Abstract
Background:
Hemorrhagic shock (HS) is a life-threatening condition resulting from rapid and significant loss of intravascular volume, leading to hemodynamic instability and death. Inflammation contributes to the multiple organ injury in HS. Type I interferons (IFNs), such as IFN-α and IFN-β, are a family of cytokines that regulate the inflammatory response through binding to IFN-α receptor (IFNAR) which consists of IFNAR1 and IFNAR2 chains. We hypothesized that type I IFNs provoke inflammation and worsen organ injury in HS.
Methods:
Male C57BL/6 mice (20–25 g) underwent hemorrhage by controlled bleeding via the femoral artery to maintain a mean arterial pressure (MAP) of 27±2.5 mmHg for 90 min, followed by resuscitation for 30 min with two times shed blood volume of Ringer’s lactate solution containing 1 mg/kg body weight of anti-IFNAR1 antibody (Ab) or control isotype-matched IgG (IgG). Blood and tissue samples were collected at 20 h after the resuscitation for various analyses.
Results:
The expression of IFN-α and IFN-β mRNAs was significantly elevated in lungs and liver of the mice after HS. IFNAR1-Ab treatment significantly decreased serum levels of organ injury markers LDH and AST, as well as improved the integrity of lung and liver morphology, compared to the IgG control. The protein levels of pro-inflammatory cytokines TNF-α and IL-6, and mRNA expression of pro-inflammatory chemokines MCP-1, MCP-2, MIP-2, and KC in the lungs of the HS mice were significantly decreased after treated with IFNAR1-Ab. Moreover, the myeloperoxidase activity and number of apoptotic cells in the lungs of HS mice treated with IFNAR1-Ab were decreased in comparison to the IgG control.
Conclusion:
Administration of IFNAR1-Ab reduce inflammation and tissue injury. Thus, type I IFN signaling may be a potential therapeutic target for mitigating organ dysfunction in patients suffering from HS.
Keywords: Hemorrhagic shock, Type I interferon, organ injury, inflammation
Background
Hemorrhagic shock (HS) is a life-threatening condition that results from a rapid and significant loss of intravascular volume, leading to hemodynamic instability and inflammation. HS plays a significant role in traumatic morbidity and mortality, which accounts for nearly 40% of trauma-related deaths in the United States each year (1). Worldwide, there are 1.9 million deaths due to HS annually (2). There have been considerable advances in understanding the pathophysiology of HS. Inadequate oxygen delivery at the cellular level leads to a surge of inflammatory mediators, such as interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) (3). However, a sustained and exacerbated inflammatory response has been shown to increase mortality (4). Furthermore, a prolonged and severe HS state leads to tissue hypoxia and cell death, thereby propagating tissue injury and creating a pool of apoptotic cells (5).
Type I IFNs, such as IFN-α and IFN-ß, are comprised of a family of cytokines that are critical to the innate immune response to infection (6), autoimmunity (7), and tumorigenesis (8, 9). IFN-α is mainly produced from plasmacytoid dendritic cells, while IFN-β can be produced by many cell types, such as fibroblast, epithelial cells, dendritic cells, phagocytes and synoviocytes (7, 10). After IFN-α/β are released into the extracellular space, they will interact with and signal through a heterodimeric IFN-α receptor (IFNAR) complex composed of the subunits IFNAR1 and IFNAR2 which are expressed on all cell types, except platelets (7, 11). Binding of IFN-α/β to IFNAR activates the receptor tyrosine kinases Jak1 and Tyk2 which phosphorylate the signal transducer and activator of transcription (STAT) protein, leading to the transcription of many IFN-stimulated genes (ISGs) (12). In addition to the classical antiviral role of IFN-α/β (6), studies in ischemia-reperfusion injury models have demonstrated that type I IFNs enhance the activation and recruitment of macrophages and neutrophils into damaged organs (13, 14) by modulating the expression of several cytokines and cytokine receptors (15).
Since type I IFNs modulate the immune response, we hypothesized that type I IFNs augment inflammation and exacerbate organ injury after HS. In this study, we used a mouse model to examine the role of type I IFNs in HS. We first measured the expression of IFN-α and IFN-β in the lung and liver of HS mice, and observed an increase of their expression in both organs after HS. We then treated HS mice with anti-IFNAR1 antibody (IFNAR1-Ab) to block the type I IFN signaling and compared their serum organ injury marker levels, pathohistologic damage of the organs, and expression of pro-inflammatory cytokines and chemokines in the lungs to the IgG-treated control and sham animals.
Methods
Mouse model of hemorrhagic shock.
Male C57BL/6 mice (20–25 g) from Jackson Laboratory (Bar Harbor, ME) were housed in a temperature-controlled environment with 12-hour light/dark cycle and fed a standard laboratory mouse chow diet and water ad libitum. After acclimation for 7 days, mice were subject to HS. All experiments were performed in accordance with the guidelines for the use of experimental animals by the National Institutes of Health (Bethesda, MD) and were approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research. The animal model of HS was performed as previously described (16). Briefly, mice were randomly assigned to undergo sham, HS treated with IgG, or IFNAR1-Ab groups. After anesthesia induction with 2.5% inhalational isoflurane, mice were placed supine and the inguinal regions were shaved and prepped with 10% povidone iodine wash. Anesthesia was maintained with 1.5 % isoflurane inhalation. The femoral arteries were subjected to bilateral cannulation with PE-10 tubing. While one catheter was used for hemorrhage and resuscitation efforts, the second catheter was connected to a blood pressure transducer for continuous mean arterial pressure (MAP) and heart rate readings. The results were recorded with a blood pressure analyzer Digi-Med (Micro-Med, Louisville, KY). The values of the MAP were averaged over 5-seconds intervals to record the oscillations in the blood pressure. Blood was withdrawn from the femoral artery to reach a MAP of 27±2.5 mmHg and maintain for 90 minutes. Mice were then resuscitated with two times of the shed blood volume with Ringer’s lactate solution during the 30-minute resuscitation phase. Mice were not heparinized during the procedure. After the resuscitation, the femoral artery was then ligated and the incision closed with non-absorbable suture. Animals were observed to recover from anesthesia prior to return to their cages. At 20 hours after the operation, all mice were euthanized by CO2 asphyxiation and organs were harvested for processing. Sham mice underwent the same procedure without induction of hemorrhage or resuscitation.
Administration of anti-IFNAR1 antibody (IFNAR1-Ab).
During the 30-minute resuscitation phase, mice were received 1 mg/kg IFNAR1-Ab or non-immunized IgG (Leinco Technologies, Fenton, MO) in Ringer’s lactate solution (17, 18). IgG is the control isotype matched with the antibody against IFNAR1. The dose of IFNAR1 is based on a previous study (17).
Analysis of serum injury markers.
Blood samples were drawn prior to euthanasia from the inferior vena cava and centrifuged at 1,000g for 10 min. The supernatant containing the serum was collected and then analyzed immediately for levels of lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) as organ injury markers using assay kits according to manufacturer’s instructions (Pointe Scientific, Canton, MI).
Histology analysis.
Segments of lung and liver tissues were collected at 20 hours after reperfusion and stored in 10% formalin before fixing in paraffin. The tissues were sectioned into 5-μm cuts, transferred to glass slides and stained with hematoxylin and eosin (H&E). Tissue injury was assessed in a blinded fashion using a semi-quantitative light microscopy evaluation. Ten fields were examined for each sample. Assessment of histological lung injury was performed using a modified version from the American Thoracic Society that assessed for parameters of injury including the infiltration of inflammatory cells into the alveolar and into the interstitial space, the presence of hyaline membranes, proteinaceous debris inside airspaces and alveolar septal thickening (19). Based on the presence of each of the parameters, scores per visual field were assessed as 0 (no injury), 1 (moderate injury), and 2 (severe injury). Using a weighted equation with a maximum score of 100 per field, presented by Matute-Bello et al. (19), the parameter scores were calculated on a scale of 0–1 and then averaged as the final lung injury score in each group. For liver injury scoring assessment, five different histological parameters were used: necrosis, sinusoidal congestion, erythrocyte stasis, vacuolization and cytoplasmic color fading (20). Injury was calculated by assigning a severity score on a scale ranged from 0 to 4 (0 = 0%, 1 = 1–10%, 2 = 10–30%, 3 = 30–60%, and 4= >60%) for each parameter, with a highest possible score of 20 as previously described (17).
Assessment of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α).
Lung tissue was homogenized in lysis buffer (10 mM Tris-HCl pH 7.5, 120 mM NaCl, 1% sodium deoxycholate, and 0.1 % sodium dodecyl sulfate) containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). The protein concentration was determined by the Bio Rad protein assay reagent (Hercules, CA). Levels of TNF-α and IL-6 in the lung tissues were analyzed with a commercial mouse enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences, San Diego, CA) according to the manufacturer protocol.
Quantitative real-time polymerase chain reaction (qPCR).
Total RNA was extracted from tissues using a Trizol reagent (Invitrogen, Carlsbad, CA) and was reverse-transcribed into cDNA using murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA). A qPCR assay was carried out in 20 μl of a final volume containing 0.25 μmol of each forward and reverse primer, cDNA, and 10 μl SYBR Green PCR master mix (Applied Biosystems). Amplification was conducted in an Applied Biosystems Step One Plus real-time PCR machine under the thermal profile of 50°C for 2 min, 95°C for 10 min followed by 45 cycles of 95°C for 15 seconds and 60°C for 1 min. The level of mouse β-actin mRNA was used for normalization. Relative expression of mRNA was expressed as the fold change in comparison with the sham tissues. The primers used for quantitative real-time PCR are: IFN-α, IFN-ß, MCP-1, MCP-2, MIP-2, KC, and β-actin (Table 1).
Table 1.
The list of RT-PCR primers used in this study.
| Name | Forward | Reverse |
| IFN-α | CTA CTG GCC AAC CTG CTC TC | AGA CAG CCT TGC CAG GTC ATT |
| IFN-ß | TGA CGG AGA AGA TGC AGA AG | ACC CAG TGC TGG AGA AAT TG |
| MCP-1 | GGA GCA TCC ACG TGT TGG C | ACA GCT TCT TTG GGA CAC C |
| MCP-2 | ACA TCA CCT GCT TGG TCT GGA AAA C | ACT AAA GCT GAA GAT CCC CCT TCG |
| MIP-2 | CGC TGT CAA TGC CTG AAG AC | ACA CTC AAG CTC TGG ATG TTC TTG |
| KC | GCT GGG ATT CAC CTC AAG AA | ACA GGT GCC ATC AGA GCA GT |
| β-actin | GTG AAA AGA TGA CCC AGA TCA | TGG TAC GAC CAG AGG CAT ACA G |
Myeloperoxidase (MPO) activity assay.
MPO activity in the lung was determined using the peroxidase-catalyzed reaction as previously described (21). Lung tissues were sonicated in 50 mM potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide (pH 6.0). After centrifugation, the supernatant was diluted in reaction solution containing o-dianisidine hydrochloride and H2O2. The rate of change in optical density (OD) for 1 min was measured at 460 nm. MPO activity (1 unit defined as change in absorbance of 1 per min) was expressed as units per gram of protein.
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay.
Lung tissue sections were immersed in 20 μg/mL of Proteinase K (Thermo Fisher Scientific, Waltham, MA) at room temperature for 20 minutes and then stained using an in situ labeling of DNA fragmentation with TUNEL assay kit (Roche Diagnostics) and counterstained with Vectashield mounting medium with 4’,6-diamidino-2’-phenylindole dihydrochloride (DAPI) (Vectorlabs, Burlingame, CA). Apoptotic cells were visualized green at 200× magnification under a fluorescence microscope (Nikon Eclipse Ti-S, Melville, NY) and were then counted in five visual fields/section. The average number of apoptotic cells/field was calculated.
Statistical analysis.
Data are expressed as mean ± standard error of the mean (SEM) and compared by one-way analysis of variance (ANOVA) using Student–Newman–Keuls (SNK) test for multiple group comparisons. Significance was considered if P < 0.05 between the experimental groups.
Results
IFNAR1-Ab treatment has no effect on the MAP during resuscitation.
Hemorrhage and resuscitation efforts were carried out in adult mice with a 100% survival rate at the end of the procedure. No differences were found in the total amount of blood withdrawn (0.7±0.15 ml per mouse) between IFNAR1-Ab- and IgG-treated mice to reduce and maintain the target MAP (27 mmHg) during the 90-min hemorrhage phase. During the fluid resuscitation phase, there was also no difference in MAP between the IFNAR1-Ab- and IgG-treated mice (Fig. 1), suggesting that the mechanism of action of IFNAR1-Ab in HS does not alter the hemodynamic parameters. Sham MAP was stable throughout the experimental procedure.
Figure 1.
Monitoring of blood pressure during HS induction period. Arterial blood pressure at the induction phase (0–20 min), the hemorrhage phase (20–110 min) and the first 30 min of resuscitation phase (110–140 min) in the IgG- and IFNAR1-Ab-treated mice was recorded from the beginning of the operation. Sham-operated mice with the same time frame were also recorded. Data presented are mean ± SEM (n = 6 mice/group).
The expression of IFN-α and IFN-ß increases after HS.
After establishing the model, we first examined the effect of HS on the expression of type I IFNs. At 20 hours after HS, the mRNA levels of IFN-α in the lung and liver were increased to 21- and 12-fold, respectively, compared to the sham (Fig. 2A). Similarly, the mRNA levels of IFN-β in the lung and liver were increased to 6.5- and 1.7-fold, respectively, compared to the sham (Fig. 2B). With the observation of increased expression of IFN-α/β, main cytokines of type I IFNs, it supports our rationale to use the antibody against their receptor to block their activity in the HS mice for the following study.
Figure 2.
The expression of type I IFNs in the lungs and liver after HS. Lung and liver tissues from mice at 20 h after HS and sham mice were harvested for total RNA isolation. The mRNA levels of (A) IFN-α and (B) IFN-ß were assessed by qPCR. Each gene expression level was normalized to β-actin. The value in the sham group is designated as 1 for comparison. Data are expressed as mean ± SEM (n = 4–6 mice/group) and compared by ANOVA and SNK test. *P < 0.05 vs. sham.
IFNAR1-Ab treatment attenuates organ injury after HS.
At 20 hours after HS, serum levels of organ injury markers LDH and AST were dramatically elevated to 2,030±88 and 708±100 IU/L, respectively, in the IgG-treated group, while they were 49.5±16 and 41±7 IU/L, respectively, in the sham group (Fig. 3). With the IFNAR1-Ab treatment, the serum levels of LDH and AST were decreased by 22% and 28%, respectively, compared to the IgG-treated mice (Fig. 3). Overall, these results showed that IFNAR1-Ab partially mitigates organ injury after HS.
Figure 3.
Serum levels of organ injury markers after HS. Blood from IgG- and IFNAR1-Ab-treated mice at 20 h after HS, and sham mice were collected for the analysis. The serum levels of (A) LDH and (B) AST were determined by enzymatic method. Data are expressed as mean ± SEM (n = 4–6 mice/group) and compared by ANOVA and SNK test. *P < 0.05 vs. sham; #P < 0.05 vs. IgG control.
IFNAR1-Ab treatment improves the integrity of the lung and liver histology after HS.
We then further examined the severity of the lung injury by histologic analysis with H&E staining. As shown in Figure 4A, alterations in the alveolar capillary barrier, alveolar septal thickening, and hyaline deposits were found in the IgG-treated animals at 20 hours after HS, while these phenomena were not observed in the sham mice. In contrast, the lung architecture and morphology of IFNAR1-Ab-treated mice were shown toward to the images like the sham group (Fig. 4A). With high magnification, we identified the neutrophils infiltrating into the lungs of IgG-treated mice and found decreased infiltration in the HS mice with IFNAR1-Ab treatment (Fig. 4A, inset). To further quantify the degree of the neutrophil infiltration, we conducted the myeloperoxidase (MPO) activity assay, a marker of neutrophil activation (22). There was a 7-fold increase in the lung MPO activity of IgG-treated HS mice in comparison to the sham, while it was decreased by 55 % in the HS mice with IFNAR1-Ab treatment in comparison to the IgG treatment (Fig. 4C). Quantification of the lung injury score exhibited a 5.3-fold increase in the IgG-treated mice compared to the sham, while with IFNAR1-Ab treatment, the lung injury score was attenuated by 46% compared to IgG-treated mice (Fig. 4D).
Figure 4.
Histology assessment of the lung tissues after HS. Lung tissues from IgG- and IFNAR1-Ab-treated mice at 20 h after HS, and sham mice were harvested, sectioned, and subjected to histologic analysis. (A) Representative images of tissue sections with H&E staining at an original magnification of 200×. The inset shows at an original magnification of 400× and arrows indicate neutrophils. (B) MPO activity measured from lung tissues. (C) Histologic injury score in each group was blindly graded as described in Materials and Methods. Data are expressed as mean ± SEM (n = 6 mice/group) and compared by ANOVA and SNK test. *P < 0.05 vs. sham; #P < 0.05 vs. IgG control. Histology assessment of the liver tissues after HS. Liver tissues from IgG- and IFNAR1-Ab-treated mice at 20 h after HS, and sham mice were harvested, sectioned, and subjected to histologic analysis. (D) Representative images of tissue sections with H&E staining at an original magnification of 200×. (E) Histologic injury score in each group was blindly graded as described in Materials and Methods. Data are expressed as mean ± SEM (n = 6 mice/group) and compared by ANOVA and SNK test. *P < 0.05 vs. sham; #P < 0.05 vs. IgG control.
We also conducted the histologic analysis on the liver. As shown in Figure 4B, the H&E stained liver tissues in the IgG-treated group exhibited a marked increase of necrosis and micro-hemorrhage in comparison to the sham. The morphology of the liver in the IFNAR1-Ab-treated animals showed a better integrity than the IgG-treated mice (Fig. 4B). Quantification of liver histologic injury score among these three groups showed that the IgG-treated group had a 4.1-fold increase in the severity of liver injury compared to the sham, while the injury score of IFNAR1-Ab-treated animals was significantly reduced by 43.5% compared to the IgG-treated mice (Fig. 4E). Taken together, these results demonstrate that IFNAR1-Ab treatment provides significant protection against lung and liver injury after HS.
IFNAR1-Ab treatment attenuates the expression of inflammatory cytokines in the lungs after HS.
To determine whether IFNAR1-Ab treatment played a role in regulating inflammation induced by HS, we measured the expression of pro-inflammatory cytokines TNF-α and IL-6 in the lungs at 20 hours after HS. The protein levels of TNF-α and IL-6 in the lungs of the IgG-treated group were significantly increased by 5.9- and 1.5-fold, respectively, compared to the sham (Fig. 5A,B). IFNAR1-Ab treatment resulted in reduction of TNF-α and IL-6 protein levels by 32% and 27%, respectively, compared to the IgG-treated group (Fig. 5A,B).
Figure 5.
Expression of pro-inflammatory cytokines in the lungs after HS. Lung tissues from IgG- and IFNAR1-Ab-treated mice at 20 h after HS, and sham mice were harvested for total protein isolation. The protein levels of (A) TNF-α and (B) IL-6 were assessed by ELISA. Data are expressed as mean ± SEM (n = 4–6 mice/group) and compared by ANOVA and SNK test. *P < 0.05 vs. sham; #P < 0.05 vs. IgG control. Expression of pro-inflammatory chemokines in the lungs after HS. Lung tissues from IgG- and IFNAR1-Ab-treated mice at 20 h after HS, and sham mice were harvested for total RNA isolation. The mRNA levels of (C) MCP-1, (D) MCP-2, (E) MIP-2, and (F) KC were assessed by qPCR. Each gene expression level was normalized to β-actin. The value in the sham group is designated as 1 for comparison. Data are expressed as mean ± SEM (n = 4–6 mice/group) and compared by ANOVA and SNK test. *P < 0.05 vs. sham; #P < 0.05 vs. IgG control.
IFNAR1-Ab treatment decreases the expression of inflammatory chemokines in the lungs after HS.
Chemokine released is another contributing factor to inflammation by attracting the immune cells to the injury or inflamed sites. We first examined the expression of MCP-1 and MCP-2 which are chemoattractants to macrophages, in the lungs at 20 hours after HS. The mRNA levels of MCP-1 and MCP-2 in the IgG-treated group were increased by 10.7- and 2.8-fold, respectively, compared to the sham (Fig. 5C,D). However, with the IFNAR1-Ab treatment, the mRNA levels of MCP-1 and MCP-2 were decreased by 79% and 38%, respectively, compared to the IgG-treated mice (Fig. 5C,D). We also examined the expression of MIP-2 and KC which are chemoattractants to neutrophils, in the lungs. Similarly, the mRNA levels of MIP-2 and KC were increased by 39- and 6.4-fold, respectively, after HS (Fig. 5E,F). In contrast, IFNAR1-Ab treatment resulted in decreased mRNA levels of MIP-2 and KC by 79% and 39%, respectively, compared to the IgG-treated mice (Fig. 5E,F).
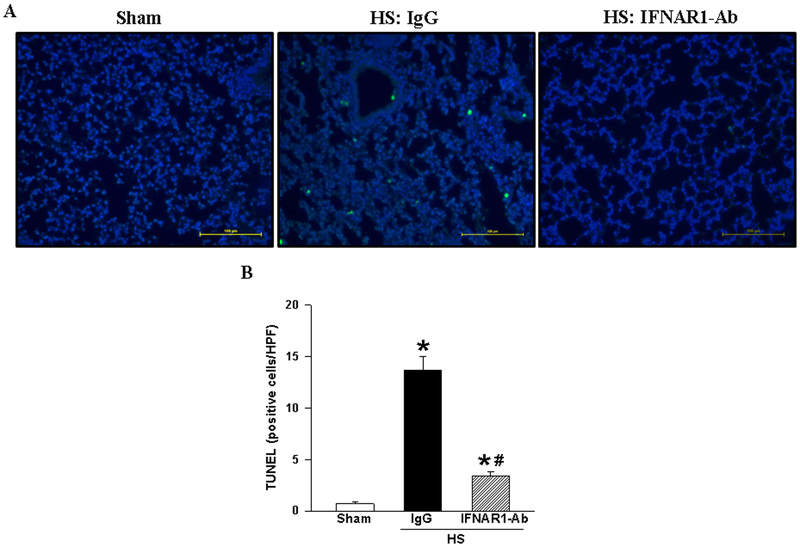
IFNAR1-Ab treatment attenuates apoptosis in the lungs after HS.
To analyze the effect of IFNAR1-Ab treatment on apoptosis, we performed the TUNEL assay in the lung tissue sections at 20 hours after HS. As shown in Figure 6, TUNEL-positive cells were barley detected in the sham group, while there were many green fluorescent cells spotted in the IgG-treated group (Fig. 6A). By counting the number of TUNEL-positive cells in the microscopic fields, it showed a markedly increase in the IgG-treated group compared to the sham, whereas in the treatment group with IFNAR1-Ab, the number of apoptotic cells was significantly decreased by 75%, compared to IgG-treated animals (Fig. 6B).
Figure 6.
Apoptosis in the lungs after HS. Lung tissues from IgG- and IFNAR1-Ab-treated mice at 20 h after HS, and sham mice were harvested, sectioned, and subjected to terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL). (A) Representative images of tissue sections stained with TUNEL (green fluorescence) and nuclear counterstaining with 4’,6-diamidino-2-phenylindole (DAPI) (blue fluorescence). Bar scale indicates 100 μm at an original magnification of 200×. (B) The number of TUNEL-positive cells was counted and averaged over 10 high power fields (HPF)/section. Data are expressed as mean ± SEM (n = 6 mice/group) and compared by ANOVA and SNK test. *P < 0.05 vs. sham; #P < 0.05 vs. IgG control.
Discussion
The pathophysiology of HS involves multiple compensatory mechanisms to maintain blood pressure and redistribution of cardiac output to sustain vital organ functions (23). However, these compensatory mechanisms are limited. Without therapeutic intervention, severe cellular hypoxia and excessive inflammatory responses occur, leading to organ injury and eventually death (2). The current management of HS relies mainly on supportive care. Thus, more research is needed to further improve our understanding of the pathophysiologic and immunologic features of HS. In this regard, we hypothesized that the administration of anti-IFNAR1-Ab during resuscitation after hemorrhage would attenuate organ injury.
In our study, we demonstrated a reduction of tissue injury markers, a significant decrease of lung pro-inflammatory cytokines and chemokines, and an improvement in the histologic architecture of the lung and liver in HS mice-treated with anti-IFNAR1-Ab. Furthermore, we showed an improvement in MPO activity and in the number of apoptotic cells in the lungs of HS mice that received anti-IFNAR1-Ab treatment. Collectively, these results suggest that the activation of type I IFN receptor induced by HS augments the inflammatory response, leading to organ damage. Therefore, the administration of anti-IFNAR1-Ab treatment has the potential mitigate these effects after HS.
Previous studies have shown that type I interferon signaling plays a key role in regulating the innate and adaptive immune responses through a wide variety of pathways, including the development and activation of neutrophils, macrophages and lymphocytes and microglia (13, 24–27). Given the autocrine and paracrine fashion used by type I IFNs to exert their biological effects once secreted by the cell (28–31), we measured the expression of IFN-α and IFN-β in the lungs and liver of mice after HS and showed an increase of both type I IFNs in these two organs. In order to inhibit the signaling of type I IFNs in stimulating the inflammatory response, we have treated the HS mice with IFNAR1-Ab that binds to type I IFN receptor to disrupt its signaling (12, 17, 18, 28), and then examined the effects of the treatment.
This study is the first to report that type I IFNs play a key role in the regulation of inflammation response in HS. Multiple mechanisms are known to cause the activation and production of type I IFN. These include the presence of bacterial DNA or LPS (14) in the circulation as well as damage associated molecular patterns such as extracellular cold-inducible RNA-binding protein (eCIRP) (32), mitochondrial DNA (33) or HMGB1 (34). Once type I IFNs are released into the extracellular space and bind to their receptor IFNAR, ISGs are induced and lead to diverse biological effects. These genes involve in multiple downstream signaling pathways such as for the production of pro-inflammatory cytokines, chemokines, pro-apoptotic proteins, and molecules involved in metabolic process (6, 35, 36). For instance, type I IFN-deficient macrophages has been shown to exhibit lower expression of MyD88-dependent pro-inflammatory genes such as TNF-α and IL-6 in response to LPS stimulation (37). It has also been demonstrated the induction of TNF-α and IL-6 in the lungs with respiratory syncytial virus infection is greatly reduced in the IFNAR1-deficient (IFNAR1−/−) mice (38). These data are consistent with our findings as demonstrated in Fig. 5 that blocking type I IFN signaling contributes to the reduction of pro-inflammatory cytokines after HS. Notably, in a liver ischemia-reperfusion model, absence of the IFNAR was shown to be protective by decreasing systemic inflammation (14).
Neutrophils play an important role in the innate immune response and in the development of the lung and hepatocellular inflammation. The recruitment of neutrophils into the lungs has been shown to exacerbate the oxidative injury and is associated with poor patient outcomes (39, 40). We found that neutrophil infiltration chemokine markers MIP-2 and KC as well as MPO activity were significantly increased after HS. Consistently, elevated levels of these markers have been shown to contribute greatly to neutrophil infiltration in the lungs and consequently, to tissue (22, 40, 41). Accordingly, antagonizing the action of type I IFNs by blocking its receptor with the administration of anti-IFNAR1-Ab, has decreased the expression levels of MIP-2 and KC, and MPO activity in the lungs of HS mice, which is associated with the reduction of lung injury as demonstrated here. Our results are in line with other studies showing that IFNAR1 knockout mice are resistant to multiple inflammatory models such as endotoxemia (42) and TNF-α-induced shock (24). These findings illustrate that type I IFN signaling plays a significant role in regulating the innate immune response to inflammation.
We have also demonstrated that the treatment with anti-IFNAR1-Ab downregulates specific chemokines markers of macrophages infiltration, such as MCP-1 and MCP-2, in the lungs of HS mice. Studies in using of MCP-1 and MCP-2 knockout mice have revealed significant defects in monocytes/macrophage recruitment and immunological responses, especially the IFN-γ, IL-1β, and IL-6 production at sites of inflammation (43–45). Consistently, these studies support the important role MCP-1 and MCP-2 in modulating the monocytes immune response and cytokine production during inflammation. Therefore, reduction of macrophage recruitment into the lung tissue by IFNAR1-Ab treatment contributes to attenuation of inflammation and lung injury in HS mice.
Prior studies have shown that apoptosis is an important mechanism of cell death in HS, which contributes to organ injury (5). Therapies focused on reducing apoptosis following HS, such as glutamine administration during the resuscitation, have proven to be protective by restoring cellular energy, improving organ function, and increasing survival (46, 47). In addition, IFNAR1-deficient mice have been shown to be protective from apoptosis in a renal ischemia-reperfusion model, suggesting that type I IFN signaling is involved in regulating pro-apoptotic pathway in the ischemic kidney (13). By blocking the IFNAR1 receptor, we have demonstrated that after HS, treated mice significantly reduce apoptosis as compared to mice treated with IgG, evidenced with the TUNEL assay.
Given the complex and diverse processes that play a role in the pathophysiology of trauma and HS, development of an a reliable experimental model of HS can be a major challenge. The experimental model used in our study is fixed-pressure hemorrhage. The primary advantage of this model is its standardization, reproducibility and ability to elucidate an animal’s hemodynamic and inflammatory response to a fixed decrease in mean arterial pressure secondary to blood loss. The authors acknowledge that this model does not completely mimic clinical hemorrhagic shock in humans (48). Other models, such as uncontrolled HS or combined HS with blunt trauma or pseudo fracture, may represent a more clinically relevant model (48, 49). However, the basis of HS evolves from an increase in systemic inflammation regardless of the clinical scenario. As such, our study focuses on the inflammatory responses underlying HS by hypothesizing that anti-IFNAR1-Ab play a beneficial role in HS at the cellular and tissue level. Although the direct clinical application of these findings is relatively limited, this study sheds some light on potential therapeutic pathways that prevent the progression of HS, specifically on the attenuation of organ injury through inhibition of type I IFN signaling. Finally, we did not assess other factors that contribute to the complexity of HS, such as the accumulation of reactive oxidative species, hyperfibrinolysis, or diffuse coagulopathy, which all can occur during HS. These complex mechanisms may explain the organ injury that was still seen in the HS mice which were treated with anti-IFNAR1-Ab. Thus, further investigation is required.
In conclusion, administration of IFNAR1-Ab during reperfusion has protective effects on mice with HS, shown by decreasing organ damage, pro-inflammatory cytokine and chemokine production, and apoptosis in the lungs. Thus, in the light of these observations, the modulation of type I IFN signaling may be a potential therapeutic target for mitigating organ injury after HS.
Acknowledgements:
We thank Dr. Fangming Zhang’s and Dr. Mahendar Ochani’s technical assistance on the initial setting of the mouse model of hemorrhagic shock and Dr. Jordan Last and Dr. Steven Gurien for their critical review of the manuscript. This study was supported by the National Institute of Health (NIH) grants, R01HL076179 and R35GM118337 (P.W).
Funding: This work was supported by National Institutes of Health grant R01HL076179 and R35GM118337 (to P.W.).
Footnotes
Declarations
Ethics approval. All experiments involving live animals were carried out in accordance with the National Institutes of Health guidelines for the use of experimental animals and were reviewed and approved by the Institutional Animal Care and Use Committee at the Feinstein Institute for Medical Research.
Availability of data and materials. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of Interest Statement: The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Contributor Information
Joaquin Cagliani, Email: jcagliani@northwell.edu.
Weng-Lang Yang, Email: wlyang@northwell.edu.
Joseph T. McGinn, Email: jmcginn3@northwell.edu.
Zhimin Wang, Email: zwang@northwell.edu.
Ping Wang, Email: pwang@northwell.edu.
References:
- 1.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31(7):1507–11. [DOI] [PubMed] [Google Scholar]
- 2.Cannon JW. Hemorrhagic Shock. N Engl J Med. 2018;378(4):370–9. [DOI] [PubMed] [Google Scholar]
- 3.Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am J Physiol Cell Physiol. 2001;280(2):C343–51. [DOI] [PubMed] [Google Scholar]
- 4.Lomas-Neira J, Perl M, Venet F, Chung CS, Ayala A. The role and source of tumor necrosis factor-α in hemorrhage-induced priming for septic lung injury. Shock. 2012;37(6):611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zacharias N, Sailhamer EA, Li Y, Liu B, Butt MU, Shuja F, Velmahos GC, de Moya M, Alam HB. Histone deacetylase inhibitors prevent apoptosis following lethal hemorrhagic shock in rodent kidney cells. Resuscitation. 2011;82(1):105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muskardin TLW, Niewold TB. Type I interferon in rheumatic diseases. Nat Rev Rheumatol. 2018;14(4):214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang KJ, Yin XF, Yang YQ, Li HL, Xu YN, Chen LY, Liu XJ, Yuan SJ, Fang XL, Xiao J, et al. A Potent In Vivo Antitumor Efficacy of Novel Recombinant Type I Interferon. Clin Cancer Res. 2017;23(8):2038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–36. [DOI] [PubMed] [Google Scholar]
- 11.de Weerd NA, Vivian JP, Nguyen TK, Mangan NE, Gould JA, Braniff SJ, Zaker-Tabrizi L, Fung KY, Forster SC, Beddoe T, et al. Structural basis of a unique interferon-beta signaling axis mediated via the receptor IFNAR1. Nat Immunol. 2013;14(9):901–7. [DOI] [PubMed] [Google Scholar]
- 12.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86. [DOI] [PubMed] [Google Scholar]
- 13.Freitas MC, Uchida Y, Lassman C, Danovitch GM, Busuttil RW, Kupiec-Weglinski JW. Type I interferon pathway mediates renal ischemia/reperfusion injury. Transplantation. 2011;92(2):131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, Kupiec-Weglinski JW. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47(1):199–206. [DOI] [PubMed] [Google Scholar]
- 15.Salazar-Mather TP, Lewis CA, Biron CA. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1alpha delivery to the liver. J Clin Invest. 2002;110(3):321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohut LK, Darwiche SS, Brumfield JM, Frank AM, Billiar TR. Fixed volume or fixed pressure: a murine model of hemorrhagic shock. J Vis Exp. 2011(52). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauch I, Muller M, Decker T. The regulation of inflammation by interferons and their STATs. Jakstat. 2013;2(1):e23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, et al. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res. 2006;26(11):804–19. [DOI] [PubMed] [Google Scholar]
- 19.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desmet VJ. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis [Hepatology 1981;1:431–435]. J Hepatol. 2003;38(4):382–6. [DOI] [PubMed] [Google Scholar]
- 21.Kuncewitch M, Yang W-L, Jacob A, Khader A, Giangola M, Nicastro J, Coppa GF, Wang P. Stimulation of Wnt/beta-catenin signaling pathway with Wnt agonist reduces organ injury after hemorrhagic shock. J Trauma Acute Care Surg. 2015;78(4):793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmekel B, Karlsson SE, Linden M, Sundström C, Tegner H, Venge P. Myeloperoxidase in human lung lavage. Inflammation. 1990;14(4):447–54. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8(5):373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huys L, Van Hauwermeiren F, Dejager L, Dejonckheere E, Lienenklaus S, Weiss S, Leclercq G, Libert C. Type I interferon drives tumor necrosis factor-induced lethal shock. J Exp Med. 2009;206(9):1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L, Zhou H, Ni M, Wang X, Busuttil R, Kupiec-Weglinski J, Zhai Y. Innate Immune Regulations and Liver Ischemia-Reperfusion Injury. Transplantation. 2016;100(12):2601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonough A, Lee RV, Noor S, Lee C, Le T, Iorga M, Phillips JLH, Murphy S, Moller T, Weinstein JR. Ischemia/Reperfusion Induces Interferon-Stimulated Gene Expression in Microglia. J Neurosci. 2017;37(34):8292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo PC, Scofield BA, Yu IC, Chang FL, Ganea D, Yen JH. Interferon-beta Modulates Inflammatory Response in Cerebral Ischemia. J Am Heart Assoc. 2016;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejager L, Vandevyver S, Ballegeer M, Van Wonterghem E, An LL, Riggs J, Kolbeck R, Libert C. Pharmacological inhibition of type I interferon signaling protects mice against lethal sepsis. J Infect Dis. 2014;209(6):960–70. [DOI] [PubMed] [Google Scholar]
- 29.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EEM, Trinchieri G, Caux C, Garrone P. A type I interferon autocrine–paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201(9):1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song J, Guan M, Zhao Z, Zhang J. Type I Interferons Function as Autocrine and Paracrine Factors to Induce Autotaxin in Response to TLR Activation. PLoS One. 2015;10(8):e0136629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatesh D, Ernandez T, Rosetti F, Batal I, Cullere X, Luscinskas FW, Zhang Y, Stavrakis G, Garcia-Cardena G, Horwitz BH, et al. Endothelial TNF receptor 2 induces IRF1 transcription factor-dependent interferon-beta autocrine signaling to promote monocyte recruitment. Immunity. 2013;38(5):1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aziz M, Brenner M, Wang P. Extracellular CIRP (eCIRP) and inflammation. J Leukoc Biol. 2019; 1–14. 10.1002/JLB.3MIR1118-443R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poli C, Augusto JF, Dauve J, Adam C, Preisser L, Larochette V, Pignon P, Savina A, Blanchard S, Subra JF, et al. IL-26 Confers Proinflammatory Properties to Extracellular DNA. J Immunol. 2017;198(9):3650–61. [DOI] [PubMed] [Google Scholar]
- 34.Chen CB, Liu LS, Zhou J, Wang XP, Han M, Jiao XY, He XS, Yuan XP. Up-Regulation of HMGB1 Exacerbates Renal Ischemia-Reperfusion Injury by Stimulating Inflammatory and Immune Responses through the TLR4 Signaling Pathway in Mice. Cell Physiol Biochem. 2017;41(6):2447–60. [DOI] [PubMed] [Google Scholar]
- 35.Thomas KE, Galligan CL, Newman RD, Fish EN, Vogel SN. Contribution of interferon-beta to the murine macrophage response to the toll-like receptor 4 agonist, lipopolysaccharide. J Biol Chem. 2006;281(41):31119–30. [DOI] [PubMed] [Google Scholar]
- 36.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25(3):361–72. [DOI] [PubMed] [Google Scholar]
- 37.Siegfried A, Berchtold S, Manncke B, Deuschle E, Reber J, Ott T, Weber M, Kalinke U, Hofer MJ, Hatesuer B, et al. IFIT2 is an effector protein of type I IFN-mediated amplification of lipopolysaccharide (LPS)-induced TNF-alpha secretion and LPS-induced endotoxin shock. J Immunol. 2013;191(7):3913–21. [DOI] [PubMed] [Google Scholar]
- 38.Goritzka M, Durant LR, Pereira C, Salek-Ardakani S, Openshaw PJ, Johansson C. Alpha/beta interferon receptor signaling amplifies early proinflammatory cytokine production in the lung during respiratory syncytial virus infection. J Virol. 2014;88(11):6128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998;161(1):440–7. [PubMed] [Google Scholar]
- 40.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154(1):335–44. [PubMed] [Google Scholar]
- 41.Dorman RB, Gujral JS, Bajt ML, Farhood A, Jaeschke H. Generation and functional significance of CXC chemokines for neutrophil-induced liver injury during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2005;288(5):G880–6. [DOI] [PubMed] [Google Scholar]
- 42.Kelly-Scumpia KM, Scumpia PO, Delano MJ, Weinstein JS, Cuenca AG, Wynn JL, Moldawer LL. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010;207(2):319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193(6):713–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148(8):2423–8. [PubMed] [Google Scholar]
- 45.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187(4):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang R, Martin-Hawver L, Woodall C, Thomas A, Qureshi N, Morrison D, Van Way C 3rd. Administration of glutamine after hemorrhagic shock restores cellular energy, reduces cell apoptosis and damage, and increases survival. JPEN J Parenter Enteral Nutr. 2007;31(2):94–100. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, Yang WL, Brenner M, Wang P. Attenuation of hemorrhage-associated lung injury by adjuvant treatment with C23, an oligopeptide derived from cold-inducible RNA-binding protein. J Trauma Acute Care Surg. 2017;83(4):690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulop A, Turoczi Z, Garbaisz D, Harsanyi L, Szijarto A. Experimental models of hemorrhagic shock: a review. Eur Surg Res. 2013;50(2):57–70. [DOI] [PubMed] [Google Scholar]
- 49.Lomas-Niera JL, Perl M, Chung CS, Ayala A. Shock and hemorrhage: an overview of animal models. Shock. 2005;24 Suppl 1:33–9. [DOI] [PubMed] [Google Scholar]