Abstract
Objective.
Obesity significantly increases the risk of the development of both endometrial hyperplasia and cancer. Our objective was to assess the feasibility of two technology-based weight loss interventions in this patient population.
Methods.
Women with obesity (BMI ≥ 30 kg/m2) and endometrial hyperplasia or Type I endometrial cancer were randomized 1:1 to a technology-based 6 month lifestyle intervention via either telemedicine or text messaging. The telemedicine arm received weekly phone calls, with weights tracked online using Withings© Wi-Fi scales. The text arm received 3–5 personalized messages daily via Text4Diet™. Participants maintained a 1200–1800 calorie/day diet, self-monitored food intake and received exercise goals. Biomarkers (IGFBP-1, adiponectin, VEGF, IL1-beta, IL2, IL6, and IL7) were assessed pre- and post-treatment.
Results.
Twenty women were randomized (Telemedicine: n = 10, Text4Diet: n = 10), and 90% lost weight. Many were early stage (70%) and grade (43.8%) disease with a median age of 60.5 years. We observed a statistically greater weight loss in the Telemedicine arm [median loss: 9.7 kg (range: 1.6–22.9 kg)] versus 3.9 kg (range: 0.3–11.4 kg) in the Text4Diet arm (p = 0.0231). Similarly, percent weight loss was greater in the Telemedicine (7.6%) as compared to the Text4Diet arm (4.1%, p = 0.014). Mean serum levels of IL-2 were significantly (27.15 pg/mL vs. 5.18 pg/mL, p = 0.0495) lower at intervention end as compared to baseline.
Conclusions.
A technology-based weight loss intervention is feasible in women with Type I endometrial cancer/hyperplasia. Both interventions produced weight loss, although more person-to-person contact produced more significant outcomes. Reductions in expression of IL-2 were related to weight loss.
Keywords: Obesity, Endometrial cancer, Weight loss intervention, Technology-based intervention, Biomarkers
1. Background
Obesity is one of the strongest risk factors for the development of endometrial cancer (EC), the most common gynecologic malignancy in the United States. Approximately 80% of new endometrial cancer cases are classified as Type 1, or estrogen dependent disease [1], and 60% of these patients are obese [2]. Type 1 endometrial cancer is on the rise [2], and is projected to increase by as much as 55% in the United States by 2030, taking into account time plus the influence of hysterectomy, severe obesity, and smoking [3]. Because estrogen exposure is the major risk factor for the development of endometrial cancer, and the major source of excess endogenous estrogen in patients comes from the conversion of androstenedione to estrone by aromatase in the peripheral fat, obesity is an important and preventable risk factor for the development of endometrial cancer. Specifically, one study showed a relative risk of developing endometrial cancer of 2.53 in patients with BMI 30–34 kg/m2 and of 6.25 for patients with BMI > 40 kg/m2 [4].
Although the mechanisms are not completely elucidated, obesity is thought to increase the risk of endometrial cancer through several endocrine pathways involving insulin, insulin-like growth factor-1, sex steroids, and adipokines [5]. These factors may play roles in cancer predilection via multiple metabolic alterations including insulin resistance, hyperglycemia, dyslipidemia, chronic inflammation, oxidative stress, altered adipocyte functioning, and immune dysfunction [5,6]. Increased levels of IGFBP-1, adiponectin, interleukins, and VEGF have all been associated with increased endometrial cancer risk [7–10]. These factors all contribute to the increased risk of endometrial cancer due to obesity and are in turn influenced by diet, weight change, body fat distribution, and physical activity [5].
Despite the knowledge by physicians that obesity is a risk factor for endometrial cancer, data suggest that many patients are unaware of this association [11], and studies have shown that endometrial cancer survivors have lower physical activity levels [12] and higher weight gain after diagnosis [13] compared to the non-cancer population. Early stage endometrial cancer has a generally favorable prognosis, but EC patients with increased BMI have lower physical, functional, and emotional well-being after diagnosis compared to patients with a normal BMI [13]. Additionally, one study of early stage EC patients demonstrated increased mortality in patients who were obese compared to normal weight patients [14]. Cardiovascular disease (for which obesity is a main risk factor) has been demonstrated as the leading cause of death of EC patients, and this risk of death surpasses that from endometrial cancer 5 years after diagnosis [15].
The role of dietary interventions and exercise on weight loss, quality of life measures, and cancer recurrence risk is being increasingly examined as an adjunct intervention for cancer survivors. In 2012 the American Cancer Society recommended that individuals maintain normal weight, engage in physical activity for at least 150 min/week, and eat a healthy diet high in fruits and vegetables to reduce cancer risk [16]. Additionally, studies of weight loss and dietary interventions in non-gynecologic cancers with increased BMI as a risk factor have suggested the utility of weight loss and dietary interventions. One review demonstrated that diet interventions led to weight loss in prostate cancer survivors [17], and recent studies in the breast cancer literature have reported favorable associations between increased physical activity and breast cancer mortality and low-fat diet on breast cancer prognosis [17–21]. Within the gynecology literature, recent studies have investigated the role of lifestyle interventions in endometrial cancer survivors and have suggested positive changes in weight, diet, and exercise, although the long-term clinical implications are still unknown [22]. In-person weight loss interventions often present a significant burden to the patient, however electronically delivered intervention (via text messaging and telephone) may reduce this burden with similar effectiveness. Further, there is a growing body of evidence that shows that weight loss interventions may change levels of cancer-associated bio-markers including leptin, VEGF, adiponectin, IL-6, IGFBP-1, IGF-1, TNF-α, CRP, estrogen, and SHBG [23–25]. This may in turn affect quality of life measures in cancer survivors [24] and suggests that weight loss may reduce cancer recurrence risk and mortality outcomes.
For this reason, a study investigating the effects of weight loss interventions in endometrial cancer survivors is warranted. As such, our primary objective was to assess both the feasibility for participation and successful weight loss utilizing technology-based weight loss interventions in patients at risk for endometrial cancer (with a diagnosis of endometrial hyperplasia) or patients with known Type I endometrial cancer. Our hypothesis was that, despite being an overall older, oncologic patient population, women would be motivated and have the technological capability to be enrolled in a trial designed to evaluate a novel intervention for weight loss. We hypothesized that women in the telemedicine arm would lose a statistically significant greater amount of weight due to a higher level of personal accountability than those in the texting arm. Our secondary objective was to assess change in potential cancer-associated biomarkers pre- and post-intervention. We hypothesized that weight loss would correlate with a decrease in inflammatory cancer-related biomarkers.
2. Materials and methods
We initially assessed a convenience sample of 81 women between August 2012 and May 2013 who had a biopsy-proven diagnosis of either endometrial hyperplasia or endometrial carcinoma and presented for outpatient care at the Gynecologic Oncology practices at the University of Pennsylvania. Women with obesity (BMI > 30 kg/m2) who were 18 years of age or older and English-speaking were asked to complete a self-administered survey to identify interest in participation. This study was reviewed and approved by the University of Pennsylvania’s Institutional Review Board (IRB Approval #815858, initial approval 7/19/12) and all participants provided written informed consent prior to any study activities.
In advance of scheduled appointments, we prescreened electronic medical records for both a history of endometrial hyperplasia/carcinoma and most current BMI. Women who met the inclusion criteria (n = 172), were provided with an overview of the study and given a survey to complete. Eighty one women (47%) agreed to complete the survey.
The survey queried demographic factors, such as age, weight, height and income. Additional questions asked about subjects’ self-perception of height, weight and BMI category, knowledge of the effect of obesity on cancer risk, diet and exercise patterns and interest in participation in a weight loss intervention. Questions were selected from a bank of previously validated questions provided by the Center for Disease Control and Prevention (CDC), World Health Organization (WHO), Behavioral Risk Factor Surveillance System and the Harvard Forums of Health Survey.
Of these 81 patients, the women were asked about their interest in participation in a formal weight loss support program and asked to quantify their weight loss goal in pounds. The questionnaire assessed the availability of access to different technologies, including text messaging and private wireless (WiFi) internet as well as patient preference on different platforms of delivery of a weight loss intervention, including in-person meetings, telephone, text messages, or internet.
Subjects were then screened for inclusion criteria for enrollment into the intervention and contacted to assess their interest in participating. Intervention inclusion criteria included: an ECOG performance status of 0–1, the ability to read and speak English and access to either wireless Internet to use the Withings Wi-Fi scales or a smart phone, such as an Android phone or Apple iPhone, to use Santech’s Text4Diet™ program. Exclusion criteria included: free of uncontrolled serious medical or psychiatric conditions that would affect their ability to participate in an intervention study, a diagnosis of any other invasive malignancy, active treatment with either cytotoxic chemotherapy or radiation, and life expectancy of less than 1 year. Twenty women elected to participate and were randomized 1:1 to the two 6-month weight loss interventions (Telemedicine arm: n = 10, Texting arm: n = 10).
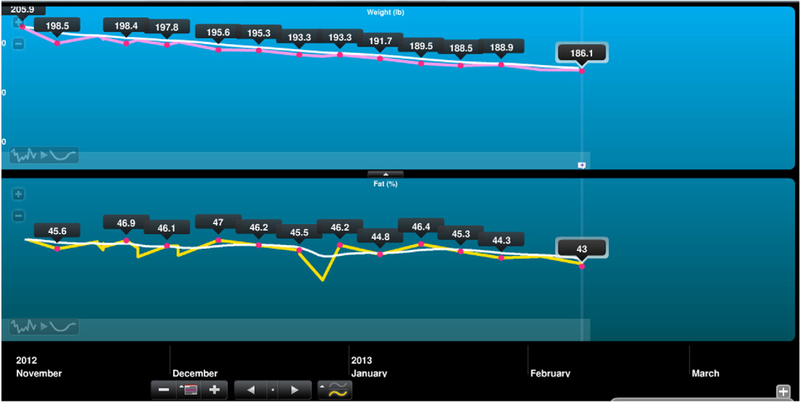
The Telemedicine arm participated in weekly telephone counseling sessions with weights being recorded via a WiFi scale from Withings©. The counseling was performed by two interventionists (a master’s level clinician and a medical doctor), supervised by a licensed psychologist, and counseling for each participant was performed by the same interventionist throughout the 6 months. A previously successful weight loss counseling program, the Diabetes Prevention Program [26], was adapted for this patient population with weekly thematic dialogues, including teaching standard weight loss skills, self-monitoring, problem-solving, enlisting social support, overcoming negative thoughts, and tracking caloric intake and exercise. The Wi-Fi scale graphed participants’ weights through an internet platform that was available to both the participant at the study staff (Fig. 1) for real-time feedback on participants’ progress, without requiring in-person visits. Phone sessions occurred weekly for 16 weeks, then bi-weekly for weeks 18–24.
Fig. 1.
Example of internet view of weights (top) and percent body fat (bottom) graphed by WiFi scale.
The Texting arm received a text messaging program called Text4Diet® that delivered 3–5 personalized text messages daily on various monthly themes. The messages included different types of interaction, such as encouraging statements and yes/no or multiple choice questions. The participants’ weights were requested weekly. The program kept the short message service (SMS) texts fresh and non-repetitive by varying the type and content of the messages sent throughout the week to so that users were engaged and aware of their behaviors. The program was personalized (used first names). SMS provided feedback, support, prompting, and strategies to encourage behaviors associated with long-term weight management. The SMS engine (SanText) used data (rules, participant information, day of week, behavioral topic, etc.) to determine the appropriate SMS to send to each user. When a user’s reply was received, it was stored in the database, and then the content of the reply was compared against the rules table to determine what (if any) additional SMS needs to be sent. The program detected database anomalies or unexpected participant responses, and errors were immediately corrected.
All subjects were instructed to limit their calories to 1200–1500 kcal/day if they weighed less than 250 lb, and 1500–1800 kcal/day if they weighed greater than 250 lb. Calorie intake was recorded daily in booklets provided by the study and reviewed weekly in the Telemedicine arm; these logs were returned at study completion in the Texting arm. The women also participated in a walking program (or an exercise of their choice), systematically walking/exercising up to 30 min a day by intervention end. Subjects had height (in.) and weight (kg) recorded at their screening visit and at study completion at six months. The primary outcome was defined as weight change [pre-enrollment weight minus post-weight (kg)] during the six-month intervention period, measured in absolute weight in kilograms.
All subjects underwent pre- and post-intervention biomarker analysis. We utilized bead based ELISA methods, which allow for the analysis of multiple analytes with a single sample, on sera that was collected on study enrollment and at the six month study completion visit. Beads were mixed and run simultaneously in a single well. This technique utilizes distinctly colored beads or microspheres created by combining two fluorescent dyes at distinct ratios. The distinct signature of each bead allows manufacturers to mix beads resulting in the multiplexing technique known as xMAP, originally developed by Luminex. We observed sera levels of cytokines IL-8, IL-6, IL-1Beta, IL-2, IL-7 and VEGF, using a Millipore 6 plex kit. Additional single-plex assays were set up for adiponectin and IGFBP. Sera was defrosted on ice and added undiluted to the beads. Incubation and washing were done according to company specifications. The secondary outcome was defined as change in bio-marker level from pre- to post-intervention.
Statistical analysis was performed with descriptive statistics. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). All demographic and categorical data were reported as means (+/−SD) or medians (IQR) and compared using χ2 tests, Fisher’s exact tests or Wilcoxon–Mann–Whitney tests as appropriate. Logistic regression was utilized to evaluate the association between dependent and independent variables. With twenty subjects, we would have 80% power to detect a difference in weight loss of 6.5 lb. Two-sided p-values of <0.05 were considered statistically significant. Statistical analyses were performed using Stata 12.1 (StataCorp., College Station, Texas). Study results are reported with adherence to CONSORT 2010 guidelines.
3. Results
Detailed demographic data is summarized in Table 1 for the initial survey respondents and in Table 2 for the participants who were subsequently enrolled in the weight loss intervention. The weight loss participants were predominantly early stage disease (70.0% stage I in both the Telemedicine arm and Texting arm respectively). The overall median BMI of the intervention cohort was 34.5 kg/m2 and the median age was 60.5 years. There was no statistically significant difference in any of the patient demographics between the two intervention arms except for a higher grade histology in the Texting arm (p = 0.009).
Table 1.
Participant demographics for initial screening survey.
| Demographic | Valuea |
|---|---|
| Age (years), mean (SD) | 59.38 (11.12) |
| BMI (kg/m2), median (IQR) | 35.4 (32.2–43.5) |
| Range: 30–82.4 | |
| Race | |
| White | 54 (68.4) |
| Black | 20 (25.3) |
| Asian | 3 (3.8) |
| Other | 2 (2.5) |
| Ethnicity | |
| Hispanic | 5 (8.5) |
| Non-Hispanic | 54 (91.5) |
| Income | |
| <$25,000 | 17 (23.0) |
| $25,000–50,000 | 13 (17.6) |
| $50,000–75,000 | 15 (20.3) |
| >$75,0000 | 24 (32.4) |
| Unknown | 5 (6.7) |
| Stage | |
| IA | 40 (54.1) |
| IB | 14 (18.9) |
| II | 4 (5.4) |
| IIIA | 7 (9.5) |
| IIIB | 0 (0) |
| IIIC | 4 (5.4) |
| IV | 3 (4.1) |
| Unstaged | 2 (2.6) |
| Grade | |
| 1 | 30 (41.7) |
| 2 | 27 (37.5) |
| 3 | 15 (20.8) |
Values reported as n (%) unless otherwise specified.
Table 2.
Patient demographics for intervention participants.
| Value (median, IQR unless noted) | Telemedicine arm (N = 10) | Texting arm (N = 10) | p-Valuea |
|---|---|---|---|
| Age (years) | 63 (58–66) | 55 (54–62) | 0.29 |
| BMI (kg/m2) | 34.4 (33.1–39.1) | 34.6 (33.1–40.7) | 0.79 |
| Starting weight (lb) | 207 (195–221) | 203 (189–246) | 0.91 |
| Histology (n, %) | Hyperplasia: 2 (20%) | Hyperplasia: 2 (20%) | - |
| Endometrioid: 8 (80%) | Endometrioid: 8 (80%) | ||
| Grade (n, %) | Grade 1:6 (75%) | Grade 1:1 (12.5%) | 0.009 |
| Grade 2: 2 (25%) | Grade 2: 4 (50%) | ||
| Grade 3: 0(0%) | Grade 3: 3 (37.5%) | ||
| Stage (n, %) | Stage 1A: 5 (50%) | Stage 1A: 6 (60%) | 0.97 |
| Stage IB: 2 (20%) | Stage 2:1 (10%) | ||
| Stage 3C: 1 (10%) | 3 A: 1 (10%) | ||
| Hyperplasia: 2 (20%) | Hyperplasia: 2 (20%) |
p-Value of <0.05 considered statistically significant.
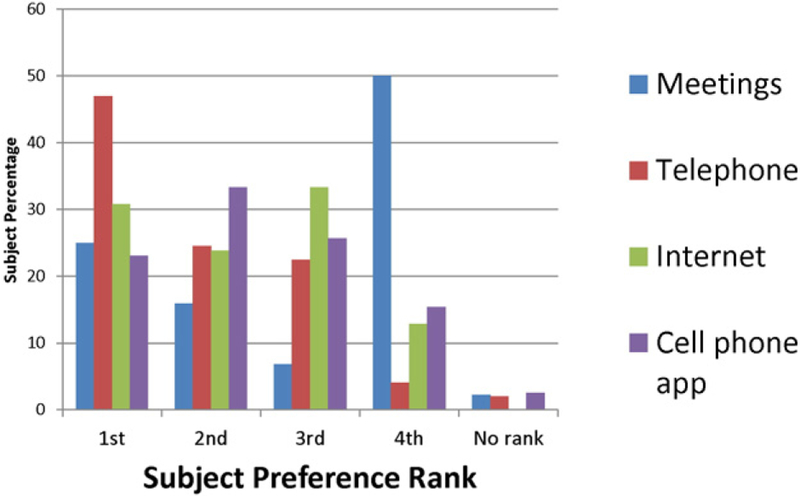
In the assessment of the feasibility of a technology-based weight loss intervention, 74.3% of survey respondents had text message capability and 65.7% had private wireless Internet access. Ultimately, 46.8% of participants were eligible for participation in our pilot intervention with both text and Internet access, and over half (59.3%) of subjects expressed interest in enrollment in an intervention. Patient interest in a variety of platforms for interventions (meetings, telephone, internet and cell phone application) was wide ranging (Fig. 2), but subjects most commonly indicated interest in telephone, with 47% ranking this option first, and Internet, with 31% ranking it first.
Fig. 2.
Subject ranking of interest in type of weight loss intervention: Ranking in order of preference for favored platform for participation in a weight loss intervention.
Ninety percent of participants lost weight (Telemedicine arm: n = 9, Texting arm: n = 9). Of those eighteen women who lost weight, we observed a statistically greater weight loss in the Telemedicine arm with a median loss of 9.7 kg (range: 1.6–22.9 kg) compared to 3.9 kg (range: 0.3–11.4 kg) in the Texting arm (p = 0.0231). Participants in the Tele-medicine arm had a statistically significant greater difference in percentage of weight change (defined as weight change divided by original weight) compared to the Texting arm, with a median decrease of 7.6% compared to 4.1%, respectively (p = 0.041). According to American Heart Association Guidelines, a weight loss greater than 5% is considered to be clinically significant [26]. Linear regression was performed to adjust for histologic grade determining that there was not a significant relationship between grade and weight loss in the overall cohort (p = 0.242).
Following completion of the program, prospective data from clinical cancer surveillance visits were abstracted to determine long-term weight changes at 3, 6, and 12 months after study completion. Participants’ weights remained stable without further weight loss at 3 months after intervention completion, but further weight loss was obtained at 6 months in 10% of participants (0.64 kg median loss from baseline in text arm only). At 12 months, 25% of the cohort was successful with continued weight loss compared to the intervention completion weight (4.0 kg vs. 2.7 kg additional weight loss in the telemedicine versus the WiFi arms). Of those individuals who initially lost weight at 6 months of follow-up, 100% were able to continue further weight loss at 12 months. Overall, 20% in the telemedicine arm and 30% in the text arm were able to maintain their weight loss at 12 months after completion of the intervention (p = 0.25).
Mean serum levels of IL-2 demonstrated a significant difference between pre- and post-intervention levels, and there was a decrease in the level of IL-1 Beta that trended towards significance (Table 3). The levels of IGFBP, VEGF, IL-6 and IL-8 decreased, and the levels of adiponectin and IL-7 increased, although these did not demonstrate statistical significance in this small sample size.
Table 3.
Mean levels of biomarkers at baseline and end of intervention, mean (SD), for the total sample.
| Biomarker | Baseline (N = 21) (Pg/mL) | 6 months (N = 18) (Pg/mL) | p-Valuea |
|---|---|---|---|
| IL-2 | 27.15 (112.65) | 5.18 (7.97) | 0.0495 |
| IL-1 beta | 18.78 (75.61) | 7.58 (14.38) | 0.0554 |
| IGFBP | 140.63 (215.81) | 109.61 (97.9) | 0.689 |
| VEFG | 1778.0 (2764.0) | 1635.93 (2654.95) | 0.57 |
| IL-6 | 28.35 (81.35) | 16.21 (30.37) | 0.3844 |
| IL-8 | 287.1 (728.18) | 272.83 (813.34) | 0.8866 |
| Adiponectin | 9,326,925 (6,429,249) | 1,060,007 (6,769,987) | 0.238 |
| IL-7 | 5.58 (103.69) | 8.85 (18.24) | 0.4611 |
p-Value of <0.05 considered statistically significant.
4. Discussion
Based on survey results, we identified a cohort of obese patients with endometrial hyperplasia/cancer who self-selected as being interested in participating in a weight loss program. Almost 60% of the women surveyed expressed interest in participating in an intervention, and almost half of the overall cohort were eligible for enrollment based on their technology capabilities, despite being older with an average age of 59 years, reflective of the endometrial cancer patient population. Additionally, patients reported a variety of interests in different platforms for delivery of a weight loss intervention, most commonly reporting interest in programs that did not require face-to-face visits (i.e. via telephone or Internet) and which were more easily integrated into daily lives. Previous studies have investigated several different methods of lifestyle interventions for endometrial cancer patients. In the SUCCEED trial, weekly group counseling sessions were provided in addition to physician face-to-face counseling and ongoing support after the intervention ended [22]. In addition, multiple trials have demonstrated the success of web- and mobile applications for weight loss in overweight individuals generally, and one recent study showed the successful use of a mobile weight-loss app with tailored multi-disciplinary team feedback for short-term weight loss specifically in endometrial and breast cancer survivors [25,27–31]. However, the variety of preferences of patients in our study speaks to the indication that a single intervention platform may not likely be broadly successful for all women, and instead that individualized programs that offer a format that is appealing and suitable for an individual patient may the one most likely to elicit success, especially in the long term.
Previous studies investigating the role of lifestyle interventions for weight loss in endometrial cancer survivors have shown efficacy. The SUCCEED trial, which randomized patients to either a lifestyle intervention which included weekly education and counseling or usual care demonstrated a significant difference in weight loss between the two groups at both 6 and 12 months, as well as significant differences in physical activity, calories consumed, and intake of fruits and vegetables [22]. Importantly, given the need for long-term health maintenance to prevent cardiovascular and other health risks associated with obesity, this trial demonstrated a positive effect on self-efficacy in the lifestyle intervention group [30].
The current study was consistent with previous research indicating the efficacy of lifestyle interventions on weight loss and also demonstrated the feasibility of the two interventions tested, despite prior concerns that older patients may not be comfortable using mobile or web-based technology. Twenty women were randomized to participate in a novel technology-based weight loss intervention and nearly all (90%) were successful in losing weight. Our study was the first to compare different types of interventions head to head. As hypothesized, there was a clinically greater weight loss in those randomized to the Telemedicine arm as compared to the Texting arm. We theorize that this was due to the personal contact with a single interventionist in weekly 20–30 minute counseling sessions. However, the participants in the Texting arm were also successful in losing weight. This has potential implications for the specific type of weight loss intervention that could be offered to patients based on their age, income, education, and access to and comfort with mobile and web-based technology. The choice of program may also be influenced by the resources available to a specific oncology or other medical practice, if such practices are able to coordinate this ancillary care. Additionally, a cancer diagnosis provides an opportunity to capitalize on a “teachable moment,” and healthcare providers may be in an optimal position to offer guidance on improved health behaviors such as diet, weight loss and exercise.
In this study, changes in expression of IL-2 were affected by weight loss. However, this was a small sample size, and thus our results warrant further study in a larger trial to test the impact of weight loss on cancer-related biomarkers. Additionally, the impact of changes in weight, expression of various inflammatory biomarkers, and clinical outcomes remains to be seen. Although multiple serum biomarkers have been associated with an increased risk in developing endometrial cancer, it is unclear whether they may be used to track disease progression or recurrence or other disease conditions. Further studies examining the long-term cancer recurrence rate, cardiovascular health, other obesity-related comorbidities, and mortality after weight loss interventions in endometrial cancer survivors are warranted.
Our study is not without limitations. We obtained a lower response rate in our initial enrollment screening survey compared to prior studies reported in the literature. However, this lower response rate does not capture those patients who qualified but were not offered a survey by the clinic administrative staff due to office work flow, and instead is calculated from a denominator of all obese women with endometrial hyperplasia or Type I endometrial cancer who were identified as eligible to be offered a survey. Another limitation is that these data were collected at a single urban tertiary care academic institution during office visits for ongoing care of endometrial cancer, and may not be generalizable to the general knowledge base of all women with endometrial cancer. The main limitation in internet access for this urban population was the restriction that participants frequently did not have “private” WiFi access that was required by the scale model used for this study (i.e. participants were using public internet and could not sign into the network to connect with the scale). However, newer models of the scale currently being utilized by these investigators for further study do not require “private” WiFi, theoretically expanding the patient population who may have access to a service provider through their cell phone. While this patient population is obese, with an overall BMI of 34.5 kg/m2, this is not necessarily fully representative of the burden of obesity in the endometrial patient population. It is unknown how the results may have differed if patients with more extreme obesity were more fully represented. Additionally, this was a patient population of predominantly early stage and grade cancer. However, it is this patient population who experience a 96% 5-year survival [15], and in whom cardiovascular adverse events related to obesity may significantly affect future morbidity and mortality, rather than the cancer itself. Thus it could be argued that this is the optimal patient population in which to enact weight loss interventions for maximal health benefit.
The effect of decreasing BMI on oncologic outcomes for obese women with endometrial hyperplasia and cancer is uncertain and warrants further study. In addition, there is a paucity of data evaluating either weight loss interventions in patients with endometrial hyperplasia/cancer or the relationship of physical activity, diet and weight with quality of life and survivorship. It may be that weight alone is not the only factor contributing to morbidity and mortality in our patient population, and future studies should investigate these variables specifically. With the growing number of mobile applications designed to track nutrition and exercise, this would seem to be an advantage of technology-based weight loss interventions. However, we have demonstrated that, despite an overall older patient population, technology-based weight loss interventions are successful and feasible. Given this high prevalence of obesity in our country, it is essential to develop appropriate interventions to reduce cancer incidence, morbidity and death among obese women at risk for endometrial cancer as well as for endometrial cancer survivors.
HIGHLIGHTS.
Obese EC survivors are interested in technology-based weight loss interventions
Obese EC survivors lost weight in 6-month weight loss interventions with telemedicine and text messaging modalities
Levels of IL-2 decreased after weight loss
Funding sources
Funding for this study was provided by pilot grant funding from the University of Pennsylvania Transdisciplinary Research on Energetics and Cancer (TREC) (U54-CA155850). Dr. Haggerty was also supported by funding from an NIH Training Grant T32 HD007440.
Footnotes
Presented as an abstract at the 45th Annual Society of Gynecologic Oncology Annual Meeting on Women’s Cancer,Tampa, FL, March 22–25, 2014.
Conflicts of interest
The authors report no con flict of interest.
References
- [1].Epplein M, Reed SD, Voight LF, Newton KM, Holt VL, Weiss NS, Risk of complex and atypical endometrial hyperplasia in relation to anthropometric measures and reproductive history, Am. J. Epidemiol 168 (6) (2008) 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Duong LM, Wilson RJ, Ajani UA, Singh SD, Eheman CR, Trends in endometrial cancer incidence rates in the United States, 1999–2006, J. Women’s Health 20 (8) (2011) 1157–1163. [DOI] [PubMed] [Google Scholar]
- [3].Sheikh MA, Althous AD, Freese KE, Soisson S, Edwards RP, Welburn S, Sukumbanich P, Comerci J, Kelley J, LaPorte RE, Linkov F, USE Endometrial Cancer Projections to 2030: should we be concerned? Future Oncol 10 (16) (2014) 2561–2568. [DOI] [PubMed] [Google Scholar]
- [4].Weiderpass E, Persson I, Adami HO, Magnusson C, Lindgren A, Baron JA, Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden), Cancer Causes Control 11 (2) (2000) 185–192 (Feb). [DOI] [PubMed] [Google Scholar]
- [5].Mohamed H, McNeill G, Haseen F, N’Dow J, Craig LC, Heys SD, The effect of dietary and exercise interventions on body weight in prostate cancer patients: a systematic review, Nutr. Cancer 67 (1) (2015) 43–60. [DOI] [PubMed] [Google Scholar]
- [6].Linkov F, Burke LE, Komaroff M, Edwards RP, Lokshin A, Styn MA, Tseytlin E, Freese KE, Bovbjerg DH, An exploratory investigation of links between changes in adipokines and quality of life in individuals undergoing weight loss interventions: possible implications for cancer research, Gynecol. Oncol 133 (1) (2014) 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Soliman PT, Wu D, Tortolero-Luna G, Schmeler KM, Slomovitz BM, et al. , Association between adiponectin, insulin resistance and endometrial cancer, Cancer 106 (11) (2006) 2376–2381. [DOI] [PubMed] [Google Scholar]
- [8].Yurkovetsky Z, Ta’asan S, Skates S, Rand A, Lomakin A, Linkov F, et al. , Development of multimarker panel for early detection of endometrial cancer. High diagnostic power of prolactin, Gynecol. Oncol 107 (1) (2007) 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bellone S, Watts K, Cane S, Palmieri M, Canon MJ, Burnett A, et al. , High serum levels of internleukin-6 in endometrial carcinoma are associated with uterine serous papillary histology, a highly aggressive and chemotherapy-resistant variant of endometrial cancer, Gynecol. Oncol 98 (1) (2005) 92–98. [DOI] [PubMed] [Google Scholar]
- [10].Park J, Morley TS, Kim M, Clegg DJ, Scherer PE, Obesity and cancer—mechanisms underlying tumour progression and recurrence, Nat. Rev. Endocrinol 10 (2014) 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Soliman PT, Bassett RL Jr., Wilson EB, Boyd-Rogers S, Schmeler KM, Milam MR, et al. , Limited public knowledge of obesity and endometrial cancer risk, Am. J. Obstet. Gynecol 112 (2008) 835–842. [DOI] [PubMed] [Google Scholar]
- [12].Kwon S, Hour N, Wang M, Comparison of physical activity levels between cancer survivors and non-cancer participants in the 2009 BRFSS, J. Cancer Surviv (2011) (Epub). [DOI] [PubMed] [Google Scholar]
- [13].Fader AN, Frasure HE, Gil KM, Berger NA, von Gruenigen VE, Quality of life in endometrial cancer survivors: what does obesity have to do with it? Obstet. Gynecol. Int 1–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].von Gruenigen VE, Tian C, Frasure H, et al. , Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma: a Gynecologic Oncology Group study, Cancer 107 (2006) 2786–2791. [DOI] [PubMed] [Google Scholar]
- [15].Ward KK, Shah NR, Saenz CC, McHale MT, et al. , Cardiovascular disease is the leading cause of death among endometrial cancer patients, Gynecol. Oncol 126 (2) (2012) 176–179. [DOI] [PubMed] [Google Scholar]
- [16].American Cancer Society (ACS) Guidelines on Nutrition and Physical Activity for Cancer Prevention. http://www.cancer.org/healthy/eathealthygetactive/acsguidelinesonnutritionphysicalactivityforcancerprevention/nupa-guidelines-toc. [PubMed]
- [17].Vitolins MZ, Milliron BJ, Hopkins JO, Fulmer A, Lawrence J, Melin S, Case D, Weight loss intervention in survivors of ER/PR-negative breast cancer, Clin. Med. Insights Womens Health 7 (2014) 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pierce JP, Stefanick ML, Flatt SW, et al. , Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity, J. Clin. Oncol 25 (2007) 2345–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Holick CN, Newcomb PA, Trentham-Dietz A, et al. , Physical activity and survival after diagnosis of invasive breast cancer, Cancer Epidemiol. Biomark. Prev 17 (2008) 379–386. [DOI] [PubMed] [Google Scholar]
- [20].Pierce JP, Natarajan L, Caan BJ, et al. , In fluence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial, JAMA 298 (2007) 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kwan ML, Weltzien E, Kushi LH, et al. , Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer, J. Clin. Oncol 27 (2009) 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].von Gruenigen V, Frasure H, Kavanagh MB, Janata J, Waggoner S, Rose P, Lerner E, Courneya KS, Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial, Gynecol. Oncol 125 (3) (June 2012) 699–704. [DOI] [PubMed] [Google Scholar]
- [23].Linkov F, Maxwell GL, Felix AS, Lin Y, Lenzner D, Bovbjerg DH, Lokshin A, Hennon M, Jakicic JM, Goodpaster BH, DeLany JP, Longitudinal evaluation of cancer-associated biomarkers before and after weight loss in RENEW study participants: implications for cancer risk reduction, Gynecol. Oncol 125 (1) (2012) 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Byers T, Sedjo RL, Does intentional weight loss reduce cancer risk? Diabetes Obes. Metab 13 (12) (2011) 1063–1072. [DOI] [PubMed] [Google Scholar]
- [25].Kushi LH, Coyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T, American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee, American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity, CA Cancer J. Clin 62 (1) (2012) 30–67. [DOI] [PubMed] [Google Scholar]
- [26].Jensen MD, Ryan DH, Apovian CM, Ard JD, Commuzzie AG, et al. , 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults, Circulation 219 (2014) S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].The Diabetes Prevention Program Research Group, Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin, N. Engl. J. Med 346 (2002) 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carter MC, Burley VJ, Nykjaer C, Elizabeth CJ, Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial, J. Med. Internet Res 15 (4) (April 2013), e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van Genugten L, van Empelen P, Boon B, Borsboom G, Visscher T, Oenema A, Results from an online computer-tailored weight management intervention for over-weight adults: randomized controlled trial, J. Med. Internet Res 14 (2) (Mar-Apr 2012), e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McCarroll ML, Armbruster S, Pohle-Krauza AJ, Lyzen AM, Min S, Nash DW, Roulette GD, Andrews SJ, von Gruenigen VE, Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application, Gynecol. Oncol 137 (3) (June 2015) 508–515. [DOI] [PubMed] [Google Scholar]
- [31].McCarroll ML, Armbruster S, Frasure HE, Gothard MD, Gil KM, Kavanagh MB, Waggoner S, von Gruenigen VE, Self-efficacy, quality of life, and weight loss in over-weight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial, Gynecol. Oncol 132 (2) (February 2014) 397–402. [DOI] [PubMed] [Google Scholar]