Abstract
Background:
Schistosomiasis caused by Schistosoma mansoni (Sm) is a chronic, debilitating and potentially deadly neglected tropical disease. The licensure of a vaccine to prevent schistosomiasis would represent a major breakthrough in public health.
Methods:
The safety and immunogenicity of a candidate Sm vaccine were assessed in this phase I, double-blind, dose-escalation trial. Seventy-two healthy Sm-naïve 18–50 year olds were randomized to receive 3 doses~ 8 weeks apart of saline placebo, or 10 μg, 30 μg, or 100 μg of recombinant Sm-Tetraspanin-2 vaccine formulated on aluminum hydroxide adjuvant (Sm-TSP-2/Al) with or without 5 μg of glucopyranosyl lipid A aqueous formulation (GLA-AF). Clinical and serologic responses were assessed for 1 year after dose 3.
Results:
Vaccines were safe and well-tolerated. The most common reactions were injection site tenderness and pain, and headache and fatigue. Tenderness and pain were more frequent in groups receiving vaccine with GLA-AF than placebo (p = 0.0036 and p = 0.0014, respectively). Injection site reactions among those given Sm-TSP-2/Al with GLA-AF lasted 1.22 and 1.33 days longer than those receiving Sm-TSP-2/Al without GLA-AF or placebo (p < 0.001 for both).
Dose- and adjuvant-related increases in serum IgG against Sm-TSP-2 were observed. Peak IgG levels occurred 14 days after dose 3. Seroresponse frequencies were low among recipients of Sm-TSP-2/Al without GLA-AF, but higher among subjects receiving 30 μg or 100 μg of Sm-TSP-2/Al with GLA-AF. More seroresponses were observed among those given 30 μg or 100 μg of Sm-TSP-2/Al with GLA-AF compared to placebo (p = 0.023 and p < 0.001, respectively). Seroresponse frequencies were 0%, 30%, 50%, and 89%, respectively, among those given placebo, or 10 μg, 30 μg or 100 μg of Sm-TSP-2/Al with GLA-AF, suggesting a dose-response relationship for Sm-TSP-2/Al with GLA-AF (p = 0.0001).
Conclusions:
Sm-TSP-2/Al with or without GLA-AF was safe and well tolerated in a Sm-naïve population. A vaccine like the one under development may represent our best hope to eliminating this neglected tropical disease.
Keywords: Schistosomiasis, Schistosoma mansoni (Sm), Sm tetraspanin 2 (Sm-TSP-2), Immune responses, Immunization, Glucopyranosyl lipid A, Adjuvant
1. Introduction
Schistosomiasis is estimated to be the second most deadly parasitic infection of humans after malaria [1,2]. A recent Global Burden of Disease report estimates that 190 million people are infected with schistosomes, with 71 million new infections occurring annually [3]. Children, adolescents, and women of reproductive age experience the highest levels of infection, as determined by fecal egg counts, leading to growth and cognitive delays as well as severe end-organ pathology in the intestines, liver, and urogenital tract. Two schistosome species [S. mansoni (Sm) and S. haematobium (Sh)] account for more than 99% of all human infections, with Sm accounting for approximately one-third of the infections (and almost one-half of the deaths) worldwide [5]. While most cases of schistosomiasis caused by Sm occur in sub-Saharan Africa, the second largest endemic focus is Brazil [1,2,4].
Praziquantel (PZQ) is the preferred treatment for schistosomiasis. However, control programs based on mass drug administration (MDA) with PZQ are difficult and expensive to maintain, fail to interrupt transmission, and are complicated by rapid and frequent re-infection following treatment. Furthermore, recent studies suggest Sm has the potential to develop resistance to this drug [6,7]. There is additional evidence for serious rebound morbidity if regular and periodic treatments with PZQ are interrupted [8,9]. The development, testing and licensure of a vaccine to prevent schistosomiasis would represent a major breakthrough in public health. An effective vaccine against schistosomiasis would overcome many of the problems associated with the current disease control strategies. Ultimately, a vaccine could become an essential biotechnology for the elimination of schistosomiasis.
Several lines of evidence indicate that vaccination with the tetraspanin 2 protein (TSP-2) of Sm (Sm-TSP-2) may elicit protective immune responses against infection: as an antigen on the surface of newly transformed schistosomula, TSP-2 is highly accessible to the host antibody response [10,11]; Sm-TSP-2 is uniquely recognized by antibodies from individuals naturally resistant to Sm infection (i.e., ‘endemic normals’) [11]; suppression of Sm-TSP-2 RNA expression results in malformed adult worm tegument [12]; and vaccination with recombinant Sm-TSP-2 protects different strains of mice against schistosomal challenge infection when formulated with aluminum hydroxide, as evidenced by significant reductions in adult worm, liver egg, and fecal egg burdens compared to controls [11].
The goals of the current study were to assess the safety, reactogenicity, and immunogenicity of Sm-TSP-2/Alhydrogel® (hereafter referred to as Sm-TSP-2/Al) with and without glucopyranosyl lipid A in aqueous formulation (GLA-AF) among healthy adults residing in a non-Sm endemic area.
2. Materials and methods
2.1. Study design
This phase 1, randomized, double-blind, placebo-controlled, dose-escalation clinical trial was conducted at the Baylor College of Medicine NIH-funded Vaccine and Treatment Evaluation Unit. The protocol was approved by the relevant ethics committee and written informed consent was provided by subjects prior to enrollment ().
2.2. Vaccines
The Sm-TSP-2 schistosomiasis vaccine tested in this study was technology transferred and produced according to current Good Manufacturing Practice at Aeras (Maryland, USA) as a recombinant protein expressed in a modified Pichia Pink system and adsorbed to an aluminum hydroxide salt adjuvant (Alhydrogel®; Al) [13,14]. Sm-TSP-2/Al (0.1 mg Sm-TSP-2 absorbed to 0.8 mg/mL Al in 10 mM imidazole, 15% sucrose, 2 mM phosphate, pH 7.4 buffer; Lot 11-69F-003) was evaluated at three dose levels of the recombinant protein: 10 μg, 30 μg, and 100 μg; these were delivered by administering different volumes (0.1 ml, 0.3 ml and 1.0 ml, respectively) of a 0.1 mg/ml suspension of recombinant Sm-TSP-2 in 0.8 mg/ml Al.. Each dose level was administered with or without 5 μg of the Toll-like receptor (TLR-4) agonist GLA-AF supplied by the Infectious Diseases Research Institute (IDRI; Seattle, WA). GLA-AF was combined with Sm-TSP-2/Al vaccine within 4 h prior to administration. Placebo was sterile normal saline for injection.
2.3. Clinical procedures
Seventy-two healthy individuals 18–50 years of age (inclusive), non-pregnant females and males with no history of past Sm infection and with no significant risk for exposure to Sm were enrolled. Detailed inclusion and exclusion criteria are available in online Supplemental materials. Three cohorts were enrolled sequentially in a dose-escalation fashion based on the Sm-TSP-2 dose level (cohort 1 = 10 μg; cohort 2 = 30 μg; and cohort 3 = 100 μg of Sm-TSP-2/Al). Within each cohort, subjects were assigned randomly to 1 of 3 groups, as follows: Sm-TSP-2/Al without GLA-AF (N = 10); Sm-TSP-2/Al with GLA-AF (N = 10); or placebo (N = 4 subjects) (Table 1). Subjects received 3 doses of the assigned vaccine delivered into the deltoid muscle using a 1 in. needle on Days 1, 57 (+/−7 days), and 113 (+/−7 days). Subjects were followed for 12 months after the third dose of vaccine.
Table 1.
Schematic of Study Design.
| Cohort | Group | Study Product | Number |
|---|---|---|---|
| 1* | A | 10 μg Sm-TSP-2/Alhydrogel® | 10 |
| B | 10 μg Sm-TSP-2/Alhydrogel®/GLA-AF | 10 | |
| C | Placebo† | 4 | |
| 2* | D | 30 μg Sm-TSP-2/Alhydrogel® | 10 |
| E | 30 μg Sm-TSP-2/Alhydrogel®/GLA-AF | 10 | |
| F | Placebo† | 4 | |
| 3 | G | 100 μg Sm-TSP-2/Alhydrogel® | 10 |
| H | 100 μg Sm-TSP-2/Alhydrogel®/GLA-AF | 10 | |
| I | Placebo† | 4 | |
| Total | 72 |
Saline for injection.
Dose escalation decisions were made after the last subject in the cohort completed the 7 day post dose 1 visit.
Within each cohort, the first 5 subjects were assigned randomly to receive Sm-TSP-2/Al without GLA-AF, Sm-TSP-2/Al with GLA-AF, or placebo in a 2:2:1 ratio. The subsequent 19 subjects were assigned randomly to these groups in an 8:8:3 ratio. For each cohort, the initial 5 subjects were enrolled, randomized, vaccinated, and completed the Day 3 visit before enrolling the rest of the cohort. Escalation to the next dose level cohort was determined based on evaluation of pre-defined escalation criteria by a Safety Monitoring Committee, requiring that 7-day safety data be reviewed after all subjects in the current cohort received their first dose of vaccine. As with dose-escalation decisions, evidence of significant reactogenicity required further review prior to proceeding. Recruitment and enrollment into the study occurred on an ongoing basis, with each cohort being recruited and vaccinated in sequence.
Enrollment was performed using an internet data entry system (AdvantageEDCSM). The unblinded pharmacist had exclusive access to the treatment key, which was kept in a secure location with limited access. Subjects, investigators, and study personnel performing any study-related assessments following study vaccine administration, and laboratory personnel performing antibody assays were blinded to group assignment.
Subjects were observed for at least 30 min after each vaccination to detect adverse events (AEs). Subjects recorded a daily oral temperature and the occurrence of injection site (pain, tenderness, erythema and induration) and systemic (chills, arthralgia, myalgia, headache, nausea, vomiting, dizziness, malaise, and fatigue) reactogenicity for 7 days after each vaccination. Subjects were also seen in-clinic 2 and 7 days after each immunization (i.e., study Days 3 and 8). Unsolicited AEs were assessed for 28 days after each dose. New-onset chronic medical conditions (NOCMCs), including adverse events of special interest (AESI), and serious AEs (SAEs) were recorded from the time of first study vaccination through approximately 12 months after the third study vaccination. Subjects graded the severity of their symptoms, as follows: mild (1; no interference with normal activities), moderate (2; some interference with normal activities), or severe (3; prevented normal activities). Mild fever was defined as an oral temperature of 100–101.1°F. Clinical laboratory safety evaluations [alanine aminotransferase, creatinine, white blood cell count, hemoglobin, and platelet levels] were performed on blood samples collected before each dose of vaccine, 7 days after the first and second doses of vaccine, and 14 days after the third vaccination. Serum samples for determination of IgG levels against Sm-TSP-2 were collected before and two weeks after each dose of vaccine and at approximately 3 and 6 months after the third dose of vaccine.
2.4. Laboratory Methods
2.4.1. Measurement of anti-Sm-TSP-2 IgG antibody
Sm-TSP-2-specific immunoglobulin G (IgG) antibodies were measured in serum by an indirect ELISA qualified for reproducibility, parallelism, ruggedness, and linearity, as described elsewhere for helminth vaccines [15–17]. This ELISA utilized heterologous interpolation of test values onto a standard reference serum (SRS) of IgG against Sm-TSP-2 consisting of pooled samples from BALB/c mice immunized with 9.33 μg Sm-TSP-2/74.64 μg Alhydrogel® in a prime-boost immunization regimen. The SRS was serially diluted in duplicate on each ELISA plate at a 1:400 initial dilution to generate a dilution-response curve whose values were modeled by a four-parameter logistic-log function in SOFTmax GXP PRO version 4 (Molecular Devices) [15–17]. In brief, 96-well plates (Nunc Maxisorp) were coated with recombinant Sm-TSP-2 at a concentration of 5 μg/mL and kept overnight at 2–8 °C. Plates were washed and blocked with 3% bovine serum albumin (Fitzgerald), and then serum samples added in duplicate at a 1:4000 dilution in the same blocking buffer. Following overnight incubation at 2–8 °C, plates were washed and a horseradish peroxidase conjugated secondary mouse anti-human IgG was added (ThermoFisher). Plates were further incubated at room temperature, washed, and developed using O-phenylenediamine dihydrochloride (OPD) (Sigma Aldrich) and read at OD492nm following the addition of 2 N H2SO4 on a SpectraMax Plus 384 Microplate Reader (Molecular Devices), with data collected using SOFTmax GXP PRO version 4. The mean OD492nm of duplicates of test sera were heterologously interpolated onto the 4-PL model of the SRS to derive the Arbitrary Units (AU) of anti-Sm-TSP-2 IgG.
2.4.2. Western blot Reactivity of Human Sera to Sm-TSP-2
To qualitatively discern the reactivity of subjects’ sera with Sm-TSP-2, Western blot screening was performed with a selected set of blinded samples. Recombinant Sm-TSP-2 [13,14,18] was loaded (2 μg) under non-reducing and reducing (with addition of 2-mercaptoethanol, βME) conditions on a 4–20% SDS-PAGE gel (Invitrogen) at 135 V for 80 min. An irrelevant control recombinant protein (Na-GST-1, also expressed in a Pichia Pink system) was loaded as a negative control. Gels were then either stained with SimplyBlue™ (Thermo) or transferred on to 0.2 μm nitrocellulose membranes for 60 min at 30 V. Following transfer, the blots were stained with Ponceau S (Sigma) to confirm protein transfer and protein loads, followed by blocking with 2% milk diluted in 1X-PBST-20 (0.05%). Serum samples were then diluted at 1:100 in blocking buffer and incubated for 60 min at room temperature. Blots were washed 3 times with 1X-PBST-20 (0.05%) and then incubated with alkaline phosphatase secondary antibody, goat antihuman IgG (Southern Biotech) at a dilution of 1:1000 in blocking buffer. Following subsequent 3 washes with 1X-PBST-20 (0.05%), the blots were developed for 15 min with BCIP/NBT substrate (Invitrogen), then stopped with multiple washes with ultra-pure water. Blots were scanned together on a flatbed scanner (Epson) for visual documentation.
2.5. Statistical methods
This phase 1 study was not designed to test a specific hypothesis. The primary objective was to assess the safety and reactogenicity of ascending doses of Sm-TSP-2/Al vaccine with or without GLA-AF in healthy adults, and to obtain data to properly design future trials. Primary safety endpoints were the occurrence of vaccine-associated SAEs and NOCMCs from the time of first vaccination through 12 months after the last study vaccination; solicited injection site and systemic reactogenicity during the 7 days after each vaccination; and clinical safety laboratory AEs related to the vaccine.
All subjects who received at least one dose of vaccine were included in the safety analyses. All subjects who received all 3 doses of study product were included in the per protocol (PP) immunogenicity population. The antibody intention-to-treat (ITT) population included subjects who received at least one dose and contributed antibody results for at least one post-baseline time point.
Safety data were summarized graphically and numerically. Proportions of subjects experiencing solicited symptoms were graphed by maximum severity, symptom, and treatment group, combining subjects who received placebo from the three cohorts into a single group. Fisher’s Exact Tests were used to compare the proportion of subjects experiencing at least one systemic event, and at least one injection site event, between treatment groups. For the analyses of solicited event data described below, subjects were combined across dose levels into three groups (Sm-TSP-2/Al without GLA-AF, Sm-TSP-2/Al with GLA-AF, and placebo) in order to maximize power to detect a signal.
To assess whether systemic reactogenicity increased with each successive dose, mixed effects logistic regression (i.e., a logistic-normal model; [19]) was used to estimate and test for the effect of dose number on the odds of experiencing a systemic symptom. The model included dose number as the fixed effect, random intercepts, and an independence correlation structure. The random intercepts account for correlation arising from the fact that each subject contributed up to three observations to the model (one for each dose). This model was fit using SAS PROC GLIMMIX with SAS version 9.4 and was repeated for the Sm-TSP-2/Al with GLA-AF group. These analyses were repeated to assess whether injection site symptoms increased with dose number.
The proportions of subjects experiencing solicited systemic symptoms within each group were graphed by post-vaccination day to assess differences in the timing of these events. In addition, a model was created to test whether the duration of systemic symptoms differed by treatment group. The duration of systemic symptoms was calculated as the number of days within the 7-day follow-up period that the subject experienced at least one systemic symptom. Mixed effects linear regression was used to model the mean duration of systemic symptoms by treatment group (Sm-TSP-2/Al without GLA-AF, Sm-TSP-2/Al with GLA-AF, and placebo). The model included treatment group indicators as predictors, random intercepts, and an independence correlation structure. The random intercepts account for correlation arising from the fact that the duration was measured up to three times on each subject (post-doses 1, 2, and 3). The analyses were repeated to assess whether the duration of injection site symptoms differed between treatment groups.
Serum IgG levels against Sm-TSP-2 were measured on the day of each dose, 14 days after each dose, and approximately 3 and 6 months following the third dose. Individual time trends of IgG against Sm-TSP-2 were plotted by cohort and treatment group. Levels of IgG against Sm-TSP-2 were summarized by their geometric mean level (GML) as well as by the number and percentage of responders (defined as at least a four-fold rise from baseline level) by treatment group and study day for the PP population and repeated for the ITT population. Proportions of responders at each time point were compared using Fisher’s Exact Tests within each cohort for each time point.
A Cochran Armitage test of trend was performed to assess for a possible dose-response relationship among subjects receiving Sm-TSP-2/Al with GLA-AF on Day 127, the day with the peak antibody response. The placebo group was included in the test with a dose level of zero. The test was also performed for Day 127 for those who receiving Sm-TSP-2/Al without GLA-AF, again including the placebo group as zero dose. For this latter test, event rates were too low for the test statistic to follow a chi-square distribution [20], so the p-value was instead calculated by a randomization test, as follows: treatment assignments were randomly permuted, and the chi-square test statistic was calculated for each permuted data set. This process was repeated 1000 times to approximate the null distribution of the test statistic. The estimated p-value was the proportion of simulated statistics greater than or equal to the observed statistic.
3. Results
3.1. Enrollment and Study Population
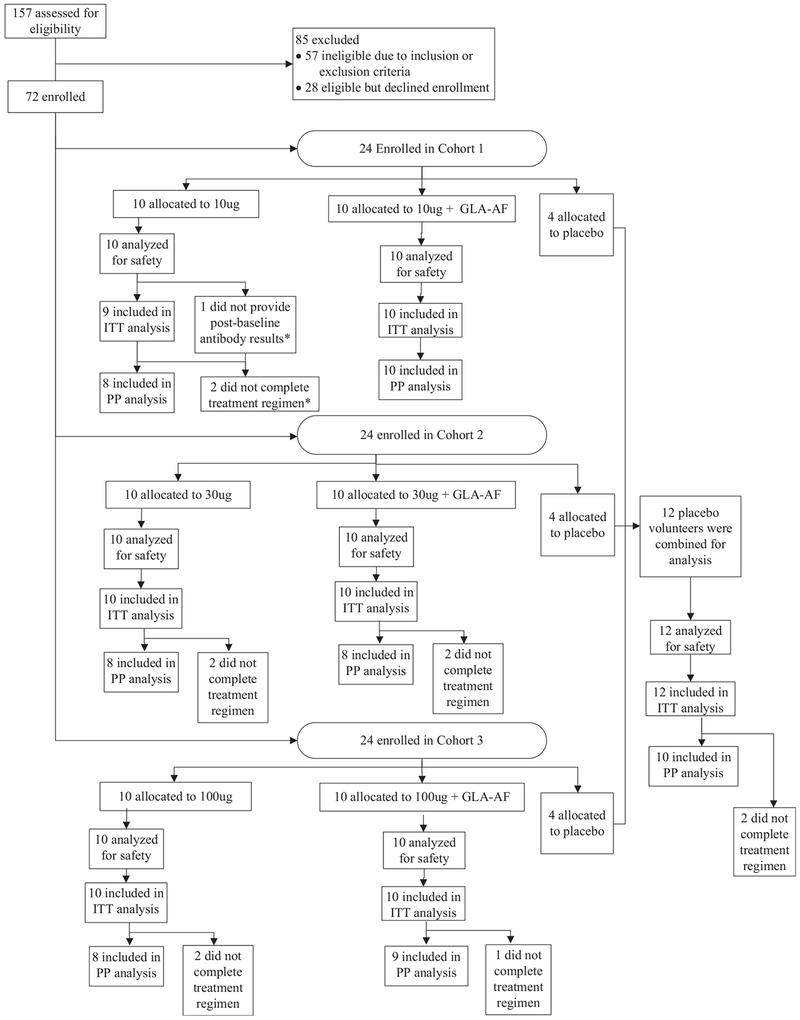
157 subjects were screened to enroll 72 subjects between 11 February 2015 and 29 October 2015. Of the 72 subjects who were enrolled, 72 (100%) received the first vaccination, 67 (93%) received the second vaccination, and 61 (85%) received all vaccinations (Fig. 1). For cohorts 1, 2 and 3, 3 subjects, 4 subjects and 4 subjects, respectively, did not receive all study vaccinations (they were either discontinued from vaccination with continued safety follow-up, N = 7; or discontinued from vaccination and withdrawn, N = 4). One additional subject in cohort 1 was lost to follow-up after receipt of all 3 doses of vaccine, for a total of 5 withdrawals. The reasons for dropping out were as follows: Lost to follow up (3 subjects); and voluntary withdrawal by 2 subjects who moved out of the area.
Fig. 1.
Disposition of Subjects. *The sole volunteer who contributed no post-baseline antibody results also did not complete the treatment regimen. The reason this person was excluded from the ITT population was lack of post-baseline antibody results, but both reasons were exclusion criteria for the PP population.
Most subjects were female (58%), not Hispanic or Latino (83%), and white (67%). Race and ethnicity did not vary substantially between arms. In each cohort, more females were assigned to the groups receiving GLA-AF than expected. Cohorts 1, 2, and 3 included 80%, 70%, and 80% females in the GLA-AF groups, respectively, as compared to 30%, 50%, and 20% in the group receiving vaccine without GLA-AF. The mean age of subjects was 30.4 years (range: 18 to 50 years) with a median age of 27 years. Age distributions were similar across treatment groups. Subject demographic data are summarized in Supplemental Table S1.
3.2. Clinical safety and reactogenicity
The vaccines were safe and well tolerated. No deaths were reported. One SAE unrelated to vaccination was reported: an ectopic pregnancy diagnosed at Day 61 requiring hospitalization and surgical removal of the right ovary and fallopian tube. One NOCMC unrelated to vaccination was reported: Grave’s disease, documented to be an unrecognized preexisting condition.
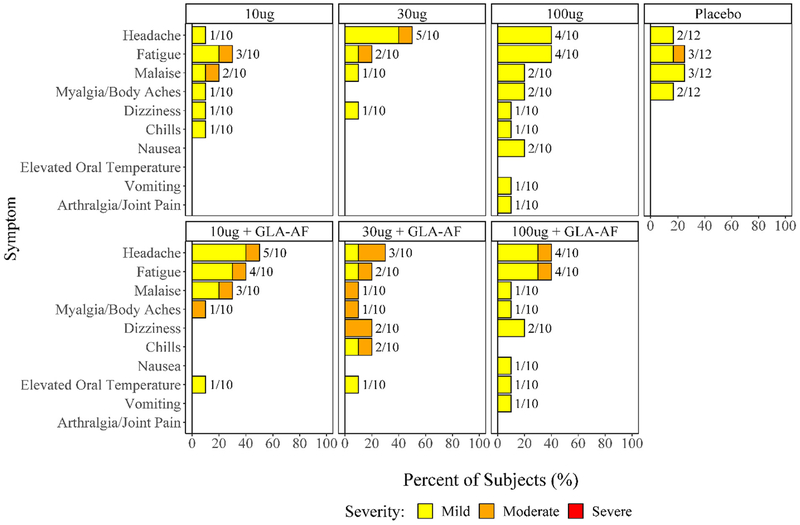
Proportions of subjects experiencing systemic reactions are summarized in Fig. 2 and were similar across treatment groups. Thirty-seven subjects across all 3 cohorts reported a solicited systemic reactogenicity event during the 7 days after any vaccination. There were no severe events. The most common systemic reactogenicity events were headache and fatigue; most reactions were mild. Three subjects, all of whom received Sm-TSP-2/Al with GLA-AF, reported mild fever on a single day during the week after vaccination. Fisher’s Exact tests comparing the proportion of subjects with fever in the groups given GLA-AF to the groups given vaccine without GLA-AF, and comparing the GLA-AF groups to the placebo control group, were not significant (p = 0.237 and p = 0.545 respectively). Furthermore, Fisher’s Exact Tests for pairwise comparisons of proportions of subjects experiencing at least one systemic event between these 3 groups found no significant differences.
Fig. 2.
Number and Percentage of Subjects Experiencing Solicited Systemic Events Post any Dose by Symptom, Maximum Severity, and Treatment Group.
Fig. 3 displays the proportions of subjects experiencing injection site reactions by symptom, treatment group, and maximum severity. Fifty-seven subjects across all 3 cohorts reported a solicited injection site reaction during the 7 days after vaccination. No severe reactions were reported. The most common injection site reactions were tenderness and pain. Tenderness was reported more frequently among subjects receiving Sm-TSP-2/Al with GLA-AF (28 of 30 subjects) than controls (6 of 12 subjects, p = 0.0036, Fig. 3). Pain was reported more frequently among those receiving Sm-TSP-2/Al with GLA-AF than controls (24 of 30 vs. 3 of 12 subjects, p = 0.0014). Rates of other injection site symptoms were similar across groups (Fig. 3). More subjects receiving Sm-TSP-2/Al with GLA-AF experienced at least one injection site reaction than controls (p = 0.046) or those receiving vaccine without GLA-AF (p = 0.042).
Fig. 3.
Number and Percentage of Subjects Experiencing Solicited Injection Site Events Post any Dose by Symptom, Maximum Severity, and Treatment Group.
The linear mixed model relating dose number to the occurrence of an injection site symptom estimated an odds ratio of 0.58 for the Sm-TSP-2/Al with GLA-AF group, meaning that the odds of an injection site symptom is decreased by a factor of 0.58 for each successive dose. However, this estimate was not significant (p = 0.169). The odds ratio comparing the Sm-TSP group to placebo and the analogous estimates for systemic symptoms were not significant, either. As such, no evidence was found for an association between dose number and the odds of experiencing a systemic or injection site symptom.
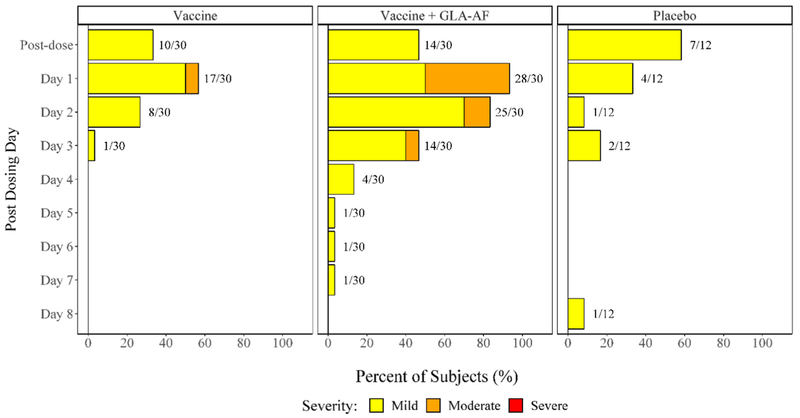
Fig. 4 displays the proportions of subjects experiencing injection site events by day post vaccination, treatment group, and maximum severity. Most injection site reactions occurred within a day or two after vaccination. The mixed effects model estimated the mean duration of injection site symptoms for a person receiving vaccine with GLA-AF to be 1.33 days longer than that of one receiving placebo (p < 0.001) and 1.22 days longer than one who received vaccine without GLA-AF (p < 0.001). No evidence for a difference in duration of systemic symptoms between groups was found.
Fig. 4.
Percent of Subjects Experiencing Solicited Injection Site Symptoms by Treatment Group, Severity, and Day Post-Vaccination, Post Any Dose. NOTE: A linear mixed effects model estimated the mean duration of injection site symptoms for a person receiving vaccine with GLA-AF to be 1.33 days longer than that of one receiving placebo (p < 0.001) and 1.22 days longer than one who received vaccine alone (p < 0.001).
3.3. Unsolicited adverse events
Forty-six subjects experienced 80 non-serious AEs, of which five (all mild) were considered related to study treatment. Of these, one (mild itching at the injection site) occurred in a subject in cohort 1 who received 10 μg Sm-TSP-2/Al with GLA-AF; and 4 subjects experienced mild decreased heart rates judged to be related to study product due to lack of clear alternative etiology (1 in a placebo recipient; one in a subject given a 30 μg dose; and 2 in the group that received a 100 μg dose). Note that these episodes of slow heart rate occurred before a blood draw on a day that vaccine was not administered. Although judged related, these slow pulse rates are more likely related to physiological variation and were not considered clinically significant.
3.4. Laboratory safety
Overall, 17 subjects experienced mild clinical laboratory abnormalities after receiving study product. There were no specific trends or patterns with regard to laboratory abnormalities, but the numbers are small. Three subjects developed mild changes in platelet counts considered related to study vaccine: Two subjects had mild increases in platelet counts (10 μg and 100 μg Sm-TSP-2/Al with GLA-AF dose level groups), and one subject had a mild decrease in platelet count; this subject received 10 μg Sm-TSP-2/Al without GLA-AF. None of these changes in platelet counts were considered clinically significant. A single subject in the 10 μg Sm-TSP-2/Al with GLA-AF group developed 2 mild increases in serum creatinine levels on days 8 and 15 after the first dose considered related to vaccine administration. All laboratory abnormalities resolved.
3.5. Immunogenicity
The immunology results described below are restricted to the PP population. All comparisons between vaccine groups and placebo controls included the placebo recipients from all 3 cohorts (Table 2; Fig. 5).
Table 2.
ELISA IgG Geometric Mean Levels, Fraction and Percent of Responders (Subjects with Fourfold Rise), by Cohort and Treatment Group, Per Protocol Population.
| Cohort | Study Day | 10 μg |
10 μg with GLA-AF |
Placebob |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GML | 95% CI | n/N (%) | 95% CIa | GML | 95% CI | n/N (%) | 95% CIa | GML | 95% CI | n/N (%) | 95% CIa | ||
| Cohort1 | 1 | 1.60 | 0.95, 2.72 | NA | NA | 1.39 | 0.90, 2.15 | NA | NA | 2.06 | 0.55, 2.15 | NA | NA |
| 15 | 1.50 | 0.98, 2.29 | 0/8 (0) | 0, 32 | 1.56 | 0.97, 2.51 | 0/10 (0) | 0, 28 | 1.93 | 0.59, 2.51 | 0/10 (0) | 0, 28 | |
| 57 | 1.49 | 0.97, 2.30 | 0/8 (0) | 0, 32 | 1.53 | 0.97, 2.41 | 0/10 (0) | 0, 28 | 1.93 | 0.59, 2.41 | 0/10 (0) | 0, 28 | |
| 71 | 1.41 | 0.86, 2.30 | 0/8 (0) | 0, 32 | 1.50 | 0.95, 2.37 | 0/10 (0) | 0, 28 | 1.99 | 0.57, 2.37 | 0/10 (0) | 0, 28 | |
| 113 | 1.39 | 0.88, 2.21 | 0/8 (0) | 0, 32 | 1.37 | 0.92, 2.04 | 0/10 (0) | 0, 28 | 1.92 | 0.60, 2.04 | 0/10 (0) | 0, 28 | |
| 127 | 1.63 | 0.93, 2.85 | 0/8 (0) | 0, 32 | 3.29 | 1.42, 7.61 | 3/10 (30) | 11, 60 | 2.03 | 0.56, 7.61 | 0/10 (0) | 0, 28 | |
| 203 | 1.67 | 0.91, 3.07 | 0/8 (0) | 0, 32 | 1.88 | 1.04, 3.38 | 1/10 (10) | 2, 40 | 2.08 | 0.54, 3.38 | 0/10 (0) | 0, 28 | |
| 293 | 1.45 | 0.83, 2.55 | 0/8 (0) | 0, 32 | 1.50 | 0.99, 2.29 | 0/10 (0) | 0, 28 | 2.07 | 0.54, 2.29 | 0/10 (0) | 0, 28 | |
| Study Day | 30 μg | 30 μg with GLA-AF | Placebob | ||||||||||
| GML | 95% CI | n/N (%) | 95% CIa | GML | 95% CI | n/N (%) | 95% CIa | GML | 95% CI | n/N (%) | 95% CIa | ||
| Cohort2 | 1 | 1.75 | 0.90, 3.40 | NA | NA | 1.87 | 0.59, 5.93 | NA | NA | 2.06 | 0.55, 7.77 | NA | NA |
| 15 | 1.64 | 0.92, 2.92 | 0/8 (0) | 0, 32 | 1.94 | 0.56, 6.71 | 0/8 (0) | 0, 32 | 1.93 | 0.59, 6.31 | 0/10 (0) | 0, 28 | |
| 57 | 1.58 | 0.96, 2.60 | 0/8 (0) | 0, 32 | 1.87 | 0.59, 5.96 | 0/8 (0) | 0, 32 | 1.93 | 0.59, 6.30 | 0/10 (0) | 0, 28 | |
| 71 | 1.53 | 0.97, 2.42 | 0/8 (0) | 0, 32 | 4.53 | 1.17, 17.52 | 3/8 (38) | 14, 69 | 1.99 | 0.57, 6.92 | 0/10 (0) | 0, 28 | |
| 113 | 1.71 | 0.90, 3.28 | 0/8 (0) | 0, 32 | 2.61 | 0.70, 9.76 | 1/8 (13) | 2, 47 | 1.92 | 0.60, 6.19 | 0/10 (0) | 0, 28 | |
| 127 | 1.86 | 0.88, 3.94 | 0/8 (0) | 0, 32 | 17.71 | 2.20, 142.68 | 4/8 (50)c | 22, 78 | 2.03 | 0.56, 7.41 | 0/10 (0)c | 0, 28 | |
| 203 | 1.55 | 0.97, 2.47 | 0/8 (0) | 0, 32 | 5.49 | 1.46, 20.59 | 3/8 (38) | 14, 69 | 2.08 | 0.54, 8.00 | 0/10 (0) | 0, 28 | |
| 293 | 1.37 | 0.89, 2.11 | 0/8 (0) | 0, 32 | 2.84 | 0.79, 10.16 | 1/8 (13) | 2, 47 | 2.07 | 0.54, 7.87 | 0/10 (0) | 0, 28 | |
| Study Day | 100 μg | 100 μg with GLA-AF | Placebob | ||||||||||
| GML | 95% CI | n/N (%) | 95% CIa | GML | 95% CI | n/N (%) | 95% CIa | GML | 95% CI | n/N (%) | 95% CIa | ||
| Cohort3 | 1 | 1.15 | NA | NA | NA | 1.15 | NA | NA | NA | 2.06 | 0.55, 7.77 | NA | NA |
| 15 | 1.73 | 0.91, 3.27 | 2/8 (25) | 7, 59 | 1.15 | NA | 0/9 (0) | 0, 30 | 1.93 | 0.59, 6.31 | 0/10 (0) | 0, 28 | |
| 57 | 1.43 | 0.85, 2.39 | 1/8 (13) | 2, 47 | 1.15 | NA | 0/9 (0) | 0, 30 | 1.93 | 0.59, 6.30 | 0/10 (0) | 0, 28 | |
| 71 | 1.68 | 0.93, 3.02 | 2/8 (25) | 7, 59 | 1.65 | 0.94, 2.90 | 2/9 (22) | 6, 55 | 1.99 | 0.57, 6.92 | 0/10 (0) | 0, 28 | |
| 113 | 1.41 | 0.86, 2.29 | 1/8 (13) | 2, 47 | 1.33 | 0.94, 1.86 | 0/9 (0) | 0, 30 | 1.92 | 0.60, 6.19 | 0/10 (0) | 0, 28 | |
| 127 | 3.54 | 0.61, 20.61 | 2/8 (25)d | 7, 59 | 13.25 | 4.41, 39.86 | 8/9 (89)d,e | 57, 98 | 2.03 | 0.56, 7.41 | 0/10 (0)e | 0, 28 | |
| 203 | 1.59 | 0.73, 3.48 | 1/8 (13) | 2, 47 | 3.17 | 1.21, 8.26 | 4/9 (44)f | 19, 73 | 2.08 | 0.54, 8.00 | 0/10 (0)f | 0, 28 | |
| 293 | 1.42 | 0.86, 2.34 | 1/8 (13) | 2, 47 | 1.91 | 1.02, 3.60 | 2/9 (22) | 6, 55 | 2.07 | 0.54, 7.87 | 0/10 (0) | 0, 28 | |
n = number of responders (subjects with a 4-fold increase in ELISA IgG relative to Day 1).
N = number of subjects in the PP population with results available.
% = Percentage of responders.
P-values for significantly different (Fisher’s Exact Test) proportions of responders between pairs of treatment groups within the same cohort are included in footnotes.
95% CI-95% confidence interval from Wilson score.
Placebo subjects are pooled across cohorts.
p = 0.023.
p = 0.015.
p < 0.001.
p = 0.033.
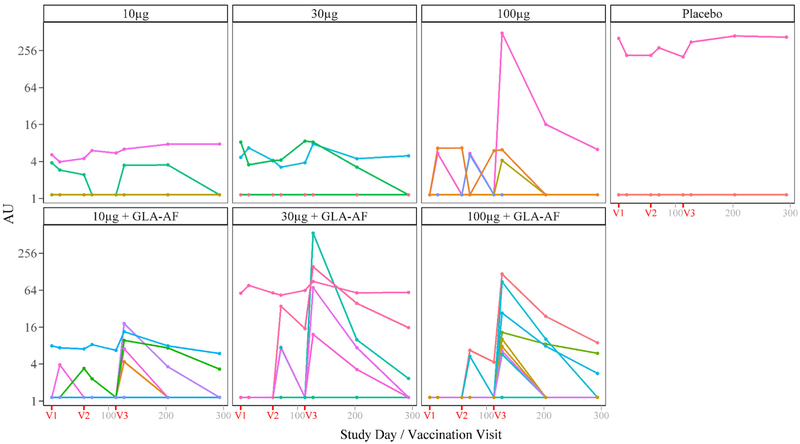
Fig. 5.
Individual Time Trends of ELISA IgG Antibody Levels by Treatment Group, Per Protocol. Population. NOTE: V1, V2, and V3 indicate the scheduled days for vaccinations 1,2, and 3, respectively (days 1,57, and 113). Of 61 volunteers in the PP population, antibody levels for 25 vaccinated and 9 control volunteers were undetectable for all time points.
Serum IgG ELISA Levels against Sm-TSP-2.
Although most PP subjects had undetectable levels of IgG against Sm-TSP-2 at baseline, several subjects in each cohort did have detectable levels at baseline (3 vaccine recipients in cohort 1; 3 vaccine recipients in cohort 2; no vaccine recipients in cohort 3; and 1 placebo recipient, Fig. 5). Because the baseline anti-IgG-Sm-TSP-2 levels were unexpected, a second run of the qualified indirect ELISA was performed to confirm these results. The correlation between the log-transformed levels from the first and second indirect ELISAs was 0.95. As no evidence was found for a lab error, the first set of results was used for analysis.
Peak levels of IgG against Sm-TSP-2 in vaccinated subjects receiving Sm-TSP-2 with and without GLA-AF were observed at Day 127, which is two weeks after the third dose, with the exception of the 10 μg dose group (Fig. 5 and Table 2), whose GML was similar at all of the time points. While significant GML differences between groups receiving Sm-TSP-2 with or without GLA-AF were not observed, groups that received Sm-TSP-2/Al with GLA-AF had higher levels of IgG against Sm-TSP-2. Levels of IgG against Sm-TSP-2 declined considerably by Day 293 in all groups.
No placebo recipients developed an IgG seroresponse against Sm-TSP-2 at any time point in the study. The proportions of responders after vaccination were highest in subjects receiving 30 μg of Sm-TSP-2/Al with GLA-AF and 100 μg of Sm-TSP-2/Al with GLA-AF. Interestingly, no vaccine recipients with IgG against Sm-TSP-2 detected at baseline developed IgG responses to Sm-TSP-2 after immunization. No significant differences were observed in cohort 1 between the vaccine groups and placebo group, whereas there were significantly more responders in the 30 μg Sm-TSP-2/Al with GLA-AF and the 100 μg Sm-TSP-2/Al with GLA-AF groups than in the placebo group (p = 0.023 and p < 0.001, respectively) at Day 127. The proportion of responders in the 30 μg Sm-TSP-2 / Al with GLA-AF group (3 of 8) was higher than the 30 μg Sm-TSP-2/Al without GLA-AF group (0 of 8) at Day 71. Results are similar for the ITT analyses (Supplemental Table S2) but, in those analyses, significantly more responders were found on Day 203 between those who received 30 μg of Sm-TSP-2/Al with GLA-AF than controls (4/9 vs. 0/11, p = 0.026). At Day 127, response frequencies were 0%, 0%, and 25%, respectively, for the 10 μg, 30 μg, and 100 μg Sm-TSP-2/Al without GLA-AF groups, suggesting a dose-response relationship for Sm-TSP-2/Al (p = 0.024; Cochran Armitage test for linear trend). For the groups given 10 μg, 30 μg and 100 μg of Sm-TSP-2/Al with GLA-AF, the frequencies of responders were 30%, 50%, and 89%, respectively, also suggesting a dose-response relationship for Sm-TSP-2/Al with GLA-AF (p = 0.0001; Cochran Armitage test for linear trend).
Western blot analysis to confirm presence of IgG against Sm-TSP-2
To confirm the reactivity of sera from subjects either vaccinated with Sm-TSP-2/Al with or without GLA-AF or placebo, Western blots were performed on a randomly selected set of sera. In brief, the drug substance contained in the vaccine (recombinant Sm-TSP-2) and an irrelevant recombinant hookworm protein (Na-GST-1) [15], which was also produced in a Pichia Pink system, were loaded on SDS-PAGE gels. Both non-reducing and reducing conditions were utilized to determine disulfide or reduction seropositivity of IgG against Sm-TSP-2, as the recombinant version of the Sm-TSP-2 protein contains four cysteines. Both the Coomassie stained (SimplyBlue™) gel images and Ponceau S staining post transfer confirmed that the antigen was pure, intact, and present in equal loads that successfully transferred to nitrocellulose on the SDS-PAGE and Western blots (Supplementary Figure S1). In brief, test sera that reacted positively in the qualified indirect ELISA for IgG against Sm-TSP-2 also demonstrated positive reactivity by Western blot (Supplementary Figure S2). Sera from several subjects reacted to both the non-reduced and reduced forms of Sm-TSP-2. Sera from two subjects showed reactivity specifically to the reduced form of Sm-TSP-2 but not the non-reduced form of Sm-TSP-2, which is most likely due to the ablation of epitopes presented specifically under reducing conditions in the absence of disulfide bond formation. Lastly, negative controls (irrelevant recombinant antigen) as well as secondary antibody alone (bottom right panel, Supplementary Figure S2) showed that the sera did not contain any non-specific reactivity. In summary, from the 26 sera analyzed-including sera from all subjects who had positive IgG at baseline-sera that demonstrated reactivity of an OD495 nm greater than 0.200 in the indirect ELISA showed positive reactivity by Western blotting.
4. Discussion
The results of this phase 1, double-blind, randomized, dose-escalation clinical trial of a candidate recombinant protein vaccine against S. mansoni are summarized herein. When administered to healthy young adults who reside in an area with no active Sm transmission, the Sm-TSP-2 schistosomiasis vaccine was found to be safe and well tolerated. Mild injection site tenderness and pain, headache, and fatigue were the most common reactions. Tenderness and pain were reported significantly more frequently among subjects receiving Sm-TSP-2/Al with GLA-AF than control subjects. Rates of other injection site symptoms were similar between groups, as were systemic symptom rates, although an indication of a possible trend for the occurrence of mild fever among recipients of Sm-TSP-2/Al with GLA-AF compared to the other groups across all cohorts was observed. While the proportion of subjects experiencing fever in those receiving Sm-TSP-2/Al with GLA-AF did not differ significantly from either Sm-TSP-2/Al without GLA-AF or the placebo control group, the relatively small sample size limited our power to detect a trend. The estimated duration of injection site symptoms for GLA-AF recipients at any dose level was longer than that for recipients of placebo or Sm-TSP-2/Al without GLA-AF. The frequencies of clinical laboratory events judged to be associated with vaccination were low, and these events were mild, transient, and clinically insignificant. Larger studies would be needed to determine the true relationship between Sm-TSP-2/Al vaccine with or without GLA-AF and these reactogenicity events. We recommend investigating the association of fever with receipt of Sm-TSP-2/Al with GLA-AF in future studies.
Dose- and adjuvant-related increases in serum IgG levels against Sm-TSP-2 were noted. The GMLs of IgG against Sm-TSP-2 tended to be highest in the groups given 30 μg of Sm-TSP-2/Al with GLA-AF and 100 μg of Sm-TSP-2/Al with GLA-AF, suggesting the advantage of adding a second immunostimulant such as GLA to this aluminum hydroxide-adjuvanted recombinant protein, but again the group sizes were too small to detect significant differences between the different groups. The frequencies of anti-Sm-TSP-2 IgG responses were low among recipients of Sm-TSP-2/Al without GLA-AF, whereas the majority of subjects given the highest two dose levels of Sm-TSP-2/Al with GLA-AF (30 μg and 100 μg) developed significant increases in IgG to Sm-TSP-2. The highest levels of IgG against Sm-TSP-2 were noted at day 127, 14 days following receipt of the third dose of vaccine. IgG levels declined significantly and rapidly after the peak in all groups, which may present a challenge for long-term vaccine efficacy given the presumed antibody-mediated mechanism of protection of this vaccine. However, the magnitude and longevity of vaccine-induced antibody responses in populations living in endemic areas may be different given the background of prior and ongoing exposure to the parasite. A Phase 1 trial is underway in an endemic area of Brazil to evaluate this (). To address this potential limitation, additional vaccine adjuvants and delivery platforms are being explored to both increase the magnitude and duration of the antibody response. For instance, reformulation of the tetraspanin-2 molecule (TSP-2), which has a predicted molecular weight of only 9.1 kDa (kDa), as a conjugate protein with carrier proteins such as CRM197 or Exoprotein A (EPA) is being explored [21,22]. Reformulation by these methods would increase the immunogenicity of Sm-TSP-2 as well as increase the “loading” ratio of Sm-TSP-2 in a total protein dose.
While no subjects in the placebo group developed an antibody response to Sm-TSP-2, a single subject in the placebo group had high levels of IgG against Sm-TSP-2 at enrollment, with these levels remaining stable for the duration of the study. Seven subjects in the various vaccine groups/cohorts also had detectable levels of IgG against Sm-TSP-2 at baseline, and none of 6 of these who received vaccine responded following vaccination. Additional studies will be needed to determine whether these subjects had antibodies that specifically react with the vaccine antigen versus potentially cross-reacting tetraspanins or other proteins. Notably, S. japonicum cercariae have been shown to express transcripts of a related tetraspanin [23], raising the possibility that other avian or mammalian schistosomes responsible for cercarial dermatitis or ‘swimmer’s itch’ in the United States [see 24 for a review] could be responsible for the antibody detected at baseline. While subjects enrolled in the current trial were not queried regarding a past history of cercarial dermatitis, consideration could be given to this issue in future studies. It is unknown is antibodies against Sm-TSP-2 from cercarial dermatitis would confer protection.
Currently, the WHO estimates that almost 70% of at-risk school-aged children have access to PZQ [25]. Despite this success, a recent Global Burden of Disease Study (2017) observed that the years lived with disability from schistosomiasis decreased only 20–30% over the last decade [26], an observation that reflects ongoing transmission of Schistosooma spp and continued, new post-treatment infections. A recent modeling manuscript found that a partially efficacious vaccine could interrupt schistosomiasis transmission during the next decade [27]. The Sm-TSP-2 schistosomiasis vaccine evaluated herein is potentially suitable for industrial-scale manufacture and delivery in endemic areas of Africa, Brazil, and the Middle East. Potential large-scale manufacturers could include multinational pharmaceutical companies or members of the Developing Country Vaccine Manufacturers Network. The vaccine is designed to be used in health systems in which it can be delivered following MDA with PZQ in a program of “vaccine-linked chemotherapy” or as part of early childhood vaccination programs. Ultimately, it would become a key technology and our best hope for eliminating this neglected tropical disease.
Based on the results of this phase I clinical trial, we conclude that the vaccine was safe and immunogenic in a Sm-naïve population.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of the study coordinator, Coni Cheesman, PA-C, and other administrative, clinical and laboratory staff at Baylor College of Medicine Vaccine and Treatment Evaluation Unit and Texas Children’s Hospital Center for Vaccine Development; members of the Clinical Immunology Laboratory at George Washington University (Jin Peng, Jill Brelsford, and Guanzhao Li); the members of the Safety Monitoring Committee [Kirsten E. Lyke, M.D. (Chair), Jason W. Bennett, M.D., LTC, MC, and Thomas L. Richie, M.D., Ph.D]; our DMID colleagues [Soju Chang, M.D., Suzanne Murray, R.N., and Blossom Smith, M.S.]; and our study subjects.
Funding
This work was supported in whole or in part with federal funds from the NIAID/NIH/Health and Human Services under Contract Numbers HHSN272200800002C, HHSN272201300015I (Baylor College of Medicine); HHSN272201500002C (Emmes); and by intramural funding from Texas Children’s Center for Vaccine Development.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: PJH, MEB, DD and JB are patent holders for a multivalent anthelminthic vaccine including schistosomiasis. WJ and GAD are employees of the Sponsor, NIAID. RLA, JMB, DD, HES, JKK, WAK, SMP, GEP: None.
Publisher's Disclaimer: Disclaimer
The views expressed here do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.08.075.
References
- [1].Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis 2009;3(9):e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hotez PJ, Bethony JM, Oliveira SC, Brindley PJ, Loukas A. Multivalent anthelminthic vaccine to prevent hookworm and schistosomiasis. Expert Rev Vaccines 2008;7(6):745–52. [DOI] [PubMed] [Google Scholar]
- [3].Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Lancet. 2017. September 16; 390 (10100):1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 2006;6(7):411–25. [DOI] [PubMed] [Google Scholar]
- [5].van der Werf MJ, Borsboom GJ, de Vlas SJ. No effect of recall period length on prevalence of self-reported haematuria in Schistosoma haematobium-endemic areas. Trans R Soc Trop Med Hyg 2003;97(4):373–4. [DOI] [PubMed] [Google Scholar]
- [6].Butterworth AE, Dunne DW, Fulford AJ, et al. Human immunity to Schistosoma mansoni: observations on mechanisms, and implications for control. Immunol Invest 1992;21(5):391–407. [DOI] [PubMed] [Google Scholar]
- [7].Mäder P, Rennar GA, Ventura AMP, Grevelding CG, Schlitzer M. Chemotherapy for fighting schistosomiasis: past, present and future. Chem Med Chem. 2018. September 13 10.1002/cmdc.201800572 [Epub ahead of print] Review. [DOI] [PubMed] [Google Scholar]
- [8].McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev 2008;21(1):225–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lammie PJ, Lindo JF, Secor WE, Vasquez J, Ault SK, Eberhard ML. Eliminating lymphatic filariasis, onchocerciasis, and schistosomiasis from the Americas: breaking a historical legacy of slavery. PLoS Negl Trop Dis 2007;1(2):e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jia X, Schulte L, Loukas A, Pickering D, Pearson M, Mobli M, et al. Solution structure, membrane interactions, and protein binding partners of the tetraspanin Sm-TSP-2, a vaccine antigen from the human blood fluke Schistosoma mansoni. J Biol Chem 2014;289(10):7151–63. 10.1074/jbc.M113.531558 Epub 2014 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med 2006;12(7):835–40. Epub 2006 Jun 18. [DOI] [PubMed] [Google Scholar]
- [12].Tran MH, Freitas TC, Cooper L, Gaze S, Gatton ML, Jones MK, et al. Suppression of mRNAs encoding tegument tetraspanins from Schistosoma mansoni results in impaired tegument turnover. PLoS Pathog 2010;6(4):e1000840 10.1371/journal.ppat.1000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Curti E, Kwityn C, Zhan B, Gillespie P, Brelsford J, Deumic V, et al. Expression at a 20L scale and purification of the extracellular domain of the Schistosoma mansoni TSP-2 recombinant protein: a vaccine candidate for human intestinal schistosomiasis. Hum Vaccin Immunother. 2013;9(11):2342–50. Epub 2013 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cheng W, Curti E, Rezende WC, Kwityn C, Zhan B, Gillespie P, et al. Biophysical and formulation studies of the Schistosoma mansoni TSP-2 extracellular domain recombinant protein, a lead vaccine candidate antigen for intestinal schistosomiasis. Hum Vaccin Immunother. 2013;9(11):2351–61. Epub 2013 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Diemert DJ, Freire J, Valente V, Fraga CG, Talles F, Grahek S, Campbell D, Jariwala A, Periago MV, Enk M, Gazzinelli MF, Bottazzi ME, Hamilton R, Brelsford J, Yakovleva A, Li G, Peng J, Correa-Oliveira R, Hotez P, Bethony J. Safety and immunogenicity of the Na-GST-1 hookworm vaccine in Brazilian and American adults. PLoS Negl Trop Dis. 2017. May 2; 11(5):e0005574 10.1371/journal.pntd.0005574 eCollection 2017 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brelsford JB, Plieskatt JL, Yakovleva A, Jariwala A, Keegan BP, Peng J, Xia P, Li G, Campbell D, Periago MV, Correa-Oliveira R, Bottazzi ME, Hotez PJ, Diemert D, Bethony JM. Advances in neglected tropical disease vaccines: Developing relative potency and functional assays for the Na-GST-1/Alhydrogel hookworm vaccine. PLoS Negl Trop Dis. 2017. February 13; 11(2): e0005385 10.1371/journal.pntd.0005385 eCollection 2017 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jariwala AR, Oliveira LM, Diemert DJ, Keegan B, Plieskatt JL, Periago MV, et al. Potency testing for the experimental Na-GST-1 hookworm vaccine. Expert Rev Vaccines 2010;9(10):1219–30. 10.1586/erv.10.107. [DOI] [PubMed] [Google Scholar]
- [18].Merrifield M, Hotez PJ, Beumier CM, et al. Advancine a vaccine to prevent human schistosomiasis. Vaccine. 2016;34(26):2988–91. 10.1016/j.vaccine.2016.03.079 Epub 2016 Mar 29. [DOI] [PubMed] [Google Scholar]
- [19].Robert Stiratelli, Nan Laird, Ware James H. Random-effects models for serial observations with binary response. Biometrics 1984:961–71. [PubMed] [Google Scholar]
- [20].Peter Armitage. Tests for linear trends in proportions and frequencies. Biometrics 1955;11(3):375–86. [Google Scholar]
- [21].Broker M, Berti F, Schneider J, Vojtek. Pollysaccharide conjugate vaccine protein carriers as a ‘neglected valency’. Potential limitations. Vaccine 2017;35:3286–94. [DOI] [PubMed] [Google Scholar]
- [22].Scaria PV, Rowe CG, Chen BB, et al. Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. NPH Vaccines 2019;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang L, Giri BR, Chen Y, et al. Molecular characterization, expression profile, and preliminary evaluation of diagnostic potential of CD63 in Schistosoma japonicum. Parasit Res 2018;117:3625–31. 10.1007/s00436-018-6063-8.24. [DOI] [PubMed] [Google Scholar]
- [24].Brandt SV, Loker ES. Schistosomes in the southwest United States and their potential for causing cercarial dermatitis or “swimmer’s itch”. J Helminthol 2009;83:191–8. 10.1017/S0022149X09308020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].World Health Organization. Schistosomiasis and soil-transmitted helminthiases: numbers of people treated in 2017. Weekly Epidemiol Rec. 14 December 2018; 93(50): 681–92. https://www.who.int/wer/2018/wer9350/en/. [Google Scholar]
- [26].GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392: 1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stylianou A, Hadjichrysanthou C, Truscott JE, Anderson RM. Developing a mathematical model for the evaluation of the potential impact of a partially efficacious vaccine on the transmission dynamics of Schistosoma mansoni in human communities. Parasit Vectors 2017;10(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.