar]
- Morciano G, Preti D, Pedriali G, Aquila G, Missiroli S, Fantinati A, Caroccia N, Pacifico S, Bonora M, Talarico A, et al. 2018. Discovery of novel 1,3,8-triazaspiro[4.5]decane derivatives that target the c subunit of F1/F0-adenosine triphosphate (ATP) synthase for the treatment of reperfusion damage in myocardial infarction. J Med Chem 61: 7131–7143. 10.1021/acs.jmedchem.8b00278 [DOI] [PubMed] [Google Scholar]
- Murphy MP, Hartley RC. 2018. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov 10.1038/nrd.2018.174 [DOI] [PubMed] [Google Scholar]
- Naon D, Scorrano L. 2014. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim Biophys Acta 1843: 2184–2194. 10.1016/j.bbamcr.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Nemani N, Shanmughapriya S, Madesh M. 2018. Molecular regulation of MCU: Implications in physiology and disease. Cell Calcium 74: 86–93. 10.1016/j.ceca.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Chalmers S. 2004. The integration of mitochondrial calcium transport and storage. J Bioenerg Biomembr 36: 277–281. 10.1023/B:JOBB.0000041753.52832.f3 [DOI] [PubMed] [Google Scholar]
- Oster A, Thomas B, Terman D, Fall C. 2011. The low conductance mitochondrial permeability transition pore confers excitability and CICR wave propagation in a computational model. J Theor Biol 273: 216–231. 10.1016/j.jtbi.2010.12.023 [DOI] [PubMed] [Google Scholar]
- Paillard M, Csordás G, Szanda G, Golenár T, Debattisti V, Bartok A, Wang N, Moffat C, Seifert EL, Spät A, et al. 2017. Tissue-specific mitochondrial decoding of cytoplasmic Ca2+ signals is controlled by the stoichiometry of MICU1/2 and MCU. Cell Rep 18: 2291–2300. 10.1016/j.celrep.2017.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, et al. 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci 107: 436–441. 10.1073/pnas.0908099107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Muallem S. 2011. Acidic Ca2+ stores come to the fore. Cell Calcium 50: 109–112. 10.1016/j.ceca.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabò I, de Stefani D, Rizzuto R. 2014. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell 53: 726–737. 10.1016/j.molcel.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna E, Espino J, de Stefani D, Rizzuto R. 2018. The MCU complex in cell death. Cell Calcium 69: 73–80. 10.1016/j.ceca.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Pérez MJ, Quintanilla RA. 2017. Development or disease: Duality of the mitochondrial permeability transition pore. Dev Biol 426: 1–7. 10.1016/j.ydbio.2017.04.018 [DOI] [PubMed] [Google Scholar]
- Pinton P. 2018. Mitochondria-associated membranes (MAMs) and pathologies. Cell Death Dis 9: 413 10.1038/s41419-018-0424-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Ward EN. 1942. Mitochondria in lymphocytes of normal and leukemic mice. Cancer Res 2: 655–659. [Google Scholar]
- Qi H, Li L, Shuai J. 2015. Optimal microdomain crosstalk between endoplasmic reticulum and mitochondria for Ca2+ oscillations. Sci Rep 5: 7984 10.1038/srep07984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasol A, Sciacovelli M, Chiara F, Pantic B, Brusilow WS, Bernardi P. 2010. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc Natl Acad Sci 107: 726–731. 10.1073/pnas.0912742107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repsold L, Joubert AM. 2018. Eryptosis: An erythrocyte's suicidal type of cell death. BioMed Res Int 2018: 1–10. 10.1155/2018/9405617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766. 10.1126/science.280.5370.1763 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, de Stefani D, Raffaello A, Mammucari C. 2012. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13: 566–578. 10.1038/nrm3412 [DOI] [PubMed] [Google Scholar]
- Samanta K, Douglas S, Parekh AB. 2014. Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+ oscillations, store-operated Ca2+ entry and Ca2+-dependent gene expression in response to receptor stimulation. PLoS ONE 9: e101188 10.1371/journal.pone.0101188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta K, Mirams GR, Parekh AB. 2018. Sequential forward and reverse transport of the Na+ Ca2+ exchanger generates Ca2+ oscillations within mitochondria. Nat Commun 9: 156 10.1038/s41467-017-02638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satrústegui J, Pardo B, del Arco A. 2007. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev 87: 29–67. 10.1152/physrev.00005.2006 [DOI] [PubMed] [Google Scholar]
- Sedova M, Dedkova EN, Blatter LA. 2006. Integration of rapid cytosolic Ca2+ signals by mitochondria in cat ventricular myocytes. Am J Physiol Cell Physiol 291: C840–C850. 10.1152/ajpcell.00619.2005 [DOI] [PubMed] [Google Scholar]
- Shao J, Fu Z, Ji Y, Guan X, Ding Z, Yang X, Cong Y, Shen Y. 2016. Leucine zipper-EF-hand containing transmembrane protein 1 (LETM1) forms a Ca2+/H+ antiporter. Sci Rep 6: 34174 10.1038/srep34174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Schredelseker J, Huang J, Lu K, Naghdi S, Lu F, Franklin S, Fiji HDG, Wang K, Zhu H, et al. 2015. Mitochondrial Ca2+ uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. eLife 4: e04801 10.7554/eLife.04801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Krelin Y, Shteinfer-Kuzmine A. 2018. VDAC1 functions in Ca2+ homeostasis and cell life and death in health and disease. Cell Calcium 69: 81–100. 10.1016/j.ceca.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y, Iino M. 2014. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun 5: 4153 10.1038/ncomms5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Várnai P, de Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. 2006. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175: 901–911. 10.1083/jcb.200608073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Zoratti M. 2014. Mitochondrial channels: Ion fluxes and more. Physiol Rev 94: 519–608. 10.1152/physrev.00021.2013 [DOI] [PubMed] [Google Scholar]
- Tadic V, Prell T, Lautenschlaeger J, Grosskreutz J. 2014. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis. Front Cell Neurosci 8: 147 10.3389/fncel.2014.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Colombini M. 2007. VDAC closure increases calcium ion flux. Biochim Biophys Acta 1768: 2510–2515. 10.1016/j.bbamem.2007.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Territo PR, Mootha VK, French SA, Balaban RS. 2000. Ca2+ activation of heart mitochondrial oxidative phosphorylation: Role of the F0/F1-ATPase. Am J Physiol Cell Physiol 278: C423–C435. 10.1152/ajpcell.2000.278.2.C423 [DOI] [PubMed] [Google Scholar]
- Tinel H, Cancela JM, Mogami H, Gerasimenko JV, Gerasimenko OV, Tepikin AV, Petersen OH. 1999. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J 18: 4999–5008. 10.1093/emboj/18.18.4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MF, Jiang D, Zhao L, Clapham D, Miller C. 2014. Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. J Gen Physiol 143: 67–73. 10.1085/jgp.201311096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere J-P, Piette J, Linehan C, Gupta S, Samali A, et al. 2012. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Diff 19: 1880–1891. 10.1038/cdd.2012.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacquier B, Combettes L, Van Nhieu GT, Dupont G. 2016. Interplay between intracellular Ca2+ oscillations and Ca2+-stimulated mitochondrial metabolism. Sci Rep 6: 19316 10.1038/srep19316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacquier B, Romero Campos HE, González-Vélez V, Combettes L, Dupont G. 2017. Mitochondrial Ca2+ dynamics in cells and suspensions. FEBS J 284: 4128–4142. 10.1111/febs.14296 [DOI] [PubMed] [Google Scholar]
- Wang S, Xiao W, Shan S, Jiang C, Chen M, Zhang Y, Lü S, Chen J, Zhang C, Chen Q, et al. 2012. Multi-patterned dynamics of mitochondrial fission and fusion in a living cell. PLoS ONE 7: e19879 10.1371/journal.pone.0019879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Karamanlidis G, Tian R. 2016. Novel targets for mitochondrial medicine. Sci Transl Med 8: 326rv3 10.1126/scitranslmed.aac7410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Fernandez-Sanz C, Wang W, Sheu SS. 2018. Why don't mice lacking the mitochondrial Ca2+ uniporter experience an energy crisis? MCU and heart bioenergetics. J Physiol 10.1113/JP276636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER, Stäubli W, Gnägi HR, Hess FA. 1969. Correlated morphometric and biochemical studies on the liver cell. J Cell Biol 42: 68–91. 10.1083/jcb.42.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GSB, Boyman L, Lederer WJ. 2015. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 78: 35–45. 10.1016/j.yjmcc.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove DE, Gunter TE. 1986. Kinetics of mitochondrial calcium transport. II: A kinetic description of the sodium-dependent calcium efflux mechanism of liver mitochondria and inhibition by ruthenium red and by tetraphenylphosphonium. J Biol Chem 261: 15166–15171. [PubMed] [Google Scholar]
- Xie A, Zhou A, Liu H, Shi G, Liu M, Boheler KR, Dudley SC Jr. 2018. Mitochondrial Ca2+ flux modulates spontaneous electrical activity in ventricular cardiomyocytes. PLoS ONE 13: e0200448 10.1371/journal.pone.0200448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Xiang G, Zheng L, Tang H, Duan L, Lin X, Zhao Q, Chen K, Wu Y, Xing G, et al. 2018. Short-term mitochondrial permeability transition pore opening modulates histone lysine methylation at the early phase of somatic cell reprogramming. Cell Metab 10.1016/j.cmet.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Yu S, Zheng S, Leng J, Wang S, Zhao T, Liu J. 2016. Inhibition of mitochondrial calcium uniporter protects neurocytes from ischemia/reperfusion injury via the inhibition of excessive mitophagy. Neurosci Lett 628: 24–29. 10.1016/j.neulet.2016.06.012 [DOI] [PubMed] [Google Scholar]
- Zemirli N, Morel E, Molino D. 2018. Mitochondrial dynamics in basal and stressful conditions. Int J Mol Sci 19: 564 10.3390/ijms19020564 [DOI] [PMC free article] [PubMed] [Google Scholar]